Abstract
Non-technical summary
Ca2+-regulated axon terminal exocytosis is essential for neuronal communication in the CNS. Besides, the somata may also undergo Ca2+-dependent exocytosis in some neurons in the brain. In the present work we recorded activity/Ca2+-dependent secretion in somata of the mesencephalic trigeminal nucleus (MeV). Following nerve activity, somatic secretion signals are recorded as changes in membrane surface area, as measured by the membrane capacitance. The depolarization-induced somatic exocytosis correlates with transient reduction in the GABA-mediated postsynaptic currents. These results implicated that the somatic secretion may play a role in bidirectional communication between pre- and post-connected neurons in the sensory MeV brain slice neurons.
Abstract
The neurons in the mesencephalic trigeminal nucleus (MeV) play essential roles in proprioceptive sensation of the face and oral cavity. The somata of MeV neurons are generally assumed to carry out neuronal functions but not to play a direct role in synaptic transmission. Using whole-cell recording and membrane capacitance (Cm) measurements, we found that the somata of MeV neurons underwent robust exocytosis (Cm jumps) upon depolarization and with the normal firing of action potentials in brain slices. Both removing {Ca2+}o and buffering {Ca2+}i with BAPTA blocked this exocytosis, indicating that it was completely Ca2+ dependent. In addition, an electron microscopic study showed synaptic-like vesicles approximated to the plasma membrane in somata. There was a single Ca2+-dependent releasable vesicle pool with a peak release rate of 1912 fF s-1. Importantly, following depolarization-induced somatic exocytosis, GABA-mediated postsynaptic currents were transiently reduced by 31%, suggesting that the somatic vesicular release had a retrograde effect on afferent GABAergic transmission. These results provide strong evidence that the somata of MeV neurons undergo robust somatic secretion and may play a crucial role in bidirectional communication between somata and their synaptic inputs in the central nervous system.
Introduction
The mesencephalic trigeminal nucleus (MeV) in mammals, forming a narrow band of cells extending from the lateral margin of the periaqueductal grey at the level of the superior colliculus rostrally to the trigeminal motor nucleus, innervates the muscle spindles of the jaw-closing muscles and receives input from the mechanoreceptors of the periodontal ligaments (Taylor & Davey, 1968; Goodwin & Luschei, 1974). MeV neurons are derived from the neural crest and migrate into the central nervous system, retaining some characteristics of neural crest cell derivatives, i.e. dorsal root ganglion (DRG) neurons and trigeminal ganglion neurons (TGNs). In contrast to other primary proprioceptive neurons of the DRG, MeV neurons are situated in the brainstem and receive numerous axosomatic contacts from various regions which regulate feeding behaviour (Dessem & Taylor, 1989; Ishii et al. 2005; Lazarov, 2007). Neurons utilize Ca2+-dependent exocytosis for fast synaptic transmission at presynaptic terminals. Although much less studied, somatic regions also undergo vesicular release in both central and peripheral neurons, including those of the locus coeruleus (Huang et al. 2007), DRG (Huang & Neher, 1996; Zhang & Zhou, 2002) and TGN (Matsuka et al. 2007). However, it is unclear whether vesicular exocytosis occurs in the somata of MeV neurons and whether it plays a role in synaptic transmission.
To determine whether somatic exocytosis occurs in the somata of MeV neurons, a real-time measurement is needed. Several methods have been developed to monitor exocytosis, including membrane capacitance (Cm) measurement (Neher & Zucker, 1993; Sun & Wu, 2001; Zhang et al. 2011) and electrochemical amperometry (Wightman et al. 1991; Zhou & Misler, 1995; Huang et al. 2007). In this report, using Cm as a selective assay for somatic exocytosis in neurons in brain slices, we found that the somata of MeV neurons underwent robust Ca2+-dependent vesicular exocytosis, which retrogradely regulated afferent GABAergic inputs. An electron microscopy (EM) study also provided morphological evidence supporting the occurrence of somatic exocytosis in MeV neurons.
Methods
Slice preparation
Experiments were performed on brain slice preparations obtained from young rats on postnatal days 8–10. To avoid the known effects that anaesthetics have on GABAA-mediated postsynaptic currents, rats were killed by decapitation using a small animal guillotine as authorised by the Peking University Animal Care and Use Committee. The brains were immediately dissected and transverse brain slices (200–300 μm) were cut with a slicer (DTK-1000, Dosaka EM, Kyoto, Japan) in ice-cold artificial cerebrospinal fluid (aCSF) with reduced Ca2+ (0.1 mm) and increased Mg2+ (3 mm). The standard aCSF contained (in mm): 125 NaCl, 2.5 KCl, 10 glucose,1.25 NaH2PO4, 2 sodium pyruvate, 3 myo-inositol, 0.5 ascorbic acid, 26 NaHCO3, 1 MgCl2 and 2 CaCl2 (pH 7.4, when oxygenated with 95% O2 and 5% CO2). Slices were incubated for 1 h at 35°C in the standard aCSF and maintained thereafter at room temperature (20–25°C).
Isolation of MeV neurons
MeV neurons were isolated by a method described in a previous report (Yoshida & Oka, 1998) with modification. Briefly, rats from postnatal day 8–10 were decapitated with a small guillotine and transverse slices (500–600 μm) were cut with a slicer. The MeV nucleus was trimmed off from a slice with fine injection needles (26G) under a stereomicroscope. The block containing the MeV nucleus was then incubated for 25 min at 35°C in dissociation buffer with 4 mg ml−1 papain (Merck 6000 USP-u/mg). Dissociation buffer contained (in mm): 82 Na2SO4, 30 K2SO4, 10 Hepes, 5 MgCl2 and 10 glucose. During these procedures, 100% O2 was continuously bubbled into the bathing solution. Subsequently, the blocks were placed in fresh dissociation buffer containing 1 mg ml−1 bovine serum albumin to terminate the enzymatic digestion. Tissue blocks were mechanically dissociated with fire-polished glass Pasteur pipettes. Following that, isolated cells were plated on polystyrene dishes 35 mm in diameter (3801, Falcon) and maintained in a plastic container gassed with 100% O2 for 1–1.5 h at room temperature (20–25°C) to allow the cells to recover from injury and settle to the bottom of the dish.
Electrophysiology
Patch electrodes were fabricated from glass with filaments and coated with dental wax. The resistances of these pipettes were 2–3 MΩ and compensated to 70–90%. The mean access series resistance of the neurons included in the study was <10 MΩ. Cells showing higher resistances (>15 MΩ) were not used for analysis. For Ca2+ current, TTX (1 μm) and 4-AP (0.5 mm) were added to the aCSF; the intracellular recording solution contained (in mm): 100 CsCl, 40 Hepes, 0.5 EGTA, 1 MgCl2, 2 ATP, 0.5 GTP, 12 phosphocreatine, 20 TEA and 3 potassium glutamate, and pH adjusted to 7.3 with CsOH. For current-clamp recording, the intracellular solution contained (in mm): 97.5 potassium gluconate, 32.5 KCl, 0.5 EGTA, 40 Hepes, 1 MgCl2, 2 ATP and 0.5 GTP (pH adjusted to 7.3 with KOH). All chemicals were from Sigma (St Louis, MO, USA).
Cm measurements were performed using an EPC/9 amplifier (HEKA Electronic, Lambrecht/Pfalz, Germany) and the Sine + DC technique (Lindau & Neher, 1988). A sine wave (20 mV, 1000 Hz) was superimposed onto a holding potential of −60 mV. The Cm amplitude was measured as the difference between baseline and 100 ms after depolarization. The Cm of single vesicles was calculated from the vesicle area using the equation: Cm= 1 (pF μm−2) × (π× diameter2). The Cm of a single synaptic-like vesicle (SLV) 50 nm in diameter is equal to ∼75 aF.
During field stimulation experiments, PSCs were induced by electrical stimuli (0.2 ms, 3–10 V) at 0.2 Hz via a bipolar electrode (MS303 Plastics One Inc., Roanoke, VA, USA). The stimulating electrode was placed in the slice (∼50 μm depth) at a site immediately dorso- or ventro-lateral to the MeV and 150 μm away from the recording pipette (Yokomizo et al. 2005). The PSC was an average of two sequentially recorded PSCs both before and after somatic stimulation (Fig. 6).
Figure 6. Depolarization evoked exocytosis and modulated GABAergic inputs.
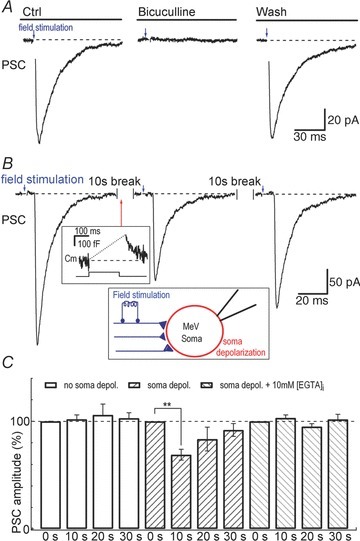
A, field stimulation-induced currents recorded from the soma of a MeV neuron. Bicuculline (10 μm) abolished the stimulation-induced inward current, demonstrating that the inward current was mediated by GABAergic inputs. B, representative experiment showing postsynaptic currents (PSC) from the soma of an MeV neuron following 0.2 Hz field stimulation. The whole-cell soma depolarization (from −60 mV to 0 mV, 200 ms), which triggers somatic release (267 fF in the example trace, similar to Figs 1 and 4), was delivered in the interval between two PSCs (at the time marked by the arrow during field stimulation). The PSCs shown here were averaged from two sequential recordings during 10 s both before and after soma depolarization. C, field stimulation-evoked PSCs at 0, 10, 20 and 30 s following no somatic depolarization (open columns, n = 5), somatic depolarization (cis-stripe, n = 6), and somatic depolarization plus 10 mm{EGTA}i dialysis (trans-stripe, n = 5).
{Ca2+}i imaging
For intracellular Ca2+ imaging, MeV neurons in slices were whole-cell dialysed with 0.1 mm fura-2-K (Invitrogen, Carlsbad, CA, USA) and photographed. Individual neurons were imaged using a 60× water-immersion lens with differential interference contrast (DIC) optics on a fixed-stage upright microscope (BX51 WI; Olympus Optical, Tokyo, Japan). Ca2+ imaging was performed using the TILLvisION Imaging System (TILL Photonics, Martinsried, Germany). We used a ratiometric measure (F340/F380) by alternating between the wavelengths 340 and 380 nm for {Ca2+}i (Chen et al. 2005). Baseline correction of the fluorescence was not used.
Dopamine loading and amperometry
MeV slices were loaded in a bath solution containing 40 mm dopamine (DA) for 40 min at 37°C, similar to our previous reports (Zhang & Zhou, 2002; Chen et al. 2005). After DA loading, the slices were washed five times with standard aCSF. Highly sensitive, low-noise, carbon fibre electrodes (ProCFE; Dagan, Minneapolis, MN, USA) were used for electrochemical monitoring of quantal DA release from MeV neurons. The amperometric current (Iamp) was measured at 780 mV. Amperometric signals were low-pass filtered at 0.3 kHz and digitized at 1kHz.
Electron microscopy
Electron microscopy was performed as described previously (Luo et al. 2001). In brief, horseradish peroxidase (HRP) was used to retrogradely label the MeV neurons. Male Sprague–Dawley rats (300–360 g) were anaesthetized with sodium pentobarbital (40 mg kg-1, i.p.). A glass micropipette with a tip diameter of 30–50 μm filled with 20% HRP (type VI, Sigma) admixed with 1% wheat-germ agglutinated HRP (WGA-HRP, Sigma) was injected into the masseteric nerve. The animals were given an analgesic (bupronorphine 0.05–0.1 mg kg−1, s.c.) after recovery and were given food and water ad libitum. Five days after HRP injection, the animals were re-anaesthetized and perfused transcardially with 0.9% saline, followed by 1% glutaraldehyde and 2% paraformaldehyde in 0.1 m phosphate buffer (pH 7.4). The brain was removed and the MeV was sliced transversely at 50 μm with a Vibratome, followed by addition of 1% osmium tetroxide. After block-staining with uranyl acetate, dehydration and embedding, sections were cut, counterstained with lead citrate, and examined in a JEOL 1200EX electron microscope as described in our previous studies. SLVs were identified by their 40–60 nm cross-sectional diameter, round-to-pleomorphic shape, and electron-lucent lumen.
Data analysis
Data were acquired on-line, filtered at 2.9 kHz, digitized at 10 kHz, and analysed off-line using Igor and Excel 2003 (Microsoft, Redmond, WA, USA). Statistical tests of significance with two-tailed unpaired or paired Student's t tests were used with a P value cut-off of <0.05. Data are expressed as the mean ± SEM.
Results
Ca2+-dependent exocytosis with depolarization in somata of MeV neurons
Somata of MeV neurons are easily identified by their location lateral to the locus coeruleus (Huang et al. 2007), by their large monopolar morphology (Fig. 1A), and by their large Ih currents (Supplementary Fig. S1; see also Khakh & Henderson, 1998). To determine whether somatic exocytosis occurs in MeV neurons in brain slices, we measured Cm jump, Ca2+ current and the associated {Ca2+}i in response to pulse depolarization (from −60 mV to +10 mV, 100 ms) (Fig. 1A). Following the depolarization-induced Ca2+ current, Cm jumped abruptly with 120 fF Cm increases. Since the baseline Cm of this neuron was 53 pF, the Cm increase was about 0.2% of total capacitance (Fig. 1A, right). After the Cm increase, Cm decayed to baseline, indicating the net retrieval of vesicular membrane (endocytosis). The simultaneous {Ca2+}i rise and Cm jump were reminiscent of Ca2+-dependent exocytosis in other exocytotic cells (Neher & Zucker, 1993; Zhou & Misler, 1995), suggesting that the somata of MeV neurons also undergo {Ca2+}i-dependent exocytosis. Most of the neurons examined in this study were large MeV neurons (Cm≥ 40 pF).
Figure 1. Ca2+ dependence of exocytosis in somata of MeV neurons.
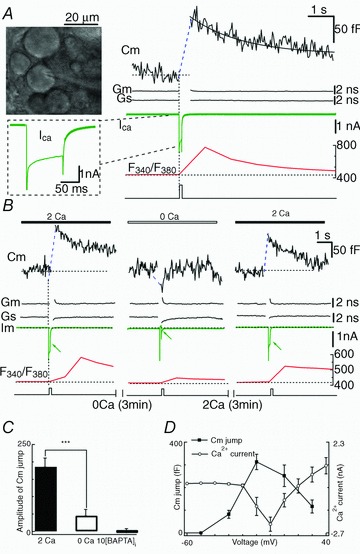
A, left upper: photomicrograph of MeV neurons in a rat brain slice. Right: Cm jump evoked by a depolarizing pulse (from −60 mV to +10 mV, 100 ms). The Ca2+ currents (ICa) and {Ca2+}i with Ca2+ imaging (F340/F380) recorded simultaneously. The baseline Cm of the neuron was 53.02 pF (dotted line). The decay of the Cm response was fitted by a single exponential function with τ= 2.1 s (continuous line). B, pre-puffing 0 mm Ca2+ attenuated the Cm response (triggered by a depolarizing pulse to +10 mV, 100 ms). Both Ca2+ currents and {Ca2+}i decreased. C, statistical bar graph showing pulse stimulation-triggered Cm jumps as in B, that were abolished by 10 mm BAPTA in the pipette. D, Cm jump and Ca2+ current as a function of membrane potential. The Cm response was observable at membrane potentials positive to −30 mV, and peaked round 0 mV (n = 8). The Ca2+ current was recorded at different depolarizing potentials (100 ms, from −60 mV to 60 mV, +10 mV steps) in different cells (n = 8).
To determine the Ca2+ dependence of vesicle release, we puffed the slice with Ca2+-free solution (3 min) and found that the depolarization-induced Cm jump was dramatically attenuated (186 ± 25 fF to 45 ± 18 fF, P < 0.001, n = 14, Fig. 1C), accompanied by a decreased Ca2+ current and {Ca2+}i (Fig. 1B and C). The Cm jump was abolished by 10 mm BAPTA, a Ca2+ chelator, in the pipette solution (1 ± 8.6 fF, n = 5, Fig. 1C). These data indicated that the Cm response was fully dependent on the {Ca2+}i. Next, we sought to determine whether the membrane potential itself regulated somatic release (Zhang & Zhou, 2002). With a given pulse duration, the Ca2+ current and Cm signals increased with membrane depolarization from −60 mV and reached a maximum at ∼0 mV, and then the Cm jump and Ca2+ current declined (Fig. 1D). Thus, depolarization-induced exocytosis occurs via a {Ca2+}i rise (but not the membrane potential per se) in the somata of MeV neurons.
To confirm that the evoked Cm jumps in MeV slice neurons were not artifacts due to gap junction conductance, we examined freshly isolated MeV neurons. As in the MeV brain slice, a Cm jump occurred in the somata of isolated MeV neurons in response to pulse depolarization (Fig. 2). Based on their similarity to the exocytotic Cm changes described in the somata of other cells, including DRG neurons (Huang & Neher, 1996; Zhang & Zhou, 2002) and chromaffin cells (Duan et al. 2003), we interpreted the Cm jump in the soma of MeV neurons (both in the slice and freshly isolated neurons) as a membrane surface area increase that occurs during vesicle fusion/exocytosis.
Figure 2. Depolarization-induced Cm jump in somata of isolated MeV neurons.
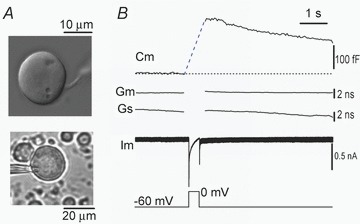
A, photomicrograph of two isolated MeV neurons under bright field DIC, one with a typical monopolar axon (upper) and the other being whole-cell patch-clamped (lower). B, a Cm jump occurred abruptly in response to 500 ms depolarization (to 0 mV). Similar results were obtained in four other neurons.
In order to ‘visualize’ exocytosis in MeV neurons, we preloaded a subset of neurons with dopamine as a false transmitter. This method is used in pancreatic β cells and DRG neurons where dopamine is first taken up by the cells and then released via somatic exocytosis (Zhou & Misler, 1996; Zhang & Zhou, 2002). Indeed, depolarization-induced quantal release of preloaded dopamine was readily detected on the soma of MeV neurons in brain slices by amperometry (Fig. 3). This is consistent with the interpretation that the depolarization-induced Cm signals in Fig. 1 represent somatic exocytosis. We note that the false transmitter (dopamine) and the native transmitter(s) may not necessary share comparable underlying mechanisms of release.
Figure 3. Amperometric spikes in DA-preloaded MeV neurons in brain slices.
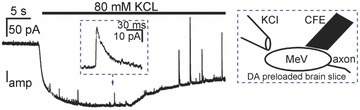
High potassium (KCl, 80 mm)-induced amperometric spikes in a MeV soma. The slowly descending background current is an artifact caused by puffing KCl (Chen et al. 2005). Inset: a single expanded amperometric spike. Similar amperometric signals were obtained from 3 slices.
Exocytosis from a single releasable pool in MeV neurons
In order to verify vesicular exocytosis from Cm recording (Fig. 1), we did an EM study. As it was possible to inject HRP into the masseteric nerve with a micropipette in adult rats, we carried out the study in adults. The HRP-labelled somata received an afferent bouton to form an asymmetric synapse, which was identified by the widened synaptic cleft and the presence of postsynaptic membrane thickening and postsynaptic densities (Fig. S2). The afferent synaptic bouton was not labelled by HRP and showed predominantly spherical small synaptic vesicles (∼50 nm) and mitochondria (Fig. S2). We found that the MeV somata contained synaptic-like vesicles (SLVs) (∼50 nm) apposed to the plasma membrane, some of which resembled clathrin-coated vesicles (blue arrow, Fig. S2A), and might represent exocytosis-coupled endocytosis. Interestingly, the SLVs in somata located opposite to the presynaptic active zone might form a functional ‘reciprocal synapse’ similar to that in the olfactory system (Isaacson & Strowbridge, 1998). These SLVs might be responsible for the evoked Cm signals in Fig. 1. However, some of the SLVs could also be Ca2+-independent vesicles following turnover of postsynaptic receptors (Wang et al. 2006).
To determine the pool size and the release rate of the releasable vesicles, we measured Cm jumps elicited by a series of step depolarizations (to +10 mV) of increasing duration to deplete the releasable vesicle pool. The Cm response was visible following the 20 ms pulse. As the duration was lengthened, the Cm response became larger (Fig. 4A). In the plot of Cmversus pulse duration, the Cm response was best fitted with a single exponential equation: f(t) =fmax× (1 − exp(−t/τ)) with an fmax (the maximal Cm response) of 749 and a τ of 321 ms, suggesting that a single vesicle pool was responsible for the Ca2+-dependent Cm changes (Fig. 1A). Linear regression of the first 100 ms showed the peak release rate had a value of 1912 fF s−1 (Fig. 4B). The SLVs had a diameter of ∼50 nm (Fig. 4A and B), corresponding to 75 aF. Therefore, the theoretical maximal Cm response (749 fF) was equal to ∼10000 SLVs. It should be noted that, however, this theoretical estimation may underestimate the pool size owing to variations in the Ca2+ current elicited by repeated pulses or inactivation of the Ca2+ channel.
Figure 4. Vesicular release rate in somata of MeV neurons.
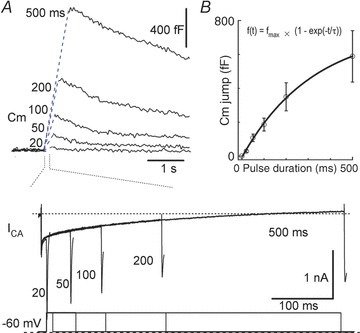
A, Cm responses induced by depolarization (to +10 mV) with pulse durations from 20 to 500 ms. Lower traces: Ca2+ currents induced by pulse stimulation. B, Cm as a function of depolarization duration. The Cm increased as a single exponential function (fmax= 749, τ= 321 ms) against pulse duration (n = 8).
Cm responses to natural firing patterns
Neurons in MeV slices exhibited two action potential (AP) patterns in response to depolarizing current: phasic firing (77.5%, 31/40), which discharged 1 to 3 APs with elevated current injection (mean spike number 1.54 ± 0.25, n = 31); and tonic firing, which produced many APs depending on the injection current (Fig. 5A; see also Henderson et al. 1982). Both phasic and tonic patterns showed strong afterhyperpolarization following the current injection (Fig. 5A). To determine whether Cm responses can be activated by native AP patterns, we applied the recorded phasic and tonic firing patterns (Fig. 5A) to whole-cell clamped neurons and recorded their Cm responses. These experiments demonstrated that the native tonic AP pattern elicited a much greater Cm change (212 ± 49 fF) than the phasic AP pattern (21 ± 27 fF, n = 8, P < 0.001, Fig. 5B and C), similar to somatic release in locus coeruleus neurons (Huang et al. 2007). This result is in contrast to adrenal chromaffin cells, where the native phasic AP pattern is more potent than the tonic pattern (Duan et al. 2003), probably because the phasic plateau is less depolarized for Ca2+ influx in MeV neurons.
Figure 5. Natural firing patterns modulate Cm responses of MeV neurons.
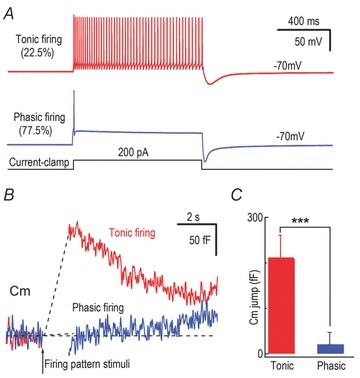
A, representative firing patterns, phasic and tonic, in MeV neurons in response to depolarization pulses (1 s, 200 pA current injection) under current clamp. B, using the phasic and tonic firing patterns recorded by current clamp in A as stimulation templates, we recorded Cm responses with voltage clamp in a MeV neuron soma. C, statistics of averaged Cm responses in B.
GABAergic inputs were inhibited after somatic depolarization
MeV neurons receive numerous axosomatic contacts from various regions (Lazarov, 2007) and the SLVs in the somata of MeVs located opposite to the afferent synaptic inputs (Fig. S2A and S2B) prompted us to investigate the effect of the somatic exocytosis in MeV neurons on their afferent inputs. Axosomatic postsynaptic currents (PSCs) (Fig. 6A, left trace) in MeV somata were triggered by field stimulation. Similar to a previous report (Yokomizo et al. 2005), field stimulation triggered PSCs in parts of the MeV slices (in brain slices, only a fraction of nerve–soma links is intact for field stimulation). These currents may originate from either glutamatergic inputs (Verdier et al. 2004) or GABAergic inputs (Hayar et al. 1997; Yokomizo et al. 2005). Perfusion with the GABAA receptor blocker bicuculline (10 μm) abolished the currents, demonstrating that the PSCs originated from GABAergic synapse(s). A 200 ms depolarization (to 0 mV) delivered to the soma between two evoked PSCs (at 0.2 Hz) triggered a large Cm jump (267 fF, Fig. 6B, similar to Figs 1 and 5). Ten seconds after this somatic depolarization, the PSC amplitude declined to 69 ± 5% (P < 0.01, n = 6, Fig. 6B and C) and then recovered within 30 s. As a control, the PSC amplitude did not significantly change at 10 s following the first PSC without somatic depolarization (102 ± 4% of that at 0 s, P > 0.05, n = 5, Fig. 6C). To further confirm that the depolarization-induced Cm jumps were responsible for the PSC inhibition, we dialysed 10 mm of the Ca2+ chelator EGTA into MeV somata and found that the intracellular EGTA abolished both the somatic depolarization-induced inhibition of PSCs (10 s: 103 ± 3% of that at 0 s, P > 0.05, n = 5) and the Cm change (control, 186 ± 25 fF, n = 14; EGTA, 2 ± 6 fF, n = 5; P < 0.001) (Fig. S3 and Fig. 6C). Further, we investigated the involvement of retrograde endocannabinoid and its type-1 receptor (CB1R) in the somatic PSC inhibition (Fig. S4). The CB1R antagonist AM251 (Diana & Marty, 2004; Kano et al. 2009) abolished the somatic depolarization-induced PSC inhibition (Fig. S4), suggesting the involvement of CB1R in the PSC inhibition. All together, these results demonstrated that Ca2+-dependent somatic release transiently inhibited GABAergic inputs in MeV neurons.
Discussion
In this study, we demonstrated that, in response to pulse depolarization and native tonic APs, MeV neurons underwent robust Ca2+-dependent somatic vesicular exocytosis. To our knowledge, this is the first capacitance recording of somatic exocytosis in a brain slice. We found only one somatic vesicle pool and a maximum release rate of 1912 fF s−1 in MeV neurons. Furthermore, the Ca2+-dependent somatic release from MeV neurons produced a retrograde inhibition of GABAergic inputs at the same soma.
Capacitance recording of somatic exocytosis in a brain slice
Membrane capacitance, Cm, has been used to record exocytosis in endocrine cells (Neher & Zucker, 1993; Duan et al. 2003), isolated neuronal somata (Huang et al. 2007; Matsuka et al. 2007) and the giant presynaptic terminals in the calyx of Held brain slice (Sun & Wu, 2001; Zhang et al. 2011). In this study, for the first time that we are aware of, we have recorded somatic exocytosis in neurons of a brain slice. Cm selectively records exocytosis in voltage-clamped plasma membrane in the somatic area in MeV, and not the cell processes (Jackson, 1992; Hsu & Jackson, 1996). However, we cannot exclude release from small processes and rudimentary dendrites in some MeV somata (Alley, 1973). Cm has the advantage that it measures exocytosis of any vesicle type. MeV neurons in the caudal regions are considered to be electrotonically coupled (Hinrichsen & Larramendi, 1970; Baker & Llinas, 1971). Fortunately, these gap junctions did not prevent our Cm recording of somatic exocytosis (Figs 1–6). However, Cm measures the total change in membrane surface including both exocytosis and endocytosis. It may underestimate the amount of total exocytosis. Although Cm can record exocytosis in several types of brain slice neurons, including the calyx of Held synapse (Sun & Wu, 2001; Zhang et al. 2011) and the soma of MeV neurons, it is necessary to determine whether Cm is applicable to other types of neurons in brain slices.
Ca2+-dependent somatic exocytosis in MeV neurons
In the present work, we demonstrated that MeV neurons underwent Ca2+-dependent somatic vesicular exocytosis in brain slices (Figs 1, 4 and 5). We have previously reported two types of exocytosis in the soma of sensory DRG neurons: Ca2+-dependent secretion and Ca2+-independent but voltage-dependent secretion (Zhang & Zhou, 2002; Zheng et al. 2009). In contrast, only Ca2+-dependent secretion occurred in the soma of sensory MeV neurons (Fig. 1).
Despite the physiological evidence for vesicular release of neurotransmitter from the somata of DRG and TGN neurons, there are no direct anatomical data to complement these findings (Huang & Neher, 1996; Zhang & Zhou, 2002; Matsuka et al. 2007). Existing EM data about the organelles in sensory neuron somata do not reveal the presence of vesicles in proximity to the plasma membrane of the type that are commonly observed at the synaptic terminals of these neurons in the sympathetic ganglia (Heym et al. 1993) and the spinal cord (Valtschanoff et al. 1994). Our EM data (Fig. S2) showed vesicles in proximity to the plasma membrane in the somata of MeV neurons. These SLVs might easily have been missed in previous EM studies (Hinrichsen & Larramendi, 1970; Alley, 1973; Luo et al. 1995) due to their low density and the possible depletion of vesicles in the vicinity of the plasma membrane during fixation procedures in native tissue. The EM methods used here cannot discriminate between Ca2+-dependent exocytosis and reluctantly releasable vesicles (such as turnover of postsynaptic receptors). The vesicle density and location could also be different between cells in younger and adult rats. Nevertheless, our EM results in light of the Cm recordings in brain slices (Fig. 1) and isolated MeV neurons (Fig. 2) provide first evidence for somatic exocytosis in MeV neurons, as far as we are aware.
Somatic exocytosis in MeV neurons and GABAergic inputs
Synaptic boutons contact the soma of MeV neurons from various regions (Lazarov, 2007). Field stimulation near the MeV triggered primarily GABAergic responses (Fig. 6), similar to a previous report that intensive GABAergic input depolarizes the MeV soma (Yokomizo et al. 2005). As shown in Fig. 6, the somatic exocytosis might release transmitter(s) and retrogradely provide negative feedback to inhibit the afferent GABAergic activity in MeV neurons. In addition to GABAergic terminals, glutamatergic nerve terminals also contact MeV soma and trigger glutamatergic synaptic responses (Verdier et al. 2004). The difference of evoked synaptic (glutamatergic or GABAergic) responses in those studies may be caused by different planes in which the brain slices were cut and different stimulation sites. It remains to be determined whether the somatic release retrogradely modulates other types of synaptic inputs (i.e. glutamatergic inputs; Luo & Dessem, 1996; Mineff et al. 1998; Verdier et al. 2004) and/or recurrent axon collaterals (Luo & Dessem, 1996).
Retrograde signalling in classic synapses has been intensively studied in many brain regions including cerebellum, hippocampus and striatum (Diana & Marty, 2004; Kano et al. 2009). Following neuronal activity in cerebellar GABAergic synapses, endocannabinoids are released from postsynaptic neurons, activate presynaptic endocannabinoid receptors (CB1Rs) and cause a transient reduction of neurotransmitter release (Kano et al. 2009). In addition, the activation of G-protein-coupled receptors, i.e. metabotropic glutamate receptors, can induce CB1R signalling in the postsynaptic neuron as well (Maejima et al. 2001; Ohno-Shosaku et al. 2002; Wilson & Nicoll, 2002; Yoshida et al. 2002). In the present study, we demonstrated another type of retrograde signalling in the GABAergic synapses on MeV neuron somata, where neuronal activity triggered robust exocytosis as well. We found that blockade of somatic secretion by intracellular EGTA removed the PSC inhibition (Fig. 6C and Fig. S3). In addition, endocannabinoid signalling was also involved in the PSC inhibition, because AM251, a selective antagonist of CB1R (Diana & Marty, 2004; Kano et al. 2009), abolished the somatic depolarization-induced PSC inhibition (Fig. S4). At present we cannot determine the fraction of the PSI produced by either endocannabinoids or somatic exocytosis, which might release unknown retrograde signalling to inhibit GABAergic transmission in MeV neurons.
Somatic exocytosis permits MeV neurons to integrate and modulate different inputs prior to the trigeminal motor nucleus. Thus, MeV neurons provide an excellent model for studying the functions and mechanisms of somatic secretion in brain slices. Future work should determine the identity of the neurotransmitter(s) in the somatic vesicles and their roles in physiological and pathological conditions, including sleep bruxism (Kato et al. 2003).
Acknowledgments
We thank Drs Bairen Wang and Iain C. Bruce for helpful comments. This work was supported by grants from the National Basic Research Program of China (2012CB518006, 2007CB512100, 2006CB500800), the National Natural Science Foundation of China (31171026, 31100597, 30970660, 30911120491 and 30830043) and a ‘985’ grant from the Department of Education of China.
Glossary
Abbreviations
- CB1R,
endocannabinoid receptor
- DA,
dopamine
- DRG,
dorsal root ganglion
- MeV,
mesencephalic trigeminal nucleus
- PSC,
postsynaptic current
- SLV,
synaptic-like vesicle
- TGN,
trigeminal ganglion neuron.
Author contributions
Experiments were done by B.Z., X.-Y.Z., W.H., P.-F.L., F.-P.Z., ., T.L., Y.-R.D., Q.-H.W., J.L., Y.X., H.-P.H., S.G. and H.Z.; Z.Z., B.Z. and C.X.Z. designed the work; and Z.Z., B.Z. and C.X.Z. wrote the paper. All authors have approved the final version.
References
- Alley KE. Quantitative analysis of the synaptogenic period in the trigeminal mesencephalic nucleus. Anat Rec. 1973;177:49–59. doi: 10.1002/ar.1091770106. [DOI] [PubMed] [Google Scholar]
- Baker R, Llinas R. Electrotonic coupling between neurones in the rat mesencephalic nucleus. J Physiol. 1971;212:45–63. doi: 10.1113/jphysiol.1971.sp009309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen X, Wang L, Zhou Y, Zheng LH, Zhou Z. “Kiss-and-run” glutamate secretion in cultured and freshly isolated rat hippocampal astrocytes. J Neurosci. 2005;25:9236–9243. doi: 10.1523/JNEUROSCI.1640-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dessem D, Taylor A. Morphology of jaw-muscle spindle afferents in the rat. J Comp Neurol. 1989;282:389–403. doi: 10.1002/cne.902820306. [DOI] [PubMed] [Google Scholar]
- Diana MA, Marty A. Endocannabinoid-mediated short-term synaptic plasticity: depolarization-induced suppression of inhibition (DSI) and depolarization-induced suppression of excitation (DSE) Br J Pharmacol. 2004;142:9–19. doi: 10.1038/sj.bjp.0705726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duan K, Yu X, Zhang C, Zhou Z. Control of secretion by temporal patterns of action potentials in adrenal chromaffin cells. J Neurosci. 2003;23:11235–11243. doi: 10.1523/JNEUROSCI.23-35-11235.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodwin GM, Luschei ES. Effects of destroying spindle afferents from jaw muscles on mastication in monkeys. J Neurophysiol. 1974;37:967–981. doi: 10.1152/jn.1974.37.5.967. [DOI] [PubMed] [Google Scholar]
- Hayar A, Poulter MO, Pelkey K, Feltz P, Marshall KC. Mesencephalic trigeminal neuron responses to γ-aminobutyric acid. Brain Res. 1997;753:120–127. doi: 10.1016/s0006-8993(97)00002-4. [DOI] [PubMed] [Google Scholar]
- Henderson G, Pepper CM, Shefner SA. Electrophysiological properties of neurons contained in the locus coeruleus and mesencephalic nucleus of the trigeminal nerve in vitro. Exp Brain Res. 1982;45:29–37. doi: 10.1007/BF00235760. [DOI] [PubMed] [Google Scholar]
- Heym C, Liu N, Gleich A, Oberst P, Kummer W. Immunohistochemical evidence for different pathways immunoreactive to substance P and calcitonin gene-related peptide (CGRP) in the guinea-pig stellate ganglion. Cell Tissue Res. 1993;272:563–574. doi: 10.1007/BF00318563. [DOI] [PubMed] [Google Scholar]
- Hinrichsen CF, Larramendi LM. The trigeminal mesencephalic nucleus. II. Electron microscopy. Am J Anat. 1970;127:303–319. doi: 10.1002/aja.1001270306. [DOI] [PubMed] [Google Scholar]
- Hsu SF, Jackson MB. Rapid exocytosis and endocytosis in nerve terminals of the rat posterior pituitary. J Physiol. 1996;494:539–553. doi: 10.1113/jphysiol.1996.sp021512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang HP, Wang SR, Yao W, Zhang C, Zhou Y, Chen XW, Zhang B, Xiong W, Wang LY, Zheng LH, Landry M, Hokfelt T, Xu ZQ, Zhou Z. Long latency of evoked quantal transmitter release from somata of locus coeruleus neurons in rat pontine slices. Proc Natl Acad Sci U S A. 2007;104:1401–1406. doi: 10.1073/pnas.0608897104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang LY, Neher E. Ca2+-dependent exocytosis in the somata of dorsal root ganglion neurons. Neuron. 1996;17:135–145. doi: 10.1016/s0896-6273(00)80287-1. [DOI] [PubMed] [Google Scholar]
- Isaacson JS, Strowbridge BW. Olfactory reciprocal synapses: dendritic signaling in the CNS. Neuron. 1998;20:749–761. doi: 10.1016/s0896-6273(00)81013-2. [DOI] [PubMed] [Google Scholar]
- Ishii T, Furuoka H, Itou T, Kitamura N, Nishimura M. The mesencephalic trigeminal sensory nucleus is involved in the control of feeding and exploratory behavior in mice. Brain Res. 2005;1048:80–86. doi: 10.1016/j.brainres.2005.04.038. [DOI] [PubMed] [Google Scholar]
- Jackson MB. Cable analysis with the whole-cell patch clamp. Theory and experiment. Biophys J. 1992;61:756–766. doi: 10.1016/S0006-3495(92)81880-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kano M, Ohno-Shosaku T, Hashimotodani Y, Uchigashima M, Watanabe M. Endocannabinoid-mediated control of synaptic transmission. Physiol Rev. 2009;89:309–380. doi: 10.1152/physrev.00019.2008. [DOI] [PubMed] [Google Scholar]
- Kato T, Thie NM, Huynh N, Miyawaki S, Lavigne GJ. Topical review: sleep bruxism and the role of peripheral sensory influences. J Orofac Pain. 2003;17:191–213. [PubMed] [Google Scholar]
- Khakh BS, Henderson G. Hyperpolarization-activated cationic currents (Ih) in neurones of the trigeminal mesencephalic nucleus of the rat. J Physiol. 1998;510:695–704. doi: 10.1111/j.1469-7793.1998.00695.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lazarov NE. Neurobiology of orofacial proprioception. Brain Res Rev. 2007;56:362–383. doi: 10.1016/j.brainresrev.2007.08.009. [DOI] [PubMed] [Google Scholar]
- Lindau M, Neher E. Patch-clamp techniques for time-resolved capacitance measurements in single cells. Pflugers Arch. 1988;411:137–146. doi: 10.1007/BF00582306. [DOI] [PubMed] [Google Scholar]
- Luo P, Dessem D. Morphological evidence for recurrent jaw-muscle spindle afferent feedback within the mesencephalic trigeminal nucleus. Brain Res. 1996;710:260–264. doi: 10.1016/0006-8993(95)01439-x. [DOI] [PubMed] [Google Scholar]
- Luo P, Haines A, Dessem D. Elucidation of neuronal circuitry: protocol(s) combining intracellular labeling, neuroanatomical tracing and immunocytochemical methodologies. Brain Res Brain Res Protoc. 2001;7:222–234. doi: 10.1016/s1385-299x(01)00065-4. [DOI] [PubMed] [Google Scholar]
- Luo P, Wong R, Dessem D. Ultrastructural basis for synaptic transmission between jaw-muscle spindle afferents and trigeminothalamic neurons in the rostral trigeminal sensory nuclei of the rat. J Comp Neurol. 1995;363:109–128. doi: 10.1002/cne.903630110. [DOI] [PubMed] [Google Scholar]
- Maejima T, Hashimoto K, Yoshida T, Aiba A, Kano M. Presynaptic inhibition caused by retrograde signal from metabotropic glutamate to cannabinoid receptors. Neuron. 2001;31:463–475. doi: 10.1016/s0896-6273(01)00375-0. [DOI] [PubMed] [Google Scholar]
- Matsuka Y, Edmonds B, Mitrirattanakul S, Schweizer FE, Spigelman I. Two types of neurotransmitter release patterns in isolectin B4-positive and negative trigeminal ganglion neurons. Neuroscience. 2007;144:665–674. doi: 10.1016/j.neuroscience.2006.09.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mineff EM, Popratiloff A, Usunoff KG, Marani E. Immunocytochemical localization of the AMPA receptor subunits in the mesencephalic trigeminal nucleus of the rat. Arch Physiol Biochem. 1998;106:203–209. doi: 10.1076/apab.106.3.203.4383. [DOI] [PubMed] [Google Scholar]
- Neher E, Zucker RS. Multiple calcium-dependent processes related to secretion in bovine chromaffin cells. Neuron. 1993;10:21–30. doi: 10.1016/0896-6273(93)90238-m. [DOI] [PubMed] [Google Scholar]
- Ohno-Shosaku T, Shosaku J, Tsubokawa H, Kano M. Cooperative endocannabinoid production by neuronal depolarization and group I metabotropic glutamate receptor activation. Eur J Neurosci. 2002;15:953–961. doi: 10.1046/j.1460-9568.2002.01929.x. [DOI] [PubMed] [Google Scholar]
- Sun JY, Wu LG. Fast kinetics of exocytosis revealed by simultaneous measurements of presynaptic capacitance and postsynaptic currents at a central synapse. Neuron. 2001;30:171–182. doi: 10.1016/s0896-6273(01)00271-9. [DOI] [PubMed] [Google Scholar]
- Taylor A, Davey MR. Behaviour of jaw muscle stretch receptors during active and passive movements in the cat. Nature. 1968;220:301–302. doi: 10.1038/220301a0. [DOI] [PubMed] [Google Scholar]
- Valtschanoff JG, Phend KD, Bernardi PS, Weinberg RJ, Rustioni A. Amino acid immunocytochemistry of primary afferent terminals in the rat dorsal horn. J Comp Neurol. 1994;346:237–252. doi: 10.1002/cne.903460205. [DOI] [PubMed] [Google Scholar]
- Verdier D, Lund JP, Kolta A. Synaptic inputs to trigeminal primary afferent neurons cause firing and modulate intrinsic oscillatory activity. J Neurophysiol. 2004;92:2444–2455. doi: 10.1152/jn.00279.2004. [DOI] [PubMed] [Google Scholar]
- Wang LC, Xiong W, Zheng J, Zhou Y, Zheng H, Zhang C, Zheng LH, Zhu XL, Xiong ZQ, Wang LY, Cheng HP, Zhou Z. The timing of endocytosis after activation of a G-protein-coupled receptor in a sensory neuron. Biophys J. 2006;90:3590–3598. doi: 10.1529/biophysj.105.069476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wightman RM, Jankowski JA, Kennedy RT, Kawagoe KT, Schroeder TJ, Leszczyszyn DJ, Near JA, Diliberto EJ, Jr, Viveros OH. Temporally resolved catecholamine spikes correspond to single vesicle release from individual chromaffin cells. Proc Natl Acad Sci U S A. 1991;88:10754–10758. doi: 10.1073/pnas.88.23.10754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson RI, Nicoll RA. Endocannabinoid signaling in the brain. Science. 2002;296:678–682. doi: 10.1126/science.1063545. [DOI] [PubMed] [Google Scholar]
- Yokomizo Y, Murai Y, Tanaka E, Inokuchi H, Kusukawa J, Higashi H. Excitatory GABAergic synaptic potentials in the mesencephalic trigeminal nucleus of adult rat in vitro. Neurosci Res. 2005;51:463–474. doi: 10.1016/j.neures.2004.12.016. [DOI] [PubMed] [Google Scholar]
- Yoshida S, Oka H. Membrane properties of dissociated trigeminal mesencephalic neurons of the adult rat. Neurosci Res. 1998;30:227–234. doi: 10.1016/s0168-0102(98)00003-0. [DOI] [PubMed] [Google Scholar]
- Yoshida T, Hashimoto K, Zimmer A, Maejima T, Araishi K, Kano M. The cannabinoid CB1 receptor mediates retrograde signals for depolarization-induced suppression of inhibition in cerebellar Purkinje cells. J Neurosci. 2002;22:1690–1697. doi: 10.1523/JNEUROSCI.22-05-01690.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang B, Sun L, Yang YM, Huang HP, Zhu FP, Wang L, Zhang XY, Guo S, Zuo PL, Zhang CX, Ding JP, Wang LY, Zhou Z. Action potential bursts enhance transmitter release at a giant central synapse. J Physiol. 2011;589:2213–2227. doi: 10.1113/jphysiol.2010.200154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang C, Zhou Z. Ca2+-independent but voltage-dependent secretion in mammalian dorsal root ganglion neurons. Nat Neurosci. 2002;5:425–430. doi: 10.1038/nn845. [DOI] [PubMed] [Google Scholar]
- Zheng H, Fan J, Xiong W, Zhang C, Wang XB, Liu T, Liu HJ, Sun L, Wang YS, Zheng LH, Wang BR, Zhang CX, Zhou Z. Action potential modulates Ca2+-dependent and Ca2+-independent secretion in a sensory neuron. Biophys J. 2009;96:2449–2456. doi: 10.1016/j.bpj.2008.11.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou Z, Misler S. Action potential-induced quantal secretion of catecholamines from rat adrenal chromaffin cells. J Biol Chem. 1995;270:3498–3505. [PubMed] [Google Scholar]
- Zhou Z, Misler S. Amperometric detection of quantal secretion from patch-clamped rat pancreatic beta-cells. J Biol Chem. 1996;271:270–277. doi: 10.1074/jbc.271.1.270. [DOI] [PubMed] [Google Scholar]


