Abstract
Non-technical summary
Clonidine, a noradrenaline-like drug, facilitates the recovery of hindlimb walking in adult cats following a complete spinal cord injury.We show that clonidine improves operation of reflex pathways that coordinate bilateral hindlimb activity in cats after spinal cord injury. In addition, sensory signals from the periphery more easily activate the spinal network involved in producing locomotion following clonidine injection.These results show that spinal neurons that produce locomotion and coordinate bilateral activity become more excitable with clonidine.These data could help identify neurons of the spinal locomotor network and lead to a better pharmacotherapy in spinal cord-injured humans.
Abstract
Clonidine, an α-noradrenergic agonist, facilitates hindlimb locomotor recovery after complete spinal transection (i.e. spinalization) in adult cats. However, the mechanisms involved in clonidine-induced functional recovery are poorly understood. Sensory feedback from the legs is critical for hindlimb locomotor recovery in spinalized mammals and clonidine could alter how spinal neurons respond to peripheral inputs in adult spinalized cats. To test this hypothesis we evaluated the effect of clonidine on the responses of hindlimb muscles, primarily in the left hindlimb, evoked by stretching the left triceps surae muscles and by stimulating the right tibial and superficial peroneal nerves in eight adult decerebrate cats that were spinalized 1 month before the terminal experiment. Cats were not trained following spinalization. Clonidine had no consistent effect on responses of ipsilateral muscles evoked by triceps surae muscle stretch. However, clonidine consistently potentiated the amplitude and duration of crossed extensor responses. Moreover, following clonidine injection, stretch and tibial nerve stimulation triggered episodes of locomotor-like activity in approximately one-third of trials. Differential effects of clonidine on crossed reflexes and on ipsilateral responses to muscle stretch indicate an action at a pre-motoneuronal site. We conclude that clonidine facilitates hindlimb locomotor recovery following spinalization in untrained cats by enhancing the excitability of central pattern generating spinal neurons that also participate in crossed extensor reflex transmission.
Introduction
The control of locomotion and posture relies on complex interactions between neuronal circuits within the spinal cord, descending commands, and sensory inputs from the periphery (reviewed in Rossignol et al. 2006). Impaired motor functions following complete spinal cord injury are due to the loss of descending excitatory inputs and to functional changes within spinal circuits caudal to the injury (reviewed in Frigon & Rossignol, 2006; Nielsen et al. 2007; Rossignol & Frigon, 2011). However, with time, training and/or pharmacological stimulation, most mammalian species recover hindlimb locomotion following spinal transection (i.e. spinalization) (reviewed in Edgerton et al. 2001, 2008; Rossignol & Frigon, 2011). Training and pharmacology are thought to reorganize spinal circuits so they can operate more autonomously without descending inputs. How adaptive plasticity ensues with training and/or pharmacotherapy following spinal cord injury is largely unknown.
A potential physiological mechanism is that feedback from the periphery regulates adaptive plasticity within spinal locomotor pattern generators located caudal to the injury. Indeed, providing sensory cues consistent with normal walking is the basis for locomotor training in spinal cord-injured animals and humans (Edgerton et al. 2001; Harkema, 2001; Rossignol & Frigon, 2011). Locomotor training was shown to modify interactions between spinal pattern generators and afferent inputs from the hindlimbs in spinalized adult cats (Cote et al. 2003; Cote & Gossard, 2004). Due to the importance of sensory feedback in the recovery of hindlimb motor functions following spinalization, it is possible that drugs known to facilitate functional recovery also modify reflex pathways. In the present study we tested the hypothesis that clonidine, an α-noradrenergic receptor agonist that initiates and facilitates hindlimb locomotion in spinal cord-injured cats (Forssberg & Grillner, 1973; Naftchi, 1982; Barbeau et al. 1987; Pearson & Rossignol, 1991; Chau et al. 1998a,b), enhances reflex excitability.
Several studies have already tested this hypothesis and found that clonidine either reduces or produces no change in reflex excitability. For instance, ipsilateral responses to mechanical or electrical stimulation of the foot dorsum in chronic spinalized cats were reduced with clonidine during spinal locomotion (Barbeau et al. 1987; Chau et al. 1998b). In spinal cord-injured humans, short- or longer-latency polysynaptic reflex responses evoked by stimulating cutaneous nerves were also depressed by clonidine (Remy-Neris et al. 1999). Other studies showed that clonidine or tizanidine depressed transmission in group II reflex pathways in anaesthetized cats with an intact spinal cord (Bras et al. 1990), in decerebrate cats with high cervical spinalization (Schomburg & Steffens, 1988), and in spinal cord-injured humans (Remy-Neris et al. 2003). Group I pathways were unaffected by clonidine in anaesthetized cats (Bras et al. 1990) and in spinal cord-injured humans (Remy-Neris et al. 1999; Remy-Neris et al. 2003). In decerebrate paralysed cats spinalized approximately 1 month before the terminal experiment, clonidine had no effect on group I-evoked monosynaptic excitatory postsynaptic potentials but depressed disynaptic inhibition (Cote et al. 2003). However, another study in anaesthetized cats reported that the effects of stimulating the contralateral quadriceps nerve at group II strength were facilitated by noradrenaline in neurons that received monosynaptic inputs from the reticular formation but depressed in neurons that received ipsilateral group II inputs (Hammar et al. 2004). Thus it is possible that noradrenergic agonists, such as clonidine, also potentiate crossed reflex pathways following spinal transection.
Due to the multitude of sensory inputs that project to the spinal cord and regulate locomotor activity through several interneuronal relays (Jankowska, 1992; Rossignol et al. 2006) a more thorough investigation of the effect of clonidine on various reflex pathways after spinal transection is required. In the present study, we evaluated the effect of clonidine on responses of hindlimb muscles, primarily in the left hindlimb, evoked by stretching the left triceps surae muscles and by stimulating the right tibial (Tib) nerve at the ankle. We hypothesized that the generation of a locomotor state by injecting clonidine in chronic spinal cats would be reflected by an enhancement of some reflex responses. The results show that clonidine increased the amplitude and duration of crossed extensor reflex actions and enhanced the effectiveness of sensory inputs in triggering locomotor-like activity in untrained chronic spinal cats. Such information is required to guide future investigations at the cellular level pertaining to the pharmacological regulation of interneuronal pathways involved in reflex transmission and pattern generation within the injured spinal cord, as well as the identification of putative central pattern generator neurons. Our results also highlight potential targets for evaluating a drug's effectiveness in facilitating locomotor recovery in spinal cord-injured humans.
Methods
Ethical information, animal care and surgical procedures
All procedures were approved by the Institutional Animal Care and Use Committee of Northwestern University. All animals were obtained from a designated breeding establishment for scientific research. Before the experiments, animals were housed and fed within designated areas, which were monitored daily by veterinarians and trained personnel. The current data set is compiled from eight adult cats weighing between 2.5 and 5.0 kg. All experimental procedures meet the guidelines set-out in The Journal of Physiology for ethical matters (Drummond, 2009).
Terminal experiment
During the terminal experiment, cats were first placed in a clear plastic cylinder and anaesthetized with 1.5–3% isoflurane in a 1:3 mixture of O2 and NO2. After approximately 15 min the animal was transferred from the cylinder to a surgical table and anaesthesia was continued with a mask. Once the animal was deeply anaesthetized, a tracheotomy was performed and cats were intubated to deliver the anaesthesia. The right common carotid artery and right jugular vein were cannulated to monitor blood pressure and for fluid administration, respectively. The level of anaesthesia was confirmed and adjusted by monitoring blood pressure, applying pressure to the paw to detect limb withdrawal, and by verifying the size and reactivity of the pupils. The animal was then transferred to a stereotaxic frame for further surgery. Following a craniotomy, the cortex and all neural tissue rostral to the colliculi were removed (i.e. a precollicular decerebration). At this point, animals are considered to have complete lack of sentience (Silverman et al. 2005) and anaesthesia was discontinued. A lethal injection of potassium chloride (2 mg kg−1) was administered at the end of the experiment through the right jugular vein.
Survival surgery
The spinal cord was completely transected at low thoracic levels approximately 1 month before the terminal experiment. The spinalization was performed under aseptic conditions in an operating room with sterilized equipment. Prior to surgery, cats were sedated (butorphanol, 0.4 mg kg−1, i.m.; acepromazine, 0.05–0.1 mg kg−1, i.m.; glycopyrrolate, 0.01 mg kg−1, subcutaneous). Induction was done with propofol (2–3 mg kg−1, i.v.) or ketamine/diazepam (0.11 ml kg−1 in 1:1 ratio, i.v.). Once anaesthetized, the cat was quickly intubated with a flexible endotracheal tube and anaesthesia was maintained by adjusting isoflurane concentration as needed (1.5–3%). The fur overlying the back was shaved with electric clippers and loose hair was vacuumed. A forelimb was shaved and an i.v. line was placed in a cephalic vein. The level of anaesthesia was confirmed and adjusted throughout the surgery by monitoring cardiac rate and respiratory rate, by applying pressure to the paw to detect limb withdrawal, and by evaluating jaw tone. Body temperature was monitored using a rectal thermometer. Monitoring of the parameters was done on a continuous basis (i.e. cardiac and respiratory rates) and recorded every 15 min. Cats received fluids i.v. (warmed lactated Ringer solution or saline + 2.5% dextrose) at a rate of 5–10 ml kg−1 throughout the surgery to maintain haemodynamic stability. Ophthalmic ointment was also applied to the eyes.
A laminectomy was performed at T12–T13, the dura was removed, and after local lidocaine application (xylocaine, 2%), the spinal cord was completely transected with surgical scissors. Haemostatic material (Surgicel) was inserted within the gap, and muscles and skin were sewn back to close the opening in anatomical layers. A transdermal fentanyl patch (25 mcg h−1) was taped to the back of the animal 2–3 cm from the base of the tail. During surgery and approximately 7 h later, an analgesic (buprenorphine, 0.01 mg kg−1) was administered subcutaneously. An oral antibiotic (Baytril, 5 mg kg−1) was given once a day for 5 days after spinalization to prevent infection. The bladder was manually emptied 1–2 times each day up to the terminal experiment. The animals were monitored daily by experienced personnel and veterinarians. The hindlimbs of the cats were frequently cleaned by placing the lower half of the body in a warm soapy bath.
Experimental design
A schematic illustration of the experimental set-up is shown in Fig. 1. The Achilles tendon of the left hindlimb was freed from surrounding tissue, leaving only a small piece of the calcaneal bone attached. The tendon was connected to a force transducer attached in series to a linear motor (Copley Controls) controlled by custom-made software to alter muscle length. Force signals were low-pass filtered at 1000 Hz and sampled at 10,000 Hz. Both hindfeet were held with a clamp. The left knee joint was also fixed with a custom-made clamp attached to the femoral epicondyles. Hip, knee and ankle joint angles were ∼120 deg, 160 deg and 90 deg for both hindlimbs. The hindpaws were not contacting the table surface.
Figure 1. Experimental preparation.
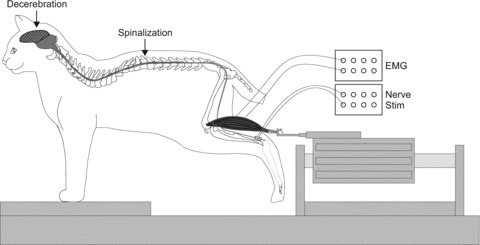
The schematic diagram illustrates the experimental set-up during the terminal experiment with the Achilles tendon attached to a force transducer connected in series to a muscle puller that can lengthen the muscle. Muscle force was always recorded as indicated in Fig. 1 and muscle lengths referred to both in the text and in all subsequent figures pertain to the left triceps surae.
Bipolar wire electrodes were inserted into the soleus (Sol, ankle extensor), lateral gastrocnemius (LG, ankle extensor/knee flexor), semitendinosus (St, knee flexor/hip extensor), anterior sartorius (Srt, hip flexor/knee extensor), and tibialis anterior (TA, ankle flexor) of the left hindlimb and in the right St for electromyography (EMG). EMG signals were amplified (×1000) with a multi-channel amplifier (AM Systems Model 3500), bandpass filtered (300–3000 Hz), and sampled at 10,000 Hz simultaneously with force data. Bipolar stimulating cuff electrodes were placed around the right tibial (Tib, n = 8) and right superficial peroneal (SP, n = 5) nerves near the ankle joint. The SP nerve cuff electrode was placed just proximal to its four cutaneous divisions in the Y-shape of the dorsal arterial arch on the dorsum of the foot. At this level the SP nerve is purely cutaneous (Bernard et al. 2007).
Experimental protocol
Stretch reflex
Several ramp-hold-return stretches of 4 s duration of the left triceps surae muscles were performed. Stretches consisted of a 5 mm ramp in 0.25 s, a 3.5 s hold, and a return to the initial length in 0.25 s, for a total of 4 s. Such stretches are similar in terms of time course and amplitude to postural perturbations used in rabbits (Musienko et al. 2010). The left triceps surae muscles were stretched at two starting lengths. The ‘short’ muscle length was the length that corresponded to a 90 deg ankle joint angle. The ‘long’ muscle length was +5 mm longer than the short length. It was previously shown that the intrinsic electrical properties of motoneurons are modulated by joint angle or muscle length due to varying levels of reciprocal inhibition (Hyngstrom et al. 2007). We wanted to evaluate the activation patterns of multiple hindlimb muscles with triceps surae muscle stretch and Tib nerve stimulation at two different levels of reciprocal inhibition and/or tonic group II feedback in chronic spinal cats with and without clonidine. Evidently, force production will be directly affected by muscle length due to the well-known length–tension relationship of muscles (Rack & Westbury, 1969). Muscle stretches were performed at intervals of no less than 1 min.
Crossed reflexes
The motor threshold (T) for right Tib nerve stimulation was first determined. The motor threshold was the stimulation intensity required to evoke a small plantar flexion of the right toes. In five cats the right SP nerve was also stimulated. The motor threshold for SP nerve stimulation was the stimulation intensity required to evoke a small flexion response of the right knee flexors. The SP nerve stimulation was performed to evaluate a pure cutaneous contribution to crossed reflex pathways and data are not included in Tib nerve group averages or statistical analyses. The nerves were stimulated at 40 Hz for 2.5 s or 20 s (0.2 ms pulse width) at 2T or 5T at short and long triceps surae muscle lengths. The stimulation intensities required to evoke minimal reflex responses (i.e. motor thresholds) were verified throughout the experiment and adjusted accordingly. Nerve stimulations were performed at intervals of no less than 1 min. Reflex testing was performed before and after injecting clonidine through the right jugular vein (250–500 μg kg−1) using the same stretch and stimulation parameters.
Measurements
All measurements were done with Spike2 v. 6.0 (Cambridge Electronic Design, Cambridge, UK). The windows used for force and EMG (right Tib stimulation only) measures are shown in Fig. 2. For stretch-evoked responses (Fig. 2A), the peak force evoked during stretch was calculated as the maximal value between cursors c and e minus the mean baseline force between cursors a and b. The mean force evoked during the 3.5 s hold portion of the stretch was calculated as the mean value between cursors d and e minus the mean baseline force between cursors a and b. The peak and mean passive forces were measured after sciatic nerve transection using the same windows. Peak and mean passive forces were subtracted from the trials obtained before sciatic nerve transection to determine peak and mean reflex forces, respectively. The passive force is calculated to provide an indication of the reflex contribution to the force produced.
Figure 2. Measurements of triceps surae force and muscle activity.
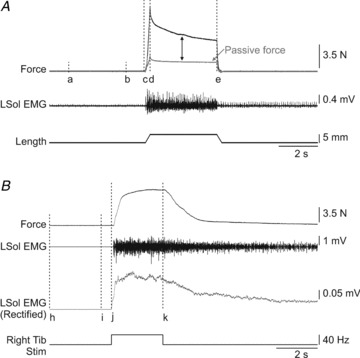
A, a single stretch of the left triceps surae muscles lasted 4 s (5 mm ramp up in 0.25 s followed by a 3.5 s hold and a 5 mm ramp down in 0.25 s). A window from 1 to 4 s (cursors a and b) was used to measure baseline force. Peak force was measured as the maximal value between cursors c and e minus the mean baseline force. The mean force was measured as the average force during the hold period of the stretch from cursors d to e minus the mean baseline force. Passive force was measured following transection of the left sciatic nerve. Passive force values were subtracted from pre-sciatic nerve transection trials to obtain reflex force (represented by bidirectional arrow). B, force and EMG evoked during stimulation of the right Tib nerve. Peak force was measured as the maximal value between cursors j and k minus the mean baseline force between cursors h and i. The force area and the EMG (rectified and smoothed with a 0.1 s time constant) during stretch were measured as the area under the curve (modulus area function in Spike2 6.0) from cursors j to k minus the area under the curve from cursors h to i. L: left.
For responses evoked by stimulating the right Tib nerve for 2.5 s (Fig. 2B), the peak force elicited during the stimulation was measured as the maximal value between cursors j and k minus the mean baseline force between cursors h and i. EMGs were rectified and smoothed with a 0.1 s time constant. The force and EMG areas were measured as the area under the force or integrated EMG curves (modulus area function in Spike2 v. 6.0) between cursors j and k minus the area between cursors h and i. A negative value in force indicates that the force during stimulation is reduced compared to baseline. The ratio of the EMG during stimulation as a function of baseline EMG was calculated and compared across conditions. This measure is very sensitive to any change in activity evoked by the stimulation and accounts for the presence of activity before stimulation. A ratio around 1 indicates no activity above baseline. It assumes a similar amount of noise before and throughout the stimulation. Trials in which stimulation artifacts were present during the stimulation were excluded from analysis. Each EMG channel in all trials was visually inspected.
Statistical analysis
All statistical tests were done with SPSS v. 18.0 for measures of force and/or EMG. Student's t test for paired data was used to determine statistical differences before and after clonidine on peak and mean reflex forces during stretch reflex trials, as well as on peak force and force area during right Tib nerve stimulation trials. A three-factor ANOVA was used to evaluate the effect of clonidine (i.e. before and after), muscle length (i.e. short and long), and stimulation intensity (i.e. 2T, 5T) on the EMG evoked during right Tib nerve stimulation. Statistical significance was set at P < 0.05. All values are the mean ± standard error of the mean (SEM).
Results
Effect of clonidine on responses evoked by triceps surae muscle stretch
We recently showed that responses evoked by stretching triceps surae muscles were markedly altered in acute and chronic spinalized cats (Frigon et al. 2011). Specifically, stretching triceps surae muscles consistently failed to activate Sol and LG while producing activity in muscles not normally activated in the intact state, such as St and Srt, particularly in chronic spinal cats. In the present study, we report the effect of clonidine on responses evoked by stretching the left triceps surae muscles in chronic spinal cats.
Triceps surae muscle stretches were performed before and after clonidine administration in seven chronic spinal cats. Figure 3 shows force and EMG responses with stretch of triceps surae muscles in two cats before and after clonidine injection. Before clonidine, stretch activated the left St, whereas stretched muscles, such as left Sol and LG, were mostly inactive (Fig. 3A and C). The effect of clonidine on force and EMG patterns differed between cats. For instance, in some cats the force produced during stretch was similar to before clonidine (Fig. 3B) while in others the force increased (Fig. 3D) or decreased (not shown). In some cases, triceps surae muscle stretch triggered episodes of locomotor-like activity that started during or immediately following stretch (Fig. 3B). The example shown in Fig. 3D shows that clonidine could restore the activation of Sol and LG that is normally observed in the intact state (Frigon et al. 2011) while abolishing the activity in the left St present before clonidine. However, restoration of extensor activity with parallel decreases in flexor activity was not consistently observed.
Figure 3. Effects of stretching the left triceps surae muscles in chronic spinal cats before and after clonidine injection.
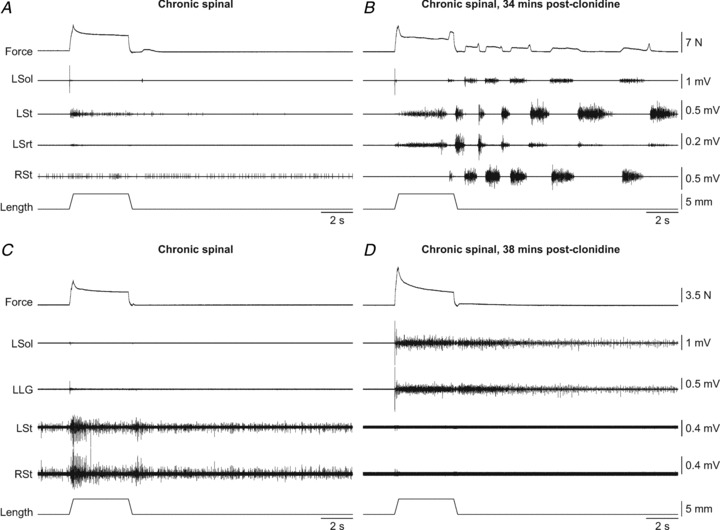
A and B, triceps surae muscle stretch at the long length in cat 715. C and D, triceps surae muscle stretch at the short length in cat 7152. L: left, R: right.
Figure 4 shows peak and mean reflex forces produced by stretching triceps surae muscles before and after clonidine at short and long triceps surae muscle lengths for the group of chronic spinal cats, as well as individually for each cat. For the group, there were no significant differences for peak (Fig. 4A) and mean (Fig. 4C) reflex forces before and after clonidine (paired t tests, P > 0.05). As can be seen from individual data, the effect of clonidine on force produced during stretch varied between cats (Fig. 4B and D).
Figure 4. Group and individual data for triceps surae peak and mean reflex forces evoked by stretching triceps surae muscles in chronic spinal cats before and after clonidine injections.
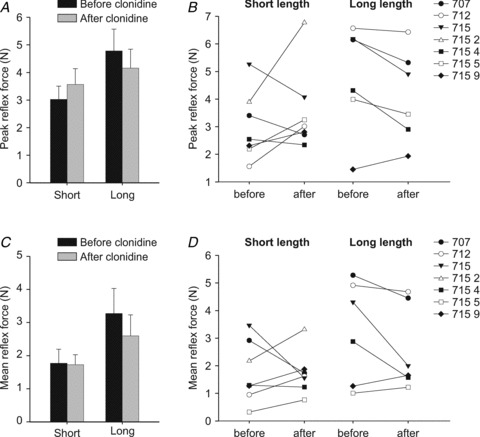
A and C, group data for peak and mean reflex forces in the different conditions. Each bar is the mean ± the standard error of the mean in 6–7 cats in each condition. B and D, individual data for peak and mean reflex forces. Each data point is the mean of 2–5 stretch reflex trials before and after clonidine. Note that data for cat 7152 is available at the short length only due to stretch-evoked rhythmic activity at the long length.
No quantitative analysis of EMG activities was done before and after clonidine injection in chronic spinal cats because stretch often induced rhythmic activity during the stretch. Indeed, triceps surae muscle stretch triggered episodes of locomotor-like activity in 6 of 8 cats (17 of 44 trials, or 38.6%). Therefore, with clonidine the spinal rhythm-generating circuitry becomes more responsive to stretch-related inputs from the left triceps surae, even though responses of hindlimb muscles to stretch are not consistently altered.
Effect of clonidine on crossed reflex pathways
Crossed responses were evoked before and after clonidine injection by stimulating the right Tib and SP nerves in eight and five chronic spinal cats, respectively. Figure 5 shows the effect of clonidine on crossed pathways in two of these cats. Stimulating the right Tib nerve often produced a small force from the left triceps surae muscles and activity in left Sol and LG (Fig. 5A). In this cat, clonidine considerably potentiated the crossed extensor force generated by the left triceps surae muscles due to strong and sustained activation of left Sol and LG (Fig. 5B). In 2 of 8 chronic spinal cats, the force produced during stimulation of the right Tib nerve decreased from baseline levels, which could be due to activation of the left TA (e.g. Fig. 5C) or the activation of crossed inhibitory pathways (Arya et al. 1991; Aggelopoulos & Edgley, 1995; Aggelopoulos et al. 1996; Frigon & Rossignol, 2008c). Crossed flexion has been reported in the cat following acute spinalization (Rossignol & Gauthier, 1980). Similar to stretching the left triceps surae muscles, stimulating the right Tib nerve could trigger locomotor-like activity in chronic spinal cats after clonidine administration (see Fig. 5D). Stimulating the right Tib nerve triggered locomotor-like activity during or after stimulation in 7 of 8 cats (24 of 82 trials, or 29.3% of trials). Clonidine also enhanced crossed extensor reflex responses evoked by stimulating the SP nerve (compare Fig. 5E and F), thus showing that neurons interposed in crossed extensor pathways that respond to a pure cutaneous input are also more excitable following clonidine injection.
Figure 5. Stimulation of the right tibial nerve in chronic spinal cats before and after clonidine injection.
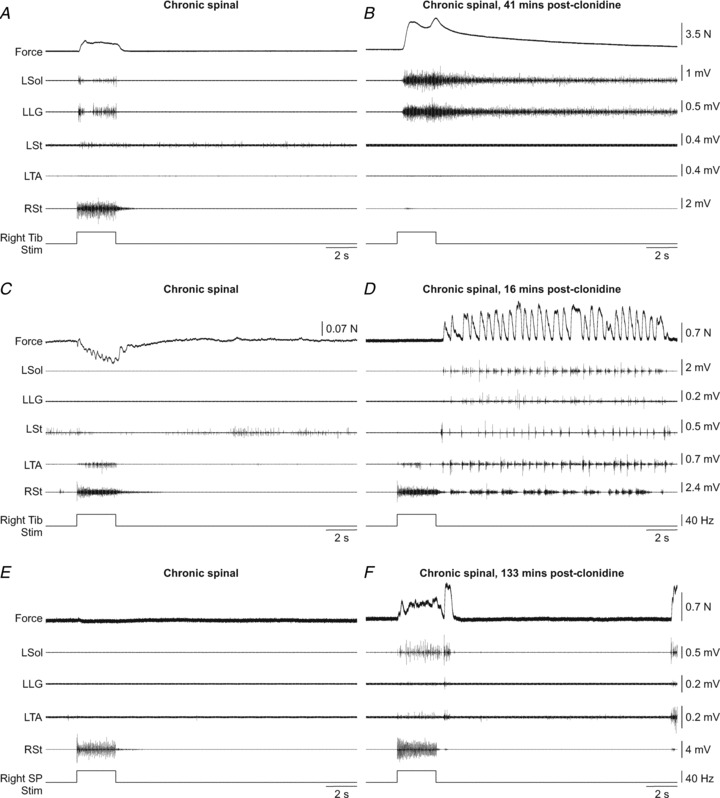
A and B, right tibial (Tib) nerve stimulation at long length in cat 7152. C and D, right Tib nerve stimulation at short length in cat 71510. E and F, right SP nerve stimulation at long length in cat 71510. The force produced by the left triceps surae muscles was recorded along with the activity of muscles of the left hindlimb and the right semitendinosus. L: left, R: right.
Across cats, triceps surae peak force and force area during stimulation of the right Tib nerve were consistently increased after clonidine injection, particularly at 5T (Fig. 6B, C, E and F). The only exception is in cat 7155 at 2T and long length (Fig. 6C and F). For the group, triceps surae peak force significantly increased with clonidine at 5T only, whereas triceps surae force area significantly increased for the short length at 2T and for both muscle lengths at 5T (paired t tests, P ≤ 0.05). Triceps surae peak force and force area during stimulation of the right SP were also consistently enhanced by clonidine (not shown).
Figure 6. Group and individual data for peak force and force area during stimulation of the right Tib nerve in chronic spinal cats before and after clonidine injection.
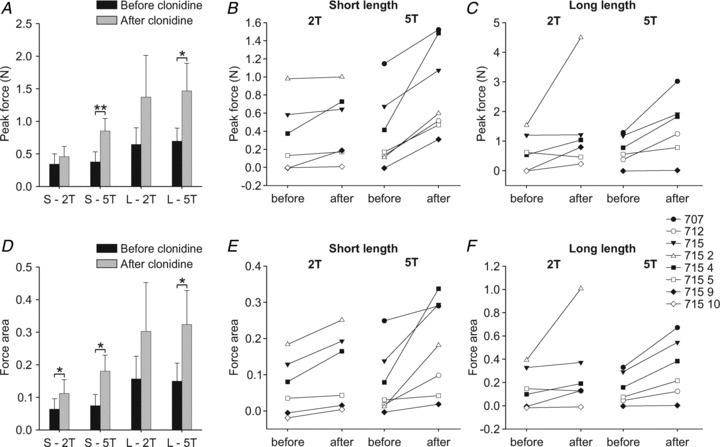
A and D, group data for peak force and force area in the different conditions. Each bar is the mean ± the standard error of the mean of 6–7 cats in each condition. B and C, individual data for peak force and E and F, force area at short and long lengths at 2T and 5T. Each data point is the mean of 2–6 stimulation trials in each condition. *P≤ 0.05, **P ≤ 0.01.
The ratio of EMG activity evoked during stimulation as a function of baseline EMG (see Methods) was calculated for right Tib nerve stimulation trials (5–16 trials per cat) before and after clonidine. A three-factor (i.e. triceps surae muscle length, clonidine, stimulation intensity) ANOVA was performed for each muscle. There was no significant effect of triceps surae muscle length on EMG activity for any muscle. Figure 7 shows the EMG activities evoked during right Tib stimulation at 2T and 5T before and after clonidine with triceps surae muscle lengths combined. Note that the bars in Fig. 7 are arranged according to the results of the three-factor ANOVA and the ratio is expressed as a percentage of pre-stimulation activity. Clonidine significantly enhanced EMG activities during right Tib stimulation in right St, left Sol, left St, and left TA. Stimulation intensity (2T, 5T) significantly influenced EMG activities of the right St, left Sol, and left Srt. There was a significant interaction between clonidine and stimulation intensity for the right St and left Sol. As a result, independent samples t tests were performed to determine significant differences between conditions for these two muscles. Fig. 7 shows that the increase in triceps surae muscle force with clonidine could be due to increased activation of the left Sol (Fig. 7C) combined with decreased activation of the left TA (Fig. 7F).
Figure 7. Muscle activity evoked during right tibial nerve stimulation across chronic spinal cats before and after clonidine injection.
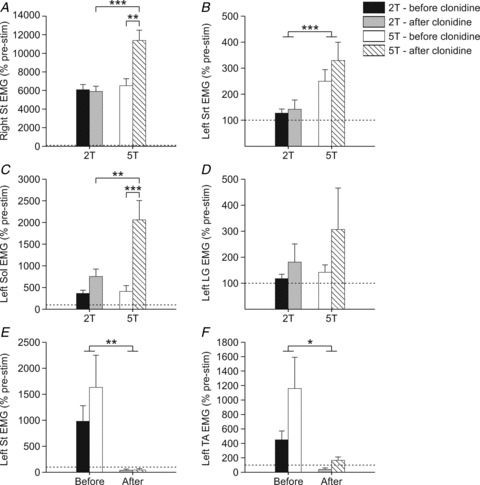
EMG signals were rectified and smoothed with a 0.1 s time constant. The area under the rectified EMG curve (modulus area function in Spike2 v. 6.0) was measured between cursors j (stim onset) and k (stim offset) minus the area between cursors h and i (i.e. baseline EMG). The ratio of the EMG during stimulation as a function of baseline EMG was calculated and compared across conditions. B, E and F show significance of three-factor ANOVA (*P≤ 0.05), while A and C show significance of independent samples t tests P ≤ 0.05). Each bar is the mean ± standard error of the mean in each condition. Horizontal dashed lines are at the 100% mark as a reference. *P ≤ 0.05, **P ≤ 0.01, ***P ≤ 0.001.
Therefore, in chronic spinal cats, clonidine consistently enhanced the excitability of crossed pathways from the right Tib and SP nerves to extensors of the left hindlimb (i.e. Sol), thus leading to larger and more sustained crossed extensor force output from triceps surae muscles. At the same time clonidine reduced the excitability of crossed pathways to flexors (i.e. St and TA). Spinal rhythm-generating circuitry also became more responsive to sensory inputs from the right Tib nerve following clonidine injection.
Effect of clonidine on responses evoked during prolonged stimulation of the right tibial nerve in chronic spinal cats
Before clonidine, stimulating the Tib nerve for 20 s produced a small force from the left triceps surae muscles and brief activity in the left Sol and LG (Fig. 8A). However, after clonidine, the same stimulation produced sustained force from the left triceps surae muscles and activity in the left Sol and LG (Fig. 8B). During prolonged stimulation, activity in the left Sol and LG was sustained with small bursts present in these muscles. A few seconds after stimulation a strong episode of locomotor-like activity was initiated. Sustained forces were present in 6 of 7 chronic spinal cats but only after clonidine injection (0 of 23 trials before clonidine and 14 of 18 trials after clonidine). Thus, clonidine can help sustain crossed extensor force and activity during prolonged stimulation in chronic spinal cats.
Figure 8. Prolonged stimulation of the right Tib nerve in chronic spinal cats.
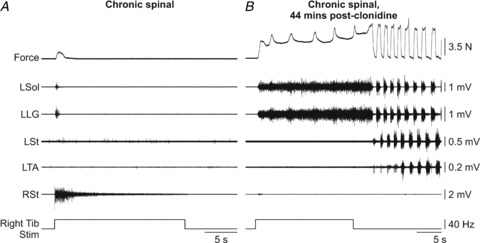
A–C, stimulation of the right Tib nerve for 20 s in the intact and acute spinal states before and after clonidine injection in cat 7158 at long length. D–E, stimulation of the right Tib nerve for 20 s before and after clonidine injection in a chronic spinal cat (7152) at long length. L: left, R: right.
Effect of sensory stimulation on the ongoing clonidine-induced rhythm in chronic spinal cats
Eleven stretch reflex trials in three cats and 20 right Tib nerve stimulation trials in six cats were excluded from group data analysis in chronic spinal cats following clonidine injection because rhythmic activity was present before the stretch or stimulation. However, this data set provided an opportunity to evaluate the effect of sensory stimulation on the ongoing rhythm. Figure 9 shows an example of triceps surae muscle stretch and right Tib nerve stimulation in the same cat during rhythmic activity. Stretching triceps surae muscles during rhythmic activity did not sustain activity in the left Sol (0/11 trials, Fig. 9A). Instead, triceps surae muscle stretch could produce sustained activity in the left Srt (6/9 trials), left St (7/11 trials), or not perturb the rhythm at all (4/11 trials). In some trials left Srt EMG was not available.
Figure 9. Effect of sensory inputs on the ongoing rhythm in a chronic spinal cat treated with clonidine.
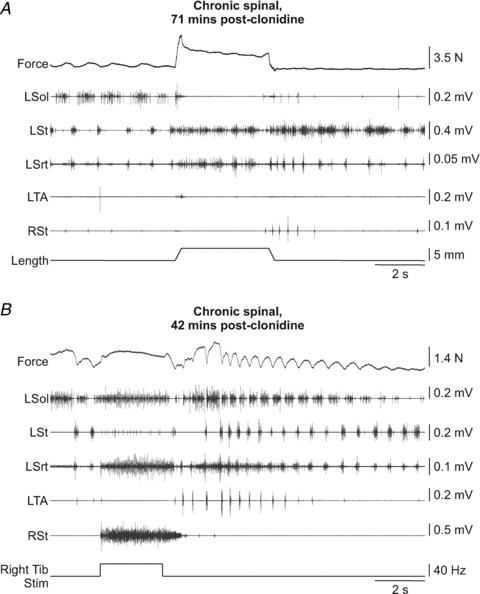
A, stretch of the left triceps surae muscles in cat 7155 at long length of triceps surae. B, stimulation of the right Tib nerve in cat 7155 at long length of triceps surae. L: left, R: right.
Stimulating the right Tib nerve during rhythmic activity (Fig. 9B) could sustain activity in left Sol (13/20 trials), left Srt (15/16 trials), and the left TA (8/18 trials). In some trials left Srt or TA EMGs were not available. Another effect of right Tib stimulation in about half the trials (11/20 trials) was an acceleration of the post-stimulation rhythm (Fig. 9B). Therefore, inputs from triceps surae stretch-sensitive afferents and from Tib nerve afferents interact differently with the clonidine-induced rhythm in chronic spinal cats.
Discussion
In the present study clonidine, a drug known to facilitate hindlimb locomotor recovery following spinalization in the adult cat, altered interactions between peripheral sensory inputs and spinal circuits involved in posture and locomotion. Specifically, crossed extensor pathway excitability was consistently enhanced in chronic spinal cats, unlike ipsilateral responses to triceps surae muscle stretch that showed variable changes with clonidine. Moreover, spinal rhythm-generating circuitry became more responsive to sensory inputs from stretch receptors of the triceps surae muscles and from afferents contained within the tibial nerve. Therefore, we propose that clonidine facilitates hindlimb locomotion following complete spinal cord injury in untrained cats partly by facilitating the organizing effect of sensory inputs on spinal locomotor pattern generators and by strengthening actions of neurons coordinating bilateral activity. These neuronal elements could be shared by crossed extensor reflex pathways and by spinal locomotor pattern generators.
Effect of clonidine on crossed and uncrossed reflex pathways
In contrast to previous studies that showed reduced or no change in ipsilateral reflex responses with clonidine after spinal cord injury (see Introduction), we report that crossed extensor reflex responses were potently enhanced by clonidine in chronic spinal cats. This extends the findings of Hammar et al. (2004) who reported enhanced effects of contralateral group II inputs by noradrenaline in neurons that received monosynaptic inputs from the reticular formation in deeply anaesthetized cats with intact spinal cords. In the present study, crossed extensor force of triceps surae muscles was increased in magnitude and more sustained after clonidine injection, even outlasting the stimulation (e.g. Fig. 5B). Enhanced crossed extensor reflex force of the triceps surae muscles with clonidine was paralleled by increased activity in the left Sol and reduced activity in the left TA (Fig. 7). We suggest that clonidine produces an extensor bias in spinal motor pools thus facilitating crossed extension, at least at rest. Following spinal cord injury, there is a greater degree of co-activation between ankle extensors and flexors during locomotion, which can be reduced with locomotor training (Dietz et al. 1995). Thus, clonidine and locomotor training might alter the spinal network through similar mechanisms, by shifting the balance of inhibition and excitation to favour extensor excitability and/or by restoring normal activation patterns.
A previous study reported that soleus force evoked by contralateral stimulation of the foot could be modulated in acutely spinalized cats following clonidine injection (Nichols et al. 1978). It thus appears that clonidine can also enhance crossed extensor reflex excitability even after acute spinalization. However, the results of that study are not directly comparable to the present results. First, in Nichols et al. (1978) the tested leg (i.e. where the force was measured) was extensively denervated except for the soleus muscle. It is known that denervation induces widespread compensation in spared pathways in cats with intact (Pearson et al. 1999; Frigon & Rossignol, 2007) or transected spinal cords (Bouyer et al. 2001; Frigon & Rossignol, 2008b). Second, Nichols et al. (1978) evoked crossed extension reflexes by squeezing the contralateral hindlimb. Such a technique simultaneously activates a variety of afferents from different regions, which can sum to enhance crossed extensor reflexes. Further experiments are required to determine if specific crossed extensor pathways are influenced by clonidine after acute spinalization.
Pre-motoneuronal site of action
Responses in a given muscle (force or EMG) by stimulating the right Tib nerve compared to those evoked by triceps surae muscle stretch were not similarly affected by clonidine in the same cats (compare individual data from Figs. 4 and 6), indicating that clonidine-induced reflex changes were mediated at a pre-motoneuronal site. The effect of clonidine on the commissural interneuronal circuitry mediating crossed reflex pathways has not been specifically studied in intact or spinalized cats. However, it was shown that bilateral locomotor responses evoked by intraspinal electrical stimulation were more frequent after clonidine injection in spinalized cats (Barthelemy et al. 2006) and that noradrenaline facilitated effects from contralateral group II inputs in neurons that received monosynaptic inputs from the reticular formation (Hammar et al. 2004). Taken together with the results of the present study it is clear that clonidine strengthens pathways that coordinate bilateral activity after spinalization. It is probable that noradrenergic inputs are important regulators of interlimb coordination in the intact state. Furthermore, commissural interneurons interposed in crossed reflex pathways are located in laminae VI–VIII in the cat (Edgley et al. 2003; Hammar et al. 2004, 2007; Jankowska et al. 2009), regions that are densely packed with locomotor-related α2-noradrenergic receptors (Noga et al. 2011).
Crossed inhibitory pathways are active within the cat spinal cord, at rest (Arya et al. 1991; Aggelopoulos & Edgley, 1995; Aggelopoulos et al. 1996) and during locomotion (Frigon & Rossignol, 2008c). Edgley and colleagues showed that crossed inhibition of extensor motoneurons elicited by group II afferents was replaced by crossed excitation following spinal transection or bilateral lesions of the dorsolateral funiculi in anaesthetized cats, indicating descending control of the commissural interneuronal circuitry. Aggelopoulos et al. (1996) demonstrated that crossed inhibition could be restored by a serotonergic receptor agonist. Therefore, it appears that neuromodulatory inputs from both noradrenergic and serotonergic pathways are important regulators of crossed pathways within the cat spinal cord.
Afferents involved in reflex responses
Triceps surae muscle stretch activates afferents from primary (group Ia) and secondary (group II) muscle spindles. The involvement of group II inputs from secondary muscle spindles in cat and human stretch reflexes could be key in the observed stretch reflex changes (Jankowska, 1992; Schieppati & Nardone, 1999; Grey et al. 2001; Jankowska & Hammar, 2002; Frigon et al. 2011). Moreover, contributions from group II, III and/or IV afferents from free nerve endings activated by muscle stretch (Cleland et al. 1990; Cleland & Rymer, 1990) cannot be excluded. However, it does not appear that noradrenergic inputs tightly regulate pathways activated by stretch-related inputs to homonymous muscles.
On the other hand, neurons interposed in crossed reflex pathways appear to be tightly controlled by noradrenergic inputs. Stimulating the Tib nerve at the ankle activates cutaneous afferents from the skin of the plantar surface but also muscle afferents from intrinsic foot muscles. Although responses evoked by stimulating the Tib nerve at the ankle are most often ascribed to cutaneous afferents (Loeb, 1993; Frigon & Rossignol, 2008a), a contribution from muscle afferents cannot be excluded, as indicated by the group II effect with noradrenaline in anaesthetized cats (Hammar et al. 2004). However, the fact that clonidine also enhanced crossed extensor reflex responses evoked by stimulating the SP nerve (Fig. 5F) indicates that neurons receiving cutaneous inputs are also involved. The lack of differences between 2T and 5T stimulation on crossed reflex responses evoked by the right Tib nerve, with the exception of responses in left Srt, suggests that large diameter afferents are involved, such as Aβ (cutaneous) and/or group II (muscle) afferents, and that smaller diameter afferents make a minimal contribution.
Inter-animal variability in reflex changes
One of the most interesting findings was that clonidine-induced directional changes (i.e. increase or decrease) in triceps surae force responses to homonymous muscle stretch were variable between cats, whereas those evoked by stimulating the right Tib nerve showed a high degree of consistency (i.e. an increase in magnitude with clonidine). As stated, this suggests that under normal circumstances noradrenergic inputs tightly regulate neurons interposed in crossed extensor pathways as opposed to those neurons receiving stretch-related inputs from triceps surae muscles. Group and individual changes were presented because addressing the issue of inter-animal or inter-individual variability is important from a fundamental neurophysiological perspective and from a clinical perspective, which is often lost or ignored by simply reporting group data (recently reviewed in Marder & Goaillard, 2006; Frigon, 2011). Results from the present study suggest that this variability could arise in part by differing levels of responsiveness to neuromodulatory inputs within specific spinal neuronal circuits. What gives rise to inter-animal variability within the CNS and how various types of injuries modify this variability is largely unknown.
Facilitation of locomotor activity with clonidine in chronic spinal cats
After spinalization, descending inputs can no longer configure the spinal circuitry to produce locomotion and signals from the periphery are required. In trained chronic spinal cats placing the hindlimbs on a moving treadmill belt is sufficient to initiate hindlimb locomotion (Lovely et al. 1986; Barbeau & Rossignol, 1987; Frigon, 2012). In untrained cats treated with clonidine, placing the hindlimbs on a treadmill will also initiate hindlimb locomotion (Barbeau et al. 1987). In the present study, sensory inputs evoked by stretching triceps surae muscles or by stimulating the Tib nerve elicited episodes of locomotor-like activity after clonidine injection, even though the legs were mechanically immobilized. As such, phasic sensory feedback from moving hindlimbs could not entrain spinal locomotor pattern generators. Therefore, clonidine appears to facilitate the emergence of a locomotor state by altering interactions between sensory inputs from the periphery and neurons participating in rhythm generation and bilateral coordination (i.e. crossed extension). Spinal interneurons mediating rhythmic activity can participate in other types of movements, such as limb withdrawal in the red-eared turtle (Berkowitz, 2005) and we hypothesize that crossed extensor reflex pathways share common neuronal elements with spinal locomotor rhythm-generating circuitry.
Additionally, various types of sensory inputs seem sufficient to initiate hindlimb locomotion with clonidine, indicating that rhythm-generating neurons are overall more excitable. As a result, clonidine probably also facilitates the emergence of a locomotor state by direct actions on intrinsic neuronal properties, such as persistent inward currents (PICs). Such properties were shown to sustain spinal locomotor rhythm generation in neonatal rodent preparations (Tazerart et al. 2007; Zhong et al. 2007). Hindlimb paralysis after complete spinal cord injury is partly due to the loss of descending monoaminergic inputs that regulate PICs and increased spinal neuronal excitability is thought to be a pre-requisite for locomotor recovery (Heckman et al. 2003, 2005). A study showed that clonidine restored sustained motoneuronal discharge following acute spinalization in decerebrate cats (Conway et al. 1988) and the same scenario probably occurs in chronic spinal cats. The role of PICs in sustaining discharge of spinal interneurons is less well understood but clonidine could enhance crossed extensor reflexes and locomotor rhythm generation by modulating intrinsic properties of shared commissural interneurons. In support of this hypothesis, sustained activity was observed during and following right Tib nerve stimulation in clonidine-treated chronic spinalized cats (examples in Figs. 5B and 8) and post-stimulation locomotor-like activity was observed in about one-third of all trials.
Functional and clinical considerations
It is important to consider that the drugs used to facilitate motor recovery after complete spinal cord injury differ between species (reviewed in Parker, 2005). Although noradrenergic agonists facilitate hindlimb locomotion in spinalized cats (Forssberg & Grillner, 1973; Naftchi, 1982; Barbeau et al. 1987; Pearson & Rossignol, 1991; Chau et al. 1998a,b), in spinal transected rodents serotonergic agonists are more effective in promoting hindlimb locomotor recovery (Antri et al. 2002, 2003; Ung et al. 2008; Courtine et al. 2009; Musienko et al. 2011). In the cat, serotonergic agonists do not initiate hindlimb locomotion following spinalization; instead they modulate the pattern once established with treadmill training (Barbeau & Rossignol, 1990). Despite striking results in spinalized animal models, noradrenergic or serotonergic agonists have weakly beneficial or even detrimental effect on walking in humans with clinically complete spinal cord injuries (Fung et al. 1990; Stewart et al. 1991; Dietz et al. 1995; Remy-Neris et al. 1999). The inter-individual variability in the efficacy of pharmacotherapy in humans with spinal cord injury is also high. As such, no pharmacological treatment to facilitate locomotor recovery in spinal cord-injured humans currently exists. Drug effectiveness can be influenced by multiple factors, such as the type of spinal cord injury (partial or complete), lesion level, time post-injury, and/or by species-dependent differences in pre-existing or injury-induced distributions of receptor types within the spinal cord (reviewed in Parker, 2005).
Studies have shown that serotonergic agonists enhanced the excitability of interlimb postural reflexes in rabbits following acute or chronic spinalization, whereas noradrenergic agonists had no effect (Lyalka et al. 2008, 2011). Thus, drugs that facilitate hindlimb locomotor recovery in cats and rodents enhance interlimb pathways. From a clinical perspective, if the physiological mechanisms leading to the emergence of a locomotor state are conserved across species, then focusing on changes in interlimb pathways would constitute an ideal target for evaluating a drug's effectiveness in humans with complete spinal cord injury.
Conclusion
In summary, clonidine enhances the excitability of neurons activated by contralateral sensory inputs from the foot in chronic spinal cats. Spinal commissural interneurons that coordinate left–right alternation during walking are thought to be integral components of the spinal locomotor central pattern generator (Kiehn, 2011) and could also be interposed in crossed extensor pathways. As such, spinal interneurons activated by contralateral inputs that also receive noradrenergic inputs could be an interesting target to identify putative central pattern generator neurons. Finally, a drug's potential effectiveness in facilitating locomotor recovery in spinal cord-injured humans could be assessed by evaluating changes in crossed extensor reflexes.
Acknowledgments
The authors would like to thank Marin Manuel and Jack Miller for technical assistance. The present research was funded by an individual grant from the Wings for Life Foundation and by a postdoctoral fellowship from the Canadian Institutes of Health Research to A.F., as well as by an NIH grant (NS034382) to C.J.H.
Glossary
Abbreviations
- EMG
electromyography
- LG
lateral gastrocnemius
- PICs
persistent inward currents
- Srt
sartorius; St, semitendinosus
- Sol
soleus
- SP
superficial peroneal
- Tib
tibial
- TA
tibialis anterior
Author contributions
Experiments were performed in the laboratory of Dr Heckman at Northwestern University. All authors were involved in the conception and design of the experiments; collection, analysis, and interpretation of data; and drafting the article or revising it critically for important intellectual content. All authors approved the final version of the manuscript.
References
- Aggelopoulos NC, Burton MJ, Clarke RW, Edgley SA. Characterization of a descending system that enables crossed group II inhibitory reflex pathways in the cat spinal cord. J Neurosci. 1996;16:723–729. doi: 10.1523/JNEUROSCI.16-02-00723.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aggelopoulos NC, Edgley SA. Segmental localisation of the relays mediating crossed inhibition of hindlimb motoneurones from group II afferents in the anaesthetized cat spinal cord. Neurosci Lett. 1995;185:60–64. doi: 10.1016/0304-3940(94)11225-8. [DOI] [PubMed] [Google Scholar]
- Antri M, Mouffle C, Orsal D, Barthe JY. 5-HT1A receptors are involved in short- and long-term processes responsible for 5-HT-induced locomotor function recovery in chronic spinal rat. Eur J Neurosci. 2003;18:1963–1972. doi: 10.1046/j.1460-9568.2003.02916.x. [DOI] [PubMed] [Google Scholar]
- Antri M, Orsal D, Barthe JY. Locomotor recovery in the chronic spinal rat: effects of long-term treatment with a 5-HT2 agonist. Eur J Neurosci. 2002;16:467–476. doi: 10.1046/j.1460-9568.2002.02088.x. [DOI] [PubMed] [Google Scholar]
- Arya T, Bajwa S, Edgley SA. Crossed reflex actions from group II muscle afferents in the lumbar spinal cord of the anaesthetized cat. J Physiol. 1991;444:117–131. doi: 10.1113/jphysiol.1991.sp018869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barbeau H, Julien C, Rossignol S. The effects of clonidine and yohimbine on locomotion and cutaneous reflexes in the adult chronic spinal cat. Brain Res. 1987;437:83–96. doi: 10.1016/0006-8993(87)91529-0. [DOI] [PubMed] [Google Scholar]
- Barbeau H, Rossignol S. Recovery of locomotion after chronic spinalization in the adult cat. Brain Res. 1987;412:84–95. doi: 10.1016/0006-8993(87)91442-9. [DOI] [PubMed] [Google Scholar]
- Barbeau H, Rossignol S. The effects of serotonergic drugs on the locomotor pattern and on cutaneous reflexes of the adult chronic spinal cat. Brain Res. 1990;514:55–67. doi: 10.1016/0006-8993(90)90435-e. [DOI] [PubMed] [Google Scholar]
- Barthelemy D, Leblond H, Provencher J, Rossignol S. Nonlocomotor and locomotor hindlimb responses evoked by electrical microstimulation of the lumbar cord in spinalized cats. J Neurophysiol. 2006;96:3273–3292. doi: 10.1152/jn.00203.2006. [DOI] [PubMed] [Google Scholar]
- Berkowitz A. Physiology and morphology indicate that individual spinal interneurons contribute to diverse limb movements. J Neurophysiol. 2005;94:4455–4470. doi: 10.1152/jn.00229.2005. [DOI] [PubMed] [Google Scholar]
- Bernard G, Bouyer L, Provencher J, Rossignol S. Study of cutaneous reflex compensation during locomotion after nerve section in the cat. J Neurophysiol. 2007;97:4173–4185. doi: 10.1152/jn.00797.2006. [DOI] [PubMed] [Google Scholar]
- Bouyer LJ, Whelan PJ, Pearson KG, Rossignol S. Adaptive locomotor plasticity in chronic spinal cats after ankle extensors neurectomy. J Neurosci. 2001;21:3531–3541. doi: 10.1523/JNEUROSCI.21-10-03531.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bras H, Jankowska E, Noga B, Skoog B. Comparison of effects of various types of NA and 5-HT agonists on transmission from group ii muscle afferents in the cat. Eur J Neurosci. 1990;2:1029–1039. doi: 10.1111/j.1460-9568.1990.tb00015.x. [DOI] [PubMed] [Google Scholar]
- Chau C, Barbeau H, Rossignol S. Early locomotor training with clonidine in spinal cats. J Neurophysiol. 1998a;79:392–409. doi: 10.1152/jn.1998.79.1.392. [DOI] [PubMed] [Google Scholar]
- Chau C, Barbeau H, Rossignol S. Effects of intrathecal α1- and α2-noradrenergic agonists and norepinephrine on locomotion in chronic spinal cats. J Neurophysiol. 1998b;79:2941–2963. doi: 10.1152/jn.1998.79.6.2941. [DOI] [PubMed] [Google Scholar]
- Cleland CL, Hayward L, Rymer WZ. Neural mechanisms underlying the clasp-knife reflex in the cat. II. Stretch-sensitive muscular-free nerve endings. J Neurophysiol. 1990;64:1319–1330. doi: 10.1152/jn.1990.64.4.1319. [DOI] [PubMed] [Google Scholar]
- Cleland CL, Rymer WZ. Neural mechanisms underlying the clasp-knife reflex in the cat. I. Characteristics of the reflex. J Neurophysiol. 1990;64:1303–1318. doi: 10.1152/jn.1990.64.4.1303. [DOI] [PubMed] [Google Scholar]
- Conway BA, Hultborn H, Kiehn O, Mintz I. Plateau potentials in α-motoneurones induced by intravenous injection of L-dopa and clonidine in the spinal cat. J Physiol. 1988;405:369–384. doi: 10.1113/jphysiol.1988.sp017337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cote MP, Gossard JP. Step training-dependent plasticity in spinal cutaneous pathways. J Neurosci. 2004;24:11317–11327. doi: 10.1523/JNEUROSCI.1486-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cote MP, Menard A, Gossard JP. Spinal cats on the treadmill: changes in load pathways. J Neurosci. 2003;23:2789–2796. doi: 10.1523/JNEUROSCI.23-07-02789.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Courtine G, Gerasimenko Y, van den Brand R, Yew A, Musienko P, Zhong H, Song B, Ao Y, Ichiyama RM, Lavrov I, Roy RR, Sofroniew MV, Edgerton VR. Transformation of nonfunctional spinal circuits into functional states after the loss of brain input. Nat Neurosci. 2009;12:1333–1342. doi: 10.1038/nn.2401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dietz V, Colombo G, Jensen L, Baumgartner L. Locomotor capacity of spinal cord in paraplegic patients. Ann Neurol. 1995;37:574–582. doi: 10.1002/ana.410370506. [DOI] [PubMed] [Google Scholar]
- Drummond GB. Reporting ethical matters in The Journal of Physiology: standards and advice. J Physiol. 2009;587:713–719. doi: 10.1113/jphysiol.2008.167387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edgerton VR, Courtine G, Gerasimenko YP, Lavrov I, Ichiyama RM, Fong AJ, Cai LL, Otoshi CK, Tillakaratne NJ, Burdick JW, Roy RR. Training locomotor networks. Brain Res Rev. 2008;57:241–254. doi: 10.1016/j.brainresrev.2007.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edgerton VR, Leon RD, Harkema SJ, Hodgson JA, London N, Reinkensmeyer DJ, Roy RR, Talmadge RJ, Tillakaratne NJ, Timoszyk W, Tobin A. Retraining the injured spinal cord. J Physiol. 2001;533:15–22. doi: 10.1111/j.1469-7793.2001.0015b.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edgley SA, Jankowska E, Krutki P, Hammar I. Both dorsal horn and lamina VIII interneurones contribute to crossed reflexes from feline group II muscle afferents. J Physiol. 2003;552:961–974. doi: 10.1113/jphysiol.2003.048009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forssberg H, Grillner S. The locomotion of the acute spinal cat injected with clonidine i.v. Brain Res. 1973;50:184–186. doi: 10.1016/0006-8993(73)90606-9. [DOI] [PubMed] [Google Scholar]
- Frigon A. Interindividual variability and its implications for locomotor adaptation following peripheral nerve and/or spinal cord injury. Prog Brain Res. 2011;188:101–118. doi: 10.1016/B978-0-444-53825-3.00012-7. [DOI] [PubMed] [Google Scholar]
- Frigon A. Central pattern generators of the mammalian spinal cord. Neuroscientist. 2012 doi: 10.1177/1073858410396101. (in press; doi: 10.1177/1073858410396101) [DOI] [PubMed] [Google Scholar]
- Frigon A, Johnson MD, Heckman CJ. Altered activation patterns by triceps surae stretch reflex pathways in acute and chronic spinal cord injury. J Neurophysiol. 2011;106:1669–1678. doi: 10.1152/jn.00504.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frigon A, Rossignol S. Functional plasticity following spinal cord lesions. Prog Brain Res. 2006;157:231–260. doi: 10.1016/s0079-6123(06)57016-5. [DOI] [PubMed] [Google Scholar]
- Frigon A, Rossignol S. Plasticity of reflexes from the foot during locomotion after denervating ankle extensors in intact cats. J Neurophysiol. 2007;98:2122–2132. doi: 10.1152/jn.00490.2007. [DOI] [PubMed] [Google Scholar]
- Frigon A, Rossignol S. Adaptive changes of the locomotor pattern and cutaneous reflexes during locomotion studied in the same cats before and after spinalization. J Physiol. 2008a;586:2927–2945. doi: 10.1113/jphysiol.2008.152488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frigon A, Rossignol S. Locomotor and reflex adaptation after partial denervation of ankle extensors in chronic spinal cats. J Neurophysiol. 2008b;100:1513–1522. doi: 10.1152/jn.90321.2008. [DOI] [PubMed] [Google Scholar]
- Frigon A, Rossignol S. Short-latency crossed inhibitory responses in extensor muscles during locomotion in the cat. J Neurophysiol. 2008c;99:989–998. doi: 10.1152/jn.01274.2007. [DOI] [PubMed] [Google Scholar]
- Fung J, Stewart JE, Barbeau H. The combined effects of clonidine and cyproheptadine with interactive training on the modulation of locomotion in spinal cord injured subjects. J Neurol Sci. 1990;100:85–93. doi: 10.1016/0022-510x(90)90017-h. [DOI] [PubMed] [Google Scholar]
- Grey MJ, Ladouceur M, Andersen JB, Nielsen JB, Sinkjaer T. Group II muscle afferents probably contribute to the medium latency soleus stretch reflex during walking in humans. J Physiol. 2001;534:925–933. doi: 10.1111/j.1469-7793.2001.00925.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hammar I, Bannatyne BA, Maxwell DJ, Edgley SA, Jankowska E. The actions of monoamines and distribution of noradrenergic and serotoninergic contacts on different subpopulations of commissural interneurons in the cat spinal cord. Eur J Neurosci. 2004;19:1305–1316. doi: 10.1111/j.l460-9568.2004.03239.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hammar I, Stecina K, Jankowska E. Differential modulation by monoamine membrane receptor agonists of reticulospinal input to lamina VIII feline spinal commissural interneurons. Eur J Neurosci. 2007;26:1205–1212. doi: 10.1111/j.1460-9568.2007.05764.x. [DOI] [PubMed] [Google Scholar]
- Harkema SJ. Neural plasticity after human spinal cord injury: application of locomotor training to the rehabilitation of walking. Neuroscientist. 2001;7:455–468. doi: 10.1177/107385840100700514. [DOI] [PubMed] [Google Scholar]
- Heckman CJ, Lee RH, Brownstone RM. Hyperexcitable dendrites in motoneurons and their neuromodulatory control during motor behavior. Trends Neurosci. 2003;26:688–695. doi: 10.1016/j.tins.2003.10.002. [DOI] [PubMed] [Google Scholar]
- Heckmann CJ, Gorassini MA, Bennett DJ. Persistent inward currents in motoneuron dendrites: implications for motor output. Muscle Nerve. 2005;31:135–156. doi: 10.1002/mus.20261. [DOI] [PubMed] [Google Scholar]
- Hyngstrom AS, Johnson MD, Miller JF, Heckman CJ. Intrinsic electrical properties of spinal motoneurons vary with joint angle. Nat Neurosci. 2007;10:363–369. doi: 10.1038/nn1852. [DOI] [PubMed] [Google Scholar]
- Jankowska E. Interneuronal relay in spinal pathways from proprioceptors. Prog Neurobiol. 1992;38:335–378. doi: 10.1016/0301-0082(92)90024-9. [DOI] [PubMed] [Google Scholar]
- Jankowska E, Bannatyne BA, Stecina K, Hammar I, Cabaj A, Maxwell DJ. Commissural interneurons with input from group I and II muscle afferents in feline lumbar segments: neurotransmitters, projections and target cells. J Physiol. 2009;587:401–418. doi: 10.1113/jphysiol.2008.159236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jankowska E, Hammar I. Spinal interneurones; how can studies in animals contribute to the understanding of spinal interneuronal systems in man? Brain Res Brain Res Rev. 2002;40:19–28. doi: 10.1016/s0165-0173(02)00185-6. [DOI] [PubMed] [Google Scholar]
- Kiehn O. Development and functional organization of spinal locomotor circuits. Curr Opin Neurobiol. 2011;21:100–109. doi: 10.1016/j.conb.2010.09.004. [DOI] [PubMed] [Google Scholar]
- Loeb GE. The distal hindlimb musculature of the cat: interanimal variability of locomotor activity and cutaneous reflexes. Exp Brain Res. 1993;96:125–140. doi: 10.1007/BF00230446. [DOI] [PubMed] [Google Scholar]
- Lovely RG, Gregor RJ, Roy RR, Edgerton VR. Effects of training on the recovery of full-weight-bearing stepping in the adult spinal cat. Exp Neurol. 1986;92:421–435. doi: 10.1016/0014-4886(86)90094-4. [DOI] [PubMed] [Google Scholar]
- Lyalka VF, Hsu LJ, Karayannidou A, Zelenin PV, Orlovsky GN, Deliagina TG. Facilitation of postural limb reflexes in spinal rabbits by serotonergic agonist administration, epidural electrical stimulation, and postural training. J Neurophysiol. 2011;106:1341–1354. doi: 10.1152/jn.00115.2011. [DOI] [PubMed] [Google Scholar]
- Lyalka VF, Musienko PE, Orlovsky GN, Grillner S, Deliagina TG. Effect of intrathecal administration of serotoninergic and noradrenergic drugs on postural performance in rabbits with spinal cord lesions. J Neurophysiol. 2008;100:723–732. doi: 10.1152/jn.90218.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marder E, Goaillard JM. Variability, compensation and homeostasis in neuron and network function. Nat Rev Neurosci. 2006;7:563–574. doi: 10.1038/nrn1949. [DOI] [PubMed] [Google Scholar]
- Musienko P, van den Brand R, Marzendorfer O, Roy RR, Gerasimenko Y, Edgerton VR, Courtine G. Controlling specific locomotor behaviors through multidimensional monoaminergic modulation of spinal circuitries. J Neurosci. 2011;31:9264–9278. doi: 10.1523/JNEUROSCI.5796-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Musienko PE, Zelenin PV, Orlovsky GN, Deliagina TG. Facilitation of postural limb reflexes with epidural stimulation in spinal rabbits. J Neurophysiol. 2010;103:1080–1092. doi: 10.1152/jn.00575.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naftchi NE. Functional restoration of the traumatically injured spinal cord in cats by clonidine. Science. 1982;217:1042–1044. doi: 10.1126/science.6126002. [DOI] [PubMed] [Google Scholar]
- Nichols TR, Stein RB, Bawa P. Spinal reflexes as a basis for tremor in the premammillary cat. Can J Physiol Pharmacol. 1978;56:375–383. doi: 10.1139/y78-056. [DOI] [PubMed] [Google Scholar]
- Nielsen JB, Crone C, Hultborn H. The spinal pathophysiology of spasticity – from a basic science point of view. Acta Physiol (Oxf) 2007;189:171–180. doi: 10.1111/j.1748-1716.2006.01652.x. [DOI] [PubMed] [Google Scholar]
- Noga BR, Johnson DM, Riesgo MI, Pinzon A. Locomotor-activated neurons of the cat. II. Noradrenergic innervation and colocalization with NEα1a or NEα2b receptors in the thoraco-lumbar spinal cord. J Neurophysiol. 2011;105:1835–1849. doi: 10.1152/jn.00342.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parker D. Pharmacological approaches to functional recovery after spinal injury. Curr Drug Targets CNS Neurol Disord. 2005;4:195–210. doi: 10.2174/1568007053544192. [DOI] [PubMed] [Google Scholar]
- Pearson KG, Fouad K, Misiaszek JE. Adaptive changes in motor activity associated with functional recovery following muscle denervation in walking cats. J Neurophysiol. 1999;82:370–381. doi: 10.1152/jn.1999.82.1.370. [DOI] [PubMed] [Google Scholar]
- Pearson KG, Rossignol S. Fictive motor patterns in chronic spinal cats. J Neurophysiol. 1991;66:1874–1887. doi: 10.1152/jn.1991.66.6.1874. [DOI] [PubMed] [Google Scholar]
- Rack PM, Westbury DR. The effects of length and stimulus rate on tension in the isometric cat soleus muscle. J Physiol. 1969;204:443–460. doi: 10.1113/jphysiol.1969.sp008923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Remy-Neris O, Barbeau H, Daniel O, Boiteau F, Bussel B. Effects of intrathecal clonidine injection on spinal reflexes and human locomotion in incomplete paraplegic subjects. Exp Brain Res. 1999;129:433–440. doi: 10.1007/s002210050910. [DOI] [PubMed] [Google Scholar]
- Remy-Neris O, Denys P, Daniel O, Barbeau H, Bussel B. Effect of intrathecal clonidine on group I and group II oligosynaptic excitation in paraplegics. Exp Brain Res. 2003;148:509–514. doi: 10.1007/s00221-002-1313-4. [DOI] [PubMed] [Google Scholar]
- Rossignol S, Dubuc R, Gossard JP. Dynamic sensorimotor interactions in locomotion. Physiol Rev. 2006;86:89–154. doi: 10.1152/physrev.00028.2005. [DOI] [PubMed] [Google Scholar]
- Rossignol S, Frigon A. Recovery of locomotion after spinal cord injury: some facts and mechanisms. Annu Rev Neurosci. 2011;34:413–440. doi: 10.1146/annurev-neuro-061010-113746. [DOI] [PubMed] [Google Scholar]
- Rossignol S, Gauthier L. An analysis of mechanisms controlling the reversal of crossed spinal reflexes. Brain Res. 1980;182:31–45. doi: 10.1016/0006-8993(80)90828-8. [DOI] [PubMed] [Google Scholar]
- Schieppati M, Nardone A. Group II spindle afferent fibers in humans: their possible role in the reflex control of stance. Prog Brain Res. 1999;123:461–472. doi: 10.1016/s0079-6123(08)62882-4. [DOI] [PubMed] [Google Scholar]
- Schomburg ED, Steffens H. The effect of DOPA and clonidine on reflex pathways from group II muscle afferents to α-motoneurones in the cat. Exp Brain Res. 1988;71:442–446. doi: 10.1007/BF00247505. [DOI] [PubMed] [Google Scholar]
- Silverman J, Garnett NL, Giszter SF, Heckman CJ, 2nd, Kulpa-Eddy JA, Lemay MA, Perry CK, Pinter M. Decerebrate mammalian preparations: unalleviated or fully alleviated pain? A review and opinion. Contemp Top Lab Anim Sci. 2005;44:34–36. [PubMed] [Google Scholar]
- Stewart JE, Barbeau H, Gauthier S. Modulation of locomotor patterns and spasticity with clonidine in spinal cord injured patients. Can J Neurol Sci. 1991;18:321–332. doi: 10.1017/s0317167100031887. [DOI] [PubMed] [Google Scholar]
- Tazerart S, Viemari JC, Darbon P, Vinay L, Brocard F. Contribution of persistent sodium current to locomotor pattern generation in neonatal rats. J Neurophysiol. 2007;98:613–628. doi: 10.1152/jn.00316.2007. [DOI] [PubMed] [Google Scholar]
- Ung RV, Landry ES, Rouleau P, Lapointe NP, Rouillard C, Guertin PA. Role of spinal 5-HT2 receptor subtypes in quipazine-induced hindlimb movements after a low-thoracic spinal cord transection. Eur J Neurosci. 2008;28:2231–2242. doi: 10.1111/j.1460-9568.2008.06508.x. [DOI] [PubMed] [Google Scholar]
- Zhong G, Masino MA, Harris-Warrick RM. Persistent sodium currents participate in fictive locomotion generation in neonatal mouse spinal cord. J Neurosci. 2007;27:4507–4518. doi: 10.1523/JNEUROSCI.0124-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]


