Abstract
Aggressive leukemias arise in both children and adults as a result of rearrangements to the Mixed Lineage Leukemia (MLL) gene located on chromosome 11q23. The MLL gene encodes a large histone methyltransferase that directly binds and positively regulates gene transcription including HOX genes. MLL is involved in chromosomal translocations, partial tandem duplication and amplifications, all of which result in hematopoietic malignancies due to sustained HOX expression and stalled differentiation. MLL lesions are associated with both acute myeloid leukemia (AML) and acute lymphoid leukemia (ALL) and are usually associated with a relatively poor prognosis despite improved treatment options like allogeneic hematopoietic stem cell transplantation underscoring the need for new treatment regimens. Recent advances have begun to reveal the molecular mechanisms driving MLL associated leukemias which have provided opportunities for therapeutic development. Here we discuss the etiology of MLL leukemias and potential directions for therapeutic development.
Keywords: Epigenetics, Translocation, Transcription, Therapeutics, Histone Methylation
Introduction
Epigenetic modifications of chromatin constitute a form of cellular memory that allows gene expression programs to remain intact through cell division. Transcriptional reprogramming and change in epigenetic signatures due to alterations in transcription factors can have catastrophic effects on cellular development including oncogenic transformation. In this review we focus on the clinical characteristics and mechanisms of transformation of leukemias associated with rearrangement of the epigenetic modifier MLL.
1. Cytogenetics and clinical characteristics of MLL associated leukemias
Chromosomal rearrangements at 11q23 are associated with pediatric, adult and therapy-related leukemias and led to the discovery of the Mixed Lineage Leukemia (MLL) gene (1-3). MLL rearrangements fuse the N-terminus of MLL to a fusion partner protein (Figure 2) and constitute >70% of infant acute lymphoid leukemias (ALL) and between 35-50% of infant acute myeloid leukemia (AML); MLL translocations also occur in leukemias of older children and adults, overall accounting for 10% of cases (4). Secondary or therapy-related leukemias that arise in patients treated with topoisomerase II inhibitors for other malignancies also display MLL rearrangements and account for about 5-10% of MLL associated leukemias (4). Together, about 10% of human acute leukemias harbor MLL translocations (4). MLL is also affected by abnormalities other than chromosomal translocations including partial tandem duplications (PTDs) of exons encoding the N-terminus of MLL in about 10% of AML (Figure 2). Non-rearranged MLL is also occasionally amplified in myelodysplasia and AML (5). In general, patients with MLL rearrangements have a poor prognosis and are treated according to high-risk protocols, however this can vary depending on the translocation partner (6). MLL is involved in over 100 recurrent translocations and greater than 60 different partner genes have been identified (6). Despite the vast number of partner genes, MLL is predominantly recombined to nine translocation partners accounting for almost 90% of MLL rearrangements (Figure 1) (6). These include AF4, AF9, ENL, AF10, AF6, ELL, AF1P, AF17 and SEPT6 (Figure 1).
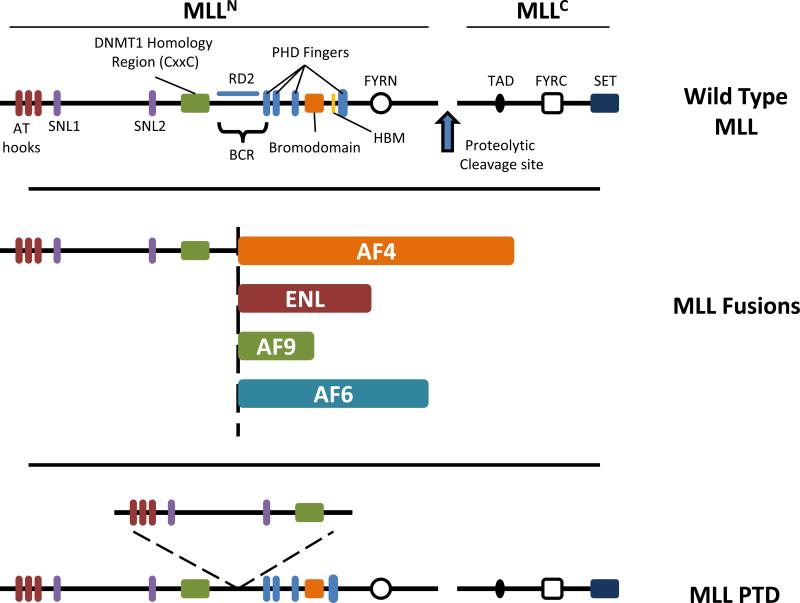
Figure 2.
Structure of wild type and leukemia associated MLL proteins. Top: Domain architecture of wild type MLL. MLL is a large multi-domain protein of about 4000 amino acids. Cleavage of MLL (denoted by the blue arrow) results in 320 kDa MLLN and 180 kDa MLLC fragments that non-covalently associate. Domains within MLLN include three AT hooks (red), two subnuclear localization motifs (SNL) (purple), a DNMT1 homology region (CxxC) (Green), four plant homeodomain (PHD) fingers (blue), an atypical bromodomain (orange) and a FYRN domain (open circle). The Breakpoint Cluster Region (BCR) spans an 8.3 kb region bound by BamH1 restriction sites and encompasses exons 5-11 or 7-13 using old or new nomenclature respectively and is the site of chromosomal translocations involving MLL. Between the CxxC and first PHD finger is repression domain 2 (RD2) that is rich in basic amino acids. A HCF binding motif (HBM) (yellow) is found between the bromodomain and PHD3. MLLC contains a transactivation domain (TAD) (filled oval), a FYRC domain (open square) and C-terminal SET domain (dark blue). Middle: Chromosomal translocations involving MLL result in chimeric MLL fusion proteins that include N-terminal sequence of MLL up to the BCR (dotted vertical line) followed by one of several different fusion partners. Examples of fusion partner proteins including AF4, ENL, AF9 and AF6 are shown. MLL fusion proteins invariably retain AT-hooks, SNL1/2 and the CxxC domain of MLLN while losing the downstream PHD fingers and beyond. Bottom: The MLL gene is also prone to internal tandem duplications (MLL-PTD) resulting in duplication of MLL sequences comprising the AT-hooks, SNL1/2 and the CxxC domain which are inserted at the BCR.
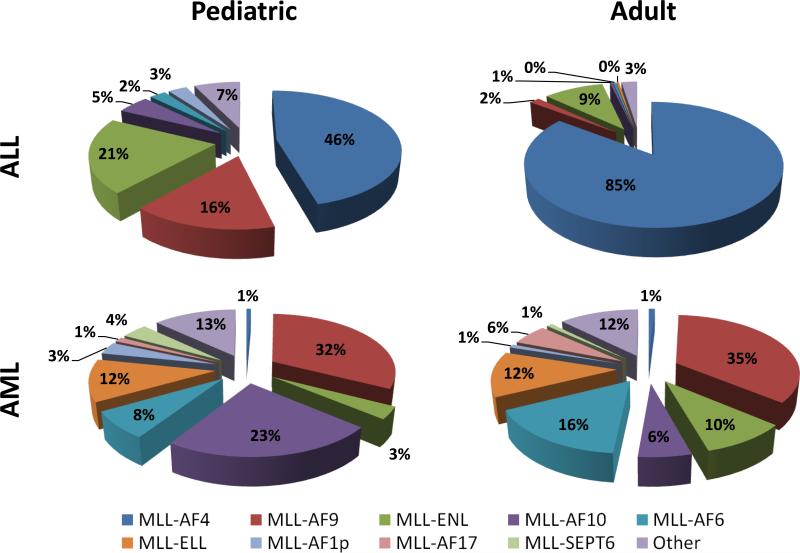
Figure 1.
Translocations with MLL occur with a large and diverse group of partner genes. The frequency of some of the most common translocations with MLL are shown for both pediatric and adult ALL or AML. In general, MLL is fused with a more diverse group of partner proteins in AML compared to ALL, which is primarily composed of AF4, AF9 and ENL translocations (6).
In both pediatric and adult ALL cases involving MLL, the most common translocation partner is AF4 (AFF1) followed by AF9 and ENL (Figure 1). The t(4;11) translocation results in the MLL-AF4 fusion protein and is mostly associated with cases of CD19+ B-lineage ALL. Infants (under the age of 1) with t(4;11) have a particularly poor 5-year event free survival (EFS) rate of about 19%, which increases to about 42% in children over the age of 1 (7, 8). This is striking given a 74-96% EFS in infants with ALL lacking MLL rearrangements (9). Although great strides have been made in infant ALL treatment, including allogeneic hematopoietic stem cell transplantation, transplantation results in worsened EFS compared to chemotherapy alone (8).
Patients with AML have a more broad distribution of translocation partners compared with the ALL, with the most common being the AF9 and ENL translocations. The t(9;11) arrangement produces the MLL-AF9 fusion protein which is found in about 2-5% of all AML and up to 25% of de novo AML in children (7, 10). The prognosis for patients carrying the t(9;11) is more favorable than other 11q23 rearrangements, however the median survival for de novo cases is only ~4 years (10, 11). The MLL-ENL fusion protein associated with the t(11;19) rearrangement is associated with both AML and ALL. Most MLL-ENL cases are found in infants of less than 1 year of age with a bi-phenotypic or B-cell ALL. The median survival for these patients is very poor with a median survival of < 1 year (8). The differences in disease phenotype and patient survival rates based on translocation partner and MLL lesion suggests a better understanding of the molecular events surrounding these various translocation events may translate to better treatment of MLL-associated leukemias.
2. The functions and structure of wild type MLL
MLL (3969 amino acids) is the mammalian homolog of the trithorax (trx) protein found in Drosophila. MLL is a member of the evolutionarily-conserved trithorax group (trxG) family of proteins that positively regulate gene transcription and act antagonistically to the Polycomb group (PcG) proteins (12). The trxG family was first identified through studies of mutant flies, which show homeotic transformations (phenotype where body segment is transformed into a different segment) due to improper expression of the homeobox (Hox) genes (13). These defects were rescued by mutations to PcG genes. It was latter shown that the PcG proteins function as transcriptional repressors of the same targets as trxG proteins, namely the Hox genes. Hox genes are transcriptional regulators that are integral to the formation of the body plan during embryogenesis. Hox genes are regulated in a strict spatiotemporal manner that is critical for tissue development including the hematopoietic system (14). Recent work has demonstrated that many developmentally regulated genes, including Hox genes, display “bivalent” epigenetic signatures, including H3K4 methylation (associated with active transcription) and K3K27 methylation (associated with a transcriptionally repressed locus). These epigenetic marks are deposited, in part, by the MLL complex and the EZH2 containing Polycomb Repressive Complex 2 (PRC2) respectively and allow the target genes to be “poised” for transcriptional activation by RNA polymerase II (15-17).
MLL is expressed in most tissues, including myeloid and lymphoid cells, and positively regulates expression of the clustered Hox genes through histone H3 lysine 4 (H3K4) methyltransferase activity (18, 19). Knockout studies in mice show that Mll is important for the maintenance of Hox gene expression. Deletion of Mll leads to embryonic lethality at ~E10.5 indicating that Mll is essential for proper development. In keeping with its role as a trxG protein disruptions of Mll result in homeotic transformations in Mll knockout mice including posterior shifts in Hox expression patterns as well as defects of the axial skeleton and the hematopoietic system (20, 21). The expression of Hox genes is initiated normally in Mll knockout mice but is not properly maintained. Detailed analysis of the hematopoietic system revealed that Mll is necessary for the proliferation and/or survival of the hematopoietic stem cell (HSC) and progenitor compartment in both the developing fetus and adult mice (20-24).
MLL is proteolytically cleaved by the threonine-aspartase TASPASE1 into a larger 320 kDa N-terminal fragment (MLLN) and smaller 180 kDa C-terminal fragment (MLLC) (Figure 2) (25, 26). Similar to Mll-/- mice, Taspase1-/- mice display homeotic defects due to improper Hox expression (27). After cleavage the two fragments non-covalently associate, via interaction between the FYRN and FYRC domains on MLLN and MLLC respectively, and translocate into the nucleus (Figure 2). Two subnuclear localization signals in MLLN localize MLL to subnuclear punctuate spots. MLLN contains several functional domains involved in binding DNA including three N-terminal AT-hooks, which nonspecifically bind the minor groove of DNA and a DNA methyltransferase homology region (or CxxC domain) that specifically binds unmethylated DNA (Figure 2) (5, 28-31). This region, along with the lysine rich RD2 region, which lies immediately downstream of the CxxC domain, has been reported to have inherent transcriptional repression activity (Figure 2) (32). A group of four plant homeodomain (PHD) zinc fingers with an embedded bromodomain are also present within MLLN. PHD finger 3 binds to tri-methylated H3K4, which also aids in MLL recruitment to target loci (33, 34). Little is understood about the other PHD fingers. Although bromodomains on other epigenetic regulators (like GCN5) bind strongly to acetylated histones, the bromodomain on MLL does not appear to have this affinity (35). Within MLLC lies a transcriptional activation domain that recruits the histone acetyltransferase CREB-binding protein (CBP) (36). At the C-terminus of MLLC is a SET (Su(var)3-9, enhancer of zeste, trithorax) domain which is responsible for the H3K4 methyltransferase activity of MLL (Figure 2) (18, 19).
3. The interacting proteins and molecular biology of MLL and MLL fusion proteins
MLL functions within the context of a large multi-protein complex including MLL, WDR5, RbBP5 and ASH2L, which is required for maximal enzymatic activity. This core complex is shared with other H3K4 methyltransferases including SET1, MLL2 and MLL3. Each component is required for full activity of the complex (37, 38). Detailed analysis demonstrated that WDR5 mediates interaction between the MLLC catalytic unit and the core complex as well as the histone H3 substrate (39). In addition to the core complex, MLLC associates with the histone H4K16 acetyltransferase MOF. Full transcriptional activation of the MLL target gene Hoxa9 requires both MLL associated H3K4 methylation and MOF associated H4K16 acetylation and explains the correlated distribution of these marks on active genes (40). As mentioned above, the CBP/p300 histone acetyltransferase also associate with MLLC and contributes to transcriptional activation (Figure 3A) (36).
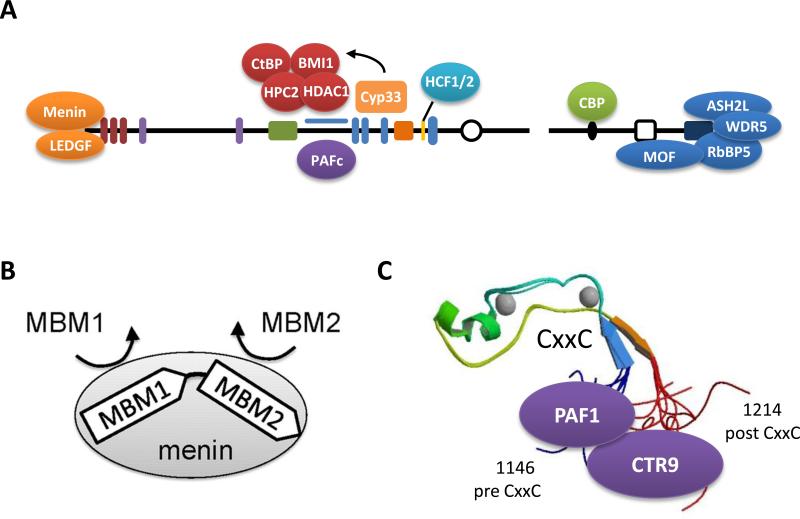
Figure 3.
MLL interacting proteins. A) MLL is known to interact with a variety of partner proteins. Sequences at the extreme N-terminus of MLL are necessary for interaction with Menin and LEDGF. Together these proteins form a trimeric complex that is necessary for leukemogenesis. PAFc interacts with sequences of MLL retained in MLL fusion proteins and is also critically required for MLL fusion leukemogenesis. The RD2 region of MLL interacts with the co-repressors CtBP, the PcG proteins HPC2 and BMI-1, and the histone deacetylase HDAC1 and appears to be regulated by the binding of the cyclophilin Cyp33 to the third PHD finger of MLL. Host Cell Factor 1 and 2 (HCF1/2) interacts with a HBM consensus sequence found between the bromodomain and PHD4 and links MLL to the function of E2F proteins. The transactivation domain of MLL recruits the HAT CBP, which promotes histone acetylation and gene transcription. MOF associates with MLLC and delivers H4K16 acetyltransferase activity to MLL target genes. MLLC also associates with a core complex of proteins including RbBP5, WDR5 and ASH2L, which are necessary for MLL methyltransferase activity. B) Two Menin Binding Motifs (MBM) with varied binding affinities are found at the N-terminus of MLL and necessary for proper interactions with Menin. C) MLL associates with the PAF complex through two interaction sites. The crystal structure of the MLL CxxC domain shows how the pre and post CxxC domain are in close proximity because of the hairpin folding of the CxxC domain. This likely creates a single binding surface of MLL for making direct interactions with the PAF1 and CTR9 components of PAFc.
Although MLL functions as a transcriptional activator, MLL contains a repression domain adjacent to the CxxC domain that interacts with the co-repressors CtBP, the PcG proteins HPC2 and BMI-1, and the histone deacetylase HDAC1 (41). The binding of these repressive proteins is mediated by the binding of Cyp33, an RNA-binding nuclear cyclophilin with peptidyl-prolyl isomerase activity. The third PHD finger of MLL mediates the binding of Cyp33 that isomerizes a proline in the PHD3-Bromodomain linker region. The isomerization facilitates binding to Cyp33 which in turn mediates the recruitment of HDAC1 (35). C-terminal to the PHD3 and bromodomain is a HBM consensus sequence that is found in proteins that associate with the G1 phase regulator Host Cell Factor (HCF) proteins 1 and 2. HCF1/2 associates with both activator and repressor E2F proteins in a cell cycle-dependent manner. HCF1/2, in turn, recruits both SET1 and MLL HMT's to induce histone methylation and transcriptional activation (Figure 3A) (42, 43).
The protein-protein interactions described thus far are largely dependent on MLL sequences that are invariably lost in MLL-fusion proteins and consequently do not contribute to MLL-fusion protein transactivation. Although the AT-hooks and the CxxC domain are retained in MLL fusion proteins and are likely involved in DNA binding, these domains do not provide sequence specific targeting. Thus, alternative interactions must be in play for the proper function and targeting of MLL fusion proteins. At the extreme N-terminus of MLL is a Menin Binding Motif (MBM) (Figure 3A and B). The tumor suppressor protein Menin is coded by the MEN1 gene that is mutated in multiple endocrine neoplasia type 1. The N-terminal 43 amino acids of MLL contain two MBM sequences that mediate the interaction with Menin. Formation of a tri-molecular complex with both MLL and the chromatin-associated protein Lens Epithelium-Derived Growth Factor (LEDGF) is critical for the proper targeting of MLL or MLL fusion proteins to specific target genes, such as HOXA9, and for transcription upregulation required for leukemogenesis (44-47). Another interaction that is preserved in MLL fusion proteins and necessary for leukemogenesis is with the Polymerase Associated Factor complex (PAFc). This interaction is mediated between sequences before and after the CxxC domain that are consistently retained in MLL fusion proteins (Figure 3A and C) (34, 48). PAFc is a transcriptional activation complex that associates with RNA polymerase II and which facilitates the deposition of a mono-ubiquitination mark on histone H2B lysine 120, a pre-requisite for both H3K4 and K3K79 methylation (49-52). Like Menin, PAFc is required for MLL and MLL fusion protein recruitment to target loci. Thus it appears that MLL fusion proteins employ both protein-DNA interactions involving the AT-hooks and the CxxC domain and protein-protein interactions with Menin and PAFc for proper targeting to gene loci.
4. MLL target genes
The best understood targets of MLL and MLL fusion proteins are the HOX genes. As discussed, MLL and MLL fusions bind directly to the promoters and coding regions of HOX loci via DNA and PAFc and Menin/LEDGF interactions (18, 19, 53). The central role of HOX genes in MLL leukemogenesis is well documented and described in detail below. Another important direct target of MLL fusion proteins is EVI-1 (Ecotropic Viral Integration site-1), a nuclear transcription factor with essential roles in hematopoietic stem cell regulation. EVI-1 is upregulated in up to 10% of AML cases as a result of MLL translocation or by other mechanisms and is usually associated with a poor outcome (54). MLL is also known to regulate the cell cycle regulatory genes Cyclins and Cyclin Dependent Kinase Inhibitors (CDKIs). This is consistent with an MLL-E2F axis mediated by HCF-1 that targets MLL to S phase promoters, including Cyclins, which are regulated by E2F proteins (27, 43). The CDK inhibitors p27 and p18 are both regulated by MLL in a Menin-dependent manner (55, 56). The failed induction of these CDKIs as a result of inactivating Menin mutations is thought to be an important oncogenic pathway in these endocrine tumors (55). It has been difficult to reconcile how MLL is involved in regulating both cell cycle activators (Cyclins) and inhibitors (CDKIs). The observed temporal regulation of Cyclins by cell cycle specific recruitment of MLL may be due to a bi-phasic degradation of MLL protein through the ubiquitin-proteasome system (UPS) (57). The MLL protein is degraded during S phase and M phase by the SCFSKP2 and APCCDC20 E3 ubiquitin ligase complexes. Indeed, it was recognized in earlier studies that after proteolytic cleavage, the MLLN fragment was extremely unstable when not in complex with MLLC and that the MLL N-terminus may be stabilized in MLL fusion proteins (25, 58). Failure to properly degrade MLL at these cell cycle checkpoints results in defective cell cycle checkpoint response (59). Although ubiquitination of MLL is mediated through the N-terminus, MLL fusion proteins display resistance to degradation and thus may be a contributing mechanism in leukemogenesis.
Telomerase activity is upregulated in up to 90% of cancers and is instrumental in protecting chromosomes from telomere shortening. Recent work has established that MLL directs H3K4 methyltransferase activity to telomeres and induces transcription of telomere repeat-containing RNAs (60). Further, MLL fusion proteins have been shown to influence the transcription of a major subunit of the telomerase enzyme TERT, apparently through upregulation of HOXA7, which binds directly to the TERT promoter to induce expression (61).
5. Transcriptional pathways deregulated by MLL fusion proteins
MLL rearranged acute lymphoid and myeloid leukemias show highly characteristic gene expression patterns, most notably high level expression of HOX genes. The preponderance of data suggest that MLL fusion protein transformation is primarily mediated through direct upregulation of the A cluster HOX genes (18, 62, 63). Early evidence for a HOX gene role in leukemia came from studies of BXH2 mice. These mice developed spontaneous leukemia as a result of ecotropic retroviral integration. One of the most common integration sites in these leukemias was at Hoxa7 or a9, resulting in their over expression (64, 65). Subsequently, it was determined that human leukemias with MLL rearrangements consistently express high levels of HOXA7 and HOXA9 (66-69) with the rare exception of low level A cluster HOX expression in a subset of ALL cases with the t(4;11), (70). In the studies reported to date, continual expression of both Hoxa9 and Meis1 is critical for maintaining MLL fusion protein mediated immortalization (63, 71). In addition, MLL amplification with HOXA9 upregulation also appears to be an important mechanism in both MDS and AML (72-74).
When over expressed by itself, Hoxa9 is only weakly oncogenic. Only rare mice transplanted with Hoxa9 transduced bone marrow develop leukemia, and those that do arise have a latency period of 6 months or more. However, overexpression of Meis1, a homeodomain containing cofactor, greatly increases the leukemogenicity of Hoxa9 (75). More than 90% of leukemias arising in BXH2 mice with Hoxa7 or a9 overexpression have a second integration site at Meis1 resulting in its over expression (64, 65). In addition, microarray studies of human leukemias show that Meis1 is consistently expressed in leukemias that express high levels of Hoxa9, either with or without MLL rearrangement (66-69). Another potential contributor to MLL mediated transformation is the microRNA mir-196b, which is located 5’ to the Hoxa9 transcription start site (76). Mir-196b is over expressed in the majority of ALL and AML cases with MLL rearrangements. Over expression of mir-196b leads to enhanced colony forming ability and a partial block in hematopoiesis in replating assays, while mir-196b specific antagomir treatment markedly decreased replating activity (76).
In addition to deregulated HOX gene expression, MLL rearranged leukemias have been found to have a transcription profile that closely resembles embryonic (ES) as opposed to hematopoietic stem cells (HSC) (77). In particular, expression of just three ES signature genes Myb, Hmgb3, and Cbx5 is sufficient for immortalization of hematopoietic progenitors in the absence of upregulated HOX gene expression (77). The WNT signaling pathway has also been implicated in the establishment of leukemic stem cells. MLL fusion proteins are capable of transforming not only hematopoietic stem cells (HSC) but also more differentiated granulocyte-macrophage progenitors (GMP). In contrast, Hoxa9 and Meis1 transform only HSC (78). The crucial difference appears to be upregulation of prostaglandin-endoperoxide synthetase cyclooxygenase 1, and the prostaglandin receptor Ptger1 and the activation of the β-catenin pathway by the MLL fusion protein (78). Interestingly, inhibition of the WNT signaling pathway through β-catenin knockout or inhibition of β-catenin synthesis with the COX2 inhibitor indomethacin differentially suppressed growth of leukemia initiating cells and not normal HSC (78).
6. Mechanisms of transformation by MLL fusion proteins: nuclear translocation partners
More than 60 different MLL translocation partners have been identified, making it challenging to develop a single model for MLL fusion protein transformation. Nonetheless, some unifying principles have emerged. The great majority of acute leukemias with MLL translocations show highly upregulated HOXA9 and MEIS1 expression. In addition, all MLL fusion proteins retain the amino terminal part of MLL that is required for MLL association with chromatin while deleting the PHD fingers, which have an inhibitory effect on transformation (79). Furthermore, in-frame fusion of MLL to a translocation partner is required for transformation, indicating that MLL mediated transformation is not a loss of function mechanism. Finally, biochemical studies show that a number of the most common translocation partners are physically associated in complexes that are involved in transcriptional elongation(80-86).
Some of the most common MLL translocation partners including AF4, AF9, ENL, and ELL, along with the less common partner AF5q31 (also known as AFF4), have been found to associate in a large complex termed “Super Elongation Complex” by Shilatifard and colleagues that also includes the elongation factors ELL2, ELL3, the elongation factors EAF1 and EAF2 and the p-TEFb complex, which is composed of CDK9 and Cyclin T1 or T2 (82). P-TEFb is required to phosphorylate the RNA Pol II C terminal domain, which promotes transcriptional elongation. A related complex AEP (AF4/ENL/P-TEFb) reported by Cleary and colleagues lacks the ELL and EAP subunits (Figure 4) (82). Several of the subunits in these complexes have been implicated in the regulation of transcriptional initiation or elongation. AF4 and AF5q31 are members of a family of related proteins including FMR2 and LAF4, the latter of which are associated with pro-B-cell leukemia. ENL and AF9 are homologous nuclear proteins that both contain a YEATs domain implicated in histone binding and transcriptional regulation. An 84 amino acid C-terminal domain of ENL, which is highly conserved with AF9 and is retained in all MLL-ENL fusions, is sufficient for transformation when fused to MLL (87). Interestingly, a short sequence shared between the AF9 and ENL C termini has been shown to interact with other SEC/AEC components including AF4 and AF5q31 (88). ELL, for example, was originally identified as a transcription elongation factor (89) and AFF4 has been shown to directly interact with P-TEFb and to regulate its kinase activity (80).
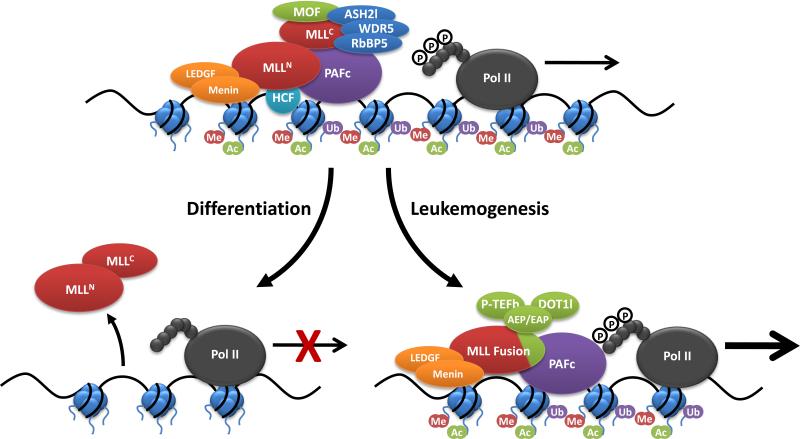
Figure 4.
MLL complex proteins during normal and malignant hematopoiesis. MLL interacts with a variety of protein complexes in hematopoietic stem and progenitor cells to promote transcription of critical target genes like HOXA9 and MEIS1. The PAF complex associates with RNA pol II and recruits the RAD6/BRE1 E2/E3 ubiquitin ligase, which promotes mono-ubiquitination of histone H2B (Ub). H2B mono-ubiquitination is a histone mark associated with transcriptional activation. PAFc, along with Menin/LEDGF, recruit the MLL complex to target genes which delivers H3K4 (Me) methyltransferase activity and promotes gene transcription. MLL associates with the HAT MOF, which promotes further gene transcription through histone H4K16 acetylation (Ac). During hematopoietic differentiation, MLL is not recruited to target genes in part due to decreased transcription of PAFc. Insufficient recruitment of MLL leads to decreased expression of target genes. Chromosomal translocations involving MLL generate MLL fusion proteins that can recruit transcriptional activation complexes dependent on the fusion partner. MLL translocation partners including AF4, AF9, ENL, ELL and AF5q31 can form a transcriptional activation complex and a related complex AEP (AF4/ENL/P-TEFb) has also been reported. These complexes involve the recruitment of pTEFb, which is required to phosphorylate the RNA Pol II C terminal domain, which promotes transcriptional elongation. The H3K79 methyltransferase DOT1l is also recruited to some MLL fusion proteins (MLL-AF10), which can further promote transcriptional activation.
AF9 and ENL have also been shown to be components of another complex, termed Dot.Com, which includes the MLL translocation partners AF10 and AF17 along with WNT signaling components TRAPP and SKP1 (85). AF10 and AF17 are highly homologous and show a number of motifs including a PHD finger, AT hook and leucine zipper domain. Importantly, both interact with Dot1l (85, 90). Studies with Dot1l knockout mice indicate that Dot1l recruitment is important for transformation by at least a subset of MLL fusion proteins (91, 92). Dot1l is required for HOXA9 and MEIS1 upregulation, which is critical to transformation, however Dot.com is also important for WNT signaling, which may be a second pathway important for leukemogenesis (78, 85). At this point, it appears that both p-TEFb and DOT1L contribute to leukemogenesis, however, their relative contributions and to what extent non-HOX target genes are important for this remain to be determined (Figure 4).
Emerging evidence suggests that there are a number of alternative mechanisms that can result in HOX deregulation and leukemogenesis. For example in rare cases, MLL is fused to translocation partners with intrinsic histone-modifying activity including the known histone acetyltransferases (HATs) CBP or P300. In these translocation products, the CBP HAT domain, as well as an adjacent bromodomain are required for transformation (93). Transformation by another infrequent MLL translocation partner EEN has been reported to involve recruitment of the histone H4 specific arginine methyltransferase PRMT1 (94).
Increasing evidence suggests that wild type MLL plays a role in MLL fusion protein leukemogenesis. The HOX A loci in MLL rearranged leukemias have high levels of both histone H3 lysine 79 and histone H3 lysine 4 methylation (53) and activation of a conditional MLL fusion protein MLL-ENL increases MLL association and H3K4 methylation at MLL target genes (53). In keeping with this, wild type MLL has recently been shown to be required for MLL-AF9 transformation (95). This is significant as it suggest that MLL methyltransferase activity may also be a therapeutic target in leukemias with MLL rearrangements. Another intriguing and controversial finding is the possible role of reciprocal translocations, which are uncommonly expressed, in MLL rearranged leukemias. For unclear reasons the MLL-AF4 fusion protein is not leukemogenic in retroviral transduction models, however, expression of the reciprocal AF4-MLL chimera did result in development of pro B cell ALL (96) apparently via a mechanism involving recruitment of DOT1L and P-TEFb (97).
7. Mechanisms of transformation by MLL fusion proteins: dimerizing translocation partners
Despite similarities between some of the more common MLL translocations, many MLL translocation partners are cytoplasmic proteins lacking intrinsic transcriptional activity. The finding of self-association motifs in many cytoplasmic MLL translocation partners suggested that MLL dimerization is transforming. The first evidence of this was the development of leukemia in Mll–lacZ mice (58). β-Galactosidase occurs as a tetramer in solution, suggesting that MLL dimerization or oligomerization by the translocation partner is oncogenic. Subsequent experiments have shown that the dimerization of MLL contributes to transformation by a number of MLL fusion proteins including AF1p, GAS7, GEPHYRIN and AF6 (62, 98). The mechanisms by which the dimerization of truncated MLL makes it transforming are unknown. Given the multivalent nature of the MLL-menin interaction, it is possible that wild type MLL dimerizes. It is attractive to speculate that this promotes coactivator (such as Pafc or the AEP complex) recruitment. In addition, dimerized MLL fusions might alter the binding activity of wild-type MLL, thereby disrupting normal MLL regulation (53). Regardless of the mechanism, the end result of MLL fusion protein dimerization appears to be upregulation of HOXA7, HOXA9 and MEIS1 (62, 98). The dimerizing MLL fusion proteins are relatively weak oncogenes, however, so that secondary genetic events such as FLT3 internal tandem duplication appear to be particularly important for transformation by these fusion proteins (99).
8. MLL amplification and partial tandem duplication in acute leukemia
Some myelodysplastic syndrome (MDS) and AML cases are associated with increased MLL copy number, either as a result of double minute chromosomes or homogenous staining region (72-74). MLL amplification appears to be more common in older patients with complex karyotypes, often with 5q- and poor outcome (100). Unlike MLL PTD cases, MLL amplification is associated with the upregulation of at least some of the genes that are consistently expressed in leukemias with MLL rearrangements, suggesting similar mechanisms of transformation as MLL fusion proteins. These include confirmed direct targets of MLL, such as HOXA7, HOXA9 and MEIS1, as well as other potential direct or indirect targets, including PROML1, ADAM10, NKG2D and ITPA (101).
About 7.5% of AML cases with normal cytogenetics harbor internal tandem duplications of MLL spanning sequences encoding the AT hook through CXXC DNA-binding domains (102). Most of these cases occur in adults in association with trisomy II or FLT3-ITD and appear to be associated with a worse prognosis than those without MLL rearrangements (103). Studies to date indicate that the other MLL allele is silenced by DNA methylation (104). The crucial oncogenic alteration is not known but may be the duplication of DNA binding motifs in MLL, as previously we showed that molecular mimics of the MLL-PTD increase the affinity for MLL binding at a target site (62). Another possibility is that the exon-duplicated form of MLL has a conformation that interferes with its normal regulation. As is the case for dimerizing MLL fusion proteins, FLT3 activation appears to be an important cooperative event in MLL PTD-associated leukemias. About half of MLL PTD cases show FLT3-ITD or point mutation. Murine knockin models expressing the MLL-PTD show Hoxa9, but not Meis1, upregulation (105). These mice do not develop leukemia on their own but rapidly succumb to AML when crossed with Flt3-ITD transgenic mice (Caliguiri, M. unpublished data). Thus far microarray expression profiling of human leukemias with MLL PTD has not revealed this characteristic signature of HOX over expression (106) raising the possibility that PTD cases transformed through mechanisms that are different to the balanced translocations of MLL and that this probably involves genes other than Hox genes.
9. Potential therapeutic targets in MLL rearranged leukemias
Inhibiting recruitment of MLL fusion proteins to target loci
A number of different strategies to target mechanisms used by MLL fusion proteins are currently under investigation. In addition to these, targeting wild type MLL may be effective in other types of leukemia with high-level HOX expression. One potential strategy is to block MLL fusion protein or associated coactivator recruitment to target genes. Small molecular inhibitors are under development to block MLL and menin or MLL, menin and LEDGF interactions, which is required for transformation by MLL fusion proteins (44, 47, 107). Another potential therapeutic target is the CXXC zinc finger domain of MLL, a region retained in all MLL fusion proteins that is crucial for MLL binding to unmethylated CpG-rich DNA (108). The crystal structure of the CXXC domain has been determined (28, 30), which will facilitate development of small molecule inhibitors that block DNA binding. Disruption of interaction between the MLL pre CXXC and RD2 regions and the PAF complex, which are also required for MLL recruitment, is another strategy for therapeutic intervention that is currently under development (34, 48).
Inhibiting the activity or recruitment of MLL fusion protein coactivators
Recruitment of P-TEFb is a critical step in transcriptional deregulation leading to leukemia (86). For leukemias involving the common nuclear translocation partners (ENL, AF4, AF9, AF5q31) inhibition of P-TEFb CDK9 activity is one possible therapeutic strategy. This is particularly feasible given the potent and apparently specific inhibitory activity of flavinoids such as flavopiridol, which is currently in phase II trials for a variety of hematologic and solid tumors. To date, use of these compounds has been limited by both their limited efficacy and high toxicity (109, 110). We, and others, have recently shown that Dot1l is required for MLL transformation and therefore represents another attractive therapeutic target (91, 92). The finding that MLL is required for transformation by MLL-AF9 and likely other MLL fusion proteins, also raises the possibility that inhibiting MLL methyltransferase activity, either directly via the SET domain or through blocking interactions with essential core components such as WDR5, may hold promise for leukemia therapy. Remarkably, mice lacking the MLL SET domain are relatively healthy, which is an encouraging sign that this will be an efficacious drug target. Specific inhibitors of MLL methyltransferase activity might also find application in MDS and AML cases showing MLL amplification or tandem duplication as well as other leukemias with deregulation of HOX gene expression.
Inhibition of downstream or cooperating pathways
The effectors of transformation by A cluster HOX genes and MEIS1 are actively being explored and a small number of potentially important targets have been identified. One of the promising classes of compounds are GSK3β inhibitors, which by inhibiting phosphorylation of CREB1, blocks its association with the HOX/PBX/MEIS transcription complex resulting in a loss of coactivator activity (111). Work is also ongoing to test the efficacy of FLT3 inhibitors, however the results of clinical trials thus far have generally been disappointing, largely because of the acquisition of resistance in leukemic blasts (112, 113).
Acknowledgements
Jay Hess is supported in part through funding from the National Institutes of Health and the Leukemia and Lymphoma Society of America.
Glossary
- Epigenetics
the study of heritable changes in gene expression caused by mechanisms other than DNA sequence
- MLL
Mixed Lineage Leukemia, histone methyltransferase
- ALL
Acute Lymphoid Leukemia
- AML
Acute Myeloid Leukemia
- MDS
Myelodysplastic Syndrome
- AF4
ALL1-fused gene from chromosome 4 (MLL translocation partner)
- AF9
ALL1-fused gene from chromosome 9 (MLL translocation partner)
- ENL
Eleven Nineteen Leukemia (MLL translocation partner)
- AF10
ALL1-fused gene from chromosome 10 (MLL translocation partner)
- AF6
ALL1-fused gene from chromosome 6 (MLL translocation partner)
- ELL
RNA polymerase elongation factor (MLL translocation partner)
- AF1p
Epidermal growth factor receptor pathway substrate 15 (MLL translocation partner)
- AF17
ALL1-fused gene from chromosome 17 (MLL translocation partner)
- SEPT6
Septin 6 (MLL translocation partner)
- AT-Hooks
Evolutionarily conserved domain of MLL that binds to AT rich DNA
- CBP
cAMP response-element binding protein (CREB) binding protein. A transcriptional coactivator with histone acetyltransferase activity (MLL translocation partner)
- Cyp33
Cyclophilin 33, an RNA binding protein with proline isomerase activity
- HDAC
Histone Deactetylase. Enzymes that remove acetyl groups from histone and associated with transcriptional repression.
- PHD finger
Plant Homeodomain finger. Structural domain that can bind methylated histones
- PcG
Polycomb group proteins. Involved in transcriptional repression
- SNL
Subnuclear localization domain
- ASH2l
absent, small, or homeotic-like (Drosophila), interacts with MLL
- WDR5
WD repeat-containing protein 5, interacts with MLL
- RbBP5
Retinoblastoma-binding protein 5, interacts with MLL
- MOF
(MYST1) ortholog of Drosophila males absent on the first, histone acetyltransferase that interacts with MLL
- HCF
Host Cell Factor, interacts with MLL
- Menin
(MEN1) Tumor suppressor associated with multiple endocrine neoplasia type 1
- LEDGF
(Psip1) Lens epithelium derived growth factor, chromatin associated transcriptional co-activator, interacts with MLL
- PAFc
Polymerase Associated Factor complex, interacts with MLL
- DOT1l
Disruptor of telomeric silencing (Yeast) Histone H3 methyltransferase
- p-TEFb
Positive Transcription Elongation Factor b, composed of CDK9 and one of either Cyclin T1, T2 or K. Kinase activity phosphorylates serine 2 of RNA pol II CTD
References
- 1.Djabali M, Selleri L, Parry P, Bower M, Young BD, Evans GA. A trithorax-like gene is interrupted by chromosome 11q23 translocations in acute leukaemias. Nat Genet. 1992;2:113–8. doi: 10.1038/ng1092-113. [DOI] [PubMed] [Google Scholar]
- 2.Gu Y, Nakamura T, Alder H, Prasad R, Canaani O, Cimino G, Croce CM, Canaani E. The t(4;11) chromosome translocation of human acute leukemias fuses the ALL-1 gene, related to Drosophila trithorax, to the AF-4 gene. Cell. 1992;71:701–8. doi: 10.1016/0092-8674(92)90603-a. [DOI] [PubMed] [Google Scholar]
- 3.Tkachuk DC, Kohler S, Cleary ML. Involvement of a homolog of Drosophila trithorax by 11q23 chromosomal translocations in acute leukemias. Cell. 1992;71:691–700. doi: 10.1016/0092-8674(92)90602-9. [DOI] [PubMed] [Google Scholar]
- 4.Krivtsov AV, Armstrong SA. MLL translocations, histone modifications and leukaemia stem-cell development. Nat Rev Cancer. 2007;7:823–33. doi: 10.1038/nrc2253. [DOI] [PubMed] [Google Scholar]
- 5.Hess JL. MLL: a histone methyltransferase disrupted in leukemia. Trends Mol Med. 2004;10:500–7. doi: 10.1016/j.molmed.2004.08.005. [DOI] [PubMed] [Google Scholar]
- 6.Meyer C, Kowarz E, Hofmann J, Renneville A, Zuna J, Trka J, Ben Abdelali R, Macintyre E, De Braekeleer E, De Braekeleer M, Delabesse E, de Oliveira MP, Cave H, Clappier E, van Dongen JJ, Balgobind BV, van den Heuvel-Eibrink MM, Beverloo HB, Panzer-Grumayer R, Teigler-Schlegel A, Harbott J, Kjeldsen E, Schnittger S, Koehl U, Gruhn B, Heidenreich O, Chan LC, Yip SF, Krzywinski M, Eckert C, Moricke A, Schrappe M, Alonso CN, Schafer BW, Krauter J, Lee DA, Zur Stadt U, Te Kronnie G, Sutton R, Izraeli S, Trakhtenbrot L, Lo Nigro L, Tsaur G, Fechina L, Szczepanski T, Strehl S, Ilencikova D, Molkentin M, Burmeister T, Dingermann T, Klingebiel T, Marschalek R. New insights to the MLL recombinome of acute leukemias. Leukemia. 2009;23:1490–9. doi: 10.1038/leu.2009.33. [DOI] [PubMed] [Google Scholar]
- 7.Huret JL, Dessen P, Le Minor S, Bernheim A. The “Atlas of genetics and cytogenetics in oncology and haematology” on the internet and a review on infant leukemias. Cancer Genet Cytogenet. 2000;120:155–9. doi: 10.1016/s0165-4608(99)00250-2. [DOI] [PubMed] [Google Scholar]
- 8.Raimondi S. 11q23 rearrangements in childhood acute lymphoblastic leukemia. Atlas Genet Cytogenet Oncol Haematol. 2004 [Google Scholar]
- 9.Mohan M, Lin C, Guest E, Shilatifard A. Licensed to elongate: a molecular mechanism for MLL-based leukaemogenesis. Nat Rev Cancer. 10:721–8. doi: 10.1038/nrc2915. [DOI] [PubMed] [Google Scholar]
- 10.Huret J. t(9;11)(p22;q23). Atlas Genet Cytogenet Oncol Haematol. 1997 [Google Scholar]
- 11.Huret JL. 11q23 rearrangements in leukaemia. Atlas Genet Cytogenet Oncol Haematol. 2003 [Google Scholar]
- 12.Ringrose L, Paro R. Epigenetic regulation of cellular memory by the Polycomb and Trithorax group proteins. Annu Rev Genet. 2004;38:413–43. doi: 10.1146/annurev.genet.38.072902.091907. [DOI] [PubMed] [Google Scholar]
- 13.Brock HW, van Lohuizen M. The Polycomb group--no longer an exclusive club? Curr Opin Genet Dev. 2001;11:175–81. doi: 10.1016/s0959-437x(00)00176-3. [DOI] [PubMed] [Google Scholar]
- 14.Argiropoulos B, Humphries RK. Hox genes in hematopoiesis and leukemogenesis. Oncogene. 2007;26:6766–76. doi: 10.1038/sj.onc.1210760. [DOI] [PubMed] [Google Scholar]
- 15.Bernstein BE, Mikkelsen TS, Xie X, Kamal M, Huebert DJ, Cuff J, Fry B, Meissner A, Wernig M, Plath K, Jaenisch R, Wagschal A, Feil R, Schreiber SL, Lander ES. A bivalent chromatin structure marks key developmental genes in embryonic stem cells. Cell. 2006;125:315–26. doi: 10.1016/j.cell.2006.02.041. [DOI] [PubMed] [Google Scholar]
- 16.Chopra VS, Hong JW, Levine M. Regulation of Hox gene activity by transcriptional elongation in Drosophila. Curr Biol. 2009;19:688–93. doi: 10.1016/j.cub.2009.02.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Muse GW, Gilchrist DA, Nechaev S, Shah R, Parker JS, Grissom SF, Zeitlinger J, Adelman K. RNA polymerase is poised for activation across the genome. Nat Genet. 2007;39:1507–11. doi: 10.1038/ng.2007.21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Milne TA, Briggs SD, Brock HW, Martin ME, Gibbs D, Allis CD, Hess JL. MLL targets SET domain methyltransferase activity to Hox gene promoters. Mol Cell. 2002;10:1107–17. doi: 10.1016/s1097-2765(02)00741-4. [DOI] [PubMed] [Google Scholar]
- 19.Nakamura T, Mori T, Tada S, Krajewski W, Rozovskaia T, Wassell R, Dubois G, Mazo A, Croce CM, Canaani E. ALL-1 is a histone methyltransferase that assembles a supercomplex of proteins involved in transcriptional regulation. Mol Cell. 2002;10:1119–28. doi: 10.1016/s1097-2765(02)00740-2. [DOI] [PubMed] [Google Scholar]
- 20.Yu BD, Hanson RD, Hess JL, Horning SE, Korsmeyer SJ. MLL, a mammalian trithorax-group gene, functions as a transcriptional maintenance factor in morphogenesis. Proc Natl Acad Sci U S A. 1998;95:10632–6. doi: 10.1073/pnas.95.18.10632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Yu BD, Hess JL, Horning SE, Brown GA, Korsmeyer SJ. Altered Hox expression and segmental identity in Mll-mutant mice. Nature. 1995;378:505–8. doi: 10.1038/378505a0. [DOI] [PubMed] [Google Scholar]
- 22.Hess JL, Yu BD, Li B, Hanson R, Korsmeyer SJ. Defects in yolk sac hematopoiesis in Mll-null embryos. Blood. 1997;90:1799–806. [PubMed] [Google Scholar]
- 23.Jude CD, Climer L, Xu D, Artinger E, Fisher JK, Ernst P. Unique and independent roles for MLL in adult hematopoietic stem cells and progenitors. Cell Stem Cell. 2007;1:324–37. doi: 10.1016/j.stem.2007.05.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.McMahon KA, Hiew SY, Hadjur S, Veiga-Fernandes H, Menzel U, Price AJ, Kioussis D, Williams O, Brady HJ. Mll has a critical role in fetal and adult hematopoietic stem cell self-renewal. Cell Stem Cell. 2007;1:338–45. doi: 10.1016/j.stem.2007.07.002. [DOI] [PubMed] [Google Scholar]
- 25.Hsieh JJ, Ernst P, Erdjument-Bromage H, Tempst P, Korsmeyer SJ. Proteolytic cleavage of MLL generates a complex of N- and C-terminal fragments that confers protein stability and subnuclear localization. Mol Cell Biol. 2003;23:186–94. doi: 10.1128/MCB.23.1.186-194.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hsieh JJ, Cheng EH, Korsmeyer SJ. Taspase1: a threonine aspartase required for cleavage of MLL and proper HOX gene expression. Cell. 2003;115:293–303. doi: 10.1016/s0092-8674(03)00816-x. [DOI] [PubMed] [Google Scholar]
- 27.Takeda S, Chen DY, Westergard TD, Fisher JK, Rubens JA, Sasagawa S, Kan JT, Korsmeyer SJ, Cheng EH, Hsieh JJ. Proteolysis of MLL family proteins is essential for taspase1-orchestrated cell cycle progression. Genes Dev. 2006;20:2397–409. doi: 10.1101/gad.1449406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Allen MD, Grummitt CG, Hilcenko C, Min SY, Tonkin LM, Johnson CM, Freund SM, Bycroft M, Warren AJ. Solution structure of the nonmethyl-CpG-binding CXXC domain of the leukaemia-associated MLL histone methyltransferase. Embo J. 2006;25:4503–12. doi: 10.1038/sj.emboj.7601340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Birke M, Schreiner S, Garcia-Cuellar MP, Mahr K, Titgemeyer F, Slany RK. The MT domain of the proto-oncoprotein MLL binds to CpG-containing DNA and discriminates against methylation. Nucleic Acids Res. 2002;30:958–65. doi: 10.1093/nar/30.4.958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Cierpicki T, Risner LE, Grembecka J, Lukasik SM, Popovic R, Omonkowska M, Shultis DD, Zeleznik-Le NJ, Bushweller JH. Structure of the MLL CXXC domain-DNA complex and its functional role in MLL-AF9 leukemia. Nat Struct Mol Biol. 17:62–8. doi: 10.1038/nsmb.1714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Erfurth FE, Popovic R, Grembecka J, Cierpicki T, Theisler C, Xia ZB, Stuart T, Diaz MO, Bushweller JH, Zeleznik-Le NJ. MLL protects CpG clusters from methylation within the Hoxa9 gene, maintaining transcript expression. Proc Natl Acad Sci U S A. 2008;105:7517–22. doi: 10.1073/pnas.0800090105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zeleznik-Le NJ, Harden AM, Rowley JD. 11q23 translocations split the “AT-hook” cruciform DNA-binding region and the transcriptional repression domain from the activation domain of the mixed-lineage leukemia (MLL) gene. Proc Natl Acad Sci U S A. 1994;91:10610–4. doi: 10.1073/pnas.91.22.10610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Chang PY, Hom RA, Musselman CA, Zhu L, Kuo A, Gozani O, Kutateladze TG, Cleary ML. Binding of the MLL PHD3 Finger to Histone H3K4me3 Is Required for MLL-Dependent Gene Transcription. J Mol Biol. doi: 10.1016/j.jmb.2010.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Milne TA, Kim J, Wang GG, Stadler SC, Basrur V, Whitcomb SJ, Wang Z, Ruthenburg AJ, Elenitoba-Johnson KS, Roeder RG, Allis CD. Multiple interactions recruit MLL1 and MLL1 fusion proteins to the HOXA9 locus in leukemogenesis. Mol Cell. 38:853–63. doi: 10.1016/j.molcel.2010.05.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wang Z, Song J, Milne TA, Wang GG, Li H, Allis CD, Patel DJ. Pro isomerization in MLL1 PHD3-bromo cassette connects H3K4me readout to CyP33 and HDAC-mediated repression. Cell. 141:1183–94. doi: 10.1016/j.cell.2010.05.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ernst P, Wang J, Huang M, Goodman RH, Korsmeyer SJ. MLL and CREB bind cooperatively to the nuclear coactivator CREB-binding protein. Mol Cell Biol. 2001;21:2249–58. doi: 10.1128/MCB.21.7.2249-2258.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Steward MM, Lee JS, O'Donovan A, Wyatt M, Bernstein BE, Shilatifard A. Molecular regulation of H3K4 trimethylation by ASH2L, a shared subunit of MLL complexes. Nat Struct Mol Biol. 2006;13:852–4. doi: 10.1038/nsmb1131. [DOI] [PubMed] [Google Scholar]
- 38.Wysocka J, Swigut T, Milne TA, Dou Y, Zhang X, Burlingame AL, Roeder RG, Brivanlou AH, Allis CD. WDR5 associates with histone H3 methylated at K4 and is essential for H3 K4 methylation and vertebrate development. Cell. 2005;121:859–72. doi: 10.1016/j.cell.2005.03.036. [DOI] [PubMed] [Google Scholar]
- 39.Dou Y, Milne TA, Ruthenburg AJ, Lee S, Lee JW, Verdine GL, Allis CD, Roeder RG. Regulation of MLL1 H3K4 methyltransferase activity by its core components. Nat Struct Mol Biol. 2006;13:713–9. doi: 10.1038/nsmb1128. [DOI] [PubMed] [Google Scholar]
- 40.Dou Y, Milne TA, Tackett AJ, Smith ER, Fukuda A, Wysocka J, Allis CD, Chait BT, Hess JL, Roeder RG. Physical association and coordinate function of the H3 K4 methyltransferase MLL1 and the H4 K16 acetyltransferase MOF. Cell. 2005;121:873–85. doi: 10.1016/j.cell.2005.04.031. [DOI] [PubMed] [Google Scholar]
- 41.Xia ZB, Anderson M, Diaz MO, Zeleznik-Le NJ. MLL repression domain interacts with histone deacetylases, the polycomb group proteins HPC2 and BMI-1, and the corepressor C-terminal-binding protein. Proc Natl Acad Sci U S A. 2003;100:8342–7. doi: 10.1073/pnas.1436338100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Yokoyama A, Wang Z, Wysocka J, Sanyal M, Aufiero DJ, Kitabayashi I, Herr W, Cleary ML. Leukemia proto-oncoprotein MLL forms a SET1-like histone methyltransferase complex with menin to regulate Hox gene expression. Mol Cell Biol. 2004;24:5639–49. doi: 10.1128/MCB.24.13.5639-5649.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Tyagi S, Chabes AL, Wysocka J, Herr W. E2F activation of S phase promoters via association with HCF-1 and the MLL family of histone H3K4 methyltransferases. Mol Cell. 2007;27:107–19. doi: 10.1016/j.molcel.2007.05.030. [DOI] [PubMed] [Google Scholar]
- 44.Caslini C, Yang Z, El-Osta M, Milne TA, Slany RK, Hess JL. Interaction of MLL amino terminal sequences with menin is required for transformation. Cancer Res. 2007;67:7275–83. doi: 10.1158/0008-5472.CAN-06-2369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Yokoyama A, Somervaille TC, Smith KS, Rozenblatt-Rosen O, Meyerson M, Cleary ML. The menin tumor suppressor protein is an essential oncogenic cofactor for MLL-associated leukemogenesis. Cell. 2005;123:207–18. doi: 10.1016/j.cell.2005.09.025. [DOI] [PubMed] [Google Scholar]
- 46.Grembecka J, Belcher AM, Hartley T, Cierpicki T. Molecular basis of the mixed lineage leukemia-menin interaction: implications for targeting mixed lineage leukemias. J Biol Chem. 285:40690–8. doi: 10.1074/jbc.M110.172783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Yokoyama A, Cleary ML. Menin critically links MLL proteins with LEDGF on cancer-associated target genes. Cancer Cell. 2008;14:36–46. doi: 10.1016/j.ccr.2008.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Muntean AG, Tan J, Sitwala K, Huang Y, Bronstein J, Connelly JA, Basrur V, Elenitoba-Johnson KS, Hess JL. The PAF complex synergizes with MLL fusion proteins at HOX loci to promote leukemogenesis. Cancer Cell. 17:609–21. doi: 10.1016/j.ccr.2010.04.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Dover J, Schneider J, Tawiah-Boateng MA, Wood A, Dean K, Johnston M, Shilatifard A. Methylation of histone H3 by COMPASS requires ubiquitination of histone H2B by Rad6. J Biol Chem. 2002;277:28368–71. doi: 10.1074/jbc.C200348200. [DOI] [PubMed] [Google Scholar]
- 50.Krogan NJ, Dover J, Wood A, Schneider J, Heidt J, Boateng MA, Dean K, Ryan OW, Golshani A, Johnston M, Greenblatt JF, Shilatifard A. The Paf1 complex is required for histone H3 methylation by COMPASS and Dot1p: linking transcriptional elongation to histone methylation. Mol Cell. 2003;11:721–9. doi: 10.1016/s1097-2765(03)00091-1. [DOI] [PubMed] [Google Scholar]
- 51.Ng HH, Dole S, Struhl K. The Rtf1 component of the Paf1 transcriptional elongation complex is required for ubiquitination of histone H2B. J Biol Chem. 2003;278:33625–8. doi: 10.1074/jbc.C300270200. [DOI] [PubMed] [Google Scholar]
- 52.Sun ZW, Allis CD. Ubiquitination of histone H2B regulates H3 methylation and gene silencing in yeast. Nature. 2002;418:104–8. doi: 10.1038/nature00883. [DOI] [PubMed] [Google Scholar]
- 53.Milne TA, Martin ME, Brock HW, Slany RK, Hess JL. Leukemogenic MLL fusion proteins bind across a broad region of the Hox a9 locus, promoting transcription and multiple histone modifications. Cancer Res. 2005;65:11367–74. doi: 10.1158/0008-5472.CAN-05-1041. [DOI] [PubMed] [Google Scholar]
- 54.Arai S, Yoshimi A, Shimabe M, Ichikawa M, Nakagawa M, Imai Y, Goyama S, Kurokawa M. Evi-1 is a transcriptional target of MLL oncoproteins in hematopoietic stem cells. Blood. doi: 10.1182/blood-2009-07-234310. [DOI] [PubMed] [Google Scholar]
- 55.Milne TA, Hughes CM, Lloyd R, Yang Z, Rozenblatt-Rosen O, Dou Y, Schnepp RW, Krankel C, Livolsi VA, Gibbs D, Hua X, Roeder RG, Meyerson M, Hess JL. Menin and MLL cooperatively regulate expression of cyclin-dependent kinase inhibitors. Proc Natl Acad Sci U S A. 2005;102:749–54. doi: 10.1073/pnas.0408836102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Xia ZB, Popovic R, Chen J, Theisler C, Stuart T, Santillan DA, Erfurth F, Diaz MO, Zeleznik-Le NJ. The MLL fusion gene, MLL-AF4, regulates cyclin-dependent kinase inhibitor CDKN1B (p27kip1) expression. Proc Natl Acad Sci U S A. 2005;102:14028–33. doi: 10.1073/pnas.0506464102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Liu H, Cheng EH, Hsieh JJ. Bimodal degradation of MLL by SCFSkp2 and APCCdc20 assures cell cycle execution: a critical regulatory circuit lost in leukemogenic MLL fusions. Genes Dev. 2007;21:2385–98. doi: 10.1101/gad.1574507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Dobson CL, Warren AJ, Pannell R, Forster A, Rabbitts TH. Tumorigenesis in mice with a fusion of the leukaemia oncogene Mll and the bacterial lacZ gene. Embo J. 2000;19:843–51. doi: 10.1093/emboj/19.5.843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Liu H, Takeda S, Kumar R, Westergard TD, Brown EJ, Pandita TK, Cheng EH, Hsieh JJ. Phosphorylation of MLL by ATR is required for execution of mammalian S-phase checkpoint. Nature. 467:343–6. doi: 10.1038/nature09350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Caslini C, Connelly JA, Serna A, Broccoli D, Hess JL. MLL associates with telomeres and regulates telomeric repeat-containing RNA transcription. Mol Cell Biol. 2009;29:4519–26. doi: 10.1128/MCB.00195-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Gessner A, Thomas M, Castro PG, Buchler L, Scholz A, Brummendorf TH, Soria NM, Vormoor J, Greil J, Heidenreich O. Leukemic fusion genes MLL/AF4 and AML1/MTG8 support leukemic self-renewal by controlling expression of the telomerase subunit TERT. Leukemia. 24:1751–9. doi: 10.1038/leu.2010.155. [DOI] [PubMed] [Google Scholar]
- 62.Martin ME, Milne TA, Bloyer S, Galoian K, Shen W, Gibbs D, Brock HW, Slany R, Hess JL. Dimerization of MLL fusion proteins immortalizes hematopoietic cells. Cancer Cell. 2003;4:197–207. doi: 10.1016/s1535-6108(03)00214-9. [DOI] [PubMed] [Google Scholar]
- 63.Zeisig BB, Milne T, Garcia-Cuellar MP, Schreiner S, Martin ME, Fuchs U, Borkhardt A, Chanda SK, Walker J, Soden R, Hess JL, Slany RK. Hoxa9 and Meis1 are key targets for MLL-ENL-mediated cellular immortalization. Mol Cell Biol. 2004;24:617–28. doi: 10.1128/MCB.24.2.617-628.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Nakamura T, Largaespada DA, Shaughnessy JD, Jr., Jenkins NA, Copeland NG. Cooperative activation of Hoxa and Pbx1-related genes in murine myeloid leukaemias. Nat Genet. 1996;12:149–53. doi: 10.1038/ng0296-149. [DOI] [PubMed] [Google Scholar]
- 65.Moskow JJ, Bullrich F, Huebner K, Daar IO, Buchberg AM. Meis1, a PBX1-related homeobox gene involved in myeloid leukemia in BXH-2 mice. Mol Cell Biol. 1995;15:5434–43. doi: 10.1128/mcb.15.10.5434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Rozovskaia T, Feinstein E, Mor O, Foa R, Blechman J, Nakamura T, Croce CM, Cimino G, Canaani E. Upregulation of Meis1 and HoxA9 in acute lymphocytic leukemias with the t(4 : 11) abnormality. Oncogene. 2001;20:874–8. doi: 10.1038/sj.onc.1204174. [DOI] [PubMed] [Google Scholar]
- 67.Ferrando AA, Armstrong SA, Neuberg DS, Sallan SE, Silverman LB, Korsmeyer SJ, Look AT. Gene expression signatures in MLL-rearranged T-lineage and B-precursor acute leukemias: dominance of HOX dysregulation. Blood. 2003;102:262–8. doi: 10.1182/blood-2002-10-3221. [DOI] [PubMed] [Google Scholar]
- 68.Casas S, Nagy B, Elonen E, Aventin A, Larramendy ML, Sierra J, Ruutu T, Knuutila S. Aberrant expression of HOXA9, DEK, CBL and CSF1R in acute myeloid leukemia. Leuk Lymphoma. 2003;44:1935–41. doi: 10.1080/1042819031000119299. [DOI] [PubMed] [Google Scholar]
- 69.Armstrong SA, Staunton JE, Silverman LB, Pieters R, den Boer ML, Minden MD, Sallan SE, Lander ES, Golub TR, Korsmeyer SJ. MLL translocations specify a distinct gene expression profile that distinguishes a unique leukemia. Nat Genet. 2002;30:41–7. doi: 10.1038/ng765. [DOI] [PubMed] [Google Scholar]
- 70.Stam RW, Schneider P, Hagelstein JA, van der Linden MH, Stumpel DJ, de Menezes RX, de Lorenzo P, Valsecchi MG, Pieters R. Gene expression profiling-based dissection of MLL translocated and MLL germline acute lymphoblastic leukemia in infants. Blood. 2010;115:2835–44. doi: 10.1182/blood-2009-07-233049. [DOI] [PubMed] [Google Scholar]
- 71.Ayton PM, Cleary ML. Transformation of myeloid progenitors by MLL oncoproteins is dependent on Hoxa7 and Hoxa9. Genes Dev. 2003;17:2298–307. doi: 10.1101/gad.1111603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Avet-Loiseau H, Godon C, Li JY, Daviet A, Mellerin MP, Talmant P, Harousseau JL, Bataille R. Amplification of the 11q23 region in acute myeloid leukemia. Genes Chromosomes Cancer. 1999;26:166–70. [PubMed] [Google Scholar]
- 73.Reddy KS, Parsons L, Mak L, Dighe P, Saphner T, Crow MK, Scott M. Segmental amplification of 11q23 region identified by fluorescence in situ hybridization in four patients with myeloid disorders: a review. Cancer Genet Cytogenet. 2001;126:139–46. doi: 10.1016/s0165-4608(00)00406-4. [DOI] [PubMed] [Google Scholar]
- 74.Poppe B, Vandesompele J, Schoch C, Lindvall C, Mrozek K, Bloomfield CD, Beverloo HB, Michaux L, Dastugue N, Herens C, Yigit N, De Paepe A, Hagemeijer A, Speleman F. Expression analyses identify MLL as a prominent target of 11q23 amplification and support an etiologic role for MLL gain of function in myeloid malignancies. Blood. 2004;103:229–35. doi: 10.1182/blood-2003-06-2163. [DOI] [PubMed] [Google Scholar]
- 75.Kroon E, Krosl J, Thorsteinsdottir U, Baban S, Buchberg AM, Sauvageau G. Hoxa9 transforms primary bone marrow cells through specific collaboration with Meis1a but not Pbx1b. Embo J. 1998;17:3714–25. doi: 10.1093/emboj/17.13.3714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Popovic R, Riesbeck LE, Velu CS, Chaubey A, Zhang J, Achille NJ, Erfurth FE, Eaton K, Lu J, Grimes HL, Chen J, Rowley JD, Zeleznik-Le NJ. Regulation of mir-196b by MLL and its overexpression by MLL fusions contributes to immortalization. Blood. 2009;113:3314–22. doi: 10.1182/blood-2008-04-154310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Somervaille TC, Matheny CJ, Spencer GJ, Iwasaki M, Rinn JL, Witten DM, Chang HY, Shurtleff SA, Downing JR, Cleary ML. Hierarchical maintenance of MLL myeloid leukemia stem cells employs a transcriptional program shared with embryonic rather than adult stem cells. Cell Stem Cell. 2009;4:129–40. doi: 10.1016/j.stem.2008.11.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Wang Y, Krivtsov AV, Sinha AU, North TE, Goessling W, Feng Z, Zon LI, Armstrong SA. The Wnt/beta-catenin pathway is required for the development of leukemia stem cells in AML. Science. 327:1650–3. doi: 10.1126/science.1186624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Muntean AG, Giannola D, Udager AM, Hess JL. The PHD fingers of MLL block MLL fusion protein-mediated transformation. Blood. 2008;112:4690–3. doi: 10.1182/blood-2008-01-134056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Bitoun E, Oliver PL, Davies KE. The mixed-lineage leukemia fusion partner AF4 stimulates RNA polymerase II transcriptional elongation and mediates coordinated chromatin remodeling. Hum Mol Genet. 2007;16:92–106. doi: 10.1093/hmg/ddl444. [DOI] [PubMed] [Google Scholar]
- 81.Monroe SC, Jo SY, Sanders DS, Basrur V, Elenitoba-Johnson KS, Slany RK, Hess JL. MLL-AF9 and MLL-ENL alter the dynamic association of transcriptional regulators with genes critical for leukemia. Exp Hematol. 2011;39:77–86. e1–5. doi: 10.1016/j.exphem.2010.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Lin C, Smith ER, Takahashi H, Lai KC, Martin-Brown S, Florens L, Washburn MP, Conaway JW, Conaway RC, Shilatifard A. AFF4, a component of the ELL/P-TEFb elongation complex and a shared subunit of MLL chimeras, can link transcription elongation to leukemia. Mol Cell. 2010;37:429–37. doi: 10.1016/j.molcel.2010.01.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Mohan M, Lin C, Guest E, Shilatifard A. Licensed to elongate: a molecular mechanism for MLL-based leukaemogenesis. Nat Rev Cancer. 2010;10:721–8. doi: 10.1038/nrc2915. [DOI] [PubMed] [Google Scholar]
- 84.Mueller D, Garcia-Cuellar MP, Bach C, Buhl S, Maethner E, Slany RK. Misguided transcriptional elongation causes mixed lineage leukemia. PLoS Biol. 2009;7:e1000249. doi: 10.1371/journal.pbio.1000249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Mohan M, Herz HM, Takahashi YH, Lin C, Lai KC, Zhang Y, Washburn MP, Florens L, Shilatifard A. Linking H3K79 trimethylation to Wnt signaling through a novel Dot1-containing complex (DotCom). Genes Dev. 2010;24:574–89. doi: 10.1101/gad.1898410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Yokoyama A, Lin M, Naresh A, Kitabayashi I, Cleary ML. A higher-order complex containing AF4 and ENL family proteins with P-TEFb facilitates oncogenic and physiologic MLL-dependent transcription. Cancer Cell. 2010;17:198–212. doi: 10.1016/j.ccr.2009.12.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Zeisig DT, Bittner CB, Zeisig BB, Garcia-Cuellar MP, Hess JL, Slany RK. The eleven-nineteen-leukemia protein ENL connects nuclear MLL fusion partners with chromatin. Oncogene. 2005;24:5525–32. doi: 10.1038/sj.onc.1208699. [DOI] [PubMed] [Google Scholar]
- 88.Slany RK, Lavau C, Cleary ML. The oncogenic capacity of HRX-ENL requires the transcriptional transactivation activity of ENL and the DNA binding motifs of HRX. Mol Cell Biol. 1998;18:122–9. doi: 10.1128/mcb.18.1.122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Shilatifard A, Lane WS, Jackson KW, Conaway RC, Conaway JW. An RNA polymerase II elongation factor encoded by the human ELL gene. Science. 1996;271:1873–6. doi: 10.1126/science.271.5257.1873. [DOI] [PubMed] [Google Scholar]
- 90.Okada Y, Feng Q, Lin Y, Jiang Q, Li Y, Coffield VM, Su L, Xu G, Zhang Y. hDOT1L links histone methylation to leukemogenesis. Cell. 2005;121:167–78. doi: 10.1016/j.cell.2005.02.020. [DOI] [PubMed] [Google Scholar]
- 91.Jo SY, Granowicz EM, Maillard I, Thomas D, Hess JL. Requirement for Dot1l in murine postnatal hematopoiesis and leukemogenesis by MLL translocation. Blood. 2011 doi: 10.1182/blood-2010-12-327668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Chang MJ, Wu H, Achille NJ, Reisenauer MR, Chou CW, Zeleznik-Le NJ, Hemenway CS, Zhang W. Histone H3 lysine 79 methyltransferase Dot1 is required for immortalization by MLL oncogenes. Cancer Res. 2010;70:10234–42. doi: 10.1158/0008-5472.CAN-10-3294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Lavau C, Du C, Thirman M, Zeleznik-Le N. Chromatin-related properties of CBP fused to MLL generate a myelodysplastic-like syndrome that evolves into myeloid leukemia. Embo J. 2000;19:4655–64. doi: 10.1093/emboj/19.17.4655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Cheung N, Chan LC, Thompson A, Cleary ML, So CW. Protein arginine-methyltransferase-dependent oncogenesis. Nat Cell Biol. 2007;9:1208–15. doi: 10.1038/ncb1642. [DOI] [PubMed] [Google Scholar]
- 95.Thiel AT, Blessington P, Zou T, Feather D, Wu X, Yan J, Zhang H, Liu Z, Ernst P, Koretzky GA, Hua X. MLL-AF9-induced leukemogenesis requires coexpression of the wild-type Mll allele. Cancer Cell. 17:148–59. doi: 10.1016/j.ccr.2009.12.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Bursen A, Schwabe K, Ruster B, Henschler R, Ruthardt M, Dingermann T, Marschalek R. The AF4.MLL fusion protein is capable of inducing ALL in mice without requirement of MLL.AF4. Blood. 115:3570–9. doi: 10.1182/blood-2009-06-229542. [DOI] [PubMed] [Google Scholar]
- 97.Benedikt A, Baltruschat S, Scholz B, Bursen A, Arrey TN, Meyer B, Varagnolo L, Muller AM, Karas M, Dingermann T, Marschalek R. The leukemogenic AF4-MLL fusion protein causes P-TEFb kinase activation and altered epigenetic signatures. Leukemia. 2011;25:135–44. doi: 10.1038/leu.2010.249. [DOI] [PubMed] [Google Scholar]
- 98.So CW, Lin M, Ayton PM, Chen EH, Cleary ML. Dimerization contributes to oncogenic activation of MLL chimeras in acute leukemias. Cancer Cell. 2003;4:99–110. doi: 10.1016/s1535-6108(03)00188-0. [DOI] [PubMed] [Google Scholar]
- 99.Ono R, Nakajima H, Ozaki K, Kumagai H, Kawashima T, Taki T, Kitamura T, Hayashi Y, Nosaka T. Dimerization of MLL fusion proteins and FLT3 activation synergize to induce multiple-lineage leukemogenesis. J Clin Invest. 2005;115:919–29. doi: 10.1172/JCI22725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Herry A, Douet-Guilbert N, Gueganic N, Morel F, Le Bris MJ, Berthou C, De Braekeleer M. Del(5q) and MLL amplification in homogeneously staining region in acute myeloblastic leukemia: a recurrent cytogenetic association. Ann Hematol. 2006;85:244–9. doi: 10.1007/s00277-005-0059-z. [DOI] [PubMed] [Google Scholar]
- 101.Worrell RT, Oghene J, Matthews JB. Ammonium effects on colonic Cl- secretion: anomalous mole fraction behavior. Am J Physiol Gastrointest Liver Physiol. 2004;286:G14–22. doi: 10.1152/ajpgi.00196.2003. [DOI] [PubMed] [Google Scholar]
- 102.Basecke J, Whelan JT, Griesinger F, Bertrand FE. The MLL partial tandem duplication in acute myeloid leukaemia. Br J Haematol. 2006;135:438–49. doi: 10.1111/j.1365-2141.2006.06301.x. [DOI] [PubMed] [Google Scholar]
- 103.Steudel C, Wermke M, Schaich M, Schakel U, Illmer T, Ehninger G, Thiede C. Comparative analysis of MLL partial tandem duplication and FLT3 internal tandem duplication mutations in 956 adult patients with acute myeloid leukemia. Genes Chromosomes Cancer. 2003;37:237–51. doi: 10.1002/gcc.10219. [DOI] [PubMed] [Google Scholar]
- 104.Whitman SP, Hackanson B, Liyanarachchi S, Liu S, Rush LJ, Maharry K, Margeson D, Davuluri R, Wen J, Witte T, Yu L, Liu C, Bloomfield CD, Marcucci G, Plass C, Caligiuri MA. DNA hypermethylation and epigenetic silencing of the tumor suppressor gene, SLC5A8, in acute myeloid leukemia with the MLL partial tandem duplication. Blood. 2008;112:2013–6. doi: 10.1182/blood-2008-01-128595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Dorrance AM, Liu S, Yuan W, Becknell B, Arnoczky KJ, Guimond M, Strout MP, Feng L, Nakamura T, Yu L, Rush LJ, Weinstein M, Leone G, Wu L, Ferketich A, Whitman SP, Marcucci G, Caligiuri MA. Mll partial tandem duplication induces aberrant Hox expression in vivo via specific epigenetic alterations. J Clin Invest. 2006;116:2707–16. doi: 10.1172/JCI25546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Frederiksen J, Juul K, Grande P, Jensen GB, Schroeder TV, Tybjaerg-Hansen A, Nordestgaard BG. Methylenetetrahydrofolate reductase polymorphism (C677T), hyperhomocysteinemia, and risk of ischemic cardiovascular disease and venous thromboembolism: prospective and case-control studies from the Copenhagen City Heart Study. Blood. 2004;104:3046–51. doi: 10.1182/blood-2004-03-0897. [DOI] [PubMed] [Google Scholar]
- 107.Kocks C, Cho JH, Nehme N, Ulvila J, Pearson AM, Meister M, Strom C, Conto SL, Hetru C, Stuart LM, Stehle T, Hoffmann JA, Reichhart JM, Ferrandon D, Ramet M, Ezekowitz RA. Eater, a transmembrane protein mediating phagocytosis of bacterial pathogens in Drosophila. Cell. 2005;123:335–46. doi: 10.1016/j.cell.2005.08.034. [DOI] [PubMed] [Google Scholar]
- 108.Bach C, Mueller D, Buhl S, Garcia-Cuellar MP, Slany RK. Alterations of the CxxC domain preclude oncogenic activation of mixed-lineage leukemia 2. Oncogene. 2009;28:815–23. doi: 10.1038/onc.2008.443. [DOI] [PubMed] [Google Scholar]
- 109.Wang LM, Ren DM. Flavopiridol, the first cyclin-dependent kinase inhibitor: recent advances in combination chemotherapy. Mini Rev Med Chem. 2010;10:1058–70. doi: 10.2174/1389557511009011058. [DOI] [PubMed] [Google Scholar]
- 110.Rizzolio F, Tuccinardi T, Caligiuri I, Lucchetti C, Giordano A. CDK inhibitors: from the bench to clinical trials. Curr Drug Targets. 2010;11:279–90. doi: 10.2174/138945010790711978. [DOI] [PubMed] [Google Scholar]
- 111.Wang Z, Iwasaki M, Ficara F, Lin C, Matheny C, Wong SH, Smith KS, Cleary ML. GSK-3 promotes conditional association of CREB and its coactivators with MEIS1 to facilitate HOX-mediated transcription and oncogenesis. Cancer Cell. 2010;17:597–608. doi: 10.1016/j.ccr.2010.04.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Kindler T, Lipka DB, Fischer T. FLT3 as a therapeutic target in AML: still challenging after all these years. Blood. 2010;116:5089–102. doi: 10.1182/blood-2010-04-261867. [DOI] [PubMed] [Google Scholar]
- 113.Wiernik PH. FLT3 inhibitors for the treatment of acute myeloid leukemia. Clin Adv Hematol Oncol. 2010;8:429–36. 44. [PubMed] [Google Scholar]