Abstract
Non-technical summary
Reaction times of planned movements can be reduced to less than 100 ms when a startling acoustic stimulus (SAS) is presented immediately prior to, or coincident with, the imperative ‘go’ cue. Based on the short latency of these reaction times, it has been suggested that the early release of planned movements by a SAS is mediated by shorter pathways that pass through the brainstem instead of via the primary motor cortex. Here we show that the application of high intensity transcranial magnetic stimulation over the primary motor cortex, a method that suppresses the excitability of the motor cortex and blocks voluntary drive, caused a significant delay in the onset of SAS-released movements. These findings provide evidence that the early release of planned movements by a SAS is mediated, in part, by pathways that pass through the primary motor cortex.
Abstract
Previous studies have shown that preplanned movements can be rapidly released when a startling acoustic stimulus (SAS) is presented immediately prior to, or coincident with, the imperative signal to initiate movement. Based on the short latency of the onset of muscle activity (typically in less than 90 ms) and the frequent co-expression of startle responses in the neck and eye muscles, it has been proposed that the release of planned movements by a SAS is mediated by subcortical, possibly brainstem, pathways. However, a role for cortical structures in mediating these responses cannot be ruled out based on timing arguments alone. We examined the role of the cortex in the mediation of these responses by testing if a suprathreshold transcranial magnetic stimulation applied over the primary motor cortex, which suppresses voluntary drive and is known to delay movement initiation, could delay the release of movement by a SAS. Eight subjects performed an instructed-delay task requiring them to make a ballistic wrist movement to a target in response to an acoustic tone (control task condition). In a subset of trials subjects received one of the following: (1) suprathreshold TMS over the contralateral primary motor cortex 70 ms prior to their mean response time on control trials (TMSCT), (2) SAS 200 ms prior to the go cue (SAS), (3) suprathreshold TMS 70 ms prior to the mean SAS-evoked response time (TMSSAS), or (4) TMSSAS and SAS presented concurrently (TMS+SAS). Movement kinematics and EMG from the wrist extensors and flexors and sternocleidomastoid muscles were recorded. The application of TMSCT prior to control voluntary movements produced a significant delay in movement onset times (P < 0.001) (average delay = 37.7 ± 12.8 ms). The presentation of a SAS alone at −200 ms resulted in the release of the planned movement an average of 71.7 ± 2.7 ms after the startling stimulus. The early release of movement by a SAS was significantly delayed (P < 0.001, average delay = 35.0 ± 12.9 ms) when TMSSAS and SAS were presented concurrently. This delay could not be explained by a prolonged suppression of motor unit activity at the spinal level. These findings provide evidence that the release of targeted ballistic wrist movements by SAS is mediated, in part, by a fast conducting transcortical pathway via the primary motor cortex.
Introduction
Over the past decade, a variety of experiments have shown that voluntary movements can be consistently initiated with premotor reaction times (time from ‘go’ stimulus presentation to EMG onset) of well less than 90 ms when the imperative stimulus to initiate movement is replaced by a startling acoustic stimulus (SAS) (Valls-Solé et al. 1999, 2008; Rothwell et al. 2002; Carlsen et al. 2003, 2004a,b, 2007, 2009; Kumru & Valls-Solé, 2006; Rothwell, 2006; Castellote et al. 2007). The seminal experiments of Valls-Solé et al. (1995, 1999) were the first to show that the reaction time of a ballistic wrist movement could be reduced from a mean of 171 ± 51 ms to 77 ± 51 ms when a 130 dB SPL sound was presented at the time of the ‘go’ cue. This extraordinary reduction in reaction time evoked by a SAS has been termed the ‘StartReact’ phenomenon (Valls-Solé et al. 2008). Comparable reductions in reaction times with SAS have also been shown for other tasks such as rising onto the toes (Valls-Solé et al. 1999), head movement (Siegmund et al. 2001) and step initiation (MacKinnon et al. 2007). A salient feature of the StartReact paradigm is that, despite the short latency of the reaction time, the spatial and temporal features of the intended movement remain unchanged. In addition, the marked shortening of reaction times is only observed during simple reaction time paradigms (Carlsen et al. 2004a) suggesting that the SAS evokes the rapid release of the preplanned and prepared movement from some, as yet unknown, site of temporary storage.
Based on conduction time arguments and the co-expression of movement release with the activation of muscles associated with the generalized classic startle reflex, it has been hypothesized that the SAS releases the pre-planned movement from the same pontomedullary-reticulospinal pathways that mediate the startle reflex (Valls-Solé et al. 1999, 2008; Carlsen et al. 2004). Indirect support for this hypothesis comes from experiments showing that the very fast reactions to a SAS occur almost exclusively when the response is accompanied by a short-latency burst in the sternocleidomastoid muscle, an indicator of a startle response (Carlsen et al. 2007). Valls-Solé et al. (1999) suggested that there may be insufficient time for these rapid responses to be mediated by a transcortical pathway since the minimum sensorimotor conduction time for responses in the wrist muscles were estimated to be near 55 ms. Yet, conduction time arguments are not sufficient to rule-out a contribution of a transcortical pathway in light of evidence that long-latency responses to imposed stretch of the wrist muscle have onset latencies in the 55− 70 ms range and are known to be mediated, in part, by a transcortical pathway (Cheney & Fetz, 1984; Matthews, 1991; Palmer & Ashby, 1992).
The purpose of this paper was to examine the contribution of the contralateral primary motor cortex to the phenomenon of the rapid release of planned movements by a SAS. Activity in the primary motor cortex was modulated by applying suprathreshold transcranial magnetic stimulation (TMS) at intensities that evoked a delay in the onset of voluntary wrist extension movements. Day et al. (1989) were the first to show that the initiation of ballistic targeted movements could be markedly delayed when suprathreshold TMS (above resting motor threshold) was applied during the interval between the imperative ‘go’ signal and the onset of agonist muscle activity, yet the spatial and temporal characteristics of the triphasic electromyographic (EMG) pattern (a sequence of agonist–antagonist–agonist bursts) were unchanged. The mechanisms mediating the delay in onset of movement are considered to be related to the long lasting changes in spinal and cortical excitability evoked by suprathreshold TMS during rest or active contractions of the target muscles (Day et al. 1989; Ziemann et al. 1997; Voss et al. 2006). When suprathreshold TMS is applied during an isometric contraction, the stimulus evokes a prolonged suppression of EMG activity, termed the ‘silent period’ (SP). The balance of evidence suggests that the first part of the SP is principally mediated by a reduction in spinal motor neuron excitability for up to 50 ms (Fuhr et al. 1991; Ziemann et al. 1993) while the later part of the SP (after 50 ms) is associated with a marked suppression of motor cortical excitability, alterations in intracortical inhibition and facilitation, and an accompanying interruption of voluntary drive (Inghilleri et al. 1993; Uncini et al. 1993; Chen et al. 1999; Tergau et al. 1999; Ni et al. 2007). Comparable corticospinal and intracortical effects are produced over a 50–200 ms time period when suprathreshold TMS is applied at rest (Valls-Solé et al. 1992; Tergau et al. 1999; Ni et al. 2007). Thus, when suprathreshold TMS is applied more than 50 ms prior to agonist EMG onset, the induced delay in movement onset is considered to be mediated by the suppression of voluntary drive and interruption of the command to initiate movement at the level of the cortex. We used this protocol to explore the hypothesis that the primary motor cortex does not contribute to the rapid release of movement by a SAS. If the TMS-induced suppression of voluntary drive at the level of the cortex does not delay the rapid release of the planned movement by a SAS then this would provide compelling evidence that the release of movement by a SAS is mediated by subcortical pathways.
Methods
Subjects
Eight neurologically healthy individuals (3 males, 5 females, age range = 22–31 years) participated in the study. All subjects gave written informed consent to the experimental procedures which had been reviewed and approved by the Institutional Review Board of Northwestern University. The study complied with the Declaration of Helsinki. All subjects were right handed by self-report and the experiments were performed with the right arm in all subjects.
Experimental task
Subjects sat in a chair with their shoulder abducted to 30 deg, elbow flexed to 90 deg and forearm braced. They grasped the handle of a manipulandum with the centre of rotation aligned to the flexion–extension axis of the wrist. The task required subjects to perform a wrist extension movement from 10 deg of flexion to 15 deg of extension ‘as fast as possible’. Online visual feedback of wrist position and the start and end target positions were provided on a computer monitor. An instructed-delay task was used which consisted of an acoustic pre-cue tone (40 ms, 1000 Hz, 80 dB) followed 2.5 s later by an imperative ‘go’ tone (40 ms, 1000 Hz, 80 dB). Subjects were instructed to initiate the movement as close in time as possible to, but not before, the ‘go’ cue, and were provided with visual feedback of their response times (red block =‘too early or too late’, green = correct) after the completion of each trial. Response time feedback was provided using an analog threshold detector that generated a trigger during the rising phase of the rectified EMG signal in the wrist extensor muscle. The threshold was adjusted to signal EMG onset with an accuracy of between 10 and 20 ms. Trials with EMG activity that was initiated before the ‘go’ cue in conditions 1 and 2 (see below), or before SAS or TMS application in conditions 3, 4 and 5 were considered ‘too early’. Trials in which EMG activity was initiated later than 150 ms after the ‘go’ cue were considered ‘too late’. Trials with early or late EMG onset times were discarded and repeated later in the experiment. The average number of errant trials, expressed as a percentage of all trials, was 11.6 ± 6.7%. This design was derived from previous studies showing that a fixed delay period is associated with an early and large build-up of movement-related cortical activity (Cui & MacKinnon, 2009) and that planned movements can be reliably released (>90% of trials) when SAS is presented less than 250 ms prior to the ‘go’ cue (MacKinnon et al. 2007; Carlsen & MacKinnon, 2010). This protocol ensures that subjects prepare the intended movement well in advance, rather than reacting to the imperative ‘go’ cue.
Kinematic and EMG recordings
Bipolar surface electromyographic (EMG) signals were recorded from the extensor digitorum (ED), extensor carpi radialis longus (ECRL), flexor carpi radialis (FCR), and sternocleidomastoid (SCM) muscles using a Bagnoli system (Delsys, Boston, MA, USA; amplification = 1000–10,000, filter = 30–1 kHz). Wrist angular displacement signals were recorded from the manipulandum using an optically encoded potentiometer. All signals were sampled at 2000 Hz using a Power 1401 data acquisition board and Signal 3.1 software (Cambridge Electronic Design (CED), Cambrdge, UK).
Transcranial magnetic stimulation
TMS was delivered using a Magstim 200 stimulator (Magstim, Whitland, Carmarthenshire, UK) with a figure-of-eight coil (7 cm outer diameter of wings). The coil was positioned over the contralateral primary motor cortex at the optimal site for eliciting motor evoked potentials (MEPs) in the wrist extensor muscles and oriented at an angle to induce a posterior-to-anterior current approximately perpendicular with the central sulcus. The optimal site and coil orientation for stimulation were marked on the scalp with a wax marker and the coil was held in place by an experimenter. Resting motor threshold (RMT) in the wrist extensors (ED) was defined as the intensity in which a 50 μV MEP was evoked in 5 of 10 trials. Stimulation intensity was adjusted to produce a minimum of a 100 ms SP in the wrist extensors during a 10% maximal voluntary isometric contraction. The SP duration was defined as the time from TMS to the onset of muscle activity after EMG silence.
Startling acoustic stimulus
The SAS was delivered via a loudspeaker placed 50 cm directly behind the head of the participant. The stimulus (1 kHz, 40 ms, <1 ms rise time) was generated using a tone generator (model S10CTCMA, Grass Technologies/Astro-Med Inc., West Warwick, RI, USA) and custom amplifier and was calibrated to produce a peak intensity of 124 dB using a sound level meter (Brüel & Kjær Impulse Precision Sound Level Meter, type 2204; Brüel & Kjær, Nærum, Denmark).
Experimental protocol
Subjects performed at least 25 trials of practice until responses times were consistently within a 70–150 ms range and the movements were fast and accurate. They subsequently performed 20 trials of the wrist extension task without TMS or SAS. The mean response time for these trials was calculated and served as a reference for the timing of TMS for the TMSCT trials (see below). Response times were calculated from the onset of the ‘go’ tone to the onset of ED activity. Subjects then performed another block of 20 trials during which SAS was applied 200 ms before the onset of the ‘go’ tone during a subset of five of the trials. SAS at this time point consistently resulted in the early release of the wrist extension movement at latencies of less than 100 ms after the startle stimulus. The mean response time to SAS was calculated and served as the SAS response time reference.
The main experiment included five conditions:
Condition 1, Control Trials (100 trials). These trials consisted of movements performed with no SAS or TMS stimulation (Fig. 1A).
Condition 2, TMSCT (10 trials). TMS was delivered 70 ms before the mean within-subject response time (as described above) (Fig. 1B). This timing of stimulation was chosen so that the time period of suppression of motor cortical excitability and interruption of voluntary drive (70–100 ms after TMS) (Valls-Solé et al. 1992; Tergau et al. 1999; Ni et al. 2007) immediately preceded the onset of initial agonist muscle activity and consistently delayed the onset of movement (see results below).
Condition 3, SAS (10 trials). SAS was delivered 200 before the imperative ‘go’ tone (Fig. 1C). This timing has been shown to consistently result in the early release of the wrist extension movement within EMG onset latencies of less than 100 ms after the SAS (Carlsen & MacKinnon, 2010). The early timing of SAS also ensured that the initiation of the movement was mediated by SAS and was unlikely to reflect an early voluntary response prior to the ‘go’ tone.
Condition 4, TMSSAS (10 trials). TMS was delivered 70 ms before the mean within-subject SAS response time reference (as described above) (Fig. 1D). Similar to Condition 2, this timing of stimulation was chosen so that the time period of suppression of motor cortical excitability and interruption of voluntary drive (70–100 ms after TMS) (Valls-Solé et al. 1992; Tergau et al. 1999; Ni et al. 2007) immediately preceded the onset of wrist extensor muscle activity observed during SAS trials. This trial condition tested whether the presentation of TMS before the ‘go’, in the absence of SAS, evoked the early release of the planned movement.
Condition 5, TMSSAS+SAS (10 trials). This condition combined the stimuli from Conditions 3 and 4 (Fig. 1E). TMS was applied 70 ms prior to the estimated onset of the initial agonist burst evoked by the SAS. The SAS was applied at −200 ms. TMS was applied before SAS in some subjects, and after SAS in others, depending upon their individual SAS response time reference.
Figure 1. Diagram showing the timing of cues and stimuli for the five experimental conditions.
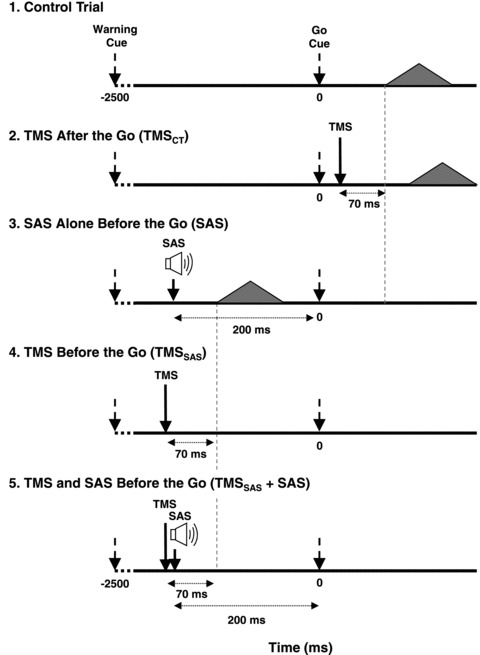
Arrows indicate when TMS or SAS was applied during each condition. The filled triangles represent the EMG activity associated with the first agonist burst and the time that this burst was hypothesized to appear in each condition.
The order of the conditions was pseudo-randomized across the experiment with the exception that a SAS condition was not presented during the first five trials and there were never two consecutive SAS trials. A total of 140 movement trials were performed. The experiment included 10 blocks of randomly assigned tasks. Each block contained 14 random trials. Each trial took 3.5 s and was followed by a 10 s interval of rest. To minimize the possible effects of fatigue, all subjects were provided a minimum of 1 min of rest after each block.
Hoffman reflexes evoked in the wrist flexor muscles
In a subset of subjects (n = 3), Hoffman reflexes (H-reflexes) were tested in the wrist flexors (FCR) to examine the state of excitability of spinal motor neurons during the delay period between TMS and the onset of the voluntary movement. H-reflexes were evoked by electrically stimulating the median nerve just proximal to the elbow crease. Stimuli were administered using a 500 μs pulse delivered by a constant current stimulator (Model DS7A, Digitimer Ltd, Welwyn Garden City, UK) through a pair of surface electrodes placed 2 cm apart with the cathode located proximal to the anode. Stimulus current was adjusted at rest to evoke a clear H-wave with an amplitude near 50% of maximal H-reflex amplitude. The same experimental protocol as described above was used with the exception that the movement direction was reversed so that FCR was the agonist muscle. Two trial conditions were added for this experiment:
Control-H (10 trials). For this trial condition, median nerve stimulation was applied alone at −200 ms.
TMSSAS+SAS+H-reflex (10 trials). This condition was similar to Condition 5 described above but with the addition of median nerve stimulation delivered 60 ms after the TMSSAS pulse. This stimulus timing was selected to ensure that the evoked H-wave arrived during the period of EMG silence associated with a presumed suppression of motor cortical excitability produced by suprathreshold TMS.
Data analysis
Data analysis was performed using Signal 3.10 software (CED). EMG signals were DC offset corrected and full-wave rectified. Agonist EMG onset times were calculated based on changes of more than three standard deviations from the mean signal recorded prior to the go cue or acoustic stimuli and were verified by visual inspection and adjusted manually as needed to coincide with the initial deflection of the signal from the baseline level (Hodges & Bui, 1996). The end of the EMG burst was estimated based on a maintained reduction of activity below 10% of the peak amplitude of the burst and was verified by visual inspection and adjusted manually as needed. For control and TMSCT trials, EMG onset time was calculated as the time interval between the ‘go’ tone and the onset of the first agonist burst in the wrist extensors. Agonist EMG onsets for the SAS and TMSSAS+SAS trials were defined as the time interval between the SAS presentation and the onset of agonist burst. For the TMSSAS condition, the response time was defined as the interval between TMS and onset of the agonist burst.
The temporal characteristics of the triphasic EMG pattern across conditions were examined by comparing the duration of the first agonist (AG1), antagonist (ANTAG) and second agonist (AG2) bursts and the time interval between the bursts (AG1–ANTAG, AG1–AG2). Since the onset and offset times of the AG1 and AG2 bursts were clearer in ED than ECRL, we only report the measurements from the ED muscle. The silent period following TMS was defined as the time from TMS to the onset of the EMG burst that followed the period of silence. For the H-reflex experiments, the peak-to-peak amplitude of the H-wave observed during the TMS-evoked delay period was measured and compared to the amplitude of the H-wave observed when median nerve stimulation alone was applied at −200 ms.
Statistics
The dependent variables were analysed using repeated measures ANOVA to test for the main effects across task conditions. Planned contrasts were used to test our two main a priori hypotheses: (1) that TMSCT would delay the onset of the control trial movements and (2) that TMSSAS would not delay the onset of the movements evoked by a SAS. Estimates of the effect size are provided using partial eta-squared values (ηp2). Student's t test for paired samples was used to examine additional post hoc differences between conditions. Differences at the P < 0.05 level were considered to be significant.
Results
TMS summary
The average TMS intensity used across all subjects was 63 ± 7% of maximal stimulator output and 183 ± 23% of resting motor threshold. The average timing of TMSCT was 25 ± 6 ms and TMSSAS was −202 ± 7 ms.
Response time differences across conditions
Examples of the changes in the timing of the wrist extensor muscle (ED) across trials in a representative individual are shown in Fig. 2. Control trials for this subject (Fig. 2A) were characterized by a triphasic EMG pattern consisting of an initial burst in the wrist extensors, beginning about 100 ms after the ‘go’ cue, followed by a burst in the antagonist muscle (FCR, not shown), then a second burst in the agonist. As shown in Fig. 2B, the application of TMS after the ‘go’ tone resulted in a marked delay in the onset of the first agonist burst. When SAS was applied at −200 ms there was an early release of the triphasic EMG pattern (onset =−140 ms; 60 ms after SAS; Fig. 2C). The application of TMS at −200 ms also resulted in the release of the movement before the ‘go’ cue but the onset of extensor EMG activity was considerably delayed (onset =−100 ms; 100 ms after TMS) relative to the trials with a SAS alone (Fig. 2D). Similarly, when TMS and SAS were applied concurrently at −200 ms, the planned movement was released before the ‘go’ cue and the onset of the agonist burst was delayed by approximately 40 ms relative to the timing of EMG onset with a SAS alone (Fig. 2E).
Figure 2. Representative trials across each experimental condition.
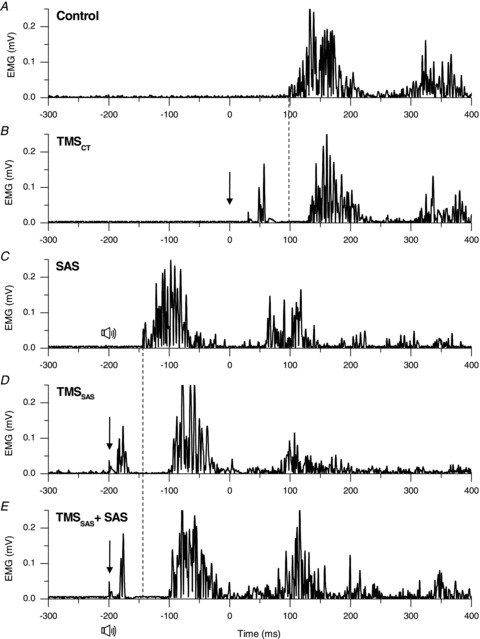
Representative trials (A–E) showing rectified EMG in the wrist extensor agonist muscle (extensor digitorum) in a single subject. The imperative go cue was presented at 0 ms. The downward arrow represents the timing of TMS and the speaker symbol represents the timing of the SAS. Note the delay in the onset of the agonist burst when TMS was applied immediately after the go cue (Fig. 2B) compared to the control trial (Fig. 2A). Similarly, note the delay in onset of the agonist burst (EMG onset =−103 ms) when TMS and SAS were applied together (Fig. 2E) at −200 ms compared to SAS alone at −200 ms (EMG onset =−140 ms).
Similar findings were obtained in all subjects. A summary of the changes in agonist EMG onset times across conditions is shown in Fig. 3. Onset times are referenced relative to the timing of the ‘go’ tone for the Control and TMSCT conditions, relative to the timing of SAS for the SAS and TMSSAS+SAS conditions, and relative to the timing of TMS for the TMSSAS alone condition. The average onset of the agonist EMG during control trials was 95.6 ± 14.0 ms. These relatively short latency responses demonstrate that the subjects appropriately predicted the onset of the imperative ‘go’ cue due to the fixed time interval between the warning tone and ‘go’ tone. The application of TMSCT after the ‘go’ tone resulted in a significant delay in the onset of agonist EMG activity (F(1) = 69.1, P < 0.001, ηp2= 0.91; average EMG onset = 133.4 ± 13.3 ms; Fig. 3A). Presentation of a SAS at −200 ms resulted in the rapid release of the planned movement sequences prior to the ‘go’ cue in 100% of trials in all subjects. The mean onset latency of the agonist EMG was 71.8 ± 2.7 ms after the SAS. When TMSSAS was applied near the onset of SAS there was a significant delay in the onset of agonist muscle activity relative to the SAS alone trials (F(1) = 58.1, P < 0.001, ηp2= 0.89; average EMG onset for the TMSAS+SAS condition = 106.8 ± 14.4 ms after SAS). There was no significant difference in the relative delay in agonist EMG onset produced during TMSCT or TMSSAS+ SAS trial conditions (TMSCT delay = 37.7 ± 12.8 ms; TMSSAS+SAS delay = 35.0 ± 12.9 ms; F(1) = 0.19, P > 0.675, ηp2= 0.027; Fig. 3B). When TMS was applied alone near the onset time of the SAS stimulus (TMSSAS condition) the planned movement was released prior to the ‘go’ tone in an average of 96 ± 8% (range = 78 – 100%) of trials, but the onset timing of the agonist burst relative to the timing of TMSSAS (EMG onset = 122.7 ± 18.7 ms after TMSSAS) was significantly longer than the onset timing observed with SAS alone (F(1) = 70.2, P < 0.001, ηp2= 0.91).
Figure 3. Comparison of response times and TMS-induced delays.
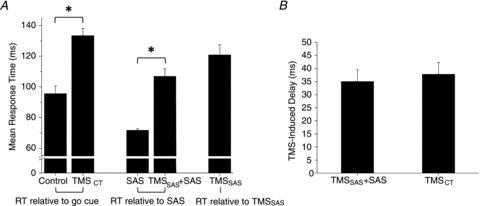
Mean and standard error for response time across the five experimental conditions (A) and the TMS-induced delay for the TMSCT and TMSSAS+SAS conditions (B). *Differences between conditions were significant (P < 0.05); RT, response time
Triphasic pattern of EMG activity
Analysis of the triphasic EMG pattern across all five conditions showed no significant main effect of task condition for the initial agonist burst duration (F(4) = 2.656, P = 0.058, ηp2= 0.31), antagonist burst duration (F(4) = 2.072, P = 0.116, ηp2= 0.26), second agonist burst duration (F(4) = 2.025, P = 0.123, ηp2= 0.25), time interval between the onset of the first agonist and the onset of the antagonist bursts (F(4) = 2.444, P = 0.074, ηp2= 0.29) and time interval between the onset of the first and the second agonist bursts (F(4) = 2.222, P = 0.097, ηp2= 0.27). The average values of these variables are shown in Table 1. The triphasic EMG pattern associated with the TMSCT condition tended to be shorter than the other conditions, but this difference did not reach significance. This analysis shows that the task condition only significantly affected the onset timing of the triphasic pattern but not the spatial or temporal characteristics of the movement performed.
Table 1.
Comparison of triphasic pattern measurements of EMG activity
| Condition | |||||
|---|---|---|---|---|---|
| Control | TMSCT | SAS | TMSSAS | TMSSAS+SAS | |
| AG1 duration | 90.0 ± 13.9 | 83.5 ± 9.4 | 89.5 ± 11.1 | 88.7 ± 9.4 | 84.5 ± 10.0 |
| ANTAG duration | 109.5 ± 18.1 | 100.9 ± 17.8 | 107.6 ± 22.2 | 107.8 ± 16.9 | 106.7 ± 25.9 |
| AG2 duration | 87.3 ± 12.4 | 79.3 ± 12.0 | 85.8 ± 7.7 | 83.8 ± 11.3 | 81.3 ± 14.4 |
| AG1–ANTAG interval | 51.1 ± 10.6 | 48.3 ± 11.6 | 50.6 ± 7.9 | 47.3 ± 12.1 | 47.4 ± 11.6 |
| AG1–AG2 interval | 128.2 ± 20.5 | 119.4 ± 18.4 | 128.5 ± 18.7 | 127.8 ± 21.4 | 122.1 ± 25.7 |
AG1, first agonist burst (ED); ANTAG, antagonist burst (FCR); AG2, second agonist burst (ED); AG1–ANTAG interval, the time interval between AG1 onset and ANT onset; AG1–AG2, the time interval between AG1 onset and AG2 onset.
SCM activity
A burst of EMG activity in the SCM muscle frequently accompanied the early release of the planned movement. The average incidence of a SCM burst was 64 ± 16% for the SAS alone condition and 71 ± 16% for the TMSSAS+SAS condition. There was no significant difference in incidence between these conditions. The ANOVA of the onset latencies of the SCM burst between the SAS alone and TMSSAS+SAS conditions across all subjects showed no significant differences between conditions (P = 0.794). However, inspection of the data revealed three different response patterns in SCM to TMSSAS+SAS across subjects. For this reason, a within-subject analysis (using paired t tests) was conducted to examine the change in SCM onset latency across conditions. In 5 of the 8 subjects, the TMSSAS+SAS condition was associated with a significant shortening of the latency of the SCM EMG burst (Fig. 4). The average onset latency of the SCM burst in these subjects was 50.3 ± 3.0 ms for the TMSSAS+SAS trials and 68.8 ± 8.0 ms for the SAS alone trials. Figure 4Bdemonstrates the marked change in both the latency of the SCM burst and width of the distribution of responses for the TMSSAS+SAS condition. The within-subject latency shift was significant in all five subjects (paired t tests, P < 0.022). In a sixth subject, a clear SCM burst was observed in all TMSSAS+SAS trials (onset = 45.2 ± 2.8 ms), but not during the SAS alone trials. Two subjects showed no significant shortening of SCM latency.
Figure 4. Changes in the onset of SCM activity between the SAS alone and TMSSAS+ SAS conditions.
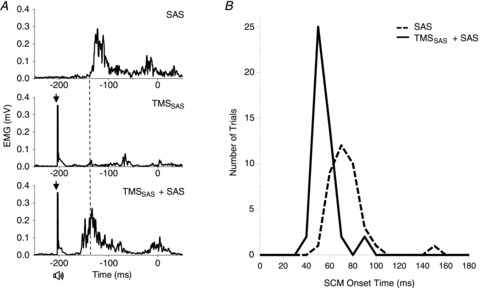
A, three representative trials from a single subject showing rectified EMGs in the sternocleidomastoid (SCM) muscle for the SAS alone (left upper plot), the TMSSAS (left middle plot) and combined stimuli (TMSSAS+SAS) (left lower plot) conditions. Note that the latency of EMG activity was shortened when TMS was applied in combination with SAS. B, histogram of the number of trials observed with response times within 10 ms bins across five subjects. Note the shift in latency of the SCM burst and narrowing of the width of the distribution for the TMSSAS+SAS condition.
Hoffman reflexes evoked in FCR during the movement delay interval
A potential confound that might explain our findings is that the TMS-evoked delay in the onset of the triphasic EMG pattern following SAS was mediated by a prolonged time period of suppressed spinal motor neuron activity. To test this possibility, a second experiment was conducted in a subset of three subjects. In addition to the trial conditions described above, a triple stimulation condition was included during which median nerve stimulation was applied 60 ms after TMSSAS+SAS to examine if H-reflexes in the agonist muscle (FCR) were suppressed during the later part of the EMG silent period associated with the delay in movement onset. Similar to the findings from the first experiment, the presentation of a SAS alone at −200 ms evoked the rapid release of the targeted wrist flexion movement with a mean agonist (FCR) onset latency of 94.2 ± 7.3 ms after the startle stimulus. When the SAS was paired with TMSSAS, the onset of the agonist burst was delayed by an average of 34.4 ± 24.5 ms. Figure 5 shows examples of the H-reflex evoked when median nerve stimulation was applied alone at −200 ms (Control-H condition) (Fig. 5A) and when applied 60 ms after TMSSAS+SAS (Fig. 5B). The H-reflex evoked during the EMG silence was similar in magnitude and waveform to the Control-H trial. Paired t tests showed that the amplitude of the H-wave evoked during the TMSSAS+SAS trials was not significantly different from the Control-H trials in two subjects (t < 2.0, P > 0.093) and the H-wave associated with the TMSSAS+SAS condition was significantly larger (t = 8.037, P < 0.001) in one subject. We also observed that the application of median nerve stimulation often evoked the release of planned movement before the ‘go’ cue in two of the three subjects (incidence = 50–60% of trials). The average onset of the agonist EMG burst for these subjects was 116.0 ± 7.3 ms and 131.2 ± 46.3 ms after median nerve stimulation. These latencies were similar to the values observed for the TMSSAS condition (122.7 ± 18.7 ms).
Figure 5. Comparison of the amplitude of the H-reflex between the Control-H and TMSSAS+SAS conditions.
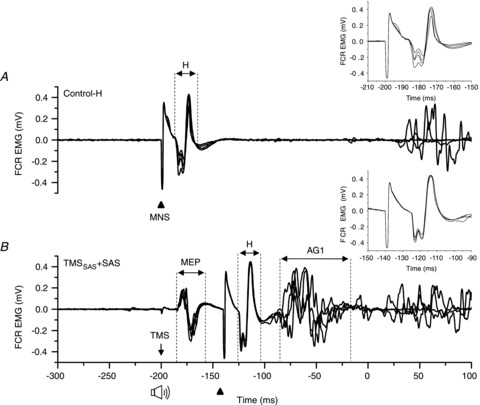
Example of EMG responses evoked in the flexor carpi radialis (FCR) muscle by electrical stimulation of the median nerve (MNS). Four consecutive trials in a single subject are shown in each plot. A, Control-H trials: the MNS was delivered at 200 ms before the go cue. B, TMSSAS+ SAS trials: the MNS was delivered 60 ms after TMS and SAS (during cortical silent period). Note that the latency and the amplitude of H-reflex (H) were similar in both conditions. The inset figures highlight the H-reflex size and shape across trials.
Discussion
The main finding of this experiment was that the rapid release of a ballistic wrist movement by a SAS could be significantly delayed when suprathreshold TMS was applied over the contralateral primary motor cortex 70 ms before the expected timing of the first agonist EMG burst. The duration of the TMS-induced delay in the response to a SAS was similar to the delay produced by TMS during control trials, suggesting that the mechanisms mediating the response delay were the same for both task conditions. In contrast, the onset latency of the SCM activity, an indicator of an evoked startle response, was either unchanged or significantly decreased when TMS and SAS were co-administered. Based on evidence that the late component of the EMG silent period induced by TMS is mediated by the suppression of motor cortical excitability, alterations in intracortical inhibition and facilitation, and an accompanying interruption of voluntary drive (Fuhr et al. 1991; Inghilleri et al. 1993; Uncini et al. 1993; Chen et al. 1999; Tergau et al. 1999; Ni et al. 2007), these findings provide evidence that the StartReact effect for ballistic movements of the wrist is mediated, in part, by a fast conducting transcortical pathway. The following sections will discuss these results with respect to pathways that are hypothesized to mediate the release of movement by a SAS.
The prevailing hypothesis to explain the extraordinarily short reaction times associated with the StartReact effect is that the movement sequence is planned and prepared at the level of the cortex and then stored and subsequently released by afferent input to the brainstem (Valls-Solé et al. 1999, 2008; Sanegre et al. 2004; Carlsen et al. 2004a). This hypothesis was first posed in the seminal papers by Valls-Solé et al. (1995, 1999) who reported average reaction times of less than 80 ms when the imperative cue was replaced by a SAS. Based on these short reaction times and the co-expression of movement release with a generalized startle response, Valls-Solé and colleagues proposed that the movement was released from similar pontomedullary-reticulospinal pathways that mediate the startle reflex. It was argued that there was insufficient time for the response to be mediated by a transcortical pathway since such a pathway would be expected to take a minimum of 55 ms. When additional delays were added to account for the conduction of sound to the ears (6 ms), only 4 ms would be available for cortico-cortical activation in trials with reaction times of 65 ms. The reaction times associated with the StartReact could not be accounted for by intersensory facilitation since this mechanism is associated reductions in reaction times of 20–50 ms (Nickerson, 1973; Carlsen et al. 2007) and not the 70–90 ms reduction observed with a SAS. Thus, a shorter, fast-conducting pathway via brainstem circuits, similar to those that mediate the startle reflex, seemed most likely.
Indirect support for the brainstem hypothesis comes from experiments showing that trials with very short reaction times (<90 ms) are usually accompanied by short-latency activation of the SCM muscle, indicative of a startle response, whereas trials without a SCM burst usually had significantly slower reaction times (Carlsen et al. 2007). These data suggest that the generalized startle reflex and StartReact are initiated by the same volley and mediated by largely parallel and linked pathways. However, experiments using prepulse inhibition have shown that the generalized startle reflex can be suppressed without affecting the StartReact (Valls-Solé et al. 2005), suggesting that the two pathways are, in many respects, separate. Additional indirect support for the subcortical hypothesis was provided by an experiment showing that a SAS resulted in a significant shortening of reaction times for an elbow extension task, but not for a simple finger abduction task (Carlsen et al. 2009). These findings were interpreted to show that muscles that are largely controlled by direct corticospinal projections from the motor cortex are less susceptible to the StartReact effect. However, this conclusion must be interpreted with caution in light of the fact that finger abduction movements could be evoked by SAS with reaction times of less than 90 ms, albeit with less frequency than elbow movements, suggesting that the threshold for the StartReact effect is higher for more distal arm movements but the fast conducting pathway that mediates the effect is present for these muscles. Descending projections from medial pontomedullary reticular formation (PMRF) brainstem regions are convergent with corticospinal inputs to motor regions of the intermediate zone of the spinal cord that are involved in the control of grasp (Riddle & Baker, 2010). Subsets of neurons within the PMRF show movement-related preparatory activity during an instructed delay task (Buford & Davidson, 2004) and many of these neurons contribute to both postural and focal components of a reaching task (Schepens & Drew, 2004). Thus, there may be sufficient motor apparatus within the brainstem to mediate these fast responses in humans. We hypothesized that if the StartReact phenomenon is mediated by such a pathway, then disruption of a transcortical pathway at the level of the primary motor cortex should have no influence on the response times of the first agonist muscle. This hypothesis was not supported by the results of our experiments.
In the present study, the contribution of a transcortical pathway via the primary motor cortex to the release of movement was tested by applying an appropriately timed suprathreshold TMS pulse over the hand representation of the motor cortex. The experimental manipulation used in our experiment was based on the premise that the latter portion of the TMS-evoked silent period in the EMG is generated by a period of cortical inhibition and an interruption of voluntary cortical drive. This idea is supported by studies showing that H-reflexes are significantly attenuated for up to 50 ms following TMS, but recover during the late phase of the EMG silent period (Fuhr et al. 1991; Inghilleri et al. 1993; Roick et al. 1993; Ziemann et al. 1993). In contrast, the EMG silent period observed approximately 50 ms after TMS is associated with a marked suppression of corticospinal excitability, an increase in motor threshold, decreased intracortical inhibition and increased intracortical facilitation (Chen et al. 1999; Tergau et al. 1999; Ni et al. 2007). GABAB agonists or uptake blockers prolong the duration of the silent period (Siebner et al. 1998; Werhahn et al. 1999). The fact that MEP thresholds and latencies during the silent period are the same as at rest, and 2 ms slower than during the active state, provides compelling evidence that the EMG silence is also mediated by an interruption of cortical voluntary drive (Tergau et al. 1999; Ni et al. 2007). Day et al. (1989) were the first to use this experimental protocol to show that targeted ballistic movements could be significantly delayed with suprathreshold TMS. The remarkable feature of this protocol is that TMS does not disrupt the spatial and temporal pattern of the planned movement (Day et al. 1989; Romaiguère et al. 1997; Ziemann et al. 1997; Hashimoto et al. 2004; Voss et al. 2006). Similar results were obtained in the present experiment. Suprathreshold TMS delayed the onset of the first agonist burst by an average of 38 ms during control trials (TMSCT) and 35 ms during SAS trials (TMSSAS+SAS). Moreover, the triphasic EMG pattern observed was unchanged across conditions. A recent study showed that sham TMS (the coil was oriented perpendicular to the scalp surface) does not affect the StartReact (Stevenson et al. 2011), suggesting that stimulation of cortical inhibitory pathways is necessary to produce the delay in the reaction time. Taken together, these findings suggest that the delivery of suprathreshold TMS delayed the StartReact via an interruption of cortical voluntary drive, similar to the mechanisms mediating the delay produced during control trials.
An assumption made in the first experiment described in this paper was that the mechanisms of action that mediate the EMG silent period evoked by TMS prior to a ballistic wrist movement are the same as those described during tonic isometric contractions. As described above, evidence showing that the late phase of the EMG silent period is mediated by a suppression of cortical activity and interruption of voluntary drive has been obtained exclusively during isometric contraction. Paired-pulse TMS experiments that have examined long-interval intracortical inhibition in resting muscle have shown changes in corticospinal excitability and threshold comparable to the effects observed during the EMG silent period evoked in isometrically contracting muscles (Tergau et al. 1999; Ni et al. 2007). Nonetheless, the possibility remained that the delay in movement onset observed in the present experiment was due to a prolonged period of suppressed motor neuron excitability at the level of the spinal cord. The results of our second experiment did not support this idea. In our second experiment, afferent volleys evoked by median nerve stimulation were timed to arrive at the spinal cord during the period of presumed motor cortical suppression evoked by suprathreshold TMS. We found no significant suppression of H-reflexes during this time period. Although our sample size was small (n = 3), the findings were the same across subjects. Thus, a prolonged period of spinal motor neuron inhibition or suppressed excitability could be excluded as factors contributing to our results.
It could also be argued that the delay in movement onset produced by suprathreshold TMS over the primary cortex was produced by disruption or suppression of activity in the pontomedullary pathways that have been hypothesized to mediate the rapid release of movement by a SAS. Although this possibility cannot be discarded, it is unlikely in light of the data showing that combined TMSSAS and SAS was associated with facilitation of the pathway that mediates the generalized startle reflex. This was shown by a significant reduction in the onset latency and a marked narrowing of the response time distribution of the SCM EMG burst in 5 of 8 subjects. A hypothesis for the mechanisms of facilitation of the startle reflex is shown in the model presented in Fig. 6A and C. The model proposes that, in addition to a corticospinal volley, suprathreshold TMS evokes a descending cortico-reticular volley (orange arrow in Fig. 6A) that has input to the regions of the PMRF that mediate the generalized startle reflex, such as the nucleus reticularis pontis caudalis (Kably & Drew, 1998). Since the cortico-reticular volleys evoked by high intensity TMS are likely to have a much shorter latency (Fisher et al. 2010) than the sensory afferent pathways to the PMRF evoked by a SAS, the nearly coincident timing of TMSSAS with the SAS would ensure that the TMS-evoked volleys arrived first and thus could depolarize the response network and increase the rise time to threshold that triggers a startle response (Fig. 6C). Alternatively, the bone vibration produced by contact of the TMS coil on the scalp could have activated vestibular pathways with input to the PMRF (Fisher et al. 2010). Irrespective of the whether the source of input to the PMRF was via cortico-reticular or vestibular pathways, the early arrival of these inputs to the startle pathway would result in an increase in rise time to threshold, the shortening of the response time in SCM and narrowing of the response time distribution (Fig. 4). These results add to evidence demonstrating that the pathways mediating the startle reflex and rapid release of movement (StartReact) are separate (Valls-Solé et al. 2005).
Figure 6. Model of the pathways mediating the release of movement in response to an 80 dB tone (Control trials), a SAS, TMSSAS alone and TMSSAS+SAS.
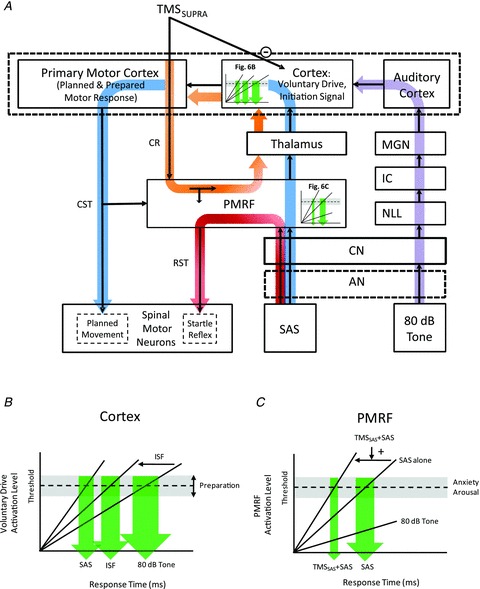
This model shows two primary pathways (blue and purple) by which planned and prepared movements can be released by a sensory stimulus. The right portion of the model (purple line) shows the classic pathway by which a low intensity acoustic tone (e.g. 80 dB) travels to the auditory cortex. The auditory cortex then has input to a voluntary drive or initiating signal that triggers the release of the planned and prepared movement sequence at the level of the cortex. The timing and distribution of the response time is dependent upon the rate of rise of the input and the threshold to triggering the response, both of which can be modulated by intersensory facilitation or the level of preparation (Fig. 6B). Disruption of voluntary drive by suprathreshold TMS delays the release of the movement (TMSCT condition). The presentation of a SAS results in a synchronous afferent volley travelling to the PMRF and releasing a generalized startle reflex (SCM burst) via known pathways (shown in red). However, the timing of the generalized startle response can be altered by descending cortico-reticular input produced by TMS (orange line and Fig. 6C). The TMS-evoked volley to the PMRF increases the rise time of the startle triggering signal and produces both a reduction in startle onset latency and narrowing of the distribution of response times. Note that a decrease in threshold would not explain our results since this would not produce a narrowing of the response time distribution. Similar to the SAS response mechanism (shown in blue loop), the TMSSAS alone can trigger the early release of the planned movement through cortico-reticular inputs to the PMRF and a subsequent ascending volley via reticulo-thalamocortical pathways to the voluntary drive/initiation signal region of the cortex (shown in orange loop). Abbreviations: AN, auditory nerve; CN, cochlear nucleus; CR, corticoreticular projection; CST, corticospinal tract; IC, inferior colliculus; ISF, intersensory facilitation; MGN, medial geniculate nucleus; NLL, nuclei of the lateral lemniscus; PMRF, pontomedullary reticular formation; SAS, startling acoustic stimulus; SCM, sternocleidomastoid muscle; SUPRA, suprathreshold; RST, reticulospinal tract; TMS, transcranial magnetic stimulation.
If the rapid release of ballistic wrist movements by a SAS is mediated by a transcortical pathway, then how are the rapid response times generated? A theoretical model of the pathways mediating the responses observed in this experiment is presented in Fig. 6. According to this model, movement planning and preparation is performed during the instructed-delay interval via iterative cortical-basal ganglia thalamocortical loops, and that the resulting spatial and temporal elements of the motor response are held in readiness for triggering from a voluntary drive or initiating signal. Since TMS of the primary motor cortex does not result in the immediate release of the planned movement via the corticospinal tract (a response that would have the same latency as a MEP), it is unlikely that the initiating signal resides within the primary motor cortical network stimulated by TMS. It is more probable that the initiating signal is mediated by cortico-cortical inputs from premotor, sensory or parietal regions. The threshold for triggering the planned response is dependent upon the timing and level of preparation (high threshold early and low threshold late in the preparation period) (Carlsen & MacKinnon, 2010). The response time distribution is therefore dependent upon the rise time of the triggering input (variable rate hypothesis) and the threshold for activation of the movement triggering signal (variable threshold) (Hanes & Schall, 1996). Accordingly, alterations in the transmission efficacy of sensory stimuli (e.g. intersensory facilitation, increased stimulus intensity, increased synchrony of the input volley) may be sufficient to trigger the early release of the movement. A similar schema was recently proposed by Maslovat et al. (2011). They hypothesized that a reduction in threshold likely accounts for the decreases in reaction time associated with practice of a skilled movement whereas the marked reduction in reaction time observed with the StartReact is best explained by a dramatic increase in the rate of rise to threshold.
The red line shown in Fig. 6 represents the pathway mediating the auditory startle reflex. The SAS evokes a synchronous sensory volley that travels via the auditory nerve and cochlear nucleus to the PMRF (Yeomans & Frankland, 1995) and descending commands from the PMRF evoke activity in startle-related spinal motor neurons (e.g. SCM) via reticulospinal pathways (Valls-Solé et al. 2008). In contrast to the idea that the stored movement plan is similarly released from networks in the PMRF, we propose that activation of the PMRF also results in a synchronous ascending volley that projects to the cortex via the thalamus (blue line in Fig. 6). It is this reticulo-thalamocortical volley that provides the input to rapidly trigger the voluntary drive or initiating signal and thereby releases the planned movement from the motor cortex via the corticospinal tract. The transcortical conduction time of such a pathway would be compatible with the response times associated with the StartReact in light of evidence showing that loud acoustic stimuli can alter the excitability of corticospinal projections when delivered between 30–60 ms prior to TMS (Furubayashi et al. 2000; Fisher et al. 2004), and the conduction time from primary motor cortex to the wrist flexors and extensors is approximately 17–20 ms. Note that, according to this schema, the startle reflex and StartReact pathways initially share a common driving input up to the level of the PMRF, but are thereafter separate pathways. This would explain why the startle reflex is amendable to modulation by prepulse inhibition, while the StartReact is not (Valls-Solé et al. 2005). Nonetheless, the spinal motor neurons mediating the release of movement and startle reflex do not have to be mutually exclusive. Under specific conditions, the reticulospinal volley evoked by the startle pathway could converge onto the same motor neurons associated with release of the planned movement resulting muscle activity and movement that is modified by the startle response (Carlsen et al. 2004).
It is important to note that the transcortical pathway supported by our findings, and the theoretical framework hypothesized to mediate the release of planned movements (Fig. 6), may hold for only a subset of the movement repertoires executed by humans. To date, the majority of research on the StartReact phenomenon has focused on discrete single degree of freedom movements. However, it has been shown that complex, multi-segmental and whole body movements that are preceded by anticipatory postural adjustments can also be readily evoked using the StartReact protocol (Valls-Solé et al. 1999; MacKinnon et al. 2007; Rogers et al. 2011). In light of evidence from experiments in cats that regions of the PMRF contain neurons that encode temporal and kinematic aspects of postural adjustments preceding target-directed movements (Schepens & Drew, 2004), it is feasible that task-related postural or balance elements of the planned movement can be rapidly evoked by a SAS via reticulospinal, rather than transcortical pathways. Support for this idea comes from experiments showing that the onset latencies of anticipatory postural responses evoked by a SAS are shorter than those observed in the distal arm, despite considerably longer conduction times (Valls-Solé et al. 1999; Queralt et al. 2010). Thus, it is still plausible that some parts of a complex and multi-segmental movement can be released by SAS via subcortical pathways.
An unexpected result from these experiments was the observation that the delivery of suprathreshold TMSSAS alone during the late stages of movement preparation frequently evoked the early release of the planned movement before the ‘go’ cue. A variety of studies have previously shown that the application of subthreshold TMS at times between −50 and +50 ms relative to the imperative ‘go’ cue can significantly reduce reaction times (Pascual-Leone et al. 1994; Terao et al. 1997; Molineuveo et al. 2000; Hashimoto et al. 2004). Stimulation at intensities between 90 and 100% of resting motor threshold reduces reaction times by as much as 50 ms. This shortening of reaction times is considered to be mediated by intersensory facilitation mechanisms since the response time shortening is in 50 ms range (Nickerson, 1973) and comparable reductions in reaction times can be produced irrespective of the location of stimulation on the scalp, and even when the coil is not held on the scalp (Terao et al. 1997). We propose that the release of movement by median nerve stimulation (Experiment no. 2) was mediated by a similar mechanism (Valls-Solé et al. 2005). In contrast to subthreshold TMS, stimulation at suprathreshold intensities near the timing of the ‘go’ cue has been shown to increase reaction times (Pascual-Leone et al. 1994; Terao et al. 1997; Hashimoto et al. 2004). The amount of response delay increases with stimulation intensity (Pascual-Leone et al. 1994) and thus likely reflects the influence of an increasingly prolonged cortical silent period. In the present study, the delivery of suprathreshold TMSSAS near −200 ms evoked the release of the planned movement an average of 123 ± 18.7 ms after the stimulus (77 ms before the ‘go’ cue). The fact that the triphasic EMG pattern was not significantly altered relative to control trials suggests that TMS evoked the release of the motor plan in a manner similar to SAS, but with a greater latency. Molinuevo et al. (2000) have reported that TMS at 95% of resting motor threshold could evoke the release of a movement sequence with onsets comparable to those observed with a SAS (mean reaction times: TMS = 92 ms, SAS = 80 ms). They argued that since the change in reaction time was much greater than the reductions usually associated with intersensory facilitation (Nickerson, 1973), the release of the movement was likely to be mediated by cortico-reticulo-spinal pathways. Indirect support for this hypothesis was provided by data showing that reaction times could not be shortened by TMS or SAS in patients with progressive supranuclear palsy, a disease associated with degeneration and dysfunction of reticular nuclei that play a role in the startle reflex. Our data are consistent with this idea with the exception that our response latencies were considerably slower than those reported by Molineuvo et al. (2000). A model of how TMSSAS alone might evoke the early release of movement is shown in Fig. 6A (orange loop). As suggested by Molineuvo et al. (2000), TMS evokes a descending cortico-reticular volley that synapses onto neurons that mediate the acoustic startle reflex. However, rather than the movement being released directly by the reticulospinal pathways, we propose that activation of the PMRF evokes an ascending reticulo-thalamo-cortical volley that triggers the release of the planned movement in a manner similar to a SAS. Accordingly, the timing of release of the planned movement at the level of the cortex would be dependent upon the duration of the suppression of cortical drive produced by TMS. Thus, the increased response latencies of the movements evoked by TMS in the present study compared to Molineuvo et al. (2000) would be explained by the higher intensity of TMS, the accompanying prolongation of the cortical silent period and suppression of voluntary drive, and delayed triggering of movement release.
It remains to be seen if similar mechanisms mediate the SAS-evoked responses observed in proximal, axial or lower limb muscles or during more complex whole body movements requiring postural adjustments. Questions also remain regarding whether planned movements can be released at StartReact latencies by stimuli that do not evoke a generalized startle reflex. For example, it has been shown that voluntary reach-to-grasp movements can be triggered at a short latency by whole-body perturbations of posture and balance (Gage et al. 2007; Lakhani et al. 2011). Similarly, it has been shown that task-related changes in reflex modulation can be evoked by imposed joint perturbations at latencies comparable to the StartReact, and that these responses are not mediated by a transcortical pathway (Shemmel et al. 2009). Experimental protocols similar to those used in the present study will help to answer these questions.
In conclusion, the data presented in this paper provide evidence that the release of targeted ballistic movements of the wrist by a SAS is mediated, in part, by a transcortical pathway via the primary motor cortex and that this mechanism differs from the pathways that mediate the generalized startle reflex.
Acknowledgments
We thank Dr Maria Knikou for her invaluable input for the H-reflex study, Dr Anthony Carlsen for his review of the manuscript and the volunteers for their participation.
Glossary
Abbreviations
- ECRL
extensor carpi radialis longus
- ED
extensor digitorum
- FCR
flexor carpi radialis
- MEP
motor evoked potentials
- PMRF
pontomedullary reticular formation
- RMT
resting motor threshold
- SAS
startling acoustic stimulus
- SCM
sternocleidomastoid
- SP
silent period
Author contributions
Both authors contributed equally to the conception and design of experiments, generation and analysis of data as well as the drafting and revision of the manuscript. All recordings were performed at the Human Sensorimotor Neurophysiology and Movement Disorders Laboratory of Department of Physical Therapy and Human Movement Sciences, Northwestern University.
References
- Buford JA, Davidson AG. Movement-related and preparatory activity in the reticulospinal system of the monkey. Exp Brain Res. 2004;159:284–300. doi: 10.1007/s00221-004-1956-4. [DOI] [PubMed] [Google Scholar]
- Carlsen AN, Chua R, Inglis JT, Sanderson DJ, Franks IM. Can prepared responses be stored subcortically? Exp Brain Res. 2004a;159:301–309. doi: 10.1007/s00221-004-1924-z. [DOI] [PubMed] [Google Scholar]
- Carlsen AN, Chua R, Inglis JT, Sanderson DJ, Franks IM. Prepared movements are elicited early by startle. J Mot Behav. 2004b;36:253–264. doi: 10.3200/JMBR.36.3.253-264. [DOI] [PubMed] [Google Scholar]
- Carlsen AN, Chua R, Inglis JT, Sanderson DJ, Franks IM. Differential effects of startle on reaction time for finger and arm movements. J Neurophysiol. 2009;101:306–314. doi: 10.1152/jn.00878.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carlsen AN, Dakin CJ, Chua R, Franks IM. Startle produces early response latencies that are distinct from stimulus intensity effects. Exp Brain Res. 2007;176:199–205. doi: 10.1007/s00221-006-0610-8. [DOI] [PubMed] [Google Scholar]
- Carlsen AN, Hunt MA, Inglis JT, Sanderson DJ, Chua R. Altered triggering of a prepared movement by a startling stimulus. J Neurophysiol. 2003;89:1857–1863. doi: 10.1152/jn.00852.2002. [DOI] [PubMed] [Google Scholar]
- Carlsen AN, MacKinnon CD. Motor preparation is modulated by the resolution of the response timing information. Brain Res. 2010;1322:38–49. doi: 10.1016/j.brainres.2010.01.076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castellote JM, Kumru H, Queralt A, Valls-Solé J. A startle speeds up the execution of externally guided saccades. Exp Brain Res. 2007;177:129–136. doi: 10.1007/s00221-006-0659-4. [DOI] [PubMed] [Google Scholar]
- Chen R, Lozano AM, Ashby P. Mechanism of the silent period following transcranial magnetic stimulation. Evidence from epidural recordings. Exp Brain Res. 1999;128:539–542. doi: 10.1007/s002210050878. [DOI] [PubMed] [Google Scholar]
- Cheney PD, Fetz EE. Corticomotoneuronal cells contribute to long-latency stretch reflexes in the rhesus monkey. J Physiol. 1984;349:249–272. doi: 10.1113/jphysiol.1984.sp015155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cui R, MacKinnon CD. The effect of temporal accuracy constraints on movement-related potentials. Exp Brain Res. 2009;194:477–488. doi: 10.1007/s00221-009-1725-5. [DOI] [PubMed] [Google Scholar]
- Day BL, Rothwell JC, Thompson PD, Maertens de Noordhout A, Nakashima K, Shannon K, Marsden CD. Delay in the execution of voluntary movement by electrical or magnetic brain stimulation in intact man. Evidence for the storage of motor programs in the brain. Brain. 1989;112:649–663. doi: 10.1093/brain/112.3.649. [DOI] [PubMed] [Google Scholar]
- Fisher KM, Zaaimi B, Baker SN. 2010 Abstract Viewer/Itinerary Planner. San Diego, CA: Society for Neuroscience; 2010. Transcranial magnetic stimulation activates neurons in the reticular formation at surprisingly long latencies. Programme No. 592.6. [Google Scholar]
- Fisher RJ, Sharott A, Kuhn AA, Brown P. Effects of combined cortical and acoustic stimuli on muscle activity. Exp Brain Res. 2004;157:1–9. doi: 10.1007/s00221-003-1809-6. [DOI] [PubMed] [Google Scholar]
- Fuhr P, Agostino R, Hallett M. Spinal motor neuron excitability during the silent period after cortical stimulation. Electroencephalogr Clin Neurophysiol. 1991;81:257–262. doi: 10.1016/0168-5597(91)90011-l. [DOI] [PubMed] [Google Scholar]
- Furubayashi T, Ugawa Y, Terao Y, Hanajima R, Sakai K, Machii K, et al. The human hand motor area is transiently suppressed by an unexpected auditory stimulus. Clin Neurophysiol. 2000;111:178–183. doi: 10.1016/s1388-2457(99)00200-x. [DOI] [PubMed] [Google Scholar]
- Gage WH, Zabjek KF, Hill SW, McIlroy WE. Parallels in control of voluntary and perturbation-evoked reach-to-grasp movements: EMG and kinematics. Exp Brain Res. 2007;181:627–637. doi: 10.1007/s00221-007-0959-3. [DOI] [PubMed] [Google Scholar]
- Hanes DP, Schall JD. Neural control of voluntary movement initiation. Science. 1996;274:427–430. doi: 10.1126/science.274.5286.427. [DOI] [PubMed] [Google Scholar]
- Hashimoto T, Inaba D, Matsumura M, Naito E. Two different effects of transcranial magnetic stimulation to the human motor cortex during the pre-movement period. Neurosci Res. 2004;50:427–436. doi: 10.1016/j.neures.2004.08.002. [DOI] [PubMed] [Google Scholar]
- Hodges PW, Bui BH. A comparison of computer-based methods for the determination of onset of muscle contraction using electromyography. Electroencephalogr Clin Neurophysiol. 1996;101:511–519. doi: 10.1016/s0013-4694(96)95190-5. [DOI] [PubMed] [Google Scholar]
- Inghilleri M, Berardelli A, Cruccu G, Manfredi M. Silent period evoked by transcranial stimulation of the human cortex and cervicomedullary junction. J Physiol. 1993;466:521–534. [PMC free article] [PubMed] [Google Scholar]
- Kably B, Drew T. Corticoreticular pathways in the cat. I. Projection patterns and collaterization. J Neurophysiol. 1998;80:389–405. doi: 10.1152/jn.1998.80.1.389. [DOI] [PubMed] [Google Scholar]
- Kumru H, Valls-Solé J. Excitability of the pathways mediating the startle reaction before execution of a voluntary movement. Exp Brain Res. 2006;169:427–432. doi: 10.1007/s00221-005-0156-1. [DOI] [PubMed] [Google Scholar]
- Lakhani B, Van Ooteghem K, Miyasike-Dasilva V, Akram S, Mansfield A, McIlroy WE. Does the movement matter? Determinants of the latency of temporally urgent motor reactions. Brain Res. 2011;1416:35–43. doi: 10.1016/j.brainres.2011.08.013. [DOI] [PubMed] [Google Scholar]
- MacKinnon CD, Bissig D, Chiusano J, Miller E, Rudnick L, Jager C, et al. Preparation of anticipatory postural adjustments prior to stepping. J Neurophysiol. 2007;97:4368–4379. doi: 10.1152/jn.01136.2006. [DOI] [PubMed] [Google Scholar]
- Maslovat D, Hodges NJ, Chua R, Franks IM. Motor preparation and the effects of practice: evidence from startle. Behav Neurosci. 2011;125:226–240. doi: 10.1037/a0022567. [DOI] [PubMed] [Google Scholar]
- Matthews PBC. The human stretch reflex and the motor cortex. Trends Neurosci. 1991;14:87–91. doi: 10.1016/0166-2236(91)90064-2. [DOI] [PubMed] [Google Scholar]
- Molinuevo JL, Valls-Sole J, Valldeoriola F. The effect of transcranial magnetic stimulation on reaction time in progressive supranuclear palsy. Clin Neurophysiol. 2000;111:2008–2013. doi: 10.1016/s1388-2457(00)00443-0. [DOI] [PubMed] [Google Scholar]
- Ni Z, Gunraj C, Chen R. Short interval intracortical inhibition and facilitation during the silent period in human. J Physiol. 2007;583:971–982. doi: 10.1113/jphysiol.2007.135749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nickerson RS. Intersensory facilitation of reaction time: energy summation or preparation enhancement? Psychol Rev. 1973;80:489–509. doi: 10.1037/h0035437. [DOI] [PubMed] [Google Scholar]
- Palmer E, Ashby P. Evidence that a long latency stretch reflex in humans is transcortical. J Physiol. 1992;449:429–440. doi: 10.1113/jphysiol.1992.sp019094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pascual-Leone A, Valls-Sole J, Brasil-Neto JP, Cohen LG, Hallett M. Akinesia in Parkinson's disease. I. Shortening of simple reaction time with focal, single-pulse transcranial magnetic stimulation. Neurology. 1994;44:884–891. doi: 10.1212/wnl.44.5.884. [DOI] [PubMed] [Google Scholar]
- Queralt A, Valls-Solé J, Castellote JM. Speeding up gait initiation and gait-pattern with a startling stimulus. Gait Posture. 2010;31:185–190. doi: 10.1016/j.gaitpost.2009.10.003. [DOI] [PubMed] [Google Scholar]
- Riddle CN, Baker SN. Convergence of pyramidal and medial brain stem descending pathways onto macaque cervical spinal interneurons. J Neurophysiol. 2010;103:2821–2832. doi: 10.1152/jn.00491.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rogers MW, Kennedy R, Palmer S, Pawar M, Reising M, Martinez KM, et al. Postural preparation prior to stepping in patients with Parkinson's disease. J Neurophysiol. 2011;106:915–924. doi: 10.1152/jn.00005.2010. [DOI] [PubMed] [Google Scholar]
- Roick H, von Giesen HJ, Benecke R. On the origin of the postexcitatory inhibition seen after transcranial magnetic brain stimulation in awake human subjects. Exp Brain Res. 1993;94:489–498. doi: 10.1007/BF00230207. [DOI] [PubMed] [Google Scholar]
- Romaiguere P, Possamai CA, Hasbroucq T. Motor cortex involvement during choice reaction time: a transcranial magnetic stimulation study in man. Brain Res. 1997;755:181–192. doi: 10.1016/s0006-8993(97)00095-4. [DOI] [PubMed] [Google Scholar]
- Rothwell JC. The startle reflex, voluntary movement, and the reticulospinal tract. Suppl Clin Neurophysiol. 2006;58:223–231. doi: 10.1016/s1567-424x(09)70071-6. [DOI] [PubMed] [Google Scholar]
- Rothwell JC, MacKinnon CD, Valls-Solé J. Role of brainstem-spinal projections in voluntary movement. Mov Disord. 2002;17(Suppl 2):S27–29. doi: 10.1002/mds.10054. [DOI] [PubMed] [Google Scholar]
- Sanegre MT, Castellote JM, Haggard P, Valls-Solé J. The effects of a startle on awareness of action. Exp Brain Res. 2004;155:527–531. doi: 10.1007/s00221-004-1849-6. [DOI] [PubMed] [Google Scholar]
- Shemmell J, An JH, Perreault EJ. The differential role of motor cortex in stretch reflex modulation induced by changes in environmental mechanics and verbal instruction. J Neurosci. 2009;29:13255–13263. doi: 10.1523/JNEUROSCI.0892-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schepens B, Drew T. Independent and convergent signals from the pontomedullary reticular formation contribute to the control of posture and movement during reaching in the cat. J Neurophysiol. 2004;92:2217–2238. doi: 10.1152/jn.01189.2003. [DOI] [PubMed] [Google Scholar]
- Siebner HR, Dressnandt J, Auer C, Conrad B. Continuous intrathecal baclofen infusions induced a marked increase of the transcranially evoked silent period in a patient with generalized dystonia. Muscle Nerve. 1998;21:1209–1212. doi: 10.1002/(sici)1097-4598(199809)21:9<1209::aid-mus15>3.0.co;2-m. [DOI] [PubMed] [Google Scholar]
- Siegmund GP, Inglis JT, Sanderson DJ. Startle response of human neck muscles sculpted by readiness to perform ballistic head movements. J Physiol. 2001;535:289–300. doi: 10.1111/j.1469-7793.2001.00289.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stevenson AJ, Maslovat D, Chua R, Franks IM. Transcranial magnetic stimulation to the primary motor cortex can influence the early release of a preplanned movement by a startling acoustic stimulus. 2011. Proceedings of the 21st Meeting of the Society for the Neural Control of Movement.
- Terao Y, Ugawa Y, Suzuki M, Sakai K, Hanajima R, Gemba-Shimizu K, et al. Shortening of simple reaction time by peripheral electrical and submotor-threshold magnetic cortical stimulation. Exp Brain Res. 1997;115:541–545. doi: 10.1007/pl00005724. [DOI] [PubMed] [Google Scholar]
- Tergau F, Wanschura V, Canelo M, Wischer S, Wassermann EM, Ziemann U, et al. Complete suppression of voluntary motor drive during the silent period after transcranial magnetic stimulation. Exp Brain Res. 1999;124:447–454. doi: 10.1007/s002210050640. [DOI] [PubMed] [Google Scholar]
- Uncini A, Treviso M, Di Muzio A, Simone P, Pullman S. Physiological basis of voluntary activity inhibition induced by transcranial cortical stimulation. Electroencephalogr Clin Neurophysiol. 1993;89:211–220. doi: 10.1016/0168-5597(93)90098-a. [DOI] [PubMed] [Google Scholar]
- Valls-Solé J, Kofler M, Kumru H, Castellote JM, Sanegre MT. Startle-induced reaction time shortening is not modified by prepulse inhibition. Exp Brain Res. 2005;165:541–548. doi: 10.1007/s00221-005-2332-8. [DOI] [PubMed] [Google Scholar]
- Valls-Solé J, Kumru H, Kofler M. Interaction between startle and voluntary reactions in humans. Exp Brain Res. 2008;187:497–507. doi: 10.1007/s00221-008-1402-0. [DOI] [PubMed] [Google Scholar]
- Valls-Solé J, Pascual-Leone A, Wassermann EM, Hallett M. Human motor evoked responses to paired transcranial magnetic stimuli. Electroencephalogr Clin Neurophysiol. 1992;85:355–364. doi: 10.1016/0168-5597(92)90048-g. [DOI] [PubMed] [Google Scholar]
- Valls-Solé J, Rothwell JC, Goulart F, Cossu G, Munoz E. Patterned ballistic movements triggered by a startle in healthy humans. J Physiol. 1999;516:931–938. doi: 10.1111/j.1469-7793.1999.0931u.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valls-Solé J, Solé A, Valldeoriola F, Munoz E, Gonzalez LE, Tolosa ES. Reaction time and acoustic startle in normal human subjects. Neurosci Lett. 1995;195:97–100. doi: 10.1016/0304-3940(94)11790-p. [DOI] [PubMed] [Google Scholar]
- Voss M, Ingram JN, Haggard P, Wolpert DM. Sensorimotor attenuation by central motor command signals in the absence of movement. Nat Neurosci. 2006;9:26–27. doi: 10.1038/nn1592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Werhahn KJ, Kunesch E, Noachtar S, Benecke R, Classen J. Differential effects on motorcortical inhibition induced by blockade of GABA uptake in humans. J Physiol. 1999;517:591–597. doi: 10.1111/j.1469-7793.1999.0591t.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yeomans JS, Frankland PW. The acoustic startle reflex: neurons and connections. Brain Res Brain Res Rev. 1995;21:301–314. doi: 10.1016/0165-0173(96)00004-5. [DOI] [PubMed] [Google Scholar]
- Ziemann U, Netz J, Szelenyi A, Homberg V. Spinal and supraspinal mechanisms contribute to the silent period in the contracting soleus muscle after transcranial magnetic stimulation of human motor cortex. Neurosci Lett. 1993;156:167–171. doi: 10.1016/0304-3940(93)90464-v. [DOI] [PubMed] [Google Scholar]
- Ziemann U, Tergau F, Netz J, Hömberg V. Delay in simple reaction time after focal transcranial magnetic stimulation of the human brain occurs at the final motor output stage. Brain Res. 1997;744:32–40. doi: 10.1016/s0006-8993(96)01062-1. [DOI] [PubMed] [Google Scholar]


