Abstract
Theta burst stimulation (TBS) protocols of repetitive transcranial magnetic stimulation (rTMS) have after-effects on excitability of motor areas thought to be due to LTP- and LTD-like processes at cortical synapses. The present experiments ask whether, despite the low intensities of stimulation used and the anatomy of the posterior fossa, TBS can also influence the cerebellum. Acquisition and retention of eyeblink classical conditioning (EBCC) was examined in 30 healthy volunteers after continuous theta burst stimulation (cTBS) over the right cerebellar hemisphere. In subjects who received cerebellar cTBS, conditioned responses were fewer and their onsets were earlier (in the last half of the acquisition blocks) than those from control subjects. There was, however, no effect of cerebellar cTBS on the re-acquisition of EBCC in another session of EBCC 7–10 days later. There was also no effect of cerebellar cTBS on the re-acquisition of EBCC in subjects not naïve to EBCC when the stimulation was delivered immediately before a re-acquisition session. Control experiments verified that suppressive effects of cTBS on EBCC were not due to changes in motor cortical excitability or sensory disturbance caused by cTBS. Based on previous EBCC studies in various cerebellar pathologies, our data are compatible with the hypothesis that cerebellar cTBS has a focal cerebellar cortical effect, and are broadly in line with data from studies of EBCC in various animal models. These results confirm that cerebellar TBS has measurable effects on the function of the cerebellum, and indicate it is a useful non-invasive technique with which to explore cerebellar physiology and function in humans.
Key points
Theta burst stimulation (TBS) protocols of repetitive transcranial magnetic stimulation (rTMS) have after-effects on excitability of motor areas thought to be due to LTP- and LTD-like processes at cortical synapses. TBS protocols have significant advantages over other rTMS techniques in time and intensities used.
Eyeblink classical conditioning (EBCC) is a form of associative motor learning in which paired presentation of a conditioned (CS) and unconditioned stimulus (US) leads to the production of a conditioned eyeblink response (CR). EBCC, with its heavy dependence on cerebellar function, is an ideal protocol with which to assess and potentially quantify the possible influence of TBS on the cerebellum.
We show that cerebellar TBS has clear effects on EBCC in humans, providing evidence that TBS can influence cerebellar function despite the low intensities of stimulation used and the anatomical constraints of the posterior fossa.
Introduction
Theta burst stimulation (TBS) is a protocol of repetitive transcranial magnetic stimulation (rTMS) where bursts of 50 Hz stimuli are given at a rate of 5 Hz (Huang et al. 2005). This stimulation pattern was based on work in animal preparations demonstrating that direct electrical stimulation with a theta burst pattern of stimulation was more efficient in producing long-term depressive (LTD) and long-term potentiation (LTP) effects at stimulated synapses than regular repetitive stimulation (Hess et al. 1996). TBS has significant advantages over other rTMS techniques in the brevity of the protocol (40–180 s) and the low intensities of stimulation used (typically 80% of active motor threshold) (Huang et al. 2005). TBS has mainly been applied to study of motor areas, where assessment of its effects is relatively simple. Here, we wished to assess its possible effects on the cerebellum. One previous study using cerebellar TBS has reported effects on the excitability of the motor cortex and intracortical motor circuits (Koch et al. 2008), but in the absence of a more direct test of cerebellar function, the degree to which cerebellar TBS can truly influence cerebellar function remains unclear. Previous modelling studies of the effect of TMS show a decrease of 50% in induced current in tissue 10 mm away from the coil surface compared to tissue adjacent to the coil (Barker, 2002). With the low intensities of stimulation used in TBS protocols and the anatomy of the posterior fossa, it seems quite possible that there would be insufficient penetration of current to have any effect on the cerebellum. This is of considerable experimental interest because it would be very beneficial to have a quick comfortable technique capable of inducing plastic changes in the cerebellum as a replacement for more lengthy and high-intensity protocols previously used (Miall & Christensen, 2004; Torriero et al. 2004; Del Olmo et al. 2007; Fierro et al. 2007).
Eyeblink classical conditioning (EBCC) is a protocol of associative motor learning in which paired presentation of a conditioned (CS) and unconditioned stimulus (US) leads to the production of a conditioned eyeblink response (CR). Studies using classical conditioning of the third eyelid, or nictitating membrane (NM), response of rabbits and EBCC in rodents and ferrets have revealed cerebellar circuitry underlying EBCC in which the cerebellar cortical Purkinje cell (PC) receives convergent afferent information about the CS and US via two separate pathways (Yeo & Hesslow, 1998) with an additional potential convergence upon the underlying interpositus nucleus (IN) (Christian & Thompson, 2003; Linden, 2003; De Zeeuw & Yeo, 2005). EBCC, with its heavy dependence on cerebellar function, is an ideal paradigm with which to assess and potentially quantify the possible influence of rTMS on the cerebellum. The wide variety of patient and animal studies that have assessed EBCC create an opportunity to contrast the effects of acute TBS-induced disruption of cerebellar function with those seen after cerebellar structural lesions.
In this study, we applied cTBS (continuous theta burst stimulation) over the right cerebellar hemisphere and measured its after-effects on acquisition and retention of EBCC.
Methods
Subjects
Thirty volunteers (8 men and 22 women; mean age 29.38 ± 4.94; range: 22–44 years) participated in this study. All participants had no history of neurological, psychiatric or hearing disorders and did not take any medication acting on the central nervous system when studied. Informed consent was obtained from all participants and the study was approved by the local Ethics Committee and conducted in accordance with regulations laid down in the Declaration of Helsinki.
The majority of healthy volunteers were woman; however, no gender differences have previously been reported in literature in terms of effect of cTBS or EBCC.
Electromyographic recordings
Electromyographic (EMG) activity was recorded from both the orbicularis oculi (OO) and first dorsal interosseous (FDI) muscles using Ag–AgCl cup electrodes. OO EMG activity was recorded with the active electrode on the lower eyelid and the reference electrode approximately 3 cm distant on the lateral canthus (Kimura et al. 1985). FDI EMG activity was recorded with the active and the reference electrodes arranged in a classical belly-tendon montage. EMG raw signals were amplified and band-pass filtered (20 Hz to 3 kHz) using a Digitimer D360 amplifier (Digitimer Ltd, Welwyn Garden City, Herts, UK), digitized at a sampling rate of 5 kHz (CED 1401 laboratory interface; Cambridge Electronic Design, UK) and stored on a laboratory computer for on-line visual display. Data were analysed offline with dedicated software (SIGNAL software; Cambridge Electronic Design).
Eyeblink classical conditioning
The right supraorbital nerve was stimulated percutaneously through a pair of Ag–AgCl cup electrodes with the cathode over the supraorbital foramen and the anode 2 cm above. We used single, constant-current, square-wave electrical stimuli with a pulse width of 200 μs delivered through an electrical stimulator Digitimer DS7 (Digitimer Ltd). The electrical stimulus intensity was adjusted to obtain stable R2 responses (defined as reflex blink components at latency greater than 22 ms from stimulus onset). Typically, stimulus levels were 7 to 10 times the sensory threshold. This electrical supraorbital nerve stimulus was preceded by a tone (the CS) of 2 kHz and 400 ms duration produced by a tone generator (Grass Instruments, Quincy, MA, USA) and presented bilaterally to the subject via binaural headphones at an intensity 50–70 dB above the individual hearing threshold (minimal sound pressure level of 80 dB). CS intensities were kept identical across sessions for individual subjects. The CS inconsistently produced an acoustic startle response (alpha blink) occurring within 200 ms after CS onset. Repeated pairs of CS and US caused CRs to develop with onsets within 200 ms before US onset.
EBCC sessions consisted of seven blocks: six acquisition blocks followed by one within-session extinction block at the end of each session. The first nine trials of each EBCC induction block consisted of nine CS–US pairs, the 10th trial was US only and trial 11 was CS only. The trials with CS only were given to verify that CRs were acquired independently of the US. The EBCC within-session extinction block consisted of 11 trials with only the CS. The inter-trial interval was randomized between 10 and 30 s.
Latencies to onset and peak of conditioned eyeblink responses were visually identified. CR onset was marked at the earliest point at which EMG activity began to rise from pre-CS EMG baseline level. In cases where the CR had multiple peaks, the amplitude and latency of peak amplitude were identified for largest amplitude peak.
CRs were defined as EMG activity lasting at least 50 ms or merging into superimposed UR of at least twice the amplitude of mean EMG baseline activity and clear rising slope (Gerwig et al. 2003). We calculated CR amplitudes only where responses above baseline were detected, which is commonly referred to as ‘CR magnitude’.
In subjects receiving neck cTBS or cerebellar cTBS, EBCC sessions started approximately 5 min after receiving rTMS. EBCC sessions lasted for approximately 25 min, a time frame over which plasticity effects of cTBS on motor cortex are active (Huang et al. 2005).
Transcranial magnetic stimulation
Single-pulse TMS was delivered through a monophasic Magstim 200 stimulator (Magstim Company Ltd, Whitland, Dyfeld, UK) connected to a figure-of-eight coil (external wing 9 cm in diameter) placed tangentially over the left primary motor cortex (M1) in the optimal position (hot spot) for eliciting motor-evoked potentials (MEPs) in the right FDI muscle. The right FDI hot spot (defined over the left M1 as the optimal position for eliciting MEPs in the right FDI muscle) was marked to ensure identical coil positioning throughout the experiment. Single-pulse TMS was delivered at the intensity able to evoke at baseline MEPs of ∼1 mV peak-to-peak amplitude.
Repetitive TMS was delivered through a high-frequency biphasic magnetic stimulator (Magstim SuperRapid, Magstim) connected to a figure-of-eight coil (external wing 9 cm in diameter), placed tangentially over the right cerebellum with the handle pointing superiorly, 1 cm inferior and 3 cm right to the inion, a scalp position by former studies defined to predominantly target the superior and posterior lobules of the lateral cerebellum (Koch et al. 2008). Repetitive TMS was delivered according to the cTBS protocol described by Huang et al. (2005). cTBS consisted of bursts of three pulses delivered at 50 Hz, repeated at intervals of 200 ms given in a continuous train lasting 40 s (600 pulses in total). The stimulation intensity of cTBS was set at 80% of active motor threshold (AMT). The AMT was defined as the lowest intensity evoking five MEPs of at least 200 μV in 10 consecutive trials while subjects maintained a low-level tonic contraction (20% of maximal voluntary contraction) in first dorsal interosseus and the coil was placed over the motor cortical ‘hot-spot’ for this muscle (Rossini et al. 1994). Sham rTMS was achieved by the delivery of cTBS with the same intensity as that used in the cerebellar stimulation but with the coil placed tangentially over the cervical muscles.
We recorded 30 MEPs from the right FDI at three time points: immediately before, 5 min after and 45 min after cerebellar cTBS and sham cTBS, to observe any influence cTBS might have on M1 excitability.
Experimental design
Subjects were studied while they were comfortably seated on a chair in a quiet room with normal indoor lighting. Two main experiments were performed to assess the effect of cerebellar cTBS on EBCC acquisition and retention. All participants were naïve to EBCC at the start of the study. The designs of experiments 1 and 2 are summarized in Fig. 1.
Figure 1.
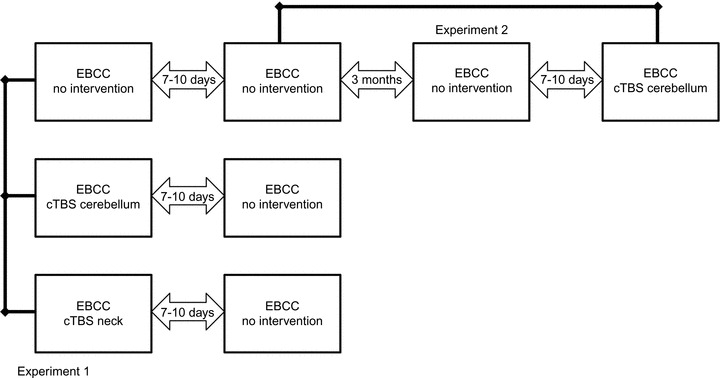
Experimental designs of experiment 1 and experiment 2
Experiment 1
In this experiment we assessed EBCC on two occasions separated by 7–10 days. The first group (no intervention, 12 participants) received no intervention in addition to these two sessions of EBCC. The second group (cTBS cerebellum, 10 participants) received cTBS over the cerebellum prior to the first session of EBCC, and no additional intervention prior to the second session of EBCC. The third group (cTBS neck, 8 participants) received cTBS over the neck muscles prior to the first session of EBCC, and no additional intervention prior to the second session of EBCC.
Experiment 2
Here we examined the effect of cerebellar cTBS on the re-acquisition of EBCC in participants who were not naïve to EBCC. We performed EBCC again in 7 of the 12 participants who had received EBCC without additional intervention in experiment 1. This EBCC session took place approximately 3 months after their last session of EBCC. Seven to ten days later, six of these participants had cTBS delivered over the cerebellum followed immediately by another session of EBCC.
Data analysis and statistics
Data were analysed using SPSS for Windows (version 16.0). The percentage of CRs, magnitude of CRs, the onset and peak latency of the CRs, the peak latency and magnitude of the URs, the number of alpha blinks and MEP peak-to-peak amplitude were used as dependent variables. Distribution of data was assessed using standard tests of normality (P value for the Shapiro–Wilk test of normality was 0.1; normality rejected). As the percentage of CRs over different blocks were not normally distributed these were analysed using non-parametric tests. We used repeated-measures ANOVA to compare magnitude and latencies of conditioned and unconditioned responses and number of alpha blinks. We also used repeated-measures ANOVA to assess whether cerebellar or neck cTBS affected the size of MEPs elicted from stimulation of the motor cortex. In all tests, the level of statistical significance was preset to P < 0.05. Unless otherwise stated all results are indicated as mean values ± the standard error of the mean (SEM). Bonferroni corrections were used in case of multiple comparisons.
Results
None of the subjects reported adverse effects related to the experimental procedures. The mean electrical threshold and electrical stimuli intensities used in the study sample were 1.69 ± 0.48 mA (range 0.60–3.0 mA) and 15.63 ± 4.80 mA (range 7.0–26.0 mA), respectively and were similar in all the experimental sessions (F(7,53) = 0.48; P = 0.85 and F(7,51) = 0.2; P = 0.98, respectively).
The AMT stimulator output in this study was 44.3 ± 6.24 (range 32–53) and did not differ between the three sessions: neck cTBS, cerebellar cTBS naïve participants and cerebellar cTBS second session (F(2,23) = 0.72; P = 0.5).
Experiment 1: EBCC acquisition and retention comparing no intervention, cerebellar cTBS and neck cTBS
Results of experiment 1 are visualized in Fig. 2. A significant difference in mean percentage of CRs over the two consecutive sessions between the three different groups (no cTBS, cerebellar cTBS, neck cTBS) was disclosed using the Kruskal–Wallis test (H(2) = 7.023, P = 0.030). There was no difference in mean number of CRs between the neck cTBS and the no intervention groups (Z =−0.85; P = 0.40). No additional differences were present between these two groups in timing and magnitude of conditioned eyeblink responses, timing and magnitude of unconditioned eyeblink responses and number of alpha blinks during additional analysis. Neck cTBS and no intervention subjects were therefore combined as a single control group.
Figure 2. Experiment 1.
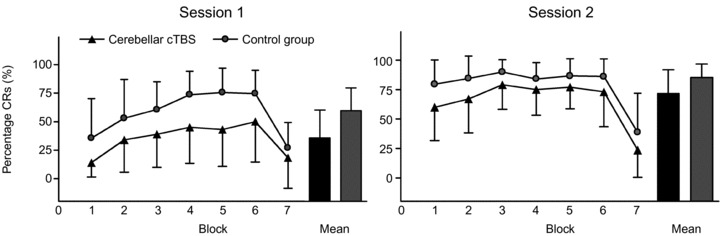
The percentages of conditioned responses in each block of testing (including block 7, the extinction block) are shown on the y axis. Data for the two groups of participants are plotted as black triangles (cerebellar cTBS) and shaded circles (control group). The figure shows data for the first session of EBCC and data for the second session of EBCC performed 7–10 days later. Mean percentage CR incidence over the six acquisition blocks is also shown to visualize overall performance. Error bars represent standard deviation.
A significantly lower mean number of CRs over these two consecutive sessions was observed in the cerebellar cTBS group compared with the control group (Z =−2.49; P = 0.013).
A significant learning effect could be confirmed for both groups as the number of conditioned responses increased over the different sessions. A statistically significant effect for percentage of conditioned responses by BLOCK was confirmed using Friedman tests for the cerebellar cTBS and the control group in session 1 and session 2 (cerebellar cTBS group session 1: X2(6) = 18.09, P = 0.006; control group session 1: X2(6) = 24.31, P < 0.001; cerebellar cTBS group session 2: X2(6) = 60.99, P < 0.001; control group session 2: X2(6) = 44.52, P < 0.001). A significant higher number of mean responses for both groups in session 2 was additionally confirmed for the cerebellar cTBS (Z =−2.60, P = 0.009) and control group (Z =−3.81, P < 0.001) using Wilcoxon signed rank tests for mean percentage of conditioned responses between SESSION 1 and SESSION 2.
Level of retention of EBCC
As a measure of retention in session 2, the percentage of CRs in the last block of paired trials (block 6) in the first EBCC session was compared with the percentage of CRs in the first block of the second. Retention appeared normal for both groups as no significant difference between these two BLOCKS for either group (cerebellar cTBS: Z =−0.97, P = 0.33; control group: Z =−0.73, P = 0.46) could be confirmed using a Wilcoxon signed rank test.
Timing and magnitude of conditioned eyeblink responses
Onset and peak timing of conditioned responses are shown in Fig. 3. A difference in timing of CRs was confirmed between the cerebellar cTBS group and control group for SESSION 1 but not SESSION 2. A repeated-measures ANOVA in the first conditioning session comparing the timing of both onset and peak latency of the conditioned eyeblink responses with main factors GROUP (control group, cerebellar cTBS) and BLOCK (blocks with paired trials) disclosed no significant effect for the between subjects factor GROUP (F(1,19) = 0.13; P = 0.73), (F(1,19) = 0.97; P = 0.34) or BLOCK (F(5,95) = 1.71; P = 0.14), (F(5,95) = 0.27; P = 0.94). A significant GROUP × BLOCK interaction was observed for latency of peak (F(5,95) = 2.37; P = 0.045) but not for onset of CRs (F(5,95) = 2.13; P = 0.07).
Figure 3. Onset and peak timing of conditioned responses (CR) are shown on the y axis.
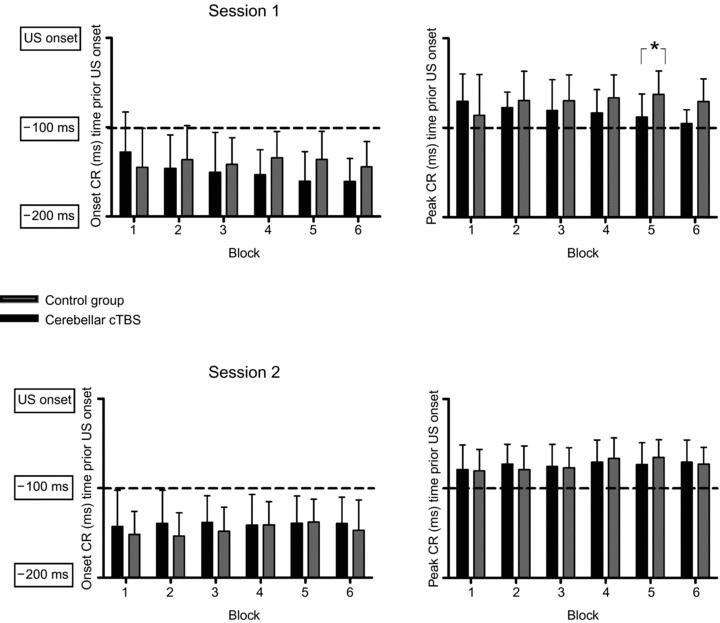
Data for the two groups of participants are plotted for participants receiving cerebellar cTBS (black) and control group (grey) for both sessions. Error bars represent standard deviation. *Significant differences between groups (P < 0.05).
We explored the effect of cTBS on timing of CRs. We only examined blocks 5 and 6 in this regard as the majority of CRs occurred in these blocks. We therefore performed two one-way ANOVAs for block 5 and block 6 with between subject factor GROUP (control group and cerebellar cTBS) that displayed significantly shorter CR peak latency for the cerebellar cTBS group in block 5 (F(1,26) = 6.20; P = 0.020).
A repeated-measure ANOVA in the second conditioning session comparing the timing of both onset and peak latency of conditioned eyeblink responses with factor GROUP (control group, cerebellar cTBS) and BLOCK (6 blocks with paired trials) disclosed no significant effect for the between-factor GROUP (F(1,25) = 0.46; P = 0.50), (F(1,25)< 0.001; P = 0.99) or BLOCK (F(5,125) = 0.97; P = 0.44), (F(5,125) = 1.98; P = 0.09) and no significant GROUP × BLOCK interaction (F(5,125) = 0.82; P = 0.54), (F(5,125) = 0.67; P = 0.65).
Two-way repeated-measures ANOVA for mean CR magnitude revealed no significant effect for the between-group factor GROUP (control group, cerebellar cTBS) (F(1,24) = 1.68; P = 0.21) nor for the within-group factor SESSION (first session, second session) (F(1,24) = 3.63; P = 0.07). The interaction GROUP × SESSION was also not significant (F(1,24) < 0.001; P = 1).
Timing and amplitude of unconditioned responses and mean number of alpha blinks
A general deficit in the performance of both learned and unlearned eyeblink responses was not present as timing and amplitude of URs and mean number of alpha blinks did not differ between the two groups. Two-way repeated-measures ANOVA for mean amplitude and mean peak latency of the URs disclosed no significant effects for the between-group factor GROUP (control group, cerebellar cTBS) (F(1,28) = 2.32; P = 0.14), (F(1,24) = 0.56; P = 0.46) and the within group factor SESSION (first session, second session) (F(1,28) < 0.001; P = 1), (F(1,24) = 1.34; P = 0.26). The interaction GROUP × SESSION was also not significant (F(1,28) = 1.49; P = 0.23), (F(1,24) = 2.24; P = 0.15).
Two-way repeated-measures ANOVA for mean number of alpha blinks revealed no significant effect for the between-group factor GROUP (control group, cerebellar cTBS) (F(1,25) = 0.024; P = 0.88) and the within-group factor SESSION (first session, second session) (F(1,25) = 0.41; P = 0.53). The interaction GROUP × SESSION was also not significant (F(1,25) = 0.41; P = 0.53).
Experiment 2: Retention of EBCC and response to cerebellar cTBS in subjects not naïve to EBCC
At 3 months following EBCC, participants appeared to have returned to baseline with respect to their response to a subsequent EBCC session. No significant retention of EBCC was seen in subjects receiving a further session of EBCC 3 months after their last session. The percentage of CRs in the first acquisition block of the EBCC session 3 months later was significantly lower compared with the last acquisition block (block 6) of their previous EBCC using a Wilcoxon signed rank tests between these two BLOCKs (Z =−2.21, P = 0.027). In addition no significant differences between the percentage of CRs over the complete EBCC session 3 months later and their first EBCC session were revealed using Mann–Whitney U tests (Acquisition Block 1 to 6: Z =−0.97, P = 0.33; Z =−0.78, P = 0.44; Z =−0.59, P = 0.56; Z =−1.36, P = 0.18; Z =−0.78, P = 0.44; Z =−0.07, P = 0.95; Extinction Block 7: Z < 0.001 P = 1).
cTBS over the cerebellum did not significantly change retention, re-acquisition and expression of EBCC when given 7–10 days following this session of EBCC.
Figure 4 reveals a trend towards overall lower percentages of CRs for a second EBCC session with cerebellar cTBS (experiment 2, session 2) from the second EBCC session of the no intervention group from experiment 1, but statistical analysis using Mann–Whitney U tests did not disclose any significant differences between the different BLOCKS (Acquisition Block 1 to 6: Z =−1.22, P = 0.22; Z =−0.16, P = 0.87; Z =−1.53, P = 0.13; Z =−0.98, P = 0.33; Z =−1.27, P = 0.20; Z =−1.15 P = 0.94; Extinction Block 7: Z =−0.081 P = 0.94).
Figure 4. The percentages of conditioned responses in each block of testing (including block 7, the extinction block) are shown on the y axis.
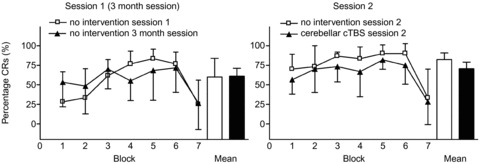
The left side illustrates participants first session of EBCC (open squares) and their third session of EBCC performed at least 3 months following their last session of EBCC. On the right, data from a fourth session of EBCC before which participants received cTBS (filled triangles) and for comparison, data from their second session of EBCC given without cTBS in experiment 1 is illustrated (open squares). Mean percentage CR incidence over the six acquisition blocks is also shown to visualize overall performance. Error bars represent standard deviation.
The percentage of CRs in the last acquisition block of the fourth EBCC session and the first block of the EBCC session 7–10 days previously were comparable as well. We found no significant difference between these two BLOCKS for either group (Z =−0.95, P = 0.34) using a Wilcoxon signed rank test.
Timing and magnitude of conditioned eyeblink responses
No significant differences in timing and magnitude of CRs were found between the second EBCC session of the no cTBS group from experiment 1 and the second session from experiment 2 where subjects who had received an EBCC session 7–10 days previously received cTBS before a final EBCC session.
A repeated-measures ANOVA was conducted to compare changes in timing of conditioned eyeblink responses over conditioning blocks with factors GROUP (no intervention, cerebellar cTBS) and BLOCK (6 blocks with paired trials). No significant effect for the between-subjects factor GROUP (F(1,9) = 3.28; P = 0.10), (F(1,9) = 2.47; P = 0.15) or BLOCK (F(5,45) = 2.07; P = 0.087), (F(5,45) = 1.83; P = 0.13) and no significant GROUP × BLOCK interaction (F(5,45) = 0.35; P = 0.88), (F(5,45) = 1.03; P = 0.41) was observed for both onset and peak latency of conditioned responses.
A one-way ANOVA comparing mean magnitude of CRs with between-subject factor GROUP (cTBS and no intervention) disclosed no significant difference in magnitude of conditioned responses between these two groups (F(1,11) = 3.08; P = 0.11).
Effects of cerebellar and sham cTBS on the MEP peak-to-peak amplitude
In order to assess the effect of cerebellar and neck cTBS on motor cortical excitability we performed a two-way ANOVA with STIMULATION (cerebellar cTBS, neck cTBS) and TIME (before cTBS, 5 min post cTBS, 45 min post cTBS) as main factors. There was no significant effect for STIMULATION (F(1,17) = 3.68; P = 0.072), TIME (F(2,34) = 0.023; P = 0.98) and no STIMULATION × TIME interaction (F(2,34) = 0.067; P = 0.94).
Discussion
The present results show that cTBS delivered over the cerebellar hemisphere had measurable after-effects on EBCC. When applied to naïve subjects before their first session of EBCC, it impaired the acquisition and timing of CRs but did not affect retention when EBCC was tested 1 week later. If cTBS was applied immediately before a second EBCC session, it had no effect on re-acquisition of EBCC. As reported by others, there was good retention of EBCC in subjects after 7–10 days that was not present at 3 months (Gerwig et al. 2010).
Our results strongly suggest that cTBS can interfere with cerebellar function. Theta burst stimulation can induce lasting changes in corticospinal excitability thought to involve LTP-/LTD-like effects on cortical synapses. The pattern of delivery of TBS determines the direction of change and cTBS results in an LTD-like effect. Different animal studies have investigated the role of LTD in cerebellar motor learning by studying different types of LTD expression-deficient mutant mice. Most of these studies found general impairments in motor learning (Koekkoek et al. 2003; Boyden et al. 2006; Hansel et al. 2006). However, a recent study, with a mouse model thought to affect LTD in a more specific way, did not reproduce this impairment and suggested that previous models did not only affect LTD at the PC level but also affected other forms of cerebellar plasticity (Schonewille et al. 2011). In our study, cTBS is likely to only directly stimulate the cerebellar cortex, as it would be unlikely that the intensities used with cTBS could directly affect the deep cerebellar nuclei. However, this does not exclude an effect on deep cerebellar structures (e.g. deep cerebellar nuclei) or extracerebellar structures (e.g. olivary nuclei) as remote secondary effects can occur secondary to rTMS (Stefan et al. 2008).
How do our results fit with previous studies of EBCC in patients with cerebellar lesions? The majority of studies in patients show that unilateral cerebellar lesions cause unilateral reduction of EBCC (Lye et al. 1988; Solomon et al. 1989; Woodruff-Pak et al. 1996; Timmann et al. 1998). Gerwig et al. (2003) examined EBCC in patients with lesions of the superior cerebellar artery (SCA) territory (the SCA supplies hemisphere lobule VI and IN) and found that although unilateral lesions impaired CR acquisition there was no difference between the effect of pure cortical lesions and lesions that also affected deep cerebellar nuclei. In contrast, Woodruff-Pak et al. (1996) observed greater impairment of acquisition if lesions involved the cerebellar nuclei as well as the cortex. Gerwig et al. (2010) recently compared multiple EBCC sessions between patients with degenerative cerebellar disorders affecting cortex and patients with focal cortical lesions of lobules VI and/or Crus I. Acquisition deficits were less marked in patients with focal cortical lesions. Patients with focal cortical lesions were able to retain conditioned responses when tested in consecutive sessions but showed no further improvement in these additional EBCC sessions. Patients with degenerative cerebellar disorders did not acquire, retain or improve conditioned responses over repeated sessions, in accordance with previous results of Timmann et al. (2005).
The clinical evidence above is limited because of the lack of uniform lesion localization and the confounding factors of compensatory change following chronic cerebellar lesions (Brickley et al. 2001). However, they do support the hypothesis that cTBS in healthy subjects predominantly targets the cerebellar cortex as the EBCC deficits in our cTBS subjects are similar to those seen in focal cerebellar cortical lesions, in which acquisition is reduced and retention retained, rather than the picture in degenerative cerebellar disorders where there is a loss of acquisition and retention.
As further support for this hypothesis, cerebellar cortical lesions have also been reported to affect the timing of CRs, particularly if they affect the anterior lobe (Perrett et al. 1993; Gerwig et al. 2005). Gerwig et al. (2010) evaluated delay eyeblink conditioning over multiple sessions in patients with focal cerebellar cortical lesions including lobules VI and Crus I. Only few lesions in this study extended into the anterior lobe and in these cases only a small area of the anterior lobe was lesioned. Patients with focal cortical lesions nevertheless exhibited earlier timing of CRs in their first conditioning session but improved timing close to control values over the two subsequent conditioning sessions. Similarly, in the experiments reported here cTBS produced a subtle shortening of the CR latency in the last half of the conditioning blocks.
Overall, cTBS was more effective in disrupting the acquisition of EBCC compared with the retention and re-acquisition of EBCC. This may reflect the general finding that acquisition is often more easily disturbed than retention by a range of interventions, and that overtraining can make retention particularly resistant (for example, Harvey et al. 1993). However, there are other possibilities: longer-term storage of memory for EBCC might be more dependent upon extracerebellar circuits. Alternatively, if the main effect of cTBS is upon the cerebellar cortex, then the findings are consistent with the suggestion that cerebellar cortex is more involved in the acquisition of EBCC than in the retention of EBCC. There is continued debate about the roles of the cerebellar cortex and nuclei in the acquisition and retention of cerebellum-dependent forms of motor learning in general and EBCC in particular (De Zeeuw & Yeo, 2005; Ohyama et al. 2006; Thompson & Steinmetz, 2009; Kellett et al. 2010). We accept (see below) that our results with regard to the effect of cTBS on retention of EBCC need to be treated with caution given the low subject numbers in this part of the experiment. However, our results in humans are broadly in line with previous animal and human studies and, like the majority of them, do not clearly dissociate the roles of cerebellar cortex and nuclei in the acquisition and retention of EBCC. The lack of significant effect of cTBS on retention of EBCC in a subsequent session may be due to the memory trace being either localized in the deep cerebellar nuclei which are not affected by cTBS or outside the cerebellum. Alternatively, the memory trace may substantially involve the cerebellar cortex but be more resistant to the effects of cTBS than the mechanisms involved in acquisition. Nonetheless, despite these limitations, our study demonstrates for the first time reversible inhibition of EBCC in humans.
We had only a small number of subjects for the second experiment assessing the effect of cTBS given prior to a re-acquisition EBCC session. We found no effect of the stimulation on number of CRs recorded in the subsequent EBCC session, but we cannot exclude the possibility that we have missed a small effect due to insufficient numbers of subjects. The subjects taking part in this part of the experiment received four sessions of EBCC over a 4 month period, and therefore it was difficult to secure sufficient participants. We therefore accept that the conclusions we can draw from these data must be tentative. We have not, in this study, fully explored the temporal profile of the interaction between EBCC and cTBS. Further experiments in humans or in animal models may be appropriate to explore such interactions. As cTBS over the neck muscles had no effect on EBCC, we suggest that the effects of cerebellar rTMS cannot be explained by distraction effects due to the sensory stimulation. A study by Koch et al. (2008) reported that cerebellar cTBS decreases motor cortical excitability; no significant difference in motor cortex excitability was, however, observed after cerebellar cTBS in a recent study by Popa et al. (2010). We looked at changes in MEP size before applying cTBS, 1 min after cTBS and after finishing the conditioning session (approximately 40 min after cTBS) but did not observe any reduction in MEP size. Methodological differences between our experiments and those of Koch et al. (2008) such as estimation of active motor threshold, could account for these different findings on the effect of cerebellar cTBS on motor cortical excitability.
Conclusions
The fact that cerebellar TBS has clear effects on EBCC in humans is strong evidence that cTBS can influence cerebellar function. It suggests that despite the low intensities of stimulation used and the anatomical constraints of the posterior fossa, cTBS can stimulate cerebellar cortex with measurable effects on behaviour. This simple, quick and comfortable rTMS protocol has advantages over traditional rTMS protocols, and the results with regard to EBCC demonstrate the potential usefulness of this technique in studying cerebellar physiology non-invasively in humans.
Acknowledgments
M.J.E. is supported by a grant from the National Institutes of Health Research. B.S.H. and B.P.v.d.W. are supported by a grant from the Prinses Beatrix Fonds, B.P.v.d.W. also receives or has received research support from the Dutch Brain Foundation, European Union, and Ipsen Pharmaceuticals. J.C.R. received funds from the Dystonia Medical Research Foundation. M.B. is supported by a ENS fellowship 2009. The authors declare that they have no conflict of interest.
Glossary
Abbreviations
- AMT
active motor threshold
- CR
conditioned eyeblink response
- CS
conditioned stimulus
- cTBS
continuous theta burst stimulation
- EBCC
eyeblink classical conditioning
- FDI
first dorsal interosseous
- IN
interpositus nucleus
- MEP
motor-evoked potential
- NM
nictitating membrane
- OO
orbicularis oculi
- PC
Purkinje cell
- rTMS
repetitive transcranial magnetic stimulation
- SCA
superior cerebellar artery
- TBS
theta burst stimulation
- US
unconditioned stimulus
Author contributions
B.S.H.: Conception and design of the experiments. Collection, analysis and interpretation of data. M.B.: Conception and design of the experiments. Collection, analysis and interpretation of data. P.K.: Collection, analysis and interpretation of data. J.T.H.: Conception and design of the experiments. J.C.R.: Conception and design of the experiments. B.P.v.d.W.: Conception and design of the experiments. M.J.E.: Conception and design of the experiments. All authors were involved in drafting the article or revising it critically for important intellectual content, and all authors approved the final version. Experiments were performed at the Sobell Department of Motor Neuroscience and Movement Disorders, UCL Institute of Neurology, UCL, UK.
References
- Barker AT. The history and basic principles of magnetic nerve stimulation. In: Pascual-Leone A, Davey NJ, Rothwell JC, Wasserman EM, Puri BK, editors. Handbook of Transcranial Magnetic Stimulation. London: Oxford UP; 2002. pp. 3–17. [Google Scholar]
- Boyden ES, Katoh A, Pyle JL, Chatila TA, Tsien RW, Raymond JL. Selective engagement of plasticity mechanisms for motor memory storage. Neuron. 2006;51:823–834. doi: 10.1016/j.neuron.2006.08.026. [DOI] [PubMed] [Google Scholar]
- Brickley SG, Revilla V, Cull-Candy SG, Wisden W, Farrant M. Adaptive regulation of neuronal excitability by a voltage-independent potassium conductance. Nature. 2001;409:88–92. doi: 10.1038/35051086. [DOI] [PubMed] [Google Scholar]
- Christian KM, Thompson RF. Neural substrates of eyeblink conditioning: acquisition and retention. Learn Mem. 2003;10:427–455. doi: 10.1101/lm.59603. [DOI] [PubMed] [Google Scholar]
- De Zeeuw CI, Yeo CH. Time and tide in cerebellar memory formation. Curr Opin Neurobiol. 2005;15:667–674. doi: 10.1016/j.conb.2005.10.008. [DOI] [PubMed] [Google Scholar]
- Del Olmo MF, Cheeran B, Koch G, Rothwell JC. Role of the cerebellum in externally paced rhythmic finger movements. J Neurophysiol. 2007;98:145–152. doi: 10.1152/jn.01088.2006. [DOI] [PubMed] [Google Scholar]
- Fierro B, Palermo A, Puma A, Francolini M, Panetta ML, Daniele O, Brighina F. Role of the cerebellum in time perception: a TMS study in normal subjects. J Neurol Sci. 2007;263:107–112. doi: 10.1016/j.jns.2007.06.033. [DOI] [PubMed] [Google Scholar]
- Gerwig M, Dimitrova A, Kolb FP, Maschke M, Brol B, Kunnel A, Boring D, Thilmann AF, Forsting M, Diener HC, Timmann D. Comparison of eyeblink conditioning in patients with superior and posterior inferior cerebellar lesions. Brain. 2003;126:71–94. doi: 10.1093/brain/awg011. [DOI] [PubMed] [Google Scholar]
- Gerwig M, Guberina H, Esser AC, Siebler M, Schoch B, Frings M, Kolb FP, Aurich V, Beck A, Forsting M, Timmann D. Evaluation of multiple-session delay eyeblink conditioning comparing patients with focal cerebellar lesions and cerebellar degeneration. Behav Brain Res. 2010;212:143–151. doi: 10.1016/j.bbr.2010.04.007. [DOI] [PubMed] [Google Scholar]
- Gerwig M, Hajjar K, Dimitrova A, Maschke M, Kolb FP, Frings M, Thilmann AF, Forsting M, Diener HC, Timmann D. Timing of conditioned eyeblink responses is impaired in cerebellar patients. J Neurosci. 2005;25:3919–3931. doi: 10.1523/JNEUROSCI.0266-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hansel C, de JM, Belmeguenai A, Houtman SH, Buitendijk GH, Andreev D, De Zeeuw CI, Elgersma Y. αCaMKII is essential for cerebellar LTD and motor learning. Neuron. 2006;51:835–843. doi: 10.1016/j.neuron.2006.08.013. [DOI] [PubMed] [Google Scholar]
- Harvey JA, Welsh JP, Yeo CH, Romano AG. Recoverable and nonrecoverable deficits in conditioned responses after cerebellar cortical lesions. J Neurosci. 1993;13:1624–1635. doi: 10.1523/JNEUROSCI.13-04-01624.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hess G, Aizenman CD, Donoghue JP. Conditions for the induction of long-term potentiation in layer II/III horizontal connections of the rat motor cortex. J Neurophysiol. 1996;75:1765–1778. doi: 10.1152/jn.1996.75.5.1765. [DOI] [PubMed] [Google Scholar]
- Huang YZ, Edwards MJ, Rounis E, Bhatia KP, Rothwell JC. Theta burst stimulation of the human motor cortex. Neuron. 2005;45:201–206. doi: 10.1016/j.neuron.2004.12.033. [DOI] [PubMed] [Google Scholar]
- Kellett DO, Fukunaga I, Chen-Kubota E, Dean P, Yeo CH. Memory consolidation in the cerebellar cortex. PLoS One. 2010;5:e11737. doi: 10.1371/journal.pone.0011737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kimura J, Wilkinson JT, Damasio H, Adams HR, Jr, Shivapour E, Yamada T. Blink reflex in patients with hemispheric cerebrovascular accident (CVA). Blink reflex in CVA. J Neurol Sci. 1985;67:15–28. doi: 10.1016/0022-510x(85)90018-8. [DOI] [PubMed] [Google Scholar]
- Koch G, Mori F, Marconi B, Codeca C, Pecchioli C, Salerno S, Torriero S, Lo GE, Mir P, Oliveri M, Caltagirone C. Changes in intracortical circuits of the human motor cortex following theta burst stimulation of the lateral cerebellum. Clin Neurophysiol. 2008;119:2559–2569. doi: 10.1016/j.clinph.2008.08.008. [DOI] [PubMed] [Google Scholar]
- Koekkoek SK, Hulscher HC, Dortland BR, Hensbroek RA, Elgersma Y, Ruigrok TJ, De Zeeuw CI. Cerebellar LTD and learning-dependent timing of conditioned eyelid responses. Science. 2003;301:1736–1739. doi: 10.1126/science.1088383. [DOI] [PubMed] [Google Scholar]
- Linden DJ. Neuroscience. From molecules to memory in the cerebellum. Science. 2003;301:1682–1685. doi: 10.1126/science.1090462. [DOI] [PubMed] [Google Scholar]
- Lye RH, Oboyle DJ, Ramsden RT, Schady W. Effects of a unilateral cerebellar lesion on the acquisition of eye-blink conditioning in man. J Physiol. 1988;403:58. [Google Scholar]
- Miall RC, Christensen LO. The effect of rTMS over the cerebellum in normal human volunteers on peg-board movement performance. Neurosci Lett. 2004;371:185–189. doi: 10.1016/j.neulet.2004.08.067. [DOI] [PubMed] [Google Scholar]
- Ohyama T, Nores WL, Medina JF, Riusech FA, Mauk MD. Learning-induced plasticity in deep cerebellar nucleus. J Neurosci. 2006;26:12656–12663. doi: 10.1523/JNEUROSCI.4023-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perrett SP, Ruiz BP, Mauk MD. Cerebellar cortex lesions disrupt learning-dependent timing of conditioned eyelid responses. J Neurosci. 1993;13:1708–1718. doi: 10.1523/JNEUROSCI.13-04-01708.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Popa T, Russo M, Meunier S. Long-lasting inhibition of cerebellar output. Brain Stimul. 2010;3:161–169. doi: 10.1016/j.brs.2009.10.001. [DOI] [PubMed] [Google Scholar]
- Rossini PM, Barker AT, Berardelli A, Caramia MD, Caruso G, Cracco RQ, Dimitrijevic MR, Hallett M, Katayama Y, Lucking CH. Non-invasive electrical and magnetic stimulation of the brain, spinal cord and roots: basic principles and procedures for routine clinical application. Report of an IFCN committee. Electroencephalogr Clin Neurophysiol. 1994;91:79–92. doi: 10.1016/0013-4694(94)90029-9. [DOI] [PubMed] [Google Scholar]
- Schonewille M, Gao Z, Boele HJ, Veloz MF, Amerika WE, Simek AA, De Jeu MT, Steinberg JP, Takamiya K, Hoebeek FE, Linden DJ, Huganir RL, De Zeeuw CI. Reevaluating the role of LTD in cerebellar motor learning. Neuron. 2011;70:43–50. doi: 10.1016/j.neuron.2011.02.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Solomon PR, Stowe GT, Pendlbeury WW. Disrupted eyelid conditioning in a patient with damage to cerebellar afferents. Behav Neurosci. 1989;103:898–902. doi: 10.1037//0735-7044.103.4.898. [DOI] [PubMed] [Google Scholar]
- Stefan K, Gentner R, Zeller D, Dang S, Classen J. Theta-burst stimulation: remote physiological and local behavioral after-effects. Neuroimage. 2008;40:265–274. doi: 10.1016/j.neuroimage.2007.11.037. [DOI] [PubMed] [Google Scholar]
- Thompson RF, Steinmetz JE. The role of the cerebellum in classical conditioning of discrete behavioral responses. Neuroscience. 2009;162:732–755. doi: 10.1016/j.neuroscience.2009.01.041. [DOI] [PubMed] [Google Scholar]
- Timmann D, Baier C, Dlener HC, Kolb FP. Impaired acquisition of limb flexion reflex and eyeblink classical conditioning in a cerebellar patient. Neurocase: The Neural Basis of Cognition. 1998;4:207–217. [Google Scholar]
- Timmann D, Gerwig M, Frings M, Maschke M, Kolb FP. Eyeblink conditioning in patients with hereditary ataxia: a one-year follow-up study. Exp Brain Res. 2005;162:332–345. doi: 10.1007/s00221-004-2181-x. [DOI] [PubMed] [Google Scholar]
- Torriero S, Oliveri M, Koch G, Caltagirone C, Petrosini L. Interference of left and right cerebellar rTMS with procedural learning. J Cogn Neurosci. 2004;16:1605–1611. doi: 10.1162/0898929042568488. [DOI] [PubMed] [Google Scholar]
- Woodruff-Pak DS, Papka M, Ivry RB. Cerebellar involvement in eyeblink classical conditioning in humans. Neuropsychology. 1996;10:443–458. [Google Scholar]
- Yeo CH, Hesslow G. Cerebellum and conditioned reflexes. Trends Cogn Sci. 1998;2:322–330. doi: 10.1016/s1364-6613(98)01219-4. [DOI] [PubMed] [Google Scholar]


