Abstract
Animals, including humans, can achieve precise regulation of caloric intake by adjusting consumption in response to covert changes in energy density. It remains unknown, however, whether the presence of flavour cues are required for the ability to maintain constant caloric intake. Also unknown are the brain circuits that may function as the central calorie monitors that control adaptive adjustments in energy intake. Here we show that mice trained to lick a dry spout in order to receive intra-gastric infusions of a fat emulsion maintained constant hourly caloric intake by adjusting the number of dry licks in response to changes in caloric density. Animals also increased dry licking according to hunger levels, and developed conditioned preferences for dry sippers associated with high calorie infusions. Importantly, striatal dopamine levels were closely associated with the amount of calories ingested, rather than with the number of dry licks produced. Dopamine levels in dorsal and ventral striatum also reflected caloric density in mice passively receiving intra-gastric infusions of fat emulsions. Consistent with the above, systemic administration of the dopamine receptor blocker haloperidol markedly increased the production of dry licks needed to obtain high-calorie infusions, as if the caloric density of the infusions had been diluted. Conversely, haloperidol markedly decreased the production of dry licks needed to obtain low-calorie infusions. Taken together, our results support the proposition that brain dopamine circuits function as one central sensor of calorie ingestion, since (1) extracellular striatal dopamine levels fluctuate in proportion to the caloric density of nutrients infused in the gut; and (2) inhibiting dopamine receptor signalling disrupts the animals’ ability to maintain constant caloric intake across experimental sessions.
Key points
The hedonic orosensory properties of fats strongly promote intake, but it remains unknown whether fat intake stimulates brain reward circuits in the absence of orosensory cues.
We developed a behavioral assay that allows for the dissociation between orosensory versus post-oral influences on fat intake.
Mice trained to lick a dry spout to receive intra-gastric infusions of fat emulsions maintained constant caloric intake in response to changes in energy density or hunger levels.
Dopamine levels in dorsal and ventral striatum were responsive to gut infusions of fat emulsions, in such a way that (1) extracellular striatal dopamine levels fluctuate in proportion to the caloric density of nutrients infused in the gut; and (2) inhibiting dopamine receptor signalling disrupts the animals’ ability to maintain constant caloric intake across experimental sessions.
Our results support the existence of a gut–brain dopamine axis that functions as a flavour-independent central sensor of fat calories.
Introduction
It has been established that body weight is either stable or fluctuates within minimal ranges in most adult individuals (Fox, 1973; Weigle, 1994; Friedman, 2004), implying the existence of a physiological system acting to maintain body weight. In fact, animals, including humans, are capable of precise regulation of caloric intake in order to maintain homeostasis. In 1947 E. Adolph systematically manipulated the energy density of diets given to adult rats and, upon observing their adaptive consummatory responses, readily concluded that ‘within limits, rats eat for calories’ (Adolph, 1947). Later investigations confirmed this finding by showing that several species adjust food intake in response to variations in nutritive density, including rodents (Peterson & Baumgardt, 1971; Booth, 1972; Smutz et al. 1975; Chi & Powley, 2007), pigs (Owens & Ridgman, 1968), monkeys (McHugh et al. 1975; McHugh & Moran, 1978; Hansen et al. 1981), and humans (Campbell et al. 1971; Wooley et al. 1972). Overall, these studies revealed that behavioural caloric compensation allows for body weight to be constantly maintained within narrow ranges (Peterson & Baumgardt, 1971; McHugh & Moran, 1978).
But how are calories sensed, or more specifically ‘counted’, during active feeding? One fundamental question left unanswered is whether detecting orosensory signals such as taste, odour or texture is required for rapid caloric compensation to occur. Seminal work by C. Richter (1941, 1943) in rats and by McHugh, Moran et al. (1975, 1978) in monkeys demonstrates that these animals are remarkably capable of maintaining constant caloric intake by reducing oral feeding following a passive intra-gastric caloric preload (see also Booth, 1972; Chi & Powley, 2007). However, it remains unknown whether animals would display the more general capacity of maintaining constant caloric intake when oral flavour stimulation is denied throughout the entire feeding episode. More specifically, while it is well established that post-oral cues have the ability to promote long-last food preferences in both animals (Sclafani, 2001) and humans (Yeomans et al. 2009), it remains to be shown that such post-ingestive signals should be sufficient to allow for the discrimination between different caloric densities and the consequent adjustments in intake.
Neither is it known which brain circuits would continuously keep track of (i.e. ‘count’) the amounts of calories being ingested using flavour-independent physiological cues. While peripheral and hypothalamic peptides have been shown to inform the brain on the physiological status of the organism (Berthoud, 2002; Elmquist et al. 2005), the identities of the brain systems regulating behavioural caloric compensation remain unknown. Brain dopamine systems constitute one strong candidate given its robust efflux during active feeding (Hernandez & Hoebel, 1988; Small et al. 2003). Interestingly, while the orosensory properties of palatable foods are by themselves sufficient to stimulate brain dopamine release (Hajnal et al. 2004; Liang et al. 2006), significant efflux is also observed during sugar intake in animals lacking sweet taste transduction (de Araujo et al. 2008; Touzani et al. 2008) as well as following intra-gastric infusions of metabolizable sugars (Ren et al. 2010). Accordingly, brain dopamine cells may work to continuously monitor ingested calories independently of oropharingeal sensations.
In this study we address three unresolved yet crucial questions linked to the regulatory capacity of maintaining caloric intake. First, we aimed at determining whether adult mice are capable of compensating for covert changes in caloric density even if denied the perception of flavour cues throughout the entire feeding episode. Second, we evaluated the possibility that extracellular dopamine levels in striatum not only increase upon intra-gastric infusions of caloric nutrients, but do so to reflect the caloric density of the infusate. Finally, we tested the hypothesis that brain dopamine systems mediate these flavour-independent adaptive responses by inquiring whether inhibition of dopamine signalling disrupts the regulated intake of calories. Accordingly, we designed a behavioural preparation, which was ultimately coupled to brain microdialysis, in which licks to dry sippers triggered intra-gastric infusions of a fat emulsion prepared at different caloric densities across experimental sessions.
Methods
Subjects
All experiments were approved by the J. B. Pierce Laboratory Institutional Animal Care and Use Committee (IACUC) and were in accordance with the J. B. Pierce Laboratory and Yale University regulations on usage of animals in research. A total of 56 C57BL/6J (purchased from The Jackson Laboratory, Bar Harbor, ME, USA) male mice were used. At the time of the experiments animals were 10–16 weeks old. Animals having performed the behavioural tasks were returned to the colony after the end of the experiments, where they resumed their usual intake patterns. Animals used in brain microdialysis experiments were killed with an intraperitoneal overdose of Euthasol after the end of the experiments, and their brains preserved for further histological assessment of probe location.
Surgical procedure for implantation of gastric catheters and microdialysis guiding cannulae
Once animals had been anaesthetized with an intraperitoneal injection of ketamine/xylazine (100/15 mg kg−1), a midline incision was made into the abdomen. The stomach was exteriorized through the midline incision and a purse string suture was placed in its non-glandular region, into which the tip of MicroRenathane tubing (Braintree Scientific Inc., Braintree, MA, USA) was inserted. The purse string was tightened around the tubing, which was then tunnelled subcutaneously to the dorsum via a small hole made into the abdominal muscle; a small incision to the dorsum between the shoulder plates was then made to allow for catheter exteriorization. Incisions were sutured and thoroughly disinfected and the exterior end of the catheter plugged. For animals used in the microdialysis experiments targeting the dorsal striatal region, immediately after the above procedure, the animal was placed on a stereotaxic apparatus (David Kopf) under constant flow of ∼1% isoflurane anaesthesia (1.5 l min−1) and a circular craniotomy was drilled at AP = 1.3 mm, ML =±1.3 mm implantation of a guide cannulae (DV =−0.5 mm from brain surface) for posterior insertion of a microdialysis probe (final probe tip positions, DV =−2.5 mm from brain surface). For microdialysis experiments targeting the nucleus accumbens of the ventral striatum, coordinates were AP =+1.65, ML =±0.6, DV =−3.25 final probe tip position.
Post-operative care
Immediately following surgery, animals were treated with buprenorphine (0.05 mg kg−1i.m.), and subsequently with ibuprofen (30 mg kg−1 in drinking water, 5 days). During this period animals were treated with Baytril (85 mg kg−1 day −1, in drinking water). During the post-operative recovery period (14 days) animals were monitored for signs of pain, distress or morbity every 12 h. Whenever any of these signs were detected animals were immediately killed with an intraperitoneal overdose of Euthasol.
Stimuli and calculation of caloric densities
Mice were trained to obtain intra-gastric infusions of a fat emulsion (30% Intralipid, Baxter Healthcare, Deerfield, IL, USA). The emulsion contains as main components 30% soybean oil, 1.2% egg yolk phospholipids, 1.7% glycerin, and water. The caloric density of 30% Intralipid is 3.0 kcal ml−1, with 2.7 kcal ml−1 accounted for by soybean oil and 0.3 kcal ml−1 by phospholipids + glycerin. The original 30% emulsion was diluted into an emulsifying control solution (1.2% phospholipids + 1.7% glycerine, in water) in order to prepare the 5% and 15% dilutions. The resulting caloric densities were as follows. For 30% emulsions: 3 kcal ml−1, equivalent to 0.09 kcal per intra-gastric infusion (see below details on behavioural protocol). For 15% emulsions: 1.65 kcal ml−1 (1.5 kcal ml−1 from Intralipid + 0.15 kcal ml−1 from control), equivalent to 0.0495 kcal per infusion. For 5% emulsions: 0.75 kcal ml−1 (0.5 kcal ml−1 from Intralipid + 0.25 kcal ml−1 from control), equivalent to 0.0225 kcal per infusion. For the control solutions: 0.3 kcal ml−1, equivalent to 0.009 kcal per infusion. Additional experiments involved infusions of d-glucose (Sigma, St Louis, MO, USA), which was diluted into the control solution to one of three concentrations that exactly matched the caloric densities of the 5%, 15% and 30% emulsions.
Behavioural apparatus
Behavioural experiments were conducted in either one of three identical mouse behaviour chambers enclosed in a ventilated and sound-attenuating cubicle (Med Associates Inc., St Albans, VT, USA). Each chamber was equipped with two slots for sipper tubing placements, at symmetrical locations on one of the cage walls. All sippers are connected to a contact-based licking detection device allowing for measurements of licking responses with 10 ms resolution. All lick timestamps were saved in a computer file for posterior analysis. Software-controlled infusion pumps equipped with TTL input devices were connected to the behavioural chambers and programmed to automatically trigger infusions in response to the detection of licks.
Behavioural protocol
Mice were trained to produce licks to a dry metallic spout in order to receive intra-gastric infusions of the fat emulsions. The exterior part of the gastric catheter was connected to a segment of MicroRenathane tubing secured to the tip of a 3 ml standard syringe containing the solutions to be infused and mounted on the syringe pump. The syringe pump was placed near a small hole made on the superior part of the sound attenuating box in such a way that mice could move freely inside the behavioural chambers. During the task, a detected dry lick triggered an intra-gastric infusion of the fat emulsion that lasted for 3 s at a rate of 0.6 ml min−1. However, licks detected while an infusion was taking place had no programmed consequences (i.e. did not result in additional infusions). Experimental tests typically lasted for 1 h, although 3 h-long additional tests were performed in a subgroup of animals. Animals were tested once a day in their responses to one single concentration chosen from a pre-determined random distribution, with all animals being tested in all concentrations across days. Specifically, the concentration of the emulsion being infused during any given experimental session (i.e. 5, 15 or 30%) was randomly assigned (so that the actual concentration of the infused solution in any given session could not be predicted by the animal), with the assignment sequence being counterbalanced across animals. In order to train the animals in this task, once mice had recovered from surgery and been habituated to the behavioural chambers, a small amount of standard chow was placed behind the spout's orifice (so that they could be smelled but not reached) to prime naive animals to dry lick and obtain intra-gastric infusions. Training sessions lasted for 1 h and were performed daily under food (20 h) deprivation. Typically the animals had acquired stable responses after two or three priming sessions, when the spouts containing chow were replaced by clean odourless ones. Animals were considered trained to perform the experiments once they showed less than 20% between-session variability.
Conditioning protocol
In addition to the one-spout tests described above, we also designed a conditioning protocol to assess whether mice are able to associate a sipper position in the behavioural chamber with a particular concentration (i.e. a caloric density) of the emulsions. Conditioning experiments were conducted following 20 h food deprivation and were performed in animals that had been trained in the task as described above. Initially, side-biases favouring the side of the chamber where the sipper was placed during training sessions were observed in all animals. Then, 1 h-long conditioning sessions were conducted daily for 10 days. The 10 daily conditioning sessions consisted of one dry-sipper forced choice sessions where animals were allowed for five sessions to dry lick a sipper placed on the same side of previous training (i.e. bias) to obtain intra-gastric infusions of 5% fat emulsions, intercalated with five sessions when animals were allowed to dry lick the sipper placed on the opposite side of the chamber to obtain intra-gastric infusions of 30% fat emulsions. To avoid possible associations between caloric value and orosensory detection of textural properties of the sippers, the same metallic sipper was used in all conditioning sessions. After conditioning, side-preferences were tested in 10 min two dry-sipper tests and determined according to the expression
where n(Sipper x) denotes the total number of licks produced to dry sipper x during the two dry-sipper preference tests. Post-conditioning preference tests were performed in extinction (i.e. only control solutions were infused during these tests). To confirm that no conditioning-independent spatial biases were accounting for the animals’ behaviours, the same experiments were performed again on the same mice but this time with the contingencies reversed, that is the 5% solutions were now being associated with the side of the chamber previously associated with 30% solutions and vice versa. Preferences tests were once again performed as in the first conditioning series.
Dopamine measurements during behavioural performance and passive infusions
To assess the potential role of the neurotransmitters dopamine and serotonin in mediating intra-gastric fat self-administration, a subgroup of mice performed the behavioural task as described above concomitantly to collecting microdialysate samples from dorsal striatum. Specifically, during the experimental sessions microdialysate samples from the freely moving mice were collected, separated and quantified by HPLC coupled to electrochemical detection methods (‘HPLC-ECD’). Briefly, after recovery from surgery and behavioural training as above, a microdialysis probe (2 mm CMA-7, cut off 6 kDa, CMA Microdialysis, Stockholm, Sweden) was inserted into the striatum through the guide cannula (the corresponding CMA-7 model). After insertion, probes were connected to a syringe pump and perfused at 1.2 μl min−1 with artificial CSF (Harvard Apparatus). After a 30 min washout period, dialysate samples were collected every 10 min and immediately manually injected into a HTEC-500 HPLC unit (Eicom, Japan). Analytes were then separated via an affinity column (PP-ODS, Eicom), and compounds subjected to redox reactions within an electrochemical detection unit (amperometric DC mode, applied potential range from 0 to ∼2000 mV, 1 mV steps). Resulting chromatograms were analysed using the software EPC-300 (Eicom, Japan), and actual sample concentrations were computed based on peak areas obtained from 0.5 pg μl−1 dopamine + serotonin standards (Sigma) and expressed as percentage changes with respect to the mean dopamine concentration associated with baseline (i.e. behavioural task) samples. For microdialysis experiments performed independently of behavioural tasks, the same procedure as above was employed, with the exception that animals were left freely moving in their home cages and following a period of 30 min baseline, 0.6 ml intra-gastric infusions were performed. Locations of microdialysis probes were confirmed histologically.
Dopamine receptor inhibition
To evaluate the effects of disrupting dopamine signalling during the behavioural task described above, following training animals were tested daily on their dry licking responses for 30% or 5% emulsion infusions after an intraperitoneal dose of either haloperidol (Sigma, 0.075 mg kg−1 prepared in saline + 5% DMSO) or vehicle (saline + 5% DMSO) injected 45 min before the beginning of the experimental sessions. Animals were initially tested on two vehicle sessions, followed by four consecutive haloperidol sessions and then on further additional four vehicle sessions. The same group of animals was subjected to a control experiment where they received vehicle only for 10 sessions. This protocol was based on previous work performed in rats self-administering cocaine, where repeatedly injecting moderate doses of haloperidol was shown to minimize potential motoric confounds (Roberts & Vickers, 1987).
Gastric emptying assessment
A separate cohort of mice implanted with gastric catheters was used for analysis of the remaining stomach content, an index of gastric emptying, after passive infusions of either 5% or 30% emulsions (n = 6 per group). The procedure was similar to that described previously (Almeida et al. 2011). Briefly, 15 min after the end of the infusions (performed as described above), the animals were killed with an intraperitoneal overdose of Euthasol and the esophagus (just proximal to the gastric fundus) and the duodenum (just distal to the pylorus) clamped and securely tied with suture threads. Transverse cuts distal to the stomach were made in the vicinity of each tie to allow for stomach removal and weighing. Then, contents were removed and stomachs desiccated (∼65°C for 72 h) and weighed a second time to determine dry tissue weight. Gastric emptying was calculated as follows: Gastric Emptying (% of total infused) ={Weight Post-dissection/Weight Pre-dissection}× 100.
Statistical analyses
Data analyses were performed using SPSS (PASW Statistics Release 18.0.0) or Matlab (R14, The MathWorks, Inc., Natick, MA, USA) and made use of linear mixed regression analyses as well as two- and one-way repeated measures ANOVAs. In order to assess the strength of the associations between emulsion density and intra-gastric self-feeding, mixed linear models were employed in order to account for correlated variability associated with repeated measures taken across the different emulsion concentrations. Numbers of infusions or calories infused were defined as dependent variables, with emulsion concentration and subjects as fixed and random effects factors, respectively. Model estimation was based on maximum likelihood methods and fixed effects estimation is presented as F statistics. The corresponding (repeated measures) ANOVA models were also computed; post hoc pairwise tests were based on Student's paired within-subject t test with resulting P values being subsequently subjected to Bonferroni's correction for multiple comparisons. Similar analyses were performed to quantify the strength of the associations between dopamine and serotonin efflux as measured by microdialysis and both the numbers of dry licks and total calories ingested. Data are reported as means ± SEM unless stated otherwise.
Results
Mice rapidly and precisely regulate caloric intake in the absence of flavour cues
We started by assessing the ability of adult mice to regulate the amounts of calories ingested in the absence of flavour cues. In our task, male mice were fitted with gastric catheters and trained to lick a dry spout in order to receive intra-gastric infusions of fat emulsions. Infusions were delivered at a rate of 0.6 ml min−1 and lasted for 3 s. For each daily 1 h session the emulsion was prepared at a caloric density that had been determined from a random sequence (so that the concentration being infused could not be predicted by the animal, see Methods for details). We hypothesized that mice have the ability to maintain constant caloric intake by regulating the number of dry licks according to the caloric density of the emulsion being infused.
The number of dry lick-triggered infusions of the most caloric 30% emulsion was 18 ± 3 and this increased to 51 ± 8 and 92 ± 7 infusions for emulsions diluted at 15% and 5%, respectively. In agreement with our hypothesis, a negative linear relationship between the numbers of infusions and the caloric densities of the emulsions was found (see Fig. 1A; the corresponding repeated measures ANOVA and pairwise results are shown in Supplemental Table 1). Accordingly, the numbers of infusions reflected the number of dry licks detected during these sessions (also displayed in Supplemental Table 1). Note that numbers of dry licks are not exactly proportional to the numbers of infusions because licks produced during infusion pump activity had no programmed consequence.
Figure 1. Mice rapidly and precisely regulate caloric intake in the absence of flavour cues.
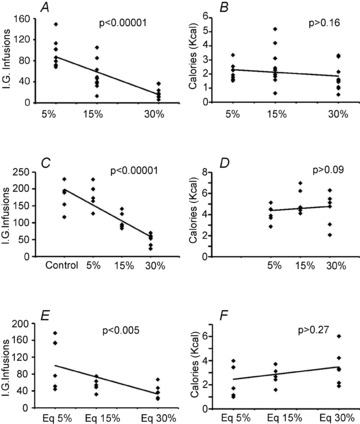
Adult male mice (n = 10) licked a dry spout to obtain intra-gastric infusions of caloric solutions prepared at different caloric densities. Scatter plots and trend lines reveal the relationships between the numbers of intra-gastric (i.g.) infusions (or amounts of calories ingested via these infusions) and emulsion density. A, during 1 h sessions mice adjusted the number of infusions of fat emulsions according to caloric density, linearly decreasing the number of infusions as emulsion caloric density increased (mixed linear regression model, F2,30= 30.6, P < 0.00001). B, however, no statistical evidence for effects of emulsion density was detected on the actual amount of calories ingested during these sessions (F2,30= 1.9, P = 0.16), suggesting that mice were able to regulate dry licking in order to maintain constant caloric intake. C, during 3 h-long sessions, mice (n = 6) once again decreased the number of infusions as emulsion caloric density increased (a mixed model design revealed a negative linear relationship between the numbers of infusions and the caloric densities of the emulsions, F3,18= 40.3, P < 0.00001). D, once again, no statistical evidence for effects of emulsion density were detected on the actual amount of calories ingested during the 3 h sessions, suggesting once more that mice were able to regulate dry licking in order to maintain constant caloric intake (mixed linear model, F2,12= 3.1, P = 0.09). E, similar effects were obtained when glucose solutions were used instead of fat emulsions in 1 h sessions. Glucose (diluted into the phospholipid emulsifier solution) was prepared at concentrations isocaloric to 5%, 15% and 30% fat emulsions. Animals (n = 6) adjusted number of infusions according to caloric density (a mixed model design revealed a negative linear relationship between the numbers of infusions and the caloric densities of the glucose emulsions, F2,12= 8.5, P = 0.005). F, however, no statistical evidence for concentration effects was observed on caloric intake (F2,12= 1.4, P = 0.27).
Similar analyses were performed on the number of calories actually ingested (2.07 ± 0.17, 2.5 ± 0.42 and 1.7 ± 0.29 kcal for 5%, 15% and 30% emulsions, respectively). Strikingly, we found no statistical evidence that emulsion density affected caloric intake (see Fig. 1B). We interpreted these results as substantiating our hypothesis that mice are capable of regulating dry licking during 1 h sessions in order to compensate for covert dilutions in caloric density.
We also asked whether such effects would have persisted had the sessions lasted for longer periods of time. A different group of mice was trained on the same behaviour task as above with the exception that sessions now lasted for 3 h. The number of dry lick-triggered infusions of the 30% emulsion was 49 ± 7, and this increased to 104 ± 9 and 181 ± 14 for emulsions diluted at 15% and 5%, respectively. In addition, we added a control condition where infusions contained the emulsifying but not the soy oil components of the emulsions. Number of infusions associated with the control solution was 179 ± 15. Likewise for the 1 h-long experiments, a negative linear relationship was observed between the numbers of infusions and the caloric densities of the emulsions (Fig. 1C). Interestingly, while the number of infusions for the control solution significantly differed from the number of infusions for the 15% and 30% emulsions, this was not the case for the 5% emulsion (see Supplemental Table 1).
When similar analyses were performed on the number of calories actually ingested, no evidence for influences of emulsion density on caloric intake was found (Fig. 1D). Therefore, when session duration was increased to 3 h, the ability of the animals to compensate for caloric dilutions was maintained, although limited to emulsions prepared at concentrations equal or higher than 5% (i.e. the weak caloric density of the control solution did not allow for caloric compensation).
Finally, we inquired whether the caloric compensation effect was specific to fat emulsions or, alternatively, could be extended to other nutrients. Accordingly, we trained a separate group of mice to perform the task during 1 h sessions as above, but this time animals infused the monosaccharide d-glucose at concentrations that were isocaloric to the 5%, 15% and 30% fat emulsions. Whereas the number of dry lick-triggered infusions of the most caloric solution (i.e. isocaloric to 30% fat emulsions) was 38 ± 6, it increased to 57 ± 7 and 109 ± 23 for the dilutions with caloric densities equivalent to 15% and 5% fat emulsions, respectively. Likewise for the lipid experiments, a negative linear relationship between the numbers of infusions and the caloric densities of the glucose emulsions was found (Fig. 1E). Dry lick numbers are shown in Supplemental Table 1. Furthermore, similar analyses performed on the number of calories actually ingested revealed no glucose concentration effects on caloric intake (Fig. 1F), suggesting that, like the case of fat emulsions, mice are capable of regulating intra-gastric infusions of glucose solutions in order to maintain constant caloric intake.
Overall, the results above reveal that, within limits, mice can maintain constant caloric intake in the absence of flavour cues. One potential physiological limit to this ability may relate to a minimal number of calories that must be present in the infusions, so that low-calorie solutions (such as our control solution) may produce extinction-like effects during extended sessions (see Discussion for further considerations on this issue).
Mice regulate caloric intake according to hunger levels in the absence of flavour cues
The results described above led to the question of whether mice are capable of regulating caloric intake by increasing dry licking in response to hunger levels. A group of mice was trained to dry lick the spout to obtain intra-gastric infusions of the 30% fat emulsion during 1 h daily sessions following 0, 18 or 24 h food deprivation (order of conditions was counterbalanced across animals). We hypothesized that mice would increase the numbers of infusions, and therefore caloric intake, in proportion to the degree of food deprivation. As predicted a positive linear relationship between deprivation times and the number of intra-gastric infusions was observed: numbers of infusions were 12 ± 2, 26 ± 3 and 40 ± 2 after 0, 18 and 24 h of food deprivation, respectively (Fig. 2A). Because the number of calories obtained is a direct reflection of the number of infusions (given that all experiments involved infusions of 30% emulsions), the equivalent statistical results were associated with the numbers of calories ingested (1.2 ± 0.1, 2.4 ± 0.2 and 3.5 ± 0.1 kcal obtained after 0, 18 and 24 h of food deprivation, respectively). Finally, we note that the increases in infusion rates according to hunger levels were observed throughout the 1 h sessions, with number of infusions significantly diverging between conditions within the last 30 min of the session (Fig. 2B). These results show that mice are capable of rapidly increasing caloric intake as a function of deprivation magnitude without the support of flavour cues.
Figure 2. Mice regulate caloric intake according to hunger levels in the absence of flavour cues.
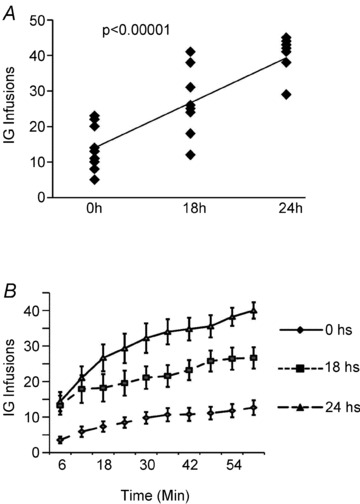
Mice (n = 9) were trained to dry lick a spout to obtain intra-gastric infusions of the 30% fat emulsion following 0, 18 or 24 h food deprivation. A, the scatter plot and trend line reveal the relationship between the numbers of intra-gastric (i.g.) infusions and emulsion density. Mice infused linearly increased the numbers of dry licks and intra-gastric infusions as a function of deprivation time (a mixed model design revealed a positive linear relationship between deprivation times and the number of intra-gastric infusions, F2,18= 33.4, P < 0.00001). B, cumulative sum of intra-gastric infusions throughout the deprivation experiments. Increases in infusion rates according to hunger levels were observed throughout the 1 h sessions, with the number of infusions significantly diverging between conditions within the first 30 min of the session.
Mice are capable of developing preferences for dry sippers associated with high-caloric intra-gastric infusions
While the experiments described above allowed us to conclude that animals regulate caloric intake in the absence of flavour, it remained unclear whether they would be able to discriminate and develop preferences for higher vs. lower caloric densities in the absence of flavour. We therefore designed a conditioning protocol to assess whether mice are capable of associating a dry sipper position in the behavioural chamber with the caloric density of the associated infusions. First, mice were trained in the dry lick task as before, a procedure that resulted in the formation of side-biases favouring the side of the chamber where the sipper was placed during training (measured during preliminary two dry-sipper preference tests). The 10 daily conditioning sessions consisted of five sessions to dry lick a sipper placed on the same side of previous training (i.e. bias) to obtain intra-gastric infusions of 5% fat emulsions, intercalated with five sessions when animals were allowed to dry lick the sipper placed on the opposite side of the chamber to obtain intra-gastric infusions of 30% fat emulsions. After conditioning, side-preferences were tested for 10 min with both sippers dry (see Methods for details). Finally, to confirm that no conditioning-independent spatial biases accounted for the animals’ preferences, the same experiments were once again performed on the same mice but this time with the contingencies reversed, that is the 5% solutions were now being associated with the side of the chamber previously associated with 30% solutions and vice versa. Preferences tests were once again performed as after the first conditioning series.
During the first conditioning series, one-way repeated measures ANOVAs revealed no significant effects of conditioning day on dry licks produced to infuse either the 30% or the 5% emulsions (both P > 0.5). A similar pattern was observed during conditioning sessions performed after contingencies had been reversed. For the first conditioning series the average number of dry licks was 563 ± 87 and 231 ± 26 for the 5% and 30% emulsions, respectively, a difference found to be significant (Fig. 3A). After contingencies reversal, dry licks were on average 907 ± 90 and 297 ± 30 for 5% and 30% emulsions, respectively, a difference once again found to be significant (Fig. 3A). As expected, the same patterns held true when similar analyses were performed on the numbers of infusions: no conditioning day effects were observed during either the first conditioning series or upon reversal (all P > 0.55). For the first conditioning series the overall average of infusions was 59 ± 7 and 28 ± 2 for 5% and 30% emulsions, respectively, a difference found to be significant (Fig. 3B). After contingencies reversal, infusions were on average 85 ± 5 and 30 ± 2 for 5% and 30% emulsions, respectively, a difference once again found to be significant (Fig. 3B). Now, for the case of the numbers of calories ingested, besides the expected lack of effects produced by conditioning day (all P > 0.5), during the first conditioning series the overall average of ingested calories was 1.3 ± 0.1 and 2.5 ± 0.1 kcal for 5% and 30% emulsions, respectively; unexpectedly, this difference was found to be significant (Fig. 3C). After contingencies reversal, ingested calories were on average 1.9 ± 0.1 and 2.7 ± 0.2 for 5% and 30% emulsions, respectively, a difference once again found to be significant (Fig. 3C). Therefore, caloric compensation did not occur when animals were allowed to lick two different dry sippers (that had been unambiguously associated with two emulsions, each diluted to a specific caloric density).
Figure 3. Mice are capable of developing preferences for dry sippers associated with high-calorie intra-gastric infusions.
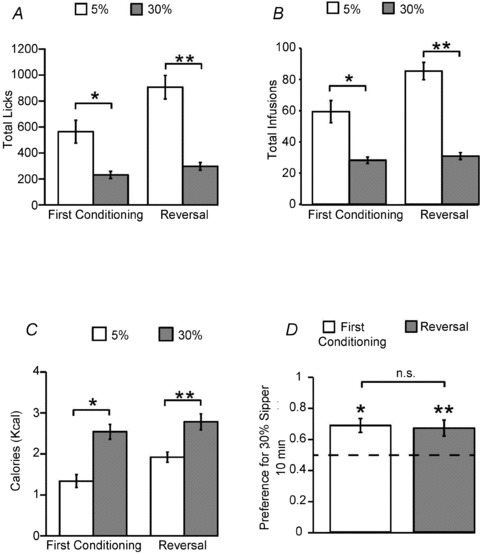
We designed a conditioning protocol to assess whether mice are capable of associating a sipper position in the behavioural chamber with the caloric density of the corresponding infusions. The 10 daily conditioning sessions consisted of one-sipper forced choice sessions where animals were allowed for five sessions to dry lick a sipper placed on the same side of previous training (i.e. bias) to obtain intra-gastric infusions of 5% fat emulsions, intercalated with five sessions when animals were allowed to dry lick the sipper placed on the opposite side of the chamber to obtain intra-gastric infusions of 30% fat emulsions. After conditioning, side-preferences were tested in 10 min two dry-sipper tests. To confirm that no conditioning-independent spatial biases accounted for the animals’ preferences, the same experiments were performed for a second time on the same mice after nevertheless reversing the contingencies. A, during conditioning sessions, mice (n = 5) produced significantly more dry licks to obtain infusions of 5% emulsions compared to dry licks to obtain infusions of 30% emulsions during both the first conditioning series (within-subject session-paired t test t21= 4.0, Bonferroni corrected *P < 0.004) and after contingencies reversal (t21= 7.2, **P < 0.002). B, the same results as in A held true for the numbers of infusions obtained (t21= 4.5, *P < 0.004 Bonferroni corrected, t21= 10.6, **P < 0.002). C, unlike the case of sessions associated with a single sipper position, during conditioning sessions mice failed to compensate for the differences in caloric densities between the emulsions, ingesting significantly more calories from infusing 30% emulsions compared to infusing 5% emulsions both during the first conditioning series (within-subject session-paired t test t21= 5.7, *P < 0.002 Bonferroni-corrected) and following reversal (t21= 4.9, **P < 0.002). D, during 10 min two dry-sipper preference tests, mice displayed significant preferences for dry sipper positions associated with the delivery of 30% emulsions compared to dry sipper positions associated with 5% emulsions following both the first conditioning series (one-sample t test against the indifference ratio of 0.5, t4= 4.1, *P < 0.02) and reversal (t4= 3.2, **P < 0.04). There was no significant difference between these two quantities (within-subject paired t test t4= 0.32, P > 0.7, n.s. = non-significant). The dashed line represents the indifference ratio of 0.5.
However, the key question to be resolved by these conditioning experiments was whether mice are capable of developing preferences for dry sippers associated with the higher caloric densities. Two dry-sipper 10 min-long preference tests were performed in extinction (i.e. control solutions only were infused during the tests) immediately after the first conditioning series. Results from these preference tests reveal that mice did in fact dry lick significantly more often the sipper associated with infusions of the 30% emulsion compared to the symmetrically positioned sipper associated with the 5% emulsions (preference ratio 0.68 ± 0.04, see Fig. 3D). Consistently, upon reversal of contingencies once again mice preferred the sipper associated with 30% emulsions – note that in this latter test the positions associated with 5% and 30% infusions had been switched with respect to first test (preference ratio 0.66 ± 0.05, Fig. 3D). In addition, there was no significant difference between the preference ratios associated with the two conditioning series (Fig. 3D).
These results demonstrate that mice are capable of discriminating and developing preferences for highly caloric fat emulsions even in the absence of flavour cues. Note that this occurred despite the fact that, during conditioning sessions, mice dry-licked more often the sipper associated with the less caloric emulsions.
Extracellular levels of dopamine in striatum reflect caloric intake in the absence of flavour cues
We now raise the question of which brain circuits may mediate the ability to maintain constant caloric intake in the absence of flavour cues. Given its critical role in food reward, we were particularly interested in the role of dopamine as a regulator of caloric intake (see Introduction and Discussion). Accordingly, we employed similar behavioural protocols as above, but this time concomitantly used microdialysis to collect dialysates from dorsal striatum. Each mouse was subjected to three 1 h-long sessions where they dry-licked to obtain infusions of 5%, 15% or 30% emulsions (one animal had the 15% session discarded due to gastric catheter malfunction). Dialysates were collected every 6 min and subsequently evaluated for dopamine content using liquid chromatography coupled to electrochemical detection (see Methods for details).
We first analysed the relative changes in extracellular dopamine levels observed during the entire (1 h) dry licking task. These relative changes are expressed as percentage increases or decreases with respect to baseline measurements, which were obtained previous to the initiation of behavioural performance (see Methods). We first observed that extracellular levels of dopamine in dorsal striatum increased significantly during the dry licking sessions. A one-way repeated measures ANOVA revealed no emulsion concentration effects on the overall changes in dopamine levels (Fig. 4A). Moreover, one sample t tests against 100% (i.e. against the null hypothesis of no change with respect to baseline) revealed significant relative increases in dopamine levels during sessions in which animals self-infused the 15% and the 30% emulsions (Fig. 4A). However, this did not hold for the 5% emulsion sessions (Fig. 4A), consistent with our previous observation that 5% emulsions may not exert reinforcing effects to the same extent as the 15% and 30% emulsions.
Figure 4. Extracellular levels of dopamine in striatum reflect caloric intake in the absence of flavour cues.
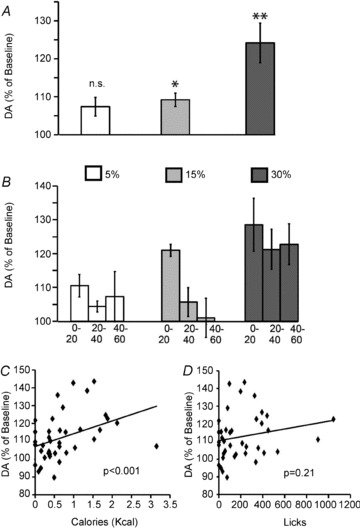
Relative changes in extracellular dopamine levels observed during the dry licking (compared to baseline measurements obtained previous to the initiation of behavioural performance) were determined by performing brain microdialysis concomitantly to the behavioural tasks (n = 5). A, a one-way repeated measures ANOVA did not reveal statistical evidence for emulsion concentration effects on the overall changes in dopamine levels (F2,6= 3.9, P = 0.08). One sample t tests against 100% (i.e. the null hypothesis of no changes with respect to baseline) revealed significant relative increases in dopamine levels during sessions in which animals self-infused the 15% (109.2 ± 1.7%, t3= 5.2, *P = 0.04 Bonferroni-corrected for multiple comparisons) and 30% (124.1%± 5.2, t4= 4.6 **P = 0.03) emulsions. However, during the 5% emulsion sessions no significant increases were detected (107.3 ± 2.4%, t4= 3.0, P = 0.09 corrected). B, experimental sessions were split into 20 min intervals, and relative changes in dopamine levels analysed accordingly. A two-way (emulsion concentration × time interval) repeated measures ANOVA model revealed no overall effects of concentration (F2,6= 3.9, P = 0.08) or interaction (F4,12= 0.6, P = 0.64) on relative changes in dopamine levels. However significant time-dependent effects on dopamine release were observed (F2,6= 14.8, P = 0.005) – with time-dependent attenuation in dopamine responses being detected during the sessions employing the 5% and 15%, but not the 30%, emulsions. C, scatter plot and linear fit displaying the relationship between caloric intake (at every 20 min interval recorded) and relative changes in striatal dopamine levels (as observed at every 20 min interval). Mixed model regression analyses reveal that caloric intake is highly predictive of relative changes in dopamine levels (P < 0.001). D, however, no such association was observed between dry licks produced and relative changes in dopamine levels (P = 0.21).
We then inquired whether the relative changes in dopamine levels were time dependent. We performed further analyses upon splitting total session duration into three equal intervals, each lasting 20 min. A two-way (emulsion concentration × time interval) repeated measures ANOVA model revealed no overall effects of concentration or interaction on relative changes in dopamine levels – an effect we ascribe to the overall increases in dopamine levels likely to be associated with the tendency of the animals to compensate for caloric dilutions. However, we did observe a significant main effect of time interval on dopamine levels (see Fig. 4B). In fact inspection of Fig. 4B reveals that, in sessions employing the 5% and 15% emulsions, dopamine release seems to have attenuated towards the last interval of the sessions. However, and rather interestingly, this pattern was not observed in the sessions employing the 30% emulsions. This led us to hypothesize that dopamine levels may have increased according to the actual amounts of calories ingested during each time interval.
To test the above hypothesis we designed a linear regression model using the relative changes in dopamine levels (at every 20 min interval) as the dependent variable and the corresponding measurements of caloric intake (in kcal) and dry licks as independent variables. These analyses were intended to account for mouse/session-specific variations in calorie intake that were independent of emulsion concentration. We found that caloric intake was strongly predictive of changes in dopamine levels (Fig. 4C). However, and rather strikingly, we found no significant associations between the corresponding numbers of dry licks and relative changes in dopamine levels (Fig. 4D), a result that in principle eliminates the possibility that purely motoric effects accounted for dopamine release during the dry licking tasks. A similar pattern was observed when the corresponding infusate volumes were entered in the linear regression model: as expected from the above, we found no significant associations between volumes infused (at every 20 min interval) and the corresponding relative changes in dopamine levels.
The above results indicate that while extracellular dopamine levels in dorsal striatum increase in proportion to the caloric density of intra-gastric infusates, this effect did not depend on flavour perception, motor-related aspects of licking, or actual volumes infused.
Extracellular levels of serotonin in striatum do not reflect caloric intake but are suppressed by low-calorie infusions
We next assessed the extent to which the calorie-dependent fluctuations in transmitter release are specific to the dopaminergic system. Accordingly, we have analysed the relative changes in extracellular levels of serotonin – another major monoamine neurotransmitter involved in food intake (Heisler et al. 2003) – based on the same samples used to assess changes in dopamine levels described above (see also Methods). As above, we first analysed the relative changes in extracellular serotonin levels observed during the entire (1 h) dry licking task. A one-way repeated measures ANOVA revealed emulsion concentration effects on the overall changes in serotonin levels. Strikingly, one sample t tests against 100% (i.e. against the null hypothesis of no change with respect to baseline) revealed significant relative decreases in serotonin levels during sessions in which animals self-infused the 5% emulsions (34.8 ± 6.0%, Fig. 5A); however, no similar effects were found for those sessions in which animals self-infused either the 15% (77.8 ± 21.1%) or 30% (111.7 ± 10.5%) emulsions (Fig. 5A).
Figure 5. Extracellular levels of serotonin in striatum do not reflect caloric intake but are suppressed by low-calorie infusions.
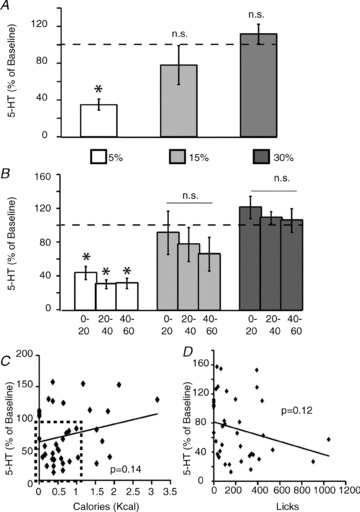
Relative changes in extracellular serotonin levels observed during the dry licking task above were determined from the same microdialysis samples used to analyse the changes in dopamine efflux shown in Fig. 4. A, a one-way repeated measures ANOVA revealed emulsion concentration effects on the overall changes in serotonin levels (F2,6= 8.1, P = 0.02). One sample t tests against 100% (i.e. the null hypothesis of no changes with respect to baseline) revealed significant relative decreases in serotonin levels during sessions in which animals self-infused the 5% (t4=−10.8, *P < 0.003 Bonferroni-corrected for multiple comparisons) emulsions. However, no significant increases were detected during either the 5% (t3=−1.0, P = 0.37) or the 15% (t4= 1.11, P = 0.32) emulsion sessions. B, experimental sessions were split into 20 min intervals, and relative changes in serotonin levels analysed accordingly. A two-way (emulsion concentration × time interval) repeated measures ANOVA model revealed significant effects of concentration (F2,6= 8.1, P = 0.02) and time (F2,16= 6.7, P = 0.03), but no interaction (F4,12= 0.57, P = 0.68), on relative changes in serotonin levels. Significant time-dependent effects on serotonin release were associated with robust suppressions in serotonin responses detected during the sessions employing the 5% (all t4≤–7.3, *P < 0.02 corrected), but not the 15% or 30%, emulsions (all P > 0.18). C, scatter plot and linear fit displaying the relationship between caloric intake (at every 20 min interval recorded) and relative changes in striatal serotonin levels (as observed at every 20 min interval). Linear regression analyses reveal no statistical evidence to suggest that serotonin levels were associated with changes in either caloric intake (P = 0.14, left panel) or numbers of dry licks (P = 0.12, right panel). Note in left panel that 5-HT levels fall abruptly for low-calorie values (highlighted as dash-bordered rectangle in scatter plot). 5-HT = serotonin.
We then inquired whether the relative changes in serotonin levels were time dependent. As above, total session duration was divided into three equal intervals, each lasting 20 min. A two-way (emulsion concentration × time interval) repeated measures ANOVA model revealed significant effects of concentration and time, but no interaction, on relative changes in serotonin levels (Fig. 5B). As expected, the effects were accounted for by significant time-related suppressions of serotonin levels during the sessions where animals self-infused the 5% emulsions (see Fig. 5B). In fact, whereas serotonin levels were found to continuously decrease during the 5% emulsion sessions, no statistical evidence supported effects associated with the 15% and 30% emulsions sessions (Fig. 5B).
To further confirm that extracellular serotonin levels, unlike the case for dopamine, did not change in response to calorie intake, we designed a linear regression model, as above, using the relative changes in serotonin levels (at every 20 min interval) as the dependent variable and the corresponding measurements of caloric intake (in kcal) and dry licks as independent variables. We found that serotonin levels were not associated with changes in either caloric intake (Fig. 5C) or numbers of dry licks (Fig. 5D). Our results support the idea that calorie-dependent transmitter efflux is not a ubiquitous finding, despite the robust suppressions in serotonin levels associated with self-infusions of lower calorie emulsions.
Extracellular levels of dopamine in dorsal and ventral striatum reflect caloric density during passive intra-gastric infusions of fat emulsions
Next, we aimed at confirming the above results by analysing changes in striatal dopamine levels during passive intra-gastric infusions of emulsions prepared at different caloric densities. This would allow us to determine whether the increases in dopamine levels described above depended on mice being actively behaving during the dry licking sessions. Accordingly, we designed an experiment where 0.6 ml infusions of highly caloric (at 30%, equivalent to a 1.8 kcal load) and less caloric (at 5%, equivalent to 0.45 a kcal load) emulsions were compared in their effects on dopamine release when intra-gastrically infused in mice food-deprived for 24 h. Baseline levels were obtained immediately previous to starting the 0.6 ml infusions (steadily administered at 0.1 ml min−1).
Consistent with our data obtained from behaving animals, calorie density heavily influenced dopamine release in dorsal striatum during passive intra-gastric infusions. In fact dopamine levels increased according to the emulsions’ caloric density (109.3 ± 3.1% with respect to baseline for 30% compared to 93.5 ± 3.2%, for 5%, Fig. 6A).
Figure 6. Extracellular levels of dopamine in striatum reflect caloric density during passive intra-gastric infusions of fat emulsions.
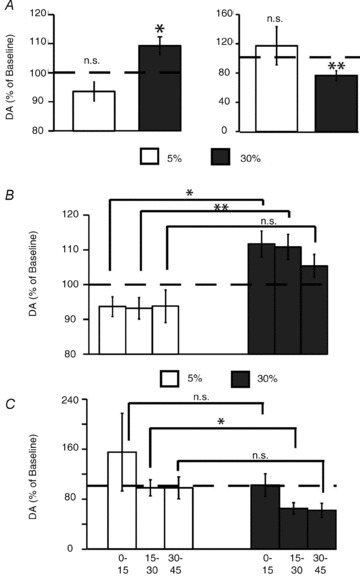
A, in dorsal striatum, intra-gastric infusions of highly caloric 30% emulsions produced significantly higher increases in dopamine levels (with respect to baseline) compared to the less caloric 5% infusions (n = 7, within-subject difference of 15.7 ± 3.6% in dopamine release favouring the more caloric emulsions was observed, paired t test t6= 4.3, *P < 0.01). In the nucleus accumbens of ventral striatum (n = 6), intra-gastric infusions of highly caloric 30% emulsions produced significant decreases in dopamine levels (with respect to baseline) compared to the less caloric 5% infusions (one sample t test t5= 3.5, **P < 0.02). No statistical evidence for similar effects associated with the less caloric 5% emulsions were found (t5= 0.65, P = 0.5, see right panel). B, a two-way (emulsion concentration × time interval) repeated measures ANOVA revealed strong effects of concentration (F1,6= 19.0, P = 0.005), but not of time interval (F2,12= 1.2, P = 0.32) or interaction (F2,12= 1.4, P = 0.26), on relative changes in dopamine levels in dorsal striatum. Post hoc within-subjects comparisons reveal that infusions of 30% emulsions resulted in significantly higher levels of dopamine compared to infusions of 5% emulsions during the 0–15 and 15–30 min (*P < 0.02 and **P < 0.01 Bonferroni-corrected, respectively), but not during the 30–45 min (P = 0.17, n.s. = non-significant effects), intervals. C, ANOVAs revealed no statistical evidence for concentration (F1,5= 2.0, P = 0.2), time interval (F2,10= 2.6, P = 0.12), or interaction (F2,10= 0.7, P = 0.92) effects on relative changes in dopamine levels in ventral striatum. Post hoc within-subjects comparisons reveal that infusions of 30% emulsions resulted in significantly higher levels of dopamine compared to infusions of 5% emulsions during the 15–30 min interval (paired t test *P < 0.04 Bonferroni-corrected), but not during the 0–15 or 30–45 min intervals (P ≥ 0.08, n.s. = non-significant effects).
We have also analysed the changes in dopamine efflux in ventral aspects of the striatum. Similar experiments to those above were performed in an additional group of mice, but this time the microdialysis probes were implanted in the nucleus accumbens region of the ventral striatum. As for the dorsal striatum case, calorie density modulated dopamine release in ventral striatum during the passive intra-gastric infusions. However, and rather unexpectedly, the more caloric 30% emulsions produced significant decreases in dopamine levels with respect to baseline (76.6 ± 6.5%) compared to no effects for the less caloric 5% emulsions (117.2 ± 26.1%, see Fig. 6A right panel). We also analysed the extent to which both findings were time dependent. We have assessed the relative changes in dorsal striatum dopamine levels observed at 15 min intervals, starting at infusion onset. A two-way (emulsion concentration × time interval) repeated measures ANOVA revealed strong effects of concentration, but not of time interval, or interaction, on relative changes in dopamine levels. In fact, infusions of 30% emulsions resulted in higher levels of dopamine release compared to 5% emulsions during the first and second time intervals (whereas they did not differ during the third time interval, Fig. 6B). For the ventral striatum case, similar ANOVAs revealed no statistical evidence for concentration (F1,5= 2.0, P = 0.2), time interval (F2,10= 2.6, P = 0.12), or interaction (F2,10= 0.7, P = 0.92) effects on relative changes in dopamine levels. Infusions of 30% emulsions resulted in lower levels of dopamine release compared to the 5% emulsions only during the second time interval (since they did not differ during the other time intervals, Fig. 6C). Taken together, the results above demonstrate that even during passive intra-gastric infusions of fat emulsions, dopamine levels seem to change in accordance to the amounts of calories being ingested.
We conclude by stressing the remarkably complementary patterns of dorsal striatal dopamine release associated with the behavioural vs. the passive microdialysis sessions. In fact, during active behavioural sessions – when animals achieve caloric compensation via intra-gastric infusions interspersed throughout the sessions – significant effects of time interval, but not of emulsion concentration, were observed on dopamine release. Conversely, during passive intra-gastric infusions – when animals cannot calorically compensate for the temporally steady intra-gastric infusions – significant effects of emulsion concentration, but not of time interval, were observed on dopamine release.
Final probe locations in dorsal and ventral striatum were verified histologically in all animals used and actual locations are shown in Supplementary Fig. 1.
Gastric emptying rates differed as a function of infusate caloric density
Because caloric density is known to regulate gastric emptying, we assessed the potential effects of passively infusing 5%vs. 30% emulsions on gastric emptying. The passive infusions were performed as in the microdialysis experiments above. We assessed gastric emptying at 15 min post-infusion since this interval was sufficient to induce significant calorie-density-dependent increases in striatum dopamine levels. As expected we found that infusions of 30% emulsions significantly slowed gastric emptying compared to 5% infusions (15.5 ± 2.4%vs. 24.8 ± 1.4% of infused contents emptied after 15 min respectively, paired two-sample t test t5= 3.6, P = 0.014). Therefore, gastric emptying rates differed as a function of caloric density concomitantly to differential dopamine release after infusions of 5%vs. 30% emulsions (see Discussion for further considerations).
Intra-gastric infusions of control solutions do not elicit dopamine release
We also performed a separate control experiment where we assessed whether passive intra-gastric infusions of the control (emulsifying) solution would be sufficient to induce significant dopamine release in striatum. Note that this control solution contains 1.2% phospholipids + 1.7% glycerine but not fat from soy bean oil. These infusions were compared against the effects produced by those of our intermediary oily emulsion, i.e. at 15%. Mice (n = 6) were infused as in the experiment above, and results reveal a within-subject increase in dopamine levels in favour of the 15% emulsion of 121.8 ± 7.5%, a change found to be significant (t5= 2.8, P < 0.04). In fact, control infusions produced significant decreases in dopamine levels with respect to baseline (81.9 ± 6.9%, t5=−2.6, P < 0.05). This result indicates that the low-caloric emulsifying components of fat emulsions do not account for increases in dopamine levels in previous experiments.
Dopamine receptor blocking disrupts caloric sensing in the absence of flavour cues
Finally, based on our results so far, we hypothesized that inhibition of dopamine signalling should impair caloric sensing. We specifically hypothesized that under the pharmacological influence of dopamine blockers, mice should treat the infused solutions as having lower caloric densities than they actually do. In other words, we hypothesized that mice would increase the numbers of dry licks to obtain infusions of caloric fat emulsions when treated with a dopamine receptor blocker.
Animals were tested daily on their dry licking responses to obtain 30% emulsion infusions after an intraperitoneal dose of either haloperidol or vehicle injected 45 min previous to initiating the experimental sessions. For the ‘neuroleptic’ condition (continuous line in Fig. 7A), mice were initially tested on two vehicle sessions, followed by four consecutive haloperidol sessions and then by four additional vehicle sessions. The same group of mice were subjected to an additional ‘control’ condition (dashed line in Fig. 7A), where animals received only vehicle for 10 sessions (one mouse was discarded due to catheter deficiency). This protocol was based on previous work performed in rats self-administering cocaine, where repeatedly injecting moderate doses of the haloperidol was shown to minimize potential motoric confounds (Roberts & Vickers, 1987).
Figure 7. Dopamine receptor blocking disrupts caloric sensing in the absence of flavour cues.
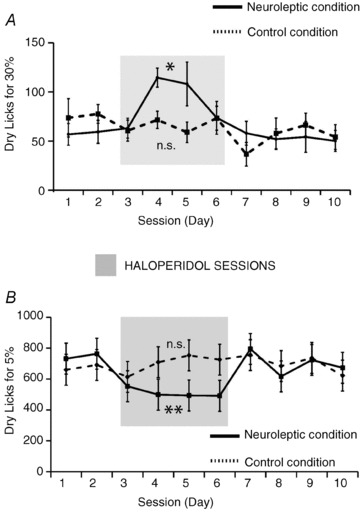
A, mice (n = 7) were tested daily on their dry licking responses to obtain 30% emulsion infusions after an intraperitoneal dose of either haloperidol or vehicle. In the ‘neuroleptic’ condition (continuous line), mice were initially tested on 2 vehicle sessions, followed by 4 consecutive haloperidol sessions and then on further additional 4 vehicle sessions. The same group of mice were subjected to an additional ‘control’ condition (dashed line) where they received only vehicle for 10 sessions. Analyses of the number of dry licks produced during the daily higher calorie 30% emulsion sessions reveal a significant effect of session day on dry licks for the neuroleptic (one-way repeated measures ANOVA F9,45= 2.4, *P = 0.02), but not for the vehicle (F9,45= 1.1, P = 0.38) sessions. In the neuroleptic condition, session 4 was associated with significant increases in the number of dry licks compared to all vehicle sessions but not to the subsequent haloperidol sessions. This session was also significantly different from its corresponding session in the control condition (P < 0.04 corrected). B, a different group of mice (n = 9) were tested on the same task as above but where animals dry-licked to self-infuse the lower calorie 5% emulsions. Analyses of the number of dry licks produced during the daily lower calorie 5% emulsion sessions reveal a significant effect of session day on dry licks for the neuroleptic (one-way repeated measures ANOVA F9,72= 2.2, **P = 0.03, continuous line in Fig. 7B), but not for the vehicle (F9,72= 0.9, P = 0.5, dotted line; n.s. = non-significant effects, HAL = haloperidol, VEH = vehicle) sessions. Darkened areas represent periods in which animals in neuroleptic condition were treated with haloperidol.
Analyses of the number of dry licks produced during the daily higher calorie 30% emulsion sessions reveal a significant effect of session day on dry licks for the neuroleptic, but not for the vehicle (Fig. 7A), condition. Note the overall increase in number of dry licks associated with the haloperidol injections (Fig. 7A), a behavioural response not unlike the one to be expected had the calorie density of the infusate been diluted. Now, if our interpretation that dopamine signalling is mediating central calorie sensing is correct, one should expect that, if lower calorie infusions are used instead, haloperidol should produce a decrease in the overall numbers of dry licks, the alternative hypothesis stating that generalized increases in dry licking should follow the neuroleptic treatment. Accordingly, we have performed the same experiment as above on a separate group of mice, but this time using the lower calorie 5% emulsions instead of the higher calorie 30% emulsions. Analyses of the number of dry licks produced during the daily lower calorie 5% emulsion sessions reveal a significant effect of session day on dry licks for the neuroleptic (continuous line in Fig. 7B), but not for the vehicle (dotted line in Fig. 7B), condition. In fact, we found that the neuroleptic condition was associated with overall lower numbers of dry licks compared to its equivalent sessions in the control condition (see Fig. 7B).
Altogether, the above results indicate that inhibiting dopamine signalling by injecting the dopamine receptor blocker haloperidol produces significant increases in dry licking to obtain intra-gastric infusions of higher calorie fat emulsions and significant decreases in dry licking to obtain intra-gastric infusions of lower calorie fat emulsions. We interpret this behavioural pattern as resulting from disrupted calorie sensing in the absence of flavour cues. More generally, we found that inhibiting dopamine receptor signalling disrupted the animals’ ability to maintain constant caloric intake across the daily experimental sessions.
Discussion
We have shown that, within limits, mice are capable of precisely regulating calorie intake even in the absence of flavour cues, by controlling intra-gastric infusions upon licking a dry sipper. The abovementioned limits relate to the actual caloric densities of the infusions, with caloric loads below certain ranges exerting relatively weak influence on the animal's behaviour. Generally, caloric regulation occurred independently of infusate volume or meaningful changes in the animals’ body weight (because caloric regulation was achieved during sessions lasting one hour). Most importantly, our microdialysis data strongly suggest that brain dopamine systems may function as the central mediators of these regulatory responses. In fact, extracellular striatal dopamine levels increased in proportion to infusate caloric density; in addition, inhibiting dopamine receptor signalling disrupted the animals’ ability to maintain constant caloric intake across experimental sessions.
In human adults as well as in most animals, body weight in maintained within exquisitely narrow ranges (Fox, 1973; Friedman, 2004), with age-related annual weight gain being equivalent to less than 0.2% of total energy intake (Weigle, 1994). Accordingly, the ability to adjust food intake in response to variations in caloric density, termed ‘caloric compensation’, has been long established, including seminal work by C. Richter (1941, 1943) and E. Adolph (1947), later extended to a number of vertebrate species (Owens & Ridgman, 1968; Dinius & Baumgardt, 1970; Peterson & Baumgardt, 1971; Booth, 1972; McHugh et al. 1975; Smutz et al. 1975; McHugh & Moran, 1978; Hansen et al. 1981) including humans (Campbell et al. 1971; Wooley et al. 1972). It is important to note that previous studies did evaluate the ability of rats to regulate food intake via intra-gastric feeding (Epstein & Teitelbaum, 1962; Epstein, 1967; Snowdon, 1969; Snowdon & Epstein, 1970). These earlier studies demonstrated that adult rats do, to some extent, regulate feeding in the absence of oropharingeal sensations. However, the overall results from these studies indicate that rats either failed to precisely regulate caloric intake (Snowdon, 1969; Snowdon & Epstein, 1970) or that they did so upon significant variations in body weight (at least for the one rat data shown, Epstein & Teitelbaum, 1962). It is nevertheless remarkable that both rodents (Richter, 1941, 1943; Booth, 1972) and monkeys (McHugh et al. 1975; McHugh & Moran, 1978) display the capacity to compensate for intra-gastric caloric passive preloads by appropriately reducing subsequent oral feeding, thereby achieving calorie intake regulation (a capacity found to be impaired in NT-4 knockout mice, Chi & Powley, 2007). Our results extend previous reports by providing the first demonstration that animals are capable of rapidly and precisely regulating calorie intake in the absence of oropharingeal sensations throughout the entire feeding episode, and in ways that were independent of infusate volume and/or changes in body composition. In particular, our findings expand the currently known repertoire of behavioural functions associated with the detection of post-ingestive physiological cues (Sclafani, 2001). Importantly, we also stress that satiety alone is unlikely to have accounted for caloric compensation, given the ability of these animals to regulate sipper choice according caloric density (see Ackroff & Sclafani, 2006).
In any event a principal finding of our studies regards a seemingly critical function exerted by brain dopamine systems, namely to detect to the amounts of calories infused using internal physiological cues. This is strongly suggested by our finding that dopamine efflux in dorsal striatum was significantly associated with the amounts of calories ingested, even more so than with the corresponding motor responses (i.e. dry licks) performed to obtain the caloric intra-gastric infusions. Regulation of dry licking according to caloric load is consistent with the fact that striatal dopamine signalling is critical for the control of instrumental responding (Jentsch et al. 2000), including the control of the vigor with which instrumental responses are produced (Salamone et al. 2007). Additionally, striatal dopamine efflux during instrumental tasks has been linked to the representation of the actual costs and benefits of responding (Day et al. 2010; Gan et al. 2010), a property that may be modulated by shifts in motivational state such as those produced by satiation (Ostlund et al. 2011).
It is unlikely, however, that the calorie-dependent dopamine efflux observed in our own study was under the control of factors such as response vigour, cost/benefit of responding, or shifts in motivational state. First, during active (dry licking) behaviour, whereas microdialysis measurements of striatal extracellular dopamine were robustly associated with ingested calories, they did not bear associations with the number of dry licks produced. These observations suggest that response cost (in our case the number of dry licks required to achieve a certain caloric load) or task requirements were not the primary drivers of dopamine release. This is further confirmed by the fact that calorie-dependent dopamine efflux was observed in animals passively receiving intra-gastric infusions of fat emulsions. Importantly, the results from experiments using passive infusions rule out the possibility that dopamine release was primarily driven by sensory stimuli predictive of the infusions, such as the dry sippers (although dopamine release produced by predictive sensory cues alone are not inconsistent with our data and should not be excluded a priori). Results from the same experiment also rule out a dominant role for motivational shift since dopamine responses to intra-gastric infusions reflected caloric density even when motivational state was fixed. We also note that calorie-dependent dopamine efflux was not, in principle, primarily related to encoding the incentive salience (Berridge, 2007) of task-related stimuli since mice significantly increased dry licking to obtain caloric infusions when pretreated with dopamine receptor blockers (an effect that we otherwise interpreted as indicative of disrupted calorie sensing). On the other hand, given that our results also showed that mice do develop preferences for dry sippers associated with the more caloric infusions, it becomes plausible to hypothesize that such dopaminergic sensitivity to caloric load may underlie the mechanisms through which striatal dopamine exerts its critical role in food reinforcement (Wise, 2006).
Calorie-dependent dopamine efflux may also underlie some of the limitations associated with the animals’ ability to regulate caloric compensation. More specifically, mice were not able to behaviourally compensate when licking for low-calorie emulsifying solutions that did not contain fatty oils. Consistently, dopamine concentrations actually decreased to levels below baseline following passive intra-gastric infusions of the low-calorie emulsifiers. Therefore, intra-gastric infusions of nutrients having caloric loads equal to or below certain values may impose physiological limits on the ability to compensate for caloric dilution, limits that are ultimately accompanied by weak dopamine efflux. Once again, extracellular dopamine levels seemingly increase in direct proportion to the reinforcement efficacy of the substance being actively self-infused by the animal (Ranaldi et al. 1999).
In addition to dopamine, we concomitantly measured efflux of serotonin, another monoamine transmitter critically involved in the regulation of appetite (Heisler et al. 2003; Zhou et al. 2007). We have not found statistical evidence for strong associations between serotonin levels and calorie intake. Such results indicate that calorie-dependent release should not be a priori attributed to other major transmitter systems involved in food intake. We do note, however, that while serotonin levels remained relatively stable during those sessions in which mice dry-licked to obtain the 15% or 30% infusions, we observed striking decreases in serotonin efflux when mice dry-licked to obtain the lower calorie 5% infusions. Therefore, rather than increasing extracellular levels in response to calorie ingestion as in the case of dopamine, central serotonin systems were strongly suppressed by the low-calorie, but not by intermediate- or high-calorie, infusions. Our data therefore depict an intriguing dissociation between dopamine and serotonin signalling, with the concept that serotonergic neurons may function as detectors of low-calorie infusates warranting future investigations.
Our decision to sample extracellular dopamine levels from dorsal striatum is also consistent with the established role of this region in both animal (Sotak et al. 2005; Palmiter, 2007) and human (Small et al. 2003; Malik et al. 2008) feeding behaviours. While seminal work by H. Grill and colleagues (Grill & Norgren, 1978; Kaplan et al. 1993) demonstrates that brainstem circuits are sufficient to produce satiation in decerebrate rats, such ability may be partially mediated by brainstem taste relays (Roper, 2006) that in principle are not under the direct control of dopamine neurons. Consistently, it is noticeable that genetically engineered dopamine-deficient mice retain the ability to prefer sucrose over water (Cannon & Palmiter, 2003) while being unable to sustain appropriate levels of feeding. Therefore, while taste-based ingestion may be preserved in the absence of forebrain dopamine tone, normal feeding behaviours are not. Because we report in our study the disruption of regulatory behaviours that do not depend on flavour perception, we believe our data actually contribute to strengthen our previous propositions that dopamine acts to regulate feeding via flavour-independent pathways (de Araujo et al. 2008; Ren et al. 2010). In addition, we also assessed changes in dopamine efflux in the nucleus accumbens of the ventral striatum, a brain circuit critically involved in appetite regulation and reward (Baldo & Kelley, 2007). We have found that accumbal dopamine levels also fluctuated in response to the caloric densities of the infusates; however, whereas accumbal dopamine was actually suppressed by the more caloric emulsions, it remained stable after infusions of less caloric emulsions. These results are reminiscent of our own previous findings showing that while intra-gastric infusions of l-amino acids significantly decreased accumbal dopamine release, sugar infusions did not (Ren et al. 2010), suggesting nutrient-specific dopaminergic coding in nucleus accumbens. Our results are also consistent with recent reports that the stomach orexigenic peptide ghrelin, whose secretion is inhibited by nutrient ingestion, acts centrally to induce dopamine release in accumbens (Abizaid et al. 2006; Jerlhag et al. 2010). Future research must determine the circuit mechanisms associated with this singular dissociation between dorsal and ventral striatum in response to calorie intake.
Our microdialysis data raise the more general question of how dopamine cells may sense the caloric density of the infusions being delivered to the gastrointestinal tract. Both pre- and post-absorptive pathways may be involved in mediating caloric sensing in dopamine cells. One strong candidate mechanism associated with pre-absorptive signals relates to the precise control exerted by caloric density on gastric emptying rates. Once again, pioneering work by P. McHugh, T. Moran, G. Schwartz and colleagues (McHugh & Moran, 1979; McHugh, 1983) as well as by Kaplan, Grill and colleagues (Kaplan et al. 1992; Kaplan et al. 1997), and Covasa, Ritter and colleagues (Covasa et al. 2000) clearly demonstrated that gastric emptying rate decreases in proportion to the caloric density of gut infusates. We have confirmed these previous observations and verified that infusions of 30% emulsions significantly reduced gastric emptying compared to 5% emulsions, placing calorie-regulated gastric distention as one candidate signal mediating the calorie-depending release of striatal dopamine. Furthermore, given the link between the intestinal peptide cholecystokinin and gastric emptying (Schwartz et al. 1993), it is also plausible to consider that the observed increases in dopamine levels may have been mediated by this gut factor, which is released in direct proportion to the amounts of lipids ingested (Beglinger & Degen, 2004) and may alter extracellular dopamine levels (Feifel et al. 2003) via currently unknown pathways. Alternatively, dopamine cells may also be modulated by lipid-sensing molecules present in the small intestine (Schwartz, 2011). From a behavioural standpoint, a role for pre-absorptive signals is reinforced by the established role of intestinal vagal afferents in mediating both fat-induced satiation (Sclafani et al. 2003) and the appetitive properties of dietary nutrients (Kondoh & Torii, 2008; Akiba & Kaunitz, 2011).
Favouring the possibility that post-absorptive signals modulate dopamine release are our own previous findings that dopamine efflux is disrupted following inhibition of glucose oxidation by systemic 2-deoxyglucose injections (Ren et al. 2010). In fact, and rather remarkably, 2-deoxyglucose injections lead to increases in numbers of licks for sugars in sweet-blind mutant mice, as if the caloric density of the glucose solution had been diluted – i.e. mice react to glucose oxidation inhibition in ways similar to those displayed after haloperidol injections in our dry licking task. This is consistent with previous findings demonstrating the existence of glucose sensing mechanisms in dopaminergic neurons of the substantia nigra (Levin, 2000). In this regard, it is of interest that post-absorptive control over dopamine release may also be triggered by pre-absorptive signals, given that lipid sensing in the upper intestine activates a brain–liver axis which ultimately regulates glucose homeostasis (Wang et al. 2008; Lam, 2010). In addition to intracellular nutrient utilization (Horvath et al. 2009), post-absorptive hormonal signals such as insulin (Figlewicz, 2003) and leptin (Figlewicz, 2003; Fulton et al. 2006; Hommel et al. 2006) may also influence dopamine activity upon fat or sugar intake. Lastly, different brain circuits may provide a link between nutrient sensing and dopamine cell activation. Hypothalamic projection fibres into midbrain dopamine cells (Zheng et al. 2007) may constitute one such circuit, as well as the forebrain-projecting catecholamine neurons that function as monitors of metabolic state (Salter & Watts, 2003; Watts & Donovan, 2009).
Finally, we note that our findings obtained with treating animals with the neuroleptic haloperidol point to a novel role for the D2-like class of dopamine receptors in food intake, namely, as mediators of calorie sensing. More specifically, our findings that haloperidol treatment led to increased dry licking to obtain higher calorie infusions but to decreased dry licking to obtain lower calorie infusions suggest that dopamine receptor downregulation, as is observed in both human and animal models of obesity (Wang et al. 2001; Volkow et al. 2008; Johnson & Kenny, 2010), may be rather associated with disrupted calorie sensing than with compensatory responses to decreased reward sensitivity. The concept that dopamine signalling via the D2-like class of receptors functions to allow central calorie sensing warrants further investigations on the currently unknown mechanisms linking dopamine alterations and obesity.
In summary, we have shown that adult mice are capable of adjusting consummatory behaviour to maintain constant hourly caloric intake. Importantly, this effect was mediated by brain dopamine systems, which seemingly function as central calorie monitors. Future research must establish the peripheral signalling pathways allowing brain dopamine systems to ‘count’ calories during active ingestive behaviours.
Acknowledgments
This work was supported by NIH grant DC009997 to I.E.A.
Author contributions
All experiments were performed at The J. B. Pierce Laboratory (Neurobiology of Feeding laboratory). All authors contributed to the design, collection, analysis and interpretation of the data. All authors approved the final manuscript.
Supplementary Material
Supplementary Figure 1
Supplemental Table 1
As a service to our authors and readers, this journal provides supporting information supplied by the authors. Such materials are peer-reviewed and may be re-organized for online delivery, but are not copy-edited or typeset. Technical support issues arising from supporting information (other than missing files) should be addressed to the authors
References
- Abizaid A, Liu ZW, Andrews ZB, Shanabrough M, Borok E, Elsworth JD, Roth RH, Sleeman MW, Picciotto MR, Tschop MH, Gao XB, Horvath TL. Ghrelin modulates the activity and synaptic input organization of midbrain dopamine neurons while promoting appetite. J Clin Invest. 2006;116:3229–3239. doi: 10.1172/JCI29867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ackroff K, Sclafani A. Energy density and macronutrient composition determine flavor preference conditioned by intragastric infusions of mixed diets. Physiol Behav. 2006;89:250–260. doi: 10.1016/j.physbeh.2006.06.003. [DOI] [PubMed] [Google Scholar]
- Adolph EF. Urges to eat and drink in rats. Am J Physiol. 1947;151:110–125. doi: 10.1152/ajplegacy.1947.151.1.110. [DOI] [PubMed] [Google Scholar]
- Akiba Y, Kaunitz JD. Luminal chemosensing in the duodenal mucosa. Acta Physiol (Oxf) 2011;201:77–84. doi: 10.1111/j.1748-1716.2010.02149.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Almeida RL, David RB, Constancio J, Fracasso JF, Menani JV, De Luca LA., Jr Inhibition of sodium appetite by lipopolysaccharide: involvement of α2-adrenoceptors. Am J Physiol Regul Integr Comp Physiol. 2011;301:R185–R192. doi: 10.1152/ajpregu.00555.2009. [DOI] [PubMed] [Google Scholar]
- Baldo BA, Kelley AE. Discrete neurochemical coding of distinguishable motivational processes: insights from nucleus accumbens control of feeding. Psychopharmacology (Berl) 2007;191:439–459. doi: 10.1007/s00213-007-0741-z. [DOI] [PubMed] [Google Scholar]
- Beglinger C, Degen L. Fat in the intestine as a regulator of appetite – role of CCK. Physiol Behav. 2004;83:617–621. doi: 10.1016/j.physbeh.2004.07.031. [DOI] [PubMed] [Google Scholar]
- Berridge KC. The debate over dopamine's role in reward: the case for incentive salience. Psychopharmacology. 2007;191:391–431. doi: 10.1007/s00213-006-0578-x. [DOI] [PubMed] [Google Scholar]
- Berthoud HR. Multiple neural systems controlling food intake and body weight. Neurosci Biobehav Rev. 2002;26:393–428. doi: 10.1016/s0149-7634(02)00014-3. [DOI] [PubMed] [Google Scholar]
- Booth DA. Satiety and behavioral caloric compensation following intragastric glucose loads in the rat. J Comp Physiol Psychol. 1972;78:412–432. doi: 10.1037/h0032291. [DOI] [PubMed] [Google Scholar]
- Campbell RG, Hashim SA, Van Itallie TB. Studies of food-intake regulation in men. Responses to variations in nutritive density in lean and obese subjects. N Engl J Med. 1971;285:1402–1407. doi: 10.1056/NEJM197112162852504. [DOI] [PubMed] [Google Scholar]
- Cannon CM, Palmiter RD. Reward without Dopamine. J Neurosci. 2003;23:10827–10831. doi: 10.1523/JNEUROSCI.23-34-10827.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chi MM, Powley TL. NT-4-deficient mice lack sensitivity to meal-associated preabsorptive feedback from lipids. Am J Physiol Regul Integr Comp Physiol. 2007;292:R2124–2135. doi: 10.1152/ajpregu.00825.2006. [DOI] [PubMed] [Google Scholar]
- Covasa M, Ritter RC, Burns GA. NMDA receptor participation in control of food intake by the stomach. Am J Physiol Regul Integr Comp Physiol. 2000;278:R1362–1368. doi: 10.1152/ajpregu.2000.278.5.R1362. [DOI] [PubMed] [Google Scholar]
- Day JJ, Jones JL, Wightman RM, Carelli RM. Phasic nucleus accumbens dopamine release encodes effort- and delay-related costs. Biol Psychiatry. 2010;68:306–309. doi: 10.1016/j.biopsych.2010.03.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Araujo IE, Oliveira-Maia AJ, Sotnikova TD, Gainetdinov RR, Caron MG, Nicolelis MA, Simon SA. Food reward in the absence of taste receptor signaling. Neuron. 2008;57:930–941. doi: 10.1016/j.neuron.2008.01.032. [DOI] [PubMed] [Google Scholar]
- Dinius DA, Baumgardt BR. Regulation of food intake in ruminants. 6. Influence of caloric density of pelleted rations. J Dairy Sci. 1970;53:311–316. [Google Scholar]
- Elmquist JK, Coppari R, Balthasar N, Ichinose M, Lowell BB. Identifying hypothalamic pathways controlling food intake, body weight, and glucose homeostasis. J Comp Neurol. 2005;493:63–71. doi: 10.1002/cne.20786. [DOI] [PubMed] [Google Scholar]
- Epstein A( Feeding without oropharyngeal sensations. In: Kare MR, Maller O, editors. The Chemical Senses and Nutrition. Baltimore: The Johns Hopkins Press; 1967. pp. 263–280. [Google Scholar]
- Epstein A, Teitelbaum P. Regulation of food intake in the absence of taste, smell and other oropharyngeal sensations. J Comp Physiol Psychol. 1962;55:753–759. [Google Scholar]
- Feifel D, Shilling PD, Kuczenski R, Segal DS. Altered extracellular dopamine concentration in the brains of cholecystokinin-A receptor deficient rats. Neurosci Lett. 2003;348:147–150. doi: 10.1016/s0304-3940(03)00767-5. [DOI] [PubMed] [Google Scholar]
- Figlewicz DP. Adiposity signals and food reward: expanding the CNS roles of insulin and leptin. Am J Physiol Regul Integr Comp Physiol. 2003;284:R882–892. doi: 10.1152/ajpregu.00602.2002. [DOI] [PubMed] [Google Scholar]
- Fox FW. The enigma of obesity. Lancet. 1973;2:1487–1488. doi: 10.1016/s0140-6736(73)92745-1. [DOI] [PubMed] [Google Scholar]
- Friedman JM. Modern science versus the stigma of obesity. Nat Medicine. 2004;10:563– 569. doi: 10.1038/nm0604-563. [DOI] [PubMed] [Google Scholar]
- Fulton S, Pissios P, Manchon RP, Stiles L, Frank L, Pothos EN, Maratos-Flier E, Flier JS. Leptin regulation of the mesoaccumbens dopamine pathway. Neuron. 2006;51:811–822. doi: 10.1016/j.neuron.2006.09.006. [DOI] [PubMed] [Google Scholar]
- Gan JO, Walton ME, Phillips PE. Dissociable cost and benefit encoding of future rewards by mesolimbic dopamine. Nat Neurosci. 2010;13:25–27. doi: 10.1038/nn.2460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grill HJ, Norgren R. Chronically decerebrate rats demonstrate satiation but not bait shyness. Science. 1978;201:267–269. doi: 10.1126/science.663655. [DOI] [PubMed] [Google Scholar]
- Hajnal A, Smith GP, Norgren R. Oral sucrose stimulation increases accumbens dopamine in the rat. Am J Physiol Regul Integr Comp Physiol. 2004;286:R31–R37. doi: 10.1152/ajpregu.00282.2003. [DOI] [PubMed] [Google Scholar]
- Hansen BC, Jen K-L, Kribbs P. Regulation of food intake in monkeys: Response to caloric dilution. Physiol Behav. 1981;26:479–486. doi: 10.1016/0031-9384(81)90177-3. [DOI] [PubMed] [Google Scholar]
- Heisler LK, Cowley MA, Kishi T, Tecott LH, Fan W, Low MJ, Smart JL, Rubinstein M, Tatro JB, Zigman JM, Cone RD, Elmquist JK. Central serotonin and melanocortin pathways regulating energy homeostasis. Ann N Y Acad Sci. 2003;994:169–174. doi: 10.1111/j.1749-6632.2003.tb03177.x. [DOI] [PubMed] [Google Scholar]
- Hernandez L, Hoebel BG. Food reward and cocaine increase extracellular dopamine in the nucleus accumbens as measured by microdialysis. Life Sci. 1988;42:1705–1712. doi: 10.1016/0024-3205(88)90036-7. [DOI] [PubMed] [Google Scholar]
- Hommel JD, Trinko R, Sears RM, Georgescu D, Liu ZW, Gao XB, Thurmon JJ, Marinelli M, DiLeone RJ. Leptin receptor signaling in midbrain dopamine neurons regulates feeding. Neuron. 2006;51:801–810. doi: 10.1016/j.neuron.2006.08.023. [DOI] [PubMed] [Google Scholar]
- Horvath TL, Andrews ZB, Diano S. Fuel utilization by hypothalamic neurons: roles for ROS. Trends Endocrinol Metab. 2009;20:78–87. doi: 10.1016/j.tem.2008.10.003. [DOI] [PubMed] [Google Scholar]
- Jentsch JD, Roth RH, Taylor JR. Role for dopamine in the behavioral functions of the prefrontal corticostriatal system: implications for mental disorders and psychotropic drug action. Prog Brain Res. 2000;126:433–453. doi: 10.1016/S0079-6123(00)26028-7. [DOI] [PubMed] [Google Scholar]
- Jerlhag E, Egecioglu E, Dickson SL, Engel JA. Ghrelin receptor antagonism attenuates cocaine- and amphetamine-induced locomotor stimulation, accumbal dopamine release, and conditioned place preference. Psychopharmacology (Berl) 2010;211:415–422. doi: 10.1007/s00213-010-1907-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson PM, Kenny PJ. Dopamine D2 receptors in addiction-like reward dysfunction and compulsive eating in obese rats. Nat Neurosci. 2010;13:635–641. doi: 10.1038/nn.2519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaplan JM, Seeley RJ, Grill HJ. Daily caloric intake in intact and chronic decerebrate rats. Behav Neurosci. 1993;107:876–881. [PubMed] [Google Scholar]
- Kaplan JM, Siemers WH, Smedh U, Schwartz GJ, Grill HJ. Gastric branch vagotomy and gastric emptying during and after intragastric infusion of glucose. Am J Physiol Regul Integr Comp Physiol. 1997;273:R1786–1792. doi: 10.1152/ajpregu.1997.273.5.R1786. [DOI] [PubMed] [Google Scholar]
- Kaplan JM, Spector AC, Grill HJ. Dynamics of gastric emptying during and after stomach fill. Am J Physiol Regul Integr Comp Physiol. 1992;263:R813–819. doi: 10.1152/ajpregu.1992.263.4.R813. [DOI] [PubMed] [Google Scholar]
- Kondoh T, Torii K. Brain activation by umami substances via gustatory and visceral signaling pathways, and physiological significance. Biol Pharm Bull. 2008;31:1827–1832. doi: 10.1248/bpb.31.1827. [DOI] [PubMed] [Google Scholar]
- Lam TK. Neuronal regulation of homeostasis by nutrient sensing. Nat Med. 2010;16:392–395. doi: 10.1038/nm0410-392. [DOI] [PubMed] [Google Scholar]
- Levin BE. Glucose-regulated dopamine release from substantia nigra neurons. Brain Res. 2000;874:158–164. doi: 10.1016/s0006-8993(00)02573-7. [DOI] [PubMed] [Google Scholar]
- Liang NC, Hajnal A, Norgren R. Sham feeding corn oil increases accumbens dopamine in the rat. Am J Physiol Regul Integr Comp Physiol. 2006;291:R1236–1239. doi: 10.1152/ajpregu.00226.2006. [DOI] [PubMed] [Google Scholar]
- Malik S, McGlone F, Bedrossian D, Dagher A. Ghrelin modulates brain activity in areas that control appetitive behavior. Cell Metab. 2008;7:400–409. doi: 10.1016/j.cmet.2008.03.007. [DOI] [PubMed] [Google Scholar]
- McHugh PR. The control of gastric emptying. J Auton Nerv Syst. 1983;9:221–231. doi: 10.1016/0165-1838(83)90143-1. [DOI] [PubMed] [Google Scholar]
- McHugh PR, Moran TH. Accuracy of the regulation of caloric ingestion in the rhesus monkey. Am J Physiol Regul Integr Comp Physiol. 1978;235:R29–34. doi: 10.1152/ajpregu.1978.235.1.R29. [DOI] [PubMed] [Google Scholar]
- McHugh PR, Moran TH. Calories and gastric emptying: a regulatory capacity with implications for feeding. Am J Physiol Regul Integr Comp Physiol. 1979;236:R254–260. doi: 10.1152/ajpregu.1979.236.5.R254. [DOI] [PubMed] [Google Scholar]
- McHugh PR, Moran TH, Barton GN. Satiety: a graded behavioural phenomenon regulating caloric intake. Science. 1975;190:167–169. doi: 10.1126/science.1166310. [DOI] [PubMed] [Google Scholar]
- Ostlund SB, Wassum KM, Murphy NP, Balleine BW, Maidment NT. Extracellular dopamine levels in striatal subregions track shifts in motivation and response cost during instrumental conditioning. J Neuroci. 2011;31:200–207. doi: 10.1523/JNEUROSCI.4759-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Owens JP, Ridgman WJ. Further studies of the effect of dietary energy content on the voluntary intake of pigs. Anim Prod. 1968:10. [Google Scholar]
- Palmiter RD. Is dopamine a physiologically relevant mediator of feeding behavior? Trends Neurosci. 2007;30:375–381. doi: 10.1016/j.tins.2007.06.004. [DOI] [PubMed] [Google Scholar]
- Peterson AD, Baumgardt BR. Food and energy intake of rats fed diets varying in energy concentration and density. J Nutr. 1971;101:1057–1067. doi: 10.1093/jn/101.8.1057. [DOI] [PubMed] [Google Scholar]
- Ranaldi R, Pocock D, Zereik R, Wise RA. Dopamine fluctuations in the nucleus accumbens during maintenance, extinction, and reinstatement of intravenous D-amphetamine self-administration. J Neuroci. 1999;19:4102–4109. doi: 10.1523/JNEUROSCI.19-10-04102.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ren X, Ferreira JG, Zhou L, Shammah-Lagnado SJ, Yeckel CW, De Araujo IE. Nutrient selection in the absence of taste receptor signaling. J Neurosci. 2010;30:8012–8023. doi: 10.1523/JNEUROSCI.5749-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richter CP. Alcohol as food. Quant J Studies Alcohol. 1941;1:650–662. [Google Scholar]
- Richter CP. Total self-regulatory functions in animals and human beings. Harvey Lecture Ser. 1943;38:63–103. [Google Scholar]
- Roberts DC, Vickers G. The effect of haloperidol on cocaine self-administration is augmented with repeated administrations. Psychopharmacology. 1987;93:526–528. doi: 10.1007/BF00207247. [DOI] [PubMed] [Google Scholar]
- Roper SD. Cell communication in taste buds. Cell Mol Life Sci. 2006;63:1494–1500. doi: 10.1007/s00018-006-6112-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salamone JD, Correa M, Farrar A, Mingote SM. Effort-related functions of nucleus accumbens dopamine and associated forebrain circuits. Psychopharmacology. 2007;191:461– 482. doi: 10.1007/s00213-006-0668-9. [DOI] [PubMed] [Google Scholar]
- Salter D, Watts AG. Differential suppression of hyperglycemic, feeding, and neuroendocrine responses in anorexia. Am J Physiol Regul Integr Comp Physiol. 2003;284:R174–182. doi: 10.1152/ajpregu.00275.2002. [DOI] [PubMed] [Google Scholar]
- Schwartz GJ. Gut fat sensing in the negative feedback control of energy balance – Recent advances. Physiol Behav. 2011;104:621–623. doi: 10.1016/j.physbeh.2011.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwartz GJ, Berkow G, McHugh PR, Moran TH. Gastric branch vagotomy blocks nutrient and cholecystokinin-induced suppression of gastric emptying. Am J Physiol Regul Integr Comp Physiol. 1993;264:R630–637. doi: 10.1152/ajpregu.1993.264.3.R630. [DOI] [PubMed] [Google Scholar]
- Sclafani A. Post-ingestive positive controls of ingestive behavior. Appetite. 2001;36:79–83. doi: 10.1006/appe.2000.0370. [DOI] [PubMed] [Google Scholar]
- Sclafani A, Ackroff K, Schwartz GJ. Selective effects of vagal deafferentation and celiac-superior mesenteric ganglionectomy on the reinforcing and satiating action of intestinal nutrients. Physiol Behav. 2003;78:285–294. doi: 10.1016/s0031-9384(02)00968-x. [DOI] [PubMed] [Google Scholar]
- Small DM, Jones-Gotman M, Dagher A. Feeding-induced dopamine release in dorsal striatum correlates with meal pleasantness ratings in healthy human volunteers. NeuroImage. 2003;19:1709–1715. doi: 10.1016/s1053-8119(03)00253-2. [DOI] [PubMed] [Google Scholar]
- Smutz ER, Hirsch E, Jacobs HL. Caloric compensation in hypothalamic obese rats. Physiol Behav. 1975;14:305–309. doi: 10.1016/0031-9384(75)90038-4. [DOI] [PubMed] [Google Scholar]
- Snowdon CT. Motivation, regulation and the control of meal parameters with oral and intragastric feeding. J Comp Physiol Psychol. 1969;69:91–100. doi: 10.1037/h0027941. [DOI] [PubMed] [Google Scholar]
- Snowdon CT, Epstein A. Oral and intragastric feeding in vagotomized rats. J Comp Physiol Psychol. 1970;71:59–67. doi: 10.1037/h0028965. [DOI] [PubMed] [Google Scholar]
- Sotak BN, Hnasko TS, Robinson S, Kremer EJ, Palmiter RD. Dysregulation of dopamine signaling in the dorsal striatum inhibits feeding. Brain Res. 2005;1061:88–96. doi: 10.1016/j.brainres.2005.08.053. [DOI] [PubMed] [Google Scholar]
- Touzani K, Bodnar R, Sclafani A. Activation of dopamine D1-like receptors in nucleus accumbens is critical for the acquisition, but not the expression, of nutrient-conditioned flavor preferences in rats. Eur J Neurosci. 2008;27:1525–1533. doi: 10.1111/j.1460-9568.2008.06127.x. [DOI] [PubMed] [Google Scholar]
- Volkow ND, Wang GJ, Telang F, Fowler JS, Thanos PK, Logan J, Alexoff D, Ding YS, Wong C, Ma Y, Pradhan K. Low dopamine striatal D2 receptors are associated with prefrontal metabolism in obese subjects: possible contributing factors. Neuroimage. 2008;42:1537–1543. doi: 10.1016/j.neuroimage.2008.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang GJ, Volkow ND, Logan J, Pappas NR, Wong CT, Zhu W, Netusil N, Fowler JS. Brain dopamine and obesity. Lancet. 2001;357:354–357. doi: 10.1016/s0140-6736(00)03643-6. [DOI] [PubMed] [Google Scholar]
- Wang PY, Caspi L, Lam CK, Chari M, Li X, Light PE, Gutierrez-Juarez R, Ang M, Schwartz GJ, Lam TK. Upper intestinal lipids trigger a gut-brain-liver axis to regulate glucose production. Nature. 2008;452:1012–1016. doi: 10.1038/nature06852. [DOI] [PubMed] [Google Scholar]
- Watts AG, Donovan CM. Sweet talk in the brain: Glucosensing, neural networks, and hypoglycemic counterregulation. Front Neuroendocrinol. 2009;31:32–43. doi: 10.1016/j.yfrne.2009.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weigle DS. Appetite and the regulation of body composition. FASEB J. 1994;8:302–310. doi: 10.1096/fasebj.8.3.8143936. [DOI] [PubMed] [Google Scholar]
- Wise RA. Role of brain dopamine in food reward and reinforcement. Philos Trans R Soc Lond B Biol Sci. 2006;361:1149–1158. doi: 10.1098/rstb.2006.1854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wooley OW, Wooley SC, Dunham RB. Can calories be perceived and do they affect hunger in obese and non-obese humans? J Comp Physiol Psychol. 1972;80:250–258. doi: 10.1037/h0033069. [DOI] [PubMed] [Google Scholar]
- Yeomans MR, Gould NJ, Leitch M, Mobini S. Effects of energy density and portion size on development of acquired flavour liking and learned satiety. Appetite. 2009;52:469–478. doi: 10.1016/j.appet.2008.12.010. [DOI] [PubMed] [Google Scholar]
- Zheng H, Patterson LM, Berthoud HR. Orexin signaling in the ventral tegmental area is required for high-fat appetite induced by opioid stimulation of the nucleus accumbens. J Neurosci. 2007;27:11075–11082. doi: 10.1523/JNEUROSCI.3542-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou L, Sutton GM, Rochford JJ, Semple RK, Lam DD, Oksanen LJ, Thornton-Jones ZD, Clifton PG, Yueh CY, Evans ML, McCrimmon RJ, Elmquist JK, Butler AA, Heisler LK. Serotonin 2C receptor agonists improve type 2 diabetes via melanocortin-4 receptor signaling pathways. Cell Metab. 2007;6:398–405. doi: 10.1016/j.cmet.2007.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


