Abstract
Although cell-based studies have shown that γ-tocotrienol (γTE) exhibits stronger anticancer activities than other forms of vitamin E including γ-tocopherol (γT), the molecular bases underlying γTE-exerted effects remains to be elucidated. Here we showed that γTE treatment promoted apoptosis, necrosis and autophagy in human prostate PC-3 and LNCaP cancer cells. In search of potential mechanisms of γTE-provoked effects, we found that γTE treatment led to marked increase of intracellular dihydroceramide and dihydrosphingosine, the sphingolipid intermediates in de novo sphingolipid synthesis pathway, but had no effects on ceramide or sphingosine. The elevation of these sphingolipids by γTE preceded or coincided with biochemical and morphological signs of cell death and was much more pronounced than that induced by γT, which accompanied with much higher cellular uptake of γTE than γT. The importance of sphingolipid accumulation in γTE-caused fatality was underscored by the observation that dihydrosphingosine and dihydroceramide potently reduced the viability of both prostate cell lines or LNCaP cells, respectively. In addition, myriosin, a specific inhibitor of de novo sphingolipid synthesis, counteracted γTE-induced cell death. In agreement with these cell-based studies, γTE inhibited LNCaP xenograft growth by 53% (P<0.05), compared with 33% (P = 0.07) by γT, in nude mice. These findings provide a molecular basis of γTE-stimulated cancer-cell death and support the notion that elevation of intracellular dihydroceramide and dihydrosphingosine is likely a novel anticancer mechanism.
Keywords: sphingolipids, vitamin E, tocopherol, autophagy, apoptosis
INTRODUCTION
Vitamin E has eight lipophilic antioxidants, i.e., α-, β-, γ- and δ-tocopherol (αT, βT, γT and δT) and α-, β-, γ- and δ-tocotrienol (αTE, βTE, γTE and δTE). Specific forms of vitamin E have been proposed to be promising anticancer agents 1, 2. For instance, γT has been shown to suppress neoplastic transformation via scavenging reactive nitrogen species 3, and inhibit growth and induce apoptosis of prostate and colon cancer cells by modulation of sphingolipid metabolism 4, down-regulation of cyclins 5 or up-regulation of peroxisome proliferator activated receptor-γ 6. Recently, γT-rich mixed tocopherols are reported to inhibit tumor development in animal models 2, 7–11. Besides tocopherols, γTE, a γT analog with an unsaturated side chain and abundant in palm oil, has been reported to exhibit potent anticancer effects in various types of cancer cells 12–16. In cell-based studies, γTE appears to show stronger efficacy than γT in the anti-proliferation and pro-apoptotic activity 14. Although several biochemical events associated with γTE-induced anticancer actions have been characterized, including its activation of caspase-8 or JNK, induction of endoplasmic reticulum (ER) stress and inhibition of PI3K-mediated AKT phosphorylation 12–17, the molecular bases that trigger these changes as a result of γTE treatment remain ambiguous.
Emerging evidence suggests that modulation of de novo synthesis of sphingolipids may play a role in cell fatality and therefore may be a useful strategy in chemoprevention and therapy 18, 19. We have demonstrated that γT treatment leads to intracellular accumulation of dihydroceramide and dihydrosphingosinge (sphinganine), two key sphingolipid intermediates in the de novo synthesis of sphingolipid pathway, in prostate LNCaP cells, and this action appears to contribute to γT-related apoptosis 4. Fenretinide, a chemotherapeutic agent being tested in clinical trails, has been shown to enhance dihydroceramide in cancer cells 19, 20. Since γTE was reported to exhibit much more potent anticancer activity than γT in prostate cancer cells 14, we hypothesize that γTE may also modulate sphingolipid metabolism, which may play a role in γTE’s pro-death effect on cancer cells. In the present study, we characterized the effect of γTE on cell fatality of human prostate PC-3 and LNCaP cancer cells, investigated whether γTE is capable of modulating intracellular sphingolipids, and examined the anticancer efficacy of γTE on LNCaP-xenograft tumor growth in nude mice. We also compared the relative effectiveness of γTE with that of γT in these activities.
MATERIALS AND METHODS
Materials and reagents
γ-Tocotrienol (>95%) was a gift from Dr. Klaus Kramer from BASF (Germany). γT (> 95%) was purchased from Sigma (St Louis, MO) or Acros Organic (New Jersey). Tissue culture reagents were from GibcoBRL (Rockville, MD). The pan-caspase inhibitor Z-Val-Ala-Asp(OMe)-CH2F (Z-VAD-fmk) and fatty acid free bovine serume albumin (BSA) were from CalBiochem (San Diego, CA). Tocopherol-stripped corn oil was obtained from Dyets Inc. (Bethlehem, PA). Dihydrosphingosine (sphinganine) was from Avanti Polar Lipids, Inc (Alabaster, AL). Myriocin from Mycelia Sterilia, C2-dihydroceramide, dimethyl sulfoxide (DMSO), [3-(4,5)-dimethylthiazol-2-yl)-2,5-diphenyl tetrazolium bromide] (MTT), mevalonate and all other chemicals were from Sigma (St Louis, MO).
Cell culture and effects of vitamin E forms and sphingolipids on cell viability
Human prostate cancer cell lines, PC-3 and LNCaP cells, were obtained from American Type Culture Collection (Manassas, VA). These cells were cultured in RPMI-1640 with 10% fetal bovine serum (FBS). At the time of experiments, cells were seeded in RPMI-1640 with 10% of FBS at a density of 3–4 × 104 cells/well in 24-well plates. Twenty-four or forty-eight hours later, media were replaced with fresh RPMI containing 1% of FBS and vitamin E forms. Before added to media with 1% FBS, γTE and γT were dissolved in DMSO at 50–100 mM and then diluted to 5 mM in fatty acid-free BSA (5 mg/ml). During preparation, samples were kept cold and exposure to light was avoided.
Dihydrosphingosine and C2-dihyceramide were dissolved in DMSO and were then added to RPMI containing 1% of FBS media. In all experiments, the final concentration of DMSO did not exceed 0.15%. The number of viable cells was quantified by the MTT assays 21.
Evaluation of apoptosis and necrosis by annexin V and propidium iodide (PI) staining
Both floating and attached cells were collected by brief trypsinization. Cells were stained with Annexin-V-Fluos staining kit from Roche Applied Science and apoptosis was evaluated using Becton Dickinson FACSort (BD Biosciences). Annexin V recognizes the externalization of phosphatidylserine of the plasma membrane, a marker of apoptosis and PI penetrates into plasma membrane of cells that have lost membrane integrity (necrosis).
Isolation of cytosolic fraction
Cells were homogenized in the buffer containing 220 mM mannitol, 68 mM sucrose, 50 mM PIPES-KOH, 50 mM KCl, 5 mM EGTA, 2 mM MgCl2, and protease inhibitors 22. The homogenate was centrifuged at x400g, 4 °C for 10 min, and the supernatant was further centrifuged at x10000g for 10 min. The resultant supernatant was used as the cytosolic fraction. Protein amount was determined by the BCA protein assay kit (Pierce, Rockford, IL).
Western Blot
Cells were lysed in Tris-EDTA, 1%SDS, 1 mM DTT with protease inhibitor cocktails (Sigma) and the resulting solution was heated at 95 °C for 5 min. To measure Akt phosphorylation, cells were collected by scraping. After a brief centrifugation, cell pellets were lysed in Tris-EDTA, 1%SDS, 1 mM DTT with 2 mM Na3VO4. 10–25 μg of proteins was loaded on 10–12% pre-cast SDS-PAGE gels (BioRad, Richmont CA), semi-dry transferred onto PVDF membrane (Millipore) and probed by antibodies. Membranes were exposed to chemiluminescent reagent (NEN, Life Science Products) and visualized on a Kodak film.
Lipid extraction
Lipids were extracted as previously described 23, 24. Briefly, cell pellets were resuspended in chloroform/methanol/1N hydrochloric acid (100:100:1; v/v/v) by tip sonicated for 20 s. After 0.25 volume of 1M sodium chloride was added to achieve phase separation, the lower organic phase was recovered. Ten percent organic phase was used to determine total choline-containing phospholipids (Ch-PL) by an enzymatic colorimetric assay (Wako Chemicals GmbH, Germany). The rest organic phase was divided into two fractions with addition of C14-sphingosine (150 pmol) as the internal standard, and dried under N2. One fraction was hydrolyzed in 0.5 mL of 1M potassium hydroxide in methanol at 90 °C for 1 h to convert ceramide or dihydroceramide to sphingoid bases. The other lipid fraction was incubated in 0.1M sodium hydroxide in methanol at 37 °C for 1 hr for measurement of free sphingoid bases.
Measurement of sphingolipid intermediates using HPLC with fluorescent detection
Sphingolipid intermediates were derivatized using o-phthalaldehyde to form fluorescent derivatives that were separated by HPLC and detected by a Shimadzu RF-10AXL spectrofluorometric detector (Shimadzu, Columbia, MD) with the excitation and emission wavelength at 340 nm and 455 nm, respectively 23, 24.
Cellular uptake of γTE and γT
Cells were incubated with γTE and γT at specified concentrations in RPMI-1640 media with 1% FBS for 6 h. After being washed twice with media, Cells were collected and homogenized by tip sonication. Vitamin E forms were extracted and analyzed by HPLC with electrochemical detection as previously described 25.
Animal study
The animal use protocol was approved by the animal care committee at Purdue University and strictly followed. LNCaP cells were collected from exponentially growing cultures that were 70–80% confluent. Collected cells were mixed with Matrigel (Becton Dickson Labware) 1:1 by volume. A total volume of 150 μL cell/Matrigel mixture containing 3×106 cells was injected subcutaneously into the right flank of 6–7 week old male BALB/c nude mice (purchased from NCI Frederick Cancer Research Center). Twenty-four hours later, mice were randomized into control (Ctrl), γT- or γTE-treated groups. γT and γTE were administrated by gavage at 125 mg/Kg body weight three times a week, using tocopherol-stripped corn oil as the vehicle (0.1 mL). Control animals were given 0.1 mL corn oil. Tumor size (L×W2×0.5), food intake and body weight were measured once a week.
RESULTS
γTE treatment induced apoptosis, necrosis and autophagy in human prostate PC-3 and LNCaP cells
γTE treatment led to a time and dose-dependent decrease in cell viability of PC-3 and LNCaP cells, as indicated by the MTT assays, and PC-3 cells appeared to be slightly more sensitive than LNCaP to the treatment (Fig. 1). Examination of cell morphology revealed a time- and dose-dependent cell detachment and vacuole formation in the cytoplasm resultant from γTE incubation, suggesting induction of cell death. Compared with our previous studies on γT 4, γTE showed much more effective anticancer activity because γTE at 20 μM decreased cell viability by 80–90% in both cell lines within 48 hours, whereas γT at 50 μM reduced cell viability by 50–60% after 72–96 h incubation and failed to induce death in PC-3 cells. These observations were also in agreement with a recent study by Yap et al 14.
Figure 1. Effects of γTE on the viability of PC-3 (A) and LNCaP (B) cells and evidence for γTE-induced apoptosis and necrosis in PC-3 cells (C).
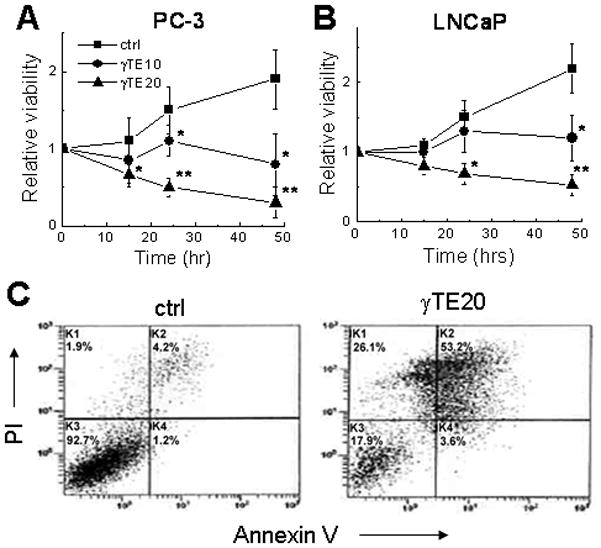
Cells were treated by γTE (indicated in μM) or DMSO (controls) and the effect on cell viability was evaluated at indicated times using MTT assays. Data are the averages of two to three independent experiments. *P<0.05 and **P<0.01 indicate a significant difference between treated and control cells. Flow cytometry was performed to evaluate apoptosis (annexin V+ and annexin V+/PI+) and necrosis (PI+) in PC-3 cells after 24-h treatment with DMSO (ctrl) or γTE at 20 μM.
The nature of γTE-induced cell death was first analyzed by propidium iodide (PI) and annexin V double staining using flow cytometry. γTE treatment appeared to cause apoptosis and necrosis in PC-3 cells (Fig. 1C) and LNCaP cells (Fig 1 in supplement). Consistent with induction of apoptosis, γTE treatment led to PPAR cleavage and the release of cytochrome C to the cytosol (Fig. 2). Activation of caspase-9 was indicated by the decrease of procaspase 9 (47 kDa) in PC-3 cells and the increase of the activated caspase 9 (37 or 35 kDa) in LNCaP cells (Fig. 2A). Caspase activation was also supported by the observation that z-VAD-fmk, an irreversible pan-caspase inhibitor, partially prevented γTE-induced activation of caspase 9 (Fig. 2B). However, z-VAD-fmk failed to counteract γTE-caused cell death (Fig. 2C), suggesting potential involvement of different types of death. Richmann et al recently reported that tocotrienols induce autophagy in pancreatic stella cells 26. Our western blotting data showed that γTE treatment led to an increase of membrane bound microtubule-associated protein light chain 3 (LC3II), a marker of autophagy 27, in both PC-3 and LNCaP cells (Fig. 2D). These results therefore indicate that γTE treatment induced autophagy, in addition to apoptosis and necrosis.
Figure 2. Biochemical events associated with cell death induced by γTE treatment.
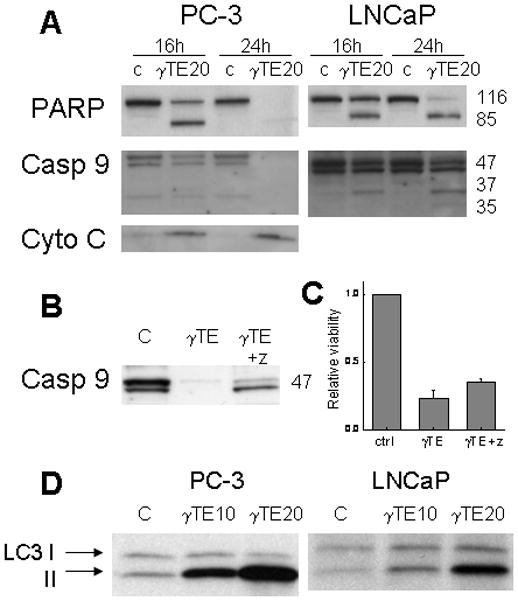
Panel A - Compared with DMSO control (c), γTE (20 μM) led to PARP cleavage, caspase-9 (casp 9) activation and cytochrome C (Cyto C) release to the cytosol. Panel B - Z-VAD-fmk (z) (30 μM) inhibited γTE-caused caspase-9 activation in PC-3 cells. Panel C - Z-VAD-fmk (z) did not counteract γTE-induced death as indicated in MTT assays. Panel D -γTE (10 or 20 μM) treatment resulted in elevation of LC-3II, a marker of autophagy.
γTE did not affect Akt phosphorylation until the late stage of cell death and mevalonate did not reverse γTE-induced effects
In search of the upstream pathway(s) that may be influenced by γTE treatment, we found that γTE had no effect on BCl-2 or BCl-xL expression (unpublished observation). Unlike observations in mouse mammal cells 15, 17, γTE did not activate caspase 8 in the prostate cancer cells (data not shown). A previous study showed that γTE treatment led to suppression of Akt phosphorylation 12. However, we found that γTE did not affect Akt phosphorylation in LNCaP and PC-3 cells after 16-h treatment when cell death was evident (comp. Fig. 2 and Fig. 3), whereas wortmannin (at 1 μM), a specific inhibitor of PI3K, effectively decreased Akt phosphorylation at both 16 and 24 h (Fig. 3). These results indicate that inhibition of PI3K signaling was not a causal factor in γTE-induced death. In addition, although γTE is known to inhibit HMG CoA reductase-mediated mevalonate formation in cholesterol biosynthesis pathway 28, co-incubation with mevalonate failed to reverse γTE-induced cell death (data no shown), suggesting that suppression of HMG-CoA does not contribute significantly to the cell fatality.
Figure 3. The effect of γTE on PI3K-mediated Akt phosphorylation in PC-3 and LNCaP cells.
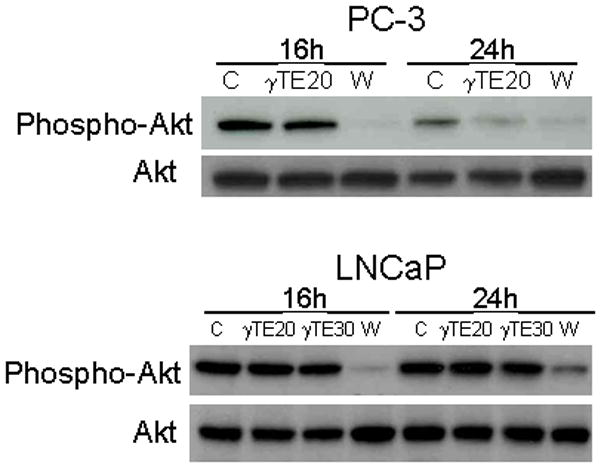
Cells were treated with γTE at 20 (γTE20) or 30 μM (γTE30) or wortmannin (W, 1 μM) for indicated times. Western blotting was performed to evaluate the effect on Akt phosphorylation.
γTE treatment induced marked accumulation of dihydrosphingosine and dihydroceramide in both PC3 and LNCaP cells, which accompanied with high cellular uptake of this vitamin E form
We have previously shown that γT treatment led to accumulation of intracellular sphingolipid intermediates, which may be responsible for γT-induced apoptosis in LNCaP cells 4. Here we found that in both LNCaP (Fig. 4A and 4B) and PC-3 cells (Fig. 4C and 4D), γTE caused marked increase of dihydrosphingosine and dihydroceramide, which are sphingolipid intermediates in de novo synthesis pathway of sphingolipids. The extent of sphingolipid accumulation induced by γTE was much more pronounced than that by γT (Fig. 4). Importantly, γTE significantly elevated dihydroceramide and dihydrosphingosine at 8 and 16 h after incubation (Fig. 4C and 4D), which preceded and/or coincided with activation of death-related biochemical events such as PARP activation (Fig. 4E). In most experiments, extensive cell detachment, a morphological indication of cell death, was evident at 16–24 h after γTE incubation. On the other hand, γTE treatment had no significant effect on ceramide or sphingosine even after 24 h incubation (data not shown).
Figure 4.
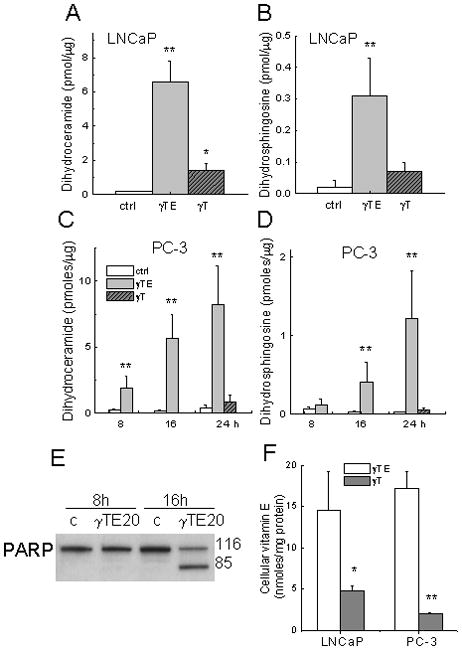
Panels A and B - γTE (20 μM) or γT (50 μM) induced accumulation of dihydroceramide and dihydrosphingosine in LNCaP cells after 24-h incubation. Panels C and D - Effects on sphingolipids by γTE (20 μM) at indicated times or γT (50 μM, 24 h) in PC-3 cells. In Panels A–D, **P<0.01 and *P<0.05 indicate difference between γTE-treated and control cells. Panel E – Effects of γTE (20 μM) on PARP cleavage at indicated times in PC-3 cells. Panel F – Cells were incubated with γTE (10 μM) or γT (50 μM) in RPMI-1640 with 1% FBS for 6 h. Vitamin E forms extracted from collected cells were analyzed by HPLC with electrochemical detection. **P<0.01 and *P<0.05 indicate difference between γTE- and γT-treated cells.
To understand potential reason(s) for the much more potent effects of γTE than γT on sphingolipid accumulation and pro-death activity, we compared the relative cellular uptake of these two vitamin E forms. The results showed that cellular accumulation of γTE in PC-3 and LNCaP cells appeared to be much higher than that of γT (Fig. 4F).
Myriosin significantly counteracted γTE-induced death; Dihydrosphingosine and dihydroceramide reduced cell viability in PC-3 and LNCaP cells
To investigate the importance of sphingolipid increase in γTE-induced cell death, we used myriosin, a specific inhibitor of serine palmitoyl CoA transferase, to block the de novo synthesis of sphingolipids, and found that myriosin significantly counteracted γTE-induced cell death in LNCaP cells (Fig. 5A). In PC-3 cells, myriosin alone caused significant reduction of cell viability (our unpublished observations), which prevents appropriate evaluation of its effect on γTE-caused cell death.
Figure 5.
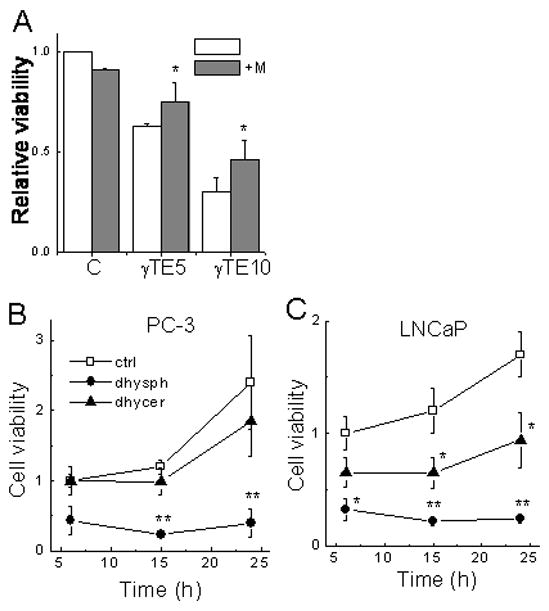
Panel A - Myriocin (6 μM) significantly protected LNCaP cells from γTE- induced death as indicated by MTT assays. *P<0.05, the difference between cells with myriocin (+M, solid bar) and without myriocin (open bar). Panel B and C - Effects of dihydrosphingosine and dihydroceramide on the viability of LNCaP and PC-3 cells. Cells were treated with dihydrosphingosine (dhysph, 25 μM) and dihydroceramide (dhycer, 25 μM) for indicated times and cell viability was analyzed by the MTT assays. **P<0.01 and *P<0.05, the difference between sphingolipid treatment and controls.
To further evaluate the role of increased sphingolipid intermediates in mediating cell death, we examined the effect of dihydrosphingosine and dihydroceramide on PC-3 and LNCaP cell proliferation. Treatment with dihydrosphingosine led to rapid and marked cell death in both PC-3 and LNCaP cells, as indicated by MTT assays (Fig. 5B and 5C) and extensive cell detachment via microscopic observations. C2-dihydroceramide also reduced the viability of LNCaP cells but had little effects on PC-3 cells after 24 h treatment (Fig. 5B and 5C). These observations, together with the fact that the elevation of these sphingolipids by γTE took place prior to the death-related molecular changes, support the notion that intracellular increase of dihydroceramide and dihydrosphingosine likely play a significant role in γTE-induced cell death.
γTE significantly suppresses tumor development in nude mice implanted with LNCaP cells, and was more effective than γT in this action
To translate the cell-based studies to an in vivo cancer model, we examined the effect of γTE and γT on the growth of LNCaP xenograft in nude mice, and compared their relative potency. Oral gavage of γTE and γT did not have any effects on the body weight (Fig. 6A) or food intake (data not shown). Compared with corn oil-fed controls, γTE supplementation significantly inhibited tumor development as indicated by 53% (P < 0.05) reduction of tumor volume, while γT reduced ~30% growth (Fig. 6B). The relative efficacy of these two vitamin E forms paralleled that observed in the cell-based studies.
Figure 6. γTE and γT inhibited LNCaP-xenograft growth in nude mice.
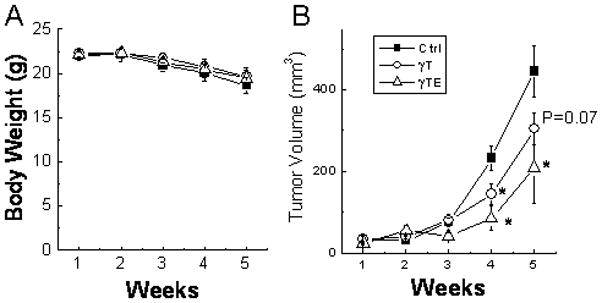
Male BALB/c nude mice were s.c. injected with LNCaP cells. Animals were gavaged with γT, γTE (at 125 mg/Kg bw in 0.1 mL corn oil) or corn oil alone (Ctrl) three times a week. Body weights were measured once a week (Panel A). Tumor volumes were assessed and expressed as mean ± SE (n = 7–8) (Panel B). *P<0.05 indicates significant difference between treated and control groups.
DISCUSSION
We showed that in human prostate cancer cells, γTE treatment led to apoptosis, necrosis and autophagy, and caused marked accumulation of dihydrosphingosine and dihydroceramide, important sphingolipids in de novo biosynthesis pathway. On the other hand, γTE did not significantly affect ceramide or sphingosine. The induction of intracellular dihydrosphingosine and dihydroceramide accumulation is likely a key molecular basis responsible for triggering γTE-induced cell death. First, the enhancement of these sphingolipids by γTE preceded or coincided with biochemical and morphological indications of cell death (Fig. 4). Secondly, since dihydroceramide and especially dihydrosphingosine appear to be potent mediators of cell death (Fig. 5), their elevation inside cells likely, at least in part, contributes to γTE-related fatality. Consistent with this notion, the extent of sphingolipid accumulation matches the relative efficacy of cell death induction, as indicated by the higher effectiveness of γTE than γT in both activities. In addition, myriosin, a specific inhibitor of the first reaction in de novo synthesis of sphingolipids, counteracted γTE-caused cell death.
Although ceramide and sphingosine have long been recognized as important modulators of apoptosis 18, 19, 29, emerging evidence suggests that sphingolipids in the de novo synthesis pathway also play significant roles in determining cell fate. In particular, dihydrosphingosine (sphinganine) has been reported to potently induce apoptosis in colon cancer cells 30 and was implicated in T cell death 31. We currently showed that dihydrosphingosine rapidly induced death of PC-3 and LNCaP cells (Fig. 5). Interestingly, safingol, the synthetic L-threo-stereoisomer of dihydrosphingosine, has recently been shown to be an inhibitor of PI3K and induce autophagy in different cancer cells 32. Since the suppressive effect of γTE on PI3K-mediated Akt phosphorylation was observed after the elevation of intracellular sphingolipids in PC-3 cells in the present study (comp. Fig. 3 with Fig. 4), it is conceivable that the enhanced dihydrosphingosine (by γTE) may lead to γTE-induced autophagy and suppression of Akt phosphorylation. In addition, C2-dihydroceramide, despite being less potent than dihydrosphingosine, inhibited the growth of neuroblastoma cells 20 and reduced the viability of LNCaP cells (Current study). Consistent with these observations, down-regulation of dihydroceramide desaturase, which results in dihydroceramide accumulation, leads to inhibition of cell growth and cell cycle arrest 20, while over-expression of this enzyme upregulates cyclin D1 and enhances metastatic efficiency in esophageal carcinoma cells 33.
Because of the significant role of dihydrosphingosine and dihydroceramide in promoting cancer cell fatality, compounds that are capable of modulation of de novo synthesis of sphingolipid may have valuable chemo-preventive or therapeutic property. In this regard, γT is the first natural compound identified to induce apoptosis by enhancing intracellular dihydrosphingolsine and dihydroceramide 4. Since then, more chemoprevention or chemotherapeutic agents, which bear different structures and specificities, have been reported to modulate intracellular levels of these sphingolipids. Fenretinide, a synthetic analog of all-trans-retinoic acid and chemotherapeutic agent that induces cancer cell death or G1/S arrest, enhances intracellular dihydroceramide 19 by inhibition of dehydroceramide desaturase activity 20. Recently, resveratrol is reported to increase dihydroceramide in gastric cancer cells 34. Celecoxib, a selective cyclooxygenase-2 inhibitor and known to cause cancer cell death via cyclooxygenase-independent mechanisms 35, is shown to increase intracellular accumulation of dihydroceramide and dihydrosphingosine by inhibiting the activity of dihydroceramide desaturase and stimulating de novo synthesis of sphingolipid pathway 36. Our current study demonstrates that γTE is much more potent than γT in induction of dihydrosphingosine and dihydroceramide. Treatment with γTE and γT also led to accumulation of these sphingolipids in colon cancer cells (Rao and Jiang, unpublished data). Based on these results, we propose that elevation of intracellular dihydrosphingosine and dihydroceramide may be a general pathway of induction of cancer cell death, although the exact mechanisms leading to this effect remains to be elucidated. Further studies should be carried out to determine whether vitamin E forms affect the expression or activity of dihydroceramide desaturase, whether they have impacts on the de novo synthesis of sphingolipids, and whether they have differential effects on different dihydroceramide or ceramide species using sphingolipodomics 19 because individual ceramide appears to have distinct bioactivities 37.
The observation that PC-3 and LNCaP cells appeared to accumulate much more γTE than γT may, in large part, explains the stronger pro-death and induction of sphingolipid accumulation by γTE. However, in contrast to its higher cellular uptake, γTE appears to be more rapidly metabolized than γT in vivo 25. It is therefore necessary to examine the relative effectiveness of these vitamin E forms in animal models. To this end, we employed LNCaP- rather than PC-3-xenograft nude mouse model because both γTE and γT treatment caused LNCaP cell death while γT was much less effective toward PC-3 cells4. Our results indicate that γTE inhibited prostate tumor growth more effectively than γT in the xenograft model, which is consistent with the cell-based observations. The paradox between rapid in vivo metabolism and high cellular uptake of γTE may suggest that γTE tends to be accumulated by tumor cells or tissues, which warrants further investigation. The mechanisms underlying differential cellular uptake between γTE and γT need to be further explored.
Although many cell-based studies have reported the anti-cancer activity of γTE, research on testing its efficacy in animal models is relatively limited and only emerged recently. In addition to our current study, Park et al showed that γTE moderately suppressed the growth of implanted-mammary tumor in female BALB/c mice 17. γTE appears to synergize with gemcitabine to inhibit pancreatic cancer growth in athymic nu/nu mice 38. These studies demonstrate that cell-based anticancer effects of γTE can be translated into animals. However, xenograft models do not always provide good clinical prediction of drug effectiveness in human due to their lack of imitation of the complexity of human cancers. In this regard, transgenic mice bearing genetic defects associated with human cancer development ought to be used to further examine chemoprevention and therapeutic potential of vitamin E forms. Interestingly, mixed tocotrienols are recently shown to inhibit prostate carcinogenesis in the TRAMP mice 39, which is in agreement with our study in LNCaP-xenograft nude mice. It is also noteworthy that in addition to the pro-death effects, γTE and γT as well as their metabolites have been demonstrated to have anti-inflammatory activities by inhibiting cyclooxygenase- and 5-lipoxygnease-catalyzed generation of eicosanoids40–42, which are well recognized pro-carcinogenic agents 43. Therefore, future studies with preclinical models including transgenic mice should examine various anticancer mechanisms exerted by γTE and other vitamin E forms including pro-death, modulation of sphingolipids and anti-inflammatory actions.
Acknowledgments
This study was in part supported by grants from NIH R01AT001821 (QJ) and R21CA133651 (QJ).
ABBREVIATIONS
- α-T,β-T, γ-T, or δ-T
α, β, γ, or δ-tocopherol
- αTE, βTE, γTE, δTE
α, β, γ, δ-tocotrienol
- PI3K
phosphoinositide 3-kinase
- PI
propidium iodide
- LC-3
microtubule-associated protein light chain 3
Footnotes
None of the authors has conflict of interest.
References
- 1.Jiang Q, Christen S, Shigenaga MK, Ames BN. gamma-tocopherol, the major form of vitamin E in the US diet, deserves more attention. Am J Clin Nutr. 2001;74:714–22. doi: 10.1093/ajcn/74.6.714. [DOI] [PubMed] [Google Scholar]
- 2.Ju J, Picinich SC, Yang Z, Zhao Y, Suh N, Kong AN, Yang CS. Cancer-preventive activities of tocopherols and tocotrienols. Carcinogenesis. 31:533–42. doi: 10.1093/carcin/bgp205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cooney RV, Franke AA, Harwood PJ, Hatch-Pigott V, Custer LJ, Mordan LJ. γ-Tocopherol detoxification of nitrogen dioxide: Superiority to α-tocopherol. Proc Natl Acad Sci USA. 1993;90:1771–5. doi: 10.1073/pnas.90.5.1771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Jiang Q, Wong J, Fyrst H, Saba JD, Ames BN. {gamma}-Tocopherol or combinations of vitamin E forms induce cell death in human prostate cancer cells by interrupting sphingolipid synthesis. Proc Natl Acad Sci U S A. 2004;101:17825–30. doi: 10.1073/pnas.0408340102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gysin R, Azzi A, Visarius T. Gamma-tocopherol inhibits human cancer cell cycle progression and cell proliferation by down-regulation of cyclins. Faseb J. 2002;16:1952–4. doi: 10.1096/fj.02-0362fje. [DOI] [PubMed] [Google Scholar]
- 6.Campbell SE, Stone WL, Whaley SG, Qui M, Krishnan K. Gamma (gamma) tocopherol upregulates peroxisome proliferator activated receptor (PPAR) gamma (gamma) expression in SW 480 human colon cancer cell lines. BMC Cancer. 2003;3:25. doi: 10.1186/1471-2407-3-25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ju J, Hao X, Lee MJ, Lambert JD, Lu G, Xiao H, Newmark HL, Yang CS. A gamma-tocopherol-rich mixture of tocopherols inhibits colon inflammation and carcinogenesis in azoxymethane and dextran sulfate sodium-treated mice. Cancer prevention research (Philadelphia, Pa) 2009;2:143–52. doi: 10.1158/1940-6207.CAPR-08-0099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Newmark HL, Huang MT, Reddy BS. Mixed tocopherols inhibit azoxymethane-induced aberrant crypt foci in rats. Nutrition and cancer. 2006;56:82–5. doi: 10.1207/s15327914nc5601_11. [DOI] [PubMed] [Google Scholar]
- 9.Barve A, Khor TO, Nair S, Reuhl K, Suh N, Reddy B, Newmark H, Kong AN. Gamma-tocopherol-enriched mixed tocopherol diet inhibits prostate carcinogenesis in TRAMP mice. Int J Cancer. 2009;124:1693–9. doi: 10.1002/ijc.24106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lee HJ, Ju J, Paul S, So JY, DeCastro A, Smolarek A, Lee MJ, Yang CS, Newmark HL, Suh N. Mixed tocopherols prevent mammary tumorigenesis by inhibiting estrogen action and activating PPAR-gamma. Clin Cancer Res. 2009;15:4242–9. doi: 10.1158/1078-0432.CCR-08-3028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lu G, Xiao H, Li GX, Picinich SC, Chen YK, Liu A, Lee MJ, Loy S, Yang CS. A gamma-tocopherol-rich mixture of tocopherols inhibits chemically induced lung tumorigenesis in A/J mice and xenograft tumor growth. Carcinogenesis. 31:687–94. doi: 10.1093/carcin/bgp332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Shah SJ, Sylvester PW. Gamma-tocotrienol inhibits neoplastic mammary epithelial cell proliferation by decreasing Akt and nuclear factor kappaB activity. Experimental biology and medicine (Maywood, NJ) 2005;230:235–41. doi: 10.1177/153537020523000402. [DOI] [PubMed] [Google Scholar]
- 13.Wali VB, Bachawal SV, Sylvester PW. Endoplasmic reticulum stress mediates gamma-tocotrienol-induced apoptosis in mammary tumor cells. Apoptosis. 2009;14:1366–77. doi: 10.1007/s10495-009-0406-y. [DOI] [PubMed] [Google Scholar]
- 14.Yap WN, Chang PN, Han HY, Lee DT, Ling MT, Wong YC, Yap YL. Gamma-tocotrienol suppresses prostate cancer cell proliferation and invasion through multiple-signalling pathways. British journal of cancer. 2008;99:1832–41. doi: 10.1038/sj.bjc.6604763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Shah S, Sylvester PW. Tocotrienol-induced caspase-8 activation is unrelated to death receptor apoptotic signaling in neoplastic mammary epithelial cells. Experimental biology and medicine (Maywood, NJ) 2004;229:745–55. doi: 10.1177/153537020422900806. [DOI] [PubMed] [Google Scholar]
- 16.Sylvester PW, Shah SJ, Samant GV. Intracellular signaling mechanisms mediating the antiproliferative and apoptotic effects of gamma-tocotrienol in neoplastic mammary epithelial cells. Journal of plant physiology. 2005;162:803–10. doi: 10.1016/j.jplph.2005.04.014. [DOI] [PubMed] [Google Scholar]
- 17.Park SK, Sanders BG, Kline K. Tocotrienols induce apoptosis in breast cancer cell lines via an endoplasmic reticulum stress-dependent increase in extrinsic death receptor signaling. Breast cancer research and treatment. 124:361–75. doi: 10.1007/s10549-010-0786-2. [DOI] [PubMed] [Google Scholar]
- 18.Hannun YA, Obeid LM. Principles of bioactive lipid signalling: lessons from sphingolipids. Nature reviews. 2008;9:139–50. doi: 10.1038/nrm2329. [DOI] [PubMed] [Google Scholar]
- 19.Zheng W, Kollmeyer J, Symolon H, Momin A, Munter E, Wang E, Kelly S, Allegood JC, Liu Y, Peng Q, Ramaraju H, Sullards MC, et al. Ceramides and other bioactive sphingolipid backbones in health and disease: lipidomic analysis, metabolism and roles in membrane structure, dynamics, signaling and autophagy. Biochimica et biophysica acta. 2006;1758:1864–84. doi: 10.1016/j.bbamem.2006.08.009. [DOI] [PubMed] [Google Scholar]
- 20.Kraveka JM, Li L, Szulc ZM, Bielawski J, Ogretmen B, Hannun YA, Obeid LM, Bielawska A. Involvement of dihydroceramide desaturase in cell cycle progression in human neuroblastoma cells. J Biol Chem. 2007;282:16718–28. doi: 10.1074/jbc.M700647200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mosmann T. Rapid colorimetric assay for cellular growth and survival: application to proliferation and cytotoxicity assays. J Immunol Methods. 1983;65:55–63. doi: 10.1016/0022-1759(83)90303-4. [DOI] [PubMed] [Google Scholar]
- 22.Li X, Marani M, Mannucci R, Kinsey B, Andriani F, Nicoletti I, Denner L, Marcelli M. Overexpression of BCL-X(L) underlies the molecular basis for resistance to staurosporine-induced apoptosis in PC-3 cells. Cancer Res. 2001;61:1699–706. [PubMed] [Google Scholar]
- 23.Fyrst H, Herr DR, Harris GL, Saba JD. Characterization of free endogenous C14 and C16 sphingoid bases from Drosophila melanogaster. J Lipid Res. 2004;45:54–62. doi: 10.1194/jlr.M300005-JLR200. [DOI] [PubMed] [Google Scholar]
- 24.Bose R, Kolesnick R. Measurement of ceramide levels by the diacylglycerol kinase reaction and by high-performance liquid chromatography-fluorescence spectrometry. Methods Enzymol. 2000;322:373–8. doi: 10.1016/s0076-6879(00)22034-x. [DOI] [PubMed] [Google Scholar]
- 25.Freiser H, Jiang Q. Gamma-tocotrienol and gamma-tocopherol are primarily metabolized to conjugated 2-(beta-carboxyethyl)-6-hydroxy-2,7,8-trimethylchroman and sulfated long-chain carboxychromanols in rats. J Nutr. 2009;139:884–9. doi: 10.3945/jn.108.103309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Rickmann M, Vaquero EC, Malagelada JR, Molero X. Tocotrienols induce apoptosis and autophagy in rat pancreatic stellate cells through the mitochondrial death pathway. Gastroenterology. 2007;132:2518–32. doi: 10.1053/j.gastro.2007.03.107. [DOI] [PubMed] [Google Scholar]
- 27.Kabeya Y, Mizushima N, Ueno T, Yamamoto A, Kirisako T, Noda T, Kominami E, Ohsumi Y, Yoshimori T. LC3, a mammalian homologue of yeast Apg8p, is localized in autophagosome membranes after processing. The EMBO journal. 2000;19:5720–8. doi: 10.1093/emboj/19.21.5720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Parker RA, Pearce BC, Clark RW, Gordon DA, Wright JJ. Tocotrienols regulate cholesterol production in mammalian cells by post-transcriptional suppression of 3-hydroxy-3-methylglutaryl-coenzyme A reductase. J Biol Chem. 1993;268:11230–8. [PubMed] [Google Scholar]
- 29.Ogretmen B, Hannun YA. Biologically active sphingolipids in cancer pathogenesis and treatment. Nat Rev Cancer. 2004;4:604–16. doi: 10.1038/nrc1411. [DOI] [PubMed] [Google Scholar]
- 30.Ahn EH, Schroeder JJ. Sphingoid bases and ceramide induce apoptosis in HT-29 and HCT-116 human colon cancer cells. Exp Biol Med (Maywood) 2002;227:345–53. doi: 10.1177/153537020222700507. [DOI] [PubMed] [Google Scholar]
- 31.Solomon JC, Sharma K, Wei LX, Fujita T, Shi YF. A novel role for sphingolipid intermediates in activation-induced cell death in T cells. Cell Death Differ. 2003;10:193–202. doi: 10.1038/sj.cdd.4401136. [DOI] [PubMed] [Google Scholar]
- 32.Coward J, Ambrosini G, Musi E, Truman JP, Haimovitz-Friedman A, Allegood JC, Wang E, Merrill AH, Jr, Schwartz GK. Safingol (L-threo-sphinganine) induces autophagy in solid tumor cells through inhibition of PKC and the PI3-kinase pathway. Autophagy. 2009;5:184–93. doi: 10.4161/auto.5.2.7361. [DOI] [PubMed] [Google Scholar]
- 33.Zhou W, Ye XL, Sun ZJ, Ji XD, Chen HX, Xie D. Overexpression of degenerative spermatocyte homolog 1 up-regulates the expression of cyclin D1 and enhances metastatic efficiency in esophageal carcinoma Eca109 cells. Molecular carcinogenesis. 2009;48:886–94. doi: 10.1002/mc.20533. [DOI] [PubMed] [Google Scholar]
- 34.Signorelli P, Munoz-Olaya JM, Gagliostro V, Casas J, Ghidoni R, Fabrias G. Dihydroceramide intracellular increase in response to resveratrol treatment mediates autophagy in gastric cancer cells. Cancer Lett. 2009;282:238–43. doi: 10.1016/j.canlet.2009.03.020. [DOI] [PubMed] [Google Scholar]
- 35.Grosch S, Tegeder I, Niederberger E, Brautigam L, Geisslinger G. COX-2 independent induction of cell cycle arrest and apoptosis in colon cancer cells by the selective COX-2 inhibitor celecoxib. Faseb J. 2001;15:2742–4. doi: 10.1096/fj.01-0299fje. [DOI] [PubMed] [Google Scholar]
- 36.Schiffmann S, Sandner J, Schmidt R, Birod K, Wobst I, Schmidt H, Angioni C, Geisslinger G, Grosch S. The selective COX-2 inhibitor celecoxib modulates sphingolipid synthesis. J Lipid Res. 2009;50:32–40. doi: 10.1194/jlr.M800122-JLR200. [DOI] [PubMed] [Google Scholar]
- 37.Senkal CE, Ponnusamy S, Bielawski J, Hannun YA, Ogretmen B. Antiapoptotic roles of ceramide-synthase-6-generated C16-ceramide via selective regulation of the ATF6/CHOP arm of ER-stress-response pathways. Faseb J. 24:296–308. doi: 10.1096/fj.09-135087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kunnumakkara AB, Sung B, Ravindran J, Diagaradjane P, Deorukhkar A, Dey S, Koca C, Yadav VR, Tong Z, Gelovani JG, Guha S, Krishnan S, et al. {Gamma}-tocotrienol inhibits pancreatic tumors and sensitizes them to gemcitabine treatment by modulating the inflammatory microenvironment. Cancer Res. 70:8695–705. doi: 10.1158/0008-5472.CAN-10-2318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Barve A, Khor TO, Reuhl K, Reddy B, Newmark H, Kong AN. Mixed tocotrienols inhibit prostate carcinogenesis in TRAMP mice. Nutr Cancer. 62:789–94. doi: 10.1080/01635581003605896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Jiang Q, Elson-Schwab I, Courtemanche C, Ames BN. gamma-tocopherol and its major metabolite, in contrast to alpha-tocopherol, inhibit cyclooxygenase activity in macrophages and epithelial cells. Proc Natl Acad Sci U S A. 2000;97:11494–9. doi: 10.1073/pnas.200357097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Jiang Q, Yin X, Lill MA, Danielson ML, Freiser H, Huang J. Long-chain carboxychromanols, metabolites of vitamin E, are potent inhibitors of cyclooxygenases. Proc Natl Acad Sci U S A. 2008;105:20464–9. doi: 10.1073/pnas.0810962106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Jiang Z, Yin X, Jiang Q. Natural forms of vitamin E and 13′-carboxychromanol, a long-chain vitamin E metabolite, inhibit leukotriene generation from stimulated neutrophils by blocking calcium influx and suppressing 5-lipoxygenase activity, respectively. J Immunology. 2011;186:1173–9. doi: 10.4049/jimmunol.1002342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Wang D, Dubois RN. Eicosanoids and cancer. Nat Rev Cancer. 2010;10:181–93. doi: 10.1038/nrc2809. [DOI] [PMC free article] [PubMed] [Google Scholar]


