Abstract
Different blends of membrane currents underlie distinct functions of neurons in the brain. A major step towards understanding neuronal function, therefore, is to identify the genes that encode different ionic currents. This study combined in situ patch clamp recordings of somatodendritic calcium currents in an identified adult Drosophila motoneuron with targeted genetic manipulation. Voltage clamp recordings revealed transient low voltage-activated (LVA) currents with activation between –60 mV and –70 mV as well as high voltage-activated (HVA) current with an activation voltage around –30 mV. LVA could be fully inactivated by prepulses to –50 mV and was partially amiloride sensitive. Recordings from newly generated mutant flies demonstrated that DmαG (Cav3 homolog) encoded the amiloride-sensitive portion of the transient LVA calcium current. We further demonstrated that the Cav2 homolog, Dmca1A, mediated the amiloride-insensitive component of LVA current. This novel role of Cav2 channels was substantiated by patch clamp recordings from conditional mutants, RNAi knock-downs, and following Dmca1A overexpression. In addition, we show that Dmca1A underlies the HVA somatodendritic calcium currents in vivo. Therefore, the Drosophila Cav2 homolog, Dmca1A, underlies HVA and LVA somatodendritic calcium currents in the same neuron. Interestingly, DmαG is required for regulating LVA and HVA derived from Dmca1A in vivo. In summary, each vertebrate gene family for voltage-gated calcium channels is represented by a single gene in Drosophila, namely Dmca1D (Cav1), Dmca1A (Cav2) and DmαG (Cav3), but the commonly held view that LVA calcium currents are usually mediated by Cav3 rather than Cav2 channels may require reconsideration.
Key points
Neurons in the brain express a diversity of ion channels to impart specialized functional properties.
At least three distinct voltage gated calcium channel gene families are known, each of which is thought to produce calcium channels with unique properties, which in turn, differently affect neuronal function.
Here we use a genetic model system to determine which genes are responsible for the calcium currents in an identified motoneuron.
Surprisingly, the same ion channel gene encodes two distinct currents with fundamentally different properties, and the data also suggest that the normal function of this calcium channel gene is affected by the expression of another one.
The results provide insights into the relationship between gene expression and ionic currents, and thus, into the regulation of normal neuronal function.
Introduction
Membrane currents are at the heart of information processing in the brain. The distinct functions of different neurons rely on different blends of ionic currents, for which different families of ion channel genes have evolved. A major challenge in understanding ion channel function is to unravel which genes underlie specific currents. The first voltage-gated ion channel identified and cloned was the Drosophila Shaker channel (Trout & Kaplan, 1970, 1973; Papazian et al. 1987), the homolog of vertebrate Kv1 potassium channels. Since then, powerful Drosophila genetic tools, combined with recent advances in patch clamping (Baines & Bate, 1998; Olsen & Wilson, 2008; Pulver & Griffith, 2010) from the small Drosophila CNS, have provided novel insights into the genetic basis, functional consequences and behavioural relevance of ion channel expression in neurons (Rohrbough et al. 2003; Baines & Pym, 2006; Cameron et al. 2010; Kang et al. 2010). Most vertebrate ion channel families are represented by a single gene in the fly model. The highly conserved nature of ion channels validates generalization of functional data obtained in invertebrate genetic model systems. By unraveling the genetic basis of adult Drosophila motoneuron somatodendritic calcium currents, this study sheds new light on the functions of voltage-gated calcium channels (VGCCs).
In vertebrates, 10 VGCC α-subunits fall into three families: Cav1, Cav2 and Cav3 (Dolphin, 2009). The Drosophila genome contains one putative homolog to each vertebrate family (Dmca1D, Cav1; Dmca1A, Cav2; DmαG, Cav3; Littleton & Ganetzky, 2000; see Table 1). In vertebrate spinal motoneurons, Cav1 α-subunits are targeted to the somatodendritic domain to mediate HVA L-type calcium currents (Hounsgaard & Mintz, 1988; Heckman et al. 2003; Carlin et al. 2009). Similarly, in Drosophila larval motoneurons, the Cav1 homolog, Dmca1D, underlies somatodendritic HVA calcium current (Worrell & Levine, 2008). Cav2 channels mediate HVA N- and P/Q-type current at presynaptic terminals throughout the vertebrate CNS (Dunlap et al. 1995; Reid et al. 2003). Accordingly, the Drosophila Cav2 homolog, Dmca1A (cacophony), encodes presynaptic calcium channels at neuromuscular (Kawasaki et al. 2000, 2002, 2004) and central synapses (Gu et al. 2009). In vertebrates, Cav3 channels mediate LVA, transient calcium currents that can contribute to neuronal pacemaker activity (Contreras, 2006; Steriade, 2006; Cain & Snutch, 2010) or synaptic integration in dendrites (Crandall et al. 2010; Isope et al. 2010). However, despite the traditional view that Cav1 and Cav2 channels mediate HVA, whereas Cav3 channels mediate LVA calcium currents, some Cav1.3 channels activate in a lower voltage range (Scholze et al. 2001; Xu & Lipscombe, 2001; Mangoni et al. 2003). In mammals, alternative splicing can cause shifts in Cav1 channel activation voltage towards more hyperpolarized potentials (Chen et al. 2009; Striessnig et al. 2010; Bock et al. 2011). In Drosophila, LVA current is present in muscle (Gielow et al. 1995) and possibly in embryonic motoneurons (Baines & Bate, 1998), but the genetic basis of such currents remains unclear. Sequence homology analysis predicts, however, that DmαG may underlie Drosophila LVA calcium current (Littleton & Ganetzky, 2000).
Table 1.
Voltage-gated calcium channel genes in vertebrates and Drosophila
| Vertebrate gene nomenclature new/old* | Currents | Activation | Drosophila homolog/annotation number | Alternative name/allele | Drosophila references |
|---|---|---|---|---|---|
| Cav1.1/α1S Cav1.2/α1C Cav1.3/α1D Cav1.4/α1F | L-type | HVA** | DmCa1D/CG4894 | Ca-α1D | Zheng et al. 1995; Worrell & Levine, 2008 |
| Cav2.1/α1A Cav2.2/α1B Cav2.3/α1E | P/Q-type N-type R-type | HVA | DmCa1A/CG43368 | cacophony (cac); nightblindA (nbA) | Smith et al. 1996; Peixoto et al. 1997{BK38} |
| Cav3.1/α1G Cav3.2/α1H Cav3.3/α1I | T-type | LVA | DmαG/CG15899 | Ca1G; Dmα1G; Dmα1T; Ca-α1T | Gielow et al. 1995; Littleton & Ganetzky, 2000 |
Vertebrate calcium channel nomenclature is according to Dolphin (2009).
For Cav1 channels activation voltages of more hyperpolarized potentials have been reported (Chen et al. 2009; Striessnig et al. 2010; Bock et al. 2011).
This study reveals somatodendritic LVA and HVA currents in adult Drosophila motoneurons. It provides novel functional data on the Cav3 homolog, DmαG, and confirms sequence predictions that DmαG encodes a LVA channel. Surprisingly, the Cav2 homolog, Dmca1A, mediates both LVA and HVA currents in adult Drosophila motoneurons in vivo. The regulation of LVA and HVA currents mediated by Dmca1A requires the presence of DmαG channels.
Methods
Animals
Drosophila melanogaster were reared in standard plastic vials with cotton plugs on a yeast–cornmeal–syrup–agar diet with a 12 h light–dark regimen (see Ryglewski & Duch, 2009). All fly lines (except cacNT27, see below) were kept at 25°C. MN5 was visualized for patch clamp recordings by expression of the UAS-mCD8-GFP reporter under the control of D42-GAL4, which drives expression in a small number of adult motoneurons, including MN1–5 and in some unidentified interneurons (Yeh et al. 1995; Sanyal et al. 2003). Expression in cholinergic interneurons was suppressed with a Cha-GAL80 construct. In one set of experiments (targeted expression of UAS-cac1, see below) the P103.3-GAL4 driver was used to drive expression in MN5. P103.3-GAL4 also expresses in a small number of adult motoneurons, including MN1–5 and in some unidentified interneurons (Consoulas et al. 2002). The controls were the progeny of homozygous+; UAS-mCD8-GFP; D42-GAL4, ChaGAL80 crossed to homozygous w1118. All UAS-RNAi lines derived from a w1118 background, the UAS-RNAi for Dmca1A (cacophony; stock 5551) and for Dmca1D (stock 51491; Worrell & Levine, 2008) were obtained from the Vienna Fly Stock Centre (Vienna, Austria). UAS-dicer constructs (stock 24666 or 24667) were crossed into the UAS-RNAi lines to enhance efficacy. For some experiments a Dmca1A temperature-sensitive mutant was used, cacNT27 (generously provided by Dr Littleton, MIT, Cambridge, MA, USA), in which synaptic transmission is strongly decreased upon a temperature shift to 38°C (Rieckhof et al. 2003). Due to reports that this line already exhibits decreased calcium currents at room temperature (Gu et al. 2009) we bred these flies at 20°C.
To test for the contribution of Dmca1A in a DmαG mutant background, we drove expression of UAS-cac1 (Kawasaki et al. 2002; 2004) in MN5 with the GAL4-driver P103.3 with a UAS-mCD8-GFP on the same chromosome as reporter. We used this GAL4-driver rather than D42-GAL4 because in the D42-GAL4 driver line the UAS-GFP construct was not on the same chromosome which led to difficulties when crossing the driver into the DmαG mutant line. Homozygous DmαG; P103.3-GAL4, UAS-mCD8-GFP;+ were crossed with the UAS-cac1 line. The UAS-Dmca1A (UAS-cac1) construct has been shown to rescue viability in otherwise embryonic lethal Dmca1A null mutant Drosophila and synaptic transmission at temperatures of 38°C in temperature-sensitive Dmca1A mutants (Kawasaki et al. 2002; 2004). Both the cacNT27 temperature-sensitive mutant and the UAS-cac1 lines were derived from a Canton S background, whereas the DmαG null mutant was derived from a w1118 background (see below). Both female and male F1 flies were used for electrophysiological experiments, except that the Dmca1A or DmαG mutants tested were all males, because both genes are located on the X chromosome.
For convenience, all transgenic fly lines are listed with source and reference in Supplementary Table 1. The authors have read and the experiments comply with the policies and regulations of The Journal of Physiology given by Drummond (Drummond, 2009).
DmαG (Ca-α1T gene) mutant generation and PCR confirmation
FLP-FRT-based deletions (Parks et al. 2004) were used to remove 45.8 kb of the putative t-type voltage-gated calcium channel DmαG gene (FlyBase annotation symbol CG15899, annotation name Ca-alpha1T; Fig. 1A). Two piggyBac elements that are located within the DmαG gene were chosen from the Exelixis Collection: PBac{WH}Ca-α1Tf06496 (stock no. f06496) and PBac{WH}f05809 (stock no. f05809). These piggyBac (WH) insertions contain a mini-white gene, a 199 bp FRT site, Su(Hw) insulator sequences, and a terminal UAS site (Thibault et al. 2004). When two piggyBac (WH) elements are within the same individual and FLP recombinase is present, genomic deletion occurs in between the FRT sites and results in a residual hybrid element that is made of the remaining partial piggyBac (WH) elements (Parks et al. 2004). This residual element can then be screened for, using two-sided PCR, as a confirmation that the deletion has occurred. As described in Parks et al. (2004) for X chromosome deletions, females carrying one piggyBac element were crossed to males carrying FLP recombinase. Of the progeny, males carrying both the piggyBac and FLP recombinase elements were then crossed to females carrying the other piggyBac element. Progeny were then heat shocked for 1 h each day in a 37°C water bath for 5 days in order to activate the FLP recombinase. Female progeny carrying both piggyBac elements were chosen and crossed to an X chromosome balancer line. Single balanced female progeny were crossed again to the X chromosome balancer line in separate vials establishing 103 putative mutant lines. Male progeny not carrying the X chromosome balancer were screened for the residual hybrid element using PCR.
Figure 1. Generation and validation of DmαG excision mutants.
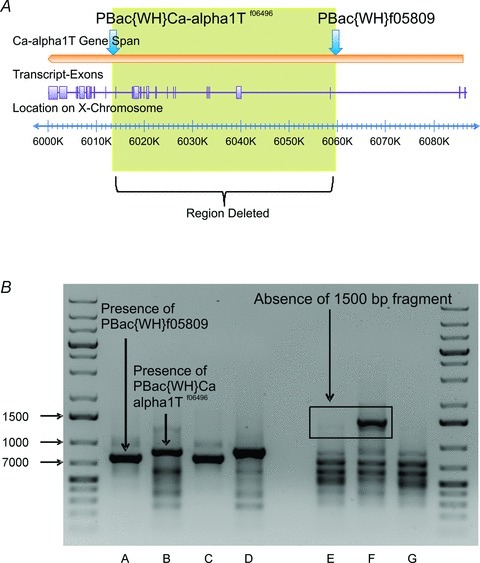
A, illustration of the Ca-alpha1T gene (DmαG) showing the placement of inserted piggyBac elements. Placement of piggyBac elements on Ca-alpha1T gene span are indicated by blue arrows. Transcript variant B is shown as an example transcript indicating the placement of the exons derived from Ca-alpha1T, seen here as purple boxes. The yellow box indicates the region of the gene that is expected to be absent after the deletion has occurred. B, confirmation of deletion on the X chromosome of a Ca-alpha 1T mutant male individual. Columns A and B show results of two-sided PCR confirming the presence of both piggyBac elements in the mutant, using transposon-specific and genomic primers designed for PBac{WH}f05809 and PBac{WH}Ca-α1Tf06496, respectively. Columns C and D show the presence of the two elements in the respective stock lines. Columns E–G show the absence of the DNA region between the piggyBac elements in the mutant individual, using primers that were designed for the PBac{WH}f05809 stock line. One primer was located within the region of DNA that was to be deleted and the other primer was located on a portion of the PBac{WH}f05809 element expected to be present after the deletion occurred. Column E shows that the putative mutant does not have a fragment at the expected band size of 1500 bp. Sample F shows that the PBac{WH}f05809 stock line does have a fragment at the expected size. Sample G shows Canton-S wildtype. Note that the residual bands that are seen in sample E are not due to the presence of the PBac{WH}f05809 element. The PCR annealing temperature and extension time for the samples that are in columns A–D were 62°C and 1 min, respectively. The PCR annealing temperature and extension time for the samples in columns E–G were 66°C and 2 min, respectively.
PCR confirmation strategies
DNA used for PCR reactions was obtained from a single male fly from each of the putative mutant lines not carrying the X chromosome balancer used in the cross. Two different PCR deletion confirmation strategies were used. The first was two-sided PCR in which all 103 putative mutant lines were screened for the residual element. The second strategy screened for the absence of the deleted region in the 14 putative mutant lines that were shown to contain the residual element.
Two-sided PCR
A genomic primer and a transposon-specific primer were generated for both inserted piggyBac elements. For piggyBac element PBac{WH}Ca-α1Tf06496 the genomic primer 5′-ACGTG TGCCTGGAGGGTCTG-3′ and transposon-specific primer 5′-TCCAAGCGGCGACTGAGATG-3′ (sequence provided by Parks et al. 2004) were generated and used. The expected fragment size for this primer pair was 862 bp. For piggyBac element PBac{WH}f05809 the transposon-specific primer 5′-CCTCGATATACAGACCGATAAAAC-3′ (sequence provided by Parks et al. 2004) and genomic primer 5′-AGTTCGCGCCGTCATCGTTATGTC-3′ were used. The expected fragment size for this primer pair was 753 bp. For each putative mutant line a single male was chosen and both primer pairs for piggyBac elements PBac{WH}Ca-α1Tf06496 and PBac{WH}f05809 were run in separate tests and then screened using agarose gel electrophoresis for the presence of both fragments (Fig. 1B).
Absence of deleted region
To determine that the expected region was indeed removed from the DmαG gene, a genomic primer and a transposon-specific primer were designed for the PBac{WH}f05809 fly line. The genomic primer 5′-ACTCGCACGCAGACAGCCAG-3′ was located within the DNA region that was to be removed. The transposon-specific primer 5′-CGCAGCTCGCGTTGCATTTTC-3′ was located 41 bp adjacent to the FRT site on the PBac{WH}f05809, a region that would be located on the residual hybrid element after the deletion occurred. The expected fragment size for this primer pair was 1432 bp. The primer pair was run on the PBac{WH}f05809 fly line to confirm that it produced a fragment of the expected length and then run on all 14 putative mutant lines that were shown to have the residual element. Putative mutant fly lines were then screened using agarose gel electrophoresis for the absence of a fragment at 1432 bp (Fig. 1B). None of the 14 lines had a fragment at the expected length.
Electrophysiology
Animals were dissected and treated with 2% protease as described elsewhere (Ryglewski & Duch, 2009). All experiments were carried out in saline of the following composition (in mm): NaCl 93.4, KCl 5, MgCl2 4, CaCl2 1.8, BaCl2 1.8, TEA-Cl 30, 4-AP 2, Hepes 5, sucrose ∼35.5. pH was adjusted to 7.24 with HCl; osmolality was adjusted to 310 mosmol kg−1 with sucrose. TEA-Cl and 4-AP were used to block potassium currents; TTX (100 nm) was applied directly into the bath to block transient sodium currents. The internal patch solution consisted of (in mm): CsCl 140, Mg-ATP 2, CaCl2 0.5, EGTA 1.1, TEA-Br 20, 4-AP 0.5, Hepes 10. The pH was adjusted to 7.24 with CsOH; osmolality was adjusted to 320 mosmol kg−1 with glucose if necessary. Patch pipettes were pulled from borosilicate glass capillaries (no filament, o.d. 1.5 mm, i.d. 1.0 mm, World Precision Instruments) with a Narishige PC-10 vertical pipette puller. Pipette tip resistance was around 3.5 MΩ with these solutions. All whole-cell calcium currents were recorded at room temperature (∼24°C) from GFP-tagged MN5 somata located in the mesothoracic neuromere in semi-intact Drosophila (Ryglewski & Duch, 2009) except in temperature-sensitive mutants (cacNT27). These flies were raised at 20°C. Acute temperature shifts during patch clamp recordings were made by controlling bath temperature with a Dagan TC10 temperature controller. In earlier studies temperature shifts to 38°C were performed (Rieckhof et al. 2003) to knock down Dmca1A currents. In our experiments a maximal decrease of calcium current upon temperature shift could be obtained already at 32°C. Further temperature shift to 38°C did not decrease current amplitude any further but resulted in unstable recording conditions. Patch clamp experiments were carried out with an Axopatch 200B amplifier (Molecular Devices, USA) digitized at 20 kHz (Digidata 1322A, Molecular Devices), filtered through a 5 kHz low-pass Bessel filter and recorded with pCLAMP 10.2 software (Molecular Devices). After break-in, 2 to 5 min were allowed for solution exchange prior to data acquisition. Only high quality recordings with little leak (input resistances ≥100 MΩ) and good access (access resistances ≤12 MΩ) were accepted for data acquisition.
Pharmacology
TEA-Cl and 4-AP were used externally and combined with CsCl internally to block most potassium current. Barium was added in addition to calcium (see above) to enhance calcium current amplitudes. In previous studies on vertebrate Cav1 and Cav2 channels anomalous mole fraction behaviour has been reported to cause decreased current amplitude when different charge carriers flow through the same channel pore (Bleakman et al. 1995; Wakamori et al. 1998). In the Drosophila motoneuron, MN5, recordings in 1.8 mm external barium and zero calcium followed by replacing the bath solution with 1.8 mm external barium and 1.8 mm calcium did not decrease calcium current amplitude. It also did not reveal any obvious calcium-dependent inactivation of either LVA or HVA calcium current. We did not quantify time courses of activation/inactivation because the variability among controls was larger than the differences recorded in barium only as compared with switching to calcium–barium solution. By contrast, current amplitude was increased on average by 24 ± 3.7% (n = 4) when replacing external solution containing 1.8 mm calcium with external solution containing 1.8 mm calcium and 1.8 mm barium. We did not use barium as sole charge carrier because we observed run-down of both LVA and HVA current over minutes in calcium-free external solution.
Barium also blocks some potassium current in MN5 (Ryglewski & Duch, 2009). Transient sodium current was blocked with TTX (100 nm). Cadmium (300 μm) was added in some recordings to block all calcium current. Dmca1A channels were selectively blocked with the Plectreurys tristis spider toxin PLTXII (10 nm; Branton et al. 1987; Leung et al. 1989; Alomone Labs, Israel). Specificity was confirmed by absence of calcium current block by PLTXII following Dmca1A knock-down (not shown).The vertebrate T-type calcium channel blocker amiloride (1 mm; Tang et al. 1988) blocks transient LVA calcium current in Drosophila muscle (Gielow et al. 1995) and embryonic neurons (Baines & Bate, 1998). Specificity of amiloride for transient LVA calcium current through DmαG channels in MN5 was confirmed by absence of block in DmαG null mutants (see Results).
Data analysis
Leak subtraction and subtraction of the capacitance artifact were routinely conducted off-line. Capacitance artifacts were subtracted by adding the on- and off-artifacts which both have the same waveforms and amplitudes but opposite polarities.
Total calcium currents were recorded by 200 ms voltage steps from –90 mV to +20 mV in 10 mV increments elicited from a holding potential of –90 mV. HVA currents were electrically isolated by 1 s pre-pulses to –50 mV, followed by test pulses of 200 ms duration from –90 mV to +20 mV in 10 mV increments. Sustained HVA calcium current amplitude was measured as mean current amplitude between 100 ms and 120 ms after pulse onset. Total calcium current amplitude was measured as the maximum inward current for all voltage steps. LVA current amplitudes were measured as maximum inward currents for voltage steps between –90 mV and –40 mV (see Fig. 2 for characterization of LVA and HVA currents in MN5). All patch clamp experiments and analysis of electrophysiological data were conducted with pCLAMP 10.2 software in combination with MS Excel 4.0. Current traces were exported to Corel Draw 10 for production of figures. Statistical difference between two samples was determined with Student's t test or with Mann–Whitney U test. Statistical significance was assumed when P < 0.05 (*), P < 0.01 (**), P < 0.001 (***). Data are represented as mean ± SEM.
Figure 2. Low voltage-activated (LVA) and high voltage-activated (HVA) calcium current in motoneuron 5.
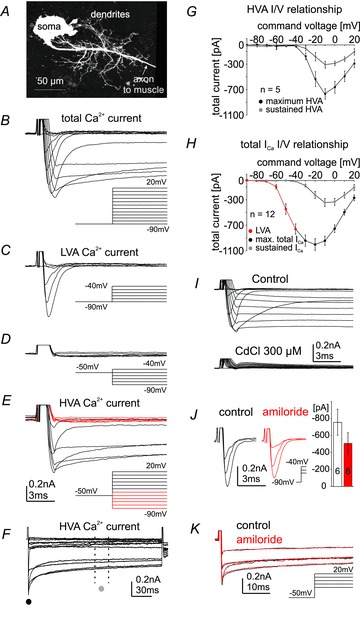
The monopolar motoneuron 5, MN5, stained intracellularly with dextran-tetramethylrhodamine reveals a complex dendritic tree (A) that is comprised of more than 6500 μm of dendrititic length. The axon projects contralaterally towards the dorsolongitudinal flight muscle. Whole-cell recordings were conducted from the soma of MN5. Command voltage steps from –90 mV to +20 mV in 10 mV increments from a holding potential of –90 mV reveal transient LVA and sustained HVA currents (B). LVA calcium current is transient (inactivates within 5 ms after pulse onset), activates between –70 mV and –60 mV (C) and is completely inactivated following a 1 s lasting prepulse to –50 mV (D). HVA is electrically isolated by a prepulse to –50 mV of 1 s duration followed by command voltage steps from –90 mV to +20 mV in 10 mV increments (E and F). HVA activates between –40 mV and –30 mV within 5 ms after pulse onset (E) and does not fully inactivate during the 200 ms lasting voltage step (F). Command voltages at which HVA does not yet activate and LVA is fully inactivated following a 1 s lasting prepulse to –50 mV are shown in red (E). Current–voltage plot in G depicts activation of isolated HVA at peak shortly after pulse onset (F and G; black circles, maximum HVA) and as mean sustained current between 100 ms and 120 ms after pulse onset (F, G; grey circles, sustained HVA). Maximum mean current amplitude is at –10 mV. Current–voltage plot in H shows isolated LVA calcium current between command voltages of –90 mV and –40 mV (H; red circles), maximum total calcium current (max. total ICa, sum of LVA and HVA) between command potentials more depolarized than –40 mV and +20 mV as measured at peak shortly after pulse onset (H; black circles). The sustained portion of HVA as measured between 100 ms and 120 ms after pulse onset is plotted for voltage steps from a holding of –90 mV (H; grey circles, sustained ICa). Cadmium (300 μm) blocks all calcium current as evoked by voltage steps from –90 mV to +20 mV from a holding potential of –90 mV (I). Amiloride (1 mm) blocks a portion of the LVA calcium current as compared with control (J, voltage steps –60, –50 and –40 mV are shown; black, control; red, amiloride). Quantification shows that amiloride reduces LVA peak current amplitude at –40 mV from –756 ± 152 pA in control to –498 ± 127 pA in amiloride (J). Amiloride has no obvious effect on electrically isolated HVA current (K). Data are represented as mean ± SEM. Insets in B–E and K depict voltage steps conducted to evoke calcium currents.
Results
This study used genetic and pharmacological tools to unravel the genetic basis of somatodendritic calcium currents in the individually identifiable adult Drosophila flight motoneuron, MN5, a large monopolar motoneuron that innervates the dorsal longitudinal flight muscle (Ikeda & Koenig, 1988; Consoulas et al. 2002). Similar to spinal motoneurons, MN5 exhibits a large dendritic tree. MN5 is comprised of more than 6500 μm total dendritic length (Fig. 2A; Vonhoff & Duch, 2010). MN5 firing properties (Duch et al. 2008), voltage-activated potassium currents (Ryglewski & Duch, 2009) and firing patterns during motor behaviour are well characterized (Wyman & Levine, 1973; Koenig & Ikeda, 1980; Gordon & Dickinson, 2006). All of these properties are tighly regulated between animals, and MN5 can be identified unambiguously from animal to animal (Vonhoff & Duch, 2010). This allows for comparison of MN5 calcium currents in controls and following genetic and pharmacological manipulation in different animals.
MN5 expresses LVA and HVA calcium currents
All calcium currents in this study were measured from the soma of MN5 (Fig. 2A) with TEA, 4-AP, barium and TTX in the external solution, and cesium, TEA and 4-AP in the internal solution to block potassium and sodium currents. In situ patch clamp recordings of MN5 in semi-intact Drosophila melanogaster revealed the existence of at least two somatodendritic calcium currents, low voltage activated (LVA) and high voltage activated (HVA) calcium current (Figs. 2B–G). Total calcium current in MN5 was measured by clamping the neuron from its resting membrane potential of –65 mV (Ryglewski & Duch, 2009) to a holding potential of –90 mV, and applying voltage steps from –90 mV to +20 mV in 10 mV increments (Fig. 2B). All calcium current was completely blocked by bath application of cadmium at 300 μm (Fig. 2I). LVA calcium current in MN5 could be measured in isolation at command voltages between –60 mV and –40 mV (Fig. 2C). LVA current was fast and transient with a peak current within 2 ms at –40 mV. However, due to uncertainties in voltage control through the large MN5 dendritic tree of more than 6500 μm length we did not attempt quantification of the activation and inactivation time constants. LVA current did fully inactivate following pre-pulses to –50 mV of 1 s duration (Fig. 2D). By contrast, HVA current activated at command voltages more positive than –40 mV (Fig. 2B). Therefore, HVA could be electrically isolated by pre-pulses to –50 mV (Fig. 2E, red traces depict voltage steps from –50 mV holding to command potentials between –90 mV and –40 mV, when LVA currents were inactivated). HVA current showed a fast onset and did not fully inactivate during the 200 ms long test potentials (Fig. 2F). To test whether HVA current could be electrically separated into a fast and a slow component conditioning depolarizing pre-pulses of 1 s duration to –50, −40 and –30 mV were applied. None of these depolarizing pre-pulses yielded electrical separation into a transient and a sustained HVA current (see Discussion). To account for the possibility that HVA current might comprise a fast and a slow component, current voltage plots for HVA were created by measuring electrically isolated HVA amplitude at two different times, first at the beginning of the voltage step when the maximum HVA current amplitude occurred (Fig. 2F, black circle), and second, as the average current amplitude between 100 ms and 120 ms (Fig. 2F, dotted lines, grey circle) after pulse onset. Both measurements of HVA current showed activation at –30 mV and maximum current at –10 mV (Fig. 2G).
For command potentials more depolarized than –40 mV, LVA current could not be cleanly isolated by subtraction routines, because capacitance artifacts elicited from a holding potential of –90 mV differed in shape and amplitude from those elicited from a holding potential of –50 mV. Therefore, we did not create I–V plots for pure LVA current over all command potentials from –90 mV to +20 mV. In order to account quantitatively for LVA current in comparisons of control calcium currents in MN5 and following genetic manipulations (Figs 3 to 7), we plotted I–V relationships for the maximal total calcium current and for the total sustained calcium current in MN5 measured between 100 ms and 120 ms after pulse onset (Fig. 2H). The sustained current represented pure HVA current because all LVA was inactivated within 5 ms (see Fig. 2C). Therefore, the I–V plots for sustained HVA as measured following electrical isolation by pre-pulses to –50 mV (Fig. 2G, grey circles) were similar to the sustained total calcium current as measured from a holding potential of –90 mV (Fig. 2H, grey circles). Maximum total calcium current represented a sum current of LVA and fast HVA for all voltage steps more depolarized than –40 mV. For command voltage steps between –90 mV and –40 mV this I–V plot resulted only from LVA calcium current (Fig. 2H, red circles), because HVA activated between –40 mV and –30 mV. LVA current activated between –70 mV and –60 mV (Fig. 2H, red circles). Maximum total calcium current occurred at –20 mV (Fig. 2H, black circles).
Figure 3. Dmca1D does not contribute to somatodendritic calcium current in MN5.
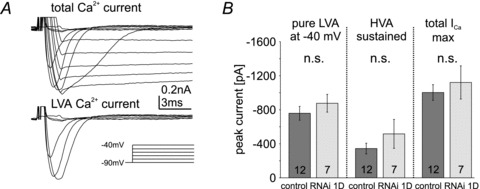
A, whole-cell total calcium current in Dmca1D RNAi knock-down animals as evoked by voltage steps from –90 mV to +20 mV from a holding potential of –90 mV in 10 mV increments (top trace) and isolated LVA current at command potentials from –90 mV to –40 mV (bottom trace). Calcium currents are not affected by Dmca1D RNAi knock-down as compared with control (compare with Fig. 2B and C). B, quantitative comparison of isolated LVA calcium current amplitudes at –40 mV (left column), sustained HVA (middle column), and maximum total calcium current amplitudes (total ICa max, right column) in controls (dark grey bars) and following Dmca1D RNAi knock-down (light grey bars) reveals no effect of Dmca1D RNAi knock-down (n.s., unpaired Student's t test). Data are shown as mean ± SEM.
Figure 7. Cacophony can produce LVA and HVA calcium current in vivo in the absence of DmαG.
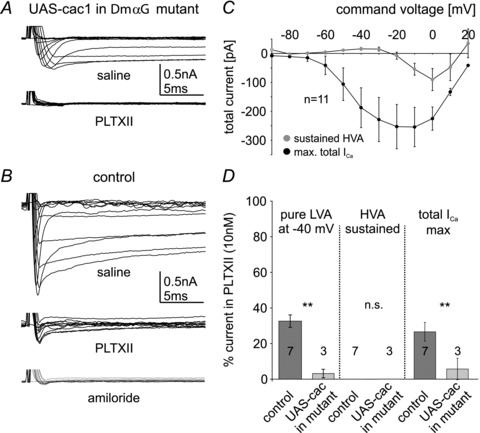
Expression of UAS-cac1 in MN5 in a DmαG mutant background yielded LVA and HVA calcium currents (A, top trace) with normal activation voltages (C) but reduced amplitudes as compared with controls (compare C and Fig. 2H). In this genotype both isolated LVA at –40 mV (D, left column, light grey bar) as well as total maximum calcium current (A, right column in D, light grey bar) were strongly reduced and HVA was abolished by bath application of PLTXII (bottom trace in A, middle column in D, light grey bar). In controls PLTXII blocks a large portion of LVA (left column in D, dark grey bar) and abolishes all HVA calcium current (middle trace in B, middle column in D). The LVA calcium current remaining after PLTXII is blocked by amiloride (1 mm, bottom trace in B). In DmαG mutants with cac1 expression in MN5 significantly more isolated LVA at –40 mV (left column in D, light grey bar) as well as total maximum calcium current (right column in D, light grey bar) are blocked by PLTXII than in control (D, left and right columns, dark grey bars; Student's t test, **P < 0.01). Data are shown as mean ± SEM.
In various preparations it has been shown that the universal calcium channel blocker cadmium did not or not fully block a LVA T-type calcium current (Mynlieff & Beam, 1992) whereas amiloride is frequently used as a blocker for LVA currents (Tang et al. 1988). In the Drosophila MN5, cadmium chloride (300 μm) blocked all TTX-insensitive inward currents, i.e. LVA and HVA calcium currents (Fig. 2I). Bath application of amiloride (1 mm) decreased LVA calcium current amplitude by 34%± 6% (Fig. 2J), but had no obvious effect on electrically isolated HVA calcium current (Fig. 2K). In fact, genetic manipulation showed that amiloride-sensitive LVA current was mediated by a different gene than amiloride-insensitive LVA and HVA currents in MN5 (see below).
Dmca1D does not contribute to calcium currents in MN5
The somatodendritic HVA calcium currents in larval motoneurons are mediated largely by Dmca1D channels (Cav1 homolog, Worrell & Levine, 2008). Similarly, mammalian motoneuron dendrites display L-type calcium currents mediated by Cav1 channels (Lee & Heckman, 2000; Heckman, 2003; Hultborn et al. 2004; Heckman et al. 2008; Hyngstrom et al. 2008). Sustained HVA calcium current in the adult MN5 exhibited I–V relationships similar to those in larval Drosophila and mammalian motoneurons (Fig. 2G and H), but was not mediated by Dmca1D channels. UAS-RNAi knock-down of Dmca1D had no effect on calcium currents when expressed in adult MN5 under the control of D42-GAL4. D42-GAL4 is expressed strongly in MN5 and other motoneurons (Yeh et al. 1995; Boerner & Duch, 2010). The UAS-Dmca1D RNAi construct effectively reduces HVA calcium current in larval Drosophila motoneurons (Worrell & Levine, 2008). We confirmed that D42-GAL4 drives expression of GFP continuously in MN5 from pupal stage 5 to adulthood, and we enhanced the potential effectiveness of Dmca1D RNAi knock-down in MN5 by co-expression of extra dicer (w1118/>; UAS-mcd8-GFP/UAS-dicer; D42-GAL4, Cha-GAL80/UAS-Dmca1D RNAi). Nevertheless, LVA, maximum total calcium current and sustained HVA calcium current amplitudes were unaffected in MN5 (Fig. 3A and B). The shapes of LVA and total calcium currents as recorded in MN5 following Dmca1D RNAi knock-down were within the variation observed in recordings from controls. However, it remains unclear whether the variation we observed in the time courses of activation and inactivation were due to uncertainties in voltage control through the large MN5 dendritic tree or to the expression of different isoforms of the same calcium channels in MN5 (see Discussion). However, the Drosophila Cav1 channel homolog, Dmca1D, did not affect LVA, total calcium current or sustained HVA calcium current amplitudes in MN5. We did not further confirm this conclusion by additional recordings in other mutant fly strains because genetic and pharmacological manipulation of the other two Drosophila calcium channels, DmαG (Cav3 homolog) and Dmca1A (Cav2 homolog), fully accounted for both LVA and HVA calcium current in MN5 (see below).
DmαG mutant flies show a variety of different LVA and HVA calcium current amplitudes in MN5
Genome sequence analysis predicts that the Cav3 homolog DmαG encodes LVA calcium channels (Littleton & Ganetzky, 2000), but no functional data exist. We generated DmαG deletion mutants (see Methods). In 14 out of 25 recordings from DmαG null mutants (56%) both the transient LVA and the sustained HVA calcium currents were abolished completely (Fig. 4A and D). By contrast, 28% of all mutant animals (7 out of 25) showed LVA and HVA calcium currents with reduced amplitudes (Fig. 4B and D), and the remaining 16% (4 out of 25) exhibited control-like calcium currents (Fig. 4C and D). On average, transient LVA, maximum total and sustained HVA calcium currents were reduced significantly in DmαG null mutants as compared with control (Fig. 4E, ***P < 0.001, Mann–Whitney U test).
Figure 4. Multiple calcium current phenotypes occur in DmαG null mutants.
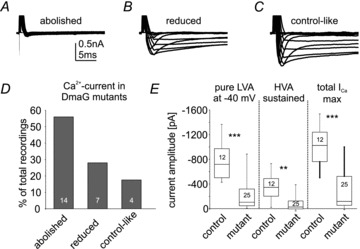
A, 56% of all DmαG mutants recorded exhibit no measurable calcium current (A, first bar in D); B, 28% show both LVA and HVA calcium current with reduced amplidues (B, second bar in D); and C, 16% exhibit control-like currents (C, third bar in D). E, on average, isolated LVA calcium current at –40 mV (left column), sustained HVA (middle column), and total calcium current at maximum (total ICa max, right column) are significantly reduced in DmαG excision mutants as compared with control (Mann–Whitney U test, ***P < 0.001; **P < 0.01). Data are shown as median with 25% and 75% quartiles. Dotted error bars depict maximum and minimum values.
In summary, most DmαG null mutants lacked transient LVA and sustained HVA currents, but some DmαG null mutant animals showed normal HVA and LVA currents. Different ages, breeding conditions or different recording conditions can be excluded as potential causes for three different current phenotypes resulting from one excision mutant. First, all animals were age matched. Second, all animals were raised under well-controlled conditions on standard food in an environmental control chamber (see Methods). Third, recordings of LVA and HVA calcium currents from all other genotypes did not show any comparable variability (Figs 2 to 7). Therefore, excision of the DmαG gene caused three distinct calcium current phenotypes, complete loss of all calcium current (56% of all animals), significant reduction of both LVA and HVA current (28% of all animals), or no effect on calcium currents (16% of all animals).
This suggests that another gene can produce both LVA and HVA currents, and may possibly be regulated by DmαG (see Discussion). Since Dmca1D does not underlie calcium currents in MN5 (see Fig. 3), Dmca1A (cacophony, Cav2 homolog) may be responsible for LVA and HVA calcium currents in the fraction of DmαG mutant animals in which these currents were present (Fig. 4B–D). This hypothesis would not account, however, for the observed absence of both LVA and HVA currents in the majority of the DmαG mutants (Fig. 4A and D). An alternative hypothesis is that DmαG channels carry a small-amplitude LVA calcium current that contributes to the establishment of high-amplitude LVA and HVA somatodendritic calcium currents, both mediated by Dmca1A channels. To address these issues we next tested whether Dmca1A knock-down affected both LVA and HVA calcium currents in MN5, whether Dmca1A could indeed produce LVA and HVA calcium currents in vivo, and what portions of adult LVA and HVA currents were normally encoded by Dmca1A and by DmαG in the adult MN5.
Dmca1A (cacophony) RNAi expression strongly reduces LVA and eliminates HVA calcium currents in MN5
To test the hypothesis that Dmca1A may underlie both LVA and HVA calcium currents, Dmca1A RNAi, along with extra UAS-dicer (w1118/>; UAS-mcd8-GFP/UAS-Dmca1A-RNAi; D42-GAL4, Cha-GAL80/UAS-dicer), was expressed in MN5. This caused a reduction in transient LVA calcium current amplitude by 70–80% (Fig. 5A–C, ***P < 0.001), and it abolished all HVA calcium currents in MN5 (Fig. 5A–C, ***P < 0.001). Consequently, the maximum total calcium current (total ICa,max) consisting of LVA and fast HVA was reduced by more than 90% (Fig. 5A–C, ***P < 0.001). The remaining small transient LVA current with an activation voltage at –60 mV showed maximum amplitudes of –100 pA to –200 pA at –40 mV (Fig. 5A and B, see also inset in Fig. 5B with enlarged amplitude scale to visualize the remaining small LVA current at command potentials to –60 mV, −50 mV and −40 mV). Therefore, the Cav2 homolog Dmca1A may encode all HVA and most LVA somatodendritic calcium current in adult MN5. However, RNAi knock-down throughout pupal development, while MN5 dendrites are forming (Consoulas et al. 2002), might cause unspecific developmental effects or compensatory regulation of other ion channels (Marder & Goaillard, 2006). This was addressed by conditional knock-down that was restricted temporally to the adult stage.
Figure 5. Dmca1A RNAi knock-down reduces LVA and eliminates HVA currents.
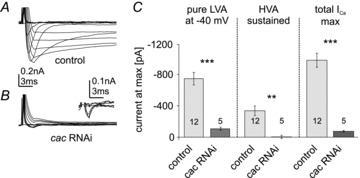
Representative recordings of MN5 calcium currents in control (A) and following targeted expression of UAS-Dmca1A RNAi; UAS-dicer (B). Remaining small outward current after pulse onset at more depolarized command voltages probably reflects potassium current that was un-masked by knock-down of inward current. Inset in B shows enlargement of current in UAS-Dmca1A RNAi for selected command voltages between –90 mV and –40 mV to show the LVA current that remained after Dmca1A RNAi knock-down. Quantification (C) shows that both isolated LVA current at –40 mV (left column) and maximum total calcium current (total ICa max, right column) are reduced by more than 80% (unpaired Student's t test, ***P < 0.001) as compared with control, and sustained HVA current is eliminated following Dmca1A knock-down (**P < 0.01). Light grey bars represent control, dark grey bars represent Dmca1A RNAi knock-down. Data represent mean ± SEM.
Conditional Dmca1A knock-down in the adult strongly reduces LVA and eliminates HVA calcium currents
For conditional knock-down of Dmca1A carried calcium currents in the adult stage we used a temperature-sensitive mutant, cacNT27. CacNT27 channels carry a point mutation in the voltage sensor of the α-subunit that allows for normal channel function at 20°C, but prohibits open state conformation at 38°C (Rieckhof et al. 2003). In initital tests we observed slight temperature-dependent reductions of calcium currents at 22°C and maximal current reductions at 32°C. Raising the temperature from 32°C to 38°C did not cause further current reduction. Therefore, for all experiments with cacNT27 animals, the control temperature was set to 20°C, and the non-permissive temperature was set to 32°C. CacNT27 animals were raised at 20°C to ensure that channel inactivation did not occur during development. In control animals, none of LVA, the maximum total calcium current or sustained HVA current amplitudes were significantly altered upon increasing the temperature from 20°C to 32°C (Fig. 6A). By contrast, in cacNT27 conditional mutants, the amplitudes of both LVA and HVA currents were strongly affected upon raising the bath temperature from 20°C to 32°C (Fig. 6B). On average (n = 6), maximal LVA current amplitude was reduced by 64%± 9% at 32°C (Fig. 6B and C). Sustained HVA current amplitude was normal at 20°C but close to zero at 32°C (Fig. 6B and C, sustained HVA). Therefore, acute temperature-sensitive knock-down of Dmca1A (cacophony) blocked nearly all HVA current and significantly reduced LVA current (Fig. 6C). Consequently, maximum total calcium current was reduced by 64 ± 6% (Fig. 6C).
Figure 6. Conditional Dmca1A knock-down in the adult MN5 reduces LVA and abolishes HVA calcium currents.
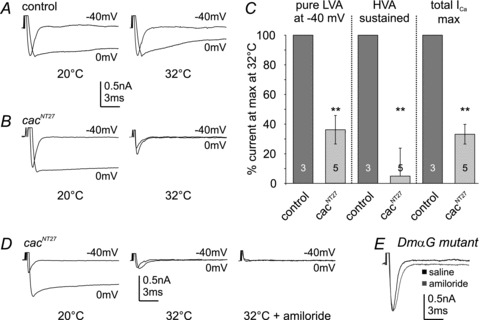
A–C, calcium currents as evoked by voltage steps to –40 mV and 0 mV before and after bath temperature shifts (20°C and 32°C) in control (A) and in conditional mutant cacNT27 animals (B). Isolated LVA at –40 mV (left column), sustained HVA (middle column), and maximum total calcium current amplitudes (right column) are not significantly affected by temperature shifts in controls (C, dark grey bars). In cacNT27 animals isolated LVA calcium current at –40 mV, sustained HVA and maximum total calcium current amplitudes are similar to controls at 20°C, but at 32°C isolated LVA at –40 mV (left column) and total calcium current amplitudes (right column) are significantly reduced (by 63.69 ± 9%, and 64.23 ± 6%, respectively; Student's t test, **P < 0.01, light grey bars). HVA calcium current is virtually abolished at 32°C (C, middle column, **P < 0.01, light grey bar). Application of amiloride (1 mm) at 32°C in cacNT27 mutants abolishes the small LVA calcium current remaining after temperature shift of cacNT27 mutants to 32°C (D). Amiloride does not affect the isolated LVA calcium current amplitude at –40 mV in DmαG mutants (E). Data are shown as mean ± SEM.
The transient LVA calcium current that remained in cacNT27 animals at 32°C (36 ± 9%) was blocked by bath application of the vertebrate T-type channel blocker amiloride (1 mm; Tang et al. 1988; Fig. 6D). Since Dmca1D channels were not present in MN5 (Fig. 3), amiloride must selectively block LVA currents that were mediated by DmαG channels. Accordingly, in control animals, with normal Dmca1A currents, amiloride reduced LVA calcium current amplitude by 34 ± 6%, but did not affect HVA current amplitude (Fig. 2J and K). This indicated that amiloride blocked only DmαG-mediated LVA current in control MN5. Accordingly, in the few DmαG excision mutants that showed control-like LVA calcium currents (16%, Fig. 4C and D) amiloride had no effect on calcium current amplitude (Fig. 6E). These genetic and pharmacological data demonstrate that Dmca1A mediates all somatodendritic HVA current and most of the LVA calcium current in the adult flight motoneuron, whereas DmαG mediates the small fraction of the LVA calcium current that is selectively blocked by amiloride.
Dmca1A channels produce LVA and HVA calcium currents in motoneurons in vivo, and DmαG encodes a small amplitude transient LVA current in vivo
Although normal LVA currents required both Dmca1A and DmαG function, and HVA currents were eliminated by Dmca1A knock-down, a confounding result was that the majority of DmαG mutant animals had no calcium current at all (Fig. 4A and D). There are many potential mechanisms by which DmαG function could influence somatodendritic calcium currents that are mediated by Dmca1A. These range from direct roles of the DmαG protein in Dmca1A splicing, targeting or membrane insertion, to activity-dependent control of Dmca1A expression via calcium-dependent mechanisms. Alternatively, the small depolarization caused by DmαG current could be necessary to activate Dmca1A channels under normal circumstances. To rule out a direct necessity of DmαG protein for somatodendritic Dmca1A calcium current, we expressed UAS-cac1, a transgene expressing GFP-tagged Dmca1A calcium channels (Kawasaki et al. 2002, 2004), in a DmαG null mutant background. In all 11 animals tested this yielded LVA, maximum total calcium current and sustained HVA calcium currents with normal activation voltages but reduced amplitudes as compared with control (Fig. 7A and B; compare also Figs 7C and 2H). Note that 56% of the DmαG mutant flies showed no calcium currents at all and 44% showed either reduced or normal LVA and HVA currents (Fig. 4). Consequently, the probability that all 11 DmαG mutant flies that were tested would have expressed at least some calcium current without UAS-cac1 expression was 0.4411, or 0.01%. Therefore, the presence of LVA and HVA currents in DmαG mutant animals (Fig. 7A and C) could be attributed to forced expression of Dmca1A (UAS-cac1). Thus, Dmca1A can encode functional LVA as well as HVA calcium channels in vivo. This was further confirmed pharmacologically by bath application of the spider toxin PLTXII (Plectreurys toxin II), a selective Dmca1A channel blocker (Branton et al. 1987). Bath application of PLTXII (10 nM) to DmαG mutants with UAS-cac1 expression in MN5 abolished nearly all LVA and HVA calcium current (Fig. 7A and D, light grey bars). By contrast, in control animals PLTXII reduced LVA by about 65% and abolished all sustained HVA current (Fig. 7B and D, dark grey bars). The 35% of LVA current amplitude remaining after PLTXII application to control MN5 matched the 34 ± 6% amiloride-sensitive LVA current in control MN5 (see Fig. 2J). Furthermore, the small LVA calcium current that remained after PLTXII application to control MN5 was blocked by bath application of 1 mm amiloride (Fig. 7B). This was consistent with amiloride block of small-amplitude LVA current that remained after Dmca1A knock-down (Fig. 6D), and with the failure of amiloride to block calcium currents in DmαG mutants (Fig. 6E). In summary, these data show that in the adult MN5 in vivo Dmca1A channels produce about 65% of the LVA and 100% of the sustained HVA calcium currents, whereas DmαG produces about 35% of LVA current.
Discussion
DmαG channels mediate LVA calcium current
Transient LVA calcium currents have previously been recorded in Drosophila muscle (Gielow et al. 1995), and indirect evidence for neuronal LVA currents came from the embryonic CNS (Baines & Bate, 1998). In other insects neuronal LVA, although not transient, has been demonstrated (Grolleau & Lapied, 1996), but the underlying gene(s) were not determined. This study demonstrates what we believe for the first time that DmαG encodes a transient LVA calcium current in Drosophila neurons that activates between –70 mV and –60 mV in vivo. Small-amplitude LVA current was absent in DmαG mutants and selectively blocked by the vertebrate T-type channel blocker amiloride (Tang et al. 1988), thus confirming genome sequence predictions that revealed strong similarity between DmαG (Littleton & Ganetzky, 2000) and vertebrate Cav3 channels (Perez-Reyes, 2003). Therefore, the three vertebrate VGCC families (Cav1, Cav2 and Cav3) each have one Drosophila homolog, namely Dmca1D, Dmca1A and DmαG.
Dmca1A (Cav2 homolog) mediates LVA and HVA currents in the same neuron in vivo
Three lines of evidence show that Dmca1A encodes somatodendritic HVA calcium currents. First, RNAi knock-down of Dmca1A abolished all HVA calcium current in MN5. Second, acute conditional knock-down of Dmca1A also abolished all HVA current, excluding the possibility that there were indirect developmental effects of Dmca1A knock-down on adult calcium currents. Third, application of the Dmca1A channel blocker PLTXII (Branton et al. 1987) blocked all HVA current. Similarly, in cultured embryonic Drosophila cytokinesis-arrested neuroblasts Dmca1A mediates sustained HVA calcium current (Peng & Wu, 2007). HVA current in MN5 has a fast onset and does not fully inactivate over the duration of the voltage step (200 ms), and the shape of the current suggests two different components. Although electrical separation with depolarizing pre-pulses into a transient and a sustained HVA component was not possible, and the genetic and pharmacological manipulations employed in this study affected all HVA current and not only a portion of it, we cannot exclude the possibility that different splice variants of Dmca1A may underlie fast and slow components of HVA in MN5. Future studies will test this by expression of different splice variants in a Dmca1A null mutant background.
However, our data demonstrate that Dmca1A encodes both HVA and LVA calcium current in vivo. Three lines of evidence proved that a large portion of MN5 LVA current was mediated by Dmca1A. First, RNAi knock-down of Dmca1A in MN5 abolished 70–80% of the LVA current. Second, acute genetic knock-down in temperature-sensitive mutants as well as acute pharmacological block of Dmca1A by PLTXII abolished about 60–70% of the LVA current in MN5. Third, the remaining PLTXII-insensitive LVA current is blocked by amiloride. This strongly indicates that Dmca1A encodes a fast, transient, PLTXII-sensitive LVA current in MN5 that activates between –70 mV and –60 mV. Therefore, Dmca1A encodes both HVA and LVA somatodendritic calcium current in the same neuron in vivo. This was further confirmed by targeted expression of a Dmca1A transgene in MN5 (along with endogenous Dmca1A) in a DmαG mutant background, which reliably produced LVA and HVA calcium currents that were blocked by PLTXII.
To the best of our knowledge this study is the first report of a Cav2-like channel mediating LVA calcium current with activation voltages between –70 mV and –60 mV. At present it remains unclear whether vertebrate Cav2 channels can mediate currents with similarly negative activation voltages, because sequence differences exist between Dmca1A and Cav2 (see below), and both may assemble with different accessory subunits. However, sustained HVA calcium current and fast inactivating calcium current with hyperpolarized activation voltages of –40 mV, both based on Dmca1A, have previously been reported in cultured Drosophila neuroblasts (Peng & Wu, 2007). Furthermore, some Cav1.3 channels activate in the LVA range (Scholze et al. 2001; Xu & Lipscombe, 2001; Mangoni et al. 2003), as alternative splicing can shift their activation voltages towards more hyperpolarized potentials (Chen et al. 2009; Striessnig et al. 2010; Bock et al. 2011).
Possible mechanism by which Dmca1A may produce LVA and HVA channels
In contrast to voltage-gated potassium and sodium channels voltage-gated calcium channels are assembled from four homologous repeats (I–IV) each containing six transmembrane domains (TMD, S1–S6), all read from a single gene. Therefore, heterotetramerization as reported to cause different current properties in potassium channels (Yuan & Cheng, 2006) is not an option. However, Dmca1A contains 34 exons, at least four of which undergo alternative splicing (Smith et al. 1996; Peixoto et al. 1997; Kawasaki et al. 2002). An attractive hypothesis is that different activation voltages of Dmca1A channels may result from alternative splicing of mutually exclusive exons coding for part of the S4/S5 extracellular loop and the voltage sensor in the S4 TMD of the first of the four homologous repeats of the gene. Other known alternative splice sites in Dmca1A affect interactions with accessory subunits as well as a small exon coding for a part of repeat IV (Peixoto et al. 1997). Additional experiments are needed to determine whether different splice variants of Dmca1A underlie LVA and HVA somatodendritic neuronal calcium current.
Dmca1A also contains at least ten known sites for A to I RNA editing which theoretically yields more than 1000 possible channel isoforms (Smith et al. 1996; Peixoto et al. 1997; Smith et al. 1998). Although genetic suppression of RNA editing causes disruption of coordinated motor behaviour including courtship (Jepson & Reenan, 2009), at present it remains speculative whether RNA editing may cause different activation voltages of Dmca1A channels. The Drosophila system offers genetic tools to directly address this possibility in future (Palladino et al. 2000).
DmαG promotes the normal development of LVA and HVA currents encoded by Dmca1A
Clearly, the Cav2 homolog Dmca1A underlies all HVA and 60 to 70% of adult MN5 LVA current. By contrast, DmαG makes up only for about 35% of all adult LVA current. What might be the function of DmαG channels if Dmca1A channels underlie most LVA current? One possibility is that DmαG channels might be localized differently from Dmca1A channels. In Purkinje and in mitral cells, Cav3 channels localize postsynaptically and interact with mGluRs (Hildebrand et al. 2009; Isope et al. 2010; Johnston & Delaney, 2010). Alternatively, DmαG channels may contribute to the normal development of LVA and HVA currents through Dmca1A channels, as indicated by the absence or reduction of adult calcium currents in the majority of all DmαG mutant animals. DmαG is not absolutely necessary for Dmca1A transcription, post-transcriptional processing or targeting of channels, because all DmαG excision mutant animals with over-expression of Dmca1A channels had both LVA and HVA calcium currents. However, this does not exclude the possibility that expression of DmαG might facilitate any of these processes, particularly transcription of the endogenous Dmca1A gene. The observed variability of calcium current amplitudes in DmαG mutants suggests that there may be activity-dependent plasticity of Dmca1A expression. Our working model is that calcium influx through dendritic LVA DmαG channels, as induced by early synaptic activity during pupal development, will facilitate Dmca1A expression. MN5 receives excitatory cholinergic synaptic drive through Dα7 nAChRs (Fayyazuddin et al. 2006). A postsynaptic localization of Cav3 channels in dendrites has been reported in several types of vertebrate neurons (Hildebrand et al. 2009; Isope et al. 2010). Therefore, it seems likely that synaptic activity will cause calcium influx through DmαG LVA channels, which in turn may facilitate Dmca1A channel expression. Since insect nAChRs conduct sodium and calcium, strong synaptic activity may be sufficient to signal Dmca1A expression without activation of DmαG channels, as was observed in 16% of all DmαG mutants. Since there are many potential ways by which activity-dependent calcium influx may regulate Dmca1A channels, ranging from transcriptional regulation to alternative splicing, targeting, translational regulation etc., the nature of the mechanisms has yet to be unraveled. Functional interactions of Cav3 and Cav2 channel expression have been reported in absence epilepsy models in mouse cortical neurons (Zamponi et al. 2010), but the underlying mechanisms remain unknown. The genetic tools available in Drosophila may facilitate future investigation of the mechanisms by which DmαG regulates Cav2 channel expression.
Acknowledgments
We gratefully acknowledge support by the National Science Foundation (NSF; 0949051 to C.D. and R.B.L.) by NIH (RO1 NS057637 to R.B.L.), and by the German Research Foundation (DFG RY117/1-1 to S.R.). We would like to thank Chris Gay, Milos Babic, Mays Imad and Konrad Zinsmaier (University of Arizona) for advice on PCR and mutagenesis strategies and Fernando Vonhoff (Arizona State University) for help with generating fly lines.
Glossary
Abbreviations
- Cav1/2/3
voltage-dependent calcium channel, family 1/2/3
- Cac
cacophony
- LVA
low voltage-activated calcium current
- HVA
high voltage-activated calcium current
- VGCC
voltage-gated calcium channel
Author contributions
All electrophysiological experiments of this study were done in the School of Life Sciences at Arizona State University. The generation and validation of DmαG excision mutants was done in the Department of Neuroscience at the University of Arizona. S.R.: Conception and design of experiments, collection, analysis, and interpretation of electrophysiological data, writing of manuscript. K.L.: Generation of DmαG excision mutants and PCR validation of mutants. R.B.L.: Conception of experiments, supervision of mutant generation and validation, data interpretation. C.D.: Conception and design of experiments, data interpretation, writing of manuscript. All authors approved the final version of the manuscript.
Supplementary Material
Supplementary Table S1
As a service to our authors and readers, this journal provides supporting information supplied by the authors. Such materials are peer-reviewed and may be re-organized for online delivery, but are not copy-edited or typeset. Technical support issues arising from supporting information (other than missing files) should be addressed to the authors
References
- Baines RA, Bate M. Electrophysiological development of central neurons in the Drosophila embryo. J Neurosci. 1998;18:4673–4683. doi: 10.1523/JNEUROSCI.18-12-04673.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baines RA, Pym EC. Determinants of electrical properties in developing neurons. Semin Cell Dev Biol. 2006;17:12–19. doi: 10.1016/j.semcdb.2005.11.003. [DOI] [PubMed] [Google Scholar]
- Bleakman D, Bowman D, Bath CP, Brust PF, Johnson EC, Deal CR, Miller RJ, Ellis SB, Harpold MM, Hans M, Grantham CJ. Characteristics of a human N-type calcium channel expressed in HEK293 cells. Neuropharmacology. 1995;34:753–765. doi: 10.1016/0028-3908(95)00078-k. [DOI] [PubMed] [Google Scholar]
- Bock G, Gebhart M, Scharinger A, Jangsangthong W, Busquet P, Poggiani C, S Sartori, Mangoni ME, Sinnegger-Brauns MJ, Herzig S, Striessnig J, Koschak A. Functional properties of a newly identified C-terminal splice variant of Cav1.3 L-type Ca2+ channels. J Biol Chem. 2011;286:42736–42748. doi: 10.1074/jbc.M111.269951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boerner J, Duch C. Average shape standard atlas for the adult Drosophila ventral nerve cord. J Comp Neurol. 2010;518:2437–2455. doi: 10.1002/cne.22346. [DOI] [PubMed] [Google Scholar]
- Branton WD, Kolton L, Jan YN, Jan LY. Neurotoxins from Plectreurys spider venom are potent presynaptic blockers in Drosophila. J Neurosci. 1987;7:4195–4200. doi: 10.1523/JNEUROSCI.07-12-04195.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cain SM, Snutch TP. Contributions of T-type calcium channel isoforms to neuronal firing. Channels. 2010;4:475–482. doi: 10.4161/chan.4.6.14106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cameron P, Hiroi M, Ngai J, Scott K. The molecular basis for water taste in Drosophila. Nature. 2010;465:91–95. doi: 10.1038/nature09011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carlin KP, Bui TV, Dai Y, Brownstone RM. Staircase currents in motoneurons: insight into the spatial arrangement of calcium channels in the dendritic tree. J Neurosci. 2009;29:5343–5353. doi: 10.1523/JNEUROSCI.5458-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng X, Pachuau J, Blaskova E, Asuncion-Chin M, Liu J, Dopico AM, Jaggar JH. Alternative splicing of Cav1.2 channel exons in smooth muscle cells of resistance-size arteries generates currents with unique electrophysiological properties. Am J Physiol Heart Circ Physiol. 2009;297:H680–H688. doi: 10.1152/ajpheart.00109.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Consoulas C, Restifo LL, Levine RB. Dendritic remodeling and growth of motoneurons during metamorphosis of Drosophila melanogaster. J Neurosci. 2002;22:4906–4917. doi: 10.1523/JNEUROSCI.22-12-04906.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Contreras D. The role of T-channels in the generation of thalamocortical rhythms. CNS Neurol Disord Drug Targets. 2006;5:571–585. doi: 10.2174/187152706779025526. [DOI] [PubMed] [Google Scholar]
- Crandall SR, Govindaiah G, Cox CL. Low-threshold Ca2+ current amplifies distal dendritic signaling in thalamic reticular neurons. J Neurosci. 2010;30:15419–15429. doi: 10.1523/JNEUROSCI.3636-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dolphin AC. Calcium channel diversity: multiple roles of calcium channel subunits. Curr Opin Neurobiol. 2009;19:237–244. doi: 10.1016/j.conb.2009.06.006. [DOI] [PubMed] [Google Scholar]
- Drummond GB. Reporting ethical matters in The Journal of Physiology: standards and advice. J Physiol. 2009;587:713–719. doi: 10.1113/jphysiol.2008.167387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duch C, Vonhoff F, Ryglewski S. Dendrite elongation and dendritic branching are affected separately by different forms of intrinsic motoneuron excitability. J Neurophysiol. 2008;100:2525–2536. doi: 10.1152/jn.90758.2008. [DOI] [PubMed] [Google Scholar]
- Dunlap K, Luebke TJ, Turner TJ. Exocytotic calcium channels in mammalian central neurons. Trends Neurosci. 1995;18:89–98. [PubMed] [Google Scholar]
- Fayyazuddin A, Zaheer MA, Hiesinger PR, Bellen HJ. The nicotinic acetylcholine receptor Dα7 is required for an escape behavior in Drosophila. PLoS Biol. 2006;4:e63. doi: 10.1371/journal.pbio.0040063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gielow ML, Gu GG, Singh S. Resolution and pharmacological analysis of the voltage-dependent calcium channels of Drosophila larval muscles. J Neurosci. 1995;15:6085–6093. doi: 10.1523/JNEUROSCI.15-09-06085.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gordon S, Dickinson MH. Role of calcium in the regulation of mechanical power in insect flight. Proc Natl Acad Sci U S A. 2006;103:4311–4315. doi: 10.1073/pnas.0510109103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grolleau F, Lapied B. Two distinct low-voltage-activated Ca2+ currents contribute to the pacemaker mechanism in cockroach dorsal unpaired median neurons. J Neurophysiol. 1996;76:963–976. doi: 10.1152/jn.1996.76.2.963. [DOI] [PubMed] [Google Scholar]
- Gu H, Jiang SA, Campusano JM, Iniguez J, Su H, Hoang AA, Lavian M, Sun X, O’Dowd DK. Cav2-type calcium channels encoded by cac regulate AP-independent neurotransmitter release at cholinergic synapses in adult Drosophila brain. J Neurophysiol. 2009;101:42–53. doi: 10.1152/jn.91103.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heckman CJ. Active conductances in motoneuron dendrites enhance movement capabilities. Exercise Sport Sci Rev. 2003;31:96–101. doi: 10.1097/00003677-200304000-00008. [DOI] [PubMed] [Google Scholar]
- Heckman CJ, Johnson M, Mottram C, Schuster J. Persistent inward currents in spinal motoneurons and their influence on human motoneuron firing patterns. Neuroscientist. 2008;14:264–275. doi: 10.1177/1073858408314986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heckman CJ, Lee RH, Brownstone RM. Hyperexcitable dendrites in motoneurons and their neuromodulatory control during motor behavior. Trends Neurosci. 2003;26:688–695. doi: 10.1016/j.tins.2003.10.002. [DOI] [PubMed] [Google Scholar]
- Hildebrand ME, Isope P, Miyazaki T, Nakaya T, Garcia E, Feltz A, et al. Functional coupling between mGluR1 and Cav3.1 T-type calcium channels contributes to parallel fiber-induced fast calcium signaling within Purkinje cell dendritic spines. J Neurosci. 2009;29:9668–9682. doi: 10.1523/JNEUROSCI.0362-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hounsgaard J, Mintz I. Calcium conductance and firing properties of spinal motoneurones in the turtle. J Physiol. 1988;398:591–603. doi: 10.1113/jphysiol.1988.sp017059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hultborn H, Brownstone RB, Toth TI, Gossard JP. Key mechanisms for setting the input-output gain across the motoneuron pool. Prog Brain Res. 2004;143:77–95. doi: 10.1016/s0079-6123(03)43008-2. [DOI] [PubMed] [Google Scholar]
- Hyngstrom A, Johnson M, Schuster J, Heckman CJ. Movement-related receptive fields of spinal motoneurons with active dendrites. J Physiol. 2008;586:1581–1593. doi: 10.1113/jphysiol.2007.149146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ikeda K, Koenig JH. Morphological identification of the motor neurons innervating the dorsal longitudinal flight muscle of Drosophila melanogaster. J Comp Neurol. 1988;273:436–444. doi: 10.1002/cne.902730312. [DOI] [PubMed] [Google Scholar]
- Isope P, Hildebrand ME, Snutch TP. Contributions of T-type voltage-gated calcium channels to postsynaptic calcium signaling within purkinje neurons. Cerebellum. 2010 doi: 10.1007/s12311-010-0195-4. DOI: 10.1007/s12311-010-0195-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jepson JEC, Reenan RA. Adenosine-to-inosine genetic recoding is required in the adult stage nervous system for coordinated behavior in Drosophila. J Biol Chem. 2009;284:31391–31400. doi: 10.1074/jbc.M109.035048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnston J, Delaney KR. Synaptic activation of T-type Ca2+ channels via mGluR activation in the primary dendrite of mitral cells. J Neurophysiol. 2010;103:2557–2569. doi: 10.1152/jn.00796.2009. [DOI] [PubMed] [Google Scholar]
- Kang K, Pulver SR, Panzano VC, Chang EC, Griffith LC, Theobald DL, Garrity PA. Analysis of Drosophila TRPA1 reveals an ancient origin for human chemical nociception. Nature. 2010;464:597–600. doi: 10.1038/nature08848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawasaki F, Collins SC, Ordway RW. A temperature-sensitive paralytic mutant defines a primary synaptic calcium channel in Drosophila. J Neurosci. 2000;20:4885–4889. doi: 10.1523/JNEUROSCI.20-13-04885.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawasaki F, Collins SC, Ordway RW. Synaptic calcium-channel function in Drosophila: analysis and transformation rescue of temperature-sensitive paralytic and lethal mutations of cacophony. J Neurosci. 2002;22:5856–5864. doi: 10.1523/JNEUROSCI.22-14-05856.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawasaki F, Zou B, Xu X, Ordway RW. Active zone localization of presynaptic calcium channels encoded by the cacophony locus of Drosophila. J Neurosci. 2004;24:282–285. doi: 10.1523/JNEUROSCI.3553-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koenig JH, Ikeda K. Interspike interval relationship among flight muscle fibres in Drosophila. J Exp Biol. 1980;87:137–147. doi: 10.1242/jeb.87.1.137. [DOI] [PubMed] [Google Scholar]
- Lee RH, Heckman CJ. Adjustable amplification of synaptic input in the dendrites of spinal motoneurons in vivo. J Neurosci. 2000;20:6734–6740. doi: 10.1523/JNEUROSCI.20-17-06734.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leung HT, Branton WD, Phillips HS, Jan L, Byerly L. Spider toxins selectively block calcium currents in Drosophila. Neuron. 1989;3:767–772. doi: 10.1016/0896-6273(89)90245-6. [DOI] [PubMed] [Google Scholar]
- Littleton JT, Ganetzky B. Ion channels and synaptic organization: analysis of the Drosophila genome. Neuron. 2000;26:35–43. doi: 10.1016/s0896-6273(00)81135-6. [DOI] [PubMed] [Google Scholar]
- Mangoni ME, Couette B, Bourinet E, Platzer J, Reimer D, Striessnig J, Nargeot J. Functional role of L-type Cav1.3 Ca2+ channels in cardiac pacemaker activity. Proc Natl Acad Sci U S A. 2003;100:5543–5548. doi: 10.1073/pnas.0935295100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marder E, Goaillard JM. Variability, compensation and homeostasis in neuron and network function. Nat Rev Neurosci. 2006;7:563–574. doi: 10.1038/nrn1949. [DOI] [PubMed] [Google Scholar]
- Mynlieff M, Beam KG. Characterization of voltage-dependent calcium currents in mouse motoneurons. J Neurophysiol. 1992;68:85–92. doi: 10.1152/jn.1992.68.1.85. [DOI] [PubMed] [Google Scholar]
- Olsen SR, Wilson RI. Lateral presynaptic inhibition mediates gain control in an olfactory circuit. Nature. 2008;45:956–960. doi: 10.1038/nature06864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palladino MJ, Keegan LP, O’Connell MA, Reenan RA. A-to-I pre-mRNA editing in Drosophila is primarily involved in adult nervous system function and integrity. Cell. 2000;102:437–449. doi: 10.1016/s0092-8674(00)00049-0. [DOI] [PubMed] [Google Scholar]
- Papazian DM, Schwarz TL, Tempel BL, Jan YN, Jan LY. Cloning of genomic and complementary DNA from Shaker, a putative potassium channel gene from Drosophila. Science. 1987;237:749–753. doi: 10.1126/science.2441470. [DOI] [PubMed] [Google Scholar]
- Parks AL, Cook KR, Belvin M, Dompe NA, Fawcett R, Huppert K, et al. Systematic generation of high-resolution deletion coverage of the Drosophila melanogaster genome. Nat Genet. 2004;36:288–292. doi: 10.1038/ng1312. [DOI] [PubMed] [Google Scholar]
- Peixoto AA, Smith LA, Hall JC. Genomic organization and evolution of alternative exons in a Drosophila calcium channel gene. Genetics. 1997;145:1003–1013. doi: 10.1093/genetics/145.4.1003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peng IF, Wu CF. Drosophila cacophony channels: a major mediator of neuronal Ca2+ currents and a trigger for K+ channel homeostatic regulation. J Neurosci. 2007;27:1072–1081. doi: 10.1523/JNEUROSCI.4746-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perez-Reyes E. Molecular physiology of low-voltage-activated t-type calcium channels. Physiol Rev. 2003;83:117–161. doi: 10.1152/physrev.00018.2002. [DOI] [PubMed] [Google Scholar]
- Pulver SR, Griffith LC. Spike integration and cellular memory in a rhythmic network from Na+/K+ pump current dynamics. Nat Neurosci. 2010;13:53–59. doi: 10.1038/nn.2444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reid CA, Bekkers JM, Clements JD. Presynaptic Ca2+ channels: a functional patchwork. Trends Neurosci. 2003;26:683–687. doi: 10.1016/j.tins.2003.10.003. [DOI] [PubMed] [Google Scholar]
- Rieckhof GE, Yoshihara M, Guan Z, Littleton JT. Presynaptic N-type calcium channels regulate synaptic growth. J Biol Chem. 2003;278:41099–41108. doi: 10.1074/jbc.M306417200. [DOI] [PubMed] [Google Scholar]
- Rohrbough J, O’Dowd DK, Baines RA, Broadie K. Cellular bases of behavioral plasticity: establishing and modifying synaptic circuits in the Drosophila genetic system. J Neurobiol. 2003;54:254–271. doi: 10.1002/neu.10171. [DOI] [PubMed] [Google Scholar]
- Ryglewski S, Duch C. Shaker and Shal mediate transient calcium-independent potassium current in a Drosophila flight motoneuron. J Neurophysiol. 2009;102:3673–3688. doi: 10.1152/jn.00693.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanyal S, Narayanan R, Consoulas C, Ramaswami M. Evidence for cell autonomous AP1 function in regulation of Drosophila motor-neuron plasticity. BMC Neurosci. 2003;4:20. doi: 10.1186/1471-2202-4-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scholze A, Plant TD, Dolphin AC, Nürnberg B. Functional expression and characterization of a voltage-gated CaV1.3 (α1D) calcium channel subunit from an insulin-secreting cell line. Mol Endocrinol. 2001;15:1211–1221. doi: 10.1210/mend.15.7.0666. [DOI] [PubMed] [Google Scholar]
- Smith LA, Peixoto AA, Hall JC. RNA editing in the Drosophila DMCA1A calcium-channel α1 subunit transcript. J Neurogenet. 1998;12:227–240. doi: 10.3109/01677069809108560. [DOI] [PubMed] [Google Scholar]
- Smith LA, Wang X, Peixoto AA, Neumann EK, Hall LM, Hall JC. A Drosophila calcium channel α1 subunit gene maps to a genetic locus associated with behavioral and visual defects. J Neurosci. 1996;16:7868–7879. doi: 10.1523/JNEUROSCI.16-24-07868.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steriade M. Grouping of brain rhythms in corticothalamic systems. Neuroscience. 2006;137:1087–1106. doi: 10.1016/j.neuroscience.2005.10.029. [DOI] [PubMed] [Google Scholar]
- Striessnig J, Bolz HJ, Koschak A. Channelopathies in Cav1.1, Cav1.3, and Cav1.4 voltage-gated L-type Ca2+ channels. Pflugers Arch. 2010;460:361–374. doi: 10.1007/s00424-010-0800-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang CM, Presser F, Morad M. Amiloride selectively blocks the low threshold (t) calcium channel. Science. 1988;240:213–215. doi: 10.1126/science.2451291. [DOI] [PubMed] [Google Scholar]
- Thibault ST, Singer MA, Miyazaki WY, Milash B, Dompe NA, Singh CM, et al. A complementary transposon tool kit for Drosophila melanogaster using P and piggyBac. Nat Genet. 2004;36:283–287. doi: 10.1038/ng1314. [DOI] [PubMed] [Google Scholar]
- Trout WE, Kaplan WD. A relation between longevity, metabolic rate, and activity in shaker mutants of Drosophila melanogaster. Exp Gerontol. 1970;5:83–92. doi: 10.1016/0531-5565(70)90033-1. [DOI] [PubMed] [Google Scholar]
- Trout WE, Kaplan WD. Genetic manipulation of motor output in shaker mutants of Drosophila. J Neurobiol. 1973;4:495–512. doi: 10.1002/neu.480040603. [DOI] [PubMed] [Google Scholar]
- Vonhoff F, Duch C. Tiling among stereotyped dendritic branches in an identified Drosophila motoneuron. J Comp Neurol. 2010;518:2169–2185. doi: 10.1002/cne.22380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wakamori M, Strobeck M, Niidome T, Teramoto T, Imoto K, Mori Y. Functional characterization of ion permeation pathway in the N-type Ca2+ channel. J Neurophysiol. 1998;79:622–634. doi: 10.1152/jn.1998.79.2.622. [DOI] [PubMed] [Google Scholar]
- Worrell JW, Levine RB. Characterization of voltage-dependent Ca2+ currents in identified Drosophila motoneurons in situ. J Neurophysiol. 2008;100:868–878. doi: 10.1152/jn.90464.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wyman JD, Levine RJ. Neurophysiology of flight in wild-type and a mutant Drosophila. Proc Natl Acad Sci U S A. 1973;70:1050–1054. doi: 10.1073/pnas.70.4.1050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu W, Lipscombe D. Neuronal CaV1.3αl L-type channels activate at relatively hyperpolarized membrane potentials and are incompletely inhibited by dihydropyridines. J Neurosci. 2001;21:5944–5951. doi: 10.1523/JNEUROSCI.21-16-05944.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yeh E, Gustafson K, Boulianne GL. Green fluorescent protein as a vital marker and reporter of gene expression in Drosophila. Proc Natl Acad Sci U S A. 1995;92:7036–7040. doi: 10.1073/pnas.92.15.7036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuan LL, Chen X. Diversity of potassium channels in neuronal dendrites. Prog Neurobiol. 2006;78:374–389. doi: 10.1016/j.pneurobio.2006.03.003. [DOI] [PubMed] [Google Scholar]
- Zamponi GW, Lory P, Perez-Reyes E. Role of voltage-gated calcium channels in epilepsy. Pflugers Arch. 2010;460:395–403. doi: 10.1007/s00424-009-0772-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng W, Feng G, Ren D, Eberl DF, Hannan F, Dubald M, Hall LM. Cloning and characterization of a calcium channel α1 subunit from Drosophila melanogaster with similarity to the rat brain type D isoform. J Neurosci. 1995;15:1132–1143. doi: 10.1523/JNEUROSCI.15-02-01132.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


