Abstract
We examined the association between certain clinical factors and aflatoxin B1–albumin adduct (AF-ALB) levels in HIV-positive people. Plasma samples collected from 314 (155 HIV-positive and 159 HIV-negative) people were tested for AF-ALB levels, viral load, CD4+ T-cell count, liver function profile, malaria parasitaemia, and hepatitis B and C virus infections. HIV-positive participants were divided into high and low groups based on their median AF-ALB of 0.93 pmol mg−1 albumin and multivariable logistic and linear regression methods used to assess relationships between clinical conditions and AF-ALB levels. Multivariable logistic regression showed statistically significant increased odds of having higher HIV viral loads (OR=2.84; 95% CI=1.17–7.78) and higher direct bilirubin levels (OR=5.47; 95% CI=1.03–22.85) among HIV-positive participants in the high AF-ALB group. There were also higher levels of total bilirubin and lower levels of albumin in association with high AF-ALB. Thus, aflatoxin exposure may contribute to high viral loads and abnormal liver function in HIV-positive people and so promote disease progression.
Keywords: aflatoxins, HIV, liver function, Ghana
Introduction
Sub-Saharan Africa is a region that is most heavily affected by the HIV/AIDS epidemic (UNAIDS 2006). It is estimated that about 22.4 million people are living with HIV/AIDS in the region (about two-thirds of the global total), and 75% of all HIV/AIDS deaths since the beginning of the HIV/AIDS pandemic have occurred in sub-Saharan Africa. Although access to antiretroviral therapy (ART) is decreasing the death toll from AIDS, fewer than half of Africans who need treatment are receiving it (WHO/UNAIDS/UNICEF 2009). Therefore, the impact of AIDS will remain severe in the region for several years to come.
Aflatoxins are a group of extremely toxic fungal metabolites (Gourama and Bullerman 1995) found in staple food crops such as groundnuts, maize, rice and other grains (Freitas and Brigido 1980; Carvajal and Arroyo 1997; Begum and Samajpati 2000; Park et al. 2002) in many developing countries at latitudes between 40°N and 40°S (Williams et al. 2004). They are potent cytotoxic, carcinogenic, immunomodulatory and immunosuppressive agents (Joint FAO/WHO Expert Committee on Food Additives Pier 1986; Pestka and Bondy 1994; (JECFA) 1998; Omer et al. 1998). We have shown that high aflatoxin B1–albumin adduct (AF-ALB) levels appeared to result in immune impairments in HIV-negative persons (Jiang et al. 2005) and to accentuate some HIV-associated changes in T-cell phenotypes and B-cells in HIV-positive people, that may facilitate HIV-associated immune hyperactivation and lead to more severe disease and disease progression (Jiang et al. 2008). Approximately 4.5 billion people are estimated to be chronically exposed to aflatoxin worldwide; therefore, the toll on human health can be considered to be enormous (Williams et al. 2004).
Aflatoxin B1 (AFB1) is usually the predominant and most toxic of the aflatoxins (B1, B2, G1, G2). It is oxidized to the AFB1-8,9-epoxide by the cytochrome P450 enzyme system (Guengerich and Shimada 1991) and this highly reactive epoxide can bind to DNA, RNA and proteins resulting in cancer and toxicity (Eaton and Gallagher 1994). The most severe effect of aflatoxin in the human body is seen in the liver, the organ generally responsible for detoxifying chemical agents and poisons. In acute aflatoxicosis, binding of the AFB1-epoxide to various cellular macromolecules leads to hepatocellular injury and death. In animal experiments and in humans acutely exposed to aflatoxin, liver specimens showed significant necrosis of parenchymal cells and extensive proliferation of bile ducts (Cullen and Newborne 1994). Previously, we found abnormal liver function (low/high total protein, low albumin and high alanine aminotransferase (AST)) levels in approximately 30–40% of HIV-negative Ghanaians exposed to aflatoxin in the diet (Jolly et al. 2007). Chronic aflatoxin exposure has been shown to interfere with metabolism of proteins (Roebuck and Maxuitenko 1994), food conversion (Shane 1993), growth (Edds 1973; Marin et al. 2002; Turner et al. 2007), and a number of micronutrients that are critical to health and immune functioning (Abdelhamid et al. 1990; Harvey et al. 1994; Williams et al. 2004).
Pre-existing liver diseases such as hepatitis B virus (HBV) or hepatitis C virus (HCV) may compromise the ability of hepatocytes to inactivate carcinogens such as aflatoxin (Zhou et al. 1997). Further, aflatoxin and HBV have been shown to work synergistically to increase the risk of liver cancer (Peers et al. 1987; Wild et al. 1992). Previously, we found that 31% of HIV-negative Ghanaians were positive for hepatitis infection (16.4% for HBV, 14.3% for HCV). Thus, certain clinical factors may predict high aflatoxin levels in humans, especially in HIV-positive individuals who already suffer the severe compromising health effects of HIV. HIV-positive people are shown to have high rates of liver injury (indicated by elevation of liver enzymes and/or bilirubin levels) that may be due to medication, HBV, HCV co-infections and/or alcohol use (Bonacini 2004). Drug-induced liver injury may be due to highly active antiretroviral therapy (HAART) with drugs such as ritonavir or anti-tuberculosis medications (Sulkowski et al. 2000; Soriano et al. 2008). Malaria infection is the number one cause of morbidity accounting for 40–60% of out-patient visits in Ghana (Asante and Asenso-Okyere 2003). Thus, malaria and HIV are two major diseases of public health importance in sub-Saharan Africa. The immune suppression that occurs in HIV infection has been shown to be correlated with increases in the incidence of clinical malaria (Martin-Blonde et al. 2010). Although the role of subclinical malaria is uncertain, transient elevation of HIV viral load during febrile malaria episodes occurs.
Thus, we conducted this study to measure and compare AF-ALB levels in plasma of a group of HIV-negative and positive Ghanaians and to examine the differences in clinical factors such as viral load, CD4 count, liver function parameters, and HBV, HCV and malaria infections among the HIV-positive participants who have lower and higher AF-ALB levels.
Materials and methods
Participant recruitment, data and sample collection
A cross-sectional study of AF-ALB levels in HIV-negative and positive people was conducted in the Kumasi, a heavy maize and groundnut producing and consuming area of the Ashanti region of Ghana. Approval for the study was obtained from the Institutional Review Board, University of Alabama at Birmingham (UAB), and the Committee on Human Research, Publications and Ethics, School of Medical Sciences, Kwame Nkrumah University of Science and Technology (KNUST), Kumasi. A total of 314 (159 HIV-negative and 155 HIV-positive) people gave informed consent and participated in the study. Participants completed a survey on socio-demographic factors such as age, sex, education, occupation and household living conditions. A blood sample (20 ml) was collected from each participant in EDTA vacutainer tubes by trained clinical personnel. The blood was separated into plasma and peripheral blood mononuclear cells (PBMCs) in the laboratories of the Kumasi Center for Collaborative Research (KCCR) in Tropical Medicine, KNUST. After centrifugation the plasma was aspirated and stored frozen at −80°C. PBMCs were prepared using Ficoll-Hypaque density gradients as conducted previously (Jolly 1997) and cell viability was checked. PBMCs were stored frozen in liquid nitrogen and shipped to the UAB for determination of CD4+ T-cell counts. Plasma samples were also shipped to the UAB for determination of AF-ALB levels, liver function tests, HIV viral load, HBV surface antigen, HCV antibody and malaria antigen. All HIV-positive and some HIV-negative study participants were recruited from a hospital that cared for both HIV-positive and -negative persons. HIV-positive study participants had previously been tested for HIV and their positive test results were available in their medical charts. HIV-negative participants were either clients at the hospital or persons from the community without a HIV diagnosis. Their plasma samples were tested for HIV using the Coulter p24 antigen assay (Coulter Corporation, Miami, FL, USA) and those confirmed negative were included as HIV-negatives in the study.
Determination of AF-ALB levels in plasma by radioimmunoassay (RIA)
AF-ALB levels in plasma were determined in the laboratory of Dr J.S. Wang using RIA as published previously (Wang et al. 1996). Briefly, plasma samples were concentrated by high-speed centrifugal filtration using Microcon-50 microconcentrator with a 50,000 mol. wt. filter cut-off and resuspended in 100–150 μl phosphate-buffered saline (PBS). The amount of plasma albumin was determined in each sample by a bromocresol purple dye binding method (Sigma, St. Louis, MO, USA), and the amount of total protein determined using the Bradford procedure (Pierce Biotechnology Inc., Rockport, IL, USA). The total protein per sample was then digested with Pronase (Calbiochem, La Jolla, CA, USA) and bound aflatoxin extracted with acetone. The supernatant was dried in vacuo using a Savant Speed-Vac Concentrator and AF-ALB quantified by the RIA procedure (Wang et al. 1996). Normal human serum/plasma samples purchased from Sigma-Aldrich and authentic AFB-albumin standard were used for QC purposes. The accuracy of the analysis based on 3 days ranged from 93.3% to 96.3% for LQC (0.1 pmol AF-ALB) and from 92.2% to 97.3% for HQC (2 pmol AF-ALB). The within-day imprecision was 5.9% (n=15) for LQC and 2.9% (n=15) for HQC. The overall variation of inaccuracy and imprecision rates were within 10%. The average recovery (0.1–5.0 pmol AF-ALB) was 88.1% ± 5.2%. The values were expressed as pmol AF-ALB mg−1 albumin and the limit of detection for the assay is 0.01 pmol mg−1 albumin.
Tests of liver function (aminotransferases, bilirubin, total blood protein and plasma albumin)
Hepatic function tests were conducted on plasma from participants at the UAB Hospital Laboratory. This included tests of the liver enzyme aspartate aminotransferase (AST) and alanine aminotransferase (ALT), liver transport (total, direct and indirect bilirubin), and liver synthesis (albumin and total protein). The normal range values were based on those reported in the University of Alabama Hospital Laboratories Bulletin of Information, Revised October 2002.
Determination of CD4+ T-cell counts
The absolute CD4 count is a calculated product of the total lymphocyte count and the percentage of lymphocytes that are CD4+ T-cells are determined by flow cytometry. Absolute lymphocyte counts were derived from the white blood cell (WBC) counts and leukocyte differential counts which were performed in the laboratory of the Department of Biochemistry, KNUST. Circulating CD4+ T-cell populations were determined by flow cytometry using fluorescein FITC-labelled monoclonal antibody against CD4 (BD PharMingen, San Diego, CA, USA). Isotype-matched controls (BD PharMingen) were used in all experiments. Briefly, cells were washed and stained with monoclonal antibodies for 30 min in the dark at 4°C, washed twice with staining buffer (PBS supplemented with 0.1% sodium azide and 1% FBS pH 7.4, BD PharMingen) and fixed in 4% paraformaldehyde in buffered PBS (BD PharMingen). The cells were subsequently run on a FACS-Calibur instrument and analysed using CellQuest software. Cells were gated on a live peripheral blood lymphocyte population identified by forward- and side-scatter parameters, and at least 10,000 cells were acquired.
Quantitative HIV-1 RNA assay for HIV viral load
HIV-1 RNA was measured using a quantitative reverse transcriptase polymerase chain reaction assay (Amplicor Monitor, Roche Diagnostic System, Brandersburg, NJ, USA). Virus from 0.2 ml of plasma was lysed in the kit lysis buffer and the HIV RNA was precipitated using isopropanol and pelleted by centrifugation. After washing with ethanol, the RNA was resuspended in the kit dilution buffer. Extracted RNA was amplified and detected according to the manufacturer’s instructions, and the results were reported as HIV RNA copies ml−1. All undetectable values (below 400 copies) were assigned a value of 399.
Test for antibodies to HBV surface antigen
Antibody to HBV surface antigen (HBsAg) in plasma samples was determined using the Bio-Rad Enzyme Immunoassay according to the manufacturer’s directions (Bio-Rad, Redmont, WA, USA). Briefly, 100 μl of specimens or controls were added in duplicate to appropriate wells on a microwell strip plate coated with mouse monoclonal antibody to HBsAg (anti-HBs) and incubated for 60 min at 37°C. After washing, 100 μl of peroxidase-conjugated mouse monoclonal antibodies against HBsAg were added to each well and the plate was incubated for 60 min at 37°C. The plates were then washed and 100 μl of tetramethylbenzidine (TMB) substrate solution were added to each well and incubated in the dark for 30 min at room temperature. The reaction was stopped with the addition of 100 μl of stopping solution to each well and the plate was read on a spectrophotometer at 450 nm. A sample was considered initially reactive for anti-HBs if the absorbance was greater than or equal to the cut-off value. The cut-off value was determined by the addition of 0.070 to the mean absorbance value of the negative controls. Positive samples were determined by repeated reactivity in duplicate tests.
Test for HCV antibody in plasma
Qualitative detection of antibody to HCV in plasma was conducted using the Abbott HCV Enzyme Immunoassy according to the manufacturer’s directions (Abbott Laboratories, Abbott Park, IL, USA). Briefly, 10 μl of each specimen or control were dispensed into test tubes and diluted using 400 μl of diluent. After mixing, 200 μl of each specimen or control were transferred to appropriate wells of a reaction tray. One polystyrene bead coated with recombinant HCV antigen was added to each specimen or control and incubated at 40°C for 1 h. After washing, horseradish-conjugated goat anti-human antibody (200 μl) was added to each well and incubated at 40°C for 30 min in a water bath. The beads were then washed, transferred to assay tubes and incubated with 300 μl of o-phenylenediamine.2HCl (OPD) substrate solution at room temperature for 30 min. The reaction was stopped with 1N sulfuric acid and read on a spectrophotometer at 492 nm. Test samples with an optic density (OD) greater or equal to the mean absorbance of the three negative controls plus 0.25 times the mean absorbance of the three positive controls were considered initially reactive by the criteria of ABBOTT HCV EIA 2.0. Positive samples were determined by repeated reactivity in duplicate tests.
Determination of malaria infection
We conducted a Malaria Antigen Celisa assay (Cellabs Pty Ltd, Brookvale, Australia) for the detection of Plasmodium falciparium antigen in the plasma of study participants. The assay detects a P. falciparium merozoite antigen that circulates in the blood for up to 14 days post-infection. Briefly, microwells were precoated with anti-P. falciparium monoclonal capture antibody. Plasma samples (100 μl) were added to the coated wells and P. falciparium malaria antigen was allowed to bind (if present) for 1 h in a humid chamber at room temperature. Positive and negative samples were run in the assay. The plates were washed to remove unbound material and 100 μl horseradish peroxidase labelled anti-malaria monoclonal indicator antibody conjugate were added to each well to bind any captured P. falciparium malaria antigen. The plate was incubated for 1 h in a humid chamber at room temperature, then washed and 100 μl of fresh enzyme substrate solution (Chromogen) were added to the test wells and incubated in the dark for 15 min at room temperature. The reaction was stopped using 50 μl of stopping solution and the plate read at 450nm in a spectrophotometer blanking the machine on air. The absorbance value of the negative control should be less than 0.1 OD units for the assay to be valid. The cut-off level was determined by adding 0.1 to the value of the negative control. Specimens with an absorbance value above the cut-off were considered positive for P. falciparium antigen. This assay has been shown to detect P. falciparium infection at parasitaemias as low as 0.001% and has a sensitivity and specificity of 98% and 96%, respectively.
Statistical analysis
Data from the surveys and clinical tests were entered into Microsoft Excel and imported into the SAS software package version 9.1 (Statistical Analytical System, Gary, NC, USA) for statistical analysis. Means and standard deviations were obtained for the distributions of the selected variables by HIV status (Tables 1 and 2). The Student’s t-test was performed to evaluate the significance of the differences between the groups. Absolute and relative frequencies (N and %) were obtained for the distributions of the selected variables by aflatoxin levels above and below the HIV-positive population median of 0.93 pmol mg−1 albumin (Table 3). The mean and standard deviation (SD) for the HIV-positive groups with AF-ALB above and below the median were 1.42 ± 0.53 and 0.57 ± 0.22 pmol mg−1 of albumin, respectively. Log transformation of the AF-ALB data prior to analysis did not result in differences in the results. Thus, we present the results as shown. Logistic regression was used to determine the association between clinical results and AF-ALB (Table 3). CD4 was categorized as shown in Table 3 based on the 1993 revised classification system by the Centres for Disease Control and Prevention (CDC) (1992). The viral load categorization was based on published research that showed that people with viral loads below 10,000 copies ml−1 of blood did not show disease progression in greater than a 9-year period compared to people with higher viral loads (Rinaldo et al. 1995) and on the World Health Organization’s designation of viral loads of >10,000 copies ml−1 as evidence of virological failure (WHO 2000). Variables that were statistically significant at p<0.1 on bivariate analysis and those known to be associated with aflatoxin based on the extant literature were incorporated into models using the backward step-wise technique (Hosmer and Lemshow 2000). Crude odds ratios and 95% confidence intervals (CI) were generated as measures of association for all variables by aflatoxin levels above and below the median aflatoxin level. Multiple linear regression analysis was also employed to assess the relationship of aflatoxin levels in plasma with clinical factors (Table 4).
Table 1.
Sociodemographic characteristics of HIV-positive and -negative participants.
| Variables | HIV positive (n =155 (%)) | HIV negative (n =159 (%)) | p-value |
|---|---|---|---|
| Age (years) | |||
| <35 | 59 (38.6) | 73 (46.5) | 0.16 |
| ≥35 | 94 (61.4) | 84 (53.5) | |
| Sex | |||
| Male | 52 (33.6) | 83 (52.2) | <0.01 |
| Female | 103 (66.4) | 76 (47.8) | |
| Education | |||
| None | 22 (14.2) | 77 (48.4) | <0.01 |
| Primary | 27 (17.4) | 34 (21.4) | |
| More than secondary | 106 (68.4) | 48 (30.2) | |
| Working | |||
| No | 33 (21.4) | 4 (2.6) | <0.01 |
| Yes | 121 (78.6) | 153 (97.4) | |
| Residence | |||
| Own home | 25 (21.0) | 75 (48.1) | <0.01 |
| Rent | 29 (24.4) | 35 (22.4) | |
| Friends and relatives | 65 (54.6) | 46 (29.5) | |
| Malaria | |||
| No | 131 (87.3) | 66 (80.5) | 0.16 |
| Yes | 19 (12.7) | 16 (19.5) | |
| Hepatitis B infection | |||
| No | 138 (89.0) | 131 (83.4) | 0.15 |
| Yes | 17 (11.0) | 26 (16.6) | |
| Tuberculosis | |||
| No | 83 (59.3) | Not available | – |
| Yes | 57 (40.7) | ||
| AF-ALB median level 0.83 pmol mg−1 albumin | |||
| No | 65 (41.9) | 92 (57.9) | <0.01 |
| Yes | 90 (58.1) | 67(42.1) | |
Notes: Numbers shown in bold are statistically significant.
AF-ALB, aflatoxin–albumin adduct.
The sum of n for some variables may not equal the total n due to missing values.
Table 2.
Clinical characteristics of HIV positive and HIV negative participants. Variables
| Variables | HIV positive (n =155), mean; SD | HIV negative (n =159), mean; SD | p-value |
|---|---|---|---|
| Total protein (NR =6.0–7.9 g dl−1) | 8.5; 1.6 | 7.3; 0.9 | <0.01 |
| Albumin (NR =3.4–5.0 g dl−1) | 3.2; 0.8 | 3.5; 0.5 | <0.01 |
| Total bilirubin (NR =0.0–1.0) | 0.6; 0.4 | 0.5; 0.2 | 0.06 |
| Direct bilirubin (NR =0.1–0.3 mg dl−1) | 0.1; 0.2 | 0.1; 0.1 | 0.58 |
| Indirect bilirubin (NR =0.0–0.9 mg dl−1) | 0.5; 0.3 | 0.4; 0.2 | 0.02 |
| ALT (NR =6–45 U l−1) | 25.9; 7.8 | 17.3; 8.0 | <0.01 |
| AST (NR =0–37 U l−1) | 65.1; 16.6 | 40.3; 12.7 | <0.01 |
| AFB1 (pmol mg albumin) | 1.1; 0.60 | 0.9; 0.5 | 0.01 |
| CD4+ T-cell count (cells/cc blood) | 308; 103.9 | 1101.39; 106.9 | <0.01 |
| HIV viral load (copies/ml) | 84,987; 22,072 | –; – |
Notes: Numbers shown in bold are statistically significant.
SD, standard deviation; ALT, alanine aminotransferase; AST, aspartate aminotransferase; NR, normal range.
Range for HIV viral load=138–1,492,562 copies/ml.
The sum of n for some variables may not equal the total n due to missing values.
Table 3.
Bivariate and multivariate analysis of clinical factors associated with median aflatoxin B1 albumin adduct level (0.93 pmol mg−1 albumin) among HIV positive participants.
| Variables | Aflatoxin ≥ median, n =78, number (%) | Aflatoxin<median, n =77, number (%) | Total, n =155, number (%) | Crude point estimate (95% CI) | Adjusted point estimate (95% CI) | p-value | |||
|---|---|---|---|---|---|---|---|---|---|
| CD4+ cell count | |||||||||
| <200 | 23 | 35.4 | 29 | 41.4 | 52 | 38.5 | Reference | Reference | |
| 200–499 | 32 | 49.2 | 31 | 44.3 | 63 | 46.7 | 0.79 (0.28, 2.23) | 0.96 (0.37, 2.52) | 0.74 |
| ≥500 | 10 | 15.4 | 10 | 14.3 | 20 | 14.8 | 1.03 (0.38, 2.82) | 1.27 (0.30, 5.34) | 0.70 |
| HIV viral load | |||||||||
| ≥10,000 | 30 | 39.0 | 30 | 38.5 | 60 | 38.7 | 1.02 (0.54, 1.95) | 2.84 (1.17, 7.78) | 0.03 |
| 0–9999 | 47 | 61.0 | 48 | 61.5 | 95 | 61.3 | Reference | Reference | |
| Total protein | |||||||||
| Low (0–5.9 g dl−1) | 5 | 6.4 | 2 | 2.6 | 7 | 4.5 | 2.26 (0.41, 12.57) | 1.67 (0.18, 15.86) | 0.71 |
| Normal (6.0–7.9 g dl−1) | 31 | 39.7 | 28 | 35.9 | 59 | 37.8 | Reference | Reference | |
| High (>7.9 g dl−1) | 42 | 53.9 | 48 | 61.5 | 90 | 57.7 | 0.79 (0.41, 1.53) | 1.22 (0.46, 3.25) | 0.89 |
| Albumin low (<3.4 g dl−1) | |||||||||
| Yes | 46 | 59.7 | 37 | 47.4 | 83 | 53.5 | 1.64 (0.87, 3.11) | 1.49 (0.61, 3.67) | 0.38 |
| No | 31 | 40.3 | 41 | 52.6 | 72 | 46.5 | Reference | Reference | |
| Total bilirubin high (>1.0 mg dl−1) | |||||||||
| Yes | 9 | 11.5 | 3 | 3.9 | 12 | 7.7 | 3.26 (0.85, 12.54) | 1.53 (0.05, 8.68) | 0.81 |
| No | 69 | 88.5 | 75 | 96.1 | 144 | 92.3 | Reference | Reference | |
| Direct bilirubin | |||||||||
| Low (<0.1 mg dl−1) | 11 | 14.1 | 11 | 14.3 | 22 | 14.1 | 1.12 (0.45, 2.77) | 0.91 (0.25, 3.21) | 0.35 |
| Normal (0.1–0.3 mg dl−1) | 59 | 75.6 | 65 | 84.4 | 125 | 80.1 | Reference | Reference | Reference |
| High (>0.3 mg dl−1) | 8 | 10.3 | 1 | 1.3 | 9 | 5.8 | 8.95 (1.09, 73.69) | 5.47 (1.03, 22.85) | 0.046* |
| Indirect bilirubin high (>0.9 mg dl−1) | |||||||||
| Yes | 6 | 7.8 | 2 | 2.6 | 8 | 5.2 | 3.21 (0.63, 16.43) | 1.1.5 (0.03, 43.69) | 0.94 |
| No | 71 | 92.2 | 76 | 97.4 | 147 | 94.8 | Reference | Reference | |
| AST levels high (>37 U l−1) | |||||||||
| Yes | 65 | 83.3 | 59 | 75.6 | 124 | 79.5 | 1.61 (0.73, 3.54) | 1.55 (0.51, 4.70) | 0.44 |
| No | 13 | 16.7 | 19 | 24.4 | 32 | 20.5 | Reference | Reference | |
| ALT levels high (>45 U l−1) | |||||||||
| Yes | 7 | 9.0 | 9 | 11.7 | 16 | 10.3 | 0.75 (0.26, 2.11) | 0.72 (0.16, 3.29) | 0.67 |
| No | 71 | 91.0 | 68 | 88.3 | 139 | 89.7 | Reference | Reference | |
| Hepatitis B | |||||||||
| Positive | 11 | 14.1 | 6 | 7.7 | 17 | 10.9 | 1.94 (0.68, 5.55) | 1.93 (0.66, 5.70) | 0.23 |
| Negative | 67 | 85.9 | 72 | 92.3 | 139 | 89.1 | Reference | Reference | |
| Hepatitis C | |||||||||
| Positive | 2 | 2.6 | 0 | 0.0 | 2 | 1.3 | – | – | – |
| Negative | 76 | 97.4 | 78 | 100.0 | 154 | 98.7 | |||
| Malaria | |||||||||
| Yes | 12 | 16.0 | 7 | 9.2 | 19 | 12.6 | 1.85 (0.69, 5.00) | 1.68 (0.55, 5.70) | 0.36 |
| No | 63 | 84.0 | 69 | 90.8 | 132 | 87.4 | Reference | Reference | |
Notes: Values are adjusted for age, education and sex.
Text shown in bold is significant at p<0.05.
The sum of n for some variables may not be equal to the total n due to missing values.
Table 4.
Final linear regression model for selected clinical characteristics and aflatoxin B1 albumin (AF-ALB) adduct levels for HIV-positive patients.
| Variables | Parameter estimate | Standard error | Pr > |
|---|---|---|---|
| Intercept | 0.91553 | 0.38521 | 0.02 |
| CD4 count | 0.0042502 | 0.38521 | 0.05 |
| Viral load | 2.717581E–7 | 3.025393 E–7 | 0.37 |
| Total protein | 0.03644 | 0.03218 | 0.26 |
| Albumin | −0.16850 | 0.05979 | 0.01 |
| Total bilirubin | 0.36126 | 0.10645 | <0.01 |
| Direct bilirubin | 0.91790 | 0.20157 | <0.01 |
| Indirect bilirubin | 0.58444 | 1.52884 | 0.37 |
| ALT | 0.00144 | 0.00373 | 0.70 |
| AST | −0.00114 | 0.00120 | 0.34 |
| F-value 2.90 | |||
| Pr>F<0.0028 | |||
| R2=0.1973 | |||
| Adjusted R2=0.1292 |
Notes: Values are adjusted for age, education and sex
Text shown in bold is significant at p<0.05.
Results
Table 1 shows differences between the sociodemographic characteristics of the HIV-positive and -negative groups. The two groups did not differ by age. However, HIV-positive participants had significantly (p<0.01) higher number of total persons and children in their households and received significantly higher monthly incomes. There was no significant difference between the HIV-positive and -negative participants in terms of HBV infection and malaria parasitaemia. HIV-positive individuals were more likely to have levels of aflatoxins above the median level compared with HIV-negative persons (p=0.01). Other characteristics of the participants are shown in Table 1.
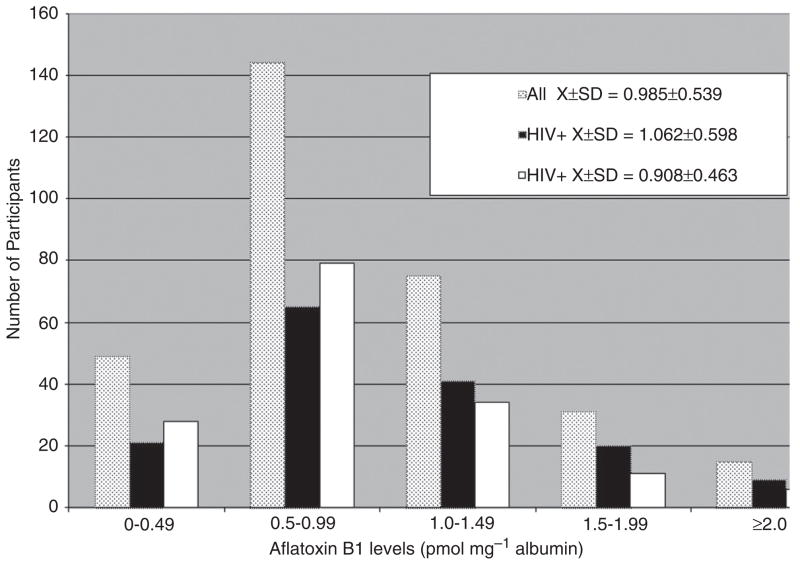
Table 2 shows that HIV-positive participants had significantly higher total protein, lower serum albumin, higher indirect bilirubin, and higher ALT and AST levels than HIV-negative participants (although indirect bilirubin and ALT were within the normal reference range for both groups). AST was above the normal range for both groups (especially the HIV-positive group) but elevated AST is not exclusive to liver damage and might reflect damage to other tissues. The mean AF-ALB level for the HIV-positive group (mean ± SD=1.06 ± 0.60 pmol mg−1 albumin) was significantly higher (p=0.01) than that of the HIV-negative group (mean ± SD=0.91 ± 0.46 pmol mg−1 albumin) (Table 2 and Figure 1). AF-ALB levels for the total group ranged from 0.00 to 3.48 pmol mg−1 albumin (mean ± SD=0.99 ± 0.54 pmol mg−1; median=0.86 pmol mg−1). For the HIV-positive group AF-ALB ranged from 0.00 to 3.48 pmol mg−1 albumin; median=0.93 pmol mg−1), and in the HIV-negative group from 0.12 to 3.00 pmol mg−1 albumin (median=0.81 pmol mg−1).
Figure 1.
Distribution of aflatoxin B1–albumin adduct (AF-ALB) levels (pmolmg−1 albumin) among the HIV-positive, HIVnegative and total study participants. AF-ALB levels for both the total study group and the HIV-positive group ranged from 0.00 to 3.48 pmolmg−1 albumin. The mean ± SD for the total and HIV-positive groups were 0.99 ± 0.54 and 1.06 ± 0.60 pmol mg−1, respectively. For the HIV-negative group AFB1 levels ranged from 0.12 to 3.00 pmol mg−1 albumin (mean ± SD=0.91 ± 0.46 pmol mg−1).
HIV-positive study participants were divided into low and high AF-ALB groups based on the group median AF-ALB level of 0.93 pmol mg−1 albumin (low AF-ALB=<0.93 pmol mg−1; high AF-ALB= ≥0.93 pmol mg−1) for bivariate and multivariable analyses. The difference in CD4+ T-cell counts between the two groups was not statistically significant by either analysis (Table 3). By bivariate analysis there was no significant association between HIV viral load and AF-ALB level (OR=1.02; 95% CI=0.54–1.95). Participants with high AF-ALB levels were more likely to have low levels of total protein though this was not statistically significant (OR=2.26; 95% CI=0.41–12.57). Also, there were non-significant increased odds of having low albumin, high AST, high total and indirect bilirubin levels, and of being positive for hepatitis B and malaria infections with higher AF-ALB levels. However, on the contrary, there was a statistically significant likelihood of having high direct bilirubin levels with high aflatoxin levels (OR=8.95; 95% CI=1.09–73.69).
In multivariate analysis, while the associations between the dependent variables maintained the direction of their relationships with AF-ALB levels as seen in the bivariate analysis, the statistically significant findings were the increased odds of having higher HIV viral loads (OR=2.84; 95% CI=1.17–7.78) and having higher direct bilirubin levels (OR=5.47; 95% CI=1.03–22.85) with higher aflatoxin levels.
When the dependent variables and AF-ALB levels were entered into linear regression models as continuous variables (Table 4), higher levels of AF-ALB were associated with lower levels of albumin (p=0.01). On the other hand, participants with higher AF-ALB levels were more likely to have higher total (p=0.01) and direct bilirubin (p=0.01) levels.
Discussion
We investigated the association between certain clinical parameters and AF-ALB levels among HIV-positive and -negative individuals. Almost all (99.4%) of the HIV-positive and all (100%) of the HIV-negative populations were positive for AF-ALB in blood. This is in agreement with a previous report of AF-ALB levels in the Ashanti Region of Ghana (Jolly et al. 2006). The results suggest that in regions of aflatoxin exposure HIV-positive people are more likely to have higher levels of AF-ALB in their blood compared with HIV-negative individuals. This observation may be connected to the impaired liver function that has been demonstrated in HIV-positive individuals (Feczko 1994). It is possible that HIV-positive individuals have a decreased ability to detoxify aflatoxin metabolites. Studies in bovine hepatocytes show that AFB1 biotransformation which results in hydroxylated and demethylated metabolites as well as AFB1 epoxides involves the cytochrome P450 enzyme system (Kuilman et al. 2000). In human hepatic cells it is thought that the cytochrome P450 isoenzymes CYPIA and CYP3A contribute to the formation of these metabolites (Kuilman et al. 2000). The high AF-ALB results are consistent with the outcome of liver injury, i.e., increased CYPs (phase I metabolism) and decreased GSTs (phase II metabolism). AF-ALB is the product of phase I metabolism mediated by CYPs. We believe that HIV-induced inflammation and liver injury may stimulate CYPs for AFB metabolic activation (phase I metabolism) and decrease GSTs, which further accumulate AFB-epoxide and an increased AF-ALB level in the blood. On the other hand, both HIV-induced malnutrition and liver injury can decrease overall albumin (made in liver) concentration, which serves as denominator for the AF-ALB assay. Even at the similar AFB dietary exposure, it is reasonable that HIV patients had higher level of AF-ALB than non-HIV patients. However, it is also possible that aflatoxin is responsible for the liver disease since aflatoxins induce injury to both hepatic parenchyma and the biliary tract (Becker 2004). Antiretrovirals (ARVs) could also play a major role in liver toxicity in HIV-positive patients on treatment (Sulkowski et al. 2000; Aceti et al. 2002; Bonacini et al. 2002) with resulting aflatoxin build up in their blood. Thirty per cent of the study participants were on ARVs.
It was not surprising to find that the HIV-positive participants had significantly higher levels of total protein, higher total and indirect bilirubin, higher ALT and AST levels and lower serum albumin levels than the HIV-negative participants (Table 2). Several antiretrovirals have been shown to be associated with elevations in liver enzymes (Bonacini et al. 2002). Indeed serum aminotransferases ALT and AST have been identified as useful markers of liver cell injury (Wu et al. 2004). Lower plasma albumin levels indicate that the synthesis function of the liver is compromised in these HIV-positive participants. Bilirubin is a specific signal of hepatic injury and elevated serum bilirubin was found in up to 5% of HIV patients treated with HAART (Bonacini 2004). Previously, Tao et al. (2005) reported a close association between AF-ALB levels and direct bilirubin levels in non-HIV infected minors, a close association of AF-ALB with albumin and other liver function tests in HIV infected minors (less than 18 years) and a close association between AF-ALB and indirect bilirubin in non-HIV-infected adults. Other authors have reported increase in plasma/serum bilirubin as a result of aflatoxin treatment in rats (Rastogi et al. 2000) and rabbits (Raval et al. 1993; Guerre et al. 1997). In the latter study increase in conjugated (direct) bilirubin was comparatively higher than increase in unconjugated (indirect) bilirubin. Higher total bilirubin levels indicate liver cell damage or bile duct damage within the liver while elevated direct or conjugated bilirubin indicate decreased bilirubin secretion from the liver or bile duct obstruction. It is likely that aflatoxin may contribute to the suboptimal liver function test results we obtained, since aflatoxins have also been shown to be independently associated with impaired liver function and hepatocellular carcinoma (Coulter et al. 1986; CDC 2004; Strosnider et al. 2006). The findings of significantly higher levels of direct bilirubin in association with high AF-ALB by logistic regression and of significantly higher levels of total and direct bilirubin by linear regression indicate that bilirubin levels are elevated with high aflatoxin exposure and that bilirubin may prove to be a useful indicator of high AF-ALB in HIV-positive people in aflatoxin prone areas. Also, the linear regression results showed an association between lower levels of albumin and high AF-ALB levels indicating that aflatoxin also compromises the synthesis function of the liver in HIV-positive people.
The most interesting finding was that of significantly higher viral load levels (almost three-fold) among HIV-positive participants with high AF-ALB levels compared with those with low AF-ALB levels by multivariate analysis. AFB1 has been reported to cause significant (five-fold) increase in the chloramphenicol acetyltransferase (CAT) reporter gene linked to the promoter sequences in the long terminal repeat (LTR) of HIV-1 (Yao et al. 1994). This increase in the rate of proviral transcription is determined by interaction between cellular transcription factors and their cognate sequences in the LTR. The mechanism by which AFB1 increased HIV-1 transcription has not been reported. TCDD (2,3,7,8-tetrachlorodibenzo-p-dioxin) has been shown to increase infectious HIV-1 titres in experimental systems (Pokrovsky et al. 1991; Tsyrlov and Pokrovsky 1993). AFB1 was more potent in increasing CAT activity than TCDD. Although the study is cross-sectional, this finding that HIV-positive participants with higher AF-ALB levels also have higher viral load is significant given that aflatoxins have previously been shown to be associated with immune suppression in numerous animal studies (Pier 1986; Pestka and Bondy 1994; Gabal and Azzam 1998; Marin et al. 2002), in Gambian children (Turner et al. 2003), and HIV-negative and positive Ghanaians (Jiang et al. 2005, 2008). Perhaps aflatoxins and HIV may act synergistically to suppress immunity and consequently lead to higher viral loads.
It is also possible that aflatoxins by their effect on the liver and in suppressing the immune system contribute to other subtle differences in the presentation of HIV/AIDS in sub-Saharan Africa and other regions of the developing world compared with developed countries where aflatoxin contamination of food crops is not as rampant. Given the significance of these findings, research is urgently needed which would employ more rigorous study designs to shed more light on the possible role of aflatoxins on liver disease and viral load in HIV-positive people. This finding of increased viral load in association with high AF-ALB levels is poignant given that with the advent of HAART, HIV is now largely a chronic disease. If aflatoxins truly act in synergy with HIV and HAART to damage liver function, the taking of HAART would only be a part measure towards a holistic management of HIV/AIDS disease. A comprehensive approach would require a multidisciplinary strategy towards managing HIV patients that involves ways to reduce their exposure to aflatoxins in meals. This would imply educating affected communities on adopting better pre-harvest and crop storage methods, providing an enterosorbent such as NovaSil (Wang et al. 2008) or drugs such as oltipraz (Wang et al. 1999) which have been demonstrated to reduce serum AF-ALB, and implementing policy changes that would improve agricultural methods and enforcement of known allowable limits for aflatoxins in food meant for human consumption.
The study has several potential limitations. The most apparent is its cross-sectional nature which prevents establishment of ‘cause and effect’ relationships. Therefore, the study is invariably a preliminary report of associations and should only be interpreted as such. The findings should lead the way for the use of more vigorous study designs to test the observed associations. Since HIV-positive individuals have a myriad of clinical conditions which have similar or overlapping pathophysiological pathways, it is difficult to extricate if observed associations are as a result of HIV infection per se, HIV treatment, or a result of other accompanying or opportunistic infections. Thirdly, since we measured only AF-ALB, due cognizance must be taken of the fact that there are other mycotoxins such as fumonisins which occur in food (Kpodo et al. 2000) that may have similar actions. The practical implications of the findings relate to the possible role aflatoxins may play in the progression of liver disease and HIV/AIDS and how aflatoxins may impact outcome of HIV treatment and management in areas where exposure to the toxin especially in food is ubiquitous.
Acknowledgments
The authors thank the study participants for making this study possible. They also thank Professor Ohene Adjei and Dr Thomas Kruppa, and other laboratory personnel at the Kumasi Center for Collaborative Research (KCCR) in Tropical Medicine, KNUST, for assistance with blood separation and shipping of samples. This research was supported by USAID grant LAG-G-00-96-90013-00 for the Peanut Collaborative Support Research Program and Minority Health International Research Training (MHIRT) grant number MD001448-09 from the National Center on Minority Health and Health Disparities, National Institutes of Health, USA.
References
- Abdelhamid AM, el-Shawaf I, el-Ayoty SA, Ali MM, Gamil T. Effect of low level of dietary afaltoxins on baladi rabbits. Arch Tierernahr. 1990;40:517–537. doi: 10.1080/17450399009421084. [DOI] [PubMed] [Google Scholar]
- Aceti A, Pasquazzi C, Zechini B, De Bac C Liverhaart Group. Hepatotoxicity development during antiretroviral therapy containing protease inhibitors in patients with HIV: the role of hepatitis B and C virus infection. Acquired Imm Defici Syn. 2002;29:41–48. doi: 10.1097/00042560-200201010-00005. [DOI] [PubMed] [Google Scholar]
- Asante AF, Asenso-Okyere K. Technical report submitted to the World Health Organization (WHO) African Regional Office (AFRO); 2003. Economic burden of malaria in Ghana. [Google Scholar]
- Becker S. Liver toxicity in epidemiological cohorts. Clin Infect Dis. 2004;38(Suppl 2):S49–S55. doi: 10.1086/381447. [DOI] [PubMed] [Google Scholar]
- Begum F, Samajpati N. Mycotoxin production on rice, pulses, and oilseeds. Naturwissenchaften. 2000;87:275–277. doi: 10.1007/s001140050720. [DOI] [PubMed] [Google Scholar]
- Bonacini M. Liver injury during highly active antirectroviral therapy: the effect of hepatitis C coinfection. Clin Infect Dis. 2004;38(S2):S104–S108. doi: 10.1086/381453. [DOI] [PubMed] [Google Scholar]
- Bonacini M, Louis S, Weisman K. Drug-induced liver disease. In: Kaplowitz N, DeLeve L, editors. Hepatotox Antiv Med. New York (NY): Marcel Dekker; 2002. pp. 519–548. [Google Scholar]
- Carvajal M, Arroyo G. Management of aflatoxin contaminated maize in Tamaulipas, Mexico. J Agri Food Chem. 1997;45:1301–1305. [Google Scholar]
- Centres for Disease Control and Prevention (CDC) 1993 revised classification system for HIV infection and expanded surveillance case definition for AIDS among adolescents and adults. MMWR. 1992 Dec 18;(RR-17) 1992/41. [PubMed] [Google Scholar]
- Centres for Disease Control and Prevention (CDC) Outbreak of aflatoxin poisoning – Eastern and Central Province, Kenya, January–July 2004. Mor Mor Weekly Rep. 2004;53(34):790–792. [PubMed] [Google Scholar]
- Coulter JBS, Suliman GI, Lamplugh SM, Mukhtar BI, Hendrickse G. Aflatoxins in liver biopsies from Sudanese children. Am J Trop Meat Hyg. 1986;35(2):360–365. doi: 10.4269/ajtmh.1986.35.360. [DOI] [PubMed] [Google Scholar]
- Cullen JM, Newborne PM. Acute hepatoxicity of aflatoxins. In: Eaton DL, Groopman JD, editors. The toxicology of aflatoxins: Human health, veterinary and agricultural significance. San Diego (CA): Academic Press; 1994. pp. 3–25. [Google Scholar]
- Eaton DL, Gallagher EP. Mechanisms of aflatoxin carcinogenesis. Ann Rev. 1994;34:135–172. doi: 10.1146/annurev.pa.34.040194.001031. [DOI] [PubMed] [Google Scholar]
- Edds GT. Acute aflatoxicosis: a review. J Am Vet Med Assoc. 1973;162:304–309. [PubMed] [Google Scholar]
- Feczko PJ. Gastrointestinal complications of human immunodeficiency virus (HIV) infection. Sem Roentgenol. 1994;29(3):275–287. doi: 10.1016/s0037-198x(05)80040-9. [DOI] [PubMed] [Google Scholar]
- Freitas VPS, Brigido BM. Occurrence of aflatoxin B1, B2, G1, and G2, in groundnuts and their products marketed in the region of Campinas, Brazil in 1995 and 1996. Food Addit Contam. 1980;15:807–811. doi: 10.1080/02652039809374714. [DOI] [PubMed] [Google Scholar]
- Gabal MA, Azzam AH. Interaction of aflatoxin in the feed and immunization against selected infectious diseases in poultry. II. Effect on one-day-old layer chicks simultaneously vaccinated against Newcastle disease, infectious bronchitis and infectious bursal disease. Avian Pathol. 1998;27:290–295. doi: 10.1080/03079459808419338. [DOI] [PubMed] [Google Scholar]
- Gourama H, Bullerman LB. Aspergillus flavus and Aspergillus parasiticus, aflatoxigenic fungi of concern in foods and feed – a review. J Food Prot. 1995;58:1395–1404. doi: 10.4315/0362-028X-58.12.1395. [DOI] [PubMed] [Google Scholar]
- Guengerich FP, Shimada T. Oxidation of toxic and carcinogenic chemicals by human cytochrome P-450 enzymes. Chem Res Toxicol. 1991;4:391–407. doi: 10.1021/tx00022a001. [DOI] [PubMed] [Google Scholar]
- Guerre P, Burga V, Galtier P. Dose-related increase in liver heme catabolism during rabbit aflatoxicosis. Toxicol Lett. 1997;92(2):101–108. doi: 10.1016/s0378-4274(97)00043-x. [DOI] [PubMed] [Google Scholar]
- Harvey RB, Kubena LF, Elissalde MH. Influence of vitamin E on aflatoxicosis in growing swine. Am J Vet Res. 1994;55:572–577. [PubMed] [Google Scholar]
- Hosmer D, Lemshow S. Wiley series in probability and statistics. 2. New York (NY): Wiley and Sons; 2000. Applied logistic regression; pp. 1–134. [Google Scholar]
- Jiang Y, Jolly PE, Ellis WO, Wang JS, Phillips TD, Williams JH. Aflatoxin B1 albumin adduct levels and cellular immune status in Ghanaians. Int Immunol. 2005;17:807–814. doi: 10.1093/intimm/dxh262. [DOI] [PubMed] [Google Scholar]
- Jiang Y, Jolly PE, Preko P, Baidoo J, Wang J-S, Ellis WO, Williams JH. Aflatoxin related immune dysfunction in Health and in Human immunodeficiency virus diseases[Internet] Clin Develop Immunol. 2008 doi: 10.1155/2008/790309. Available from: http://www.hindawi.com/ [DOI] [PMC free article] [PubMed]
- Joint FAO/WHO Expert Committee on Food Additives (JECFA) WHO Food Additive Series no 40. Geneva (Switzerland): WHO; 1998. Aflatoxins: Safety evaluation of certain food additives and contaminants. The Forty-ninth Meeting of the Joint FAO/WHO Expert Committee on Food Additives; pp. 359–468. [Google Scholar]
- Jolly PE. Replicative characteristics of primary isolates of the human immunodeficiency virus type 1 in peripheral blood mononuclear cells, primary macrophages and CD4+ transformed T-cell lines. Cell Mol Biol. 1997;43:1057–1065. [PubMed] [Google Scholar]
- Jolly PE, Jiang Y, Ellis WO, Appawu J, Awuah RT, Nnedu O, Adjei O, Stiles J, Person S, Jolly CM. Association between aflatoxin levels, health characteristics, liver function, hepatitis and malaria infections in Ghanaians. J Nutr Environ Med. 2007;16:1–16. [Google Scholar]
- Jolly PE, Jiang Y, Ellis WO, Awuah RT, Nnedu O, Wang J, Phillips T, Afiyie-Gyawu E, Person S, Jolly CM. Determinants of aflatoxin levels in Ghanaians: factors, knowledge of aflatoxin and food handling and consumption practices. Int J Hyg Environ Hlth. 2006;209:345–358. doi: 10.1016/j.ijheh.2006.02.002. [DOI] [PubMed] [Google Scholar]
- Kpodo K, Thrane U, Hald B. Fusaria and fumonisins in maize from Ghana and their co-occurrence with aflatoxins. Int J Food Microbiol. 2000;61:147–157. doi: 10.1016/s0168-1605(00)00370-6. [DOI] [PubMed] [Google Scholar]
- Kuilman MEM, Maas RFM, Fink-Gremmels J. Cytochrome P450-mediated metabolism and cytotoxicity of aflatoxin B1 in bovine hepatocytes. Toxicol In Vitro. 2000;14(4):321–327. doi: 10.1016/s0887-2333(00)00025-4. [DOI] [PubMed] [Google Scholar]
- Marin DE, Taranu I, Bunaciu RP, Pascale F, Tudor DS, Avram N, Sarca M, Cureu I, Criste RD, Suta V, et al. Changes in performance, blood parameters, humoral and cellular immune responses in weanling piglets exposed to low doses of aflatoxin. J Anim Sci. 2002;80:1250–1257. doi: 10.2527/2002.8051250x. [DOI] [PubMed] [Google Scholar]
- Martin-Blonde G, Soumah M, Carmara B, Chabrol A, Porte L, Delobel P, Cruzin L, Berry A, Massip P, Marchou B. Impact of malaria on HIV infection. Med Mal Infect Pub Med. 2010;40(5):256–267. doi: 10.1016/j.medmal.2009.10.008. [DOI] [PubMed] [Google Scholar]
- Omer RE, Bakker MI, Veer PV, Hoogenboom RLAP, Polman THG, Alink GM, Idris MO, Kadaru MY, Kok FJ. Aflatoxin and liver cancer in Sudan. Nutr Can. 1998;32:174–180. doi: 10.1080/01635589809514737. [DOI] [PubMed] [Google Scholar]
- Park JW, Kim EK, Shon DH, Kim YB. Natural co-occurrence of aflatoxin B1, fumonsin B1, and ochratoxin A in barley and maize foods from Korea. Food Addit Contam. 2002;19(11):1073–1080. doi: 10.1080/02652030210151840. [DOI] [PubMed] [Google Scholar]
- Peers F, Bosch X, Kaldor J, Linsell A, Pluumen M. Aflatoxin exposure, hepatitis B virus infection and liver cancer in Swaziland. Int J Canc. 1987;39:545–553. doi: 10.1002/ijc.2910390502. [DOI] [PubMed] [Google Scholar]
- Pestka JJ, Bondy GS. Mycotoxin-induced immunomodulation. In: Dean JH, Luster MI, Munson AE, Kimber I, editors. Immunotoxicology and immunopharmacology. New York (NY): Raven Press; 1994. pp. 163–182. [Google Scholar]
- Pier AC. Immunologic changes associated with mycotoxicoses. 13. Immunomodulation in aflotoxicosis. In: Richard JL, Thurston JR, editors. Diagnosis of mycotoxicosis. Boston (MA): Martinus Nijhoff; 1986. pp. 143–148. [Google Scholar]
- Pokrovsky AG, Cherykh AI, Yastrebova ON, Tsyrlov IB. 2,3,7,8-Terrachlorodibenzo-p-dioxin as a possible activator of HIV infection. Biochem Biophys Res Commun. 1991;179:46–51. doi: 10.1016/0006-291x(91)91331-6. [DOI] [PubMed] [Google Scholar]
- Rastogi R, Srivastava AK, Rastogi AK. Long term effect of aflatoxin B1 on lipid peroxidation in rat liver and kidney: effect of picroliv and silymarin. Phytother Res. 2000;15:307–310. doi: 10.1002/ptr.722. [DOI] [PubMed] [Google Scholar]
- Raval PJ, Verma RJ, Mehta DN. Alterations in bilirubin concentrations during induced aflatoxicosis in rabbits. Bull Environ Contam Toxicol. 1993;53:389–393. doi: 10.1007/BF00201757. [DOI] [PubMed] [Google Scholar]
- Rinaldo C, Huang X, Fan Z, Ding M, Beltz L, Logar A, Panicali D, Mazzara G, Liebmann J, Cottrill M, et al. High levels of anti-human immunodeficiency virus type 1 (HIV-1) memory cytotoxic T-lymphocyte activity and low viral load are associated with lack of disease in HIV-1-infected long-term nonprogressors. J Virol. 1995;69:5838–5842. doi: 10.1128/jvi.69.9.5838-5842.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roebuck BD, Maxuitenko YY. Biochemical mechanisms and biological implications of the toxicity of aflatoxins as related to aflatoxin carcinogenesis. In: Eaton DL, Groopman JD, editors. The toxicology of aflatoxins: Human health, veterinary and agricultural significance. San Diego (CA): Academic Press; 1994. pp. 27–43. [Google Scholar]
- Shane SM. Economic issues associated with aflatoxins. In: Eaton DL, Groopman JD, editors. The toxicology of aflatoxins: human health, veterinary, and agricultural significance. London: Academic Press; 1993. pp. 513–527. [Google Scholar]
- Soriano V, Puoti M, Garcia-Gascó P, Rockstroh JK, Benhamou Y, Barreiro P, McGovern B. Antiretroviral drugs and liver injury. AIDS. 2008;22(1):1–13. doi: 10.1097/QAD.0b013e3282f0e2fd. [DOI] [PubMed] [Google Scholar]
- Strosnider H, Azziz-Baumgartner E, Banziger M, Bhat RV, Breiman R, Brune MN, DeCock K, Dilley A, Groopman J, Hell K, et al. Workgroup Report: Public health strategies for reducing aflatoxin exposure in developing countries. Environ Hlth Perspect. 2006;114(12):1898–1903. doi: 10.1289/ehp.9302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sulkowski MD, Thomas DL, Chaisson RE, Moore RD. Hepatotoxicity associated with antiretroviral therapy in adults infected with human immunodeficiency virus and the role of Hepatitis C or B virus infection. J Am Med Assoc. 2000;283(1):74–80. doi: 10.1001/jama.283.1.74. [DOI] [PubMed] [Google Scholar]
- Tao P, Zhi-Ming L, Tang-Wei L, Le-Qun L, Ming-Hao P, Xue Q, Lu-Nam Y, Ren-Xiang L, Zong-Liang W, Lian-Wen W, et al. Associated factors moduating aflatoxin B1-albumin adduct level in three Chinese populations. Digest Dis Sci. 2005;50(3):525–532. doi: 10.1007/s10620-005-2468-1. [DOI] [PubMed] [Google Scholar]
- Tsyrlov IB, Pokrovsky A. Stimulatory effect of the CYP1A1 inducer, 2,3,7,8-terrachlorodibenzo-p-dioxin on the reproduction of HIV-1 in human lymphoid cell culture. Xenobiotica. 1993;23:457–467. doi: 10.3109/00498259309057034. [DOI] [PubMed] [Google Scholar]
- Turner PC, Collinson AC, Cheung BY, Gong YY, Hall AJ, Prentice AM, Wild CP. Aflatoxin exposure in utero causes growth in Gambian infants. Int J Epidemiol. 2007;36(5):1119–1125. doi: 10.1093/ije/dym122. [DOI] [PubMed] [Google Scholar]
- Turner PC, Moore SE, Hall AJ, Prentice AM, Wild CP. Modification of immune function through exposure to dietary aflatoxin in Gambian children. Environ Health Perspect. 2003;111(2):217–220. doi: 10.1289/ehp.5753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- UNAIDS. Chapter 3: Progress in countries. Geneva (Switzerland): UNAIDS; 2006. Report on the global AIDS epidemic. [Google Scholar]
- University of Alabama Hospital Laboratories Bulletin of Information, Revised October 2002.
- Wang JS, Qian GS, Zarba A, He X, Zhu YR, Zhang BC, Jacobson L, Gange SJ, Munoz A, Kensler TW, et al. Temporal patterns of aflatoxin–albumin adducts in hepatitis B surface antigen-positive and antigen-negative residents of Daxin, Qidong County, People’s Republic of China. Canc Epidemiol Biomark Prevent. 1996;5:253–261. [PubMed] [Google Scholar]
- Wang J-S, Shen X, He X, Zhu Y-R, Zhang B-C, Wang J-B, Qian G-S, Kuang S-Y, Zarba A, Egner PA, et al. Protective alterations in Phase 1 and 2 metabolism of aflatoxin B1 by oltipraz in residents of Qidong, People’s Republic of China. J Natl Canc Inst. 1999;91:347–354. doi: 10.1093/jnci/91.4.347. [DOI] [PubMed] [Google Scholar]
- Wang P, Afriyie-Gyawu E, Tang Y, Johnson N, Xu L, Tang L, Heubner HJ, Ankrah N-A, Ofori-Adjei D, Ellis WO, et al. NovaSil clay intervention in Ghanaians at high risk for aflatoxicosis: II Reduction in biomarkers of aflatoxin exposure in blood and urine. Food Addit Contam. 2008;25(5):622–634. doi: 10.1080/02652030701598694. [DOI] [PubMed] [Google Scholar]
- WHO. HIV/AIDS Antiretroviral Newsletter no 4. World Health Organization; 2000. Clinical and laboratory monitoring of antiretroviral therapy in resource-limited and unlimited settings. [Google Scholar]
- WHO/UNAIDS/UNICEF. Towards universal access: scaling up priority HIV/AIDS interventions in the health sector. Geneva (Switzerland): WHO/UNAIDS/UNICEF; 2009. [Google Scholar]
- Wild CP, Shrestha SM, Anwar WA, Montesano R. Field studies of aflatoxin exposure, metabolism and induction of genetic alterations in relation to HBV infection and hepatocellular carcinoma in the Gambia and Thailand. Toxicol Lett. 1992;64–65:455–461. doi: 10.1016/0378-4274(92)90219-a. [DOI] [PubMed] [Google Scholar]
- Williams JH, Phillips TD, Jolly P, Stiles JK, Jolly CM, Aggarwal D. Human aflatoxicosis in developing countries: a review of exposure, toxicology, potential consequences and interventions. Am J Clin Nutr. 2004;80:1106–1124. doi: 10.1093/ajcn/80.5.1106. [DOI] [PubMed] [Google Scholar]
- Wu K-L, Lu S-N, Changchien CS, Chiu KW, Kuo CH, Chuah SK, Liu JW, Lin MC, Eng HL, Chen SS, et al. Sequential changes of serum aminotransferase levels in patients with severe acute respiratory syndrome. Am J Trop Med Hyg. 2004;71(2):125–128. [PubMed] [Google Scholar]
- Yao Y, Hoffer A, Chang C, Puga A. Dioxin activates HIV-1 gene expression by an oxidative stress pathway requiring a functional cytochrome P450 CYP1A1 enzyme. Environ Hlth Perspect. 1994;103:366–371. doi: 10.1289/ehp.95103366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou T, Evans AA, London WT, Xia X, Zou H, Shen F, Clapper ML. Glutathione S-transferase expression in hepatitis B virus-associated human hepatocellular carcinogens. Canc Res. 1997;57:2749–2753. [PubMed] [Google Scholar]