Abstract
Certain essential cognitive processes require the precise temporal interplay between glutamatergic (excitatory) pyramidal cells and γ-aminobutyric acid (GABA)-releasing inhibitory interneurons in the hippocampus. Basket cells, the main class of interneurons, target pyramidal cell somata and proximal dendrites and thus are poised to modify network oscillations. Though only present in limited numbers, the impaired development of basket cells can result in changes in the hippocampal circuitry leading to neurological disorders, such as schizophrenia. The diversity of the spatial origins, neurochemical make-up, cytoarchitecture and network contributions amongst basket cells is a provocative example of interneuron heterogeneity in the hippocampus. This review discusses recent data concerned with the developmental trajectories of one subclass, the cholecystokinin-containing basket cell, and emphasizes the significance of the short-range intercellular guidance cues that have recently emerged to impact the formation and function of their inhibitory synapses.
Tibor Harkany (left) is jointly appointed as professor of Developmental Neurobiology at the Karolinska Institute (Stockholm, Sweden) and SULSA professor of Cell Biology at the University of Aberdeen (UK). After receiving his PhD from the Semmelweis Medical School in Budapest, Hungary, he worked as a post-doctoral fellow with Paul Luiten and Patrik Ernfors in the Netherlands and Sweden, respectively. Erik Keimpema (right) is a recent PhD graduate from Tibor Harkany's lab in Aberdeen who currently works as a post-doctoral fellow at the Karolinska Institute. Harkany and his colleagues investigate the molecular biology of endocannabinoid signalling in the developing brain, particularly in interneurons, with emphasis on the molecular imprint of maternal cannabis abuse in the offspring.
 |
Functional significance of basket cells in the hippocampal circuitry
Some cognitive processes, including attention and memory formation, require the precise temporal interplay between glutamatergic (excitatory) pyramidal cells and γ-aminobutyric acid (GABA)-releasing inhibitory interneurons in the hippocampus (McBain et al. 1999; Freund, 2003; Buzsaki et al. 2004; Morellini et al. 2010; Murray et al. 2011). Although vastly outnumbered by pyramidal cells, the precisely timed activity of interneurons and their innate property to entrain large principal cell ensembles are essential to set the output of the hippocampal circuitry (Freund, 2003; Freund & Katona, 2007).
Interneurons are historically considered as the ‘diverse’ cell type in the hippocampal circuitry, which have evolved to define specific network modalities through selective positioning, spike timing and input–output relationships. The presently accepted consensus to classify interneurons is based on the ‘Petilla’ terminology, which classifies GABAergic interneurons by collating their molecular, anatomical and physiological characteristics (Ascoli et al. 2008). This approach requires multiparametric analysis of the interneurons’ birth places, migratory routes, neurochemical tags, molecular make-up (Table 1), cytoarchitectural features, intrinsic membrane properties and network relationships (Pleasure et al. 2000; Anderson et al. 2002; Ascoli et al. 2008; Tricoire et al. 2011).
Table 1.
Molecular parameters of CCK- and PV-containing cells
| CCK+ small basket cell | PV+ nest/large basket cell | ||
|---|---|---|---|
| References | References | ||
| Receptors | Receptors | ||
| 5-HT3A | Morales & Bloom, 1997 | M2 muscarinic ACh | Hajos et al. 1998 |
| Nicotinic ACh | Porter et al. 1999 | μ-Opioid | Drake & Milner, 2002 |
| CB1R | Katona et al. 1999 | ||
| Oestrogen receptor α | Hart et al. 2007 | ||
| GABAB | Sloviter et al. 1999 | ||
| Peptides | Peptides | ||
| VIP | Kawaguchi & Kubota, 1997 | NPY (subset) | Wang et al. 2002 |
| CRF | Kubota et al. 2011 | ||
| Endocannabinoids | Endocannabinoids | ||
| MGL | Gulyas et al. 2004 | MGL | Gulyas et al. 2004 |
| Vesicular transporters | K+ channels | ||
| VGLUT3 | Somogyi et al. 2004 | Kv3.1b | Sekirnjak et al. 1997 |
| Kv3.2 | Hernandez-Pineda et al. 1999 | ||
| Kv3.3 | Chang et al. 2007 | ||
Using these criteria, at least 22 subtypes of GABAergic interneurons are presently discerned in the hippocampus alone. The term ‘basket cell’ alludes to the axonal field of these cells being restricted to the soma and proximal dendrites of principal cells, with their synapses enwrapping excitatory perikarya in the pyramidal layer (Gulyas et al. 1999; Papp et al. 2001). This synapse distribution confers particular power to tune pyramidal cell excitation and network oscillations (Freund, 2003). Basket cells can be divided into neurochemically and functionally distinct classes, the majority expressing parvalbumin (PV) (Nomura et al. 1997), a cytosolic fast Ca2+ buffer implicated in the generation and maintenance of high-frequency action potential trains known as ‘fast-spiking’ (>100 Hz). A second class expresses cholecystokinin (CCK), a peptide hormone, and exhibits irregular discharge patterns unusually exceeding 50 Hz (Freund & Buzsaki, 1996; Kawaguchi & Kondo, 2002; Wang et al. 2002). A division of function between PV and CCK basket cells has been proposed with PV+ interneurons controlling the rhythm of network oscillations (‘clockwork precision’), while CCK+ cells sculpt the efficacy of information flow and encoding by fine-tuning network chronosynchrony (Freund, 2003).
Ever since Ramon y Cajal's first description of local-circuit interneurons, the molecular identity and network contributions of GABAergic cells have been studied in postnatal cortical networks. It is only through recent advances of molecular genetics and cell biology that we have come to appreciate the interneurons’ different origins, migratory behaviours and cytoarchitectural features (Xu et al. 2004; Butt et al. 2005; Miyoshi et al. 2010; Tricoire et al. 2011). Although present in limited numbers, impaired development of GABAergic interneurons in the fetal cerebrum can have permanent impact on the hippocampal circuitry (Peters & Kara, 1985), precipitating neurological disorders (Di Cristo, 2007). This review discusses recent data concerned with the developmental trajectories of CCK+ basket cells, and emphasizes the significance of recently identified short-range intercellular guidance cues that impact the formation and function of GABAergic synapses.
The historical approach: retrospective neurochemical analysis of CCK+ basket cells
Do CCK+ basket cells exhibit unique cytoarchitectural and molecular signalling properties that distinguish them from other basket cells? This is an important question to address to gain insights regarding their circuit parameters, response patterns, as well as development and integration into hippocampal neuronal networks (Table 1). Electrophysiology studies combined with post hoc cellular neuroanatomy were the preferred approach to identify unique characteristics of basket cells. However, the advent of molecular array technologies and genetic tagging opened new horizons in the understanding of the birthplaces, migratory routes and terminal differentiation programs of various interneuron subtypes, facilitating the resolution of ambiguities regarding molecular identities.
CCK is the primary marker to classify this group of basket cells (Fig. 1). Recent findings have revealed dual pathways downstream from CCK receptor signalling in the two basket cell populations (Foldy et al. 2007). First, CCK inhibits GABA release from CCK+ basket cells through CCK2 receptors. This mechanism is dependent on type 1 cannabinoid receptor (CB1R) activation (see below) but independent of GABA release from other interneurons (Lee & Soltesz, 2011). Second, CCK2 receptors on PV+ basket cells respond to CCK and trigger Ca2+ release from intracellular Ca2+ stores. This event leads to depolarization by the activation of a non-selective cationic conductance enhancing GABA release (Lee et al. 2011). Therefore, the net effect is to shift inhibition from CCK+ to PV+ basket cells, leaving CCK signalling poised to modulate complex signalling networks by modifying inhibitory and excitatory signals to fine-tune precise firing patterns.
Figure 1. Characteristic molecular markers of CCK+ interneurons.
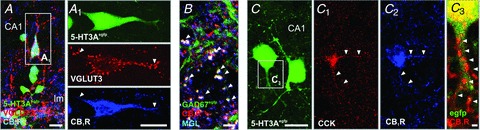
A, a subpopulation of 5-HT3A-EGFP+ cells in the hippocampus is immunoreactive for VGLUT3 and CB1Rs. A1, arrowheads point to VGLUT3/CB1R+ processes. B, CB1R+/GAD67+ boutons contain the 2-AG degrading enzyme MGL (arrowheads), C–C3, CCK+ interneurons express 5-HT3ARs and CB1Rs. Arrowheads indicate CB1R+ structures. Scale bars: 20 μm (A, A1, C), 5 μm (B) and 1 μm (C3).
An intracellular feature of hippocampal CCK+ basket cells is that a subset contains vasoactive intestinal peptide (VIP) (Freund & Buzsaki, 1996). CCK+–VIP+ basket cells can co-express corticotropin-releasing factor (CRF) (Kubota et al. 2011). Once released, CRF and CCK depolarize pyramidal cells through CRF-1 and CCK2 receptors, respectively (Gallopin et al. 2006). Conspicuously, CCK+ basket cells express vesicular glutamate transporter 3 (VGLUT3; Fig. 1) (Harkany et al. 2004; Somogyi et al. 2004). Although the precise role of VGLUT3 in basket cells remains unknown, experiments using genetic deletion of VGLUT3 indicate that glutamate co-transmission at GABA/glycinergic synapses is necessary for the developing inhibitory circuit in the auditory system (Noh et al. 2010).
CCK+ interneurons also exhibit a unique assembly of cell-surface receptors. These include postsynaptic α4/β2 nicotinic acetylcholine (nAChR) (Porter et al. 1999; Ferezou et al. 2002; Bell et al. 2011), ionotropic 5-HT3A serotonin (Morales & Bloom, 1997; Vucurovic et al. 2010) and oestrogen α receptors (Hart et al. 2007), the latter being associated with clusters of synaptic vesicles in perisomatic boutons. However, the specific role of oestrogen receptors on vesicles remains unclear. Serotonin and cholinergic signalling via 5-HT3A and α4-nAChR, respectively, elicit fast synaptic excitation of CCK+–VIP+ basket cells (Ferezou et al. 2002; Varga et al. 2009). In contrast, oestrogen α receptor signalling limits presynaptic neurotransmitter release by reducing the likelihood of GABA-laden synaptic vesicle docking (Hart et al. 2007).
CCK+ interneurons express high levels of the CB1R (Katona et al. 1999; Marsicano & Lutz, 1999; Tsou et al. 1999). CB1Rs, a primarily Gi/o protein-coupled GPCR receptor subclass, are named after their ability to bind to and transduce the psychotropic effect of Δ9-tetrahydrocannabinol (THC), the major phytocannabinoid from cannabis (Devane et al. 1988; Matsuda et al. 1990). The physiological impact of agonist activation of CB1Rs is profound since these receptors are targeted to the presynaptic terminals of many neurons (including CCK+ basket cells; Fig. 1) where they generally limit neurotransmitter release (Wilson & Nicoll, 2001; Kano et al. 2009; Regehr et al. 2009). CB1R activation under physiological conditions is achieved through the ‘on-demand’ liberation of endogenous cannabinoids (‘endocannabinoids’), such as 2-arachidonoylglycerol (2-AG) (Mechoulam et al. 1995) and anandamide (Devane et al. 1992), from postsynaptic neurons. Endocannabinoid signalling belongs to the emerging family of signalling systems that mediate feedback control of neurotransmitter release through retrograde action: for 2-AG, ligand synthesis at the postsynapse occurs through diacylgycerol lipases α/β (DAGLα/β) in pyramidal cells (Bisogno et al. 2003). However, CCK+ interneurons lack DAGLα/βin vivo during development and adulthood identifying endocannabinoids as target-derived cues (Yoshida et al. 2011). Having traversed the synaptic cleft, 2-AG engages presynaptic CB1Rs. Endocannabinoid inactivation operates both at postsynaptic and presynaptic loci through the segregated action of the serine hydrolase ABHD6 (Marrs et al. 2010) and monoacylglycerol lipase (MGL) (Dinh et al. 2002), respectively. CCK+ basket cells express and co-target MGL with CB1Rs to their presynapses (Gulyas et al. 2004; Yoshida et al. 2011). Furthermore, cyclooxygenase-2 (COX-2) has been demonstrated to degrade 2-AG (Kozak et al. 2000) and its inhibition prolongs depolarization-induced suppression of inhibition (DSI) in a CB1R-dependent manner affirming that COX-2 is involved in CB1R-mediated retrograde signalling (Kim & Alger, 2004; Straiker & Mackie, 2009) (Fig. 3).
Figure 3. Enzyme-dependent divergent time courses for DSI- and 2-AG-mediated inhibition in DSIfast- and DSIslow-expressing cultured interneurons.
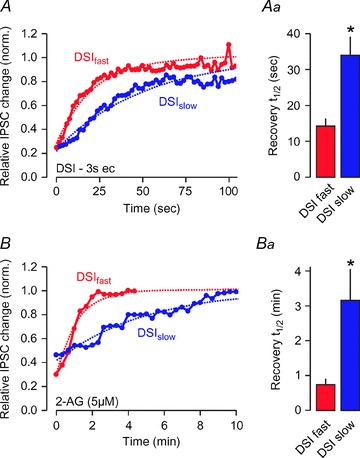
A, duration of DSI (3 s depolarization) in DSIfast (red) and DSIslow (blue) neurons, as a result of MGL acting alone (DSIslow) or in combination with COX-2 (DSIfast). Aa, bar graph shows t1/2 values with 99% confidence intervals. B, duration of 2-AG-mediated inhibition in DSIfastvs. DSIslow neurons. Ba, bar graph shows t1/2 values with 99% confidence intervals. *99% confidence intervals do not overlap. Reprinted from Neuroscience163, Straiker & Mackie (2009), Cannabinoid signaling in inhibitory autaptic hippocampal neurons, pp. 190–201, Copyright 2009, with permission from Elsevier.
CCK+ basket cells also express presynaptic GABAB receptors (Sloviter et al. 1999). Although the role of GABAB receptors remains largely unclear, a coupling to N-type Ca2+ channels (Wilson et al. 2001) in a Ca2+-dependent manner similar to CB1Rs (Neu et al. 2007) has been reported.
Should I stay or should I go: the developmental program of CCK interneurons
Understanding the mechanisms driving the development of any neuron and its network requires detailed knowledge about the genetic and environmental history of the cell. This is particularly challenging for telencephalic interneurons since it is impossible to deduce the precise birth location of a neuron based on where it is found in the adult structure. Despite these challenges, the origins and genetic programs involved in the developmental control of neocortical interneuron differentiation are beginning to be understood (Batista-Brito & Fishell, 2009). In contrast to pyramidal cells, interneurons migrate long distances tangentially to reach their final destinations by and after birth (Fig. 2). The medial ganglionic eminence (MGE) was long thought to give rise to around 85–90% of all interneurons (Nery et al. 2002). However, recent data suggest that as many as 30–40% of cortical interneurons are instead derived from the caudal ganglionic eminence (CGE, Fig. 2) (Lee et al. 2010a; Miyoshi et al. 2010). The phenotypes of CGE-derived interneurons are non-overlapping with their MGE-derived counterparts, and these neurons are generally born later during development (Butt et al. 2005; Miyoshi et al. 2010; Tricoire et al. 2011).
Figure 2. Molecular cues orchestrating CCK+ basket cell development.

Overview of the molecular cues involved in the migration and differentiation of basket cells towards the neocortex and the hippocampus. A and B, basket cells born in the CGE migrate through the marginal zone (MZ) or the intermediate zone (IZ) of the cerebral cortex (Manent et al. 2005; Morozov et al. 2009) to find their final positions either in the neocortex (A) or the hippocampus (B). Green trajectories indicate the migratory routes of prospective GABA interneurons. Molecular cues for each developmental state are noted. Question marks query the specificity of cues during CCK+ interneuron development.
CCK+ basket cells are CGE derived
All cortical CGE-derived interneurons express 5-HT3A receptors, as well as respond to nicotinergic stimulation (Lee et al. 2010a). Therefore, the use of a 5-HT3ABAC-EGFP reporter mouse line allowed detailed analyses showing that all VIP+ cells are born in the CGE (Lee et al. 2010a; Vucurovic et al. 2010). Some of these deep layer VIP cells were shown to co-express CCK. A recent genetic fate mapping study of the hippocampus using GAD65-EGFP and Mash1CreER mice substantiated these findings by showing that CGE-derived cells included most CCK+ interneurons (Tricoire et al. 2011). However, a definitive conclusion about the origin of the entire cortical/hippocampal CCK population remains elusive for a number of reasons: (1) the GAD65-EGFP and Mash1CreER transgenic models suffer from an incomplete labelling of the entire CGE-derived neuron population (Miyoshi et al. 2010; Tricoire et al. 2011). (2) The 5-HT3ABAC-EGFP mouse has not yet been shown to be as specific for CGE-derived cells in the hippocampus as it is in the cortex. (3) In contrast to available immunohistochemical data, a stringent genetic design found a higher number of GFP-tagged cells in superficial cortical laminae, when crossing CCK-IRES-Cre (Taniguchi et al. 2011) with a Dlx5/6-Flpe and a RCE-dual reporter (Miyoshi et al. 2010), which expresses EGFP only upon coincident Flpe- and Cre-driven recombination. This suggests that either VIP− CCK cells escaped detection in earlier studies or there is a population of interneurons transiently expressing CCK during development.
Genetic regulation of interneuron specification
A number of transcription factors are crucial for general cortical interneuron development. These include the Dlx gene family and the proneural gene Mash1 (Long et al. 2009). Mice lacking both Dlx1 and Dlx2 have a severe loss of tangentially migrating interneurons (Cobos et al. 2007). Similarly, Mash1 null mice have a marked loss of GABAergic cells in the neocortex and hippocampus (Anderson et al. 1997; Casarosa et al. 1999). The specific genetic program that controls the specification and generation of interneurons derived from the MGE, and involving the sequential activation of Nkx2-1 (initial specification) followed by Lhx6 and Sox6 (migration and differentiation) is beginning to be elucidated (Liodis et al. 2007; Du et al. 2008; Azim et al. 2009; Batista-Brito et al. 2009).
The search is still on for a CGE-specific factor equivalent to Nkx2-1. Such a transcription factor is probably repressed by Nkx2-1 since removal of Nkx2-1 after the final cell division of interneuron progenitors dictates the acquisition of a full CGE phenotype in many MGE-derived cells (Butt et al. 2008). One gene family that is preferentially expressed in the CGE during development includes the Coup-TF1/2 genes (Kanatani et al. 2008). Accordingly, Coup-TF1 removal specifically in interneurons reduces the amount of CGE-derived VIP+ and CR+ interneurons (Lodato et al. 2011). Coup-TF2 has also been implicated in regulating tangential migration towards the cerebral cortex, and is expressed in a subset of CB1R+, as well as CB1R−, hippocampal cells (Kanatani et al. 2008; Fuentealba et al. 2010; Antypa et al. 2011). Although the hunt for a CGE-specific ‘master regulator’ might be rewarding, the hypothesis that CGE fate may be a ‘default state’ of interneurons derived from the ganglionic eminences also seems plausible.
Developmental cues for CCK+ basket cells
Although cell-autonomous mechanisms can drive the initial engagement of CGE-derived basket cells in migratory behaviours and cytoarchitectural differentiation, short-range paracrine signals will provide critical cues to instruct the directionality of cell movement, synapse formation and functional integration into neuronal networks (Fig. 2).
Classical neurotransmitters impact interneuron development. In particular, serotonin has been identified as a cue instructing interneuron migration, differentiation and synaptogenesis (Lauder, 1990). Inhibition of serotonin synthesis in pregnant rats leads to a decrease in the migration and terminal differentiation of CCK+ interneurons in affected offspring (Vitalis et al. 2007). A gain-of-function analysis using SLC6A4 knockouts in which serotonin reuptake is blocked (‘hyperserotonergic mouse’) demonstrated an increase of CGE-derived GAD65+ interneurons in the cerebral cortex (Riccio et al. 2009). Data from organotypic slice systems suggest that serotonin's concentration is critical to define the directionality of its action: excessive bath-applied serotonin decreased interneuron migration. This effect was mediated by 5-HT6 but not 5-HT3A receptors (Riccio et al. 2009). Although its direct impact on CCK+ interneurons is less well understood, dopamine D1 receptor activation promotes interneuron migration from both the MGE and CGE. In contrast, dopamine D2 receptors (D2Rs) limit the tangential migration of interneurons (Crandall et al. 2007). Similarly, disrupted dopamine signalling by either inhibition of tyrosine hydroxylase or stimulation of D2Rs limits the size of the Dlx5a/6aIG-GFP+ GABAergic interneuron pool in zebrafish (Souza et al. 2011).
GABA signalling is thought to be excitatory during embryogenesis and early postnatal development (Ben-Ari, 2002). The conversion to an inhibitory mode of GABA action relies on the coincident and opposite expressional regulation of the neuron-specific K+–Cl− co-transporter (KCC2, increase) and the Na+–K+–Cl− co-transporter (NKCC1, decrease), resulting in a significant decrease of the intracellular Cl− concentrations (Liu et al. 2006). Manipulations that limit the loss of NKCC1 prevent the developmental switch of the Cl− gradient (Liu et al. 2006). Cholinergic neurotransmission through nAChRs is needed for the developmental switch. Accordingly, α7-nAChR−/− mice retain high NKCC1 and low KCC2 levels, precluding inhibitory GABA neurotransmission (Liu et al. 2006) associated with morphological irregularities in hippocampal neurons (Liu et al. 2007). Since CCK+ basket cells express both α7-nAChRs and GABAA receptors, it is plausible to assume that nicotine stimulation (e.g. maternal tobacco smoking) could compromise the morphological differentiation – particularly synaptogenesis – of this cell type. Short-range GABA signals, acting on GABAA receptors, are also required to maintain tangential migration through leading process elongation in vivo (Manent et al. 2005).
Neurotrophins are indispensable for interneuron development. Brain-derived neurotrophic factor (BDNF) powerfully regulates the morphological and neurochemical differentiation of GABAergic interneurons (Marty et al. 1996; Berghuis et al. 2004). GABA signalling acts upstream to BDNF by repressing BDNF synthesis (Marty et al. 1996) to decrease neurite outgrowth. Yet other signalling cassettes can hijack (‘trans-activate’) the tropomyosin-related kinase B (TrkB) receptor on interneurons in the absence of its cognate ligand. Endocannabinoids are one such class of signalling molecules using TrkB receptors to regulate CCK+ interneuron migration (Berghuis et al. 2005). The idea that endocannabinoid signalling via CB1Rs is involved in interneuron development is reinforced by several findings: firstly, by the ability of THC to induce redistribution of hippocampal CCK+ interneurons in utero (Berghuis et al. 2005). Secondly, acute exposure of the growth cones of CB1R+/CCK+ interneurons to endocannabinoid microgradients evokes chemorepulsive turning or collapsing responses (Berghuis et al. 2007). Thirdly, CB1R activation inhibits neurite outgrowth, and abolishes BDNF-induced axonal growth (Berghuis et al. 2005). Cumulatively, these data suggest an antagonistic interplay between endocannabinoid and neurotrophin signalling cassettes in the refinement of interneuron morphology and synaptic wiring. Interestingly, these effects prevail once the synapse forms with remodelled endocannabinoid and BDNF signalling networks participating in negative and positive retrograde feedback loops to control synaptic efficacy, respectively.
Endocannabinoid control of synaptic plasticity
Theta oscillations in the hippocampus occur during exploration and rapid eye movement sleep and are involved in place finding and learning and memory (O’Keefe & Nadel, 1978; Buzsaki, 2002). During these oscillations, pyramidal cells transmit information. PV+ basket cells synchronize the rhythm of the network, while endocannabinoid-sensitive CCK+ basket cells function as fine-tuning devices (Freund, 2003). In anesthetized rodents, CCK+ interneurons fire on the ascending phase of the theta wave, at the moment when hippocampal place cells become activated (Klausberger et al. 2005). In contrast, PV+ basket cells fire on the descending phase (Klausberger et al. 2003). As we discussed above, CCK+ basket cells play a critical role in feed-forward inhibition by releasing CCK, resulting in the enhancement of GABA release from PV+ interneurons (Foldy et al. 2007). Their inhibition of pyramidal cells ceases upon activation of CB1Rs on their presynaptic terminals, forming a spatial focus of activity. Coincidentally, they maintain inhibitory control over resting place cells through entrainment of PV+ interneurons (Carlson et al. 2002; Chevaleyre & Castillo, 2004; Klausberger et al. 2005).
Amongst all hippocampal neurons, CCK+ interneurons express the highest known levels of CB1Rs (Katona et al. 1999). This gives rise to diverse mechanisms of endocannabinoid-mediated short- and long-term synaptic plasticity at their synaptic inputs onto pyramidal neurons, including depolarization-induced suppression of inhibition (DSI) (Pitler & Alger, 1992; Ohno-Shosaku et al. 2001; Wilson & Nicoll, 2002), metabotropic suppression of inhibition (MSI) (Varma et al. 2001; Kim et al. 2002) and long-term depression (iLTD) (Chevaleyre & Castillo, 2003; Chevaleyre et al. 2007).
Of these, the inhibitory perisomatic inputs deriving from CCK+ basket cells are the best characterized. The CCK+–CA1 pyramidal synapse was one of the first sites shown to express DSI (Pitler & Alger, 1992; Ohno-Shosaku et al. 2001; Wilson & Nicoll, 2002). DSI is a form of synaptic plasticity that is induced postsynaptically, but acts presynaptically via a messenger traversing the synaptic cleft in a direction opposite to that of the major neurotransmitter (‘retrograde messenger’). Accordingly, 2-AG, the likely retrograde messenger (Gao et al. 2010; Tanimura et al. 2010), is produced in a Ca2+-dependent manner via postsynaptic activation of DAGLα, which cleaves the DAG precursor into 2-AG. The release of lipophilic 2-AG is facilitated, yet non-vesicular, contrasting classical vesicle-dependent neurotransmitter release (Neu et al. 2007). Once liberated, 2-AG (and other endocannabinoids) activates presynaptic CB1Rs, which subsequently reduce neurotransmitter release by inhibiting N-type Ca2+ channels (Wilson et al. 2001). DSI can last for tens of seconds. However, its duration is chiefly determined by the expression of one or more pre- or post-synaptic 2-AG-degrading enzymes (Kim & Alger, 2004; Hashimotodani et al. 2007; Straiker et al. 2009), which can act in concert to terminate 2-AG signalling. An example of enzymatic cooperativity is shown in Fig. 3, where the two temporally distinct forms of DSI (referred to as DSIfast and DSIslow) – observed in cultured neurons – are determined by the complement of MGL and COX-2 available to break down 2-AG (Straiker & Mackie, 2009).
Endocannabinoid signalling is not restricted to CCK+ basket cell to CA1 pyramidal neuron synapses. For instance, CCK+ basket cell synapses also target other CCK+ or PV+ basket cells (Karson et al. 2009). Although studying interneuron–interneuron coupling is challenging, evidence for domain-specific plasticity of outputs has begun to appear (Lee et al. 2010b), including endocannabinoid plasticity at three inputs/outputs of CCK+ Schaffer collateral-associated interneurons (Ali & Todorova, 2010).
Whilst dendritic spines of pyramidal cells are the consensus sites for DAGL's subcellular accumulation in the neocortex and hippocampus (Yoshida et al. 2006), synaptically connected CCK+ basket cells can express endocannabinoid-mediated DSI in the apparent absence of DAGLα (Daw et al. 2009). This phenomenon could be explained by a complex metabolic configuration of synaptic plasticity through the release of other endocannabinoids, such as anandamide (Ali, 2007; Puente et al. 2011), or by other forms of endocannabinoid actions like the direct potentiation of postsynaptic GABAA receptors by 2-AG (Sigel et al. 2011). Given that at least ∼40% of cultured hippocampal interneurons express the machinery to sustain endocannabinoid signalling in response to depolarization (Straiker & Mackie, 2009), this suggests that many CA1 interneurons may be more than just targets of endocannabinoid signalling.
Perisomatic endocannabinoid-mediated plasticity by CCK+ basket cells is well positioned to regulate the output of CA1 pyramidal neurons. Their role may be that of an integrator of assorted weaker inputs to support feedback inhibition at key sites of pyramidal neuron output regulation (Glickfeld & Scanziani, 2006). However, endocannabinoid-mediated synaptic plasticity is itself under modulation: endocannabinoid inhibition can be overridden by a high (>20Hz) firing rate in CCK+ basket cells (Foldy et al. 2006). In addition, indirect evidence suggests that postsynaptic CCK receptors can enhance endocannabinoid release (Foldy et al. 2007).
Conclusions: disease implications
This review provides a concise summary of the molecular and network features that make CCK+ basket cells unique, and indispensable for the proper function of hippocampal neuronal networks. If a specific class of interneurons is required to maintain a form of control over the hippocampal circuitry then its loss should manifest in altered behaviours or neuropsychiatric illness.
Endocannabinoids have also been suggested to play a role in the prevention of epileptic activity, particularly in the neonatal period when they can act as a substitute for inhibitory GABA (note that GABA is excitatory during this period; Ben-Ari, 2002). In the immature hippocampus, CB1R antagonism leads to epileptic discharges, while receptor stimulation reduces network activity (Bernard et al. 2005). However, and in accord with the role of endocannabinoids during axon guidance (Berghuis et al. 2007; Mulder et al. 2008; Keimpema et al. 2010), prenatal (‘ectopic’) stimulation of CB1Rs by full agonists results in permanent modifications of the hippocampal circuitry such that decreased glutamatergic excitation persists in the offspring (Mereu et al. 2003). Therefore, CB1R signalling appears unexpectedly fundamental for the proper development and function of neuronal networks.
Another compelling example of this lies with the molecular pathogenesis of schizophrenia. BDNF and TrkB expression are decreased in schizophrenic patients (Hashimoto et al. 2005). In addition, CCK levels are also reduced in schizophrenics (Bachus et al. 1997; Fung et al. 2010). Since BDNF influences the differentiation of CCK+ interneurons (Marty et al. 1996; Berghuis et al. 2005), the decrease in BDNF, together with a decrease in CCK content, argues for the loss of CCK+ interneurons. This hypothesis is supported by the coincident decrease of CB1R and CCK mRNAs in schizophrenia (Eggan et al. 2008). It is interesting to note that these correlative changes are not a consequence of treating the psychosis but inherent to the disease itself (Bachus et al. 1997; Eggan et al. 2008). In contrast, higher CCK (Bachus et al. 1997) and CB1R levels (Hungund et al. 2004) have been reported in suicide victims, suggesting an increased potential for modulation of GABAergic neurotransmission. These findings suggest that CCK+ basket cell functions underlie fundamental behavioural and cognitive traits, and that their dysfunction can precipitate devastating neuropsychiatric symptoms.
An emerging consensus is that most, if not all, neuropsychiatric disorders have developmental origins. A developmental component is particularly well characterized in schizophrenia, and centres around the misrouting of interneurons and inappropriate endocannabinoid signalling (Di Cristo, 2007). Further studies focusing on the birth, migration, differentiation and functions of defined subsets of interneurons integrating into the hippocampal circuitry will therefore be essential to drive the development of successful therapeutic interventions.
Acknowledgments
The authors thank C.J. McBain for his invitation to contribute to the symposium ‘Inhibitory neuron ‘basket cells’: from circuit function to disruption’, and Y. Yanagawa for GAD67-egfp mice. This work was supported by the Scottish Universities Life Science Alliance (SULSA; T.H.), European Commission (HEALTH-F2–2007-201159; T.H.), National Institutes of Health (DA023214, T.H.; DA021696, DA011322, K.M.) and the Swedish Research Council (T.H., J.H.-L.).
Glossary
Abbreviations
- 2-AG
2-arachidonoylglycerol
- ABHD6
α-β hydrolase domain 6
- BDNF
brain-derived neurotrophic factor
- CB1R
type 1 cannabinoid receptor
- CCK
cholecystokinin
- CGE
caudal ganglionic eminence
- COX-2
cyclooxygenase-2
- CRF
corticotropin releasing factor
- ctx
cortex
- D2R
dopamine receptor D2
- DAGLα
diacylglycerol lipase α
- DSI
depolarization-induced suppression of inhibition
- GABA
γ-aminobutyric acid
- hip
hippocampus
- iLTD
long-term depression of inhibition
- IZ
intermediate zone
- LGE
lateral ganglionic eminence
- MGE
medial ganglionic eminence
- MGL
monoacylglycerol lipase
- MSI
metabotropic suppression of inhibition
- MZ
marginal zone
- nAChR
nicotinic acetylcholine receptor
- NPY
neuropeptide Y
- PV
parvalbumin
- SOM
somatostatin
- TrkB
tropomyosin-related receptor kinase B
- THC
tetrahydrocannabinol
- VGLUT3
vesicular glutamate transporter 3
- VIP
vasoactive intestinal peptide
- VZ
ventricular zone
References
- Ali AB. Presynaptic Inhibition of GABAA receptor-mediated unitary IPSPs by cannabinoid receptors at synapses between CCK-positive interneurons in rat hippocampus. J Neurophysiol. 2007;98:861–869. doi: 10.1152/jn.00156.2007. [DOI] [PubMed] [Google Scholar]
- Ali AB, Todorova M. Asynchronous release of GABA via tonic cannabinoid receptor activation at identified interneuron synapses in rat CA1. Eur J Neurosci. 2010;31:1196–1207. doi: 10.1111/j.1460-9568.2010.07165.x. [DOI] [PubMed] [Google Scholar]
- Anderson SA, Eisenstat DD, Shi L, Rubenstein JL. Interneuron migration from basal forebrain to neocortex: dependence on Dlx genes. Science. 1997;278:474–476. doi: 10.1126/science.278.5337.474. [DOI] [PubMed] [Google Scholar]
- Anderson SA, Kaznowski CE, Horn C, Rubenstein JL, McConnell SK. Distinct origins of neocortical projection neurons and interneurons in vivo. Cereb Cortex. 2002;12:702–709. doi: 10.1093/cercor/12.7.702. [DOI] [PubMed] [Google Scholar]
- Antypa M, Faux C, Eichele G, Parnavelas JG, Andrews WD. Differential gene expression in migratory streams of cortical interneurons. Eur J Neurosci. 2011;34:1584–1594. doi: 10.1111/j.1460-9568.2011.07896.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ascoli GA, Alonso-Nanclares L, Anderson SA, Barrionuevo G, Benavides-Piccione R, Burkhalter A, et al. Petilla terminology: nomenclature of features of GABAergic interneurons of the cerebral cortex. Nat Rev Neurosci. 2008;9:557–568. doi: 10.1038/nrn2402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Azim E, Jabaudon D, Fame RM, Macklis JD. SOX6 controls dorsal progenitor identity and interneuron diversity during neocortical development. Nat Neurosci. 2009;12:1238–1247. doi: 10.1038/nn.2387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bachus SE, Hyde TM, Herman MM, Egan MF, Kleinman JE. Abnormal cholecystokinin mRNA levels in entorhinal cortex of schizophrenics. J Psychiatr Res. 1997;31:233–256. doi: 10.1016/s0022-3956(96)00041-6. [DOI] [PubMed] [Google Scholar]
- Batista-Brito R, Fishell G. The developmental integration of cortical interneurons into a functional network. Curr Top Dev Biol. 2009;87:81–118. doi: 10.1016/S0070-2153(09)01203-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Batista-Brito R, Rossignol E, Hjerling-Leffler J, Denaxa M, Wegner M, Lefebvre V, et al. The cell-intrinsic requirement of Sox6 for cortical interneuron development. Neuron. 2009;63:466–481. doi: 10.1016/j.neuron.2009.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bell KA, Shim H, Chen CK, McQuiston AR. Nicotinic excitatory postsynaptic potentials in hippocampal CA1 interneurons are predominantly mediated by nicotinic receptors that contain α4 and β2 subunits. Neuropharmacology. 2011;61:1379–1388. doi: 10.1016/j.neuropharm.2011.08.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ben-Ari Y. Excitatory actions of GABA during development: the nature of the nurture. Nat Rev Neurosci. 2002;3:728–739. doi: 10.1038/nrn920. [DOI] [PubMed] [Google Scholar]
- Berghuis P, Dobszay MB, Sousa KM, Schulte G, Mager PP, Hartig W, et al. Brain-derived neurotrophic factor controls functional differentiation and microcircuit formation of selectively isolated fast-spiking GABAergic interneurons. Eur J Neurosci. 2004;20:1290–1306. doi: 10.1111/j.1460-9568.2004.03561.x. [DOI] [PubMed] [Google Scholar]
- Berghuis P, Dobszay MB, Wang X, Spano S, Ledda F, Sousa KM, et al. Endocannabinoids regulate interneuron migration and morphogenesis by transactivating the TrkB receptor. Proc Natl Acad Sci U S A. 2005;102:19115–19120. doi: 10.1073/pnas.0509494102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berghuis P, Rajnicek AM, Morozov YM, Ross RA, Mulder J, Urban GM, et al. Hardwiring the brain: endocannabinoids shape neuronal connectivity. Science. 2007;316:1212–1216. doi: 10.1126/science.1137406. [DOI] [PubMed] [Google Scholar]
- Bernard C, Milh M, Morozov YM, Ben Ari Y, Freund TF, Gozlan H. Altering cannabinoid signaling during development disrupts neuronal activity. Proc Natl Acad Sci U S A. 2005;102:9388–9393. doi: 10.1073/pnas.0409641102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bisogno T, Howell F, Williams G, Minassi A, Cascio MG, Ligresti A, et al. Cloning of the first sn1-DAG lipases points to the spatial and temporal regulation of endocannabinoid signaling in the brain. J Cell Biol. 2003;163:463–468. doi: 10.1083/jcb.200305129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butt SJ, Fuccillo M, Nery S, Noctor S, Kriegstein A, Corbin JG, Fishell G. The temporal and spatial origins of cortical interneurons predict their physiological subtype. Neuron. 2005;48:591–604. doi: 10.1016/j.neuron.2005.09.034. [DOI] [PubMed] [Google Scholar]
- Butt SJ, Sousa VH, Fuccillo MV, Hjerling-Leffler J, Miyoshi G, Kimura S, Fishell G. The requirement of Nkx2-1 in the temporal specification of cortical interneuron subtypes. Neuron. 2008;59:722–732. doi: 10.1016/j.neuron.2008.07.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buzsaki G. Theta oscillations in the hippocampus. Neuron. 2002;33:325–340. doi: 10.1016/s0896-6273(02)00586-x. [DOI] [PubMed] [Google Scholar]
- Buzsaki G, Geisler C, Henze DA, Wang XJ. Interneuron diversity series: circuit complexity and axon wiring economy of cortical interneurons. Trends Neurosci. 2004;27:186–193. doi: 10.1016/j.tins.2004.02.007. [DOI] [PubMed] [Google Scholar]
- Carlson G, Wang Y, Alger BE. Endocannabinoids facilitate the induction of LTP in the hippocampus. Nat Neurosci. 2002;5:723–724. doi: 10.1038/nn879. [DOI] [PubMed] [Google Scholar]
- Casarosa S, Fode C, Guillemot F. Mash1 regulates neurogenesis in the ventral telencephalon. Development. 1999;126:525–534. doi: 10.1242/dev.126.3.525. [DOI] [PubMed] [Google Scholar]
- Chang SY, Zagha E, Kwon ES, Ozaita A, Bobik M, Martone ME, et al. Distribution of Kv3.3 potassium channel subunits in distinct neuronal populations of mouse brain. J Comp Neurol. 2007;502:953–972. doi: 10.1002/cne.21353. [DOI] [PubMed] [Google Scholar]
- Chevaleyre V, Castillo PE. Heterosynaptic LTD of hippocampal GABAergic synapses: a novel role of endocannabinoids in regulating excitability. Neuron. 2003;38:461–472. doi: 10.1016/s0896-6273(03)00235-6. [DOI] [PubMed] [Google Scholar]
- Chevaleyre V, Castillo PE. Endocannabinoid-mediated metaplasticity in the hippocampus. Neuron. 2004;43:871–881. doi: 10.1016/j.neuron.2004.08.036. [DOI] [PubMed] [Google Scholar]
- Chevaleyre V, Heifets BD, Kaeser PS, Sudhof TC, Castillo PE. Endocannabinoid-mediated long-term plasticity requires cAMP/PKA signaling and RIM1α. Neuron. 2007;54:801–812. doi: 10.1016/j.neuron.2007.05.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cobos I, Borello U, Rubenstein JL. Dlx transcription factors promote migration through repression of axon and dendrite growth. Neuron. 2007;54:873–888. doi: 10.1016/j.neuron.2007.05.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crandall JE, McCarthy DM, Araki KY, Sims JR, Ren JQ, Bhide PG. Dopamine receptor activation modulates GABA neuron migration from the basal forebrain to the cerebral cortex. J Neurosci. 2007;27:3813–3822. doi: 10.1523/JNEUROSCI.5124-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daw MI, Tricoire L, Erdelyi F, Szabo G, McBain CJ. Asynchronous transmitter release from cholecystokinin-containing inhibitory interneurons is widespread and target-cell independent. J Neurosci. 2009;29:11112–11122. doi: 10.1523/JNEUROSCI.5760-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Devane WA, Dysarz FA, III, Johnson MR, Melvin LS, Howlett AC. Determination and characterization of a cannabinoid receptor in rat brain. Mol Pharmacol. 1988;34:605–613. [PubMed] [Google Scholar]
- Devane WA, Hanus L, Breuer A, Pertwee RG, Stevenson LA, Griffin G, et al. Isolation and structure of a brain constituent that binds to the cannabinoid receptor. Science. 1992;258:1946–1949. doi: 10.1126/science.1470919. [DOI] [PubMed] [Google Scholar]
- Di Cristo G. Development of cortical GABAergic circuits and its implications for neurodevelopmental disorders. Clin Genet. 2007;72:1–8. doi: 10.1111/j.1399-0004.2007.00822.x. [DOI] [PubMed] [Google Scholar]
- Dinh TP, Carpenter D, Leslie FM, Freund TF, Katona I, Sensi SL, et al. Brain monoglyceride lipase participating in endocannabinoid inactivation. Proc Natl Acad Sci U S A. 2002;99:10819–10824. doi: 10.1073/pnas.152334899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drake CT, Milner TA. Mu opioid receptors are in discrete hippocampal interneuron subpopulations. Hippocampus. 2002;12:119–136. doi: 10.1002/hipo.1107. [DOI] [PubMed] [Google Scholar]
- Du T, Xu Q, Ocbina PJ, Anderson SA. NKX2.1 specifies cortical interneuron fate by activating Lhx6. Development. 2008;135:1559–1567. doi: 10.1242/dev.015123. [DOI] [PubMed] [Google Scholar]
- Eggan SM, Hashimoto T, Lewis DA. Reduced cortical cannabinoid 1 receptor messenger RNA and protein expression in schizophrenia. Arch Gen Psychiatry. 2008;65:772–784. doi: 10.1001/archpsyc.65.7.772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferezou I, Cauli B, Hill EL, Rossier J, Hamel E, Lambolez B. 5-HT3 receptors mediate serotonergic fast synaptic excitation of neocortical vasoactive intestinal peptide/cholecystokinin interneurons. J Neurosci. 2002;22:7389–7397. doi: 10.1523/JNEUROSCI.22-17-07389.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foldy C, Lee SY, Szabadics J, Neu A, Soltesz I. Cell type-specific gating of perisomatic inhibition by cholecystokinin. Nat Neurosci. 2007;10:1128–1130. doi: 10.1038/nn1952. [DOI] [PubMed] [Google Scholar]
- Foldy C, Neu A, Jones MV, Soltesz I. Presynaptic, activity-dependent modulation of cannabinoid type 1 receptor-mediated inhibition of GABA release. J Neurosci. 2006;26:1465–1469. doi: 10.1523/JNEUROSCI.4587-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freund TF. Interneuron diversity series: rhythm and mood in perisomatic inhibition. Trends Neurosci. 2003;26:489–495. doi: 10.1016/S0166-2236(03)00227-3. [DOI] [PubMed] [Google Scholar]
- Freund TF, Buzsaki G. Interneurons of the hippocampus. Hippocampus. 1996;6:347–470. doi: 10.1002/(SICI)1098-1063(1996)6:4<347::AID-HIPO1>3.0.CO;2-I. [DOI] [PubMed] [Google Scholar]
- Freund TF, Katona I. Perisomatic inhibition. Neuron. 2007;56:33–42. doi: 10.1016/j.neuron.2007.09.012. [DOI] [PubMed] [Google Scholar]
- Fuentealba P, Klausberger T, Karayannis T, Suen WY, Huck J, Tomioka R, Rockland K, et al. Expression of COUP-TFII nuclear receptor in restricted GABAergic neuronal populations in the adult rat hippocampus. J Neurosci. 2010;30:1595–1609. doi: 10.1523/JNEUROSCI.4199-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fung SJ, Webster MJ, Sivagnanasundaram S, Duncan C, Elashoff M, Weickert CS. Expression of interneuron markers in the dorsolateral prefrontal cortex of the developing human and in schizophrenia. Am J Psychiatry. 2010;167:1479–1488. doi: 10.1176/appi.ajp.2010.09060784. [DOI] [PubMed] [Google Scholar]
- Gallopin T, Geoffroy H, Rossier J, Lambolez B. Cortical sources of CRF, NKB, and CCK and their effects on pyramidal cells in the neocortex. Cereb Cortex. 2006;16:1440–1452. doi: 10.1093/cercor/bhj081. [DOI] [PubMed] [Google Scholar]
- Gao Y, Vasilyev DV, Goncalves MB, Howell FV, Hobbs C, Reisenberg M, et al. Loss of retrograde endocannabinoid signaling and reduced adult neurogenesis in diacylglycerol lipase knock-out mice. J Neurosci. 2010;30:2017–2024. doi: 10.1523/JNEUROSCI.5693-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glickfeld LL, Scanziani M. Distinct timing in the activity of cannabinoid-sensitive and cannabinoid-insensitive basket cells. Nat Neurosci. 2006;9:807–815. doi: 10.1038/nn1688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gulyas AI, Cravatt BF, Bracey MH, Dinh TP, Piomelli D, Boscia F, Freund TF. Segregation of two endocannabinoid-hydrolyzing enzymes into pre- and postsynaptic compartments in the rat hippocampus, cerebellum and amygdala. Eur J Neurosci. 2004;20:441–458. doi: 10.1111/j.1460-9568.2004.03428.x. [DOI] [PubMed] [Google Scholar]
- Gulyas AI, Megias M, Emri Z, Freund TF. Total number and ratio of excitatory and inhibitory synapses converging onto single interneurons of different types in the CA1 area of the rat hippocampus. J Neurosci. 1999;19:10082–10097. doi: 10.1523/JNEUROSCI.19-22-10082.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hajos N, Papp EC, Acsady L, Levey AI, Freund TF. Distinct interneuron types express m2 muscarinic receptor immunoreactivity on their dendrites or axon terminals in the hippocampus. Neuroscience. 1998;82:355–376. doi: 10.1016/s0306-4522(97)00300-x. [DOI] [PubMed] [Google Scholar]
- Harkany T, Holmgren C, Hartig W, Qureshi T, Chaudhry FA, Storm-Mathisen J, et al. Endocannabinoid-independent retrograde signaling at inhibitory synapses in layer 2/3 of neocortex: Involvement of vesicular glutamate transporter 3. J Neurosci. 2004;24:4978–4988. doi: 10.1523/JNEUROSCI.4884-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hart SA, Snyder MA, Smejkalova T, Woolley CS. Estrogen mobilizes a subset of estrogen receptor-α-immunoreactive vesicles in inhibitory presynaptic boutons in hippocampal CA1. J Neurosci. 2007;27:2102–2111. doi: 10.1523/JNEUROSCI.5436-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hashimoto T, Bergen SE, Nguyen QL, Xu B, Monteggia LM, Pierri JN, et al. Relationship of brain-derived neurotrophic factor and its receptor TrkB to altered inhibitory prefrontal circuitry in schizophrenia. J Neurosci. 2005;25:372–383. doi: 10.1523/JNEUROSCI.4035-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hashimotodani Y, Ohno-Shosaku T, Kano M. Presynaptic monoacylglycerol lipase activity determines basal endocannabinoid tone and terminates retrograde endocannabinoid signaling in the hippocampus. J Neurosci. 2007;27:1211–1219. doi: 10.1523/JNEUROSCI.4159-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hernandez-Pineda R, Chow A, Amarillo Y, Moreno H, Saganich M, Vega-Saenz de Miera EC, et al. Kv3.1-Kv3.2 channels underlie a high-voltage-activating component of the delayed rectifier K+ current in projecting neurons from the globus pallidus. J Neurophysiol. 1999;82:1512–1528. doi: 10.1152/jn.1999.82.3.1512. [DOI] [PubMed] [Google Scholar]
- Hungund BL, Vinod KY, Kassir SA, Basavarajappa BS, Yalamanchili R, Cooper TB, et al. Upregulation of CB1 receptors and agonist-stimulated {35S}GTPγS binding in the prefrontal cortex of depressed suicide victims. Mol Psychiatry. 2004;9:184–190. doi: 10.1038/sj.mp.4001376. [DOI] [PubMed] [Google Scholar]
- Kanatani S, Yozu M, Tabata H, Nakajima K. COUP-TFII is preferentially expressed in the caudal ganglionic eminence and is involved in the caudal migratory stream. J Neurosci. 2008;28:13582–13591. doi: 10.1523/JNEUROSCI.2132-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kano M, Ohno-Shosaku T, Hashimotodani Y, Uchigashima M, Watanabe M. Endocannabinoid-mediated control of synaptic transmission. Physiol Rev. 2009;89:309–380. doi: 10.1152/physrev.00019.2008. [DOI] [PubMed] [Google Scholar]
- Karson MA, Tang AH, Milner TA, Alger BE. Synaptic cross talk between perisomatic-targeting interneuron classes expressing cholecystokinin and parvalbumin in hippocampus. J Neurosci. 2009;29:4140–4154. doi: 10.1523/JNEUROSCI.5264-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katona I, Sperlagh B, Sik A, Kafalvi A, Vizi ES, Mackie K, Freund TF. Presynaptically located CB1 cannabinoid receptors regulate GABA release from axon terminals of specific hippocampal interneurons. J Neurosci. 1999;19:4544–4558. doi: 10.1523/JNEUROSCI.19-11-04544.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawaguchi Y, Kondo S. Parvalbumin, somatostatin and cholecystokinin as chemical markers for specific GABAergic interneuron types in the rat frontal cortex. J Neurocytol. 2002;31:277–287. doi: 10.1023/a:1024126110356. [DOI] [PubMed] [Google Scholar]
- Kawaguchi Y, Kubota Y. GABAergic cell subtypes and their synaptic connections in rat frontal cortex. Cereb Cortex. 1997;7:476–486. doi: 10.1093/cercor/7.6.476. [DOI] [PubMed] [Google Scholar]
- Keimpema E, Barabas K, Morozov YM, Tortoriello G, Torii M, Cameron G, et al. Differential subcellular recruitment of monoacylglycerol lipase generates spatial specificity of 2-arachidonoyl glycerol signaling during axonal pathfinding. J Neurosci. 2010;30:13992–14007. doi: 10.1523/JNEUROSCI.2126-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim J, Alger BE. Inhibition of cyclooxygenase-2 potentiates retrograde endocannabinoid effects in hippocampus. Nat Neurosci. 2004;7:697–698. doi: 10.1038/nn1262. [DOI] [PubMed] [Google Scholar]
- Kim J, Isokawa M, Ledent C, Alger BE. Activation of muscarinic acetylcholine receptors enhances the release of endogenous cannabinoids in the hippocampus. J Neurosci. 2002;22:10182–10191. doi: 10.1523/JNEUROSCI.22-23-10182.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klausberger T, Magill PJ, Marton LF, Roberts JD, Cobden PM, Buzsaki G, Somogyi P. Brain-state- and cell-type-specific firing of hippocampal interneurons in vivo. Nature. 2003;421:844–848. doi: 10.1038/nature01374. [DOI] [PubMed] [Google Scholar]
- Klausberger T, Marton LF, O’Neill J, Huck JH, Dalezios Y, Fuentealba P, et al. Complementary roles of cholecystokinin- and parvalbumin-expressing GABAergic neurons in hippocampal network oscillations. J Neurosci. 2005;25:9782–9793. doi: 10.1523/JNEUROSCI.3269-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kozak KR, Rowlinson SW, Marnett LJ. Oxygenation of the endocannabinoid, 2-arachidonylglycerol, to glyceryl prostaglandins by cyclooxygenase-2. J Biol Chem. 2000;275:33744–33749. doi: 10.1074/jbc.M007088200. [DOI] [PubMed] [Google Scholar]
- Kubota Y, Shigematsu N, Karube F, Sekigawa A, Kato S, Yamaguchi N, et al. Selective coexpression of multiple chemical markers defines discrete populations of neocortical GABAergic neurons. Cereb Cortex. 2011;21:1803–1817. doi: 10.1093/cercor/bhq252. [DOI] [PubMed] [Google Scholar]
- Lauder JM. Ontogeny of the serotonergic system in the rat: serotonin as a developmental signal. Ann N Y Acad Sci. 1990;600:297–313. doi: 10.1111/j.1749-6632.1990.tb16891.x. discussion 314. [DOI] [PubMed] [Google Scholar]
- Lee S, Hjerling-Leffler J, Zagha E, Fishell G, Rudy B. The largest group of superficial neocortical GABAergic interneurons expresses ionotropic serotonin receptors. J Neurosci. 2010a;30:16796–16808. doi: 10.1523/JNEUROSCI.1869-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee SH, Foldy C, Soltesz I. Distinct endocannabinoid control of GABA release at perisomatic and dendritic synapses in the hippocampus. J Neurosci. 2010b;30:7993–8000. doi: 10.1523/JNEUROSCI.6238-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee SH, Soltesz I. Requirement for CB1 but not GABAB receptors in the cholecystokinin mediated inhibition of GABA release from cholecystokinin expressing basket cells. J Physiol. 2011;589:891–902. doi: 10.1113/jphysiol.2010.198499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee SY, Foldy C, Szabadics J, Soltesz I. Cell-type-specific CCK2 receptor signaling underlies the cholecystokinin-mediated selective excitation of hippocampal parvalbumin-positive fast-spiking basket cells. J Neurosci. 2011;31:10993–11002. doi: 10.1523/JNEUROSCI.1970-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liodis P, Denaxa M, Grigoriou M, Akufo-Addo C, Yanagawa Y, Pachnis V. Lhx6 activity is required for the normal migration and specification of cortical interneuron subtypes. J Neurosci. 2007;27:3078–3089. doi: 10.1523/JNEUROSCI.3055-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Z, Neff RA, Berg DK. Sequential interplay of nicotinic and GABAergic signaling guides neuronal development. Science. 2006;314:1610–1613. doi: 10.1126/science.1134246. [DOI] [PubMed] [Google Scholar]
- Liu Z, Zhang J, Berg DK. Role of endogenous nicotinic signaling in guiding neuronal development. Biochem Pharmacol. 2007;74:1112–1119. doi: 10.1016/j.bcp.2007.05.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lodato S, Tomassy GS, De Leonibus E, Uzcategui YG, Andolfi G, Armentano M, et al. Loss of COUP-TFI alters the balance between caudal ganglionic eminence- and medial ganglionic eminence-derived cortical interneurons and results in resistance to epilepsy. J Neurosci. 2011;31:4650–4662. doi: 10.1523/JNEUROSCI.6580-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Long JE, Cobos I, Potter GB, Rubenstein JL. Dlx1&2 and Mash1 transcription factors control MGE and CGE patterning and differentiation through parallel and overlapping pathways. Cereb Cortex. 2009;19:i96–i106. doi: 10.1093/cercor/bhp045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McBain CJ, Freund TF, Mody I. Glutamatergic synapses onto hippocampal interneurons: precision timing without lasting plasticity. Trends Neurosci. 1999;22:228–235. doi: 10.1016/s0166-2236(98)01347-2. [DOI] [PubMed] [Google Scholar]
- Manent JB, Demarque M, Jorquera I, Pellegrino C, Ben-Ari Y, Aniksztejn L, Represa A. A noncanonical release of GABA and glutamate modulates neuronal migration. J Neurosci. 2005;25:4755–4765. doi: 10.1523/JNEUROSCI.0553-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marrs WR, Blankman JL, Horne EA, Thomazeau A, Lin YH, Coy J, et al. The serine hydrolase ABHD6 controls the accumulation and efficacy of 2-AG at cannabinoid receptors. Nat Neurosci. 2010;13:951–957. doi: 10.1038/nn.2601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marsicano G, Lutz B. Expression of the cannabinoid receptor CB1 in distinct neuronal subpopulations in the adult mouse forebrain. Eur J Neurosci. 1999;11:4213–4225. doi: 10.1046/j.1460-9568.1999.00847.x. [DOI] [PubMed] [Google Scholar]
- Marty S, Berninger B, Carroll P, Thoenen H. GABAergic stimulation regulates the phenotype of hippocampal interneurons through the regulation of brain-derived neurotrophic factor. Neuron. 1996;16:565–570. doi: 10.1016/s0896-6273(00)80075-6. [DOI] [PubMed] [Google Scholar]
- Matsuda LA, Lolait SJ, Brownstein MJ, Young AC, Bonner TI. Structure of a cannabinoid receptor and functional expression of the cloned cDNA. Nature. 1990;346:561–564. doi: 10.1038/346561a0. [DOI] [PubMed] [Google Scholar]
- Mechoulam R, Ben Shabat S, Hanus L, Ligumsky M, Kaminski NE, Schatz AR, et al. Identification of an endogenous 2-monoglyceride, present in canine gut, that binds to cannabinoid receptors. Biochem Pharmacol. 1995;50:83–90. doi: 10.1016/0006-2952(95)00109-d. [DOI] [PubMed] [Google Scholar]
- Mereu G, Fa M, Ferraro L, Cagiano R, Antonelli T, Tattoli M, et al. Prenatal exposure to a cannabinoid agonist produces memory deficits linked to dysfunction in hippocampal long-term potentiation and glutamate release. Proc Natl Acad Sci U S A. 2003;100:4915–4920. doi: 10.1073/pnas.0537849100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyoshi G, Hjerling-Leffler J, Karayannis T, Sousa VH, Butt SJ, Battiste J, et al. Genetic fate mapping reveals that the caudal ganglionic eminence produces a large and diverse population of superficial cortical interneurons. J Neurosci. 2010;30:1582–1594. doi: 10.1523/JNEUROSCI.4515-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morales M, Bloom FE. The 5-HT3 receptor is present in different subpopulations of GABAergic neurons in the rat telencephalon. J Neurosci. 1997;17:3157–3167. doi: 10.1523/JNEUROSCI.17-09-03157.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morellini F, Sivukhina E, Stoenica L, Oulianova E, Bukalo O, Jakovcevski I, et al. Improved reversal learning and working memory and enhanced reactivity to novelty in mice with enhanced GABAergic innervation in the dentate gyrus. Cereb Cortex. 2010;20:2712–2727. doi: 10.1093/cercor/bhq017. [DOI] [PubMed] [Google Scholar]
- Morozov YM, Torii M, Rakic P. Origin, early commitment, migratory routes, and destination of cannabinoid type 1 receptor-containing interneurons. Cereb Cortex. 2009;19:i78–i89. doi: 10.1093/cercor/bhp028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mulder J, Aguado T, Keimpema E, Barabas K, Ballester Rosado CJ, Nguyen L, et al. Endocannabinoid signaling controls pyramidal cell specification and long-range axon patterning. Proc Natl Acad Sci U S A. 2008;105:8760–8765. doi: 10.1073/pnas.0803545105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murray AJ, Sauer JF, Riedel G, McClure C, Ansel L, Cheyne L, et al. Parvalbumin-positive CA1 interneurons are required for spatial working but not for reference memory. Nat Neurosci. 2011;14:297–299. doi: 10.1038/nn.2751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nery S, Fishell G, Corbin JG. The caudal ganglionic eminence is a source of distinct cortical and subcortical cell populations. Nat Neurosci. 2002;5:1279–1287. doi: 10.1038/nn971. [DOI] [PubMed] [Google Scholar]
- Neu A, Foldy C, Soltesz I. Postsynaptic origin of CB1-dependent tonic inhibition of GABA release at cholecystokinin-positive basket cell to pyramidal cell synapses in the CA1 region of the rat hippocampus. J Physiol. 2007;578:233–247. doi: 10.1113/jphysiol.2006.115691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noh J, Seal RP, Garver JA, Edwards RH, Kandler K. Glutamate co-release at GABA/glycinergic synapses is crucial for the refinement of an inhibitory map. Nat Neurosci. 2010;13:232–238. doi: 10.1038/nn.2478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nomura T, Fukuda T, Aika Y, Heizmann CW, Emson PC, Kobayashi T, Kosaka T. Distribution of nonprincipal neurons in the rat hippocampus, with special reference to their dorsoventral difference. Brain Res. 1997;751:64–80. doi: 10.1016/s0006-8993(96)01395-9. [DOI] [PubMed] [Google Scholar]
- O’Keefe J, Nadel L. The Hippocampus as a Cognitive Map. Oxford University Press; 1978. [Google Scholar]
- Ohno-Shosaku T, Maejima T, Kano M. Endogenous cannabinoids mediate retrograde signals from depolarized postsynaptic neurons to presynaptic terminals. Neuron. 2001;29:729–738. doi: 10.1016/s0896-6273(01)00247-1. [DOI] [PubMed] [Google Scholar]
- Papp E, Leinekugel X, Henze DA, Lee J, Buzsaki G. The apical shaft of CA1 pyramidal cells is under GABAergic interneuronal control. Neuroscience. 2001;102:715–721. doi: 10.1016/s0306-4522(00)00584-4. [DOI] [PubMed] [Google Scholar]
- Peters A, Kara DA. The neuronal composition of area 17 of rat visual cortex. II. The nonpyramidal cells. J Comp Neurol. 1985;234:242–263. doi: 10.1002/cne.902340209. [DOI] [PubMed] [Google Scholar]
- Pitler TA, Alger BE. Postsynaptic spike firing reduces synaptic GABAA responses in hippocampal pyramidal cells. J Neurosci. 1992;12:4122–4132. doi: 10.1523/JNEUROSCI.12-10-04122.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pleasure SJ, Anderson S, Hevner R, Bagri A, Marin O, Lowenstein DH, Rubenstein JL. Cell migration from the ganglionic eminences is required for the development of hippocampal GABAergic interneurons. Neuron. 2000;28:727–740. doi: 10.1016/s0896-6273(00)00149-5. [DOI] [PubMed] [Google Scholar]
- Porter JT, Cauli B, Tsuzuki K, Lambolez B, Rossier J, Audinat E. Selective excitation of subtypes of neocortical interneurons by nicotinic receptors. J Neurosci. 1999;19:5228–5235. doi: 10.1523/JNEUROSCI.19-13-05228.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Puente N, Cui Y, Lassalle O, Lafourcade M, Georges F, Venance L, et al. Polymodal activation of the endocannabinoid system in the extended amygdala. Nat Neurosci. 2011;14:1542–1547. doi: 10.1038/nn.2974. [DOI] [PubMed] [Google Scholar]
- Regehr WG, Carey MR, Best AR. Activity-dependent regulation of synapses by retrograde messengers. Neuron. 2009;63:154–170. doi: 10.1016/j.neuron.2009.06.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riccio O, Potter G, Walzer C, Vallet P, Szabo G, Vutskits L, et al. Excess of serotonin affects embryonic interneuron migration through activation of the serotonin receptor 6. Mol Psychiatry. 2009;14:280–290. doi: 10.1038/mp.2008.89. [DOI] [PubMed] [Google Scholar]
- Sekirnjak C, Martone ME, Weiser M, Deerinck T, Bueno E, Rudy B, Ellisman M. Subcellular localization of the K+ channel subunit Kv3.1b in selected rat CNS neurons. Brain Res. 1997;766:173–187. doi: 10.1016/s0006-8993(97)00527-1. [DOI] [PubMed] [Google Scholar]
- Sigel E, Baur R, Racz I, Marazzi J, Smart TG, Zimmer A, Gertsch J. The major central endocannabinoid directly acts at GABAA receptors. Proc Natl Acad Sci U S A. 2011;108:18150–18155. doi: 10.1073/pnas.1113444108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sloviter RS, Ali-Akbarian L, Elliott RC, Bowery BJ, Bowery NG. Localization of GABAB (R1) receptors in the rat hippocampus by immunocytochemistry and high resolution autoradiography, with specific reference to its localization in identified hippocampal interneuron subpopulations. Neuropharmacology. 1999;38:1707–1721. doi: 10.1016/s0028-3908(99)00132-x. [DOI] [PubMed] [Google Scholar]
- Somogyi J, Baude A, Omori Y, Shimizu H, Mestikawy SE, Fukaya M, et al. GABAergic basket cells expressing cholecystokinin contain vesicular glutamate transporter type 3 (VGLUT3) in their synaptic terminals in hippocampus and isocortex of the rat. Eur J Neurosci. 2004;19:552–569. doi: 10.1111/j.0953-816x.2003.03091.x. [DOI] [PubMed] [Google Scholar]
- Souza BR, Romano-Silva MA, Tropepe V. Dopamine D2 receptor activity modulates Akt signaling and alters GABAergic neuron development and motor behavior in zebrafish larvae. J Neurosci. 2011;31:5512–5525. doi: 10.1523/JNEUROSCI.5548-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Straiker A, Hu SS, Long J, Arnold A, Wager-Miller J, Cravatt BF, Mackie K. Monoacyl glycerol lipase (MGL) limits the duration of endocannabinoid-mediated depolarization-induced suppression of excitation (DSE) in autaptic hippocampal neurons. Mol Pharmacol. 2009;76:1220–1227. doi: 10.1124/mol.109.059030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Straiker A, Mackie K. Cannabinoid signaling in inhibitory autaptic hippocampal neurons. Neuroscience. 2009;163:190–201. doi: 10.1016/j.neuroscience.2009.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taniguchi H, He M, Wu P, Kim S, Paik R, Sugino K, et al. A resource of cre driver lines for genetic targeting of GABAergic neurons in cerebral cortex. Neuron. 2011;71:995–1013. doi: 10.1016/j.neuron.2011.07.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanimura A, Yamazaki M, Hashimotodani Y, Uchigashima M, Kawata S, Abe M, et al. The endocannabinoid 2-arachidonoylglycerol produced by diacylglycerol lipase alpha mediates retrograde suppression of synaptic transmission. Neuron. 2010;65:320–327. doi: 10.1016/j.neuron.2010.01.021. [DOI] [PubMed] [Google Scholar]
- Tricoire L, Pelkey KA, Erkkila BE, Jeffries BW, Yuan X, McBain CJ. A blueprint for the spatiotemporal origins of mouse hippocampal interneuron diversity. J Neurosci. 2011;31:10948–10970. doi: 10.1523/JNEUROSCI.0323-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsou K, Mackie K, Sanudo-Pena MC, Walker JM. Cannabinoid CB1 receptors are localized primarily on cholecystokinin-containing GABAergic interneurons in the rat hippocampal formation. Neuroscience. 1999;93:969–975. doi: 10.1016/s0306-4522(99)00086-x. [DOI] [PubMed] [Google Scholar]
- Varga V, Losonczy A, Zemelman BV, Borhegyi Z, Nyiri G, Domonkos A, et al. Fast synaptic subcortical control of hippocampal circuits. Science. 2009;326:449–453. doi: 10.1126/science.1178307. [DOI] [PubMed] [Google Scholar]
- Varma N, Carlson GC, Ledent C, Alger BE. Metabotropic glutamate receptors drive the endocannabinoid system in hippocampus. J Neurosci. 2001;21:RC188. doi: 10.1523/JNEUROSCI.21-24-j0003.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vitalis T, Cases O, Passemard S, Callebert J, Parnavelas JG. Embryonic depletion of serotonin affects cortical development. Eur J Neurosci. 2007;26:331–344. doi: 10.1111/j.1460-9568.2007.05661.x. [DOI] [PubMed] [Google Scholar]
- Vucurovic K, Gallopin T, Ferezou I, Rancillac A, Chameau P, van Hooft JA, et al. Serotonin 3A receptor subtype as an early and protracted marker of cortical interneuron subpopulations. Cereb Cortex. 2010;20:2333–2347. doi: 10.1093/cercor/bhp310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y, Gupta A, Toledo-Rodriguez M, Wu CZ, Markram H. Anatomical, physiological, molecular and circuit properties of nest basket cells in the developing somatosensory cortex. Cereb Cortex. 2002;12:395–410. doi: 10.1093/cercor/12.4.395. [DOI] [PubMed] [Google Scholar]
- Wilson RI, Kunos G, Nicoll RA. Presynaptic specificity of endocannabinoid signaling in the hippocampus. Neuron. 2001;31:453–462. doi: 10.1016/s0896-6273(01)00372-5. [DOI] [PubMed] [Google Scholar]
- Wilson RI, Nicoll RA. Endogenous cannabinoids mediate retrograde signalling at hippocampal synapses. Nature. 2001;410:588–592. doi: 10.1038/35069076. [DOI] [PubMed] [Google Scholar]
- Wilson RI, Nicoll RA. Endocannabinoid signaling in the brain. Science. 2002;296:678–682. doi: 10.1126/science.1063545. [DOI] [PubMed] [Google Scholar]
- Xu Q, Cobos I, De La Cruz E, Rubenstein JL, Anderson SA. Origins of cortical interneuron subtypes. J Neurosci. 2004;24:2612–2622. doi: 10.1523/JNEUROSCI.5667-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshida T, Fukaya M, Uchigashima M, Miura E, Kamiya H, Kano M, Watanabe M. Localization of diacylglycerol lipase-alpha around postsynaptic spine suggests close proximity between production site of an endocannabinoid, 2-arachidonoyl-glycerol, and presynaptic cannabinoid CB1 receptor. J Neurosci. 2006;26:4740–4751. doi: 10.1523/JNEUROSCI.0054-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshida T, Uchigashima M, Yamasaki M, Katona I, Yamazaki M, Sakimura K, et al. Unique inhibitory synapse with particularly rich endocannabinoid signaling machinery on pyramidal neurons in basal amygdaloid nucleus. Proc Natl Acad Sci U S A. 2011;108:3059–3064. doi: 10.1073/pnas.1012875108. [DOI] [PMC free article] [PubMed] [Google Scholar]


