Abstract
Non-technical summary
ON and OFF pathways are major information processing channels of the visual system, and they begin at the retinal bipolar cell level. Here we show a systematic study correlating light response characteristics and cell morphology of OFF (hyperpolarizing) bipolar cells (HBCs) in the mouse retina. Results from this study provide a description of how rods, M-cones and M/S-cones mediate responses in three major types of HBCs, and how synapses in the OFF pathway are interconnected to serve various visual tasks.
Abstract
Bipolar cells are the central neurons of the retina that convey visual signals from rod and cone photoreceptors in the outer retina to higher-order neurons in the inner retina and the brain. Early anatomical studies have suggested that there are four types of cone hyperpolarizing (OFF) bipolar cells (HBCs) in the mouse retina, but no light responses have been systematically examined. By analysing light-evoked cation and chloride currents (ΔIC and ΔICl) from over 50 morphologically identified HBCs in the dark-adapted wildtype and connexin36 knockout (Cx36-/-) mouse retinas, we identified three types of HBCs, each with distinct light responses and morphological characteristics. The HBCR/MCs with axon terminals ramifying between 0% and 30% of the inner plexiform layer (IPL) receive mixed inputs from rods and M-cones, the HBCMCs with axon terminals ramifying between 10% and 50% of the IPL receive inputs primarily from M-cones, and the HBCM/SCs with axon terminals ramifying between 25% and 50% of IPL receive inputs primarily from cones with mixed M- and S-cone pigments. Moreover, we found that HBCR/MCs in the Cx36-/- mice exhibit light responses very similar to the wildtype HBCR/MCs, suggesting that the mixed rod–cone inputs are not mediated by connexin36-dependent rod–cone coupling, but rather by direct synaptic contacts from rods and M-cones. This study constitutes the first systematic investigation that correlates light response characteristics and axonal morphology of HBCs in dark-adapted mouse retina, and contributes to recently emerging evidence that revises the traditional view that mammalian HBCs only contact cone photoreceptors.
Introduction
In the visual system, retinal bipolar cells (BCs) are second-order neurons that receive light-elicited signals from rod and cone photoreceptors in the outer retina and transmit them to amacrine cells (ACs) and ganglion cells (GCs) in the inner retina (Werblin & Dowling, 1969; Dowling, 1987). Early anatomical studies have suggested that mammalian rods make synaptic contacts only with rod depolarizing bipolar cells (DBCRs), whereas cones make synaptic contacts with eight to nine types of cone ON and OFF (depolarizing or hyperpolarizing) bipolar cells (DBCCs or HBCCs) (Kolb, 1994; Boycott & Wassle, 1999). Moreover, rods and cones are electrically coupled to each other, possibly via connexin36-mediated gap junctions and such rod–cone signal mixing at the photoreceptor level may play an important role in rod–cone signalling in the visual pathways (Deans et al. 2002; Dang et al. 2004; Zhang & Wu, 2004).
The rod and cone bipolar cell signalling circuitry described above has been considered for many years as the general organizational plan for all mammals (Kolb, 1994; Boycott & Wassle, 1999; Wassle, 2004). Evidence from recent studies, however, has emerged to challenge this view. In the rabbit retina, for example, when the rod-DBCR synapses are blocked by l-AP4, rod inputs to OFF GCs persist, indicative of an alternative rod-HBC-OFF GC synaptic pathway (Devries & Baylor, 1995). Studies on normal and coneless transgenic mice and rabbits indicate that rods send signals directly to certain types of HBCs (Soucy et al. 1998; Li et al. 2004). While these results on rod–cone signal mixing may be explained by rod–cone coupling, electron microscopic analysis demonstrated that rods in the mouse retina make chemical synapses on dendrites of some HBCCs and DBCCs (Tsukamoto et al. 2001, 2007), suggesting direct chemical synapses may be involved in rod–cone signal mixing in mammalian bipolar cells. By analysing light-evoked cation currents from DBCs in the wildtype, connexin36 knockout (Cx36-/-) and other pathway-specific knockout mice, a recent study shows that a subpopulation of rod DBCs receives substantial inputs directly from cones and a subpopulation of cone DBCs receives substantial inputs directly from rods. Such rod–cone mixed inputs in DBCs are unlikely to be mediated by rod–cone coupling, as they persist in the Cx36-/- mice where rod–cone coupling is absent (Pang et al. 2010a). It is of great interest to determine whether there is physiological evidence for subpopulations of HBCCs that receive mixed rod–cone inputs and whether such HBCCs receive direct chemical synaptic inputs from rods in the mammalian retina.
In the mouse retina, one type of rod and three types of cones, the M-cones, the M/S-cones and the S-cones, have been found (Carter-Dawson & Lavail, 1979; Lyubarsky et al. 1999; Applebury et al. 2000; Haverkamp et al. 2005; Nikonov et al. 2006). All mouse cones express both M- and S-cone pigments, but the S-cone pigment in M-cones is repressed, and the ratio of M- and S-cone pigments expressed in the M/S-cones varies (Applebury et al. 2000) with very few exhibiting pure S-opsin spectral sensitivity (Nikonov et al. 2006; Breuninger et al. 2011; Wang et al. 2011). At the bipolar cell level, anatomical studies have suggested four types of cone HBCs (HBCCs), five types of cone DBCs (DBCCs) and one type of rod DBCs (DBCRs), based on their synaptic contacts in the outer retina and levels of axon terminal stratifications in the inner plexiform layer (IPL) (Ghosh et al. 2004), as well as on their selective GFP- or immuno-labelling pattern (Wassle et al. 2009). On the other hand, physiological studies revealed, based on light responses characteristics, that there are two types of DBCRs, one primarily receives rod inputs and the other receives mixed rod–M-cone inputs, and two types of DBCCs, one receives primarily M-cone inputs and the other receives mixed M-cone–rod inputs. No M/S-cone-driven DBCs were recorded (Pang et al. 2010a). Systematic analysis of light-evoked photoreceptor inputs to various types of HBCs in dark-adapted mouse retinas has not yet been performed.
In this study, we examine rod and cone synaptic inputs to various morphologically identified (by Lucifer yellow dye filling) HBCs in dark-adapted mouse retinas. In addition to studying rod, M- and M/S-cone inputs to HBCs by using the response sensitivity and paired light protocols in wildtype mice, we take advantage of the connexin36 knockout mice to determine the contribution of the connexin36-dependent rod–cone coupling to those HBCs with mixed rod–cone inputs. Results obtained suggest that in addition to M-cone- and M/S-cone-driven HBCs, some HBCs receive mixed rod and M-cone inputs and the mixed signals are not mediated by rod–cone coupling, but rather by direct synaptic inputs from rods and M-cones.
Methods
Animals
The wildtype mouse used in this study was C57Bl/6J from Jackson laboratory (Bar Harbor, MA, USA). Generation of the Cx36-/- mice was described in previous publications (Deans et al. 2002). All animals were handled in accordance with Baylor College of Medicine's policies on the treatment of laboratory animals. Mice were dark-adapted for 1–2 h prior to the experiment. To maintain the retina in the fully dark-adapted state, all further procedures were performed under infrared illumination with dual-unit Nitemare (BE Meyers, Redmond, WA, USA) infrared scopes. Animals were killed by a lethal injection of ketamine + xylazine + acepromazine (0.1 ml, 100 mg ml−1) and the eyes were immediately enucleated and placed in oxygenated Ames’ medium (Sigma, MO, USA) at room temperature. Dissection and preparation of living retinal slices followed essentially the procedures described in previous publications (Pang et al. 2004b, 2007). Oxygenated Ames solution (adjusted to pH 7.3) was introduced continuously to the recording chamber by a gravity superfusion system, and the medium was maintained at 35°C by a temperature control unit (TC 324B, Warner Instruments, CT, USA).
Light stimulus
A photostimulator was used to deliver light spots (of diameter 600–1200 μm) to the retina via the epi-illuminator of the microscope. The intensity of unattenuated (log I = 0) 500 nm light was 1.4 × 106 photons μm−2 s−1. The number of photoisomerizations per rod per second (Rh* rod−1 s−1) was calculated from a rod cross section of 0.5 μm−2 (Field & Rieke, 2002). The peak amplitude of light-evoked current responses was plotted against light stimulus intensity, and data points were fitted by the Hill equation:
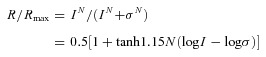 |
(1) |
where R is the current response amplitude, Rmax is the maximum response amplitude, σ is the light intensity that elicits a half-maximal response, N is the Hill coefficient, tanh is the hyperbolic tangent function and log is the logarithmic function of base 10. In this study, we used the R –log I plot for our analysis, and the light intensity span (dynamic range (DR): range of intensity that elicits responses between 5 and 95% of Rmax) of a cell equals to 2.56/N (Thibos & Werblin, 1978). We define response threshold as the intensity of light that elicits 5% of Rmax.
Electrophysiology
Voltage-clamp recordings were made with an Axopatch 700B amplifier connected to a DigiData 1200 interface and pCLAMP 6.1 software (Axon Instruments, Foster City, CA, USA). Whole-cell voltage-clamp recordings were made from HBCs in living retinal slices from central mouse retinas (Pang et al. 2004b). Since the orientations of the retinal slices were not marked, the exact retinal locations of the recorded HBCs were unknown. Patch electrodes were made with Narishige or Sutter patch electrode pullers with 5–7 MΩ tip resistance when filled with internal solution containing 118 mm caesium methanesulfonate, 10 mm CsCl, 5 mm EGTA, 0.5 mm CaCl2, 1.0 mm MgCl2, 4 mm ATP, 0.3 mm GTP, 10 mm Tris and 0.8 mm Lucifer yellow, adjusted to pH 7.2 with CsOH. The chloride equilibrium potential, ECl, with this internal solution was about –60 mV.
Morphology
Cell morphology was visualized in retinal slices through the use of Lucifer yellow fluorescence with a confocal microscope (Zeiss 510). Images were acquired with a ×40 water immersion objective (NA = 1.20), using the 458 nm excitation line of an argon laser, and a long-pass 505 nm emission filter. Consecutive optical sections were superimposed to form a single image using the Zeiss LSM-PC software, and these compressed image stacks were further processed in Adobe Photoshop 6.0 to improve the signal to noise ratio. The level at which dendritic processes stratified in the IPL was characterized in retinal vertical sections by the distance from the processes to the distal margin (0%) of the IPL.
Results
Rod and cone inputs and morphology of hyperpolarizing bipolar cells (HBCs) in dark-adapted mouse retinas
We recorded light responses from 45 HBCs in dark-adapted wildtype mouse retinal slices and 9 HBCs in dark-adapted connexin36 knockout mouse (Cx36-/-) retinal slices, and each cell was filled with Lucifer yellow to reveal the cell morphology. We found that HBCs in the wildtype mouse retina fall into three groups, each with distinct light responses and morphological characteristics. According to the response thresholds and dynamic ranges (DRs, defined as the range of light intensity that elicits responses between 5% and 95% of the cell's maximum response, see Methods section and below) to 500 nm light steps and response waveform to paired light steps (Pang et al. 2010a), 19/45 HBCs receive mixed inputs from rods and M-cones (HBCR/MC), 15/45 HBCs receive inputs primarily from M-cones (HBCMC) and 11/45 HBCs receive inputs primarily from M/S-cones (HBCM/SC). Axon terminals of HBCR/MCs ramify roughly between 0% (distal IPL margin, 1 ± 3%) and 30% (28 ± 8%) of the IPL, axon terminals of HBCMCs ramify between 10% (11 ± 4%) and 50% (53 ± 6%) of IPL, and axon terminals of HBCM/SCs ramify between 25% (23 ± 11%) and 50% (51 ± 5%) of IPL. Columns 1–3 in Fig. 1 show one example of each of the three types of HBCs. Panel A shows the cell morphology (revealed by Lucifer yellow fluorescence), panel B shows light-evoked currents at various holding potentials, panel C shows cation current responses (ΔIC, recorded at ECl) evoked by single light steps of various intensities, panel D shows chloride current responses (ΔICl, recorded at EC) evoked by single light steps of various intensities, and panel E shows ΔIC evoked by a pair of light steps.
Figure 1. Morphology and light responses of HBCs in the wildtype and Cx36 -/- mouse retinas.
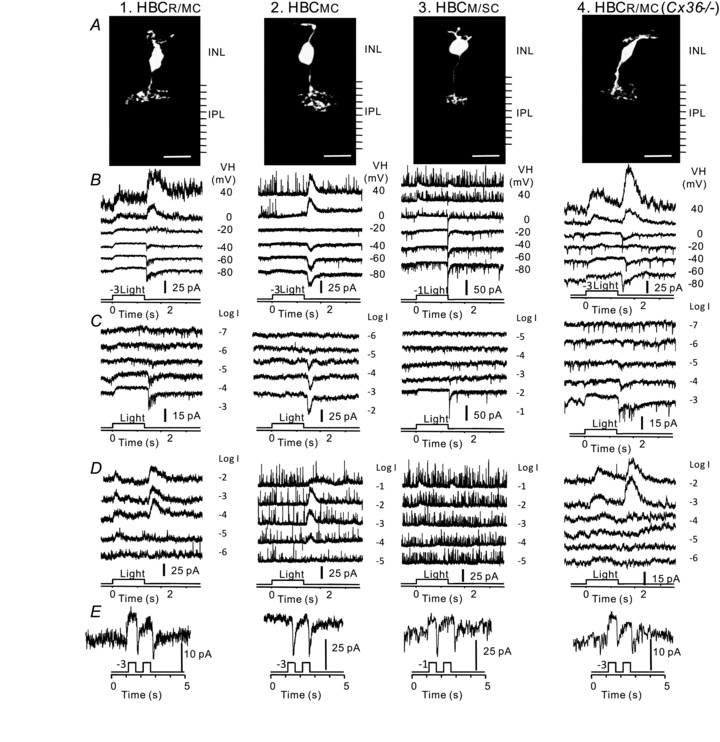
A, stacked confocal fluorescent image of a HBCR/MC, a HBCMC, a HBCM/SC in the wildtype mouse retinal slices and a HBCR/MC in a Cx36-/- mouse retinal slice (column 1–4, respectively). INL, inner nuclear layer; IPL, inner plexiform layer (with 10 divisions). B, light-evoked current responses to 500 nm light steps (1.25 s) of –3 or –1 log unit attenuation at various holding potentials; C, light-evoked cation current (ΔIC) recorded at ECl to the 500 nm, 1.25 s light steps of different intensities (marked as log units of attenuation); D, light-evoked chloride current (ΔICl) recorded at EC to the 500 nm, 1.25 s light steps of different intensities (marked as log units of attenuation); E, light-evoked cation current (ΔIC) recorded at ECl to a pair of light steps (500 nm, –3 or –1 log unit attenuation, 1.25 s in duration and 1 s apart).
In Fig. 1B, it is evident that the light-evoked cation current (ΔIC, recorded near ECl (–60 mV), see Methods section) of all three types of HBCs consists of a sustained outward current during the 1.25 s light step (ON response) and a transient inward current after the light step offset (OFF response). Light-evoked chloride current (ΔICl recorded near EC (0 mV)) of the HBCR/MC consists of a sustained outward ON current and a transient outward OFF current; ΔICl of the HBCM/SC consists of a transient outward ON current and a transient outward OFF current; and ΔICl of the HBCMC only consists of a transient outward OFF current. The outward ON ΔIC (mediated by photoreceptor synapses) is synergistic with the outward ON ΔICl (mediated by ON amacrine cell synapses), and thus the HBCs’ ON responses with both ON ΔIC and ON ΔICl (e.g. HBCR/MCs and HBCM/SCs) do not appear to have a reversal potential (Wu et al. 2001). The HBCMCs exhibit an ON response reversal potential (Fig. 1B, second column) because their ON ΔICl is negligible (Fig. 1D, second column), suggesting that these cells receive little amacrine cell inputs when light stimulus is on. The transient inward OFF ΔIC (mediated by cone synapses; Pang et al. 2004a) is of opposite sign to the transient outward OFF ΔICl (mediated by OFF amacrine cell synapses), and thus the HBCs’ transient OFF responses have a reversal potential between EC and ECl. These response waveforms are consistent with the cone-dominated HBC responses in other species (Pang et al. 2004a).
ΔIC and ΔICl evoked by 500 nm light steps of various intensities of the three HBCs are given in Fig. 1C and D, and the average response–intensity relations for the ON sustained and OFF transient components of the ΔIC of the three types of HBCs, as well as for the rods, M-cones and M/S-cones are shown in Fig. 2A (response–intensity relations of ΔICl are not shown, due to limited space, but the average DRs are displayed in panel 2Bb). The average DRs of the 500 nm light-evoked ΔIC (red) and ΔICl (black) of the three types of HBCs as well as the dynamic ranges of the rod, M-cone and M/S-cone are plotted as continuous (ON responses) and dotted (OFF responses) lines in Fig. 2Bb and Ba, respectively. The rod and M-cone DRs are derived from the photocurrents recorded with suction electrodes in dark-adapted mouse retinal slices (Pang et al. 2010a). The M/S-cone DR is estimated based on the M- and S-cone b-wave responses in a mouse ERG study (Lyubarsky et al. 1999), suction electrode recordings from cones in the presence of background light (Nikonov et al. 2006), as well as the observation that the mouse M/S-cones express mixed M- and S-cone opsins of varying portions (Applebury et al. 2000). Rod photocurrent is about 2 log units more sensitive to the 500 nm light step than the M-cone photocurrent, and M-cone responses are more than 4 log units more sensitive than the S-cone responses (thus an ensemble of M/S-cones with different M- and S-cone opsin ratios cover a wide DR from the M-cone DR to the S-cone DR, long dashed red line in Fig. 2B) (Lyubarsky et al. 1999; Pang et al. 2010a). Comparing the DRs of the three types of HBCs with the DRs of the rod, M-cone and M/S-cone suggests that the ON and OFF ΔIC of the HBCR/MCs are mediated by both rods and M-cones, the ON and OFF ΔIC components of HBCMCs are mediated by M-cones, and the ON and OFF ΔIC components of HBCM/SCs are mediated by M/S-cones. The ON and OFF ΔICl of both HBCR/MCs and HBCMCs are mediated by amacrine cells with M-cone inputs and the ON and OFF ΔICl of HBCM/SCs are mediated by amacrine cells with M/S-cone inputs (Pang et al. 2010b, 2011).
Figure 2. Response–intensity relations and average dynamic ranges of photoreceptors, HBCs, OFFGCs and OFFACs to 500 nm light steps in wildtype and Cx36-/- mice.
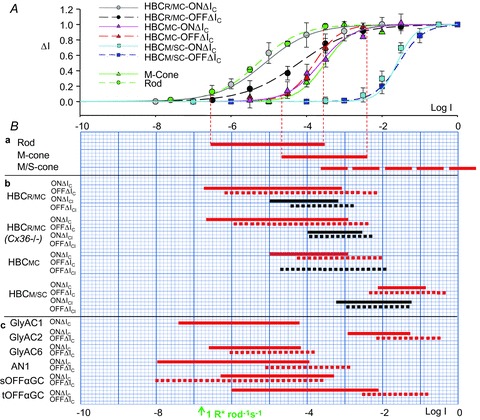
A, average response–intensity (ΔI– log I) relations of the rod (n = 8, green circles) and M-cone photocurrents (n = 2, green triangles; from Pang et al. 2010a), light-evoked cation currents (ΔIC) of the HBCR/MC s (n = 12), HBCMCs (n = 9) and HBCSCs (n = 8) in wildtype mice. HBCR/MC ON ΔIC, grey circles; HBCR/MC OFF ΔIC, black circles; HBCMC ON ΔIC, pink triangles; HBCMC OFF ΔIC, red triangles; HBCM/SC ON ΔIC, light blue squares; HBCM/SC OFF ΔIC, blue squares; Error bars are standard deviations. Ba, average dynamic ranges (DRs; red horizontal lines) of the rod (n = 8) and M-cone (n = 2) photocurrents, and the estimated DR for the M/S-cone responses (see text); Bb, average DRs and of the ON (continuous lines) and OFF (dotted lines) ΔIC (red) and ΔICl (black) of the HBCR/MCs (n = 12), HBCMCs (n = 9) and HBCM/SCs (n = 8) in wildtype mice, and HBCR/MCs in Cx36-/- mice (n = 5) in response to 500 nm light stimuli. Bc, average DRs and of the ON (red continuous lines) and OFF (red dotted lines) ΔIC of the GlyAC1s (n = 3), GlyAC2s (n = 4), GlyAC6s (n = 4), AN1s (n = 6) (from Pang et al. 2010b, 2011), sOFFαGCs (n = 18) and tOFFαGCs (n = 10) (from Pang et al. 2003).
ΔIC responses to light step pairs (Fig. 1E) also support the idea that HBCR/MCs receive mixed rod and cone inputs, as two distinguishable responses to the light step pair with the second response smaller than the first are observed. HBCMCs and HBCM/SCs received primarily cone inputs, as two separate responses of similar amplitude to the light step pair are observed. These conclusions follow an earlier study where we showed that rods and rod DBCs exhibit one continuous response to the 500 nm light step pair (due to its slow response recovery time) whereas the M-cones and cone DBCs show two separate responses of the same amplitude and DBCs with mixed rod and cone inputs exhibit two distinguishable responses to the light step pair with the second response smaller than the first (Pang et al. 2010a). Although single M/S-cone responses to light step pairs have not been recorded from the dark-adapted mouse retina, it is reasonable to assume that they exhibit two separate response peaks of the same amplitude (with brighter 500 nm light steps than for the M-cones), based on the mouse ERG records (Lyubarsky et al. 1999), and the kinetics of single S-cone photocurrents in other species (Makino & Dodd, 1996).
HBC responses in connexin36 knockout mice
We also recorded light responses from nine HBCs in dark-adapted connexin36 knockout mice (Cx36-/-) retinal slices, and each cell was filled with Lucifer yellow to reveal the cell morphology. Four HBCs exhibited HBCR/MC morphology and responses, three with HBCMC morphology and responses and two with HBCM/SC morphology and responses. Since ΔIC of the HBCMCs and HBCM/SCs are primarily mediated by M-cones and M/S-cones, respectively (Fig. 1, columns 2 and 3, and Fig. 2), the contribution of connexin36-dependent rod–cone coupling to ΔIC of these cells is minor. We therefore focused on examining the light response characteristics of HBCR/MCs in the Cx36-/- mice. Column 4 in Fig. 1 is an example of the HBCR/MCs recorded from a dark-adapted retinal slice of a Cx36-/- mouse. The cell morphology, current–voltage responses, ΔIC and ΔICl evoked by single light steps of various intensities, and ΔIC elicited by a light step pair are shown in the same way as the three wildtype HBCs. Moreover, the average response DRs of the 500 nm light-evoked ΔIC (red) and ΔICl (black) of the four HBCR/MCs in Cx36-/- mice are plotted as continuous (ON responses) and dotted (OFF responses) lines in Fig. 2Bb. The waveform, average response threshold and DRs of ΔIC, as well as the ΔIC responses to the light step pairs of the HBCR/MCs in the Cx36-/- mouse are very similar to the HBCR/MCs in the wildtype mouse. This suggests that the rod input to the HBCR/MCs is unlikely to be mediated by the connexin36-dependent rod–cone coupling, but rather by direct rod–HBCR/MC synapses (Tsukamoto et al. 2001). Our observation that the response thresholds and DRs of ΔICl shift to the right (by about 1 log unit) in Fig. 2Bb suggest that connexin36 may be involved in amacrine cells that make inhibitory synapses on HBCR/MC axon terminals (see Discussion).
Discussion
Sign-preserving synaptic inputs from rods, M-cones and M/S-cones to three types of mouse HBCs
We present evidence in this article that hyperpolarizing bipolar cells (HBCs) in dark-adapted wildtype mouse retinas can be divided into three types, according to their distinct light response and morphological characteristics. The HBCR/MCs with axon terminals ramifying between 0% (distal IPL margin) and 30% of IPL receive mixed inputs from rods and M-cones, the HBCMCs with axon terminals ramifying between 10% and 50% of IPL receive inputs primarily from M-cones, and the HBCM/SCs with axon terminals ramifying between 25% and 50% of IPL receive inputs primarily from M/S-cones. These conclusions are derived from the response thresholds and dynamic ranges (DRs) of the cells’ cation current responses (ΔIC) to 500 nm light steps (Figs 1C and 2), as compared with the response DRs of rods, M-cones and M/S-cones to the same 500 nm lights. Moreover, ΔIC response waveforms to light step pairs also support our conclusions that HBCR/MCs receive mixed rod–M-cone inputs, as they exhibit two distinguishable responses to the light step pair with the second response smaller than the first, and that HBCMCs and HBCM/SCs receive inputs primarily from M-cones and M/S-cones, respectively, as they exhibit two distinguishable responses of the same amplitudes to the light step pair (Pang et al. 2010a).
The present study constitutes the first systematic investigation that correlates light response characteristics and axonal morphology of HBCs in dark-adapted mouse retina. An early anatomical study has shown that there are four types (types 1–4) of bipolar cells in the mouse retina with axon terminals ramifying within the distal half of the IPL (presumably HBCs), but no light responses were recorded (Ghosh et al. 2004). By comparing the cell and axonal morphology and levels of axon terminal stratification of the HBCs in the present report and the early anatomical study, it is evident that the HBCR/MCs described here resemble the types 1 and 2, HBCMCs resemble type 4 and HBCM/SCs resemble type 3 in the anatomical study (Ghosh et al. 2004). It has been shown in a recent study that the cone inputs to mouse bipolar cell types 1, 2 and 3–4 (recorded in the presence of a rod-suppressing background light) are mediated by M-, most M-, and mixed M/S-opsins, respectively (Breuninger et al. 2011). This is largely consistent with our results, except that we showed significant rod inputs in types 1 and 2 in addition to the M-cone inputs, and that type 4 is more M-cone biased than type 3. A reason for these differences is that our HBCs were recorded under dark-adapted conditions (with infrared illumination) whereas HBCs in the Breuninger et al. study were recorded in the presence of background light, which suppresses the rod inputs (and perhaps slightly compromises M-cone inputs). We did not correlate the three types of HBCs in this study with the HBC subtypes distinguished by immuno- and GFP/Clomeleon-labelling techniques (Wassle et al. 2009), partially because the antibodies (against NK3R, HCN4, PKARIIβ and Calsenilin) did not clearly label the HBCs filled with Lucifer yellow, and partially because we do not have the GFP/Chomeleon transgenic mouse lines. We plan to carry out this line of investigation after resolving these technical issues.
Our results in Fig. 1B, C and D show that HBCs in the dark-adapted mouse retina exhibit a sustained outward current during the light step (ON response) and a transient inward current after light step offset (OFF responses). In the dark-adapted salamander retina, rod-dominated HBCs (HBCRs) exhibit a large sustained outward current response to a light step, mixed rod–cone HBCs (HBCMs) exhibit a medium sustained outward current and a medium transient off inward current, and the cone-dominated HBCs (HBCCs) exhibit a small sustained outward current and a large transient off inward current (Pang et al. 2004a). The response waveform of the mouse HBCR/MC in this study resembles the current responses of the salamander HBCMs, and the mouse HBCMCs and HBCM/SCs resemble the current responses of the salamander HBCCs.
In the connexin36 knockout mice, we have shown that HBCR/MCs exhibit ΔIC response thresholds, DRs and responses to light step pairs very similar to their counterparts in the wildtype mouse (Fig. 1, columns 1 and 4, and Fig. 2B). These results indicate that the mixed rod–cone inputs to HBCR/MCs, like the DBCR2s and DBCC1s described in a previous study (Pang et al. 2010a), are not mediated by the connexin36-dependent rod–cone coupling, but rather by direct synaptic contacts from rods and cones as revealed by ultrastructural studies (Tsukamoto et al. 2001, 2007). Our present results therefore add to the recently emerging evidence that challenges the dogma on mammalian retinal circuitry set forth by early anatomical studies that only rod DBCs make direct chemical synaptic contacts with rods whereas other BCs only make synaptic contacts with cones (Boycott & Wassle, 1999).
We used the connexin36 knockout (Cx36-/-) mice in this study. A major concern commonly associated with using mutant mice for studying neural circuitry is whether the mutation or gene deletion results in changes or re-organization of the neural network other than the changes caused by the specific gene products. In the retina, many gene mutations result in neuronal degeneration, which leads to re-organization of the surviving neurons nearby in order to accommodate for the degenerative changes, a process called retinal remodelling (or rewiring) (Marc et al. 2003). However, we believe that the Cx36-/- mouse strains used in this study do not exhibit significant retinal rewiring at least during the age window we used (6 to 16 weeks of age), because of three reasons: (1) The Cx36-/- mouse strain has been extensively studied and used by many research groups and there is little detectable neuronal degeneration (Guldenagel et al. 2001; Deans et al. 2002; Abd-El-Barr et al. 2009), and thus degeneration-induced rewiring should not occur in these mice; (2) The function of the retinal network and individual cells in the Cx36-/- mice has been examined and there is no evidence suggesting that re-organization of any kind occurs in these mice (Pang et al. 2010a). In fact, we have shown in a previous publication that the light response characteristics of AII amacrine cells (AIIACs) and DBCs in Cx36-/- mice agree almost completely with corresponding response characteristics isolated by pharmacological tools in the wildtype mouse (Pang et al. 2007); (3) Both wildtype and Cx36-/- mouse strains used in this study were within the same age window (6–16 weeks). This not only gives us age-matched information from all mouse strains, but also lowers the possibility of age-related retinal remodelling in the mutant strain.
Sign-inverting synaptic inputs from ON and OFF amacrine cells to mouse HBCs
Our results in Fig. 2Bb indicate that ΔICl of HBCR/MCs and HBCMCs are mediated by amacrine cells (ACs) with M-cone inputs (Pang et al. 2010b) and ΔICl of HBCM/SCs is mediated by ACs with M/S-cone inputs (Pang et al. 2011). Moreover, since ΔICl of HBCR/MCs and HBCM/SCs exhibit a transient/sustained outward current at light onset and a transient outward current at light offset, they are likely to receive inhibitory (sign-inverting) synaptic inputs from both ON ACs (for ON ΔICl in HBCs) and OFF ACs (for OFF ΔICl in HBCs). Since ΔICl in HBCMCs display transient OFF outward current without significant ON current, they may receive inhibitory (sign-inverting) synaptic inputs mainly from OFF ACs. Connexin36 has been found in AIIACs and DBCC1s in the ON AC pathway (Deans et al. 2002; Demb & Pugh, 2002). Our observation that the response DRs of ON and OFF ΔICl shift to the right (by about 1 log unit) in Fig. 2B suggests these connexin36-containing ON and OFF ACs are involved in mediating inhibitory synaptic inputs to the HBCR/MCs in the mouse retina.
Synaptic outputs from mouse HBCs to amacrine cells and ganglion cells
HBCs make glutamatergic output synapses (with a cation reversal potential) on OFF and ON–OFF ganglion cells (OFFGCs and ON–OFFGCs) and amacrine cells (OFFACs and ON–OFFACs) (Taylor et al. 1995; Gao & Wu, 1999). We have recorded light-evoked cation current responses (ΔIC) from two types of OFFGCs in dark-adpted mouse retinas: the sustained alpha OFFGCs (sOFFαGCs) and the transient alpha OFFGCs (tOFFαGCs) (Pang et al. 2003). The waveform and DRs of the sOFFαGCs ΔIC resemble the HBCR/MC+ HBCMC responses, with a sustained outward current (DR covering rod and M-cone DRs) during light step and a transient inward current at light offset (DR covering M-cone DR). The waveform and DRs of the tOFFαGCs ΔIC resemble the HBCMC+ HBCM/SC responses, with a sustained outward current (DR covering M-cone and M/S-cone DRs) during light step and a transient inward current at light offset (DR covering M/S-cone DR) (Pang et al. 2003). We have also recorded OFF ΔIC from three types of glycinergic amacrine cells: the GlyAC1, GlyAC2 and GlyAC6 (Pang et al. 2011), as well as the NOS-positive AN1 (Pang et al. 2010b). The waveform and DRs of the GlyAC1 ΔIC resemble the HBCR/MC responses, with a large sustained outward current (DR covering rod and M-cone DRs) during light step and a small transient inward current at light offset (DR covering rod and M-cone DRs). The waveform and DRs of the GlyAC2 ΔIC resemble the HBCM/SC responses, with a small sustained outward current during light step and a transient inward current at light offset (DR covering M/S-cone DR). The waveform and DRs of the GlyAC6 ΔIC resemble the HBCMC responses, with a small sustained outward current during light step and a transient inward current at light offset (DR covering M-cone DR). The waveform and DRs of the AN1 OFF ΔIC (AN1 is an ON–OFFAC) resemble the HBCR/MC+ HBCMC responses, with a sustained outward current (DR covering rod and M-cone DRs) during light step and a transient inward current at light offset (DR covering M-cone DR). The average DRs of the sOFFαGC, tOFFαGC, GlyAC1, GlyAC2, GlyAC6 and AN1 ΔIC are plotted in Fig. 2Bc, along with the DRs of the rods, M-cones, M/S-cones (in Fig. 2Ba) and the three types of HBCs (in Fig. 2Bb). A schematic diagram illustrating the major input and output synapses of the three types of mouse HBCs is given in Fig. 3. The glutamatergic synapses are labelled as red, glycinergic/GABAergic synapses as blue arrows and red zigzags are electrical synapses.
Figure 3. Schematic diagram of synaptic connections of the input and output synapses of the three types of HBCs in the mouse retina.
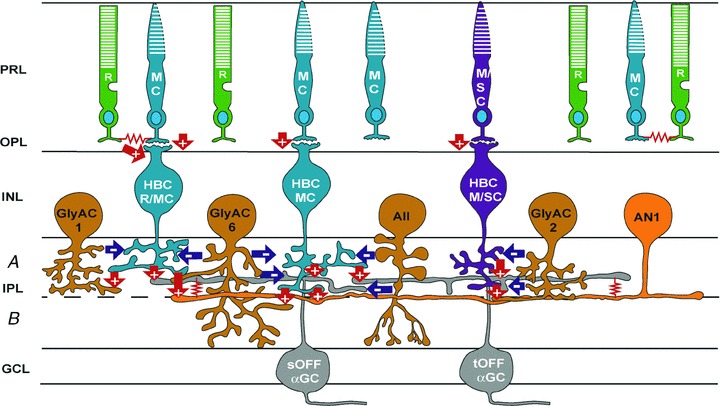
R, rod; MC, M-cone; M/SC, M/S-cone; HBCR/MC, hyperpolarizing bipolar cell with mixed rod/M-cone inputs; HBCMC, hyperpolarizing bipolar cell with M-cone inputs; HBCM/SC, hyperpolarizing bipolar cell with M/S-cone inputs; GlyAC1, type 1 glycinergic amacrine cell; AII, AII amacrine cell; GlyAC2, type 2 glycinergic amacrine cell; GlyAC6, type 6 glycinergic amacrine cell; AN1, type 1 NOS-positive amacrine cell; sOFFαGCs, sustained OFF alpha ganglion cell; and tOFFαGCs, transient OFF alpha ganglion cell; green, rods; blue, M-cones and M-cone BCs; purple, M/S-cones and M/S-cone bipolar cells; light orange, GABAergic ACs; dark orange, glycinergic ACs; grey, GCs; arrows, chemical synapses (‘+’ sign-preserving, and ‘–’ sign-inverting), glutamatergic synapses, red arrows; GABAergic/glycinergic synapses, blue arrows; red zigzags, electrical synapses. PRL, photoreceptor layer; OPL, outer plexiform layer; INL, inner nuclear layer; IPL, inner plexiform layer (a, sublamina a; b, sublamina b); GCL, ganglion cell layer.
It is important to note that the two types of GCs and four types of ACs represent only a fraction of the many types of HBC-driven GCs and ACs in the mammalian retina (Masland & Raviola, 2000). It is also possible that more physiological/morphological types of DBCs and HBCs (beyond the 4 types of DBCs (Ghosh et al. 2004; Breuninger et al. 2011) and 3 types of HBCs (in this report)) will emerge when more sophisticated recording techniques become available. Nevertheless, our results in Fig. 2B demonstrate that one may employ the physiological approach used in this study, in addition to anatomical methods, to study synaptic connectivity in the mammalian retina. By comparing the response DRs and waveforms of the presynaptic glutamatergic (GABAergic/glycinergic) neurons with the DRs and waveforms of the ΔIC (ΔICl) in the postsynaptic cells, it is possible to obtain a comprehensive synaptic circuitry scheme illustrating how visual information is processed in the entire mammalian retina.
Acknowledgments
We thank Roy Jacoby for critically reading this manuscript. This work was supported by grants from NIH (EY004446, EY019908, EY014127), NIH Vision Core (EY02520), the Retina Research Foundation (Houston), and the Research to Prevent Blindness, Inc.
Glossary
Abbreviations
- AC
amacrine cell
- BC
bipolar cell
- Cx36-/-
connexin36 knockout mouse
- DBC
depolarizing bipolar cell
- DR
dynamic range
- GC
ganglion cell
- HBC
hyperpolarizing bipolar cell
- IPL
inner plexiform layer
Author contributions
Conception and design of the experiments: J.-j.P., D.L.P. and S.M.W. Collection, analysis and interpretation of data: J.-j.P., F.G. and S.M.W. Drafting the article or revising it critically for important intellectual content: S.M.W. All authors approved the final version of the manuscript. D.L.P. provided the coonexin36 knockout mice.
References
- Abd-El-Barr MM, Pennesi ME, Saszik SM, Barrow AJ, Lem J, Bramblett DE, Paul DL, Frishman LJ, Wu SM. Genetic dissection of rod and cone pathways in the dark-adapted mouse retina. J Neurophysiol. 2009;102:1945–1955. doi: 10.1152/jn.00142.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Applebury ML, Antoch MP, Baxter LC, Chun LL, Falk JD, Farhangfar F, Kage K, Krzystolik MG, Lyass LA, Robbins JT. The murine cone photoreceptor: a single cone type expresses both S and M opsins with retinal spatial patterning. Neuron. 2000;27:513–523. doi: 10.1016/s0896-6273(00)00062-3. [DOI] [PubMed] [Google Scholar]
- Boycott B, Wassle H. Parallel processing in the mammalian retina: the Proctor Lecture. Invest Ophthalmol Vis Sci. 1999;40:1313–1327. [PubMed] [Google Scholar]
- Breuninger T, Puller C, Haverkamp S, Euler T. Chromatic bipolar cell pathways in the mouse retina. J Neurosci. 2011;31:6504–6517. doi: 10.1523/JNEUROSCI.0616-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carter-Dawson LD, Lavail MM. Rods and cones in the mouse retina. I. Structural analysis using light and electron microscopy. J Comp Neurol. 1979;188:245–262. doi: 10.1002/cne.901880204. [DOI] [PubMed] [Google Scholar]
- Dang L, Pulukuri S, Mears AJ, Swaroop A, Reese BE, Sitaramayya A. Connexin 36 in photoreceptor cells: studies on transgenic rod-less and cone-less mouse retinas. Mol Vis. 2004;10:323–327. [PubMed] [Google Scholar]
- Deans MR, Volgyi B, Goodenough DA, Bloomfield SA, Paul DL. Connexin36 is essential for transmission of rod-mediated visual signals in the mammalian retina. Neuron. 2002;36:703–712. doi: 10.1016/s0896-6273(02)01046-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Demb JB, Pugh EN. Connexin36 forms synapses essential for night vision. Neuron. 2002;36:551–553. doi: 10.1016/s0896-6273(02)01062-0. [DOI] [PubMed] [Google Scholar]
- Devries SH, Baylor DA. An alternative pathway for signal flow from rod photoreceptors to ganglion cells in mammalian retina. Proc Natl Acad Sci U S A. 1995;92:10658–10662. doi: 10.1073/pnas.92.23.10658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dowling JE. The Retina, an Approachable Part of the Brain. Harvard University Press; 1987. [Google Scholar]
- Field GD, Rieke F. Nonlinear signal transfer from mouse rods to bipolar cells and implications for visual sensitivity. Neuron. 2002;34:773–785. doi: 10.1016/s0896-6273(02)00700-6. [DOI] [PubMed] [Google Scholar]
- Gao F, Wu SM. Multiple types of spontaneous excitatory synaptic currents in salamander retinal ganglion cells. Brain Res. 1999;821:487–502. doi: 10.1016/s0006-8993(99)01067-7. [DOI] [PubMed] [Google Scholar]
- Ghosh KK, Bujan S, Haverkamp S, Feigenspan A, Wassle H. Types of bipolar cells in the mouse retina. J Comp Neurol. 2004;469:70–82. doi: 10.1002/cne.10985. [DOI] [PubMed] [Google Scholar]
- Guldenagel M, Ammermuller J, Feigenspan A, Teubner B, Degen J, Sohl G, Willecke K, Weiler R. Visual transmission deficits in mice with targeted disruption of the gap junction gene connexin36. J Neurosci. 2001;21:6036–6044. doi: 10.1523/JNEUROSCI.21-16-06036.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haverkamp S, Wassle H, Duebel J, Kuner T, Augustine GJ, Feng G, Euler T. The primordial, blue-cone color system of the mouse retina. J Neurosci. 2005;25:5438–5445. doi: 10.1523/JNEUROSCI.1117-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kolb H. The architecture of functional neural circuits in the vertebrate retina. The Proctor Lecture. Invest Ophthalmol Vis Sci. 1994;35:2385–2404. [PubMed] [Google Scholar]
- Li W, Keung JW, Massey SC. Direct synaptic connections between rods and OFF cone bipolar cells in the rabbit retina. J Comp Neurol. 2004;474:1–12. doi: 10.1002/cne.20075. [DOI] [PubMed] [Google Scholar]
- Lyubarsky AL, Falsini B, Pennesi ME, Valentini P, Pugh EN., Jr UV- and midwave-sensitive cone-driven retinal responses of the mouse: a possible phenotype for coexpression of cone photopigments. J Neurosci. 1999;19:442–455. doi: 10.1523/JNEUROSCI.19-01-00442.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Makino CL, Dodd RL. Multiple visual pigments in a photoreceptor of the salamander retina. J Gen Physiol. 1996;108:27–34. doi: 10.1085/jgp.108.1.27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marc RE, Jones BW, Watt CB, Strettoi E. Neural remodeling in retinal degeneration. Prog Retin Eye Res. 2003;22:607–655. doi: 10.1016/s1350-9462(03)00039-9. [DOI] [PubMed] [Google Scholar]
- Masland RH, Raviola E. Confronting complexity: strategies for understanding the microcircuitry of the retina. Annu Rev Neurosci. 2000;23:249–284. doi: 10.1146/annurev.neuro.23.1.249. [DOI] [PubMed] [Google Scholar]
- Nikonov SS, Kholodenko R, Lem J, Pugh EN., Jr Physiological features of the S- and M-cone photoreceptors of wild-type mice from single-cell recordings. J Gen Physiol. 2006;127:359–374. doi: 10.1085/jgp.200609490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pang JJ, Abd-El-Barr MM, Gao F, Bramblett DE, Paul DL, Wu SM. Relative contributions of rod and cone bipolar cell inputs to AII amacrine cell light responses in the mouse retina. J Physiol. 2007;580:397–410. doi: 10.1113/jphysiol.2006.120790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pang JJ, Gao F, Lem J, Bramblett DE, Paul DL, Wu SM. Direct rod input to cone BCs and direct cone input to rod BCs challenge the traditional view of mammalian BC circuitry. Proc Natl Acad Sci U S A. 2010a;107:395–400. doi: 10.1073/pnas.0907178107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pang JJ, Gao F, Wu SM. Light-evoked excitatory and inhibitory synaptic inputs to ON and OFF alpha ganglion cells in the mouse retina. J Neurosci. 2003;23:6063–6073. doi: 10.1523/JNEUROSCI.23-14-06063.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pang JJ, Gao F, Wu SM. Stratum-by-stratum projection of light response attributes by retinal bipolar cells of Ambystoma. J Physiol. 2004a;558:249–262. doi: 10.1113/jphysiol.2004.063503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pang JJ, Gao F, Wu SM. Light-evoked current responses in rod bipolar cells, cone depolarizing bipolar cells and AII amacrine cells in dark-adapted mouse retina. J Physiol. 2004b;559:123–135. doi: 10.1113/jphysiol.2003.059543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pang JJ, Gao F, Wu SM. Light responses and morphology of bNOS-immunoreactive neurons in the mouse retina. J Comp Neurol. 2010b;518:2456–2474. doi: 10.1002/cne.22347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pang JJ, Gao F, Wu SM. Physiological characterization and functional heterogeneity of narrow-field mammalian amacrine cells. J Physiol. 2011;590:223–234. doi: 10.1113/jphysiol.2011.222141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soucy E, Wang Y, Nirenberg S, Nathans J, Meister M. A novel signaling pathway from rod photoreceptors to ganglion cells in mammalian retina. Neuron. 1998;21:481–493. doi: 10.1016/s0896-6273(00)80560-7. [DOI] [PubMed] [Google Scholar]
- Taylor WR, Chen E, Copenhagen DR. Characterization of spontaneous excitatory synaptic currents in salamander retinal ganglion cells. J Physiol. 1995;486:207–221. doi: 10.1113/jphysiol.1995.sp020803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thibos LN, Werblin FS. The response properties of the steady antagonistic surround in the mudpuppy retina. J Physiol. 1978;278:79–99. doi: 10.1113/jphysiol.1978.sp012294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsukamoto Y, Morigiwa K, Ishii M, Takao M, Iwatsuki K, Nakanishi S, Fukuda Y. A novel connection between rods and ON cone bipolar cells revealed by ectopic metabotropic glutamate receptor 7 (mGluR7) in mGluR6-deficient mouse retinas. J Neurosci. 2007;27:6261–6267. doi: 10.1523/JNEUROSCI.5646-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsukamoto Y, Morigiwa K, Ueda M, Sterling P. Microcircuits for night vision in mouse retina. J Neurosci. 2001;21:8616–8623. doi: 10.1523/JNEUROSCI.21-21-08616.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang YV, Weick M, Demb JB. Spectral and temporal sensitivity of cone-mediated responses in mouse retinal ganglion cells. J Neurosci. 2011;31:7670–7681. doi: 10.1523/JNEUROSCI.0629-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wassle H. Parallel processing in the mammalian retina. Nat Rev Neurosci. 2004;5:747–757. doi: 10.1038/nrn1497. [DOI] [PubMed] [Google Scholar]
- Wassle H, Puller C, Muller F, Haverkamp S. Cone contacts, mosaics, and territories of bipolar cells in the mouse retina. J Neurosci. 2009;29:106–117. doi: 10.1523/JNEUROSCI.4442-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Werblin FS, Dowling JE. Organization of the retina of the mudpuppy, Necturus maculosus. II. Intracellular recording. J Neurophysiol. 1969;32:339–355. doi: 10.1152/jn.1969.32.3.339. [DOI] [PubMed] [Google Scholar]
- Wu SM, Gao F, Maple BR. Integration and segregation of visual signals by bipolar cells in the tiger salamander retina. Prog Brain Res. 2001;131:125–143. doi: 10.1016/s0079-6123(01)31012-9. [DOI] [PubMed] [Google Scholar]
- Zhang J, Wu SM. Connexin35/36 gap junction proteins are expressed in photoreceptors of the tiger salamander retina. J Comp Neurol. 2004;470:1–12. doi: 10.1002/cne.10967. [DOI] [PubMed] [Google Scholar]


