Abstract
Non-technical summary
Prenatal stress (PS) has been associated with a higher risk for the development of various neurological and psychiatric disorders later in life, but the underlying mechanisms are not yet fully understood. Our results support an action mode in which PS downregulates tissue plasminogen activator levels within the hippocampus, inhibiting the proteolytic conversion of pro-brain-derived neurotrophic factor (pro-BDNF) to the mature form of BDNF, thereby leading to long-lasting alterations of the properties of synaptic plasticity. Our findings bolster the idea that stressful experience during gestation or early in life may lead to long-lasting malfunction of the hippocampus and our PS model may be useful for the development of more effective intervention and prevention strategies.
Abstract
Prenatal stress (PS) has been associated with a higher risk of development of various neurological and psychiatric disorders later in life, but the underlying mechanisms are not yet fully understood. Here, using a chronic prenatal restraint stress model where the rat dams were immobilized for 45 min three times per day during the last week of pregnancy, we explored the long-lasting effects of PS on hippocampal synaptic plasticity in the offspring of both sexes. We found that PS switched the direction of synaptic plasticity in hippocampal CA1 region, favouring low-frequency stimulation-induced long-term depression (LTD) and opposing the induction of long-term potentiation (LTP) by high-frequency stimulation in young (5-week-old) rat offspring, but these changes disappeared at adult age (8 weeks old). Fostering of PS offspring to control dams did not alter the effects of PS on LTP and LTD. In addition, PS-induced changes in LTP and LTD induction were correlated with increasing endogenous pro-brain-derived neurotrophic factor (pro-BDNF) and decreasing of the mature form of BDNF (mBDNF) levels. Furthermore, PS resulted in a significant decrease in the activity and expression of tissue plasminogen activator (tPA), a key serine protease involved in the extracellular conversion of pro-BDNF to mBDNF. No significant differences were observed between the sexes for the effects of PS on hippocampal synaptic plasticity, the levels of pro-BDNF and mBDNF, and tPA expression. These results suggest that PS downregulates tPA levels within the hippocampus, inhibiting the proteolytic conversion of pro-BDNF to mBDNF, thereby leading to long-lasting alterations of the properties of synaptic plasticity.
Introduction
Both preclinical and clinical studies have shown that exposure to stress during pregnancy has profound effects on the neurodevelopment and behaviour of the offspring, which may lead to cognitive deficits and increasing incidence of the development of neuropsychiatric disorders, including depression, anxiety, schizophrenia and autism (Gillott & Standen, 2007; Rice et al. 2007; Walker et al. 2008; Charil et al. 2010). Although the molecular mechanisms contributing to increased risk of developing these disorders remain unclear, several contributors have been proposed to be involved in changes of fetal development. One potential mechanism whereby prenatal stress (PS) can influence the fetal development is through the alteration in programming of the hypothalamus–pituitary–adrenal (HPA) axis function, a major system in controlling the organism's response to stress and regulating certain circadian activity. For example, it has been shown that chronic exposure to restrain stress in utero reprograms the fetal HPA axis (Owen et al. 2005; Weinstock, 2005). In addition, PS rats show a reduced hippocampal glucocorticoid receptor expression in adulthood, which leads to the attenuation of HPA axis feedback loop sensitivity and gives rise to increased corticosterone levels in the resting state, as well as after exposure to stressful situations (Vallée et al. 1997). Furthermore, the enduring effects of PS on HPA axis function seem to be the consequence of excessive exposure of the developing fetus to maternal corticosterone since such abnormalities can be effectively prevented by maternal adrenalectomy and restored with corticosterone administration (Maccari et al. 2003; Zagron & Weinstock, 2006), suggesting that fetal overexposure to maternal glucocorticoid might underpin the link between early life stress and the increased incidence of diseases later in life.
Although it is now clear that PS is associated with alterations in the offspring's HPA axis function, the downstream effectors of the HPA axis contributing to the effects of PS on fetal brain are still poorly understood. Beyond alterations in baseline corticosterone levels, recent studies have demonstrated that PS may perturb biosynthesis of the brain-derived neurotrophic factor (BDNF) in the hippocampus (Van den Hove et al. 2006; Zuena et al. 2008), but no consensus has thus far emerged. Van den Hove and co-workers 2006) have shown that PS resulted in a reduction in BDNF protein levels in the hippocampus at postnatal day 5, an effect that was not detected at birth or postnatal day 8 and 15. However, a significant increase of BDNF protein levels in the hippocampus has been reported in adult male PS rats (Zuena et al. 2008). The question remains, therefore, whether PS causes long-lasting changes in hippocampal BDNF expression, which will ultimately contribute to alterations in brain function leading to an increased incidence of cognitive and neuropsychiatric disorders. Using a chronic immobilization stress on pregnant mice, PS has also been shown to effectively reduce the expression of NR1 and NR2B NMDA receptor subunits at the synapses of the offspring's hippocampus, a mechanism that may contribute to impairment in hippocampus-dependent spatial memory and NMDA receptor-mediated long-term potentiation (LTP) (Son et al. 2006). In contrast, a recent study using daily varied stress from day 17–22 of gestation has shown that PS only caused a decrease in NR2B subunits in total hippocampal homogenates of juvenile male rats but did not affect amounts of either NR1 or NR2A subunits (Yaka et al. 2007). Therefore, it cannot be determined from these inconsistent results whether altered BDNF or NMDA receptor expression may represent the molecular basis for mediating the long-lasting effects of PS on fetal brain function.
Activity-dependent changes in synaptic strength, like LTP and long-term depression (LTD), are critical for the refinement of neural connections in the developing nervous system (Zhang & Poo, 2001) and for learning and memory in the mature brain (Bliss & Collingridge, 1993). Although PS has been shown to impair LTP but facilitate LTD induction in the hippocampal CA1 region (Son et al. 2006; Yang et al. 2006; Yaka et al. 2007), the underlying mechanisms remain unclear. Here, we re-examine the issue of whether PS permanently alters the induction of hippocampal CA1 LTP and LTD and explore its possible underlying mechanisms. We focused specifically on the hippocampus because it is a primary target brain area for PS (Uno et al. 1989; Lemaire et al. 2000). We used a chronic prenatal restraint stress model where the rat dams were immobilized for 45 min three times per day during the last week of pregnancy and the offspring assessed at different developmental stages in both sexes. Our results indicate that the effects of PS on LTP and LTD were correlated with increasing endogenous pro-BDNF and reducing the mature form of BDNF (mBDNF) levels.
Methods
Animals and prenatal stress
The authors have read, and the experiments comply with the policies and regulations of The Journal of Physiology, given by Drummond (2009). All procedures were performed according to NIH guidelines for animal research (Guide for the Care & Use of Laboratory Animals, NRC, 1996) and were approved by the Institutional Animal Care and Use Committee of National Cheng Kung University. Pregnant Sprague–Dawley rats (250–280 g body weight) were housed in individual cages with a 12 h light–dark cycle (lights on from 07:00 to 19:00 h) and ad libitum access to food and water. Pregnant females were randomly assigned to PS or control group and individually housed in plastic breeding cages. Prenatal restrain stress was performed according the protocol as described by Ward & Weisz (1984). Briefly, pregnant females were subjected to three 45 min daily stress sessions starting at 10:00, 14:00 and 18:00 h, in a transparent plastic cylinder (7 cm diameter, 19 cm long, with breathing holes) under intense illumination from days 15–21 of pregnancy. This stress protocol was chosen as it has an indirect influence on the fetus via a direct stress on the mother. Control pregnant females were left undisturbed in their home cages. On the day of birth, the number of pups per litter was standardized to eight with equal numbers of both sexes. In most experiments, the offspring were raised by their biological mothers until weaning (21 days after birth). In a subset of experiments, the pups were raised by non-handled control foster mothers. The pups were placed in the cage of the foster mothers within the first 3–6 h after birth. During this procedure, the foster mothers were briefly removed from their home cages. After weaning, male and female rats from each experimental group were housed in groups of four and maintained under similar environmental conditions until the experiments were started. All efforts were made to minimize animal suffering and to use only the number of animals necessary to produce reliable scientific data.
Hippocampal slice preparations and electrophysiology
Hippocampal slices were prepared from 3- to 8-week-old rats using standard procedures described previously (Lin et al. 2006). In brief, rats were killed by decapitation under isoflurane anaesthesia, and hippocampal slices (400 μm thick) were prepared using a Leica VT1200S vibrating blade microtome (Leica, Nussloch, Germany). The slices were placed in a storage chamber of artificial CSF (ACSF) oxygenated with 95% O2–5% CO2 and kept at room temperature for at least 1 h before recording. The composition of the ACSF solution was (in mm): 117 NaCl, 4.7 KCl, 2.5 CaCl2, 1.2 MgCl2, 25 NaHCO3, 1.2 NaH2PO4 and 11 glucose. For recording, one slice was transferred to a submersion-type recording chamber continually perfused with oxygenated ACSF at a flow rate of 2–3 ml min−1 at 32.0 ± 0.5°C. The extracellular field potential recordings were made using an Axoclamp-2B amplifier (Axon Instruments, Union City, CA, USA). Microelectrodes were pulled from microfibre 1.0 mm capillary tubing on a Brown-Flaming electrode puller (Sutter Instruments, San Rafael, CA, USA). The responses were low-pass filtered at 2 kHz, digitally sampled at 5–10 kHz, and analysed using pCLAMP software (version 8.0; Axon Instruments). Postsynaptic responses were evoked in CA1 stratum radiatum by stimulation of Schaffer collateral/commissural afferents at 0.033 Hz with a bipolar stimulating electrode. The stimulation strength was set to elicit response for which the amplitude was 30–40% of the maximum spike-free response. Field excitatory postsynaptic potentials (fEPSPs) were recorded with a glass pipette filled with 1 m NaCl (2–3 MΩ resistance) and the fEPSP slope was measured from approximately 20–70% of the rising phase using a least-squares regression. The LTP was induced by high-frequency stimulation (HFS), at the test pulse intensity, consisting of two 1 s trains of stimuli separated by an intertrain interval of 20 s at 100 Hz. The LTD was induced by low-frequency stimulation (LFS) delivered at 1 Hz for 15 min (900 pulses) or a brief bath application of the group I metabotropic glutamate receptor (mGluR) agonist (S)-3,5,-dihydroxyphenylglycine (DHPG; Tocris Cookson Ltd, Bristol, UK) for 5 min. To further obtain the relationship between stimulation frequency and synaptic plasticity, LTD was also evoked using 900 pulses at 5 or 10 Hz. The crossover point between LTP and LTD observed in the frequency–response curve was defined as the synaptic modification threshold (θm). The magnitudes of LTP and LTD were averaged for the responses recorded during the last 10 min of recording (50–60 min). The experiments were performed with the experimenter blind to the status of the animals from which slices were prepared. In some experiments, hippocampal slices were preincubated with the functional anti-pro-BDNF antibody (100 μg ml−1; R&D Systems, Minneapolis, MN, USA), mouse IgG (100 μg ml−1; Santa Cruz Biotechnology, Santa Cruz, CA, USA) or recombinant rat tissue plasminogen activator (tPA; 60 μg ml−1; Molecular Innovations Inc., Southfield, MI, USA) for 1 h before transfer to the recording chamber to start the experiment. The doses were selected based on previous and our pilot studies (Yang et al. 2009).
Preparation of synaptoneurosomes
Synaptoneurosomes were prepared according to the procedure of Wells et al. (2001). Briefly, the microdissected CA1 regions were homogenized in ice-cold Ca2+- and Mg2+-free buffer (in mm: 50 Hepes, 100 NaCl and 3 potassium acetate; pH 7.4) with RNase inhibitor (15 U ml−1) and was centrifuged at 2000 g for 1 min. Supernatants were passed through two 100 μm nylon mesh filters, followed by a 5 μm pore filter. The filtrate was then centrifuged at 1000 g for 10 min and then was gently resuspended in the same buffer at a protein concentration of 2 mg ml−1.
Preparation of subcellular fractions
Subcellular fractions were prepared according to the procedure of Huttner et al. (1983). The homogenate was centrifuged at 1000 g for 10 min to remove nuclei and cell debris, and the supernatant (S1) was transferred to a new tube and centrifuged at 10,000 g for 30 min to generate a crude synaptosomal fraction (P2). The supernatant (S2) was collected and spun at 165,000 g for 2 h to yield a cytosolic fraction (S3) and a light membrane/microsome-enriched fraction (P3). The P2 fraction was washed once in HBS buffer (in mm: 10 Hepes-KOH, 142 NaCl, 2.4 KCl, 1 MgCl2, 5 glucose, 0.1 EGTA and 0.3 phenylmethylsulfonyl fluoride (PMSF); pH 7.5) and centrifuged once more at 13,000 g for 15 min. It was then lysed in 10 volume of ice-cold H2O containing 0.3 mm PMSF for 30 min, buffered with 1 m Hepes-KOH, pH 7.4, and centrifuged at 25,000 g for 30 min to generate the synaptosomal membrane fraction (LP1). LP1 was resuspended in isolation buffer. All procedures were performed at 4°C.
Quantitative real-time PCR
Total RNA was isolated from hippocampal CA1 tissue lysates using a Tri Reagent kit (Molecular Research Centre, Cincinnati, OH, USA) and treated with RNase-free DNase (RQ1; Promega, Madison, WI, USA) to remove potential contamination by genomic DNA. Total RNA (1 μg) from samples was reverse transcribed using a SuperScript cDNA synthesis kit (Invitrogen, Carlsbad, CA, USA). Real-time PCR was performed on the Roche LightCycler instrument (Roche Diagnostics, Indianapolis, IN, USA) using the FastStart DNA Master SYBR Green I kit (Roche Applied Science) according to the manufacturer's instructions. The following primers were used: tPA, 5′-TCTTCTGTGGAAGAGGAAGAGG-3′ (forward) and 5′-TGAGGCCATGATTAAGAGG-3′ (reverse); β-actin, 5′-TTCTACAATGAGCTGCGTGTGGC-3′ (forward) and 5′-CTCATAGCTCTTCTCCAGGGAGGA-3′ (reverse). Samples were amplified for 40 cycles consisting of 95°C (30 s), 60°C (45 s) and 72°C (40 s), and PCR amplifications were repeated in duplicate. After amplification, equal volumes of PCR products were subjected to electrophoresis on 1.5% (w/v) agarose gels and visualized with ethidium bromide. A melting curve was created at the end of the PCR cycle to confirm that a single product had been amplified. Data were analysed by LightCycler quantification software to determine the threshold cycle above background for each reaction. The relative transcript amount of tPA, which was calculated using standard curves of serial RNA dilutions, was normalized to expression of β-actin mRNA.
Western blotting
For each experimental group, homogenates from at least three slices were pooled. The microdissected CA1 subregions were lysed in ice-cold Tris-HCl buffer solution (TBS; pH 7.4) containing a cocktail of protein phosphatase and proteinase inhibitors (50 mm Tris-HCl, 100 mm NaCl, 15 mm sodium pyrophosphate, 50 mm sodium fluoride, 1 mm sodium orthovanadate, 5 mm EGTA, 5 mm EDTA, 1 mm phenylmethylsulfonyl fluoride, 1 μm microcystin-LR, 1 μm okadaic acid, 0.5% Triton X-100, 2 mm benzamidine, 60 μg ml−1 aprotinin and 60 μg ml−1 leupeptin) to avoid dephosphorylation and degradation of proteins, and ground with a pellet pestle (Kontes glassware, Vineland, NJ, USA). Samples were sonicated and spun down at 15,000 g at 4°C for 10 min. The supernatant was then assayed for total protein concentration using a Bio-Rad Bradford Protein Assay Kit (Hercules, CA, USA). Each sample from tissue homogenate or postsynaptic density (PSD) protein was separated in 8% (for NMDA receptor subunit analysis), 10% (for tissue plasminogen activator (tPA) analysis) or 12% (for pro-BDNF and mBDNF analysis) SDS-PAGE gel. Following the transfer on nitrocellulose or polyvinylidene fluoride membranes, blots were blocked in buffer solution containing 5% milk and 0.1% Tween-20 in PBS (in mm: 124 NaCl, 4 KCl, 10 Na2HPO4 and 10 KH2PO4; pH 7.2) for 1 h and then blotted for 2 h at room temperature with the antibodies that recognize NR1 (1:4000; Santa Cruz Biotechnology), NR2A (1:1000; Santa Cruz Biotechnology), NR2B (1:1000; Santa Cruz Biotechnology), tPA (1:1000; Santa Cruz Biotechnology), BDNF (1:500, Santa Cruz Biotechnology) or β-actin (1:2000, Sigma-Aldrich, St Louis, MO, USA). It was then probed with HRP-conjugated secondary antibody for 1 h and developed using the ECL immunoblotting detection system (Amersham Biosciences, Buckinghamshire, UK), according to manufacturer's instructions. Immunoblots were analysed by densitometry using Bio-profil BioLight PC software. Only film exposures that were in the linear range of the ECL reaction were used for quantification analysis. The expression level of NR1, NR2A, NR2B or tPA was evaluated relative to that for β-actin. Background correction values were subtracted from each lane to minimize the variability across membranes.
tPA zymography
tPA activity was assayed by zymography according to the procedure of Adhami et al. (2008). Plasminogen (10 μg ml−1; American Diagnostica Inc., Stamford, CT, USA) and casein (1 mg ml−1; Sigma-Aldrich) were added to the SDS-PAGE gel. Protein samples were extracted using radio immunoprecipitation assay (RIPA) buffer and mixed with an equal volume of the sample buffer (0.5 m Tris-HCl, pH 6.8, 100% glycerol, 0.05% bromophenol blue, 10% SDS) and run at a low voltage for up to 6 h. After electrophoresis, the gel was washed twice (30 min each) with 2.5% Triton X-100 at room temperature with gentle shaking. The gel was then incubated in glycine buffer (0.1 m glycine, pH 8.0) at 37°C for 24 h to allow caseinolysis to occur, followed by staining with Coomassie blue solution and destaining with 20% methanol and 10% acetic acid. tPA activity was visualized as light bands resulting from casein degradation and recombinant rat tPA (Molecular Innovations Inc.) was used as the positive control to reveal the tPA band in the zymogram. Zymography gels were analysed by scanning densitometry and expressed as arbitrary units.
Matrix metalloprotease zymography
Matrix metalloproteinase-2 (MMP-2) and MMP-9 activity were assayed by zymography (Adhami et al. 2008). Porcine skin gelatin (0.15%, Sigma-Aldrich) was added to the SDS-PAGE gel. Protein samples were extracted and ran as mentioned above. The gels were washed twice (30 min each) with 2.5% Triton X-100 at room temperature, then incubated in reaction buffer (in mm: 50 Tris, 200 NaCl and 5 CaCl2; pH 7.5) at 37°C for 48 h to allow gelatinolysis to occur, followed by staining with Coomassie blue solution and destaining with 20% methanol and 10% acetic acid.
tPA activity assay
tPA activity was quantitatively measured using a rat tPA activity assay kit (Molecular Innovations, Novi, MI, USA) according to manufacturer's instructions. Absorbance data were obtained using a 96-well Molecular Devices Spectramax Microplate Reader (Molecular Devices, Foster City, CA, USA) with a 450 nm filter, and 492 nm as reference wavelength. All assays were run in duplicate.
MMP activity assay
Matrix metalloproteinase-2 (MMP-2) and MMP-9 activity were quantitatively measured using an InnoZyme Gelatinase (MMP-2/MMP-9) activity assay kit (Calbiochem, La Jolla, CA, USA) according to manufacturer's instructions. Absorbance data were obtained using a 96-well Molecular Devices Spectramax Microplate Reader (Molecular Devices) with an excitation wavelength of 320 nm, and an emission wavelength of 405 nm. All assays were run in duplicate. The detection limits were 6.25 and 4 ng ml−1 for MMP-2 and MMP-9, respectively.
Histology and quantification
Animals were deeply anaesthetized with sodium pentobarbital (100 mg kg−1, intraperitoneally) and perfused transcardially with 0.1 m PBS and 4% paraformaldehyde. After perfusion, brains were removed and continued to be fixed in 4% paraformaldehyde for 48 h at 4°C and were then transferred to the solution containing 30% sucrose and immersed at 4°C for at least 48 h before slicing. Coronal hippocampal slices (25 μm) were collected, washed with 0.3% Triton X-100, and then incubated for blocking with a solution containing 3% goat serum in PBS. For quantitative evaluation of neuronal numbers with Nissl staining, sections were mounted directly on gelatin-coated glass slides and dried. The slides were stained with 1.0% cresyl violet, dehydrated through a series of ethanol, cleared and coverslipped with Permount (Fisher Scientific, Electron Microscopy Sciences, Washington, PA, USA). Nissl staining within the hippocampal CA1 region was quantified in images from about 2.5 to 4.5 mm posterior to Bregma for every sixth coronal section captured at 200× magnification and digitized with an Olympus BX51 microscope coupled to an Olympus DP70 digital camera (Olympus, Tokyo, Japan). Then, all images were imported into NIH ImageJ software for analysis. All counting was performed in a blind manner.
Golgi impregnation
Rats were deeply anaesthetized with sodium pentobarbital (100 mg kg−1 intraperitoneally) and transcardially perfused with ice-cold PBS and then brains were removed and immersed in Golgi–Cox solution (5% potassium dichromate, 5% mercuric chloride and 5% potassium chromate. pH = 6.5) at room temperature for 2 weeks. After transfer to a 30% sucrose solution for 48 h, 200-μm-thick coronal sections were prepared on a Leica VT1200S vibrating blade microtome. The slices were subsequently alkalinized in 18.7% ammonia, fixed in Kodak Rapid Fix solution, dehydrated in alcohol, cleared with xylene, mounted onto gelatinized slides, and coverslipped under Permount (Fisher Scientific). At least 10 pyramidal neurons from each animal (three animals per group) were chosen for analysis. Only those neurons that were impregnated in their entirety and displaying complete dendritic trees within the 200 μm section and that were relatively isolated from neighbouring cells were selected for the analysis. Hippocampal CA1 pyramidal neurons were reconstructed with a computer-assisted neuron tracing system (Neurolucida, Microbrightfield, Inc., Williston, VT, USA) attached to an Olympus BX51 microscope with a 20× objective and analysed with NIH software ImageJ running the three-dimensional Sholl analysis. Total dendritic length was determined and the shell interval was set at 10 μm. Images of dendritic spines were taken from the secondary and tertiary branches of basilar dendrites (30–100 μm from the soma) of hippocampal CA1 pyramidal neurons by using the Olympus microscope equipped with a 100× 1.25 NA oil immersion objective. Spines were defined as dendritic protrusions ≤2 μm in length, with an obvious head structure. The number of spines were counted with 50 μm dendrite segments and presented as the number of dendritic spines in 50 μm. All counting was performed in a blind manner.
Data analysis
All data are expressed as means ± SEM. Data of body weight was analysed by two-way repeated-measures analysis of variance (ANOVA), followed by Fisher's least significance difference test when appropriate. For LTP and LTD experiments, statistical analysis was performed using the Mann–Whitney U test. Western blot, real-time PCR and activity assay data were analysed using unpaired Student's t test. Number of animals used is indicated by n. Probability values of P < 0.05 were considered to represent significant differences.
Results
Effect of PS on fetal somatic growth
We first evaluated the influence of PS on litter size. No significant difference was observed in litter size between control- (12.4 ± 0.5 pups per litter, n = 10) and PS-treated groups (12.1 ± 0.6 pups per litter, n = 10; P > 0.05). To determine whether PS affected somatic growth rate, we compared the body weights of pups obtained from control- and PS-treated groups at different postnatal ages. As shown in Fig. 1, there were no significant differences in the body weights over the time period examined between control- and PS-treated groups of both sexes (male: F(1,719)= 0.02, P = 0.92; female: F(1,767)= 0.03, P = 0.96). In both control- and PS-treated groups, male rats weighed significantly more than female rats at 6–8 weeks of age (control: F(2,185)= 5.91, P < 0.05; PS: F(2,185)= 4.73, P < 0.05).
Figure 1. Effect of PS treatment on body weight.
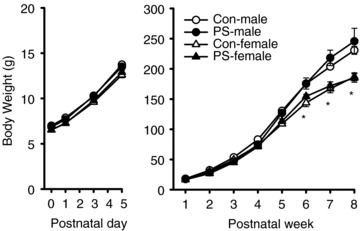
There was no significant difference in body weight gain over the time period examined between control (Con) and PS-treated groups of both sexes. In both Con and PS-treated groups, female rats gained significantly less weight than male rats at 6–8 weeks of age. Data represent the mean ± SEM. *P < 0.05 compared with Con male rats.
Effect of PS on glutamatergic synaptic transmission
To determine whether the basal glutamatergic synaptic transmission at the Schaffer collateral–CA1 synapses in young (5-week-old) pups was altered by PS, stimulus–response relationships for extracellular fEPSPs obtained from control- and PS-treated rat slices were compared. As shown in Supplementary Fig. S1A and B, stimulus–response curves of fEPSPs were similar in slices from control- and PS-treated groups of both sexes. To determine whether PS alters the presynaptic function, we examined the paired-pulse facilitation (PPF), a transient form of presynaptic plasticity in which the second of two closely spaced stimuli elicits enhanced transmitter release (Zucker, 1989). As shown in Supplementary Fig. S1C and D, pairs of presynaptic fibre stimulation pulses delivered at interpulse intervals of 20, 40, 60, 80, 150 and 200 ms evoked nearly identical amounts of PPF in slices from control- and PS-treated groups of both sexes. These results suggest that the basal synaptic transmission and presynaptic glutamate release at the Schaffer collaterial–CA1 synapses remain normal after PS treatment.
PS impairs LTP but enhances LTD induction
To examine the influence of PS on long-term synaptic plasticity, we examined the induction of hippocampal CA1 LTP and LTD in slices from control- and PS-treated pups at 3–8 weeks of age. In slices from control 3- or 5-week-old rats, conditioning HFS induced a robust LTP of fEPSPs (50–60 min after HFS: 3-week-old control male, 52.4 ± 6.5%, n = 6; 3-week-old control female, 47.3 ± 4.3%, n = 9; 5-week-old control male, 47.5 ± 5.3%, n = 7; 5-week-old control female, 53.3 ± 5.4%, n = 6), whereas in slices from PS-treated rats, the magnitude of LTP was significantly reduced in both sexes (3-week-old PS male, 29.4 ± 4.3%, n = 10; 3-week-old control female, 28.7 ± 4.4%, n = 11; 5-week-old PS male, 24.9 ± 5.4%, n = 9; 5-week-old PS female, 31.6 ± 5.4%, n = 8; P < 0.05; Fig. 2A–F). In contrast to the differences observed in the 3- or 5-week-old groups, there were no differences between the 8-week-old groups: both showed normal LTP (control male, 54.5 ± 6.5%, n = 9; control female, 59.5 ± 8.5%, n = 9; PS male, 47.5 ± 8.1%, n = 6; PS female, 48.6 ± 6.5%, n = 6; P > 0.05; Fig. 2G–I). These results suggest that PS impairs the induction of hippocampal CA1 LTP later in life of both sexes but such effects do not persist into adulthood.
Figure 2. PS impairs LTP induction in the CA1 region of the hippocampus at 3 or 5 weeks old but not at 8 weeks old.
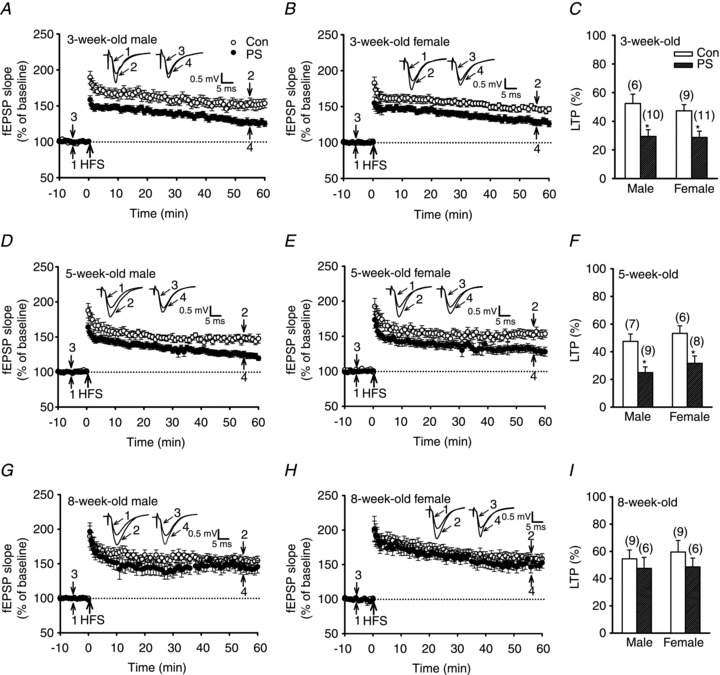
A and B, summary graphs of LTP induced with a HFS (two 1 s trains of 100 Hz stimuli separated by an intertrain interval of 20 s) in slices from 3-week-old male (A) or female (B) rats that received Con or PS treatment. C, summary bar graphs depicting levels of potentiation measured 50–60 min after HFS in slices from Con or PS-treated rats at 3 weeks old. D and E, summary graphs of LTP induced with a HFS in slices from 5-week-old male (D) or female (E) rats that received Con or PS treatment. F, summary bar graphs depicting levels of potentiation measured 50–60 min after HFS in slices from Con or PS-treated rats at 5 weeks old. G and H, summary graphs of LTP induced with a HFS in slices from 8-week-old male (G) or female (H) rats that received Con or PS treatment. I, summary bar graphs depicting levels of potentiation measured 50–60 min after HFS in slices from Con or PS-treated rats at 8 weeks old. Representative traces of fEPSPs were taken at the time indicated by number. Dashed lines show baseline level. The total number of animals examined is indicated by n in parentheses. Data represent the mean ± SEM. *P < 0.05 compared with Con rats.
LTD was induced by applying LFS at 1 Hz for 15 min (900 pulses) to the Schaffer collateral afferents onto CA1 hippocampal pyramidal neurons. As shown in Fig. 3A–C, the slices from control and PS-treated 3-week-old rats showed normal LTD of both sexes (50–60 min after the end of LFS: control male, 37.2 ± 4.3%, n = 5; control female, 32.8 ± 3.2%, n = 8; PS male, 36.2 ± 4.2%, n = 6; PS female, 36.1 ± 3.4%, n = 8; P > 0.05). However, in slices from PS-treated but not control 5-week-old rats showed reliable LTD (control male, 10.2 ± 3.3%, n = 6; control female, 9.7 ± 2.1%, n = 5; PS male, 24.5 ± 2.9%, n = 7; PS female, 24.8 ± 4.6%, n = 7; P < 0.05; Fig. 3D–F). In slices from control and PS-treated 8-week-old rats, no significant LTD was observed in both sexes (control male, 12.5 ± 5.6%, n = 5; control female, 10.1 ± 4.3%, n = 7; PS male, 16.5 ± 3.5%, n = 8; PS female, 18.2 ± 7.2%, n = 8; P > 0.05; Fig. 3G–I). These results suggest that PS enhances the induction of hippocampal CA1 LTD in later life of both sexes but this effect does not persist into adulthood.
Figure 3. PS facilitates LTD induction in the CA1 region of the hippocampus at 5 weeks old but not at 3 or 8 weeks old.
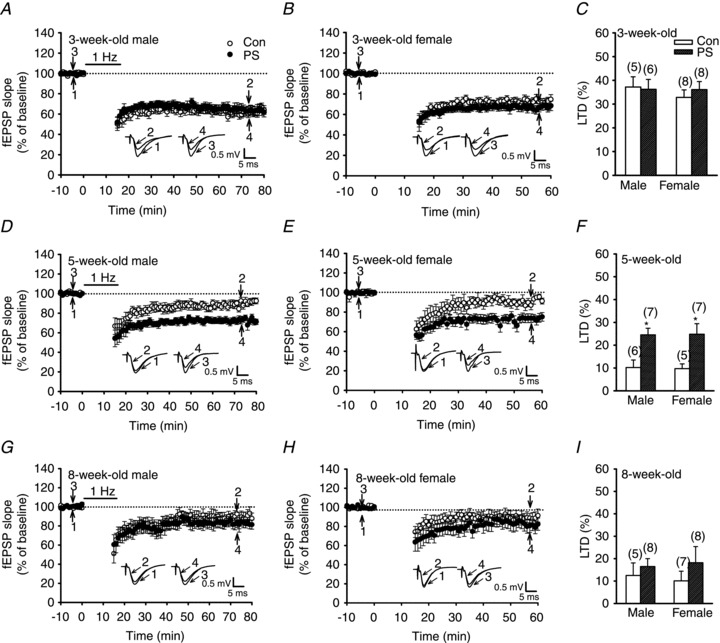
A and B, summary graphs of LTD induced with a prolonged LFS (900 stimuli delivered at 1 Hz) in slices from 3-week-old male (A) or female (B) rats that received Con or PS treatment. C, summary bar graphs depicting levels of depression measured 50–60 min after LFS in slices from Con or PS-treated rats at 3 weeks old. D and E, summary graphs of LTD induced with a LFS in slices from 5-week-old male (D) or female (E) rats that received Con or PS treatment. F, summary bar graphs depicting levels of depression measured 50–60 min after LFS in slices from Con or PS-treated rats at 5 weeks old. G and H, summary graphs of LTD induced with a LFS in slices from 8-week-old male (G) or female (H) rats that received Con or PS treatment. I, summary bar graphs depicting levels of depression measured 50–60 min after LFS in slices from Con or PS-treated rats at 8 weeks old. Representative traces of fEPSPs were taken at the time indicated by number. Horizontal bars denote the period of delivery of LFS. Dashed lines show baseline level. The total number of animals examined is indicated by n in parentheses. Data represent the mean ± SEM. *P < 0.05 compared with Con rats.
The findings that PS treatment impairs LTP but enhances LTD induction suggest the possibility that it may change synaptic properties. To explore this possibility, we stimulated the Schaffer collateral afferent inputs with other frequencies (5 Hz and 10 Hz) and examined the consequent changes in the synaptic strength in slices from 5-week-old rats of both sexes. Our results confirmed the theoretical model of synaptic plasticity postulated by Bienenstock, Cooper and Munro (Bienenstock et al. 1982); HFS leads to LTP, intermediate frequency stimulation produces only a minor or no change in synaptic strength, and LFS produces LTD (Fig. 4). In slices from control rats of both sexes, 900 pulses of 1 Hz and 5 Hz produced no significant changes in synaptic strength. In contrast, 900 pulses of 1 Hz and 5 Hz stimulation induced significant LTD in slices from PS-treated rats of both sexes. However, the slices from both control and PS-treated rats showed no significant changes in synaptic strength by 900 pulses of 10 Hz stimulation. The results obtained with a whole range of different conditioning frequencies are summarized in Fig. 4A. In these experiments, the frequency–response curve for the slices from PS-treated rats favours LTD at frequencies below 5 Hz and obliterates LTP induction at 100 Hz frequency stimulation when compared to those from control rats. PS caused no horizontal shift in the synaptic modification threshold θm but produced an apparent downward shift of the frequency–response curve (Fig. 4B).
Figure 4. Effect of PS on frequency–response function in the CA1 region of the hippocampus.
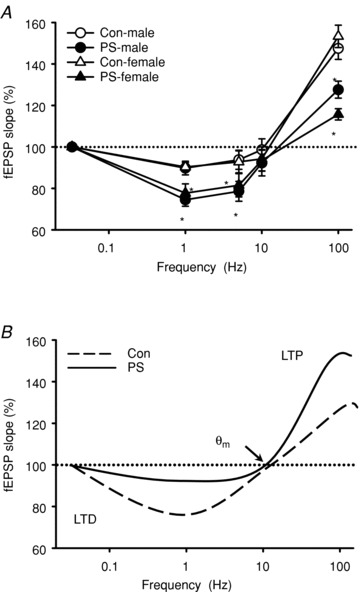
A, summary of the ability of different frequencies (0.033, 1, 3, 10 and 100 Hz) of synaptic stimulation to induce persistent changes in synaptic strength in slices from Con and PS-treated rats at 5 weeks old. The percentage change in synaptic strength from baseline in all slices was averaged for the responses recorded during the last 10 min of recording (50–60 min) at the indicated frequencies. The control experiments were not subjected to HFS, but received continual LFS at 0.033 Hz for the duration of the recording. The percentage change in synaptic strength from baseline in control experiments was averaged for the responses recorded during the last 10 min of recording (75–85 min). B, according to the BCM theory, the dynamic range of depression and potentiation is linked and the direction of the change in synaptic weight is a function of postsynaptic activity. PS does not shift the synaptic modification threshold (θm) but causes an apparent vertical shift in the curve, as compared with Con groups. Data represent the mean ± SEM (n = 4–6 for each frequency). *P < 0.05 compared with Con rats.
Synaptic plasticity in the hippocampus does not always depend on the activation of NMDA receptors. At the Schaffer collateral–CA1 synapses, NMDA receptor-dependent LTD coexists with another form of LTD mediated by the activation of group I mGluRs (Bolshakov & Siegelbaum, 1994). This mGluR-dependent LTD can be elicited by bath application of a group I mGluR agonist, DHPG, over a short period of time (Palmer et al. 1997; Huber et al. 2000; Huang et al. 2004). Moreover, these two forms of LTD differ with respect to their underlying mechanisms (Bashir, 2003; Malenka & Bear, 2004). We then examined whether the induction of mGluR-dependent LTD was also altered by PS treatment. In slices from 5-week-old control rats, a brief bath application of DHPG (50 μm) for 5 min elicited robust LTD of both sexes (50–60 min after washout of DHPG: male, 31.9 ± 2.8%, n = 6; female, 37.1 ± 2.8%, n = 4; P < 0.05; Fig. 5A and B). Slices from PS-treated rats showed no difference in the magnitude of DHPG-induction LTD (male, 30.4 ± 2.8%, n = 6; female, 33.2 ± 3.8%, n = 5; P > 0.05) when compared to slices from control rats (Fig. 5C).
Figure 5. Effect of PS on DHPG-induced LTD in the CA1 region of the hippocampus at 5 weeks old.
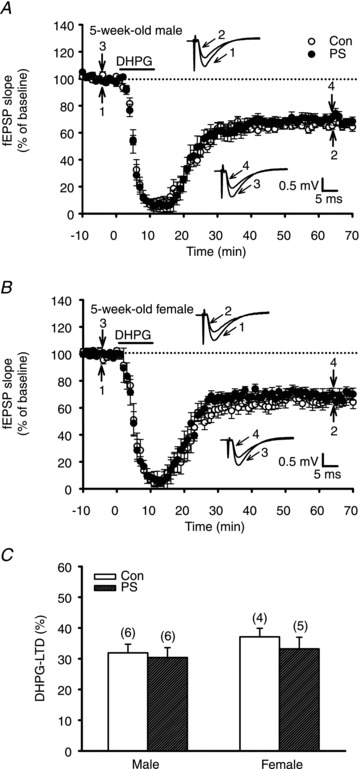
A and B, summary graphs of LTD induced with a brief application of mGluR agonist DHPG (50 μm) for 10 min in slices from 5-week-old male (A) or female (B) rats that received Con or PS treatment. C, summary bar graphs depicting levels of depression measured 50–60 min after washout of DHPG in slices from Con or PS-treated rats at 5 weeks old. Representative traces of fEPSPs were taken at the time indicated by number. Horizontal bars denote the period of delivery of DHPG. Dashed lines show baseline level. The total number of animals examined is indicated by n in parentheses. Data represent the mean ± SEM.
It has been proposed that the developmental and behavioural abnormalities in PS offspring may not only result from changes in the developmental programming during gestation but also from a difference in the levels of attention given by the stressed mother to her pups during the preweaning period (Maccari et al. 1995). To examine whether alterations of hippocampal synaptic plasticity after PS treatment were associated with the decrease in maternal care in the postnatal stages, the effects of early adoption on the induction of LTP and LTD in slices from control and PS-treated 5-week-old rats were examined. Control and PS-treated offspring were subjected to adoption procedures to assess the influence of postnatal care on hippocampal synaptic plasticity. No significant difference between control and PS-treated offspring was observed in the levels of maternal care by their foster mothers. The percentage of time spent in maternal contact during the observation period did not differ between control and PS-treated rat pups (data not shown). As shown in Fig. 6A–C, in slices from PS-treated rats raised by the foster mothers, the magnitude of LTP was still significantly less than those of controls of both sexes (control male, 46.2 ± 4.8%, n = 5; control female, 49.8 ± 5.2%, n = 5; PS male, 27.6 ± 4.1%, n = 6; PS female, 15.7 ± 2.7%, n = 5; P < 0.05). Moreover, the slices from PS-treated rats raised by the foster mothers showed enhanced LTD of both sexes when compared to those from control rats (control male, 9.1 ± 3.8%, n = 5; control female, 10.2 ± 3.2%, n = 5; PS male, 25.5 ± 3.1%, n = 6; PS female, 22.3 ± 4.5%, n = 5; P < 0.05; Fig. 6D–F).
Figure 6. Effect of postnatal adoption treatment on PS-induced alterations of LTP and LTD induction in the CA1 region of the hippocampus at 5 weeks old.
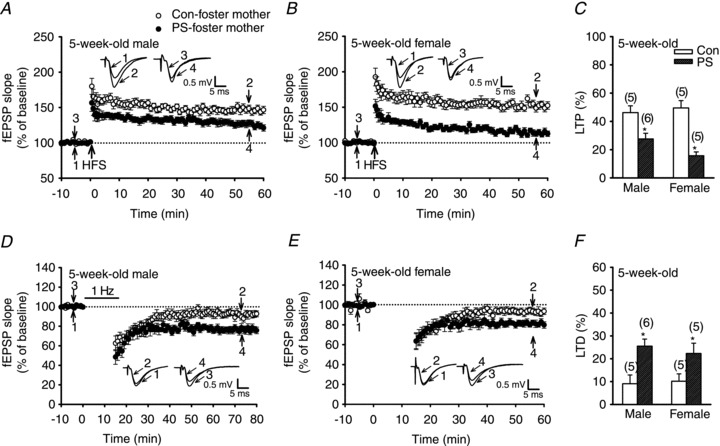
A and B, summary graphs of LTP induced with a HFS in slices from 5-week-old male (A) or female (B) rats that received Con or PS treatment and raised by a foster control mother at birth. C, summary bar graphs depicting levels of potentiation measured 50–60 min after HFS in slices from Con or PS-treated rats raised by a foster control mother. D and E, summary graphs of LTD induced with a LFS in slices from 5-week-old male (D) or female (E) rats that received Con or PS treatment and rose by a foster control mother at birth. F, summary bar graphs depicting levels of depression measured 50–60 min after LFS in slices from Con or PS-treated rats raised by a foster control mother. Representative traces of fEPSPs were taken at the time indicated by number. Horizontal bars denote the period of delivery of LFS. Dashed lines show baseline level. The total number of animals examined is indicated by n in parentheses. Data represent the mean ± SEM. *P < 0.05 compared with Con rats.
PS does not alter dendritic morphology of hippocampal CA1 pyramidal neurons
We next examined whether the effects of PS on long-term synaptic plasticity were attributed to changes in dendritic morphology of hippocampal CA1 pyramidal neurons. Histological evaluation using cresyl violet staining showed that PS does not significantly affect total number of neurons in the stratum pyramidale of hippocampal CA1 region when compared with control rats at 5 weeks of age of both sexes (P > 0.05, unpaired Student's t test) (Supplementary Fig. S2). Examination of Golgi-impregnated neurons using a Sholl analysis also revealed that PS did not significantly affect the amount and distribution of both apical and basal dendritic arbors of CA1 pyramidal neurons of both sexes (Supplementary Fig. S3). In addition, no significant difference was found in total spine density of apical dendrites between control and PS-treated rats of both sexes (P > 0.05, unpaired Student's t test).
PS does not affect the expression of NMDA receptor subunits
Given that altered NMDA receptor subunit composition may contribute to the shift in the threshold for LTP and LTD induction (Malenka & Bear, 2004), we therefore compared the protein expression patterns of NMDA receptor subunits in the hippocampal CA1 region of control and PS-treated rats at 5 weeks of age in both sexes. As shown in Fig. 7A, there were no significant differences between control and PS-treated rats of both sexes in the expression levels of the three major NMDA receptor subunits, NR1, NR2A and NR2B, in the whole tissue lysates of hippocampal CA1 regions (P > 0.05, unpaired Student's t test). As the functional NMDA receptors are thought to reside within synapses (Petralia et al. 1994), we isolated the crude synaptoneurosomal fractions from hippocampal CA1 tissues to examine the expression patterns of NMDA receptor subunits in control and PS-treated rats of both sexes. The levels of NR1, NR2A and NR2B proteins in the synaptoneurosomes were not altered by PS of both sexes when compared to those from control rats (P > 0.05, unpaired Student's t test; Fig. 7B). Considering that NMDA receptors are also targeted to the extrasynaptic and intracellular sites (Dunah & Standaert, 2003), we therefore examined whether the expression patterns of NR1, NR2A and NR2B proteins in the subcellular compartments were changed after PS treatment. Consistent with previous reports (Dunah & Standaert, 2003; Mao et al. 2009), NR1, NR2A and NR2B subunits were present in the membrane-associated compartments, specifically the crude synaptosomal membranes (P2), intracellular light membranes (P3) and synaptosomal membranes (LP1) (Fig. 7C and D). Similarly, PS treatment did not significantly alter the subcellular expression of NR1, NR2A and NR2B subunits in different compartments of hippocampal CA1 neurons of both sexes (P > 0.05, unpaired Student's t test). These results suggest that NMDA receptor distribution and density in hippocampal CA1 region were not altered by PS.
Figure 7. Effect of PS on expression of NMDA receptor subunits in hippocampal CA1 region at 5 weeks old.
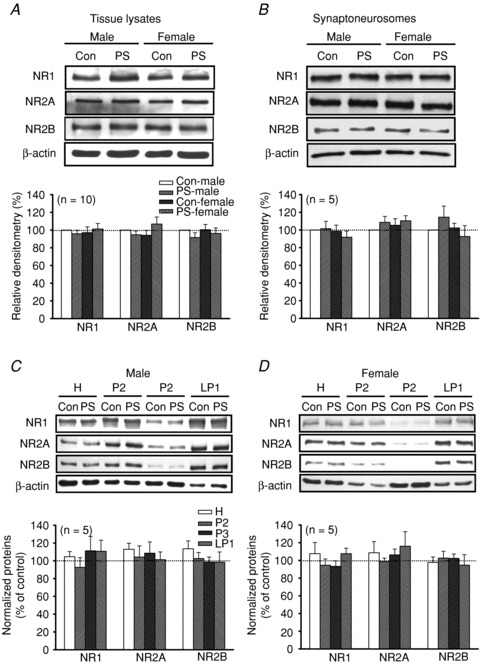
A, representative Western blot and summary bar graph depicting levels of NR1, NR2A and NR2B subunits in hippocampal CA1 tissue homogenates from Con or PS-treated male and female rats at 5 weeks old. B, representative Western blot and summary bar graph depicting levels of NR1, NR2A and NR2B subunits in hippocampal CA1 synaptoneurosomes prepared from Con or PS-treated male and female rats at 5 weeks old. C and D, representative Western blot and summary bar graph depicting levels of NR1, NR2A and NR2B subunits in different subcellular fractions from Con or PS-treated male (C) and female (D) rats at 5 weeks old. H, total homogenate; P2, crude synaptosomal membranes; P3, light membranes; LP1, synaptosomal membranes. Dashed lines show baseline level. The total number of animals examined is indicated by n in parentheses. Data represent the mean ± SEM.
PS inhibits the proteolytic conversion of pro-BDNF to mBDNF
Since BDNF can regulate the frequency–response function of hippocampal CA1 synapses for the production of both LTP and LTD (Ikegaya et al. 2002; Woo et al. 2005) and PS has been shown to interfere with BDNF biosynthesis in the hippocampus (Van den Hove et al. 2006; Zuena et al. 2008), we therefore assessed whether PS prevents the proteolytic conversion of pro-BDNF to mBDNF and whether such interference may result in the effects of PS on LTP and LTD. Western blot analysis revealed that PS reduced the steady-state levels of mBDNF in hippocampal CA1 region of both sexes, whereas the pro-BDNF levels were significantly higher in PS-treated rats when compared to those from control rats (Fig. 8A). As the proteolytic conversion of pro-BDNF to mBDNF can be achieved by extracellular proteases, such as the tPA/plasmin system and MMPs (Lee et al. 2001; Pang et al. 2004; Lu et al. 2005; Yang et al. 2009), we next determined whether PS may prevent the conversion of pro-BDNF to mBDNF through an inhibition of proteolytic enzyme activity. Plasminogen/casein zymography of the proteins extracted from hippocampal CA1 tissues showed a marked decrease of tPA activity, which activates plasmin for conversion of pro-BDNF to mBDNF, in PS-treated rats when compared to those from control rats at 5 weeks of age of both sexes (Fig. 8B). In contrast, gelatin zymography showed no significant difference in MMP-2 and MMP-9 activity between control and PS-treated rats of both sexes (Fig. 8C). The proteolytic activity of tPA, MMP-2 and MMP-9 in hippocampal CA1 tissues was also detected and quantified by ELISA assay. As shown in Fig. 8D and E, PS induced a reduction in tPA activity (P < 0.05, unpaired Student's t test) but did not affect either MMP-2 or MMP-9 activity (P > 0.05, unpaired Student's t test) of both sexes. In addition, Western blot analysis revealed a significant decrease in the amount of tPA expression in the hippocampal CA1 region of PS-treated rats when compared to those from control rats at 5 weeks of age of both sexes (P < 0.05, unpaired Student's t test; Fig. 8F). In parallel, we observed that PS caused a significant decrease in tPA mRNA levels in the hippocampal CA1 region of both sexes (P < 0.05, unpaired Student's t test; Fig. 8G).
Figure 8. PS reduces tPA activity and thereby prevents the proteolytic conversion of pro-BDNF to mBDNF.
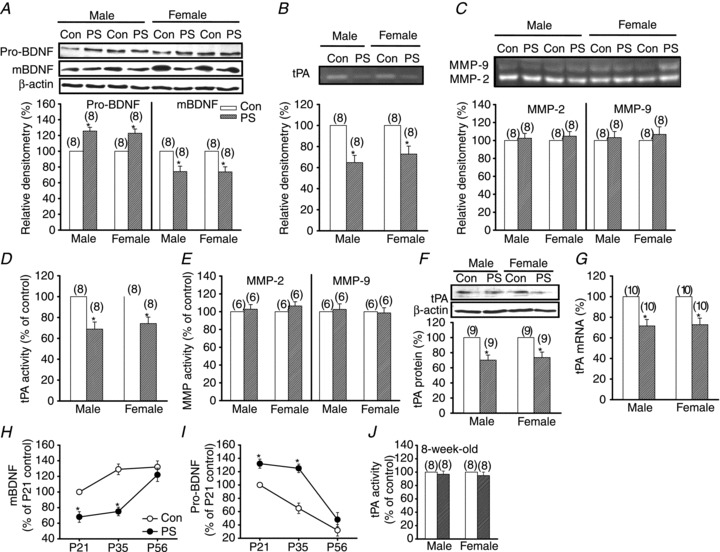
A, representative Western blot and summary bar graph depicting levels of pro-BDNF and mBDNF in hippocampal CA1 tissue homogenates from Con or PS-treated male and female rats at 5 weeks old. B, representative plasminogen/casein zymography and summary bar graph depicting tPA activity in hippocampal CA1 tissue homogenates from Con or PS-treated male and female rats at 5 weeks old. C, representative gelatin zymography and summary bar graph depicting the activity of MMP-2 and MMP-9 in hippocampal CA1 tissue homogenates from Con or PS-treated male and female rats at 5 weeks old. D, summary bar graphs depicting tPA activity in hippocampal CA1 tissue homogenates from 5-week-old Con or PS-treated male and female rats measured with ELISA kits. E, summary bar graphs depicting the activity of MMP-2 and MMP-9 in hippocampal CA1 tissue homogenates from 5-week-old Con or PS-treated male and female rats measured with ELISA kits. F, representative Western blot and summary bar graph depicting tPA protein level in hippocampal CA1 tissue homogenates from Con or PS-treated male and female rats at 5 weeks old. G, summary bar graphs depicting tPA mRNA level in hippocampal CA1 tissue homogenates from Con or PS-treated male and female rats at 5 weeks old. H, summary of experiments showing the relative postnatal developmental profile of BDNF in hippocampal CA1 region of Con or PS-treated male rats (n = 6–8 for each group). Expression level was normalized to P21 Con rats. I, summary of experiments showing the relative postnatal developmental profile of pro-BDNF in hippocampal CA1 region of Con or PS-treated male rats (n = 6–8 for each group). J, summary bar graphs depicting tPA activity in hippocampal CA1 tissue homogenates from 8-week-old Con- or PS-treated male and female rats measured with ELISA kits. Expression level was normalized to P21 Con rats. The total number of animals examined is indicated by n in parentheses. Data represent the mean ± SEM.
Having confirmed that PS induces decreased extracellular conversion of pro-BDNF to mBDNF, we next examined the correlation between the expression levels of pro-BDNF and mBDNF in the hippocampal CA1 region and the induction of LTP and LTD. A comparison of steady-state levels of mBDNF in slices from control and PS-treated male rats showed that mBDNF in the hippocampal CA1 region was significantly lower in 3- and 5-week-old PS-treated male rats compared with control rats (P < 0.05, unpaired Student's t test); however, this difference was not observed in 8-week-old male rats (P > 0.05, unpaired Student's t test; Fig. 8H). In contrast, in slices from 3- and 5-week-old PS-treated rats, the levels of pro-BDNF in hippocampal CA1 region was significantly higher than those of control male rats (P < 0.05, unpaired Student's t test; Fig. 8I). No significant difference in the levels of pro-BDNF was observed in slices from 8-week-old male rats. We further confirmed that PS-treated 8-week-old rats showed normal tPA activity of both sexes when compared to those from control rats (P > 0.05, unpaired Student's t test; Fig. 8J).
We also computed a correlation between the levels of pro-BDNF and mBDNF levels and the induction of hippocampal CA1 LTP and LTD in slices prepared from control and PS-treated male rats of both sexes. The slices were first used for electrophysiological recordings, then subsequently microdissected and used for Western blot analysis. As shown in Fig. 9A, the magnitude of LTP is highly correlated with the relative levels of mBDNF (r = 0.56; P < 0.01), whereas there was a clear inverse correlation between the relative levels of mBDNF and the magnitude of LTD (r =–0.32; P < 0.03; Fig. 9B). In contrast, an inverse correlation between the relative levels of pro-BDNF and the magnitude of LTP was observed (r =–0.42; P < 0.01; Fig. 9C). The magnitude of LTD is highly correlated with the relative levels of pro-BDNF (r = 0.52; P < 0.01; Fig. 9D). These results underscore the importance of the steady-state levels of pro-BDNF and mBDNF in determining the induction of LTP and LTD in the hippocampal CA1 region.
Figure 9. Relationships between baseline pro-BDNF/mBDNF levels and the expression of hippocampal CA1 LTP and LTD.
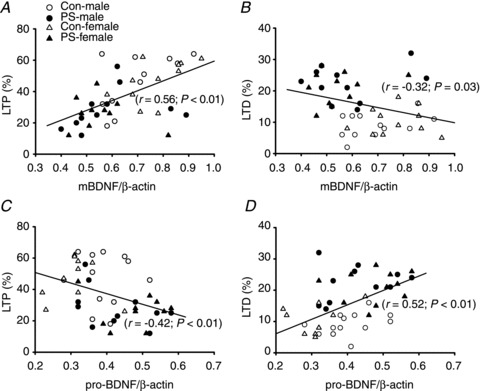
A, a significant positive correlation is evident between the mBDNF level and the extent of LTP expression in slices from Con and PS-treated rats of both sexes. B, a significant negative correlation is evident between the mBDNF level and the extent of LTD expression. C, a clear negative correlation is evident between the pro-BDNF level and the extent of LTP expression. D, a significant positive correlation is evident between the pro-BDNF level and the extent of LTD expression. The extent of LTP and LTD was calculated 50–60 min after HFS or LFS, respectively.
To further investigate whether the elevation of steady-state pro-BDNF levels is sufficient to exert the effects of PS on LTP and LTD, we used two additional approaches. First, we treated the hippocampal slices from 5-week-old PS-treated rats with the functional anti-pro-BDNF antibody (100 μg ml−1) for 1 h before the induction of LTP and LTD, and found that normal LTP (50–60 min after HFS: control male, 43.5 ± 5.3%, n = 4; control female, 54.8 ± 5.6%, n = 4; PS male, 38.5 ± 6.5%, n = 5; PS female, 46.1 ± 6.1%, n = 6; P > 0.05; Fig. 10A–C) and LTD (50–60 min after the end of LFS: control male, 6.5 ± 4.5%, n = 4; control female, 5.5 ± 4.3%, n = 4; PS male, 7.5 ± 4.3%, n = 5; PS female, 7.3 ± 4.3%, n = 6; P > 0.05; Fig. 10D–F) were induced by HFS and LFS, respectively. Second, a similar rescue effect was observed when the hippocampal slices from 5-week-old PS-treated rats were preincubated with the recombinant tPA (60 μg ml−1) for 1 h before the induction of LTP (50–60 min after HFS: control male, 50.6 ± 4.7%, n = 4; control female, 52.7 ± 4.5%, n = 4; PS male, 42.5 ± 5.3%, n = 6; PS female, 46.3 ± 4.4%, n = 6; P > 0.05; Fig. 10G–I) or LTD (50–60 min after the end of LFS: control male, 3.6 ± 4.2%, n = 4; control female, 4.5 ± 5.3%, n = 6; PS male, 8.5 ± 4.5%, n = 6; PS female, 6.7 ± 4.2%, n = 6; P > 0.05; Fig. 10J–L). Taken together, a long-lasting downregulation of tPA after PS leading to the impairment of the proteolytic conversion of pro-BDNF to mBDNF appears to be responsible for mediating the effects of PS on LTP and LTD in the hippocampal CA1 region.
Figure 10. Relationship of tPA and pro-BDNF in PS-induced alterations of LTP and LTD induction in the CA1 region of the hippocampus at 5 weeks old.
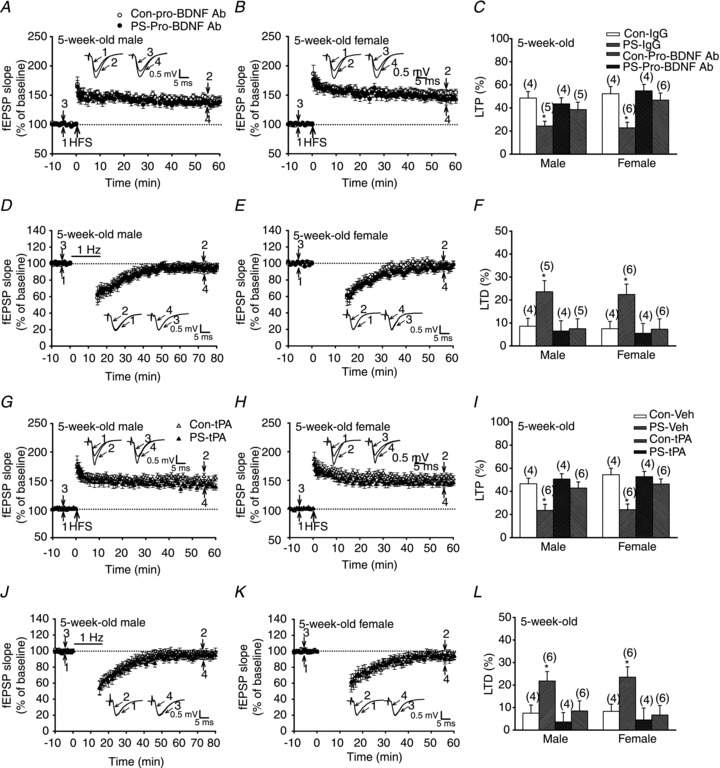
A and B, summary of experiments showing application of the functional anti-pro-BDNF antibody (100 μg ml−1) for 1 h to slices from 5-week-old male (A) or female (B) PS-treated rats completely reversed LTP deficit. C, summary bar graphs depicting levels of potentiation measured 50–60 min after HFS in slices from Con and PS-treated rats. D and E, summary of experiments showing application of the functional anti-pro-BDNF antibody (100 μg ml−1) for 1 h to slices from 5-week-old male (D) or female (E) PS-treated rats prevented PS-induced enhancement of LTD induction. F, summary bar graphs depicting levels of depression measured 50–60 min after LFS in slices from Con and PS-treated rats. G and H, summary of experiments showing application of recombinant tPA (60 μg ml−1) for 1 h to slices from 5-week-old male (G) or female (H) PS-treated rats completely reversed LTP deficit. I, summary bar graphs depicting levels of potentiation measured 50–60 min after HFS in slices from Con and PS-treated rats. J and K, summary of experiments showing application of recombinant tPA (60 μg ml−1) for 1 h to slices from 5-week-old male (J) or female (K) PS-treated rats prevented PS-induced enhancement of LTD induction. L, summary bar graphs depicting levels of depression measured 50–60 min after LFS in slices from Con and PS-treated rats. Representative traces of fEPSPs were taken at the time indicated by number. Horizontal bars denote the period of delivery of LFS. Dashed lines show baseline level. The total number of animals examined is indicated by n in parentheses. Data represent the mean ± SEM. *P < 0.05 compared with Con rats.
Discussion
Although PS has been shown to alter the properties of long-term synaptic plasticity in the hippocampal CA1 region (Son et al. 2006; Yang et al. 2006; Yaka et al. 2007), the underlying mechanisms are not yet fully understood. The results of the present study show that PS treatment during days 15–21 of gestation led to a long-lasting impairment of LTP and enhancement of LTD in the hippocampal CA1 region in young rat offspring of both sexes, but such effects did not persist into adulthood. In addition, we reveal what we believe is for the first time that the effects of PS on LTP and LTD were correlated with an impairment of proteolytic conversion of pro-BDNF to mBDNF and a constitutive decrease in tPA activity and expression. Furthermore, no significant differences were observed between the sexes for the effects of PS on hippocampal synaptic plasticity.
Consistent with a previous study (Yang et al. 2006), we found that PS impaired LTP but facilitated LTD in the hippocampal CA1 region of young rat offspring. We have, in addition, extended these previous findings by demonstrating that PS caused an apparent downward shift of the frequency–response curve without affecting the synaptic modification threshold θm, suggesting that PS modulates the induction of LTP and LTD independently and thereby produces a downward shift of the curve. What mechanisms might be responsible for alterations in the properties of hippocampal synaptic plasticity? Previous studies have shown that exogenous application of BDNF produced an upward shift of the frequency–response curve of synaptic plasticity (Ikegaya et al. 2002), whereas activation of p75 neurotrophin receptor signalling by pro-BDNF facilitated the induction of LTD (Woo et al. 2005). It is possible that changes in steady-state levels of pro-BDNF and mBDNF after PS treatment are responsible for the observed shift in the frequency–response function. Consistent with this idea, direct measurements of the levels of pro-BDNF and mBDNF in hippocampal CA1 tissue homogenates showed a substantial elevation in protein levels of pro-BDNF, but a reduction in mBDNF protein levels, from PS-treated rats when compared to those from control rats. Given evidence that the total amount of BDNF proteins (the sum of signals from pro-BDNF and mBDNF bands) was unaffected by PS treatment, a straightforward hypothesis is that PS-induced reduction in mBDNF levels is due to an inhibition of the proteolytic conversion of pro-BDNF to mBDNF. Although PS-induced changes in properties of synaptic plasticity could be associated with the elevation of pro-BDNF levels, the reduction of mBDNF levels or both, our results indicated that pro-BDNF is a key player in mediating the effects of PS on LTP and LTD. There are two lines of evidence to support this conclusion. First, neutralizing endogenous pro-BDNF with function-blocking antibody effectively rescued the effects of PS on LTP and LTD. Second, application of tPA to the slices from PS-treated rats to promote extracellular cleavage of pro-BDNF almost completely reversed the LTP deficit and the LTD enhancement. Our findings are consistent with results from a recent study showing that pro-BDNF, serving as a signalling molecule to regulate the induction of LTP and LTD, is highly dependent on whether there is extracellular cleavage (Nagappan et al. 2009). A pressing question that follows these observations is how pro-BDNF differentially modulates the induction of LTP and LTD. Although the molecular mechanisms underlying the effects of pro-BDNF on LTP and LTD remain to be established, it is possible that pro-BDNF may, by activating the p75 neurotrophin receptors, lead to changes in properties and trafficking of synaptic NR2B-containing NMDA receptors, which could, in theory, alter the inducibility of hippocampal long-term synaptic plasticity (Woo et al. 2005). Further investigation is needed to examine this possibility.
How could PS cause a long-lasting decrease in tPA activity? Given that PS-treated offspring displayed a significant reduction in tPA mRNA and protein levels in the hippocampal CA1 region, it is therefore possible that the tPA gene is transcriptionally repressed by PS treatment, causing a decrease in the expression of tPA. However, further studies are required to elucidate the exact mechanisms that cause downregulation of tPA gene transcription by PS treatment. Interestingly, a recent study indicates that PS can stimulate plasminogen activator inhibitor 1 (PAI-1), a fast-acting inhibitor of tPA (Salles & Strickland, 2002), expression in the hippocampus (Neeley et al. 2011). Hence, in addition to interfering with the process of tPA gene transcription in the hippocampus, PS may also act indirectly by increasing expression of endogenous PAI-1 and subsequently decreasing tPA activity. Additional studies are needed to clarify this possibility.
As the induction of both LTP and LTD in the hippocampal CA1 region is dependent on the activation of NMDA receptors (Malenka & Bear, 2004), another likely candidate mechanism for regulating synaptic plasticity is by altering composition or function of NMDA receptors. Previous studies have demonstrated that PS may cause long-lasting downregulation of NR2B subunit expression (Yaka et al. 2007) or inhibition of the interaction between NR1 and NR2B subunits in the hippocampus (Son et al. 2006). To our surprise, we found no significant differences in the synaptic expression of NR1, NR2A and NR2B subunits in the hippocampal CA1 region between control and PS-treated rats, suggesting that PS does not alter the total amount of synaptic NMDA receptors. In accordance with this notion, our data also indicate that the synaptic modification threshold θm was not significantly affected by PS treatment. Indeed, both theoretical and experimental studies have suggested that changes in NMDA receptor function may cause a horizontal shift in the frequency–response curve and θm value (Shouval et al. 2002; Philpot et al. 2003). The cause of these inconsistencies between our current study and previous studies is unclear, but it may be related to the use of different experimental protocols (from day 17–22 of gestation versus from day 15–21 of gestation) or animal species (mice versus rats), resulting in the activation of different cellular processes that may vary in their mode of action. Moreover, we observed that PS treatment failed to affect the induction of DHPG-induced LTD in hippocampal CA1 region of young rat offspring, suggesting a highly receptor type-specific effect of PS on the induction of hippocampal CA1 LTD. Recently, Zuena et al. (2008) have reported, in contrast to our results, that PS selectively upregulated mGluR1/5 receptor-mediated polyphosphoinositide (PI) hydrolysis in both dorsal and ventral hippocampus of female rat offspring, whereas male PS rat offspring showed a decrease in DHPG-induced PI hydrolysis in ventral hippocampus. This discrepancy in findings can be partially reconciled by accounting for differences in the selected hippocampal areas (the whole dorsal or ventral hippocampus versus the CA1 region of the dorsal hippocampus) or the ways of mGluR1/5 receptors to modulate their distinct downstream signalling cascades. Indeed, it has been demonstrated that the induction of DHPG-LTD in the hippocampal CA1 region is Ca2+ independent and does not involve the typical intracellular signalling cascade that is normally associated with mGluR1/5 receptor activation (Gladding et al. 2009).
PS has been shown to cause significant changes in spine density and dendritic complexity in pyramidal neurons of the prefrontal cortex (Murmu et al. 2006) and the hippocampus (Martínez-Téllez et al. 2009). However, in our present study, we found no significant difference in dendritic morphology of hippocampal CA1 pyramidal neurons between control and PS-treated rats. These findings are consistent with a previous study showing a selective apical dendritic atrophy of hippocampal CA3 but not CA1 pyramidal neurons by PS (Hossein-Sharifabad & Hadinedoushan, 2007). One possible explanation of these seemingly discrepant observations is the different intensity and timing of PS applied among studies. In the study by Martínez-Téllez et al. (2009), pregnant dams were exposed to restraint stress daily for 2 h from gestational day 11 until delivery. However, in our current study, repeated restraint stress was applied to the pregnant dams for 45 min three times per day during the last week of gestation. Considering the intensity and duration of stress exposure, our PS protocol seems to be relatively milder than that of Martínez-Téllez et al. (2009), thereby causing less influence on the dendritic morphology of hippocampal CA1 pyramidal neurons. Moreover, the lack of changes in basal synaptic transmission and presynaptic transmitter release property by PS treatment also suggest that the mechanisms for PS-induced changes in the properties of synaptic plasticity may not be related to structural changes in synapses of hippocampal CA1 pyramidal neurons.
Previous studies have shown that early adoption can reverse the effects of PS on glucocorticoid negative feedback (Maccari et al. 1995) and anxiogenic behaviours (Barros et al. 2006), indicating that variations in maternal care during the preweaning period could be involved in the destructive effects of PS. However, in the present study, we found no significant effect of early adoption on PS-induced alterations of LTP and LTD induction in young rat offspring. Our results are consistent with previous research showing that a cross-fostering procedure failed to prevent the disruptive effects of PS on hippocampal synaptic plasticity and spatial learning (Yang et al. 2006). The reason for these seemingly contradictory findings is unclear but may be related to differences in phenotype targeted. These observations might suggest that early adoption would be more effective in reversing the increase in HPA axis responsiveness and emotional reactivity induced by PS; however, the effects of PS on hippocampal synaptic plasticity are not related to the deficient maternal care and cannot be rescued by early adoption.
There is some evidence that PS may exert a sex-specific reprogramming of fetal HPA axis function and developing behavioural disturbances (Darnaudéry & Maccari, 2008; Mueller & Bale, 2008; Charil et al. 2010). While the increased responsiveness of the HPA axis to stress was observed in both male and female PS rats, females displayed less anxiety-like behaviours than males (Zagron & Weinstock, 2006; Zuena et al. 2008). Interestingly, PS has been shown to result in a significant increase in hippocampal levels of both pro-BDNF and mBDNF in male offspring, but has no effect in female offspring (Zuena et al. 2008). However, our data indicate that both male and female PS rats displayed increased pro-BDNF but decreased mBDNF in the hippocampal CA1 region and no significant sex differences in PS-induced alterations in LTP and LTD induction. The different prenatal period of stress exposure (day 11 of pregnancy until delivery versus days 15–21 of pregnancy) and hippocampal tissue sampling (the whole hippocampus versus the CA1 region of the dorsal hippocampus) may account, at least in part, for the differences in the pro-BDNF and mBDNF data obtained by Zuena et al. (2008) and our own. It is worth noting that Zuena et al. (2008) demonstrated pronounced gender effects in the outcome of PS on hippocampal-dependent spatial learning and anxiety-like behaviours at 3 months of age. It will be important in future studies to delineate whether long-term detrimental effects of PS may become apparent later in life in behavioural aspects and to a different extent in male and female rats.
Our results demonstrate that the effects of PS on LTP and LTD disappeared at 8 weeks of age. This finding is in contrast with observations made in a previous study, which reported that the effects of PS on LTP and LTD were still significant at 8 weeks of age (Yang et al. 2007). The reason for this discrepancy is unclear but could be attributed to the use of a different PS protocol (foot shock versus restraint condition) or animal strains (Wistar versus Sprague–Dawley rats). Future studies are required to test this possibility.
In conclusion, our work supports the notion that PS may lead to alterations of hippocampal synaptic plasticity in later life and, more importantly, identifies specific molecular mechanisms to support this contention. These effects were not maintained throughout the life span because normal synaptic plasticity was observed in 8-week-old PS-treated rats. Regardless of the mechanism, this finding raises an intriguing possibility that during postweaning development, a compensatory process to reverse the impairments of PS on hippocampal synaptic function may develop. Although it is unclear whether these findings apply directly to humans, this study supports the idea that stressful experience during gestation or early in life may lead to long-lasting malfunction of the hippocampus and our PS model may be useful for the development of more effective intervention and prevention strategies.
Acknowledgments
This work was supported by a research grant from the National Science Council (NSC98-2320-B-006-005-MY3), Taiwan. We thank Dr Chia-Yi Kuan for expert technical assistance in tPA and MMP zymography assay.
Glossary
Abbreviations
- DHPG
(S)-3,5,-dihydroxyphenylglycine
- fEPSP
field excitatory postsynaptic potentials
- HFS
high-frequency stimulation
- HPA axis
hypothalamic–pituitary–adrenal axis
- LFS
low-frequency stimulation
- LTD
long-term depression
- LTP
long-term potentiation
- mBDNF
mature form of brain-derived neurotrophic factor
- mGluR
metabotropic glutamate receptor
- MMP-2/-9
matrix metalloproteinase-2/-9
- PI
polyphosphoinositide
- PMSF
phenylmethylsulfonyl fluoride
- PPF
paired-pulse facilitation
- pro-BDNF
pro-brain-derived neurotrophic factor
- PS
prenatal stress
- PSD
postsynaptic density
- tPA
tissue plasminogen activator
Author contributions
C.M.Y. and C.C.H. contributed to data collection and analysis. All authors contributed to the conception and design of experiments, and the drafting of the article as well as revising it critically for important intellectual content and all authors critically revised the manuscript and approved the final version for publication. All experiments were performed in the Synaptic Plasticity laboratory at the Department of Pharmacology, College of Medicine, National Cheng Kung University.
Supplementary Material
Supplementary Figure S1
Supplementary Figure S2
Supplementary Figure S3
As a service to our authors and readers, this journal provides supporting information supplied by the authors. Such materials are peer-reviewed and may be re-organized for online delivery, but are not copy-edited or typeset. Technical support issues arising from supporting information (other than missing files) should be addressed to the authors
References
- Adhami F, Yu D, Yin W, Schloemer A, Burns KA, Liao G, Degen JL, Chen J, Kuan CY. Deleterious effects of plasminogen activators in neonatal cerebral hypoxia-ischemia. Am J Pathol. 2008;172:1704–1716. doi: 10.2353/ajpath.2008.070979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barros VG, Rodríguez P, Martijena ID, Pérez A, Molina VA, Antonelli MC. Prenatal stress and early adoption effects on benzodiazepine receptors and anxiogenic behavior in the adult rat brain. Synapse. 2006;60:609–618. doi: 10.1002/syn.20336. [DOI] [PubMed] [Google Scholar]
- Bashir ZI. On long-term depression induced by activation of G-protein coupled receptors. Neurosci Res. 2003;45:363–367. doi: 10.1016/s0168-0102(03)00002-6. [DOI] [PubMed] [Google Scholar]
- Bienenstock EL, Cooper LN, Munro PW. Theory for the development of neuron selectivity: orientation specificity and binocular interaction in visual cortex. J Neurosci. 1982;2:32–48. doi: 10.1523/JNEUROSCI.02-01-00032.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bliss TV, Collingridge GL. A synaptic model of memory: long-term potentiation in the hippocampus. Nature. 1993;361:31–39. doi: 10.1038/361031a0. [DOI] [PubMed] [Google Scholar]
- Bolshakov VY, Siegelbaum SA. Postsynaptic induction and presynaptic expression of hippocampal long-term depression. Science. 1994;264:1148–1152. doi: 10.1126/science.7909958. [DOI] [PubMed] [Google Scholar]
- Charil A, Laplante DP, Vaillancourt C, King S. Prenatal stress and brain development. Brain Res Rev. 2010;65:56–79. doi: 10.1016/j.brainresrev.2010.06.002. [DOI] [PubMed] [Google Scholar]
- Darnaudéry M, Maccari S. Epigenetic programming of the stress response in male and female rats by prenatal restraint stress. Brain Res Rev. 2008;57:571–585. doi: 10.1016/j.brainresrev.2007.11.004. [DOI] [PubMed] [Google Scholar]
- Drummond GB. Reporting ethical matters in The Journal of Physiology: standards and advice. J Physiol. 2009;587:713–719. doi: 10.1113/jphysiol.2008.167387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunah AW, Standaert DG. Subcellular segregation of distinct heteromeric NMDA glutamate receptors in the striatum. J Neurochem. 2003;85:935–943. doi: 10.1046/j.1471-4159.2003.01744.x. [DOI] [PubMed] [Google Scholar]
- Gillott A, Standen PJ. Levels of anxiety and sources of stress in adults with autism. J Intellect Disabil. 2007;11:359–370. doi: 10.1177/1744629507083585. [DOI] [PubMed] [Google Scholar]
- Gladding CM, Collett VJ, Jia Z, Bashir ZI, Collingridge GL, Molnár E. Tyrosine dephosphorylation regulates AMPAR internalisation in mGluR-LTD. Mol Cell Neurosci. 2009;40:267–279. doi: 10.1016/j.mcn.2008.10.014. [DOI] [PubMed] [Google Scholar]
- Hosseini-Sharifabad M, Hadinedoushan H. Prenatal stress induces learning deficits and is associated with a decrease in granules and CA3 cell dendritic tree size in rat hippocampus. Anat Sci Int. 2007;82:211–217. doi: 10.1111/j.1447-073X.2007.00186.x. [DOI] [PubMed] [Google Scholar]
- Huang CC, You JL, Wu MY, Hsu KS. Rap1-induced p38 mitogen-activated protein kinase activation facilitates AMPA receptor trafficking via the GDI.Rab5 complex. Potential role in (S)-3,5-dihydroxyphenylglycene-induced long term depression. J Biol Chem. 2004;279:12286–12292. doi: 10.1074/jbc.M312868200. [DOI] [PubMed] [Google Scholar]
- Huber KM, Kayser MS, Bear MF. Role for rapid dendritic protein synthesis in hippocampal mGluR-dependent long-term depression. Science. 2000;288:1254–1257. doi: 10.1126/science.288.5469.1254. [DOI] [PubMed] [Google Scholar]
- Huttner WB, Schiebler W, Greengard P, De Camilli P. Synapsin I (protein I), a nerve terminal-specific phosphoprotein. III. Its association with synaptic vesicles studied in a highly purified synaptic vesicle preparation. J Cell Biol. 1983;96:1374–1388. doi: 10.1083/jcb.96.5.1374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ikegaya Y, Ishizaka Y, Matsuki N. BDNF attenuates hippocampal LTD via activation of phospholipase C: implications for a vertical shift in the frequency-response curve of synaptic plasticity. Eur J Neurosci. 2002;16:145–148. doi: 10.1046/j.1460-9568.2002.02051.x. [DOI] [PubMed] [Google Scholar]
- Lemaire V, Koehl M, Le Moal M, Abrous DN. Prenatal stress produces learning deficits associated with an inhibition of neurogenesis in the hippocampus. Proc Natl Acad Sci U S A. 2000;97:11032–11037. doi: 10.1073/pnas.97.20.11032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee R, Kermani P, Teng KK, Hempstead BL. Regulation of cell survival by secreted proneurotrophins. Science. 2001;294:1945–1948. doi: 10.1126/science.1065057. [DOI] [PubMed] [Google Scholar]
- Lin HJ, Huang CC, Hsu KS. Effects of neonatal dexamethasone treatment on hippocampal synaptic function. Ann Neurol. 2006;59:939–951. doi: 10.1002/ana.20885. [DOI] [PubMed] [Google Scholar]
- Lu B, Pang PT, Woo NH. The yin and yang of neurotrophin action. Nat Rev Neurosci. 2005;6:603–614. doi: 10.1038/nrn1726. [DOI] [PubMed] [Google Scholar]
- Maccari S, Darnaudery M, Morley-Fletcher S, Zuena AR, Cinque C, Van Reeth O. Prenatal stress and long-term consequences: implications of glucocorticoid hormones. Neurosci Biobehav Rev. 2003;27:119–127. doi: 10.1016/s0149-7634(03)00014-9. [DOI] [PubMed] [Google Scholar]
- Maccari S, Piazza PV, Kabbaj M, Barbazanges A, Simon H, Le Moal M. Adoption reverses the long-term impairment in glucocorticoid feedback induced by prenatal stress. J Neurosci. 1995;15:110–116. doi: 10.1523/JNEUROSCI.15-01-00110.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malenka RC, Bear MF. LTP and LTD: an embarrassment of riches. Neuron. 2004;44:5–21. doi: 10.1016/j.neuron.2004.09.012. [DOI] [PubMed] [Google Scholar]
- Mao LM, Wang W, Chu XP, Zhang GC, Liu XY, Yang YJ, Haines M, Papasian CJ, Fibuch EE, Buch S, Chen JG, Wang JQ. Stability of surface NMDA receptors controls synaptic and behavioral adaptations to amphetamine. Nat Neurosci. 2009;12:602–610. doi: 10.1038/nn.2300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martínez-Téllez RI, Hernández-Torres E, Gamboa C, Flores G. Prenatal stress alters spine density and dendritic length of nucleus accumbens and hippocampus neurons in rat offspring. Synapse. 2009;63:794–804. doi: 10.1002/syn.20664. [DOI] [PubMed] [Google Scholar]
- Mueller BR, Bale TL. Sex-specific programming of offspring emotionality after stress early in pregnancy. J Neurosci. 2008;28:9055–9065. doi: 10.1523/JNEUROSCI.1424-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murmu MS, Salomon S, Biala Y, Weinstock M, Braun K, Bock J. Changes of spine density and dendritic complexity in the prefrontal cortex in offspring of mothers exposed to stress during pregnancy. Eur J Neurosci. 2006;24:1477–1487. doi: 10.1111/j.1460-9568.2006.05024.x. [DOI] [PubMed] [Google Scholar]
- Nagappan G, Zaitsev E, Senatorov VV, Jr, Yang J, Hempstead BL, Lu B. Control of extracellular cleavage of ProBDNF by high frequency neuronal activity. Proc Natl Acad Sci U S A. 2009;106:1267–1272. doi: 10.1073/pnas.0807322106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neeley EW, Berger R, Koenig JI, Leonard S. Prenatal stress differentially alters brain-derived neurotrophic factor expression and signaling across rat strains. Neuroscience. 2011;187:24–35. doi: 10.1016/j.neuroscience.2011.03.065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Owen D, Andrews MH, Matthews SG. Maternal adversity, glucocorticoids and programming of neuroendocrine function and behaviour. Neurosci Biobehav Rev. 2005;29:209–226. doi: 10.1016/j.neubiorev.2004.10.004. [DOI] [PubMed] [Google Scholar]
- Palmer MJ, Irving AJ, Seabrook GR, Jane DE, Collingridge GL. The group I mGlu receptor agonist DHPG induces a novel form of LTD in the CA1 region of the hippocampus. Neuropharmacology. 1997;36:1517–1532. doi: 10.1016/s0028-3908(97)00181-0. [DOI] [PubMed] [Google Scholar]
- Pang PT, Teng HK, Zaitsev E, Woo NT, Sakata K, Zhen S, Teng KK, Yung WH, Hempstead BL, Lu B. Cleavage of proBDNF by tPA/plasmin is essential for long-term hippocampal plasticity. Science. 2004;306:487–491. doi: 10.1126/science.1100135. [DOI] [PubMed] [Google Scholar]
- Petralia RS, Wang YX, Wenthold RJ. The NMDA receptor subunits NR2A and NR2B show histological and ultrastructural localization patterns similar to those of NR1. J Neurosci. 1994;14:6102–6120. doi: 10.1523/JNEUROSCI.14-10-06102.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Philpot BD, Espinosa JS, Bear MF. Evidence for altered NMDA receptor function as a basis for metaplasticity in visual cortex. J Neurosci. 2003;23:5583–5588. doi: 10.1523/JNEUROSCI.23-13-05583.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rice F, Jones I, Thapar A. The impact of gestational stress and prenatal growth on emotional problems in offspring: a review. Acta Psychiatr Scand. 2007;115:171–183. doi: 10.1111/j.1600-0447.2006.00895.x. [DOI] [PubMed] [Google Scholar]
- Salles FJ, Strickland S. Localization and regulation of the tissue plasminogen activator-plasmin system in the hippocampus. J Neurosci. 2002;22:2125–2134. doi: 10.1523/JNEUROSCI.22-06-02125.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shouval HZ, Bear MF, Cooper LN. A unified model of NMDA receptor-dependent bidirectional synaptic plasticity. Proc Natl Acad Sci U S A. 2002;99:10 831–10 836. doi: 10.1073/pnas.152343099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Son GH, Geum D, Chung S, Kim EJ, Jo JH, Kim CM, Lee KH, Kim H, Choi S, Kim HT, Lee CJ, Kim K. Maternal stress produces learning deficits associated with impairment of NMDA receptor-mediated synaptic plasticity. J Neurosci. 2006;26:3309–3318. doi: 10.1523/JNEUROSCI.3850-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uno H, Tarara R, Else JG, Suleman MA, Sapolsky RM. Hippocampal damage associated with prolonged and fatal stress in primates. J Neurosci. 1989;9:1705–1711. doi: 10.1523/JNEUROSCI.09-05-01705.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vallée M, Mayo W, Dellu F, Le Moal M, Simon H, Maccari S. Prenatal stress induces high anxiety and postnatal handling induces low anxiety in adult offspring: correlation with stress-induced corticosterone secretion. J Neurosci. 1997;17:2626–2636. doi: 10.1523/JNEUROSCI.17-07-02626.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van den Hove DL, Steinbusch HW, Scheepens A, Van de Berg WD, Kooiman LA, Boosten BJ, Prickaerts J, Blanco CE. Prenatal stress and neonatal rat brain development. Neuroscience. 2006;137:145–155. doi: 10.1016/j.neuroscience.2005.08.060. [DOI] [PubMed] [Google Scholar]
- Walker FR, Knott B, Hodgson DM. Neonatal endotoxin exposure modifies the acoustic startle response and circulating levels of corticosterone in the adult rat but only following acute stress. J Psychiatr Res. 2008;42:1094–1103. doi: 10.1016/j.jpsychires.2007.12.006. [DOI] [PubMed] [Google Scholar]
- Ward IL, Weisz J. Differential effects of maternal stress on circulating levels of corticosterone, progesterone, and testosterone in male and female rat fetuses and their mothers. Endocrinology. 1984;114:1635–1644. doi: 10.1210/endo-114-5-1635. [DOI] [PubMed] [Google Scholar]
- Weinstock M. The potential influence of maternal stress hormones on development and mental health of the offspring. Brain Behav Immun. 2005;19:296–308. doi: 10.1016/j.bbi.2004.09.006. [DOI] [PubMed] [Google Scholar]
- Wells DG, Dong X, Quinlan EM, Huang YS, Bear MF, Richter JD, Fallon JR. A role for the cytoplasmic polyadenylation element in NMDA receptor-regulated mRNA translation in neurons. J Neurosci. 2001;21:9541–9548. doi: 10.1523/JNEUROSCI.21-24-09541.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woo NH, Teng HK, Siao CJ, Chiaruttini C, Pang PT, Milner TA, Hempstead BL, Lu B. Activation of p75NTR by proBDNF facilitates hippocampal long-term depression. Nat Neurosci. 2005;8:1069–1077. doi: 10.1038/nn1510. [DOI] [PubMed] [Google Scholar]
- Yaka R, Salomon S, Matzner H, Weinstock M. Effect of varied gestational stress on acquisition of spatial memory, hippocampal LTP and synaptic proteins in juvenile male rats. Behav Brain Res. 2007;179:126–132. doi: 10.1016/j.bbr.2007.01.018. [DOI] [PubMed] [Google Scholar]
- Yang J, Han H, Cao J, Li L, Xu L. Prenatal stress modifies hippocampal synaptic plasticity and spatial learning in young rat offspring. Hippocampus. 2006;16:431–436. doi: 10.1002/hipo.20181. [DOI] [PubMed] [Google Scholar]
- Yang J, Hou C, Ma N, Liu J, Zhang Y, Zhou J, Xu L, Li L. Enriched environment treatment restores impaired hippocampal synaptic plasticity and cognitive deficits induced by prenatal chronic stress. Neurobiol Learn Mem. 2007;87:257–263. doi: 10.1016/j.nlm.2006.09.001. [DOI] [PubMed] [Google Scholar]
- Yang J, Siao CJ, Nagappan G, Marinic T, Jing D, McGrath K, Chen ZY, Mark W, Tessarollo L, Lee FS, Lu B, Hempstead BL. Neuronal release of proBDNF. Nat Neurosci. 2009;12:113–115. doi: 10.1038/nn.2244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zagron G, Weinstock M. Maternal adrenal hormone secretion mediates behavioral alterations induced by prenatal stress in male and female rats. Behav Brain Res. 2006;175:323–328. doi: 10.1016/j.bbr.2006.09.003. [DOI] [PubMed] [Google Scholar]
- Zhang LI, Poo MM. Electrical activity and development of neural circuits. Nat Neurosci. 2001;4:1207–1214. doi: 10.1038/nn753. [DOI] [PubMed] [Google Scholar]
- Zucker RS. Short-term synaptic plasticity. Annu Rev Neurosci. 1989;12:13–31. doi: 10.1146/annurev.ne.12.030189.000305. [DOI] [PubMed] [Google Scholar]
- Zuena AR, Mairesse J, Casolini P, Cinque C, Alemà GS, Morley-Fletcher S, Chiodi V, Spagnoli LG, Gradini R, Catalani A, Nicoletti F, Maccari S. Prenatal restraint stress generates two distinct behavioral and neurochemical profiles in male and female rats. PLoS ONE. 2008;3:e2170. doi: 10.1371/journal.pone.0002170. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


