Abstract
Objectives
To examine structural differences in selected anterior limbic brain regions between at-risk children of parents with bipolar I disorder and children with healthy parents. We hypothesized that at-risk children would exhibit abnormalities in brain regions that are involved in mood regulation.
Methods
Children (8–12 years old) of parents with bipolar I disorder (“at-risk”, AR, N=21) and of parents without any DSM-IV Axis I disorder (health controls, HC, N=24) were evaluated using diagnosticassessments and brain magnetic resonance imaging (MRI). Morphometric analyses were used to examine group differences in the prefrontal cortical, thalamic, striatal, and amygdalar volumes.
Results
Nine (43%) of the AR children met DSM-IV-TR criteria for a non-bipolar mood disorder at the time of assessment. AR and HC children did not demonstrate statistically significant differences across regions of interest [Wilks Lambda = 0.86, F(4,39)=1.64, p=0.18; effect size, (f)=0.19]. Post-hoc analyses of covariance showed the largest relative effect size was contributed by the prefrontal cortex [(f)=0.26].
Conclusions
8 to 12 year old children with a familial risk for mania do not exhibit any statistically significant volumetric differences in the prefrontal cortex, thalamus, striatum, or amygdala as compared to age matched children of parents without any psychopathology. Longitudinal studies examining whether structural changes over time may be associated with vulnerability for developing subsequent bipolar disorder are needed to clarify the underlying pathophysiology of this disorder.
Keywords: Bipolar disorder, offspring, amygdala, prefrontal cortex, MRI
INTRODUCTION
Bipolar disorder is associated with a high rate of familial transmission. Specifically, children of one bipolar parent have a 30% chance of developing a mood disorder, which increases to 70% if the second parent also has been diagnosed with a mood disorder.1 Furthermore, early onset bipolar disorder may be particularly associated with genetic factors.2 For example, offspring of parents with bipolar disorder have an increased risk of developing early-onset bipolar disorder, compared with other bipolar patients.3 While the familial risk of bipolar disorder is well established, potential biomarkers for later illness development in high risk children remain poorly understood. Several recent studies report evidence of physiological abnormalities in youths at high risk for developing bipolar disorder, including autonomic hyperactivity, atypically lateralized information processing, depressed affect during induced mild stress,4 supersensitivity to melatonin suppression by light,5 and elevated daytime basal cortisol levels with normal reactivity to psychosocial stress.6 However, there are few studies examining neuroanatomical abnormalities in these children and adolescents.
There is growing evidence that structural, functional and neurochemical abnormalities in the anterior limbic network (ALN),7 a network of interconnected brain regions including portions of the prefrontal cortex, as well as subcortical, medial temporal, and cerebellar structures, may underlie the neurophysiology of bipolar disorder.7 At least some of these abnormalities may occur early in the development of affective syndromes. In one study, children and adolescents (ages 9–18 years) with bipolar I disorder and a positive family history of bipolar disorder exhibited decreased amygdala size, and in another, first-episode patients showed increased thalamic volume.8,9 In addition, 8 to 12 year old children of parents with bipolar disorder diagnosed with a mood disorder showed decreased N-acetyl aspartate and phosphocreatine/creatine levels in the cerebellar vermis and elevated frontal cortical myo-inositol levels, suggesting impaired energy and cellular metabolism.10
With these considerations in mind, the aim of this study was to evaluate whether children of parents with bipolar I disorder exhibit structural abnormalities in portions of the ALN including the prefrontal cortex, thalamus, striatum, and amygdala - regions that are found to be abnormal in adolescents and adults already diagnosed with bipolar disorder. Based on previous findings, we hypothesized that compared with children of healthy parents (HC), children of parents with bipolar disorder (AR) would exhibit volumetric abnormalities in these brain regions.
METHODS
Subjects
Adults with bipolar I disorder who had children between the ages of 8–12 years were identified from ongoing studies at the University of Cincinnati and from local bipolar support groups. Their children were defined as high risk for developing mania and were, with their parents’ consent, recruited for this study (N=21). At-risk (AR) children were excluded from the study if they had a diagnosis of bipolar I or II disorder. AR children with major depression, dysthymia, and cyclothymia were included because these individuals may represent a group of children who may be at particularly high risk for developing mania. A healthy control (HC) group of 8 to 12 year old children whose parents were free of DSM-IV Axis I mood, psychotic, or anxiety disorders were also recruited for this study (N=24) from advertisements and local schools in the communities in which the AR children reside. Socioeconomic status (SES) was evaluated using a 7-point SES scale ranking income levels according to the following categories: 1=$0–10K, 2=$10–20K, 3=$20–35K, 4=$35–50K, 5=$50–75K, 6=$75–100K, and 7=more than $100K. Exclusion criteria for the HC children included the presence of any DSM-IV Axis I disorder in themselves or any first-degree relatives, and lifetime exposure to any psychotropic medications. Exclusion criteria for both groups included the presence of any major medical or neurological disorder, a history of head trauma with loss of consciousness for greater than 10 minutes, any contraindication to receiving an MRI scan (e.g. presence of metallic implants or braces), a diagnosis of mental retardation, current or prior substance abuse or dependence, or a positive pregnancy test. This study was approved by the University of Cincinnati and Cincinnati Children’s Hospital Medical Center Institutional Review Boards. All study participants and their parents provided written informed assent and consent, respectively. Demographic and clinical characteristics of the AR and HC groups are summarized in Table 1. Tanner stage was assessed using a self-report scale.11 Handedness was assessed using the Crovitz Handedness Questionnaire.12
Table 1.
Demographic and Clinical variables of Children At Risk for Bipolar Disorder (AR) and Healthy Control (HC) Children
| Variable | AR children (N=21) |
HC children (N=24) |
|---|---|---|
| Age, mean (SD), years | 9.7 (1.5) | 10.1 (1.5) |
| Sex, N (%), female | 9 (43) | 13 (54) |
| Ethnicity, N (%), White | 20 (95) | 18 (75) |
| Socioeconomic status, mean (SD), range 1–7 | 4.5 (1.5) | 5.2 (1.6) |
| Tanner stage, mean (SD) | 1.8 (0.8) | 2.2 (0.96) |
| Handedness, N (%), right | 21 (100) | 20 (83) |
| DSM-IV Axis I Diagnosis, N (%) | N/A | |
| Cyclothymia | 2 (9.5) | |
| Dysthymia | 3 (14) | |
| Major depressive disorder | 4 (19) | |
| Attention-deficit hyperactivity disorder | 4 (19) | |
| Anxiety disorder | 5 (24) | |
| Oppositional defiant disorder | 2 (9.5) | |
| At least one psychiatric disorder | 16 (76) | |
| More than one psychiatric disorder | 7 (33) | |
| C-GAS, mean (SD)a | 77 (16) | 88 (8) |
| Current Medications, N (%) | N/A | |
| Mood Stabilizers | 0 (0) | |
| Antidepressants | 4 (17) | |
| Antipsychotics | 1 (4) | |
| Psychostimulants | 2 (8) | |
t=−3.06, df=40, p=0.004
Diagnostic Assessments
The Structured Clinical Interview for DSM-IV (SCID-P)13 was administered to all parents by raters blind to diagnostic group and with established symptom and diagnostic interrater reliability (kappa>0.9). All children were evaluated for lifetime psychiatric diagnoses using the Affective and Psychotic Modules of the Washington University in St. Louis Kiddie-Schedule for Affective Disorders and Schizophrenia (WASH-U KSADS)14 and the Kiddie Schedule of Affective Disorders and Schizophrenia Present and Lifetime version (KSADS-PL),15 administered separately to parents and children by raters blind to diagnostic group and with established symptom and diagnostic reliability (kappa>0.9).16 Overall functioning was assessed using the Child Global Assessment Scale (CGAS).17 Exposure to medication was recorded by chart reviews and clinical interviews of the AR study participants and their parents.
MRI Procedures
Brain magnetic resonance imaging was acquired on a 1.5 Tesla (T) GE scanner using a three-dimensional infrared (IR) prepped Fast Spoiled Grass (FSPGR) acquisition technique (flip angle = 20°, TE=5 ms, TR=15 ms, field of view= 24 cm, and matrix 256×256 pixels), and then reformatted and resliced into 1mm-thick slices displayed in three planes (coronal, sagittal, and axial). Images were digitally transferred to a Macintosh workstation, where morphometric analyses were performed using Brain Image version 3.1.4, allowing for semiautomatic measurements. Regions of interest (ROI) included total intracranial, prefrontal cortical, thalamic, striatal and amygdalar volumes. Measurements were made by trained raters who were blinded to subject group (ICC for all regions of interest>0.9), and a detailed description of each ROI has been provided elsewhere.18,19 A brief description of each ROI based on published methods follows:
Total Intracranial Volume (TICV)
TICV was semiautomatically measured with approximately 1.5 mm thick axial slices covering the entire brain (124 image slices/subject), and included the entire intracranial cavity including cerebral gray and white matter, cerebrospinal fluid, brainstem, and cerebellum. TICV was utilized to control for variation in subject head size.
Prefrontal Cortex
Left and right prefrontal cortical volumes were semiautomatically measured (average=32 slices) and included all intracranial tissue anterior to the coronal plane at the most anterior point of the genu of the corpus callosum.18
Thalamus
Left and right thalamic volumes were measured by manually tracing (average=27 slices) with a medial boundary at the lateral ventricle and the lateral boundary at the posterior limb of the internal capsule.18
Striatum
The striatum includes the combined volumes of the caudate and putamen. The head and body of the caudate were traced in each slice until the inferolateral progression of the tail (average= 45 slices), and a line was drawn extending from the most inferior point of the ipsilateral lateral ventricle to the most inferomedial point of the internal capsule. The putamen was traced over an average of 43 slices, bounded laterally and anteriorly by the external capsule, and by a line extending inferiorly from the anterior limb of the internal capsule. The putamen was separated from the globus pallidus by the lateral medullary lamina.18
Amygdala
The amygdala was manually traced using the alveus to separate it from the hippocampus (average= 15 slices). Lateral and superior boundaries of the amygdala were defined by the white matter of the temporal lobe. The amygdala was anteriorly bound by its thickness being 2.5 times that of the adjacent entorhinal cortex, and posteriorly bound where the amygdala joined the tail of the caudate.19
DATA ANALYSIS
All statistical analyses were performed using Statistical Analysis System software, version 9.1 (SAS Institute, Cary, N.C.). T-tests and chi-square tests were used to compare demographic and clinical characteristics between groups. Morphometric data were first examined for normality using univariate analyses to conform to the assumptions of the parametric statistics employed (Shapiro-Wilks statistic, W>0.95; p>0.31). Paired t-tests identified no statistically significant ROI-laterality differences within each group; therefore, left and right measurements were combined into a total measurement for each ROI. A multivariate analysis of covariance (MANCOVA) was used to examine group comparisons in four ROIs (prefrontal cortex, striatum, thalamus, and amygdala) adjusted for TICV as a covariate. ROI volumes were the dependent variables and group (AR versus HC) was the independent variable. The assumption of equal covariance structure across groups was met (χ2=8.2, df=10, p=0.6) supporting the validity of the MANCOVA result. Post-hoc analyses of covariance (ANCOVA) were used to examine group differences in individual ROIs. Effect sizes for group differences in brain region sizes were calculated to identify the structures with the largest difference between groups based on F-values obtained from the ANCOVAs using the formula (f) = √(k-1)F/N, where (f)= effect size, k=number of groups, F=test statistic, N=total number of subjects. An effect size of (f)>0.25 is considered a medium effect.19
Additional ANCOVAs were performed in regions that showed between group differences, to assess specific TICV-adjusted ROI volume differences among the following three subgroups: healthy controls, AR with mood symptoms (N=9), and AR without mood symptoms (N=12). Since the sample size for these subgroup analyses is small, the results should be interpreted with caution. Where a large effect size was found, least squared means tests were performed to determine which groups differed significantly.
Spearman correlations were performed on those brain regions which showed significant group differences to determine if those differences were correlated with CGAS scores within each group (p-value of ≤0.05 was significant). Additionally TICV-adjusted analyses were repeated after excluding AR children with psychotropic medication exposure, to explore whether exposure to psychotropic medications contributed to significant group differences in ROI volumes,.
RESULTS
Demographics and Clinical Characteristics
There were no statistically significant group differences in age, sex, ethnicity, socioeconomic status, Tanner stage, or handedness between the AR and HC groups. AR children had lower CGAS scores than the HC group (Table 1). Sixteen (76%) of the AR children met DSM-IV-TR criteria for at least one psychiatric disorder, and one-third (N=7) had more than one psychiatric disorder. Nine (43%) of the AR children met criteria for a non-bipolar mood disorder [i.e. major depression (n=4), dysthymia (n=3), or cyclothymia (n=2)]; 4 (19%) AR children had attention-deficit with hyperactivity (ADHD); 5 (24%) AR subjects had anxiety disorders [i.e. obsessive compulsive disorder (n=2), simple phobia (n=2), separation anxiety disorder (n=1)]; and 2 (9.5%) AR subjects had oppositional defiant disorder (Table 1). HC children were free of DSM-IV Axis I mood, psychotic, disruptive behavioral, or anxiety disorders. Psychopathological characteristics of these and other high risk offspring have been previously described.20
Neuroanatomical Findings
AR and HC did not demonstrate a statistically significant difference across ROIs [Wilks Lambda = 0.86, F(4,39)=1.64, p=0.18, (f)=0.19]. Post-hoc ANCOVAs found no statistically significant ROI group differences in the prefrontal cortex, striatum, thalamus, or amygdala (lowest p= 0.09 for TICV-adjusted prefrontal cortex). Post-hoc analyses of covariance showed the largest (medium) relative effect size contributed by the prefrontal cortex [(f)=0.26], followed by low effect sizes in the amygdala [(f)=0.142], thalamus [(f)=0.139], and striatum [(f)=0.00]. CGAS scores were lower in AR versus HC [AR, mean (SD)=77(16) versus HC=88(8), t=-3.06, df=40, p=0.004].
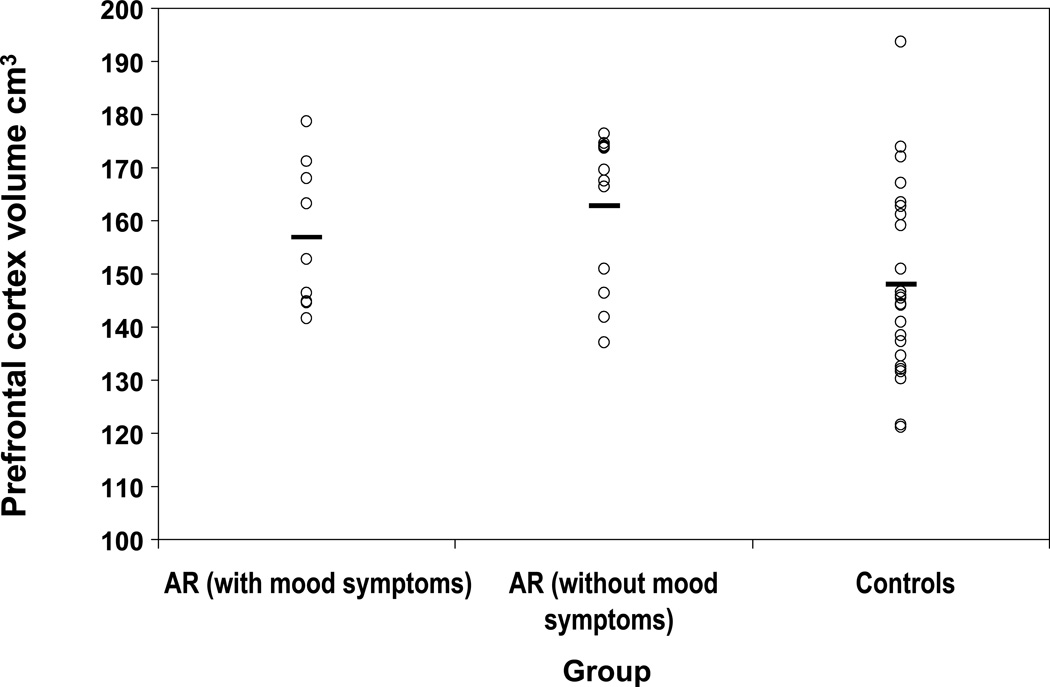
In secondary analyses examining only prefrontal cortical volumes, all subjects in the study were regrouped as AR with mood symptoms (N=9), AR without any mood symptoms (N=12), and HC (N=24). Although there were no group differences in TICV-adjusted mean prefrontal cortex volumes between AR with and without mood symptoms [F(1,19)=0.91, p<0.35, (f)=0.21] or between AR with mood symptoms and HC [F(1,30)=1.78, p<0.19; (f)=0.23], there were significant group differences between AR without mood symptoms and HC [163 ± 16.9 cm3 (SD) versus 148 ± 16.9 cm3 (SD); F(1,33)=6.12, p<0.019, (f)=0.42] (Figure 1). No statistically significant correlation was found between symptom severity (CGAS) and TICV-adjusted prefrontal cortical volume within either of the AR subgroups (highest Spearman correlation coefficient, (r)=0.21, p>0.54) or within the HC group (Spearman correlation coefficient, (r)=0.26, p>0.24).
FIGURE 1.
Adjusted prefrontal cortex volume by group [Overall: F(2,42)=3.48, p<0.04, (f)=0.28; At-risk (AR) with mood symptoms versus Controls: F(1,30)=1.78, p<0.19, (f)=0.23; AR without mood symptoms versus Controls: F(1, 34)=6.12, p<0.019; (f)=0.42]
Restricting analyses to medication naïve subjects did not change our results. Medication naïve AR children showed a medium effect size for group differences in the TICV-adjusted prefrontal cortex versus HC subjects, but these volume differences were not statistically significant [160 ± 21.7 cm3 (SD) versus 147 ± 21.7 cm3 (SD); F(1, 35)=3.12, p<0.09, (f)=0.29].
DISCUSSION
The findings from our study indicate that there were no statistically significant differences in prefrontal, striatal, thalamic, and amygdalar volumes between children at familial risk for bipolar disorder and healthy controls. Moreover, effect sizes were, for the most part, small, suggesting that it is unlikely that these negative findings are due to insufficient sample size. However, post-hoc analyses of prefrontal cortical volumes showed non-significant enlargement in AR children, with a medium effect size. Among the most consistent of neuroanatomic findings in pediatric bipolar disorder is a decrease in amygdala volume as compared to healthy controls.8,19,21 In a recent study by Rosso and colleagues,22 patients experiencing their first episode of mania were also reported to have reduced amygdalar volumes relative to healthy controls, suggesting that amygdala changes occur early in the course of illness. Differences in subject demographics, MRI scanner characteristics, and methods of analyses make it difficult to directly compare the findings of our study with this recent study. Nonetheless, the lack of difference that we observed might suggest that reduced amygdala volume represents an early marker of mania that is not present in those at risk until the onset of manic symptoms. Some,19,23 but not all8,19,24,25 studies of bipolar youth report increases in striatal volumes, whereas studies of ADHD youth report decreases in striatal volumes.26, 27 Differences in rates of co-occurring ADHD among studies may have contributed to the differences in findings. The presence of co-occurring ADHD and mood disorders may explain why the AR sample did not show any group differences in the striatum. Future examinations of high risk offspring should be repeated with larger samples, which would permit closer examinations of the effects of variables such as age, gender, medication exposure, and comorbidity on the neuroanatomy of high risk offspring.
The medium effect size for prefrontal differences between AR and HC subjects suggests that our failure to observe significant volumetric change might be related to insufficient study power for this brain region,28 or may represent a truly negative finding. Preadolescent increases in cortical gray matter have been previously observed in healthy children and adolescents, followed by a postadolescent decrease that is accompanied by a shift from subcortical to prefrontal activity during goal-dependent behavior and emotion regulation.29 Prefrontal cortical hypertrophy in preadolescent high-risk bipolar offspring might reflect an abnormality in the normal neuronal proliferation or pruning of prefrontal cortical circuits. Alternatively, this process might be related to increased activity in this region or represent a compensatory neuroprotective effect, as has been suggested in recent studies of patients with bipolar disorder and Tourette’s syndrome.30, 31
There are a number of limitations to this pilot study including its cross-sectional design and the relatively small sample size; consequently these results should be viewed as preliminary and hypothesis generating. This small sample size may have limited our ability to detect some anatomical differences, increasing the risk of type II error, and limited the generalizability of these findings. Moreover, our methodology did not allow examination of prefrontal subregions; previous work suggests that prefrontal abnormalities may be restricted to specific portions of the prefrontal cortex.32 Exposure to psychotropic medications represents another potential confound. However, no subjects received lithium, which has been associated with gray matter increases.19 Furthermore, restricting our analyses to unmedicated subjects did not affect our findings.
Sixteen (76%) of the AR subjects had an Axis I psychiatric condition, including 4 (19%) with ADHD, and one third (N=7, 33%) with more than one condition. Although exclusion of these subjects did not substantially affect our findings, a larger sample size would allow examination of the effects of comorbid findings on neuroanatomy. Further studies examining structural, functional, and neurochemical abnormalities in children at high risk for developing bipolar disorder are essential to continue to identify potential neurobiological markers for developing bipolar disorder and ultimately, to establish targets for early intervention strategies.
Supplementary Material
Table 2.
MRI Measurements of Regional Brain Volumes for Children At Risk for Bipolar Disorder and Healthy Control Children
| Region of Interest, Mean Volume in cm3 (SD) |
AR Children (N=21) | HC Children (N=24) |
|---|---|---|
| Prefrontal cortex, total | 159 (20.7) | 148 (20.7) |
| Right | 80 (10.4) | 74 (10.4) |
| Left | 80 (10.8) | 75 (10.9) |
| Thalamus, total | 13.2 (2.2) | 13.8 (2.2) |
| Right | 6.6 (1.0) | 6.9 (1.0) |
| Left | 6.6 (1.2) | 6.9 (1.2) |
| Striatum, total | 28.4 (2.8) | 28.3 (2.8) |
| Right | 14.2 (1.7) | 14.2 (1.7) |
| Left | 14.2 (1.6) | 14.1 (1.6) |
| Amygdala, total | 5.7 (0.84) | 5.4 (0.85) |
| Right | 2.8 (0.44) | 2.7 (0.44) |
| Left | 2.85 (0.42) | 2.74 (0.42) |
| TICV, Mean Volume in cm3 (SD) | 286 (29) | 283 (38) |
MRI = magnetic resonance imaging, ROI=region of interest, AR=at-risk, HC=healthy control, TICV=Total Intracranial Volume
Acknowledgements
This study was supported by the Stanley Medical Research Institute. An abstract of this paper was presented at the American Academy of Child & Adolescent Psychiatry Annual Meeting, Toronto, Canada, October 22, 2005.
Footnotes
Disclosure: The authors report no conflict of interest.
REFERENCES
- 1.Goodwin FK, Jamison KR. Manic-depressive illness. New York: Oxford University Press; 1990. [Google Scholar]
- 2.Pavuluri MN, Birmaher B, Naylor MW. Pediatric bipolar disorder: a review of the past 10 years. J Am Acad Child Adolesc Psychiatry. 2005;44(9):846–871. doi: 10.1097/01.chi.0000170554.23422.c1. [DOI] [PubMed] [Google Scholar]
- 3.DelBello MP, Geller B. Review of studies of child and adolescent offspring of bipolar parents. Bipolar Disord. 2001;3(6):325–334. doi: 10.1034/j.1399-5618.2001.30607.x. [DOI] [PubMed] [Google Scholar]
- 4.Zahn TP, Nurnberger JI, Jr, Berrettini WH. Electrodermal activity in young adults at genetic risk for affective disorder. Arch Gen Psychiatry. 1989;46(12):1120–1124. doi: 10.1001/archpsyc.1989.01810120062010. [DOI] [PubMed] [Google Scholar]
- 5.Nurnberger JI, Jr, Berrettini W, Tamarkin L, Hamovit J, Norton J, Gershon E. Supersensitivity to melatonin suppression by light in young people at high risk for affective disorder. A Preliminary Report. Neuropsychopharmacology. 1988;1(3):217–223. doi: 10.1016/0893-133x(88)90020-6. [DOI] [PubMed] [Google Scholar]
- 6.Ellenbogen MA, Hodgins S, Walker CD, Couture S, Adam S. Daytime cortisol and stress reactivity in the offspring of parents with bipolar disorder. Psychoneuroendocrinology. 2006;31(10):1164–1180. doi: 10.1016/j.psyneuen.2006.08.004. [DOI] [PubMed] [Google Scholar]
- 7.DelBello MP, Adler CM, Strakowski SM. The neurophysiology of childhood and adolescent bipolar disorder. CNS Spectr. 2006;11(4):298–311. doi: 10.1017/s1092852900020794. [DOI] [PubMed] [Google Scholar]
- 8.Chang K, Karchemskiy A, Barnea-Goraly N, et al. Reduced amygdalar grey matter volume in familial pediatric bipolar disorder. J Am Acad Child Adolesc Psychiatry. 2005;44(6):565–573. doi: 10.1097/01.chi.0000159948.75136.0d. [DOI] [PubMed] [Google Scholar]
- 9.Adler CM, DelBello MP, Jarvis K, Levine A, Adams J, Strakowski SM. Voxel-based study of structural changes in first-episode patients with bipolar disorder. Biol Psychiatry. 2007;61(6):776–781. doi: 10.1016/j.biopsych.2006.05.042. [DOI] [PubMed] [Google Scholar]
- 10.Cecil KM, DelBello MP, Sellars MC, Strakowski SM. Proton magnetic resonance spectroscopy of the frontal lobe and cerebellar vermis in children with a mood disorder and a familial risk for bipolar disorders. J Child Adolesc Psychopharmacol. 2003;13(4):545–555. doi: 10.1089/104454603322724931. [DOI] [PubMed] [Google Scholar]
- 11.Morris NM, Udry JR. Validation of a self-administered instrument to assess stage of adolescent development. J Youth Adolesc Dev. 1980;9:271–280. doi: 10.1007/BF02088471. [DOI] [PubMed] [Google Scholar]
- 12.Crovitz HF, Zener K. A group-test for assessing hand- and eye-dominance. Am J Psychol. 1962;75:271–276. [PubMed] [Google Scholar]
- 13.First MB, Spitzer RL, Gibbon M, Williams JBW. Structured clinical interview for DSM-IV axis I disorders-patient version (SCID-P) New York: New York State Psychiatric Institute, Biometrics Research Department; 1996. [Google Scholar]
- 14.Geller B, Zimerman B, Williams M, Frazier J. Washington University at St. Louis Kiddie and Young Adult Schedule for Affective Disorders and Schizophrenia (WASH-U-KSADS) St. Louis: Washington University School of Medicine; 1996. [DOI] [PubMed] [Google Scholar]
- 15.Chambers WJ, Puig-Antich J, Hirsch M, Paez P, et al. The assessment of affective disorders in children and adolescents by semistructured interview. Test-retest reliability of the schedule for affective disorders and schizophrenia for school-age children, present episode version. Arch Gen Psychiatry. 1985;42(7):696–702. doi: 10.1001/archpsyc.1985.01790300064008. [DOI] [PubMed] [Google Scholar]
- 16.Geller B, Zimerman B, Williams M, et al. Reliability of the Washington University in St Louis Kiddie Schedule for Affective Disorders and Schizophrenia (WASH-U-KSADS) mania and rapid cycling sections. J Am Acad Child Adolesc Psychiatry. 2001;40(4):450–455. doi: 10.1097/00004583-200104000-00014. [DOI] [PubMed] [Google Scholar]
- 17.Shaffer D, Gould MS, Brasic J, et al. A children's global assessment scale (CGAS) Arch Gen Psychiatry. 1983;40(11):1228–1231. doi: 10.1001/archpsyc.1983.01790100074010. [DOI] [PubMed] [Google Scholar]
- 18.Strakowski SM, DelBello MP, Sax KW, et al. Brain magnetic resonance imaging of structural abnormalities in bipolar disorder. Arch Gen Psychiatry. 1999;56(3):254–260. doi: 10.1001/archpsyc.56.3.254. [DOI] [PubMed] [Google Scholar]
- 19.DelBello MP, Zimmerman ME, Mills NP, Getz GE, Strakowski SM. Magnetic resonance imaging analysis of amygdala and other subcortical brain regions in adolescents with bipolar disorder. Bipolar Disord. 2004;6:143–152. doi: 10.1046/j.1399-5618.2003.00087.x. [DOI] [PubMed] [Google Scholar]
- 20.Singh MK, DelBello MP, Stanford KE, et al. Psychopathology in children of bipolar parents. J Affect Disord. 2007;102(1–3):131–136. doi: 10.1016/j.jad.2007.01.004. [DOI] [PubMed] [Google Scholar]
- 21.Blumberg HP, Kaufman J, Martin A, et al. Amygdala and hippocampal volumes in adolescents and adults with bipolar disorder. Arch Gen Psychiatry. 2003;60(12):1201–1208. doi: 10.1001/archpsyc.60.12.1201. [DOI] [PubMed] [Google Scholar]
- 22.Rosso IM, Killgore WD, Cintron CM, Gruber SA, Tohen M, Yurgelun-Todd DA. Reduced amygdala volumes in first-episode bipolar disorder and correlation with cerebral white matter. Biol Psychiatry. 2007;61(6):743–749. doi: 10.1016/j.biopsych.2006.07.035. [DOI] [PubMed] [Google Scholar]
- 23.Wilke M, Kowatch RA, DelBello MP, Mills NP, Holland SK. Voxel-based morphometry in adolescents with bipolar disorder: first results. Psychiatry Res. 2004;131(1):57–69. doi: 10.1016/j.pscychresns.2004.01.004. [DOI] [PubMed] [Google Scholar]
- 24.Dickstein DP, Milham MP, Nugent AC, et al. Frontotemporal alterations in pediatric bipolar disorder: results of a voxel-based morphometry study. Arch Gen Psychiatry. 2005;62(7):734–741. doi: 10.1001/archpsyc.62.7.734. [DOI] [PubMed] [Google Scholar]
- 25.Sanches M, Roberts RL, Sassi RB, et al. Developmental abnormalities in striatum in young bipolar patients: a preliminary study. Bipolar Disord. 2005;7(2):153–158. doi: 10.1111/j.1399-5618.2004.00178.x. [DOI] [PubMed] [Google Scholar]
- 26.Castellanos FX, Giedd JN, Marsh WL, et al. Quantitative brain magnetic resonance imaging attention-deficit/hyperactivity disorder. Arch Gen Psychiatry. 1996;53(7):607–616. doi: 10.1001/archpsyc.1996.01830070053009. [DOI] [PubMed] [Google Scholar]
- 27.Castellanos FX, Sharp WS, Gottesman RF, Greenstein DK, Giedd JN, Rapoport JL. Anatomic brain abnormalities in monozygotic twins discordant for attention deficit hyperactivity disorder. Am J Psychiatry. 2003;160(9):1693–1696. doi: 10.1176/appi.ajp.160.9.1693. [DOI] [PubMed] [Google Scholar]
- 28.Friedman L, Findling RL, Kenny JT, et al. An MRI study of adolescent patients with either schizophrenia or bipolar disorder as compared to healthy control subjects. Biol Psychiatry. 1999;46(1):78–88. doi: 10.1016/s0006-3223(98)00351-5. [DOI] [PubMed] [Google Scholar]
- 29.Blumberg HP, Kaufman J, Martin A, Charney DS, Krystal JH, Peterson BS. Significance of Adolescent Neurodevelopment for the Neural Circuitry of Bipolar Disorder. Ann NY Acad Sci. 2004;1021:376–383. doi: 10.1196/annals.1308.048. [DOI] [PubMed] [Google Scholar]
- 30.Najt P, Nicoletti M, Chen HH, et al. Anatomical measurements of the orbitofrontal cortex in child and adolescent patients with bipolar disorder. Neurosci Lett. 2007;413(3):183–186. doi: 10.1016/j.neulet.2006.10.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Peterson BS, Staib L, Scahill L, et al. Regional brain and ventricular volumes in Tourette syndrome. Arch Gen Psychiatry. 2001;58(5):427–440. doi: 10.1001/archpsyc.58.5.427. [DOI] [PubMed] [Google Scholar]
- 32.Lopez-Larson MP, DelBello MP, Zimmerman ME, Schwiers ML, Strakowski SM. Regional prefrontal gray and white matter abnormalities in bipolar disorder. Biol Psychiatry. 2002;52(2):93–100. doi: 10.1016/s0006-3223(02)01350-1. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.