Abstract
Objective
To compare the effect of perinatal regimens of short-course nevirapine (HIVNET 012) and zidovudine [Thai-Centers for Disease Control and Prevention (CDC) regimen] on breast milk viral shedding and perinatal transmission during the first 6 weeks postpartum in a randomized clinical trial.
Design
Randomized clinical trial.
Methods
Pregnant HIV-1 seropositive women in Nairobi, Kenya who planned to breastfeed were randomized to HIVNET 012 or Thai-CDC regimens. Two to four breast milk samples were collected each week between delivery and 6 weeks postpartum. Breast milk HIV-1 RNA was quantified using the Gen-Probe TMA assay. Infants were tested for HIV-1 DNA at birth and 6 weeks.
Results
From March to October 2003, 76 women were enrolled and 795 breast milk samples were collected from 60 women who were randomized and followed after delivery. Between 3 and 21 days postpartum, nevirapine was associated with significantly greater suppression of breast milk log10 HIV-1 RNA: days 3 to 7 (1.98 versus 2.42, P = 0.1); days 8 to 14 (1.78 versus 2.48, P = 0.005); days 15 to 21 (1.90 versus 2.97, P = 0.003). At 6 weeks, the HIV-1 perinatal transmission rate was significantly lower among those who took nevirapine than zidovudine (6.8% versus 30.3%, P = 0.02).
Conclusions
Compared to a peripartum zidovudine regimen, nevirapine was significantly more likely to decrease HIV-1 RNA in breast milk during the first week and through the third week postpartum following single-dose administration, and corresponded with decreased transmission risk at 6 weeks. Sustained breast milk HIV-1 suppression may contribute to the ability of nevirapine to decrease perinatal transmission of HIV-1.
Keywords: Africa, vertical transmission, mother-to-child transmission, HIV-1, perinatal transmission, nevirapine, zidovudine
Introduction
Mother-to-child transmission of HIV-1 is the main cause of pediatric HIV-1 infection worldwide, and is a severe problem in Africa where more than 90% of HIV-1 infected children live. Short-course antiretroviral therapy has been utilized to decrease the risk of mother-to-child transmission of HIV-1 in this setting [1–4]. Two perinatal therapies that are widely implemented are the HIVNET 012 nevirapine regimen and the Thai-Centers for Disease Control and Prevention (CDC) zidovudine regimen [1, 2]. Both regimens significantly decrease mother-tochildtransmissionofHIV-1,buthavenotbeencomparedin a randomized trial.
Breast milk HIV-1 transmission contributes to 30–50% of infant infections in Africa [5]. In a randomized clinical trial conducted in Nairobi, Kenya, 44% of mother-to-child transmission of HIV-1 was attributable to breastfeeding with the majority of breast milk transmissions occurring within the first 6 weeks of life [6]. Elevated breast milk HIV-1 RNA was associated with increased transmission, and HIV-1 RNA levels were significantly higher in breast milk obtained in the first 10 days after delivery than in breast milk obtained in the latter postpartum period [7].
Because perinatal administration of antiretroviral medications may have a significant impact on HIV-1 virus levels in breast milk and early breastfeeding transmission, we compared short-course zidovudine and nevirapine regimens in a randomized clinical trial to determine the effect on HIV-1 RNA levels in breast milk and infant transmission during the first 6 weeks postpartum.
Methods
Study population
The study protocol was approved by the institutional review boards at the University of Washington, USA and the Kenyatta National Hospital, Kenya. The study was designed to compare the effect of nevirapine (HIVNET 012 regimen) and short-course zidovudine (Thai-CDC regimen) on the quantity of HIV-1 RNA in breast milk during the first 6 weeks postpartum. The primary outcome was HIV-1 RNA viral load in breast milk and the secondary outcome was mother-to-child transmission of HIV-1 at 6 weeks. The sample size was calculated to be 50 women in each arm to detect a 0.75 log10 HIV-1 RNA difference in breast milk with greater than 90% power.
Pregnant women attending antenatal clinic at the Mathare North City Council Clinic in Nairobi, Kenya were offered HIV-1 serological testing after counseling by nurse counselors. Women who were HIV-1 seropositive were counseled on mother-to-child transmission of HIV-1 and the risks and benefits of breastfeeding versus formula feeding. Women at or below 32 weeks’ gestation who opted to breastfeed were referred to the research clinic at the same location. Women were eligible to participate in the study if they were above 18 years of age, had no previous exposure to antiretroviral medications, had hemoglobin concentrations ≥8 g/dl, agreed to home visits, and resided in the clinic catchment area.
Enrollment, randomization, and delivery
Women were enrolled after written informed consent. At enrollment, a study physician performed a physical examination and interviewed the participant. Peer counselors accompanied participants to their houses to confirm locations for future home visits. At 32 weeks gestation, blood was taken to determine CD4 cell count and HIV-1 RNA virus levels.
At 34 weeks’ gestation, women were randomized to one of two antiretroviral regimens using computer-generated block randomization that was concealed until time of randomization. Randomization was revealed through numbered envelopes by the study physician who assigned the treatment regimens. Study investigators and participants were not blinded to the interventions. One regimen (HIVNET 012) was the oral administration of 200 mg of nevirapine to the mother at the onset of labor, and a single 2 mg/kg (6 mg if birthweight > 2.5 kg) oral dose of nevirapine suspension administered to the infant within 72 h of delivery. The second regimen (Thai-CDC) was the oral administration of 300 mg of zidovudine twice daily to the mother from 34 weeks’ gestation until the onset of labor and 300 mg orally every 3 h from the onset of labor until delivery. After randomization, participants were seen weekly in clinic until delivery.
At or within 3 days of delivery, maternal blood was obtained for CD4 cell count and HIV-1 RNA virus levels and infant blood was collected on filter paper for HIV-1 DNA. For mothers and infants in whom early collection was not achieved, specimen blood collection was performed within 2 weeks of delivery during a home visit.
Postpartum follow up
Mothers and their infants were followed for 6 weeks after delivery. Each woman was scheduled to have 2–5 ml of breast milk collected at her home by peer counselors at 14 time points separated by at least one day within the first 6 weeks. In general, peer counselors scheduled to collect breast milk during home visits between 2 and 4 times per week. Peer counselors obtained breast milk by observing mothers manually express milk from a single breast into a sterile 10 ml container. For each mother, breast milk was obtained from the same breast at every time point. Information concerning breastfeeding practices was elicited through questionnaires administered at each home visit. At 6 weeks postpartum, participants returned for a clinic visit with the study physician. At this visit, maternal blood was drawn for CD4 cell count and HIV-1 RNA virus levels and infant blood was collected on filter paper for HIV-1 DNA.
Laboratory methods
Breast milk samples were centrifuged at 710 g for 20 min, the lipid layer was discarded, and the supernatant was aspirated into a separate tube [8]. The cell pellet was stored in liquid nitrogen and the breast milk supernatant samples were frozen at –70°C. The samples were subsequently shipped from Nairobi, Kenya on liquid nitrogen to Seattle, Washington, where they were stored at –70°C. The samples were thawed at the time of analysis and HIV-1 RNAvirus levels were measured using the Gen-Probe HIV-1 viral load assay (Gen-Probe Incorporated, San Diego, California, USA) as previously described [9,10]. The lower limit of detection of HIV-1 RNA in breast milk with the reagents used in this study was defined to be 100 copies/ml based on parallel testing of HIV-1 negative samples and samples spiked with low copies of HIV-1 RNA.
Quantitative viral HIV-1 RNA levels of maternal plasma were determined using the Gen-Probe HIV-1 viral load assay [9]. CD4 cell counts were determined using flow cytometry (FACScan, Becton Dickinson, Franklin Lakes, New Jersey, USA). Infant HIV-1 infections status was determined by PCR for HIV-1 DNA as previously described [11].
Statistical methods
All analyses were intent-to-treat and were performed using SPSS version 11.5 (SPSS Inc, Chicago, Illinois, USA) and S-Plus 2000 (MathSoft Inc, Seattle, Washington, USA). Viral load data were log10 transformed. Breast milk HIV-1 RNA viral load data which measured below the lower limit of detection (100 copies/ml) was assigned a value at the midpoint between the lower limit of detection and zero (50 copies/ml). Analyses included grouping breast milk samples by weekly and daily postpartum intervals. If multiple samples were available from a woman within a particular time interval, then the mean of these samples was used in analyses. Comparisons of median log10 HIV-1 RNA viral load across treatment arms were performed using the Mann–Whitney U test.
Kaplan–Meier estimation was used to calculate rates of infant HIV-1 infection at 6 weeks postpartum, and the z test was used to compare these infection rates. The time to HIV-1 infection in the infant was defined as the midpoint between the last negative and first positive HIV-1 PCR.
Results
Study population
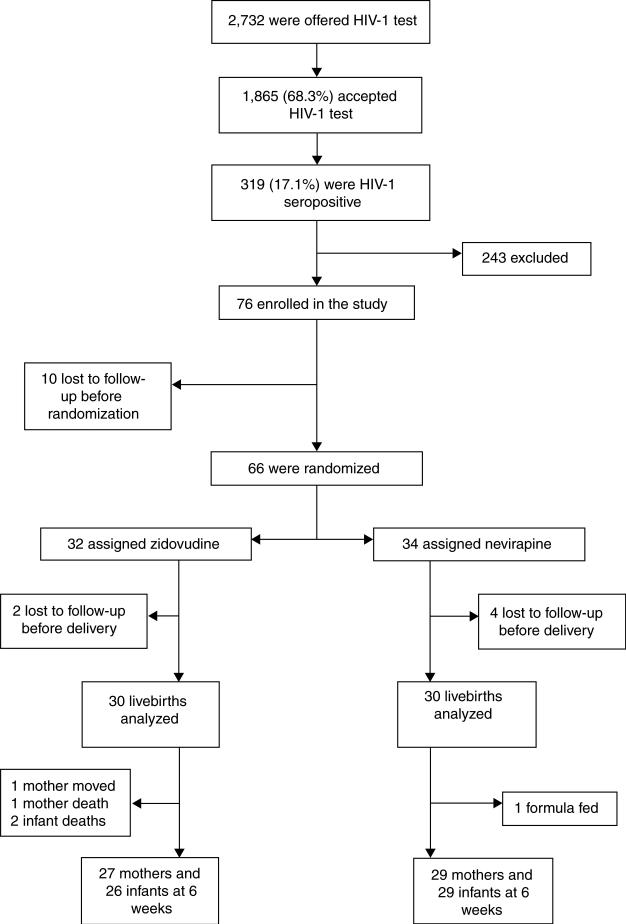
Enrollment began on 5 March 2003 and ended on 31 October 2003. During this time period, 2732 pregnant women were offered HIV-1 testing, of whom 1865 (68%) accepted. Among the women tested, 319 (17%) were HIV-1 seropositive and 76 women were enrolled in the study. Of the 243 remaining women with positive HIV-1 tests, 97 (40%) did not return to be informed of the study; 72 (29%) were ineligible due to decision to formula feed, previous exposure to antiretroviral drugs, illness, age, or plan to move from Nairobi; 53 (22%) were too late in gestation; 12 (5%) expressed no interest in participating in the study; and 9 (4%) did not receive test results. Excluded women who returned for HIV-1 results and their infants were given nevirapine during labor and delivery as per Kenyan national guidelines. Of the 76 women enrolled, 10 were lost to follow up before randomization at 34 weeks’ gestation, 34 were randomized to the nevirapine regimen, and 32 were randomized to the zidovudine regimen (Fig. 1). There were no adverse events or side effects reported in either intervention group.
Fig. 1.
Trial profile.
At enrollment and delivery, characteristics of women who gave birth were comparable across the two study arms and similar to all who were randomized (Table 1). Of the 66 women who were randomized, 60 women were followed through delivery and had breast milk collected. Thirty of these women were randomized to zidovudine and 30 to nevirapine. Characteristics of neonates were also similar at birth in the two treatment arms (Table 1).
Table 1.
Characteristics of women who gave birth and neonates by treatment arm.
| Zidovudine |
Nevirapine |
||||
|---|---|---|---|---|---|
| Characteristic | n | Value | n | Value | P |
| Women who gave birth | |||||
| Total | 30 | 30 | |||
| Median (IQR) age (years) | 30 | 25 (21–29) | 30 | 24 (21–28) | 0.45 |
| Median (IQR) schooling (years) | 30 | 8 (7–10) | 29 | 8 (7–12) | 0.31 |
| Median (IQR) number of lifetime sexual partners | 30 | 3 (2–4) | 30 | 3 (2–4) | 0.64 |
| Median (IQR) age at first sexual intercourse | 30 | 16 (15–18) | 30 | 17 (15–19) | 0.64 |
| CD4 cell count (× 106 cells/l) at 32 weeks’ gestation | 29 | 29 | |||
| Median (IQR) | 456 (291–732) | 528 (337–682) | 0.62 | ||
| ≤ 200 × 106 cells/l (%) | 3 (10.3) | 3 (10.3) | |||
| 201–499 × 106 cells/l (%) | 14 (48.3) | 10 (34.5) | |||
| ≥ 500 × 106 cells/l (%) | 12 (41.4) | 16 (55.2) | |||
| Median (IQR) HIV-1 RNA (log10 copies/ml) at 32 weeks’ gestation | 30 | 4.70 (4.43–5.41) | 28 | 4.84 (3.98–5.37) | 0.53 |
| Median (IQR) length of gestation at delivery (weeks) | 30 | 39 (37–40) | 30 | 38 (36–40) | 0.25 |
| Caesarean section | 30 | 0 (0%) | 30 | 0 (0%) | |
| Median (IQR) length of labour (h) | 29 | 10 (7–16) | 27 | 8 (6–15) | 0.34 |
| Neonates | |||||
| Birthweight (g) | 29 | 28 | |||
| Median (IQR) | 3100 (2650–3400) | 3050 (2650–3300) | 0.56 | ||
| < 2500 mg (%) | 2 (7) | 4 (14) | 0.42 | ||
IQR, Interquartile range.
At the 6-week study endpoint, 56 mothers and 55 infants were assessed and analyzed. In the zidovudine arm, one mother moved away from the study site, one mother died, and two infants died prior to 6 weeks. In the nevirapine arm, one mother decided to formula feed exclusively and chose not to participate further in the study. Overall, in the zidovudine arm 27 mothers (90%) and 26 (87%) infants were assessed while in the nevirapine arm 29 (97%) mothers and 29 (97%) infants were assessed at 6 weeks. All of these women were reported as having exclusively breastfed their infants.
Self-reported and observed adherence to treatment medications was high. The median duration of zidovu-dine therapy was 5 weeks and 97% of women in this treatment arm received at least 2 weeks of antenatal zidovudine treatment before delivery. Of women who took antenatal zidovudine, 87% attended all scheduled antenatal clinic visits. A pill count performed at 50% of clinic visits made by women in the zidovudine arm found that 83% of all zidovudine doses were taken. The median length of labor in the zidovudine treatment arm was 10 h and participants received an appropriate median number of three zidovudine (300 mg) doses during this time (Table 1). All women in the nevirapine treatment arm reported taking one dose of nevirapine during labor.
Breast milk HIV-1 RNA viral levels
A total of 795 breast milk samples were collected from 60 women over the first 6 weeks postpartum: 391 samples were collected from 30 women in the zidovudine arm and 404 samples were collected from 30 women in the nevirapine arm. The median number of breast milk samples collected per woman was 14. Breast milk was collected between 1 and 49 days postpartum with half of the total number of samples collected within the first 14 days after delivery.
During the first 2 days postpartum, there was a trend for mothers randomized to nevirapine to have higher HIV-1 RNA viral loads in breast milk compared to those in the zidovudine arm (median log10 HIV-1 RNA, 3.08 versus 1.70, P = 0.1). Between days 3 and 7, mothers randomized to nevirapine tended to have lower HIV-1 RNA viral loads in breast milk compared to those treated with zidovudine (median log10 HIV-1 RNA, 1.98 versus 2.42, P = 0.1) (Table 2). The suppressive effects of nevirapine compared to zidovudine was statistically significant in the second week postpartum (median log10 HIV-1 RNA, 1.78 versus 2.48, P = 0.005), and continued through the third week (median log10 HIV-1 RNA, 1.90 versus 2.97, P = 0.003). In the fourth, fifth, and sixth weeks postpartum there was no significant difference in breast milk HIV-1 RNA viral load between the two treatment arms (Table 2).
Table 2.
Viral load of breast milk (median of the mean log10 copies/ml) over weeks and collection days postpartum.
| n | Zidovudine treated [median (IQR)] | n | Nevirapine treated [median (IQR)] | P | |
|---|---|---|---|---|---|
| Weeks postpartum | |||||
| Week 1 (3–7 days) | 30 | 2.42 (1.79–3.58) | 28 | 1.98 (1.70–2.82) | 0.1 |
| Week 2 (8–14 days) | 28 | 2.48 (1.72–3.60) | 29 | 1.78 (1.70–2.23) | 0.005 |
| Week 3 (15–21 days) | 28 | 2.97 (2.11–4.01) | 29 | 1.90 (1.70–2.73) | 0.003 |
| Week 4 (22–28 days) | 28 | 2.70 (1.74–3.75) | 28 | 2.23 (1.70–3.46) | 0.3 |
| Week 5 (29–35 days) | 27 | 2.60 (1.70–4.30) | 29 | 2.24 (1.70–2.93) | 0.3 |
| Week 6 (36–42 days) | 26 | 2.43 (1.70–3.58) | 29 | 2.03 (1.70–2.87) | 0.2 |
| Days postpartum | |||||
| 0–2 days | 9 | 1.70 (1.70–2.46) | 15 | 3.08 (1.70–3.78) | 0.1 |
| 3–4 days | 22 | 2.75 (1.82–3.62) | 23 | 2.42 (1.70–3.12) | 0.3 |
| 5–6 days | 26 | 2.19 (1.70–3.18) | 25 | 2.05 (1.70–2.69) | 0.4 |
| 7–8 days | 26 | 2.16 (1.70–3.38) | 26 | 1.70 (1.70–2.29) | 0.04 |
| 9–10 days | 28 | 2.19 (1.70–3.67) | 26 | 1.70 (1.70–2.47) | 0.06 |
| 11–13 days | 22 | 2.63 (1.70–3.68) | 23 | 1.70 (1.70–2.41) | 0.01 |
| 14–16 days | 26 | 3.21 (2.18–3.98) | 24 | 1.70 (1.70–2.80) | 0.004 |
| 17–20 days | 22 | 2.98 (1.70–4.02) | 24 | 2.08 (1.70–2.79) | 0.06 |
| 21–24 days | 23 | 2.84 (2.02–3.89) | 26 | 2.26 (1.70–3.26) | 0.1 |
| 25–28 days | 24 | 2.84 (1.70–3.42) | 23 | 2.24 (1.70–3.47) | 0.4 |
| 29–32 days | 24 | 2.64 (1.70–4.21) | 27 | 2.61 (1.70–3.13) | 0.5 |
| 33–36 days | 22 | 2.61 (1.70–3.56) | 24 | 2.32 (1.70–3.33) | 0.4 |
| 37–40 days | 22 | 2.11 (1.70–3.54) | 24 | 1.70 (1.70–2.47) | 0.1 |
| ≥ 41 days | 9 | 3.26 (1.70–4.80) | 9 | 2.31 (1.70–3.17) | 0.5 |
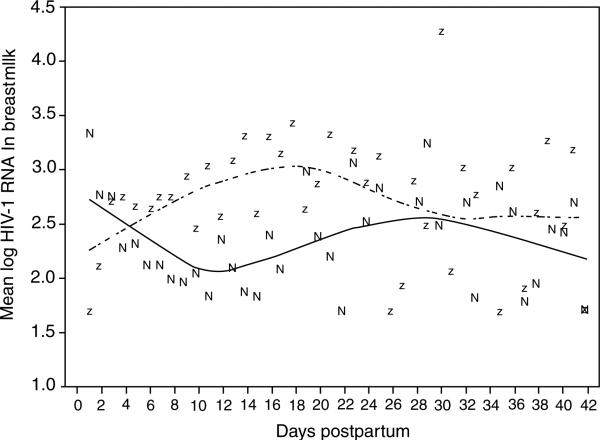
Narrowing the time analysis to daily intervals when breast milk was scheduled for collection reinforced the weekly analysis of breast milk HIV-1 RNA suppression. Between days 3 and 4, there was no longer a trend for differential HIV-1 RNA suppression by nevirapine compared to zidovudine (2.42 versus 2.75, P = 0.3) (Table 2). By days 7–8, nevirapine significantly suppressed HIV-1 RNA in breast milk compared to zidovudine (1.70 versus 2.16, P = 0.04) and this effect was found in daily analyses through days 17–20 (2.08 versus 2.98, P = 0.06). Similar to the weekly analyses, there was no significant difference between the treatment arms in breast milk HIV-1 RNA suppression after 21 days (Fig. 2).
Fig. 2. Mean log10 HIV-1 RNA in breast milk over days postpartum.
Dashed line and ‘z’ indicate the loess curve and mean values for the zidovudine group; solid line and ‘n’ indicate the loess curve and mean values for the nevirapine group.
HIV-1 transmission at 6 weeks postpartum
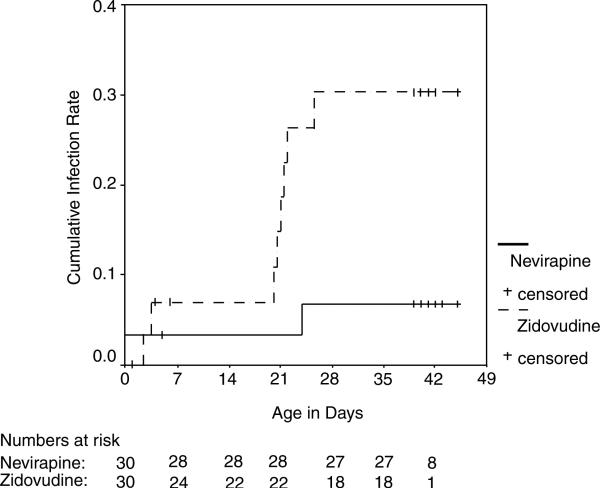
All 60 infants who were born had HIV-1 assessment performed within 2 weeks of delivery. Subsequently, three infants were lost to follow up and two died before reaching the 6-week postpartum endpoint. Blood samples for HIV-1 DNA were obtained from 55 infants (92%) at 6 weeks. Two weeks after delivery, there were two infants infected in the zidovudine arm and one infant infected in the nevirapine arm. By 6 weeks postpartum, there were eight infants infected in the zidovudine arm and two infants infected in the nevirapine arm. The cumulative perinatal HIV-1 transmission rate at 6 weeks was 6.8% [95% confidence interval (CI), 0.0–15.9%] in the nevirapine arm versus 30.3% (95% CI, 12.7–47.9%) in the zidovudine arm, and differed significantly between the two arms (P = 0.02) (Fig. 3). In sensitivity analyses considering infants with incomplete follow up, nevir apine remained significantly associated with lower transmission risk (P = 0.008, all infants infected; P = 0.04, all infants uninfected).
Fig. 3.
Kaplan–Meier estimates of cumulative HIV-1 infant infection.
Discussion
In this randomized comparison of two widely used perinatal antiretroviral regimens, breast milk HIV-1 RNA and mother-to-child HIV-1 transmission were significantly lower among mothers who received nevirapine than those who received short-course zidovudine.
Breast milk HIV-1 RNA was suppressed between 3 and 21 days after delivery in the nevirapine arm compared to the zidovudine arm in this study. Prolonged breast milk HIV-1 RNA suppression among women who received single-dose intrapartum nevirapine compared to women who received antenatal and intrapartum zidovudine most likely reflects the differing pharmacokinetics of the two drugs [12]. In a Ugandan study, the plasma half-life of nevirapine was 61 h and nevirapine concentration in breast milk was >100 ng/ml 1 week following single-dose administration [13]. In contrast, the plasma half-life of zidovudine in pregnant women is 1 h, limiting its ability to suppress breast milk virus for an extended time period [14]. Thus, while we observed a trend for intrapartum zidovudine to suppress HIV-1 RNA virus in breast milk immediately after delivery, this effect diminished compared to nevirapine after the second day.
In this study, nevirapine was associated with a profound decrease in HIV-1 RNA virus in breast milk compared to zidovudine. During the second week postpartum, nevirapine resulted in up to 1 log10 lower breast milk viral load and undetectable viral levels in over 60% of breast milk samples collected. Breast milk HIV-1 RNA levels have been found to correlate with vertical transmission of HIV-1 [15–18]. In one study among antiretroviral naive women, a 1 log10 decrease in breast milk HIV-1 RNA levels was associated with a twofold reduction in mother-to-child HIV-1 transmission [7]. Studies have suggested that transmission through breast milk may be particularly high-risk within the first month postpartum [6,19,20]. The finding of this study that nevirapine significantly suppressed breast milk HIV-1 RNA throughout most of the first month postpartum may therefore partly explain the efficacy of nevirapine in decreasing HIV-1 transmission in breast-feeding populations.
An unexpected finding in this randomized trial was that mother-to-child HIV-1 transmission risk at 6 weeks postpartum was significantly reduced in the nevirapine arm compared to the zidovudine arm. These regimens are widely considered to be similar in efficacy in reducing HIV-1 perinatal transmission [20], however they have not been compared directly in a randomized trial. Transmission risk for zidovudine was higher and for nevirapine was lower in our study than in larger previously published trials of each regimen, but most estimates from previous trials were contained in the 95% CI for our estimates [21–24]. Although our study was small and not primarily designed to evaluate effects on transmission risk, it suggests that nevirapine may be more effective than short-course zidovudine in decreasing mother-to-child transmission in breastfeeding cohorts and certainly confirms the benefit of this simple regimen.
Nevirapine added to zidovudine has been shown to significantly reduce HIV-1 transmission in non-breast-feeding mothers, suggesting that part of its effect is via exposure prophylaxis [25]. Our study suggests that in breastfeeding mothers the suppression of breast milk HIV-1 RNA contributes to decreasing HIV-1 transmission to infants. It is therefore plausible that regimens that combine zidovudine and nevirapine may be more beneficial in breastfeeding than non-breastfeeding cohorts because of the dual mechanisms of action. It also supports evidence from other studies that extending maternal antiretroviral prophylaxis into the early post-partum period may substantially reduce the risk of mother-to-child HIV-1 transmission among breastfeeding mothers [3,4].
Our study was unique in intensively delineating breast milk HIV-1 RNA shedding during the first 6 weeks postpartum. On average, 14 specimens were obtained per woman in the 6-week postpartum period with breast milk specimens obtained almost every other day for the first 2 weeks postpartum. Randomized allocation to either zidovudine or nevirapine enabled serial comparison of breast milk HIV-1 for the two regimens side by side. Frequent home visits for breast milk collection also meant close monitoring of breastfeeding practices where exclusive breastfeeding was observed. Enrollment was stopped after preliminary analyses demonstrated significant differences in the primary outcome between study arms.
The prolonged effect of nevirapine found in this study underscores the concern regarding the potential for selection of resistant virus [26]. The effect of a single dose of nevirapine was sustained for 3 weeks amounting to prolonged monotherapy. It is unknown how effective nevirapine will be for repeated prevention of HIV-1 perinatal transmission. Breast milk virus may not be suppressed for as long in a woman who had previously received perinatal nevirapine because of selection of nevirapine-resistant strains. Further studies will be important to define effects of these regimens on prevalence of viral resistance in breast milk.
We found that nevirapine resulted in significantly greater reduction of breast milk HIV-1 RNA and mother-to-child transmission of HIV-1 compared to zidovudine. Our study suggests that nevirapine exerts at least part of its effect on reducing mother-to-child transmission by lowering breast milk HIV-1, and that this regimen may be superior to short-course zidovudine in breastfeeding HIV-1 infected mothers.
Acknowledgements
The authors thank the research personnel, laboratory staff, and data management teams in Nairobi, Kenya and Seattle, Washington; the Mathare North City Council Clinic for their participation and cooperation; the Divisions of Obstetrics and Gynaecology and Paediatrics at Kenyatta National Hospital for providing facilities for laboratory and data analysis. Most of all we thank the mothers and children who participated in the trial.
Sponsorship: M.H. Chung is a scholar in the International AIDS Research and Training Program and is supported by the Fogarty International Center, National Institutes of Health (D43-TW00007). This work was supported by NIH Research Grant # D43-TW000007, the Fogarty International Center, the Office of Research on Women's Health, the Pediatric AIDS Foundation, and NIH Research Grant # AI38518. G. John-Stewart is an Elizabeth Glaser Pediatric AIDS Foundation (EGPAF) Scientist.
References
- 1.Guay LA, Musoke P, Fleming T, Bagenda D, Allen M, Nakabiito C, et al. Intrapartum and neonatal single-dose nevirapine compared with zidovudine for prevention of mother-to-child transmission of HIV-1 in Kampala, Uganda: HIVNET 012 randomised trial. Lancet. 1999;354:795–802. doi: 10.1016/S0140-6736(99)80008-7. [DOI] [PubMed] [Google Scholar]
- 2.Shaffer N, Chuachoowong R, Mock PA, Bhadrakom C, Siriwasin W, Young NL, et al. Short-course zidovudine for perinatal HIV-1 transmission in Bangkok, Thailand: a randomised controlled trial. Bangkok Collaborative Perinatal HIV Transmission Study Group. Lancet. 1999;353:773–780. doi: 10.1016/s0140-6736(98)10411-7. [DOI] [PubMed] [Google Scholar]
- 3.Efficacy of three short-course regimens of zidovudine and lamivudine in preventing early and late transmission of HIV-1 from mother to child in Tanzania, South Africa, and Uganda (Petra study): a randomised, double-blind, placebo-controlled trial. Lancet. 2002;359:1178–1186. doi: 10.1016/S0140-6736(02)08214-4. [DOI] [PubMed] [Google Scholar]
- 4.Moodley D, Moodley J, Coovadia H, Gray G, McIntyre J, Hofmyer J, et al. A multicenter randomized controlled trial of nevirapine versus a combination of zidovudine and lamivudine to reduce intrapartum and early postpartum mother-to-child transmission of human immunodeficiency virus type 1. J Infect Dis. 2003;187:725–735. doi: 10.1086/367898. [DOI] [PubMed] [Google Scholar]
- 5.John-Stewart G, Mbori-Ngacha D, Ekpini R, Janoff EN, Nkengasong J, Read JS, et al. Breast-feeding and transmission of HIV-1. J Acquir Immune Defic Syndr. 2004;35:196–202. doi: 10.1097/00126334-200402010-00015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Nduati R, John G, Mbori-Ngacha D, Richardson B, Overbaugh J, Mwatha A, et al. Effect of breastfeeding and formula feeding on transmission of HIV-1: a randomized clinical trial. JAMA. 2000;283:1167–1174. doi: 10.1001/jama.283.9.1167. [DOI] [PubMed] [Google Scholar]
- 7.Rousseau CM, Nduati RW, Richardson BA, Steele MS, John-Stewart GC, Mbori-Ngacha DA, et al. Longitudinal analysis of human immunodeficiency virus type 1 RNA in breast milk and of its relationship to infant infection and maternal disease. J Infect Dis. 2003;187:741–747. doi: 10.1086/374273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lewis P, Nduati R, Kreiss JK, John GC, Richardson BA, Mbori-Ngacha D, et al. Cell-free human immunodeficiency virus type 1 in breast milk. J Infect Dis. 1998;177:34–39. doi: 10.1086/513816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Emery S, Bodrug S, Richardson BA, Giachetti C, Bott MA, Panteleeff D, et al. Evaluation of performance of the Gen-Probe human immunodeficiency virus type 1 viral load assay using primary subtype A, C, and D isolates from Kenya. J Clin Microbiol. 2000;38:2688–2695. doi: 10.1128/jcm.38.7.2688-2695.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.DeVange Panteleeff D, Emery S, Richardson BA, Rousseau C, Benki S, Bodrug S, et al. Validation of performance of the gen-probe human immunodeficiency virus type 1 viral load assay with genital swabs and breast milk samples. J Clin Microbiol. 2002;40:3929–3937. doi: 10.1128/JCM.40.11.3929-3937.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Panteleeff DD, John G, Nduati R, Mbori-Ngacha D, Richardson B, Kreiss J, et al. Rapid method for screening dried blood samples on filter paper for human immunodeficiency virus type 1 DNA. J Clin Microbiol. 1999;37:350–353. doi: 10.1128/jcm.37.2.350-353.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mirochnick M, Capparelli E. Pharmacokinetics of antiretrovirals in pregnant women. Clin Pharmacokinet. 2004;43:1071–1087. doi: 10.2165/00003088-200443150-00002. [DOI] [PubMed] [Google Scholar]
- 13.Musoke P, Guay LA, Bagenda D, Mirochnick M, Nakabiito C, Fleming T, et al. A phase I/II study of the safety and pharmacokinetics of nevirapine in HIV-1-infected pregnant Ugandan women and their neonates (HIVNET 006). AIDS. 1999;13:479–486. doi: 10.1097/00002030-199903110-00006. [DOI] [PubMed] [Google Scholar]
- 14.Rodman JH, Flynn PM, Robbins B, Jiménez E, Bardeguez AD, Rodriguez JF, et al. Systemic pharmacokinetics and cellular pharmacology of zidovudine in human immunodeficiency virus type 1-infected women and newborn infants. J Infect Dis. 1999;180:1844–1850. doi: 10.1086/315152. [DOI] [PubMed] [Google Scholar]
- 15.Semba RD, Kumwenda N, Hoover DR, Taha TE, Quinn TC, Mtimavalye L, et al. Human immunodeficiency virus load in breast milk, mastitis, and mother-to-child transmission of human immunodeficiency virus type 1. J Infect Dis. 1999;180:93–98. doi: 10.1086/314854. [DOI] [PubMed] [Google Scholar]
- 16.Pillay K, Coutsoudis A, York D, Kuhn L, Coovadia HM. Cell-free virus in breast milk of HIV-1-seropositive women. J Acquir Immune Defic Syndr. 2000;24:330–336. doi: 10.1097/00126334-200008010-00006. [DOI] [PubMed] [Google Scholar]
- 17.Richardson BA, John-Stewart GC, Hughes JP, Nduati R, Mbori-Ngacha D, Overbaugh J, et al. Breast-milk infectivity in human immunodeficiency virus type 1-infected mothers. J Infect Dis. 2003;187:736–740. doi: 10.1086/374272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Manigart O, Crepin M, Leroy V, Meda N, Valea D, Janoff EN, et al. Effect of perinatal zidovudine prophylaxis on the evolution of cell-free HIV-1 RNA in Breast Milk and on postnatal transmission. J Infect Dis. 2004;190:1422–1428. doi: 10.1086/424569. [DOI] [PubMed] [Google Scholar]
- 19.Miotti PG, Taha TE, Kumwenda NI, Broadhead R, Mtimavalye LA, Van der Hoeven L, et al. HIV transmission through breast-feeding: a study in Malawi. JAMA. 1999;282:744–749. doi: 10.1001/jama.282.8.744. [DOI] [PubMed] [Google Scholar]
- 20.Nolan ML, Greenberg AE, Fowler MG. A review of clinical trials to prevent mother-to-child HIV-1 transmission in Africa and inform rational intervention strategies. AIDS. 2002;16:1991–1999. doi: 10.1097/00002030-200210180-00003. [DOI] [PubMed] [Google Scholar]
- 21.Garcia F, Plana M, Vidal C, Cruceta A, O'Brien WA, Pantaleo G, et al. Dynamics of viral load rebound and immunological changes after stopping effective antiretroviral therapy. AIDS. 1999;13:F79–F86. doi: 10.1097/00002030-199907300-00002. [DOI] [PubMed] [Google Scholar]
- 22.Wiktor SZ, Ekpini E, Karon JM, Nkengasong J, Maurice C, Severin ST, et al. Short-course oral zidovudine for prevention of mother-to-child transmission of HIV-1 in Abidjan, Cote d'Ivoire: a randomised trial. Lancet. 1999;353:781–785. doi: 10.1016/S0140-6736(98)10412-9. [DOI] [PubMed] [Google Scholar]
- 23.Leroy V, Karon JM, Alioum A, Ekpini ER, Meda N, Greenberg AE, et al. Twenty-four month efficacy of a maternal short-course zidovudine regimen to prevent mother-to-child transmission of HIV-1 in West Africa. AIDS. 2002;16:631–641. doi: 10.1097/00002030-200203080-00016. [DOI] [PubMed] [Google Scholar]
- 24.Jackson JB, Musoke P, Fleming T, Guay LA, Bagenda D, Allen M, et al. Intrapartum andneonatal single-dose nevirapine compared with zidovudine for prevention of mother-to-child transmission of HIV-1 in Kampala, Uganda: 18-month follow-up of the HIVNET 012 randomised trial. Lancet. 2003;362:859–868. doi: 10.1016/S0140-6736(03)14341-3. [DOI] [PubMed] [Google Scholar]
- 25.Lallemant M, Jourdain G, Le Coeur S, Mary JY, Ngo-Giang-Huong N, et al. Single-dose perinatal nevirapine plus standard zidovudine to prevent mother-to-child transmission of HIV-1 in Thailand. N Engl J Med. 2004;351:217–228. doi: 10.1056/NEJMoa033500. [DOI] [PubMed] [Google Scholar]
- 26.Jourdain G, Ngo-Giang-Huong N, Le Coeur S, Bowonwatanuwong C, Kantipong P, Leechanachai P, et al. Intrapartum exposure to nevirapine and subsequent maternal responses to nevirapine-based antiretroviral therapy. N Engl J Med. 2004;351:229–240. doi: 10.1056/NEJMoa041305. [DOI] [PubMed] [Google Scholar]