Abstract
Non-technical summary
Primary sensory neurones with unmyelinated axons signal touch, temperature, pain and itch. A peculiar feature of these axons is their pronounced slowing of axonal conduction velocity seen at low frequencies (<2 Hz) as a progressive walking of the action potential response latency to electrical stimulation. In human microneurographic recordings, the magnitude of latency walking can be used to predict an individual axon's sensitivity to thermal and mechanical stimuli. Recently, activity-dependent inactivation of voltage-gated sodium channels (NaV) has been shown to contribute to latency walking implying that NaV-dependent processes such as action potential initiation may also be affected. This was tested here using dynamic mechanical stimuli. Precise determination of mechanical activation threshold in single axons revealed that conduction velocity slowing during repetitive activity was paralleled by an increase in threshold. This suggests that activity-induced axonal slowing represents a form of accommodation limiting excessive firing in unmyelinated sensory axons.
Abstract
The passage of an action potential along a peripheral axon modulates the conduction velocity of subsequent action potentials. In C-neurones with unmyelinated axons repetitive activity progressively slows axonal conduction velocity and in microneurographic recordings from healthy human subjects the magnitude of this slowing can be used to predict the receptive properties of individual axons. Recently, a reduction in the number of available voltage-gated sodium channels (NaV) through inactivation has been implicated as the predominant factor responsible for the slowing of axonal conduction. Since NaVs are also responsible for the initiation of action potentials in sensory nerve terminals, changes in their availability may be expected to affect activation threshold for sensory stimuli. To examine this proposal, dynamic mechanical stimuli were used to make precise estimates of activation threshold in single unmyelinated axons innervating the rat cranial dura mater. Decreases in axonal conduction velocity induced by repetitive electrical stimulation were paralleled by an increase in mechanical activation threshold. Application of TTX (10–20 nm) also slowed axonal conduction velocity in all 11 fibres examined and in 9 of these this resulted in a parallel increase in mechanical activation threshold. We interpret this as indicating that a reduction in available NaV number contributes to both axonal conduction velocity slowing and the observed parallel increase in mechanical activation threshold. The slowing of axonal conduction velocity observed during repetitive activity thus represents a form of accommodation, i.e. self inhibition, which is likely to be decisive in limiting peripheral input to the spinal dorsal horn and thereby regulating processes that could otherwise lead to central sensitization.
Introduction
A prominent functional property of the unmyelinated axons of primary sensory C-neurones is the dependence of their action potential conduction velocity on the immediate firing history of the axon (Raymond et al. 1990; Thalhammer et al. 1994; Gee et al. 1996). Indeed, for individual axons, conduction velocity can be reduced by as much as half in response to a few minutes of low-frequency (ca. 2 Hz) stimulation. Axons of mechanically insensitive afferents slow considerably more than their mechanically sensitive counterparts which in turn slow more than axons of cold-sensitive units and on this basis alone, individual C-fibres recorded using human microneurography techniques can be attributed to distinct functional classes (Serra et al. 1999; Weidner et al. 1999). While this feature is valuable technically, the physiological role of axonal conduction velocity slowing is less well defined.
In unmyelinated axons, activity-induced changes in axonal conduction velocity are thought to result from an interplay between changes in axonal membrane potential, likely to hyperpolarise in response to activity-induced increases in intra-axonal sodium and subsequent Na+/K+-ATPase activity (Rang & Ritchie, 1968), and changes in the availability of voltage-gated sodium channels (NaV) (De Col et al. 2008). In isolated dorsal root ganglion (DRG) neurones, long-lasting changes in total TTX-resistant (TTX-r) current, attributable to accumulation of individual channels in slow inactivated states, can be induced by either maintained depolarization, repetitive brief depolarising pulses (Ogata & Tatebayashi, 1992; Rush et al. 1998; Fazan et al. 2001; Tripathi et al. 2006) or repetitive action potential voltage profiles (Blair & Bean, 2003). In particular, a reduction in available NaVs during repetitive activity leads to an increase in the current required to initiate an action potential in DRG neurones staining positively with the plant lectin IB4 (Snape et al. 2010). Both TTX-r and TTX-sensitive (TTX-s) NaV channel isoforms have been shown to be functionally present in the nerve terminals of polymodal and cold-sensitive afferents (Brock et al. 1998; Carr et al. 2002) and their availability in the terminal region is regulated by membrane potential (Carr et al. 2002; Carr et al. 2009). Since repetitive activity modulates both membrane potential and sodium channel availability, the sensory activation threshold for individual axons would also be expected to change during repetitive activation.
To examine this, a combined mechanical and electrical stimulator was developed allowing bimodal stimulation within the receptive field of individual meningeal afferents innervating the rat cranial dura mater. The dura was made accessible from the arachnoidal side using a hemisected preparation of the cranium described previously (De Col et al. 2008). In the dura mater, nerve bundles, comprising trigeminal and autonomic fibres, together with blood vessels form a roughly two-dimensional network between layers of connective tissue (Fricke et al. 2001). Terminal branches of trigeminal fibres leave these bundles and remain in the same plane as their parent axons to form typically club-like endings that may constitute the mechanically sensitive parts of the afferent fibres (Messlinger et al. 1993). Punctate stimulation of mechanically sensitive spots in the dura mater supported by the underlying cranial bone allowed the activation threshold of mechanosensitive afferents to be determined with high precision. We show here that in single axons innervating the dura mater, repetitive electrically evoked activity slowed axonal conduction velocity and resulted in a parallel increase in the threshold of mechanical activation.
Methods
The housing of animals and all experimental procedures were carried out in compliance with the guidelines for the welfare of experimental animals as stipulated by the Federal Republic of Germany.
Tissue preparation
Wistar rats of both sexes and with body weights ranging from 210 to 360 g were used. Rats were killed by carbon dioxide inhalation, following which the head and lower jaw were removed and the cranial vault cleared of overlying skin and muscle. The skull was divided in the sagittal plane with a scalpel. The cortex, cerebellum and brainstem were gently lifted out of each of the resulting skull halves exposing the underlying dura. One skull half was embedded in a Perspex chamber using agarose (5%) such that the dura-lined skull itself formed a tissue bath. The second skull half was kept in buffered physiological solution at room temperature. The intervening period before use of the second half-skull was typically less than 4 h and no differences in average conduction velocity or the average magnitude of slowing were found between axons innervating the two skull halves.
The tissue was perfused continuously at ∼6 ml min−1 with physiological solution of the following composition (in mm): 145 Na+; 3.5 K+, 1.53 Ca2+, 0.69 Mg2+; 1.67 PO42−; 114 Cl−; 9.64 C6H11O7; 5.55 d(+)-glucose; 7.6 d(+)-sucrose and was buffered to pH 7.4 with carbogen (95% O2, 5% CO2). The temperature of the perfusing solution was controlled with an in-line Peltier element regulated by feedback from a thermocouple positioned in the bath in close apposition to the dura. Typically the preparation was held at 33.5 ± 0.5°C.
Recording arrangement
The spinosus nerve was cut distal to its point of entry into the trigeminal ganglion and freed of surrounding tissue over a length of approximately 4 mm. A glass recording electrode filled with physiological solution and with a tip diameter of ∼10 μm was attached to the side of the isolated nerve stem by light suction to form a high-resistance seal. Signals from axons coursing within the nerve were recorded over the sealing resistance using an Axopatch 200A amplifier (Axon Instruments, USA). Signals were filtered (<5 kHz, 80 dB Bessel), digitized (20 kHz, micro 1401, Cambridge Electronic Design, UK) and stored to disk for subsequent processing with custom-written software in Igor Pro (Wavemetrics, USA).
Mechanical and electrical stimulation
A combined mechanical and electrical stimulator was developed to enable precise temporal application of the two stimulus modalities within the dimensions of a single unit's receptive field (Fig. 1A). The stimulator comprises a mechanical stimulus probe surrounding an insulated wire electrode. The mechanical stimulus probe was formed from a fire-polished glass capillary (external diameter 150 μm, internal diameter 60 μm) and attached to a metal cannula. The wire electrode was insulated with rayon (20 μm diameter, ISAOHM, Isabellenhütte, Dillenburg, Germany) except for the transverse section at the cut end. When placed upon the meninges, the electrode exerted a constant buckling load of 0.4 mN. A silver wire attached to, but electrically isolated from, the metal shaft served as the anode. Axial movements of the mechanical stimulator were driven by an electromagnet and for the experiments described here the command signal was a sinusoidal half-wave. The solenoid was constructed by hand by coiling copper wire. The steel armature was suspended between rubber diaphragms at each end of the coil and was contiguous with the shaft that formed the proximal half of the mechanical stimulator. The combined stimulator allowed electrical and actuator-driven mechanical stimuli to be delivered independently at precise interstimulus intervals.
Figure 1. Bimodal activation of a single sensory axon using a combined electrical and actuator-driven mechanical stimulator.
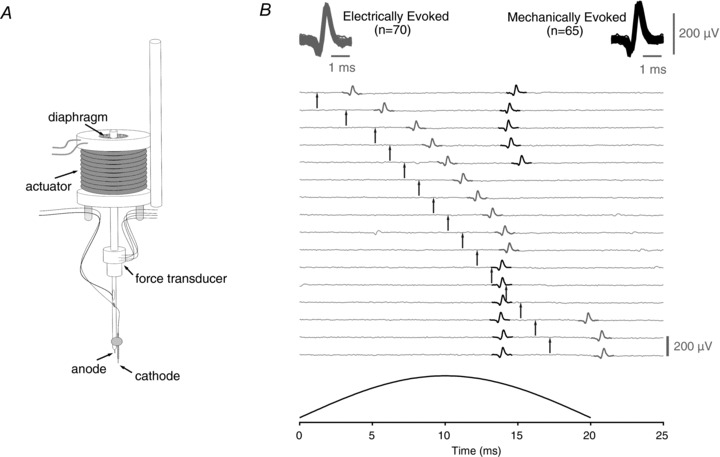
Mechanical stimuli are delivered via an actuator with the armature suspended between rubber diaphragms and a strain gauge based force transducer incorporated into the axial shaft (A). The cathode is placed in contact with the tissue while the anode is attached to the shaft and immersed in the solution perfusing the bath. The stimulator allows electrical and mechanical stimuli to be applied independently in close spatial proximity to one another (A and see Methods) such that an action potential response can be evoked by either electrical or mechanical stimulation (B, grey and black spikes, respectively). Action potential responses to mechanical and electrical stimulation can be shown to arise from a single axon by exploiting the phenomena of refractoriness. This is illustrated by the series of traces in B. Traces are aligned to the onset (i.e. 0 ms) of the 20-ms-wide mechanical stimulus pulse. In each trace, the time of electrical stimulation is indicated by an arrow. The profile of mechanical stimulation is shown in the bottom panel. For clarity, electrical stimulus artifacts have been removed by subtraction. The action potential response to mechanical stimulation occurs approximately 14–15 ms after onset of the mechanical stimulus. The latency of the response to electrical stimulation is approximately 2 ms. Electrical stimulation in the period 8–12 ms after the onset of mechanical stimulation produces an electrically evoked action potential, the absolute refractory period of which prevents generation of an action potential response to mechanical stimulation. Similarly, an action potential response to mechanical stimulation precludes the generation of an electrically evoked action potential when the electrical stimulus is delivered 14–16 ms after the onset of mechanical stimulation. Since it is not possible to evoke an action potential response with either stimulus modality during the refractory period of an action potential evoked by the other stimulus modality, both stimulus modalities must activate the same sensory axon. For each sensory ending reported on here this functional cross-modality refractory period analysis was performed to verify the singular origin of action potential responses to both stimulus modalities.
Axial force was determined using a diaphragm strain gauge (see Fig. 1A) with a gauge factor of 2.05, a resistance of 350 Ω and temperature compensation for steel (Vishay, Micro-Measurements, Wendell, USA). The gauge was glued to a thin steel disc (50 μm) with epoxy resin. The other side of the metal disc was fixed with epoxy to the distal metal shaft of the mechanical stimulator. The low compliance of the metal disc provided high dynamic sensitivity at the expense of stability under high static load. Voltage across the bridge was amplified (GSV-2ASD, ME-Messsysteme, Henningsdorf, Germany), digitised (10 kHz) and stored together with the neural signal. Calibration of the strain gauge was performed manually by pressing the tip of the stimulator on a fine balance (BP210D, Sartorius Ag, Göttingen, Germany) and recording voltages corresponding to weights (forces) from 10 mg to 1 g. The resulting relationship was linear (26.3 mV mg−1) up to 2 g with an offset of 32.7 mV which was compensated prior to amplification.
Establishing receptive fields
Receptive fields in which a single axon could be activated both mechanically and electrically were determined in a three-step process. Initially, a von Frey filament (10 mN, 0.2 mm diameter) was used to establish sites at which action potential activity could be evoked mechanically. At a likely site, the combined stimulator was gently positioned such that the central electrode made contact with the tissue. Mechanical stimuli were applied first in order to determine whether a single action potential response could be evoked. Upon establishing a constant latency action potential response to mechanical simulation the possibility of evoking a single action potential response with electrical stimulation was tested. In the event of not being able to generate an action potential response to either or both mechanical and electrical stimulation, the stimulator was repositioned and the search procedure repeated.
Determination of axonal conduction velocity
Axonal conduction velocity was calculated by dividing the latency of the action potential response to electrical stimulation by the length of axon between the stimulating and recording sites. The length of axon between the two sites was estimated visually with reference to a graticule placed in the light path of the microscope's ocular objective. It is possible to determine the distance between the stimulation and recording sites to a reasonable degree of accuracy in the meninges as it is a transparent tissue within which the path of the larger nerve bundles is easily discerned and the intervening path to the site of stimulation can be extrapolated.
For comparisons of mechanical activation threshold and axonal conduction velocity, conduction velocity was determined from the average latency of all action potential responses to electrical stimulation delivered during the period of mechanical threshold determination.
Determination of mechanical threshold
Mechanical stimulation within a unit's receptive field was performed exclusively with the actuator-driven stimulator (see Fig. 1). Mechanical activation threshold was established for individual axons by determining the likelihood of an action potential response at several discrete stimulus strengths. In practice, the procedure was automated and typically comprised three to five up–down cycles in which mechanical stimuli were given at 0.5–1 Hz in a sequence comprising 15–20 steps (i.e. stimulus strengths) each with three to five sequential stimulus presentations (see examples in Figs 2C, 3A and 4A). The probability of evoking an action potential response was determined by simply dividing the number of evoked action potential responses by the number of trials over a particular range of forces. Mechanical activation threshold was taken as the force at the inflection point of a sigmoid fit of response probability on force (see Figs 2D, 3B and 4B).
Figure 2. Determination of mechanical activation threshold in a single axon (c.v. = 2 m s−1).
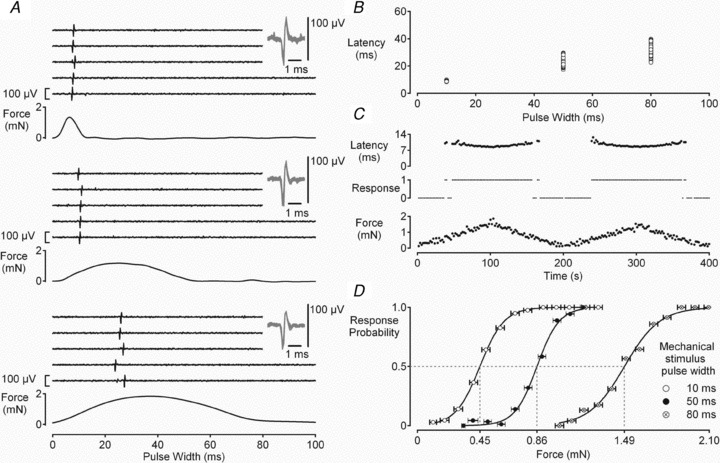
The mechanical activation threshold was established by determining the likelihood of evoking a single action potential in response to brief mechanical stimuli of varying amplitude. Single action potential responses could be evoked using a wide range of mechanical stimulus pulse widths as illustrated in A. Both the latency to the action potential response (B) and the threshold of mechanical activation (D) increase as the width of mechanical stimulus pulse increases. The mechanical activation threshold was determined at 0.5 Hz. At each pulse width the amplitude of the mechanical stimulus was varied systematically (C, lower trace) and the action potential response monitored (C, centre trace). In C, data from mechanical stimuli delivered with a pulse width of 10 ms is shown. The force at which an action potential response is evoked in 50% of trials (P = 0.5) is considered to be the threshold of mechanical activation (D). The force at P = 0.5 was determined from a Boltzmann fit of response probability on stimulus force. The horizontal error bars in D indicate the standard deviation of values contributing to each force bin. Each bin contained no less than 10 values.
Figure 3. Repetitive action potential activity increases the threshold of mechanical activation in sensory axons.
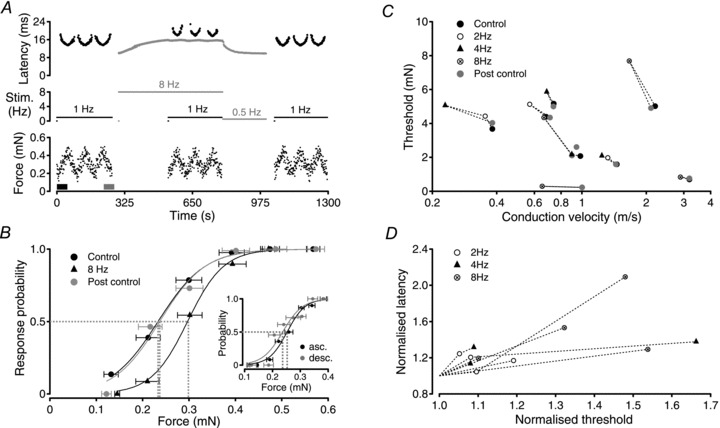
For the single C-fibre (c.v. = 1.0 m s−1) shown in A and B, the threshold of mechanical activation was determined using the method of action potential response likelihood (see Fig. 2). Using mechanical stimuli alone at 1 Hz (A, black markers) the control threshold of mechanical activation was 0.227 ± 0.11 mN (B). Electrical stimulation (A, grey markers) of the axon at 8 Hz begins at 300 s and produces a characteristic increase in response latency, i.e. slowing of axonal conduction velocity, to a stable latency of approximately 15.6 ms (A, grey trace). Using the bimodal stimulator, mechanical stimuli were interleaved instead of every eighth electrical stimulus between 534 and 790 s (A, black markers). The slowed conduction velocity was determined from the average latency to 1776 action potential responses to electrical stimulation. Monitoring the likelihood of responses to these mechanical stimuli gave a threshold for mechanical activation of 0.29 ± 0.09 mN during stimulation at 8 Hz (B). The small fluctuations in electrical response latency observed during determination of mechanical threshold results from mechanical stimulus trials in which no action potential response was evoked, allowing axonal conduction velocity to recover partially from its slowed state. Similarly, the latency of action potential responses to mechanical stimulation (A, black markers) increases when the axon is subject to electrical stimulation at 8 Hz, further confirming that both stimulus modalities activate the same axon. A reduction in the frequency of electrical stimulation from 8 Hz to 0.5 Hz at 796 s and subsequent cessation of electrical stimulation at 1000 s allows the axonal conduction velocity to return to the non-conditioned value. Re-determination of the threshold for mechanical threshold (A, beginning at 1043 s) gives a value of 0.229 ± 0.13 mN (B). To examine potential hysteresis during each sequence of mechanical stimulation, a comparison was made between threshold values determined from responses during the first ascending series (A, thick black bar in force trace) and the last descending series (A, thick grey bar in force trace) of mechanical stimuli. Thresholds calculated from these 2 subsets of mechanical stimuli are shown in the inset in B. Under control conditions, mechanical threshold determined from only those stimuli presented during the first ascending (B, inset asc.) and last descending series (B, inset desc.) are not significantly different (0.252 ± 0.007 mN ascending; 0.237 ± 0.01 mN descending). The reversible increase in mechanical activation threshold with increasing firing rate was confirmed in 8 individual axons (C and D). In each axon, the threshold for mechanical activation returned to within 10% of the control value after the period of high frequency electrical stimulation (C). Horizontal error bars represent forces at 10% and 90% response probability. In D, relative changes in mechanical activation threshold are plotted against relative changes in conduction latency. Mechanical activation threshold determined during the period of electrical stimulation was normalised to the mechanical threshold determined in the control period before electrical stimulation. Similarly, the steady-state conduction latency during the period of electrical stimulation was normalised to the control conduction latency determined from the first response after a 300 s pause.
Figure 4. TTX (10–20 nm) increases the threshold of mechanical activation in single axons.
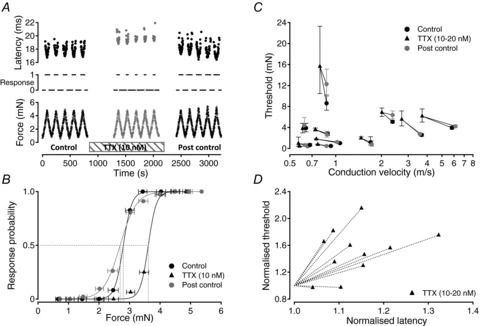
This is shown by example for a single C-fibre (c.v. = 0.9 m s−1) in A and B. The threshold of mechanical activation was determined using mechanical stimuli alone at 0.5 Hz (A). To do this, the absence or presence (A, centre trace, 0 or 1) of an action potential response to the peak force of each mechanical stimulus (A, lower trace) was determined. Response likelihood was calculated for all stimuli within each force bin (B). Threshold was determined from the inflection point of a sigmoid fit to the resulting plot of response probability on force (B). The threshold of mechanical activation determined in this way was 2.8 mN under control conditions (B). Application of TTX (10 nm) increased the latency of action potential responses to mechanical stimulation (A, centre) and the threshold of mechanical activation to 3.6 mN (B). Following washout of TTX, the latency of the response to mechanical activation returned to control values and the threshold of mechanical activation returned to 2.7 mN (A, right; B). For 9 of 11 fibres examined, the mechanical activation threshold increased in the presence of TTX (10–20 nm, C and D). In two axons, however, TTX (20 mm) was without significant effect on the threshold of mechanical activation despite producing a slowing of conduction velocity (D). In each axon, the threshold of mechanical activation returned to within 40% of the control threshold after washout of TTX (C). For visual comparison across fibres, mechanical activation thresholds and conduction latencies were normalised to their control values (D).
To determine mechanical threshold during electrically induced conduction velocity slowing, mechanical stimuli were interleaved with electrical stimuli. The frequency of mechanical stimulation was the same as that used to determine the threshold under control conditions. For example, determination of mechanical threshold at 1 Hz during electrical stimulation at 8 Hz comprised a sequence of seven electrical stimuli and one mechanical stimulus each separated by a 125 ms interstimulus interval. Mechanical stimuli that did not evoke an action potential were not compensated and consequently the response latency to electrical stimuli varied slightly during determination of mechanical threshold (see Fig. 3A).
Chemicals
Using aliquots of a frozen stock solution (1 μm), tetrodotoxin (TTX, Sigma-Aldrich, Taufkirchen, Germany) was thawed and diluted to the required concentration in the perfusing solution on the day of each experiment.
Data analysis
Electrical stimulation protocols began after a 300 s period without stimulation. Action potential response latencies were normalised to the latency of the response to the first stimulus after this pause which is referred to here as the initial latency. The force of mechanical stimulation was taken as the peak maximum of the sinusoidal force profile in each trial. For curve fitting, the Levenberg–Marquardt algorithm for iterative fitting in Igor Pro (Wavemetrics, Lake Oswego, USA) was used. Values of mechanical activation threshold are displayed as markers representing activation threshold with error bars representing the force range encompassing the range of response probabilities between 0.1 and 0.9 (see Figs 3B and 4B).
Statistics
The use of statistical tests is indicated in the text. To assess changes in threshold before, during and after a period of electrically induced slowing or TTX, repeated measures ANOVA was applied with post hoc Dunnett's tests. For comparisons, between groups Student's t test was used.
Results
Responses to mechanical stimuli were examined in 26 individual axons innervating the rat cranial meninges. Axonal conduction velocities ranged from 0.22 to 6.2 m s−1. All units were mechanically sensitive and the sensitivity of units to other stimulus modalities was not examined.
Mechanical and electrical stimulation of the same single axon
A combined mechanical and electrical stimulator (Fig. 1A) was developed to stimulate single sensory axons at a controlled position and with accurate timing of the delivery of each stimulus modality. The stimulator was used for both mechanical and electrical stimulation of all units examined here. A form of action potential collision analysis was used to determine the singularity of action potential responses evoked at a single site by both mechanical and electrical stimulation (Fig. 1B). By shifting the relative timing of electrical and mechanical stimulation it is possible to demonstrate that a mechanically evoked action potential (Fig. 1B, black) cannot be generated in the period of refractoriness subsequent to an electrically evoked action potential (Fig. 1B, grey). Reciprocally, it is not possible to evoke an action potential with an electrical stimulus in the refractory period that follows a mechanically evoked action potential (Fig. 1B). Only initiation of action potentials within the same axon in response to both electrical and mechanical stimulation can account for this observation. The singularity of units was additionally confirmed by the consistency of the response latency to either or both electrical and mechanical stimulation at low frequency (Figs 1 and 2A) as well as the consistency of spike shape (Figs 1B and 2A, insets).
Determination of mechanical threshold
Since technical limitations preclude intracellular recordings from individual unmyelinated axons and their tissue-embedded terminals, mechanical thresholds can only be determined on the basis of action potential responses to mechanical stimuli. The threshold for mechanical activation was defined as the stimulus force able to evoke an action potential response with a likelihood of 0.5, i.e. in half of the stimulus trials. To establish mechanical activation threshold the duration of the mechanical stimulus was held sufficiently short such that only a single action potential was evoked in response to each supra-maximal stimulus. This feature is illustrated in Fig. 2 and is deemed important, because it minimizes the contaminating effects of action potential activity itself on threshold (see below and Fig. 3). Mechanical stimuli were applied to the same site within the receptive field of each unit thereby reducing variability associated with inhomogeneities in activation threshold known to exist within the receptive field of individual mechanically sensitive units (Kenins, 1988).
The threshold for mechanical activation was found to vary according to the profile (width and amplitude) of the mechanical stimulus (see example in Fig. 2). A detailed analysis of seven fibres with conduction velocities ranging from 0.75 to 4.1 m s−1 revealed several interesting features. Firstly, mechanical activation threshold increased as the stimulus became less dynamic, i.e. threshold was higher for the same stimulus amplitude applied over a longer period (Fig. 2A and D). Secondly, the range of forces over which the likelihood of evoking an action potential response changed from 0 to 1 was greater for less dynamic stimuli (Fig. 2D). Finally, the variability in latency of an action potential response increased as a function of stimulus pulse width (Fig. 2A and B). Taken together, the most precise estimate of mechanical activation threshold is obtained with brief dynamic mechanical stimuli and for this reason a 10 ms pulse width was used to determine mechanical activation threshold. Using this stimulus profile, mechanical activation thresholds ranged from 0.22 to 24.86 mN in the 26 axons examined. Mechanical activation threshold was not correlated with basal axonal conduction velocity, i.e. determined immediately after a 300 s period without stimulation (r2= 0.071, P = 0.19, n = 26).
Changes in mechanical activation threshold correlate with changes in axonal conduction velocity
In peripheral C-fibres, low frequencies of repetitive action potential firing result in a reduction in the speed of conduction of subsequent action potentials, an effect referred to as activity-dependent slowing. In some axons, this slowing can be so pronounced that failures of action potential conduction occur (Raymond, 1979; Weidner et al. 1999; de Col et al. 2008). Experimentally, however, it is possible to select an appropriate electrical stimulus frequency at which the conduction velocity slows to a steady-state value and does not block (Fig. 3A, upper trace, grey markers).
Taking advantage of the stimulator's ability to deliver electrical and mechanical stimuli to a single axon (see above), the relationship between mechanical activation threshold and axonal conduction velocity was examined in eight axons. The procedure is illustrated by the example in Fig. 3A and B. The mechanical activation threshold of this axon was initially determined as 0.227 ± 0.11 mN using a mechanical stimulus frequency of 1 Hz (Fig. 3B, Control). Upon repetitive electrical activation of the axon at 8 Hz the latency of conduction of the electrically evoked action potential increased from an initial 10 ms to 15.6 ms, i.e. 1.56 times its initial value and the mechanical activation threshold increased to 0.29 ± 0.09 mN (Fig. 3B). To determine the mechanical activation threshold during 8 Hz electrical stimulation, mechanical stimuli were interposed in place of every eighth electrical stimulus. The frequency of mechanical stimulation was 1 Hz. Returning to mechanical stimuli alone, after the period of electrical stimulation at 8 Hz, the mechanical activation threshold returned to 0.229 ± 0.13 mN (Fig. 3B, Post control).
Mechanical activation threshold was determined before, during and after a period of electrically induced conduction velocity slowing in eight individual axons. In each case mechanical activation threshold increased monotonically with activity-induced increases in axonal conduction latency (i.e. decreasing conduction velocity, Fig. 3C and D) and returned to control values after the period of electrical stimulation (repeated measures ANOVA, F = 42.49, P < 0.001, df = 23; post hoc Dunnett Control vs. 2–8 Hz, mean diff. −1.059, q = 3.745, P < 0.01; Control vs. Post control, mean diff. −0.01820, q = 0.06437, P > 0.05).
The use of repeat up–down mechanical stimulus sequences to determine activation threshold could lead to a change in the efficacy of mechanical stimulation through, for example, a change in tissue compliance or inactivation of the transduction process (see Discussion). To examine this potential hysteresis, a post hoc comparison of mechanical threshold was made for each fibre (Fig. 3B, inset) using only those stimuli presented during the first and last set of ascending or descending stimulus intensities, i.e. for the first 50 stimuli (Fig. 3A, thick black bar) and similarly the last 50 mechanical stimuli (Fig. 3A, thick grey bar). Comparisons across the eight individual fibres used to examine activity-induced changes in mechanical threshold revealed no difference and thus no hysteresis between thresholds determined at the beginning and the end of a series of mechanical stimuli (average relative change 1.05 ± 0.19, P = 0.43, paired t test, n = 8).
To compare effects across fibres, mechanical activation thresholds and conduction latencies were normalised to their control values. Following normalisation activity-induced increases in conduction latency were paralleled by increases in mechanical activation threshold in all fibres examined (Fig. 3D).
Slowing of axonal conduction velocity by TTX increases mechanical activation threshold
Since use-dependent sodium channel inactivation is thought to contribute to activity-dependent changes in axonal conduction velocity, the effect of a reduction in TTX-s sodium channel number on mechanical activation threshold was examined. Mechanical activation threshold was determined initially using mechanical stimuli at 0.5 Hz and for the example shown in Fig. 4 the activation threshold was 2.78 ± 0.31 mN (Fig. 4B, Control). Application of TTX (10 nm) to the solution perfusing the bath slowed axonal conduction of the mechanically evoked action potential response from 16.4 ms to 18.9 ms (Fig. 4A). In the presence of TTX (10 nm), the mechanical activation threshold increased to 3.61 ± 0.27 mN and upon washout returned to 2.67 ± 0.58 mN (Fig. 4B).
The effect of TTX (10–20 nm) on mechanical activation threshold was examined in 11 individual units with initial mechanical activation thresholds ranging from 0.43 to 8.62 mN. Mechanical activation thresholds and conduction latencies were normalised to their control values, that is to values determined in the period before application of TTX for comparison between fibres. In all 11 units, application of TTX resulted in an increase in the latency of the action potential response to mechanical stimulation (1.04- to 1.32-fold) and in 9 of the 11 units this increase was paralleled by an increase in mechanical activation threshold (Fig. 4C and D). The increase in mechanical threshold observed in the presence of TTX was significant (repeated measures ANOVA, F = 30.6, P < 0.001, df = 32; post hoc Dunnett Control vs. TTX, mean diff. −1.526, q = 3.307, P < 0.01; Control vs. Post control, mean diff. −0.5042, q = 1.092, P > 0.05). In two units, mechanical activation threshold decreased marginally in the presence of TTX (Fig. 4D).
Discussion
TTX-r NaVs are able to support action potential initiation in the nerve terminals of sensory axons (Brock et al. 1998). During low-frequency repetitive action potential activity, NaVs in DRG neurones accumulate in slow inactivated states (Blair & Bean, 2003; Snape et al. 2010), an effect that would be expected to result in an increase in the activation threshold of sensory nerve terminals. Indeed, an increase in mechanical activation threshold paralleled the slowing of axonal conduction velocity induced by repetitive electrical activation in all axons examined here. This finding establishes an important nexus between axonal conduction velocity and sensory activation threshold in somatosensory primary afferent axons.
The physical dimensions of unmyelinated somatosensory axons preclude the use of conventional patch clamp techniques to record from their nerve terminals. Consequently, methods to determine mechanical threshold rely upon the initiation of action potentials. The mechanical force required to evoke an action potential in an axon is typically determined using hand-held von Frey filaments, a technique that can lack precision (Lambert et al. 2009). To improve this, a combined mechanical electrical stimulator was constructed. The device delivers mechanical stimuli at a fixed spatial locus with high stimulus fidelity allowing small changes in activation threshold to be determined.
In sensory nerve terminals a change in mechanical activation threshold could arise either as a result of a change in the process of mechanical stimulus transduction, which may include contributions from cells in the immediate vicinity of the terminal, or from alterations in action potential initiation. To date, studies addressing the process of mechanical stimulus transduction have used cultured dorsal root ganglion (DRG) neurones as a surrogate for the nerve terminal to examine mechanically evoked currents (Takahashi & Gotoh, 2000; Cho et al. 2002; Drew et al. 2002; Hu & Lewin, 2006; McCarter & Levine, 2006). Using this model, Rugiero et al. (2010) have recently shown that low-frequency (1 Hz) repetitive mechanical stimulation of rat DRG neurones results in a progressive reduction in the magnitude of mechanically evoked currents suggesting that the transduction process itself is subject to long-term regulation. This may reflect a form of inactivation similar to that reported for the mechanically sensitive two-pore domain potassium channels TRAAK and TREK-1 upon repetitive mechanical activation (Honore et al. 2006). A comparison of mechanical thresholds determined at the beginning and the end of a series of up–down mechanical stimuli used here revealed no difference (Fig. 3B, inset) suggesting that changes in the efficacy of mechanical stimulation and thereby the process of transduction are not prominent for the determination of mechanical threshold performed in this study.
A reduction in the number of available NaVs in the terminal region is, however, likely to contribute to the observed increase in mechanical activation threshold accompanying repetitive activity in sensory axons (Fig. 3). Using electrical stimuli, Raymond (1979) demonstrated that activation threshold correlated with activity-induced changes in axonal conduction velocity in single myelinated axons from frog. A similar increase in threshold has also been reported upon low-frequency stimulation in single human C-fibres responsive to mechanical and heat stimulation (Olausson, 1998) and mechanically sensitive joint afferents in the cat (Just & Heppelmann, 2002). Consistent with this, repetitive stimulation of isolated rodent DRG neurones with either action potential voltage profiles (Blair & Bean, 2003) or brief depolarizing current pulses (Snape et al. 2010) results in a reduction in the transient sodium action current. The magnitude of this reduction varies amongst DRG neurones (Rush et al. 1988), being most pronounced in IB4-positive neurones (Snape et al. 2010) and has been attributed to entry of NaVs into slow inactivated states (De Col et al. 2008). An activity-induced increase in the number of slow inactivated NaVs occurs without alterations in membrane potential (Snape et al. 2010) and we show here that this manifests as an increase in mechanical activation threshold in nerve terminals (Fig. 3). Repetitive mechanical stimuli have been shown to affect NaVs directly, accelerating inactivation in cell expressed NaV1.5 (Morris & Juranka, 2007). For sodium channel subtypes expressed in DRG neurones, changes in the voltage dependence and kinetics of activation and inactivation have been reported for NaV1.7 (Shcherbatko et al. 1999) and NaV1.6 (Wang et al. 2009) subject to mechanical stress, although knock-out strain phenotypes might suggest that a direct role of NaV1.8 and NaV1.7 in the process of mechanotransduction in sensory neurones is unlikely (Nassar et al. 2004; Drew et al. 2004). Nevertheless, the process of transformation contributes to stimulus encoding and elevated thresholds of mechanically evoked pain are observed in mice lacking TTX-r NaV1.8 (Akopian et al. 1996; Abrahamsen et al. 2008).
A reduction in voltage-gated sodium channel availability is a critical factor contributing to the activity-related reduction in conduction velocity observed in unmyelinated axons. Similarly, the time to action potential initiation in small-diameter DRG neurones is prolonged during low-frequency (2 Hz) repetitive activation (Snape et al. 2010). Here we show that this has an important physiological correlate with low-frequency activity leading to a progressive increase in mechanical activation threshold in individual unmyelinated sensory axons. The empirical tracking of axonal conduction velocity, such as that used in human microneurography, thereby provides an indirect means of monitoring changes in activation threshold for sensory stimuli in unmyelinated axons.
Acknowledgments
We are grateful to Birgit Vogler and Jana Schramm for technical assistance. This work was funded by grants from the Deutsche Forschungsgemeinschaft to K.M. (ME995/1) and R.W.C. (CA853/1-2).
Glossary
Abbreviations
- DRG
dorsal root ganglion (DRG)
- NaV
voltage-gated sodium channel
Author contributions
Experiments were performed in K.M.'s laboratory in Erlangen. R.D.C. and R.W.C. conceived and designed the experiments and collected, analysed and interpreted the data. R.D.C., K.M. and R.W.C. drafted the manuscript. All authors approved the final version of the manuscript.
References
- Abrahamsen B, Zhao J, Asante CO, Cendan CM, Marsh S, Martinez-Barbera JP, Nassar MA, Dickenson AH, Wood JN. The cell and molecular basis of mechanical, cold, and inflammatory pain. Science. 2008;321:702–705. doi: 10.1126/science.1156916. [DOI] [PubMed] [Google Scholar]
- Akopian AN, Sivilotti L, Wood JN. A tetrodotoxin-resistant voltage-gated sodium channel expressed by sensory neurons. Nature. 1996;379:257–262. doi: 10.1038/379257a0. [DOI] [PubMed] [Google Scholar]
- Blair NT, Bean BP. Role of tetrodotoxin-resistant Na+ current slow inactivation in adaptation of action potential firing in small-diameter dorsal root ganglion neurons. J Neurosci. 2003;23:10338–10350. doi: 10.1523/JNEUROSCI.23-32-10338.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brock JA, McLachlan EM, Belmonte C. Tetrodotoxin-resistant impulses in single nociceptor nerve terminals in guinea-pig cornea. J Physiol. 1998;512:211–217. doi: 10.1111/j.1469-7793.1998.211bf.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carr RW, Pianova S, Brock JA. The effects of polarizing current on nerve terminal impulses recorded from polymodal and cold receptors in the guinea-pig cornea. J Gen Physiol. 2002;120:395–405. doi: 10.1085/jgp.20028628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carr RW, Pianova S, McKemy DD, Brock JA. Action potential initiation in the peripheral terminals of cold-sensitive neurones innervating the guinea-pig cornea. J Physiol. 2009;587:1249–1264. doi: 10.1113/jphysiol.2008.167023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cho H, Shin J, Shin CY, Lee SY, Oh U. Mechanosensitive ion channels in cultured sensory neurons of neonatal rats. J Neurosci. 2002;22:1238–1247. doi: 10.1523/JNEUROSCI.22-04-01238.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Col R, Messlinger K, Carr RW. Conduction velocity is regulated by sodium channel inactivation in unmyelinated axons innervating the rat cranial meninges. J Physiol. 2008;586:1089–1103. doi: 10.1113/jphysiol.2007.145383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drew LJ, Rohrer DK, Price MP, Blaver KE, Cockayne DA, Cesare P, Wood JN. Acid-sensing ion channels ASIC2 and ASIC3 do not contribute to mechanically activated currents in mammalian sensory neurones. J Physiol. 2004;556:691–710. doi: 10.1113/jphysiol.2003.058693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drew LJ, Wood JN, Cesare P. Distinct mechanosensitive properties of capsaicin-sensitive and -insensitive sensory neurons. J Neurosci. 2002;22:RC228. doi: 10.1523/JNEUROSCI.22-12-j0001.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fazan R, Jr, Whiteis CA, Chapleau MW, Abboud FM, Bielefeldt K. Slow inactivation of sodium currents in the rat nodose neurons. Auton Neurosci. 2001;87:209–216. doi: 10.1016/S1566-0702(00)00281-2. [DOI] [PubMed] [Google Scholar]
- Fricke B, Andres KH, von Düring M. Nerve fibres innervating the cranial and spinal meninges: morphology of nerve fibre terminals and their structural integration. Microsc Res Tech. 2001;53:96–105. doi: 10.1002/jemt.1074. [DOI] [PubMed] [Google Scholar]
- Gee MD, Lynn B, Cotsell B. Activity-dependent slowing of conduction velocity provides a method for identifying different functional classes of C-fibre in the rat saphenous nerve. Neuroscience. 1996;73:667–675. doi: 10.1016/0306-4522(96)00070-x. [DOI] [PubMed] [Google Scholar]
- Honore E, Patel AJ, Chemin J, Suchyna T, Sachs F. Desensitization of mechano-gated K2P channels. Proc Natl Acad Sci U S A. 2006;103:6859–6864. doi: 10.1073/pnas.0600463103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu J, Lewin GR. Mechanosensitive currents in the neurites of cultured mouse sensory neurones. J Physiol. 2006;577:815–828. doi: 10.1113/jphysiol.2006.117648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Just S, Heppelmann B. Frequency dependent changes in mechanosensitivity of rat knee joint afferents after antidromic saphenous nerve stimulation. Neuroscience. 2002;112:783–789. doi: 10.1016/s0306-4522(02)00125-2. [DOI] [PubMed] [Google Scholar]
- Kenins P. The functional anatomy of the receptive fields of rabbit C polymodal nociceptors. J Neurophysiol. 1988;59:1098–1115. doi: 10.1152/jn.1988.59.4.1098. [DOI] [PubMed] [Google Scholar]
- Lambert GA, Mallos G, Zagami AS. Von Frey's hairs – a review of their technology and use – a novel automated von Frey device for improved testing for hyperalgesia. J Neurosci Methods. 2009;177:420–426. doi: 10.1016/j.jneumeth.2008.10.033. [DOI] [PubMed] [Google Scholar]
- McCarter GC, Levine JD. Ionic basis of a mechanotransduction current in adult rat dorsal root ganglion neurons. Mol Pain. 2006;2:28. doi: 10.1186/1744-8069-2-28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Messlinger K, Hanesch U, Baumgärtel M, Trost B, Schmidt RF. Innervation of the dura mater encephali of cat and rat: ultrastructure and calcitonin gene-related peptide-like and substance P-like immunoreactivity. Anat Embryol. 1993;188:219–237. doi: 10.1007/BF00188214. [DOI] [PubMed] [Google Scholar]
- Morris CE, Juranka PF. Nav channel mechanosensitivity: activation and inactivation accelerate reversibly with stretch. Biophys J. 2007;93:822–833. doi: 10.1529/biophysj.106.101246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nassar MA, Stirling LC, Forlani G, Baker MD, Matthews EA, Dickenson AH, Wood JN. Nociceptor-specific gene deletion reveals a major role for Nav1.7 (PN1) in acute and inflammatory pain. Proc Natl Acad Sci U S A. 2004;101:12706–12711. doi: 10.1073/pnas.0404915101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ogata N, Tatebayashi H. Slow inactivation of tetrodotoxin-insensitive Na+ channels in neurons of rat dorsal root ganglia. J Membr Biol. 1992;129:71–80. doi: 10.1007/BF00232056. [DOI] [PubMed] [Google Scholar]
- Olausson B. Recordings of human polymodal single C-fibre afferents following mechanical and argon-laser heat stimulation of inflamed skin. Exp Brain Res. 1998;122:55–61. doi: 10.1007/s002210050490. [DOI] [PubMed] [Google Scholar]
- Rang HP, Ritchie JM. On the electrogenic sodium pump in mammalian non-myelinated nerve fibres and its activation by various external cations. J Physiol. 1968;196:183–221. doi: 10.1113/jphysiol.1968.sp008502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raymond SA. Effects of nerve impulses on threshold of frog sciatic nerve fibres. J Physiol. 1979;290:273–303. doi: 10.1113/jphysiol.1979.sp012771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raymond SA, Thalhammer JG, Popitz-Bergez F, Strichartz GR. Changes in axonal impulse conduction correlate with sensory modality in primary afferent fibres in the rat. Brain Res. 1990;526:318–321. doi: 10.1016/0006-8993(90)91239-d. [DOI] [PubMed] [Google Scholar]
- Rugiero F, Drew LJ, Wood JN. Kinetic properties of mechanically activated currents in spinal sensory neurons. J Physiol. 2010;588:301–314. doi: 10.1113/jphysiol.2009.182360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rush AM, Brau ME, Elliott AA, Elliott JR. Electrophysiological properties of sodium current subtypes in small cells from adult rat dorsal root ganglia. J Physiol. 1998;511:771–789. doi: 10.1111/j.1469-7793.1998.771bg.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shcherbatko A, Ono F, Mandel G, Brehm P. Voltage-dependent sodium channel function is regulated through membrane mechanics. Biophys J. 1999;77:1945–1959. doi: 10.1016/S0006-3495(99)77036-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Serra J, Campero M, Ochoa J, Bostock H. Activity-dependent slowing of conduction differentiates functional subtypes of C fibres innervating human skin. J Physiol. 1999;515:799–811. doi: 10.1111/j.1469-7793.1999.799ab.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snape A, Pittaway JF, Baker MD. Excitability parameters and sensitivity to anemone toxin ATX-II in rat small diameter primary sensory neurones discriminated by Griffonia simplicifolia isolectin IB4. J Physiol. 2010;588:125–137. doi: 10.1113/jphysiol.2009.181107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takahashi A, Gotoh H. Mechanosensitive whole-cell currents in cultured rat somatosensory neurons. Brain Res. 2000;869:225–230. doi: 10.1016/s0006-8993(00)02366-0. [DOI] [PubMed] [Google Scholar]
- Thalhammer JG, Raymond SA, Popitz-Bergez FA, Strichartz GR. Modality-dependent modulation of conduction by impulse activity in functionally characterized single cutaneous afferents in the rat. Somatosens Mot Res. 1994;11:243–257. doi: 10.3109/08990229409051392. [DOI] [PubMed] [Google Scholar]
- Tripathi PK, Trujillo L, Cardenas CA, Cardenas CG, de Armendi AJ, Scroggs RS. Analysis of the variation in use-dependent inactivation of high-threshold tetrodotoxin-resistant sodium currents recorded from rat sensory neurons. Neuroscience. 2006;143:923–938. doi: 10.1016/j.neuroscience.2006.08.052. [DOI] [PubMed] [Google Scholar]
- Wang JA, Lin W, Morris T, Banderali U, Juranka PF, Morris CE. Membrane trauma and Na+-leak from Nav1.6 channels. Am J Physiol Cell Physiol. 2009;297:C823–C834. doi: 10.1152/ajpcell.00505.2008. [DOI] [PubMed] [Google Scholar]
- Weidner C, Schmelz M, Schmidt R, Hansson B, Handwerker HO, Torebjork HE. Functional attributes discriminating mechano-insensitive and mechano-responsive C nociceptors in human skin. J Neurosci. 1999;19:10184–10190. doi: 10.1523/JNEUROSCI.19-22-10184.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]


