Abstract
Deposition of extracellular plaques, consisting of amyloid β peptide (Aβ), in the brain is the confirmatory diagnostic of Alzheimer’s disease (AD); however, the physiological and pathological role of Aβ is not fully understood. Herein, we demonstrate novel Aβ activity as a putative transcription factor upon AD–associated genes. We used oligomers from 5’–flanking regions of the apolipoprotein E (APOE), Aβ–precursor protein (APP) and β–amyloid site cleaving enzyme–1 (BACE1) genes for electrophoretic mobility shift assay (EMSA) with different fragments of the Aβ peptide. Our results suggest that Aβ bound to an Aβ–interacting domain (AβID) with a consensus of “KGGRKTGGGG”. This peptide–DNA interaction was sequence specific, and mutation of the first “G” of the decamer’s terminal “GGGG” eliminated peptide–DNA interaction. Furthermore, the cytotoxic Aβ25–35 fragment had greatest DNA affinity. Such specificity of binding suggests that the AβID is worth of further investigation as a site wherein the Aβ peptide may act as a transcription factor.
Keywords: Alzheimer’s disease, amyloid beta, DNA–protein interaction, gene regulation, transcription factor
1. Introduction
Alzheimer’s disease (AD) is the most common form of dementia in the elderly (Hebert et al., 2003) and is associated with heterogeneous risks including genetic, epigenetic, dietary, and lifestyle factors (Lahiri and Maloney, 2010b). It is characterized by neuronal loss, intraneuronal tangles of hyperphosphorylated microtubule–associated τ protein (MAPT), and extracellular deposition of β–amyloid peptide plaque. This amyloid plaque is composed primarily of the amyloid β peptide (Aβ), 39–42 amino acids in length (Lahiri et al., 2003; De Strooper, 2010). Aβ is a cleavage product of the Aβ precursor protein (APP) by the β– and γ–secretases. Cleavage of APP by β–secretase (BACE1) releases the large extracellular domain of APP (Hussain et al., 1999; Sinha et al., 1999; Vassar et al., 1999; Yan et al., 1999), and subsequent cleavage by γ–secretase releases Aβ and the APP intracellular domain fragment (AICD) (Kimberly et al., 2001). Aβ can accumulate in neurons without plaque formation in both human AD cases and transgenic AD models (Gouras et al., 2000; Shie et al., 2003). The non–pathological functions of Aβ in addition to its activity in aging–related disorders, such as AD, are multifaceted (Lahiri and Maloney, 2010a), including but not necessarily limited to kinase activation (Bogoyevitch et al., 2004; Tabaton et al., 2010), protection against metal–induced oxidative damage (Zou et al., 2002; Baruch-Suchodolsky and Fischer, 2009), regulation of cholesterol transport (Yao and Papadopoulos, 2002; Igbavboa et al., 2009), and formation of ion channels (Jang et al., 2010). However, the pathological role of Aβ is not fully understood.
The greatest risk factor for AD is age (Thies and Bleiler, 2011). DNA damage and changes in gene regulation have been reported in the aging human brain (Lu et al., 2004). Specifically, aging of the human cortex is characterized by a distinct transcriptional signature that includes reduced expression of genes that mediate synaptic plasticity and correlates with age–dependent DNA damage to the promoters of these genes. Recent work also suggests an important transcriptional role of several genes and their products in AD, including APP, AICD (from APP), BACE1, and presenilin complex (Zhang et al., 2007; Checler et al., 2010). We report results herein that Aβ interacts directly with specific DNA sequences from regulatory region(s) of AD–associated genes.
Extracellular Aβ is transported into the cell under oxidative and heat stress (Ohyagi et al., 2005; Ohyagi and Tabira, 2006; Ohyagi et al., 2007), and Aβ nuclear localization occurs under the regulation of the Aβ–related death–inducing protein (AβDIP). Aβ can also induce an increase in levels of the apoptosis–associated tumor protein 53 (p53, gene name TP53) (Ohyagi et al., 2005), the transcription factor achaete–scute complex homolog 1 (ASCL1) (Uchida et al., 2007) and transcription of the BACE1 (27, 28) gene, while inducing reduction in levels of oligodendrocyte lineage transcription factor 2 (OLIG2) gene expression (Uchida et al., 2007). In the case of p53, this induction was from direct action of Aβ upon the TP53 promoter, Aβ binding centered around a known heat shock element (HSE), “GGATTGGGGT” (Ohyagi et al., 2005).
We identified similar decamers in the 5’–flanking sequences of the APOE, APP, and BACE1 genes, all of which are implicated in the pathogenesis of AD (Lahiri et al., 2002). These particular genes were chosen since polymorphisms in the genomic sequences APOE, APP, and BACE1 have been associated with AD (31–33). We generated thirteen 20–mer pairs, each containing a decamer that had at least 80% homology to the TP53 decamer. We investigated decamers’ interaction with different Aβ peptides. Six of the oligomer pairs showed interaction with Aβ, including the TP53 oligomer. The “positive” sequences were used to generate a consensus, “KGGRKTGGGG”, and a similarity matrix with the Target Explorer utility (Sosinsky et al., 2003). Negative controls consisting of an HSE within the APP 5’–flanking region (La Fauci et al., 1989) and an oligomer pair derived from APP promoter, both of which had ≤ 40% homology with the TP53 decamer, did not interact with Aβ.
Of particular interest, an oligomer pair containing a single–nucleotide polymorphism (SNP) in one of the ”positive” APP oligomer pairs significantly reduced binding with both Aβ peptides, although binding was not qualitatively reduced with the Aβ25–35 peptide. The G→A substitution is a functional APP SNP that we have previously associating with increased AD risk (Lahiri et al., 2005). We also investigated the regions of the Aβ peptide that would bind the DNA decamer and determined that maximum DNA binding was obtained with the cytotoxic Aβ25–35 peptide. Taken together, we have demonstrated DNA sequence–specific interaction with the Aβ peptide. In one important instance, this specificity was to a SNP in that APP gene that has been implicated in AD risk. This suggests functional investigation of this interaction as a potential regulatory pathway for control of Aβ and possibly in development of AD.
Portions of this work have been presented as part of the proceedings of the 21st American Peptide Symposium (Lahiri et al., 2009a).
2. Materials and Methods
2.1 Chemicals/Reagents
Unless otherwise specified, reagents were purchased from Sigma (St. Louis, MO) and were of “molecular biology” or “analytic” quality. Enzymes were purchased from Roche (Indianapolis, IN). Cell culture reagents were purchased from Invitrogen (Carlsbad, CA).
2.2 Aβ peptides and their fragments
Peptides Aβ1–42, 1–40, 1–28, 25–35, 29–40, 31–35, 42–1, 40–1, and 35–25 were purchased as trifluoroacetic acid salts from Bachem (Torrance, CA) and resuspended at stock concentrations of 1mg/ml in different solvents per manufacturer’s recommendations. Consultation with manufacturer indicated that peptides dissolved under these conditions would be dimers or larger aggregates.
2.3 Synthesis of different oligomers representing putative AβIDs in the regulatory regions of APOE, APP, BACE1, and TP53
The 5’–flanking regions of 4 genes (APOE, APP, BACE1, and TP53) were selected for investigation. TP53 was chosen to as a “positive control” for Aβ–DNA interaction (Ohyagi et al., 2005). APOE, APP, and BACE1 were selected as genes with a strong contribution to AD etiology. The TP53 HSE decamer, 5’–GGATTGGGGT–3’ (Ohyagi et al., 2005) was used to identify potential Aβ–binding decamers within the APOE (Paik et al., 1985; Du et al., 2005), APP (Lahiri and Robakis, 1991; Hattori et al., 1997), and BACE1 (Christensen et al., 2004; Sambamurti et al., 2004) 5’–flanking regions and introns upstream of the “ATG” start codon. A minimum 80% homology to the TP53 sequence was required for selection. Of the decamers located on these four sequences, twelve were selected (Fig. 1). Twenty–mer pairs (Table 1) of decamers plus flanking DNA were synthesized (Invitrogen), annealed, and radiolabeled with 32γP–ATP via polynucleotide kinase (Roche).
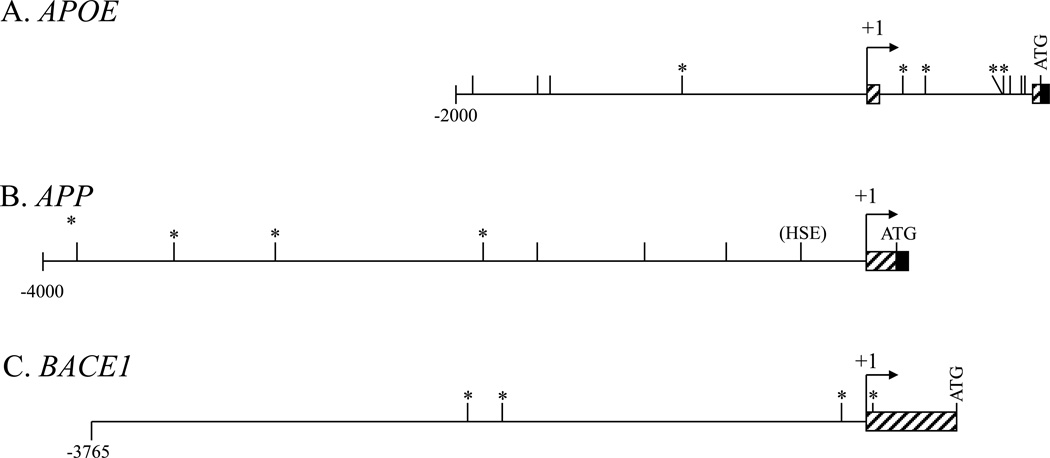
Figure 1. Presence of Aβ–binding motifs (AβID) with 80% homology to p53 HSE sequence on APOE, APP, and BACE1 5’–flanking sequences.
The 5’–flanking sequences of the APOE, APP, and BACE1 genes were searched for decamers with at least 80% homology to the “GGATTGGGGT” Aβ–binding HSE site of the TP53 promoter (Ohyagi et al., 2005). Sites marked with “*” were chosen for further study in this report. A. A region of the APOE gene from 2kb upstream of the +1 TSS to the end of the first coding exon, which contains the first intron, (Paik et al., 1985; Du et al., 2005) was searched. B. The APP 5’–flanking region from 4kb upstream of the +1 TSS to the end of the first coding exon (Lahiri and Robakis, 1991; Hattori et al., 1997) was searched. C. The BACE1 5’ flanking region from 3.8kb upstream of the +1 TSS to the “ATG” start codon (Christensen et al., 2004; Sambamurti et al., 2004) was searched.
Table 1.
Oligomers generated for electrophoretic mobility shift (EMSA) assays
| Oligo | Sequence | Oligo | Sequence |
|---|---|---|---|
| p53−1F | 5’–TGATGGGATTGGGGTTTTCC–3’ | BACE−119F | 5’–GGGCTGGAGAGGGGTCTGGG–3’ |
| p53−1R | 5’–GGAAAACCCCAATCCCATCA–3’ | BACE−119R | 5’–CCCAGACCCCTCTCCAGCCC–3’ |
| APOE+171F | 5’–GGTCGGGCTTGGGGAGAGGA–3’ | BACE−1766F | 5’–CTTATTGATTAGGGTTTTCT–3’ |
| APOE+171R | 5’–TCCTCTCCCCAAGCCCGACC–3’ | BACE−1766R | 5’–AGAAAACCCTAATCAATAAG–3’ |
| APOE+284F | 5’–CAGCTGGACTGGGATGTAAG–3’ | BACE−1939F | 5’–AAATTGGATTTGGTTTTTTT–3’ |
| APOE+284R | 5’–CTTACATCCCAGTCCAGCTG–3’ | BACE−1939R | 5’–AAAAAAACCAAATCCAATTT–3’ |
| APOE+660/+665F | 5’–AGGGAATGGGTTGGGGGCGG–3’ | BACE+36F | 5’–GAGCTGGATTATGGTGGCCT–3’ |
| APOE+660/+665R | 5’–CCGCCCCCAACCCATTCCCT–3’ | BACE+36R | 5’–AGGCCACCATAATCCAGCTC–3’ |
| APOE−899F | 5’–CACAGGTATTGTGGTTTCCA–3’ | APP−3833GFa | 5’–TGGGGGTGGGGGTACATAAT–3’ |
| APOE−899R | 5’–TGGAAACCACAATACCTGTG–3’ | APP−3833GRa | 5’–ATTATGTACCCCCACCCCCA–3’ |
| APP−1862F | 5’–AGTAGAGATGGGGGTTTCAC–3’ | APP−3833AFa | 5’–TGGGGGTGAGGGTACATAAT–3’ |
| APP−1862R | 5’–GTGAAACCCCCATCTCTACT–3’ | APP−3833ARa | 5’–ATTATGTACCCTCACCCCCA–3’ |
| APP−2871F | 5’–AACTAGGATGGGGATGCTGT–3’ | APP HSE Fb | 5’–GCTCTCGACTTTTCTAGAGC–3’ |
| APP−2871R | 5’–ACAGCATCCCCATCCTAGTT–3’ | APP HSE Rb | 5’–GCTCTAGAAAAGTCGAGAGC–3’ |
| APP−3364F | 5’–AAATAGAAATGGGGTATCTG–3’ | APP−1023Fab | 5’–GGGGATACATCTGGGCAGTT–3’ |
| APP−3364R | 5’–CAGATACCCCATTTCTATTT–3’ | APP−1023Rab | 5’–AAGTGCCCAGATGTATCCCC–3’ |
These oligomers were previously used in characterization of two APP–promoter polymorphisms implicated in late–onset familial AD (Lahiri et al., 2005). The SNP site in the APP−3833G and A oligomers is underlined.
These oligomers were selected as negative controls due to low (≤50% homology) to the TP53 HSE decamer.
2.4 Electrophoretic mobility shift assay (EMSA) with Aβ1–42 peptide and DNA oligomers
A quantity of 1µg of Aβ1–42 peptide was incubated in EMSA buffer 4 (ActiveMotif, Carlsbad, CA), supplemented with 25% glycerol, 0.04% Triton X–100, for 20 minutes at room temperature (25° C). Oligomer pairs (50,000 CPM, approximately 75pg), corresponding to sites at TP53 HSE (TP53 −1); APOE +171, +284, +660/+665 (two overlapping sites); APP −1862, −2871, −3364, −3833G; and BACE1 +36, −119, −1766, −1939 were added, and incubation proceeded for an additional 30 minutes. Reactions were analyzed on native 5% polyacrylamide tris–glycine-EDTA (TGE) gels. Gels were dried and DNA–protein interactions were visualized by autoradiography. EMSA was repeated independently with Aβ1–42 and Aβ1–40, using the APOE APP, and BACE1 oligomers pairs from the previous experiment, plus the following additions: An oligomer pair based on APP −3833G with a single–base substitution, corresponding to a naturally–occurring single–nucleotide G↔A polymorphism at −3829 in the APP promoter sequence (Lahiri et al., 2005); a pair crossing a second APP promoter polymorphism at −1023 (Lahiri et al., 2005), with 40% maximum homology to the TPE decamer; and a pair corresponding to an HSE within the APP promoter, with 40% maximum homology to the TP53 decamer. Films were densitometrically scanned, and signals were quantified with ImageJ software package (Girish and Vijayalakshmi, 2004). For consistent data, the results for Aβ1–40 and Aβ1–42 EMSA were combined. Data within each film was standardized to that film’s mean and standard deviation with the equation , where x is densitometric reading, m is mean densitometric readings within that gel, and s is sample standard deviation of that gel’s readings. Data was analyzed by Waller–Duncan multiple range test to determine a standardized densitometric cut–off between “non–binding” and “binding” levels of DNA–peptide interaction.
2.5 Competition EMSA with Aβ1–42 and Aβ1–40 peptides and DNA oligomers
Specificity of interaction between Aβ peptides and DNA sequences was tested by homocompetition EMSA, using those oligomer pairs that had previously shown interaction with Aβ. These were incubated as described, with the addition of 140× molar excess unlabeled oligomer pairs.
2.6 Concentration–dependency of Aβ peptide–DNA interactions
Reactions were performed with the oligomer pairs APP −3833G, APOE +660/+665, and BACE1 −119. The APP −3833G pair was reacted with Aβ1–40 at 10, 100, 250, 500, and 1000ng of peptide; with Aβ1–42 at 0.5, 1, 2, and 4 µg of peptide, with Aβ41–1 and 1–28 at 2, 4, and 10 µg of peptide; and with Aβ25–35 at 10, 100, 250, and 500ng of peptide. The APOE +660/+665 and BACE1 −119 oligomers were reacted with Aβ1–40, 1–42, 42–1, 1–28, and 25–35 at 500ng and 1µg of each peptide.
2.7 Determination of the protein–binding DNA consensus within oligomer sequences
Sequences of five experimental potential AβID–containing 20–mers that had greater than “non–binding” interaction by EMSA (see 3.2) plus the positive control TP53 derived 20–mer were aligned by Target Explorer (Sosinsky et al., 2003) without pre–defining the length of the conserved motif. Target Explorer determined the motif to be a decamer. Target explorer calculates weights for individual bases at specific motif positions using prior probabilities (priors) for each base, but available alternatives on the Target Explorer server were limited to Drosophila whole genome priors or estimation based on nucleotide frequencies within the submitted sequences. Neither was a desirable alternative, since appropriate priors would, in theory, be better derived from promoter sequences instead of an entire genome. Therefore, human promoter sequences were downloaded from the Eukaryotic Promoter Database (Schmid et al., 2006), version based on EMBL release 104. This database consists exclusively of experimentally confirmed PolII binding sites associated with a confirmed RNA transcript. Sequences that were not complete between −4000/+1000 were excluded, leaving 1840 sequences. Priors for each base were then calculated at each position for this database.
To determine the weight of each nucleotide (G, A, T, C) at each position, we used a position weight matrix equation: , where ln is the natural logarithm, ni,j = the number of times nucleotide i appears at position j in the alignment, pi = prior probability of nucleotide i at a specific position relative to the +1 TSS, and N = number of sequences in the alignment. Prior probabilities for individual bases are not constant along the range of human promoter sequences. Therefore, instead of deriving a static weight matrix to screen sequences of genes of interest, we used a “dynamic” calculation, where the Target Explorer equation was applied to each position in turn from −4000 to +1000, and these weights were then compared in ten–base blocks with sequences of interest, according to the position of each possible decamer within the sequences. While this is a computationally intensive method, it inherently corrects for position–dependent effects of background nucleotide frequency.
Individual bases within DNA sequences were compared to calculated weights for each base at specific positions, and the results for each decamer of bases totaled. If this total was less than the total observed for the lowest–scoring decamer in the matrix training set at that specific position between −4000/+1000, the decamer was rejected. In addition to weights based on the oligomer alignment, a sequence logo was calculated from the six aligned sequences (Schneider and Stephens, 1990).
The dynamic weight method was used to predict potential AβID sites on the APOE (Paik et al., 1985; Du et al., 2005), APP (Hattori et al., 1997), BACE1 (Sambamurti et al., 2004), BACE2 (Maloney et al., 2006), along with ASCL1; insulin–degrading enzyme (IDE), microtubule associated protein τ (MAPT), OLIG2, SLC38A1, and TP53 (Sayers et al., 2011) 5’–flanking regions.
2.8 EMSA of selected oligomers with different Aβ peptides
Selected oligomers that bound to Aβ1–42, specifically APOE +171, APOE +660/+665, APP −3833G, APP −3833A, and BACE1 −119, were used to screen binding capacity of Aβ peptides 1–42, 1–40, 1–28, 20–29, 25–35, 29–40, and 31–35 (Bachem) for both qualitative binding/non–binding and concentration–dependency. Each peptide was resuspended per manufacturer’s instructions and 1 µg of each peptide was used in EMSA vs. the oligomer pairs.
2.9 Data analysis
EMSA films were scanned at 300dpi, 14 bit–depth grayscale and densitometrically measured with ImageJ. All statistical analysis was carried out using SAS 9.1.3 (SAS Institute, Cary, NC). Expression data was tested for distribution and specific analyses applied via PROC GLIMMIX.
3. Results
3.1 Determination of potential Aβ–interacting sequences in the APOE, APP, and BACE1 5’–flanking regions
The APOE, APP, and BACE 5’–flanking regions were compared to a “GGATTGGGGT” decamer from the TP53 HSE sequence. Sites with at least 80% homology were accepted as potential AβID (Fig. 1). Within the APOE 5’–flanking region, 11 decamers were located. The APP sequence had 7 decamers, while the BACE1 sequence had 4. One site in the APP 5’–flanking region included an APP promoter SNP we have previously linked to risk of AD (Lahiri et al., 2005).
3.2 The Aβ peptide binds DNA in a sequence–specific manner, and the reverse peptide lacks binding
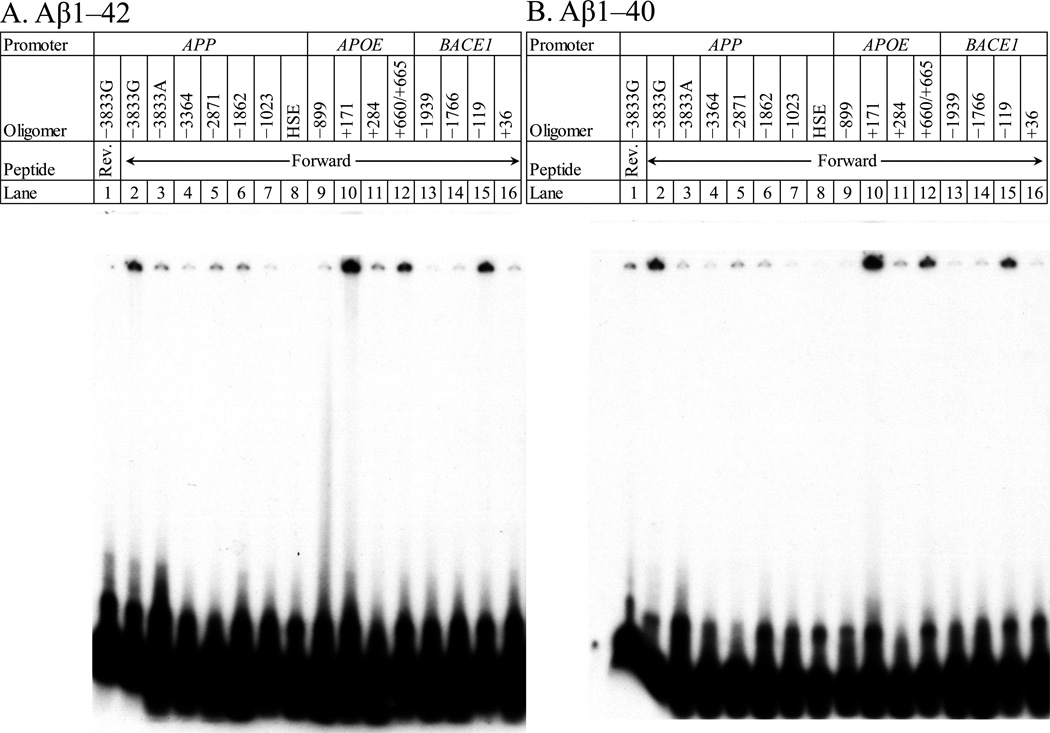
To determine specificity of a potential AβID motif based upon the TP53 decamer, Aβ1–42 and Aβ1−40 peptides were incubated with radiolabeled double–stranded oligomers corresponding to putative AβIDs in the APP (−3833G, −3364, −2871, −1682), APOE (−899, +171, +284, +660/+665), BACE1 (−1939, −1766, −119, +36) and TP53 (−1) 5’–flanking regions plus two additional oligomer pairs from the APP 5’–flanking region, specifically APPHSE and APP−1023 as controls for presumably non–specific “background” levels of DNA–peptide interaction. Mixtures were analyzed on 5% polyacrylamide–TGE gel. The gels were dried and subjected to autoradiography (Fig. 2). DNA–protein interactions were visualized as radioactive bands on the gel. Interactions were similar between Aβ1–42 and Aβ1–40. The Aβ42–1 peptide had no apparent DNA–protein interaction, while the Aβ40–1 peptide interacted with the APP −3833G DNA oligomer pair. However, the signal was less intense than the forward peptide (Fig. 2B). The −3833G APP oligomer pair is of particular interest as its AβID includes a previously–characterized APP promoter SNP at −3829 (Lahiri et al., 2005). The majority population sequence at this SNP is a “C” in the sense strand (corresponding to a “G” in the AβID if read in the same orientation as the TP53 −1 decamer). The minority SNP variant would correspond to an “A” in the decamer. Of particular note, this G→A substitution significantly reduced apparent Aβ binding. DNA–protein interactions in these and further gels migrated very slowly after entering through the well. Our consultation with the peptide manufacturer indicated that their peptides would most likely resuspend as oligomers, explaining very slow electrophoresis. Pilot tests done with peptides that the manufacturer (rPeptide) deemed more likely to resuspend as monomers produced no interaction. This is touched upon further in section 4. Discussion.
Figure 2. Electrophoretic mobility shift assay (EMSA)/gel shift assay of Aβ1–42 vs. different putative binding decamer–containing oligomers.
Figure shows representative EMSA of the A. Aβ1–42 and the B, Aβ1–40 peptides. The reverse Aβ40–1 and 42–1 peptides were independently incubated with radiolabeled oligomer containing the putative AβID at −3833 (lane 1). Aβ1–42 or 1–40 (lanes 2–16) were incubated with radiolabeled oligomers that contained the putative AβIDs in the APP (lanes 2–8) 5’–flanking region at positions −3833G (lane 2), −3833A with a “G” to “A” substitution at −3829 (lane 3), −3364 (lane 4), −2871 (lane 5) and −1862 (lane 6). In addition, an oligomer corresponding to the FAD mutation site in APP at −1023 (lane 7) and a distinct HSE–binding site within APP (lane 8) were incubated and run; in the APOE (lanes 9–12) 5’–flanking region at positions −899 (lane 9), +171 (lane 10), +284 (lane 11), +660/+665 (lane 12); and in the BACE1 (lanes 13–16) 5’–flanking region at positions −1939 (lane 13), −1766 (lane 14), −119 (lane 15), and +36 (lane 16). The gel was dried and exposed to X–ray film for autoradiography. DNA–peptide interactions appeared as dark signal near the top of the gel, unbound oligomer ran to the bottom of the gel.
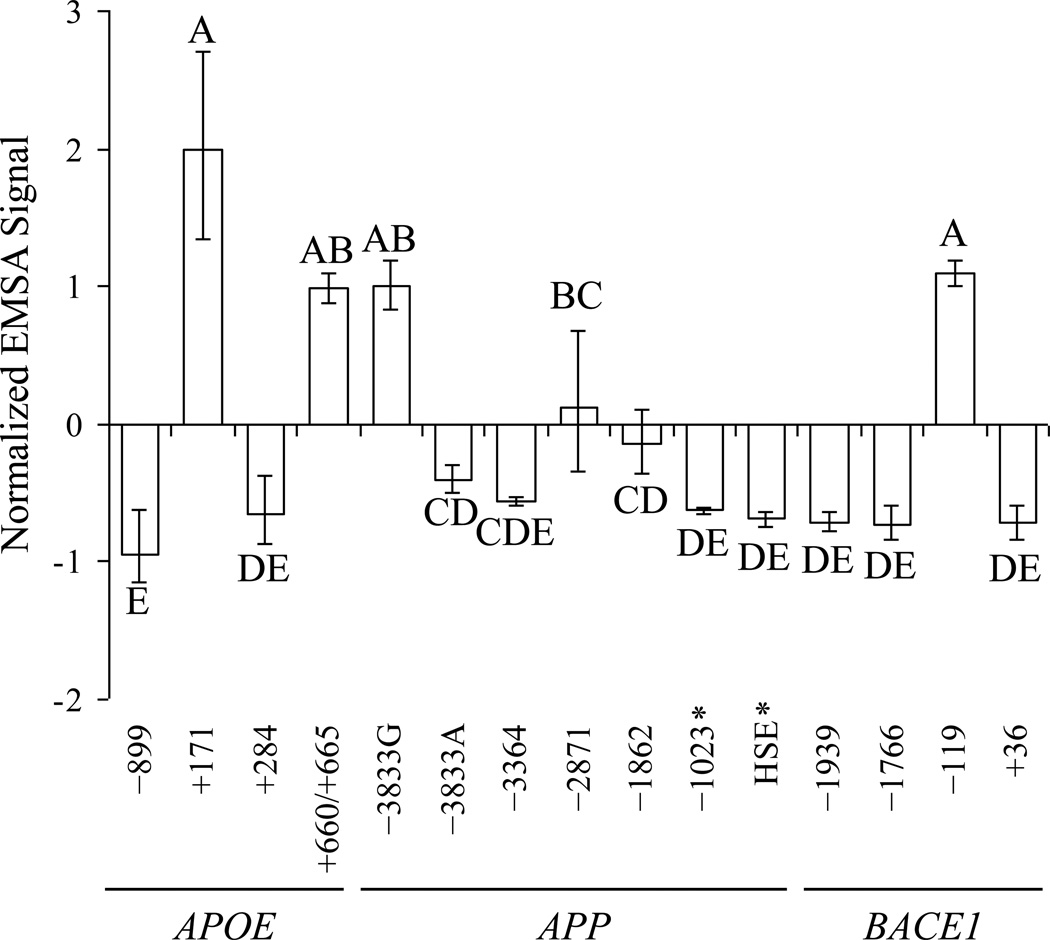
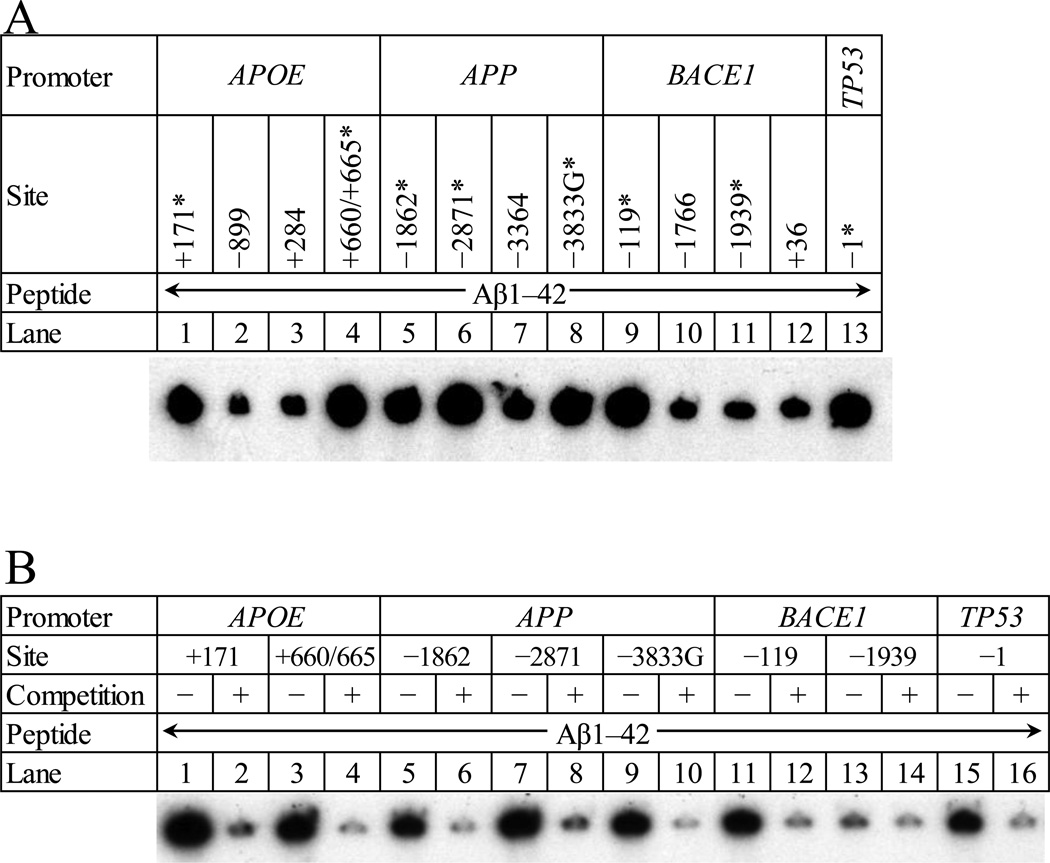
Analysis of standardized densitometric scans (Fig. 3) was based on our pre–designation of the APPHSE and APP−1023 oligomer pairs as “non–specific binding” controls from their low homology with the prototype TP53 decamer. They were used to reflect a “background signal” of non–specific DNA–protein interaction between Aβ and a given oligomer pair. The normalized EMSA signals were compared by Waller–Duncan multiple range test. Those oligomer pairs that were grouped with either of the APPHSE and APP−1023 controls by the statistical test (group “DE” in Fig. 3) were also classified as “non–specifically binding”, i.e., indistinguishable from “background”. Five oligomers were excluded from the “non–specifically binding” statistical group and were, therefore, considered to have DNA sequence specific interaction with Aβ (Table 2). Two of these were from APOE (−171 and −660/665), two from APP (–3833 and −2871), and one from BACE1 (−119). These oligomer sequences were used to determine the DNA sequence motif for the AβID. To further test the specificity of the aforementioned EMSA results, reactions were repeated in the presence of 140× molar excess of unlabeled homologous oligomer. In all cases, self–competition visibly reduced Aβ–DNA interaction (Fig. 4).
Figure 3. Determination of “binding” vs. “non–binding oligomer pairs to Aβ peptides in EMSA.
EMSA films were densitometrically scanned and signals within each film were normalized by subtracting the mean of that signal’s film from the individual signal and dividing by the standard deviation of signals. Normalized signals were then analyzed by Waller–Duncan means separation, and categories indicated with letters on the figure. Samples sharing the same letter were not significantly different at k = 100 (analogous to α = 0.05). The two “background control” oligomer pairs, APPHSE and APP−1023, indicated by “*”.
Table 2.
Aligned Aβ–binding oligomers determined by semiquantitative EMSA
| Gene | +/− | Positiona | Sequenceb | |
|---|---|---|---|---|
| Predicted | Aligned | |||
| APOE | + | +171 | +170 | – –GGTCGGGCTTGGGGAGAGGA– – – |
| APOE | + | +660/665 | +664 | AGGGAATGGGTTGGGGGCGG– – – – – |
| APP | − | −2871 | −2873 | – – – AACTAGGATGGGGATGCTGT– – |
| APP | − | −3833 | −3831 | – – – – –TGGGGGTGGGGGTACATAAT |
| BACE1 | − | −119 | −118 | – –GGGCTGGAGAGGGGTCTGGG– – – |
| TP53 | + | −1 | −2 | – –TGATGGGATTGGGGTTTTCC– – – |
Position is relative to the +1 transcription start site.
AβID decamers found by sequence alignment are italicized. Putative AβIDs predicted from 80% homology to the TP53 HSE are underlined.
Figure 4. Competition EMSA with “positive” oligomer pairs from APOE, APP, BACE1 and TP53 promoters vs. Aβ1–42 peptide.
A. Aβ1–42 peptide was incubated with radiolabeled oligomers that contained the putative AβIDs in the APOE (lanes 1–4) 5’–flanking region at positions +171 (lane 1), −899 (lane 2), +284 (lane 3), +660/+665 (lane 4); in the APP (lanes 5–8) 5’–flanking region at positions −1862 (lane 5), −2871 (lane 6), −3364 (lane 7), and −3833 (lane 8); and in the BACE1 (lanes 9–12) 5’–flanking region at positions −119 (lane 9), −1766 (lane 10), −1939 (lane 11), and +36 (lane 12). In addition, an oligomer containing the Aβ–binding element from p53 was labeled and run (lane 13). The gel was dried and exposed to X–ray film for autoradiography. Specific oligomer pairs that were selected for competition EMSA are indicated with “*”. B. Aβ1–42 was incubated with radiolabeled oligomers that had previously shown interaction with Aβ1–42 (lanes 1,3,5,7,9,11,13,15). These reactions were repeated with the addition of 140× molar excess unlabeled oligomer (lanes 2,4,6,8,10,12,14,16). Specificity of Aβ/oligomer interaction is demonstrated by a significant reduction of autoradiograph signal at top of lane. The gel was dried and exposed to X–ray film for autoradiography. DNA–peptide interactions appeared as dark signal near the top of the gel.
3.3 The Aβ–binding DNA motif/AβID is a specific degenerate decamer
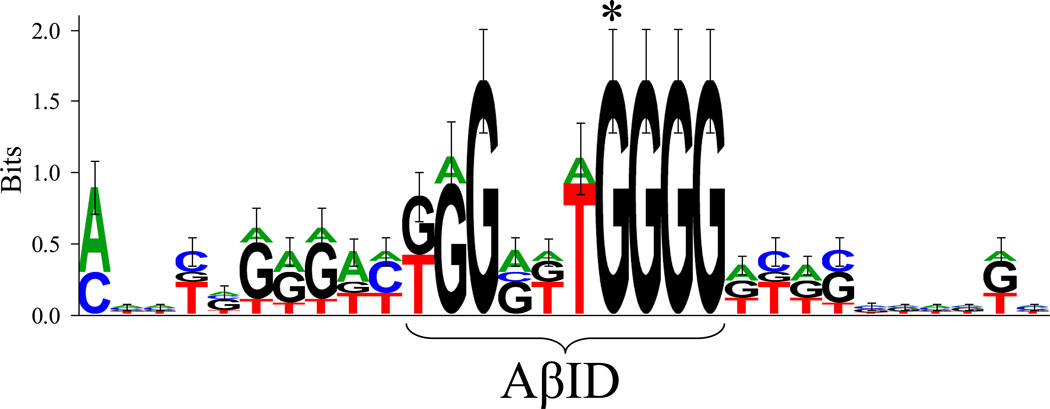
When those oligomers that showed positive DNA–peptide interaction (Table 1), along with the TP53 oligomer, were aligned by Target Explorer (Table 2), a consensus decamer of “KGGRKTGGGG” was determined. This decamer was “offset” 1 base upstream of the decamer originally chosen from the TP53 sequence, and 1 or 2 bases upstream of the predicted decamers. A sequence logo was generated (Fig. 5). Four “G” bases at the 3’ end of the decamer did not vary. Altering the “G” indicated by “*” to an “A” (APP −3833G vs. −3833A) significantly diminished Aβ binding capacity. Dynamic weight analysis predicted a decamer with a sequence of “GGGGTTGGGG” to have the maximum possible score, although this decamer did not appear in sequences of interest searched or in the database used to establish priors.
Figure 5. Structure of the Aβ–binding domain (AβID) consensus motif.
Combined height of stacked letters corresponds to bits of information (Shannon, 1997). An asterisk indicates the position of a “G” that may be critical for Aβ binding activity.
3.4 DNA binding of Aβ is strongest with the cytotoxic Aβ25–35 peptide
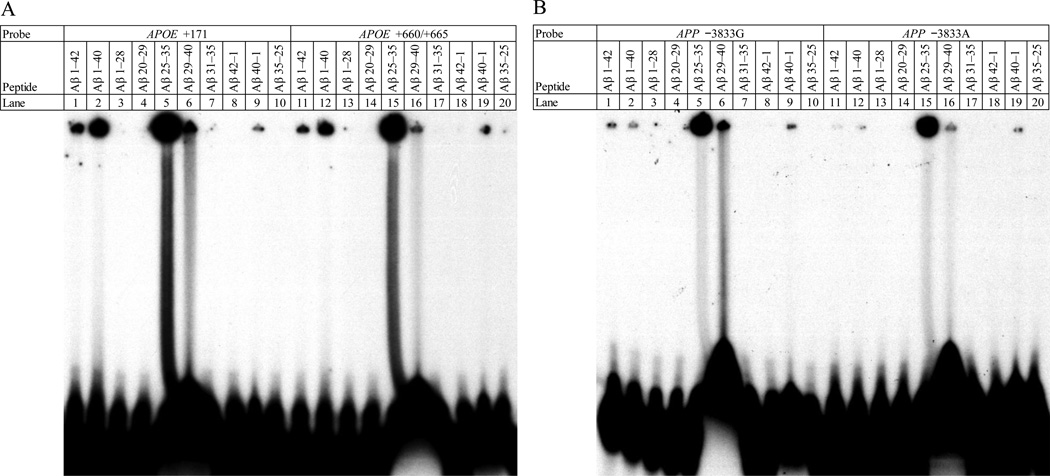
To determine the specific DNA–binding site within the Aβ peptide, various peptide fragments of Aβ, specifically 1–42, 1–40, 1–28, 20–29, 25–35, 29–40, 31–35, 42–1, 40–1, and 35–25, were reacted with the oligomers for the AβIDs at APOE +171 (Fig. 6A, lanes 1–10), APOE +660 (Fig. 6A, lanes 11–20), APP −3833G (Fig. 8B, lanes 1–10), and APP−3833A (Fig. 6B, lanes 11−20). Of the peptides tested, the 1–28, 20–29, 31–35, 35–25, and 42–1 peptides had no apparent DNA–binding capacity. Of the remaining peptides, binding capacity was, from highest to lowest, 25–35 > 29–40 > 1–40 > 1–42 > 40–1 (Figs. 6 and 7). The particularly cytotoxic (Millucci et al., 2010) Aβ25–35 peptide had the greatest DNA–binding capacity with any of the DNA oligomer pairs studied.
Figure 6. EMSA of different fragments of the Aβ peptide vs. four different DNA probes.
Fragments of Aβ peptide corresponding to residues 1–42, 1–40, 1–28, 20–29, 25–35, 29–40, and 31–35 were self–oligomerized. In addition, “reverse” fragments 42–1, 40–1, and 35–25 were prepared. A. Fragments were incubated against oligomers the contained the Aβ–binding sequence at APOE +171 (lanes 1–10) or APOE +660 (lanes 11–20). The gel was dried and exposed to X–ray film for autoradiography. DNA–peptide interactions appeared as dark signal near the top of the gel. B. Fragments were incubated against oligomers the contained the Aβ–binding sequence at APP −3833G (lanes 1–10) or APP −3833A (with a “G”→“A” substitution) (lanes 11–20). The gel was dried and exposed to X–ray film for autoradiography. DNA–peptide interactions appeared as dark signal near the top of the gel.
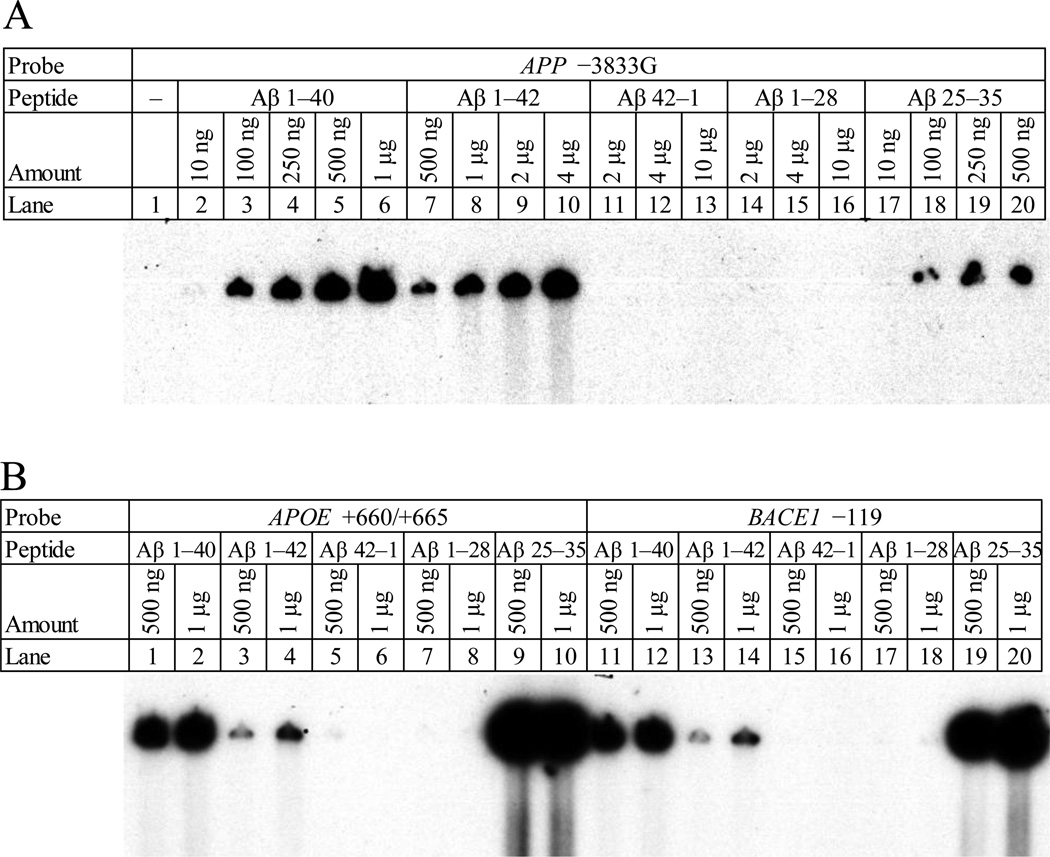
Figure 8. Concentration dependency of Aβ–DNA interaction.
A. The oligomer containing the Aβ–binding consensus at APP −3833G was incubated with fragments of the Aβ peptide, specifically Aβ1–40 (lanes 2–6), 1–42 (lanes 7–10), 42–1 (lanes 11–13), 1–28 (lanes 14–16), and 25–35 (lanes 17–20) at different peptide concentrations as indicated. The gel was dried and exposed to X–ray film for autoradiography. DNA–peptide interactions appeared as dark signal near top of gel. B. The oligomers containing the Aβ–binding consensus at APOE +660 (lanes 1–10) and at BACE1 −119 (lanes 11–20) were incubated with fragments of the Aβ peptide, specifically Aβ1–40 (lanes 1–2, 11–12), 1–42 (lanes 3–4, 13–14), 42–1 (lanes 5–6, 15–16), 1–28 (lanes 7–8, 17–18), and 25–35 (lanes 9–10, 19–20) at different peptide concentrations as indicated. The gel was dried and exposed to X–ray film for autoradiography. DNA–peptide interactions appeared as dark signal near top of gel.
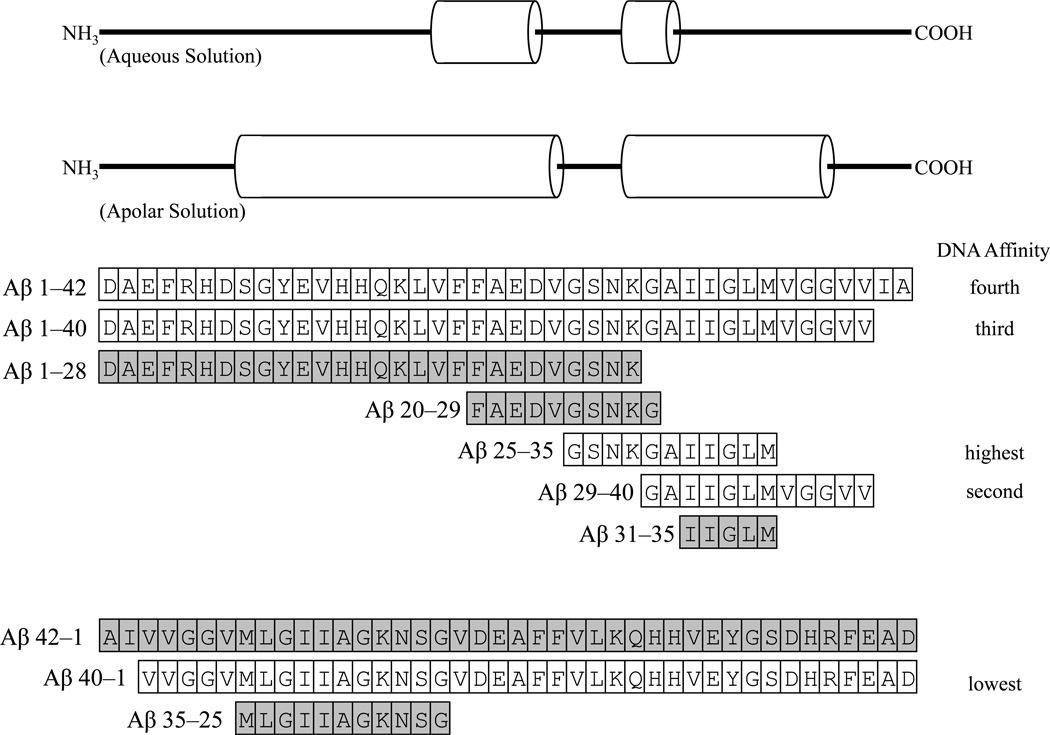
Figure 7. Sequences and alignment of different Aβ fragments used in EMSA.
All diagrams are aligned and to the same scale. A. “Helical–form” secondary structure of Aβ1–42 in aqueous environment (Tomaselli et al., 2006), helices indicated by cylinders. B. Secondary structure of Aβ1–42 in apolar solvent (Crescenzi et al., 2002), helices indicated by cylinders. C. Forward sequences. Sequences for Aβ 1–42, 1–40, 1–28, 20–29, 25–35, 29–40, and 31–35 are depicted as aligned amino acid residues. Those peptides with no apparent DNA–binding activity are in gray background. D. Reverse sequences. Sequences for Aβ42–1, 40–1, and 35–25 are depicted as aligned amino acid residues. Those peptides with no apparent DNA–binding activity are in gray background.
In addition, the polymorphic APP −3833A oligomer pair was allowed to interact with the same series of Aβ peptides. This pair did not have apparent binding with either Aβ1–42 or 1–40, although the high binding affinity of the 25–35 peptide did result in apparently strong interaction (Fig. 6B). Apparent differences between different peptide migration rates were minimal. This was probably because our particular preparations of Aβ were likely to be aggregates instead of monomers, according to the manufacturer.
3.5 Binding of Aβ1–42 and 1–40 occurred in a concentration–dependent manner
To determine if Aβ–DNA interaction occurred in a concentration–dependent manner typical of transcription factors, we selected one “positive” oligomer pair each from APOE, APP, and BACE to bind with the Aβ1–42, 1–40, 42–1, 1–28, and 25–35 peptides (Fig. 8A). The 1–40 and 1–42 peptides both showed qualitative concentration–dependent increases in interaction with the target DNA oligomer pairs. The 1–40 peptide (10ng–1µg with APP−3383G) showed a greater apparent interaction at any given amount of peptide when compared to the 1–42 peptide (500ng–4µg with APP−3383G). The interaction of the 25–35 peptide with the −3833G oligomer pair was less dependent upon peptide concentration than for the 1–40 and 1–42 peptides. The reverse peptide (and negative control) 42–1, and forward 1–28 peptide showed no DNA–protein interaction. Two different concentrations of each of these peptides was interacted with the APOE +660/+665 and BACE1 −119 oligomer pairs, with similar results (Fig. 8B).
3.6 AβID sites predicted in AD–related gene promoter sequences
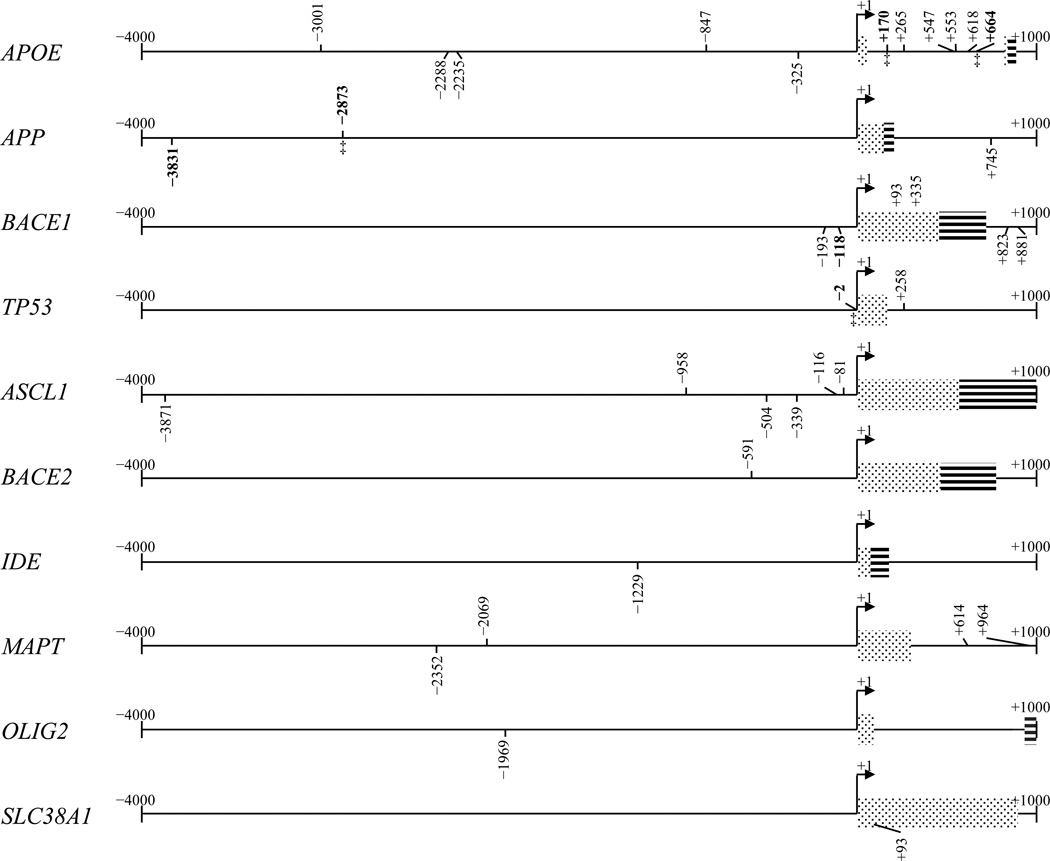
Dynamic weight probing of the APOE, APP, ASCL1, BACE1, IDE, MAPT, OLIG2, and SLC38A1 5’–flanking regions found 11 AβIDs in APOE; 3 in APP; 5 in BACE1, and 2 in TP53 and found 6 AβIDs in ASCL1; 1 in BACE2, 1 in IDE, 4 in MAPT; 1 in OLIG2; and 1 in SLC38A1 (Table 3, Fig. 9). The weight–based search of APOE, APP, and BACE1 produced no “false negatives”, and all AβIDs confirmed by EMSA had corresponding decamers from matrix–based search, with a 1 or 2–base “offset”. In addition, no AβIDs determined by matrix search corresponded with any of the decamers that had been experimentally determined to lack Aβ–binding capacity. Comparable search was performed in genes unrelated to AD pathology. Frequency of the AβID within a search of over 1,000 4kb promoter sequence was significantly different from its calculated frequency in a random 4kb sequence with the same base frequency distribution (data not shown).
Table 3.
Aβ–binding DNA decamers predicted by dynamic weight search
| Gene | Locationa | Orien. | Sequenceb | Gene | Location | Orien. | Sequence |
|---|---|---|---|---|---|---|---|
| APOE | −3001 | F | TAGGTTGGGG | BACE1 | −193 | R | GTCTGCCCTC |
| APOE | −2288 | R | GGGGTAGGAG | BACE1 | −118 | R | TCTCCCGTAG |
| APOE | −2235 | R | GGGGTAGGGG | BACE1 | 335 | R | GACCGCAGGA |
| APOE | −847 | F | TGGGGAGGGG | BACE1 | 823 | R | CCTGTCGTCC |
| APOE | −325 | R | GGGGAGAGGT | BACE1 | 881 | R | TTCACAAGGG |
| APOEc | 170 | F | GGGCTTGGGG | BACE2 | −591 | F | GGGGGTGGGG |
| APOE | 265 | F | TGGGGTGGGG | IDE | −1229 | R | GGGGTCGGGT |
| APOE | 547 | F | TGGGGAGGGG | MAPT | −2352 | R | GGGGAAGGGT |
| APOE | 553 | F | GGGGGTGGGG | MAPT | −2069 | F | GGGGTTGGGG |
| APOE | 618 | F | GGGGATGGGG | MAPT | 614 | F | GGGGGAGGGG |
| APOE | 664 | F | TGGGTTGGGG | MAPT | 964 | F | TGGGGAGGGG |
| APPd | −3831 | R | GGGGTGGGGG | OLIG2 | −1969 | R | GGGGTGGGGG |
| APP | −2873 | F | TAGGATGGGG | SLC38A1 | 93 | R | GGGGTGGGGG |
| APP | 745 | R | GGGGTAGGGG | TP53 | −2 | F | GGGATTGGGG |
| ASCL1 | −3871 | R | GGGGTGGGAG | TP53 | 258 | F | GGGGGTGGGG |
| ASCL1 | −958 | F | TGGGGAGGGG | ||||
| ASCL1 | −504 | R | GGGGTGAGGG | ||||
| ASCL1 | −339 | R | GGGGTGGGGG | ||||
| ASCL1 | −116 | F | GGGAGTGGGG | ||||
| ASCL1 | −81 | F | GGGAGTGGGG | ||||
Location is the 5’–most base of the decamer, relative to the +1 TSS.
Sequence of reverse–orientation decamers is reverse–complemented.
Boldface indicates confirmation of this sequence by EMSA.
This decamer crosses a previously characterized APP single–nucleotide polymorphism (underlined)
Figure 9. Presence of confirmed AβIDs and putative motifs as predicted by dynamic weight scores on selected gene 5’−flanking regions.
The 5’–flanking sequences of several genes were searched for decamers, using the weight matrix we generated. Matrix–matching sites are indicated on the sequences. Forward–orientation sites are above the sequence line. Reverse–orientation sites are below the sequence line. The 5’–flanking regions from 4kb upstream to 1 kb downstream of the +1 transcription start site were analyzed for the APOE, APP, BACE1, TP53, ASCL1, BACE2, IDE, MAPT, OLIG2, and SLC38A1 gene sequences. Positions that corresponded to oligomers for our EMSA herein, all positive on EMSA, are boldface. A site on the APP sequence marked with “§” crosses a previously–characterized APP polymorphism associated with late–onset familial AD.
4. Discussion
A distinctive feature of AD is the accumulation of amyloid plaque in affected brains. The primary constituent of this plaque is Aβ peptide, derived from the much larger APP protein. Aβ is neurotoxic in monomers and oligomers (Walsh et al., 2002; Morgan et al., 2004; Ono et al., 2009). Therefore, research has concentrated on the “pathogenic function” of the peptides, especially the Aβ1–42 and Aβ1–40 peptides. However, there has been ongoing work on elucidating non–pathogenic function for the Aβ peptide. For example, Aβ modulates glutamatergic transmission in the rat basal forebrain (Chin et al., 2007). Its activity in kinase induction has also been investigated (Bogoyevitch et al., 2004). Aβ has also been shown to reduce metal–induced oxidative damage (Zou et al., 2002; Baruch-Suchodolsky and Fischer, 2009) and potentially to regulate cholesterol transport (Yao and Papadopoulos, 2002; Igbavboa et al., 2009).
Aβ has been shown to locate intracellularly in response to conditions such as oxidative stress (Ohyagi and Tabira, 2006). These aspects of Aβ structure and location lead to considering whether some of Aβ’s pathological activity may be related to direct peptide–DNA interaction with genes such as those that determine apoptosis. To test this hypothesis, Ohyagi et al investigated whether or not intracellular Aβ peptide altered expression of the TP53 apoptosis–associated protein gene (Ohyagi et al., 2005). What they determined was that, not only did Aβ enhance p53 levels, but that it did so through direct interaction with a region that contains an HSE on the TP53 promoter/5’–UTR sequence. The transcription factors ASCL1 and OLIG2 have also shown to be differentially regulated in cell culture by Aβ (Uchida et al., 2007). Native BACE1 gene transcription is upregulated in cell culture by addition of Aβ1–42 (Tamagno et al., 2009)..
Another cleavage product of the APP protein, AICD, forms part of the AICD/KAT5/APBB1 transcription factory complex (ATF) complex, along with K (lysine) Acetyltransferase 5 (KAT5/TIP60) and the APP–Binding, Family B, Member 1 protein (APBB1/Fe65) (Konietzko et al., 2010). The ATF, in particular, can regulate APP gene transcription (von Rotz et al., 2004). However, it is unknown whether Aβ participates in this particular pathway as a possible transcription factor, especially since Aβ stimulation of BACE1 RNA transcription occurred independently of addition of AICD (Giliberto et al., 2009).
Surface plasmon resonance has established that soluble Aβ1–42 readily binds DNA in vitro (Barrantes et al., 2007), although specificity of its binding site was not determined by that method. It has also been determined that p53, itself, downregulates expression of the APP gene (Cuesta et al., 2009). Aβ is likely to contain a helix–loop–helix structure common to certain transcription factors (Durell et al., 1994), and this theoretical model is similar to work that demonstrates a helix–kink–helix structure (Shao et al., 1999; Crescenzi et al., 2002) in apolar environments. In an aqueous environment, the peptide can transition between a β–turn–β structure and a more amorphous form with two small helices (Tomaselli et al., 2006).
To investigate the possibility of transcription factor–like sequence specific DNA–Aβ affinity, we have determined a consensus matrix for Aβ–DNA interaction. The consensus is a “G”–rich decamer with the IUPAC sequence 5’–KGGRKTGGGG–3’. However, we urge extreme caution in favoring this for motif searching instead of the sequence logo or a frequency matrix, given the severe limitations of consensus sequences (Schneider, 2002). The empirically determined decamer was “offset” from the original we used to search for oligomers used in EMSA. This might raise questions of actual specificity for Aβ–DNA interaction. However, our EMSA was not done solely with these decamers. Instead, we used 20–mers that contained native flanking sequences. In addition, the work done by Ohyagi et al also used flanking sequences for DNA–protein binding assays. Thus, while the TP53 HSE that happens to share sequence with the AβID might have one specific sequence, the actual AβID may only share partial overlap with the TPE HSE, and our use of multiple putative oligomers was able to more finely distinguish the difference. It bears note that the majority of our test oligomers did not have significant DNA–Aβ interaction by EMSA, giving our direct empirical evidence of Aβ interaction with specific DNA sequences and “rejection” of non–specific sequences.
We readily admit that non–specific interaction between AD plaque and oligonucleotide DNA has long been known (Syrjanen et al., 1991). We do not accept that this precludes sequence–specific interaction between intracellular Aβ and DNA, specifically because: i) “Amyloid plaque” does not exclusively consist of Aβ. Other proteins such as α–synuclein and ApoE are also plaque constituents (Sheng et al., 1996; Yokota et al., 2003); ii) amyloid plaque has a very different tertiary structure than do Aβ monomers, dimers, or oligomers, and properties resulting from higher–order structures, including significant change in DNA binding specificity, cannot be excluded; and iii) interaction with DNA in a non–specific manner does not automatically preclude a protein from being a transcription factor. It has been long established that transcription factors bind DNA in both sequence specific and non–specific fashions (Postel et al., 1993; von Hippel, 1994). Likewise, non–specific binding of a transcription factor to DNA is part of the initial process of its recognition of a specific binding site (von Hippel, 1994). Thus, we accept specificity for Aβ–AβID interaction. Significantly weaker interactions with sequences such as APPHSE and APP−1023 would be “background signal” from non–specific DNA–protein interaction.
We further determined that a single “G” DNA base within this decamer was required for DNA–protein interaction. The APP −3833G AβID included a previously–characterized familial Alzheimer’s disease (FAD) SNP at −3829 (Lahiri et al., 2005). In the “sense” orientation for the APP gene, this is a C↔T polymorphism. We represent it herein as G↔A to preserve the orientation reading determined by Ohyagi et al within TP53. The difference we observed between “G” and “A” response to Aβ 25–35 treatment was not dramatic, suggesting some possibility that Aβ activity on this site may include pathways other than transcription modification.
We investigated the 5’–flanking regions of selected genes involved in AD for potential AβIDs according to our weight matrix. In addition to conducting a search on the APOE, APP, and BACE1 sequences, we also looked at the ASCL1, BACE2, IDE, MAPT, OLIG2, SLC38A1, and TP53 5’–flanking sequences. All sequences had predicted AβIDs. The MAPT sequence is of interest in AD research because it codes for microtubule–associated protein τ, the primary constituent in intraneuronal hyperphosphorylated τ tangles associated with AD (Iqbal et al., 2010). Overproduction of Aβ has already been shown to result in greater hyperphosphorylation of τ protein (Wang et al., 2006a; Wang et al., 2006b). The presence of AβIDs on the MAPT promoter suggests a further relationship between the two products. The IDE gene codes for insulysin/insulin–degrading enzyme, which has been shown to degrade Aβ in vitro and in vivo (Farris et al., 2003; Leissring et al., 2003; Eckman and Eckman, 2005). Presence of predicted AβID suggests further study in characterizing its role in AD. ASCL1 and OLIG2 have been shown to be regulated by Aβ, although the specific pathway of regulation was not suggested in that work (Uchida et al., 2007). The presence of AβIDs on both of the genes’ sequences suggest the possibility that intracellular Aβ may operate directly upon the promoters in a manner similar to that found for TP53 by Ohyagi et al (Ohyagi et al., 2005) and for BACE1 and APP herein.
We also analyzed peptides within the Aβ1–42 peptide to determine that the Aβ25–35 peptide contains the seat for strongest DNA–protein interaction. However, the DNA–binding affinity of the 25–35 peptide is so high that it may reduce target sequence specificity. For example, if the Aβ25–35 peptide was incubated with the two APP FAD oligomer pairs, no apparent change in DNA–protein interaction occurred when qualitatively comparing the “A” oligomer pair with the “G” pair. This greater DNA affinity may explain the moderate results that we had when comparing the SNP–containing clones’ responses to Aβ25–35 treatment. Additional factors such as DNA modification of DNA secondary structure and histone association by the SNP could partially “overcome” the lower specificity to produce a measurable functional difference in cell culture still dependent upon differences in overall transcription factor activity by Aβ on this site.
The EMSA results we present herein were obtained using Aβ peptides that were solubilized as aggregates. We have also performed EMSA with Aβ peptides generated from recombinant E. coli (rPeptide, Bogart, GA, USA). According to the manufacturer, these Aβ peptides were far more likely to be monomers if solubilized according to the manufacturer’s instructions. EMSA assays performed with these peptides were universally negative (data not shown). Several transcription factors, such as NF–κB (Grilli et al., 1995), must consist of homo– or heterodimers of smaller protein subunits to bind DNA in a site–specific fashion. Individual Aβ peptides could function as such subunits for a higher–order transcription factor dimer/aggregate form. Such aggregation also would explain the very slow migration of our EMSA reactions in the gels.
We propose that, whatever other functions it may have, specific DNA–peptide interaction suggests that Aβ could be investigated as a possible transcription factor. Current treatments for AD concentrate upon remediation of cholinergic loss or other specific receptor–based treatments (Sambamurti et al., 2011) However, even if restricting AD etiology to effects of Aβ, evidence exists to suggest a wide variety of pathways that are worth therapeutic exploration (Lahiri and Maloney, 2010a). Furthermore, other pathways, such as loss of synaptic markers, may not be directly caused by Aβ and could be fruitfully treated on their own (81). Given that the AβID is particularly “G” rich, oxidative damage of DNA may play a role in disrupting normal Aβ–DNA interaction. This would be compatible with a recently–proposed gene–environment interaction model of AD etiology, which proposes a specific mechanistic role for DNA oxidation and aberrant DNA methylation in early–life environmental induction of latent predisposition for AD and other idiopathic neurobiological disorders (Lahiri et al., 2009b).
We have determined herein that a degenerate DNA decamer that binds to the Aβ peptide in a sequence–specific manner. Furthermore, binding of Aβ to DNA requires a specific fragment of the Aβ peptide, between residues 25–35. This data would not be sufficient to propose that Aβ’s very broad range of potential functions would include activity as a transcription factor, modifying expression of AD–related genes, such as APP and BACE1 and glutamatergic pathway genes, such as SLC38A1. Functional studies would need to be performed. We have recently done such studies and determined that reporter clones containing the AβID respond to treatment by Aβ peptides. Furthermore, this response was reduced significantly by mutation of the −3833G AβID site to the −3833A SNP variant. In addition, chromatin immune precipitation assays on human neuroblastoma cell chromatin indicated specific binding of Aβ to AβID sequences within the APP and BACE1 promoters. (Bailey et al., 2011). The specificity of DNA interaction as we determined in the present work, coupled with the functional results we have also recently obtained, lead us to suggest the possibility that Aβ may function as a transcription factor and may further act to at least in part regulate its own precursor gene regulation.
Acknowledgements
This work was supported by the Alzheimer’s Association and National Institutes of Health AG18379 and AG18884. We sincerely thank Yuan–Wen Ge for providing excellent technical assistance.
ABBREVIATIONS
The abbreviations used are
- AD
Alzheimer’s disease
- APOE
apolipoprotein E gene
- AICD
APP intracellular domain fragment
- APP
Aβ–precursor protein
- APP
Aβ–precursor protein gene
- ASCL1
achaete–scute complex homolog 1 gene
- ATF
AICD/KAT5/APBB1 transcription factory complex
- Aβ
amyloid beta–peptide
- BACE1
β–amyloid cleaving enzyme 1/β–secretase gene
- EMSA
electrophoretic mobility shift assay
- FAD
familial AD
- HSE
heat shock element
- OLIG2
oligodendrocyte lineage transcription factor 2 gene
- p53
tumor protein 53
- SNP
single–nucleotide polymorphism
- SLC38A1
solute carrier family 38 member 1 gene
- TP53
tumor protein 53 gene.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Portions of this work have been presented as part of the proceedings of the 21st American Peptide Symposium (Lahiri et al., 2009a).
REFERENCES
- Bailey JA, Maloney B, Ge Y-W, Lahiri DK. Functional activity of the novel Alzheimer’s amyloid β–peptide interacting domain (AβID) in the APP and BACE1 promoter sequences and implications in activating apoptotic genes and in amyloidogenesis. Gene. 2011 doi: 10.1016/j.gene.2011.06.017. IN SUBMISSION. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barrantes A, Rejas MT, Benitez MJ, Jimenez JS. Interaction between Alzheimer's Abeta1-42 peptide and DNA detected by surface plasmon resonance. J. Alzheimers. Dis. 2007;12:345–355. doi: 10.3233/jad-2007-12408. [DOI] [PubMed] [Google Scholar]
- Baruch-Suchodolsky R, Fischer B. Abeta40, either soluble or aggregated, is a remarkably potent antioxidant in cell-free oxidative systems. Biochemistry. 2009;48:4354–4370. doi: 10.1021/bi802361k. [DOI] [PubMed] [Google Scholar]
- Bogoyevitch MA, Boehm I, Oakley A, Ketterman AJ, Barr RK. Targeting the JNK MAPK cascade for inhibition: basic science and therapeutic potential. Biochim. Biophys Acta. 2004;1697:89–101. doi: 10.1016/j.bbapap.2003.11.016. [DOI] [PubMed] [Google Scholar]
- Checler F, Dunys J, Pardossi-Piquard R, Alves da Costa C. p53 is regulated by and regulates members of the gamma-secretase complex. Neurodegener. Dis. 2010;7:50–55. doi: 10.1159/000283483. [DOI] [PubMed] [Google Scholar]
- Chin JH, Ma L, MacTavish D, Jhamandas JH. Amyloid beta protein modulates glutamate-mediated neurotransmission in the rat basal forebrain: involvement of presynaptic neuronal nicotinic acetylcholine and metabotropic glutamate receptors. J. Neurosci. 2007;27:9262–9269. doi: 10.1523/JNEUROSCI.1843-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christensen MA, Zhou W, Qing H, Lehman A, Philipsen S, Song W. Transcriptional regulation of BACE1, the beta-amyloid precursor protein beta-secretase, by Sp1. Molecular and Cell Biology. 2004;24:865–874. doi: 10.1128/MCB.24.2.865-874.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crescenzi O, Tomaselli S, Guerrini R, Salvadori S, D'Ursi AM, Temussi PA, Picone D. Solution structure of the Alzheimer amyloid beta-peptide (1–42) in an apolar microenvironment. Similarity with a virus fusion domain. Eur. J. Biochem. 2002;269:5642–5648. doi: 10.1046/j.1432-1033.2002.03271.x. [DOI] [PubMed] [Google Scholar]
- Cuesta A, Zambrano A, Royo M, Pascual A. The tumour suppressor p53 regulates the expression of amyloid precursor protein (APP) Biochem. J. 2009;418:643–650. doi: 10.1042/BJ20081793. [DOI] [PubMed] [Google Scholar]
- De Strooper B. Proteases and proteolysis in Alzheimer disease: a multifactorial view on the disease process. Physiol. Rev. 2010;90:465–494. doi: 10.1152/physrev.00023.2009. [DOI] [PubMed] [Google Scholar]
- Du Y, Chen X, Wei X, Bales KR, Berg DT, Paul SM, Farlow MR, Maloney B, Ge Y-W, Lahiri DK. NF-kappaB mediates amyloid beta peptide-stimulated activity of the human apolipoprotein E gene promoter in human astroglial cells. Brain Res. Mol. Brain Res. 2005;136:177–188. doi: 10.1016/j.molbrainres.2005.02.001. [DOI] [PubMed] [Google Scholar]
- Durell SR, Guy HR, Arispe N, Rojas E, Pollard HB. Theoretical models of the ion channel structure of amyloid beta-protein. Biophys. J. 1994;67:2137–2145. doi: 10.1016/S0006-3495(94)80717-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eckman EA, Eckman CB. Abeta-degrading enzymes: modulators of Alzheimer's disease pathogenesis and targets for therapeutic intervention. Biochem. Soc. Trans. 2005;33:1101–1105. doi: 10.1042/BST20051101. [DOI] [PubMed] [Google Scholar]
- Farris W, Mansourian S, Chang Y, Lindsley L, Eckman EA, Frosch MP, Eckman CB, Tanzi RE, Selkoe DJ, Guenette S. Insulin-degrading enzyme regulates the levels of insulin, amyloid beta-protein, and the beta-amyloid precursor protein intracellular domain in vivo. Proc. Natl. Acad. Sci. U. S. A. 2003;100:4162–4167. doi: 10.1073/pnas.0230450100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giliberto L, Borghi R, Piccini A, Mangerini R, Sorbi S, Cirmena G, Garuti A, Ghetti B, Tagliavini F, Mughal MR, Mattson MP, Zhu X, Wang X, Guglielmotto M, Tamagno E, Tabaton M. Mutant presenilin 1 increases the expression and activity of BACE1. J. Biol. Chem. 2009;284:9027–9038. doi: 10.1074/jbc.M805685200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Girish V, Vijayalakshmi A. Affordable image analysis using NIH Image/ImageJ. Indian J Cancer. 2004;41:47. [PubMed] [Google Scholar]
- Gouras GK, Tsai J, Naslund J, Vincent B, Edgar M, Checler F, Greenfield JP, Haroutunian V, Buxbaum JD, Xu H, Greengard P, Relkin NR. Intraneuronal Abeta42 accumulation in human brain. Am. J. Pathol. 2000;156:15–20. doi: 10.1016/s0002-9440(10)64700-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grilli M, Ribola M, Alberici A, Valerio A, Memo M, Spano P. Identification and characterization of a kappa B/Rel binding site in the regulatory region of the amyloid precursor protein gene. J Biol Chem. 1995;270:26774–26777. doi: 10.1074/jbc.270.45.26774. [DOI] [PubMed] [Google Scholar]
- Hattori M, Tsukahara F, Furuhata Y, Tanahashi H, Hirose M, Saito M, Tsukuni S, Sakaki Y. A novel method for making nested deletions and its application for sequencing of a 300 kb region of human APP locus. Nucleic Acids Res. 1997;25:1802–1808. doi: 10.1093/nar/25.9.1802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hebert LE, Scherr PA, Bienias JL, Bennett DA, Evans DA. Alzheimer disease in the US population: prevalence estimates using the 2000 census. Arch. Neurol. 2003;60:1119–1122. doi: 10.1001/archneur.60.8.1119. [DOI] [PubMed] [Google Scholar]
- Hussain I, Powell D, Howlett DR, Tew DG, Meek TD, Chapman C, Gloger IS, Murphy KE, Southan CD, Ryan DM, Smith TS, Simmons DL, Walsh FS, Dingwall C, Christie G. Identification of a novel aspartic protease (Asp 2) as beta-secretase. Mol. Cell Neurosci. 1999;14:419–427. doi: 10.1006/mcne.1999.0811. [DOI] [PubMed] [Google Scholar]
- Igbavboa U, Sun GY, Weisman GA, He Y, Wood WG. Amyloid beta-protein stimulates trafficking of cholesterol and caveolin-1 from the plasma membrane to the Golgi complex in mouse primary astrocytes. Neuroscience. 2009;162:328–338. doi: 10.1016/j.neuroscience.2009.04.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iqbal K, Liu F, Gong CX, Grundke-Iqbal I. Tau in Alzheimer disease and related tauopathies. Curr Alzheimer Res. 2010;7:656–664. doi: 10.2174/156720510793611592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jang H, Arce FT, Ramachandran S, Capone R, Azimova R, Kagan BL, Nussinov R, Lal R. Truncated beta-amyloid peptide channels provide an alternative mechanism for Alzheimer's Disease and Down syndrome. Proc. Natl. Acad. Sci. U.S.A. 2010;107:6538–6543. doi: 10.1073/pnas.0914251107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kimberly WT, Zheng JB, Guenette SY, Selkoe DJ. The intracellular domain of the beta-amyloid precursor protein is stabilized by Fe65 and translocates to the nucleus in a notch-like manner. J. Biol. Chem. 2001;276:40288–40292. doi: 10.1074/jbc.C100447200. [DOI] [PubMed] [Google Scholar]
- Konietzko U, Goodger ZV, Meyer M, Kohli BM, Bosset J, Lahiri DK, Nitsch RM. Co-localization of the amyloid precursor protein and Notch intracellular domains in nuclear transcription factories. Neurobiol. Aging. 2010;31:58–73. doi: 10.1016/j.neurobiolaging.2008.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- La Fauci G, Lahiri DK, Salton SR, Robakis NK. Characterization of the 5'-end region and the first two exons of the beta-protein precursor gene. Biochem. Biophys. Res. Commun. 1989;159:297–304. doi: 10.1016/0006-291x(89)92437-6. [DOI] [PubMed] [Google Scholar]
- Lahiri DK, Farlow MR, Greig NH, Sambamurti K. Current drug targets for Alzheimer's disease treatment. Drug Devel. Res. 2002;56:267–281. [Google Scholar]
- Lahiri DK, Farlow MR, Sambamurti K, Greig NH, Giacobini E, Schneider LS. A critical analysis of new molecular targets and strategies for drug developments in Alzheimer's disease. Curr. Drug Targets. 2003;4:97–112. doi: 10.2174/1389450033346957. [DOI] [PubMed] [Google Scholar]
- Lahiri DK, Maloney B. Beyond the signaling effect role of amyloid-beta(42) on the processing of APP, and its clinical implications. Exp. Neurol. 2010a;225:51–54. doi: 10.1016/j.expneurol.2010.04.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lahiri DK, Maloney B. The "LEARn" (Latent Early-life Associated Regulation) model integrates environmental risk factors and the developmental basis of Alzheimer's disease, and proposes remedial steps. Exp. Gerontol. 2010b;45:291–296. doi: 10.1016/j.exger.2010.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lahiri DK, Maloney B, Bailey J, Ge Y-W. Role of alzheimer's amyloid-beta peptide as a putative transcription factor. In: Lebl M, Lebl M, Lebl Ms, editors. Peptides: Breaking Away, Proceedings of the Twenty-First American Peptide Symposium; Prompt Scientific Publishing; Bloomington, Indiana, USA. 2009a. pp. 185–186. [Google Scholar]
- Lahiri DK, Maloney B, Zawia NH. The LEARn model: an epigenetic explanation for idiopathic neurobiological diseases. Mol. Psychiatry. 2009b;14:992–1003. doi: 10.1038/mp.2009.82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lahiri DK, Robakis NK. The promoter activity of the gene encoding Alzheimer beta-amyloid precursor protein (APP) is regulated by two blocks of upstream sequences. Brain Res. Mol. Brain Res. 1991;9:253–257. doi: 10.1016/0169-328x(91)90009-m. [DOI] [PubMed] [Google Scholar]
- Lahiri DK, Wavrant De-Vrieze F, Ge Y-W, Maloney B, Hardy J. Characterization of two APP gene promoter polymorphisms that appear to influence risk of late-onset Alzheimer's disease. Neurobiol. Aging. 2005;26:1329–1341. doi: 10.1016/j.neurobiolaging.2004.11.005. [DOI] [PubMed] [Google Scholar]
- Leissring MA, Farris W, Chang AY, Walsh DM, Wu X, Sun X, Frosch MP, Selkoe DJ. Enhanced proteolysis of beta-amyloid in APP transgenic mice prevents plaque formation, secondary pathology, and premature death. Neuron. 2003;40:1087–1093. doi: 10.1016/s0896-6273(03)00787-6. [DOI] [PubMed] [Google Scholar]
- Lu T, Pan Y, Kao SY, Li C, Kohane I, Chan J, Yankner BA. Gene regulation and DNA damage in the ageing human brain. Nature. 2004;429:883–891. doi: 10.1038/nature02661. [DOI] [PubMed] [Google Scholar]
- Maloney B, Ge YW, Greig NH, Lahiri DK. Characterization of the human beta-secretase 2 (BACE2) 5'-Flanking region: identification of a 268-bp region as the basal BACE2 promoter. J. Mol. Neurosci. 2006;29:81–99. doi: 10.1385/JMN:29:1:81. [DOI] [PubMed] [Google Scholar]
- Millucci L, Ghezzi L, Bernardini G, Santucci A. Conformations and biological activities of amyloid beta peptide 25–35. Curr Protein Pept Sci. 2010;11:54–67. doi: 10.2174/138920310790274626. [DOI] [PubMed] [Google Scholar]
- Morgan C, Colombres M, Nunez MT, Inestrosa NC. Structure and function of amyloid in Alzheimer's disease. Prog. Neurobiol. 2004;74:323–349. doi: 10.1016/j.pneurobio.2004.10.004. [DOI] [PubMed] [Google Scholar]
- Ohyagi Y, Asahara H, Chui DH, Tsuruta Y, Sakae N, Miyoshi K, Yamada T, Kikuchi H, Taniwaki T, Murai H, Ikezoe K, Furuya H, Kawarabayashi T, Shoji M, Checler F, Iwaki T, Makifuchi T, Takeda K, Kira JI, Tabira T. Intracellular Abeta42 activates p53 promoter: a pathway to neurodegeneration in Alzheimer's disease. FASEB. J. 2005;19:255–257. doi: 10.1096/fj.04-2637fje. [DOI] [PubMed] [Google Scholar]
- Ohyagi Y, Tabira T. Intracellular amyloid beta-protein and its associated molecules in the pathogenesis of Alzheimer's disease. Mini. Rev. Med. Chem. 2006;6:1075–1080. doi: 10.2174/138955706778560175. [DOI] [PubMed] [Google Scholar]
- Ohyagi Y, Tsuruta Y, Motomura K, Miyoshi K, Kikuchi H, Iwaki T, Taniwaki T, Kira J. Intraneuronal amyloid beta42 enhanced by heating but counteracted by formic acid. J. Neurosci. Methods. 2007;159:134–138. doi: 10.1016/j.jneumeth.2006.06.010. [DOI] [PubMed] [Google Scholar]
- Ono K, Condron MM, Teplow DB. Structure-neurotoxicity relationships of amyloid beta-protein oligomers. Proc. Natl. Acad. Sci. U. S. A. 2009;106:14745–14750. doi: 10.1073/pnas.0905127106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paik YK, Chang DJ, Reardon CA, Davies GE, Mahley RW, Taylor JM. Nucleotide sequence and structure of the human apolipoprotein E gene. Proc. Natl. Acad. Sci. U. S. A. 1985;82:3445–3449. doi: 10.1073/pnas.82.10.3445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Postel EH, Berberich SJ, Flint SJ, Ferrone CA. Human c-myc transcription factor PuF identified as nm23-H2 nucleoside diphosphate kinase, a candidate suppressor of tumor metastasis. Science. 1993;261:478–480. doi: 10.1126/science.8392752. [DOI] [PubMed] [Google Scholar]
- Sambamurti K, Greig NH, Utsuki T, Barnwell EL, Sharma E, Mazell C, Bhat NR, Kindy MS, Lahiri DK, Pappolla MA. Targets for AD treatment: conflicting messages from gamma-secretase inhibitors. J Neurochem. 2011;117:359–374. doi: 10.1111/j.1471-4159.2011.07213.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sambamurti K, Kinsey R, Maloney B, Ge YW, Lahiri DK. Gene structure and organization of the human beta-secretase (BACE) promoter. FASEB J. 2004;18:1034–1036. doi: 10.1096/fj.03-1378fje. [DOI] [PubMed] [Google Scholar]
- Sayers EW, Barrett T, Benson DA, Bolton E, Bryant SH, Canese K, Chetvernin V, Church DM, DiCuccio M, Federhen S, Feolo M, Fingerman IM, Geer LY, Helmberg W, Kapustin Y, Landsman D, Lipman DJ, Lu Z, Madden TL, Madej T, Maglott DR, Marchler-Bauer A, Miller V, Mizrachi I, Ostell J, Panchenko A, Phan L, Pruitt KD, Schuler GD, Sequeira E, Sherry ST, Shumway M, Sirotkin K, Slotta D, Souvorov A, Starchenko G, Tatusova TA, Wagner L, Wang Y, Wilbur WJ, Yaschenko E, Ye J. Database resources of the National Center for Biotechnology Information. Nucleic Acids Res. 2011;39:D38–D51. doi: 10.1093/nar/gkq1172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmid CD, Perier R, Praz V, Bucher P. EPD in its twentieth year: towards complete promoter coverage of selected model organisms. Nucleic Acids Res. 2006;34:D82–D85. doi: 10.1093/nar/gkj146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schneider TD. Consensus sequence Zen. Appl Bioinformatics. 2002;1:111–119. [PMC free article] [PubMed] [Google Scholar]
- Schneider TD, Stephens RM. Sequence logos: a new way to display consensus sequences. Nucleic Acids Res. 1990;18:6097–6100. doi: 10.1093/nar/18.20.6097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shannon CE. The mathematical theory of communication. 1963. MD Comput. 1997;14:306–317. [PubMed] [Google Scholar]
- Shao H, Jao S, Ma K, Zagorski MG. Solution structures of micelle-bound amyloid beta-(1–40) and beta-(1–42) peptides of Alzheimer's disease. J. Mol. Biol. 1999;285:755–773. doi: 10.1006/jmbi.1998.2348. [DOI] [PubMed] [Google Scholar]
- Sheng JG, Mrak RE, Griffin WS. Apolipoprotein E distribution among different plaque types in Alzheimer's disease: implications for its role in plaque progression. Neuropathology and Applied Neurobiology. 1996;22:334–341. doi: 10.1111/j.1365-2990.1996.tb01112.x. [DOI] [PubMed] [Google Scholar]
- Shie FS, LeBoeuf RC, Jin LW. Early intraneuronal Abeta deposition in the hippocampus of APP transgenic mice. Neuroreport. 2003;14:123–129. doi: 10.1097/01.wnr.0000051151.87269.7d. [DOI] [PubMed] [Google Scholar]
- Sinha S, Anderson JP, Barbour R, Basi GS, Caccavello R, Davis D, Doan M, Dovey HF, Frigon N, Hong J, Jacobson-Croak K, Jewett N, Keim P, Knops J, Lieberburg I, Power M, Tan H, Tatsuno G, Tung J, Schenk D, Seubert P, Suomensaari SM, Wang S, Walker D, John V. Purification and cloning of amyloid precursor protein beta-secretase from human brain. Nature. 1999;402:537–540. doi: 10.1038/990114. [DOI] [PubMed] [Google Scholar]
- Sosinsky A, Bonin CP, Mann RS, Honig B. Target Explorer: An automated tool for the identification of new target genes for a specified set of transcription factors. Nucleic Acids Res. 2003;31:3589–3592. doi: 10.1093/nar/gkg544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Syrjanen S, Heinonen O, Miettinen R, Paljarvi L, Syrjanen K, Riekkinen P. Short biotinylated oligonucleotides bind non-specifically to senile plaques of Alzheimer's disease. Neuroscience letters. 1991;130:89–91. doi: 10.1016/0304-3940(91)90234-k. [DOI] [PubMed] [Google Scholar]
- Tabaton M, Zhu X, Perry G, Smith MA, Giliberto L. Signaling effect of amyloid-beta(42) on the processing of AbetaPP. Exp. Neurol. 2010;221:18–25. doi: 10.1016/j.expneurol.2009.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tamagno E, Guglielmotto M, Giliberto L, Vitali A, Borghi R, Autelli R, Danni O, Tabaton M. JNK and ERK1/2 pathways have a dual opposite effect on the expression of BACE1. Neurobiol Aging. 2009;30:1563–1573. doi: 10.1016/j.neurobiolaging.2007.12.015. [DOI] [PubMed] [Google Scholar]
- Thies W, Bleiler L. 2011 Alzheimer's disease facts and figures. Alzheimers Dement. 2011;7:208–244. doi: 10.1016/j.jalz.2011.02.004. [DOI] [PubMed] [Google Scholar]
- Tomaselli S, Esposito V, Vangone P, van Nuland NA, Bonvin AM, Guerrini R, Tancredi T, Temussi PA, Picone D. The alpha-to-beta conformational transition of Alzheimer's Abeta-(1–42) peptide in aqueous media is reversible: a step by step conformational analysis suggests the location of beta conformation seeding. ChemBioChem. 2006;7:257–267. doi: 10.1002/cbic.200500223. [DOI] [PubMed] [Google Scholar]
- Uchida Y, Nakano S, Gomi F, Takahashi H. Differential regulation of basic helix-loop-helix factors Mash1 and Olig2 by beta-amyloid accelerates both differentiation and death of cultured neural stem/progenitor cells. J. Biol. Chem. 2007;282:19700–19709. doi: 10.1074/jbc.M703099200. [DOI] [PubMed] [Google Scholar]
- Vassar R, Bennett BD, Babu-Khan S, Kahn S, Mendiaz EA, Denis P, Teplow DB, Ross S, Amarante P, Loeloff R, Luo Y, Fisher S, Fuller J, Edenson S, Lile J, Jarosinski MA, Biere AL, Curran E, Burgess T, Louis JC, Collins F, Treanor J, Rogers G, Citron M. Beta-secretase cleavage of Alzheimer's amyloid precursor protein by the transmembrane aspartic protease BACE. Science. 1999;286:735–741. doi: 10.1126/science.286.5440.735. [DOI] [PubMed] [Google Scholar]
- von Hippel PH. Protein-DNA recognition: new perspectives and underlying themes. Science. 1994;263:769–770. doi: 10.1126/science.8303292. [DOI] [PubMed] [Google Scholar]
- von Rotz RC, Kohli BM, Bosset J, Meier M, Suzuki T, Nitsch RM, Konietzko U. The APP intracellular domain forms nuclear multiprotein complexes and regulates the transcription of its own precursor. J. Cell Sci. 2004;117:4435–4448. doi: 10.1242/jcs.01323. [DOI] [PubMed] [Google Scholar]
- Walsh DM, Klyubin I, Fadeeva JV, Cullen WK, Anwyl R, Wolfe MS, Rowan MJ, Selkoe DJ. Naturally secreted oligomers of amyloid beta protein potently inhibit hippocampal long-term potentiation in vivo. Nature. 2002;416:535–539. doi: 10.1038/416535a. [DOI] [PubMed] [Google Scholar]
- Wang YP, Wang XC, Tian Q, Yang Y, Zhang Q, Zhang JY, Zhang YC, Wang ZF, Wang Q, Li H, Wang JZ. Endogenous overproduction of beta-amyloid induces tau hyperphosphorylation and decreases the solubility of tau in N2a cells. J. Neural Transm. 2006a;113:1723–1732. doi: 10.1007/s00702-006-0507-5. [DOI] [PubMed] [Google Scholar]
- Wang ZF, Li HL, Li XC, Zhang Q, Tian Q, Wang Q, Xu H, Wang JZ. Effects of endogenous beta-amyloid overproduction on tau phosphorylation in cell culture. J. Neurochem. 2006b;98:1167–1175. doi: 10.1111/j.1471-4159.2006.03956.x. [DOI] [PubMed] [Google Scholar]
- Yan R, Bienkowski MJ, Shuck ME, Miao H, Tory MC, Pauley AM, Brashier JR, Stratman NC, Mathews WR, Buhl AE, Carter DB, Tomasselli AG, Parodi LA, Heinrikson RL, Gurney ME. Membrane-anchored aspartyl protease with Alzheimer's disease beta-secretase activity. Nature. 1999;402:533–537. doi: 10.1038/990107. [DOI] [PubMed] [Google Scholar]
- Yao ZX, Papadopoulos V. Function of beta-amyloid in cholesterol transport: a lead to neurotoxicity. FASEB J. 2002;16:1677–1679. doi: 10.1096/fj.02-0285fje. [DOI] [PubMed] [Google Scholar]
- Yokota O, Terada S, Ishizu H, Ujike H, Ishihara T, Namba M, Hayashi Y, Nishinaka T, Namba R, Nakashima H, Ueda K, Checler F, Kuroda S. Variability and heterogeneity in Alzheimer's disease with cotton wool plaques: a clinicopathological study of four autopsy cases. Acta neuropathol. 2003;106:348–356. doi: 10.1007/s00401-003-0737-7. [DOI] [PubMed] [Google Scholar]
- Zhang X, Zhou K, Wang R, Cui J, Lipton SA, Liao FF, Xu H, Zhang YW. Hypoxia-inducible factor 1alpha (HIF-1alpha)-mediated hypoxia increases BACE1 expression and beta-amyloid generation. J. Biol. Chem. 2007;282:10873–10880. doi: 10.1074/jbc.M608856200. [DOI] [PubMed] [Google Scholar]
- Zou K, Gong JS, Yanagisawa K, Michikawa M. A novel function of monomeric amyloid beta-protein serving as an antioxidant molecule against metal-induced oxidative damage. J. Neurosci. 2002;22:4833–4841. doi: 10.1523/JNEUROSCI.22-12-04833.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]