Abstract
Optimizing cell-material interactions is critical for maximizing regeneration in tissue engineering. Combinatorial and high-throughput (CHT) methods can be used to systematically screen tissue scaffolds to identify optimal biomaterial properties. Previous CHT platforms in tissue engineering have involved a two-dimensional (2D) cell culture format where cells were cultured on material surfaces. However, these platforms are inadequate to predict cellular response in a three-dimensional (3D) tissue scaffold. We have developed a simple CHT platform to screen cell-material interactions in 3D culture format that can be applied to screen hydrogel scaffolds. Herein we provide detailed instructions on a method to prepare gradients in elastic modulus of photopolymerizable hydrogels.
Keywords: Combinatorial methods, high-throughput screening, hydrogels, elastic modulus, gradients, osteoblast, tissue engineering
1. INTRODUCTION
Billions of dollars have been invested in tissue engineering research over the past decade and yet the industry is only starting to become profitable [1] underscoring the need to accelerate the pace of research in this field. Towards this goal, combinatorial and high-throughput (CHT) methods successfully utilized by the pharmaceutical industry for drug discovery [2] are being adapted in tissue engineering research [3]. CHT platforms can be used to systematically screen cell-material interactions and identify optimal scaffold properties to maximize desired tissue regeneration.
Several CHT methods have been developed to screen cell response on biomaterials for tissue engineering [3]. Composition and temperature gradient techniques were used to screen cell response on surfaces of poly(D,L-lactide) and poly(ε-caprolactone) blends [4]. A nanoliter assay was developed to screen libraries of polymer compositions to support human embryonic stem cells [5]. A microarray platform was used to screen response of rat hepatocytes and mouse embryonic cells on arrays of different combinations of extracellular matrix proteins [6]. Gradients of surface-immobilized peptides have been generated to study cell adhesion [7] and cell alignment [8]. Orthogonal gradients of topography and chemical composition were used to study the effect of cell-surface interactions [9]. An orthogonal approach was used to screen cellular response to dimethacrylate polymers of different properties [10]. These platforms typically involved two-dimensional (2D) cell-culture format where cells were cultured on the surface of a biomaterial often presented in the form of a thin specimen, as discrete arrays or continuous gradients.
However, most tissues and organs in the human body have a complex three-dimensional (3D) architecture and require that the biomaterial be processed as a 3D scaffold. Cells are very responsive to differences in topography between flat 2D systems and 3D scaffold systems. Moreover, there is growing evidence that cells cultured in 3D formats behave more physiologically than those cultured on a surface (2D) [11]. Therefore, an unmet need for novel CHT methods to enable rapid screening of 3D tissue scaffolds exists. A CHT technique has been developed to screen 3D porous salt-leached scaffolds [12, 13]. In this paper we provide detailed procedure to design a CHT platform to screen cell response to hydrogel scaffolds in a 3D format. This platform is technically simple, is inexpensive to assemble and use, and only requires commonly-available laboratory equipment. In recent work, we successfully utilized this platform to study the effect of scaffold stiffness (compressive modulus) on encapsulated osteoblasts towards tissue engineering of bone [14].
Poly(ethylene glycol) (PEG)-based hydrogels are commonly used as tissue scaffolds due to their high water content and bioinertness [15]. Moreover, PEG hydrogels can also be modified to present, individually or in combination, a wide variety of chemical, biological and physical cues to direct biological response [15]. Photopolymerized PEG gels are typically prepared from acrylate-functionalized PEG molecules. A simple one-step microwave-assisted reaction can be used to prepare poly(ethylene glycol) dimethacrylate (PEGDM) from PEG [16]. Photopolymerizable PEG gels are injectable and may be used for tissue regeneration in situ [17]. We have developed a methodology to fabricate hydrogels with gradients in stiffness.
Biomaterial mechanical properties have been shown to have a profound effect on a number of cellular responses and functions, such as morphology, migration, proliferation and differentiation [18–20]. It is widely believed that cell response is most physiological when the elastic modulus of the biomaterial matches that of the tissue in vivo [19, 20]. Thus, optimizing the modulus of the 3D tissue scaffold will be critical in maximizing regeneration in tissue engineering [14, 21, 22] and we describe a method to systematically and rapidly screen cell response to 3D scaffold modulus.
2. MATERIALS AND METHODS
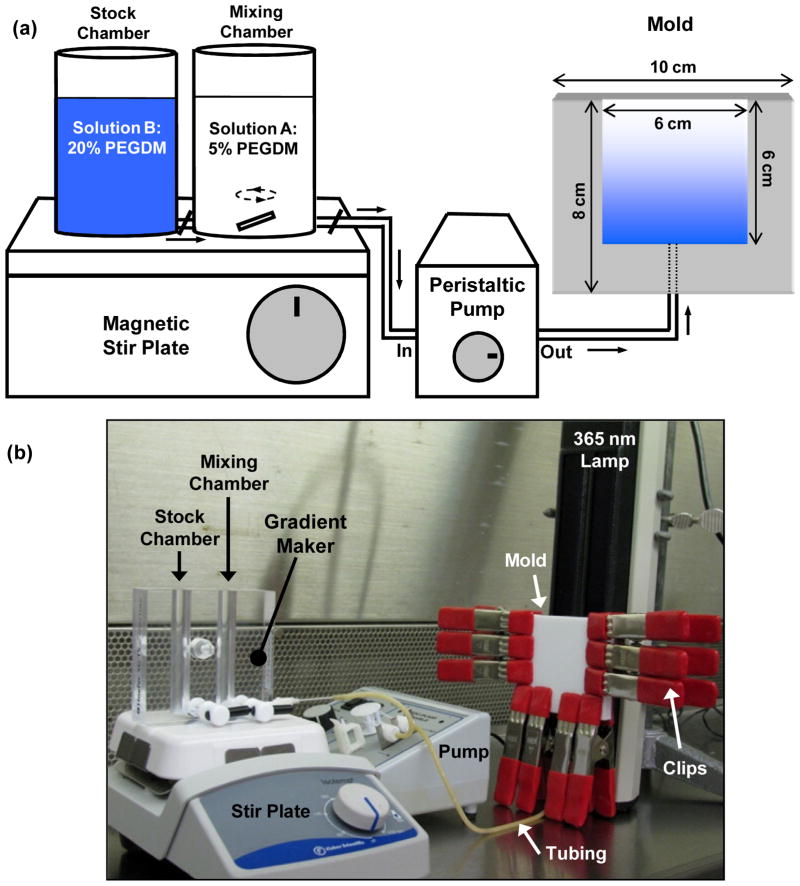
The CHT platform described here consists of four major components: a gradient maker, a peristaltic pump, a mold to cast the gradient and a 365 nm lamp (Fig. 1a, b). In this section, we describe preparation of PEGDM from PEG followed by preparation and characterization of a hydrogel with a gradient in modulus. We also describe detailed procedures for characterizing biological response of cells encapsulated in the hydrogels.
Fig. 1.
(a) Schematic illustration of the combinatorial platform used to prepare hydrogel gradient scaffolds. The output of the gradient maker is pumped in to a single-entry bottom-filling vertical mold, wherein it is cured using a 365 nm lamp. (b) Photograph of the sterilized equipment arranged in a cell culture hood for aseptic gradient fabrication.
2.1. Materials
Poly(ethylene glycol) (PEG, mass average molecular mass 4000, Sigma-Aldrich); Methacrylic anhydride (Sigma-Aldrich); Methanol (Sigma-Aldrich); Ether (Sigma-Aldrich); Consumer microwave (GE 1100 W); Gradient maker (Hoefer SG-15 Amersham Biosciences); Two needles (2.1 mm outer and 1.6 mm inner diameters, respectively, 3.8 cm long, blunt end, with Luer hub; Small Parts, Inc.); Poly(vinyl chloride) (PVC) tubing (1.4 mm inner and 3.4 mm outer diameters, respectively); Two Teflon sheets (10 cm × 8 cm × 3 mm and 10 cm × 8 cm × 5 mm); Glass slide (10 cm × 8 cm × 1 mm, Amersham Biosciences); High pressure clips (Lowes retail store); Luer connector (Sigma-Aldrich); Peristaltic pump (MMP-100, C.B.S. Scientific); Irgacure 2959 (1-[4-(2-hydroxyethoxy)-phenyl]-2-methyl-1-propane-1-one, Ciba Chemicals); Phosphate-buffered saline (PBS, 0.1 M, Invitrogen); Lamp (UVL-28EL series 8W, UVP); Razor blades; 4-well plates (Fischer Scientific); Trypan blue (Sigma-Aldrich); Plate reader (Spectra Max, Molecular Devices); Ethylene oxide sterilizer (Anprolene AN74i, Anderson Products); 0.22 μm syringe filter (Millipore); EnduraTEC compression tester (Bose); Live/dead stain (Invitrogen); Wst-1 assay kit (Dojindo); Picogreen dsDNA Quantitation Kit (Molecular Probes); Glass dounce tissue homogenizer (Kontes Glass Company); Sodium dodecyl sulfate (SDS, Sigma-Aldrich); Proteinase K (Sigma-Aldrich); Alkaline Phosphatase Detection Reagent (Stanbio Laboratory); Nonidet P-40 detergent solution [nonylphenol poly(ethylene glycol) ether, Roche Diagnostics]; Control human serum for alkaline phosphatase assay (Stanbio Laboratory); Alizarin red S (Sigma-Aldrich); Formalin (10%, neutral buffered, Sigma-Aldrich).
2.2. Preparation and Characterization of Poly(Ethylene Glycol) Dimethacrylate
In a 20 mL glass scintillation vial, react 2 g of PEG with 0.75 mL methacrylic anhydride (≈10X molar excess) in a consumer microwave at maximum power (GE, 1100 W) for a total of 5 min.
To prevent overheating, heat in 5 intervals of 1 min each interspersed with 1 min cooling periods by simply placing the vial outside the microwave at room temperature.
Unreacted methacrylic anhydride must be removed because it increases acidity (and toxicity) of the pre-polymer solution used for photopolymerization during cell encapsulation (described below). To remove unreacted methacrylic anhydride, dissolve the cooled solid (2g) in a minimum volume of methanol (≈ 4 mL).
Precipitate PEGDM in a large excess (≈500 mL) of cold ether (−20°C).
Collect by vacuum filtration.
2.3. Assembling the Combinatorial Platform
The choice of gradient maker is dictated primarily by the maximum and the minimum volumes required for use and the one described herein works for the volumes described below. A gradient maker consists of two interconnected vertical chambers referred to as the mixing and the stock chamber containing solutions A and B, respectively (Fig. 1a). The underlying principle of gradient maker procedure is that as solution A from the mixing chamber is drawn out, solution B from the stock chamber fills in to the mixing chamber and is mixed using a magnetic stir bar. Therefore, the effluent from the mixing chamber progressively becomes leaner in solution A and richer in solution B.
Assemble the gradient maker by closing the valves and connecting a needle (with Luer hub) to the gradient maker (Fig. 1c).
Place a magnetic stir bar in the mixing chamber and place on a magnetic stir plate (Fig. 1b).
Use PVC tubing to connect the gradient maker to the inlet of a peristaltic pump (Fig. 1b).
2.4. Designing the Mold
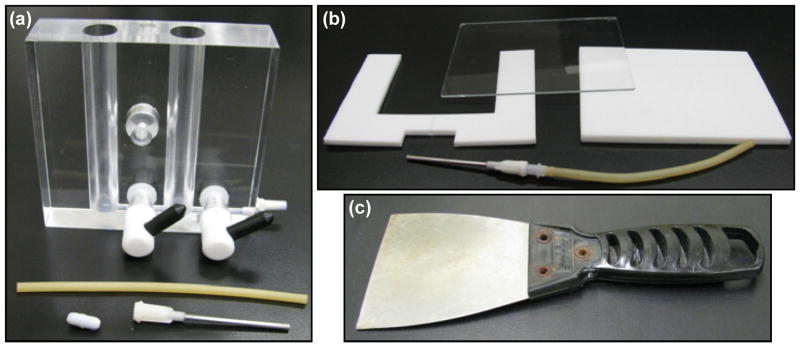
The design of the mold is critical. The mold is prepared from Teflon sheets and a glass slide (Fig. 2b). The glass slide is required for photopolymerization of the cast solution to yield a gel. The mold design is optimized in part by the maximum and minimum allowable volumes of the gradient maker and the width of the lamp window that would ensure uniform curing of the gradient gel.
Fig. 2.
Disassembled parts and equipment for making hydrogel scaffold gradients. (a) Photograph of the gradient maker is shown with a needle, tubing and a magnetic stir bar that is added to the mixing chamber. (b) Disassembled mold showing a Teflon sheet machined to create a mold that is clipped between a glass slide and a Teflon block. A needle is inserted through the drilled port at the bottom of the mold. (c) A wide spackling knife (8 cm wide) is useful to handle the gradient samples.
Machine a Teflon block (10 cm × 8 cm × 3 mm) using a router to generate a cavity of 6 cm × 6 cm × 3 mm, as shown in Fig. (1a).
Drill a port of 2.1 mm diameter to insert a needle at the bottom of the Teflon sheet for entry of the liquid into the mold (Fig. 1a).
Assemble the mold by clipping (red clips are shown in Fig. 1b) the machined Teflon sheet (with cavity) between a solid Teflon block (10 cm × 8 cm × 5 mm, this one is thicker to give support) and a glass slide (10 cm × 8 cm × 1 mm).
Set the mold vertically such that the solution from the gradient maker fills in from the bottom (Fig. 1b).
Insert a needle (with Luer hub) connected to a PVC tube via a Luer connector at the bottom port of the vertical mold.
Connect the other end of the PVC tube to the outlet of the pump.
Note: Two glass slides can be used as the supporting walls instead of combination of a glass slide and the Teflon. However, we observed that the cured PEGDM gel strongly adheres to glass making it difficult to disassemble the mold without tearing the gels if two glass slides are used (the gels easily slide off Teflon).
2.5. Fabrication of Hydrogel Gradients
Assemble the fabrication platform by connecting the gradient maker, the peristaltic pump and the mold as described above (Fig. 1b).
Set the speed of the peristaltic pump to 1 mL/min.
-
Prepare two pre-polymer solutions as follows for the two chambers of the gradient maker as follows:
For the stock chamber: Dissolve 20% by mass of PEGDM and 0.05% by mass of Irgacure 2959 in 0.1 mmol/L PBS.
For the mixing chamber: Dissolve 5% by mass of PEGDM and 0.05% by mass of Irgacure 2959 in PBS.
Add 5.8 mL of 20% PEGDM solution to the stock chamber.
Open the stopper just enough to remove any air bubbles between the two chambers. Plug the connector again.
Add 5.8 mL of the 5% PEGDM solution to the mixing chamber.
Switch on the magnetic stir plate.
Open both the stops on the gradient maker and start the peristaltic pump.
Stop the pump after the solution has completely filled the mold (≈ 15 min).
Without disturbing the mold, place the UV lamp vertically along the glass side of mold (Fig. 1b). The lamp can be clamped to a ring stand for support as shown in Fig. (1b).
Cure the solution at 2 mW/cm2 for 15 min.
Unclip the mold. The cured gel slab tends come easily off the Teflon and stick to the glass slide.
Using a sharp razor blade, cut the gels along the direction of the gradient to yield six samples of 6 cm × 1 cm × 3 mm (Fig. 3c). Adding a few drops of PBS will facilitate removal of the gels from the glass slide.
Transfer the gels to rectangular 4-well plates with 8 mL of water (or culture medium for cell-containing samples). A spackling knife with a wide blade (> 6 cm) that can fully support the length of the gel is recommended as the sample may break during handling (Fig. 2c).
Fig. 3.
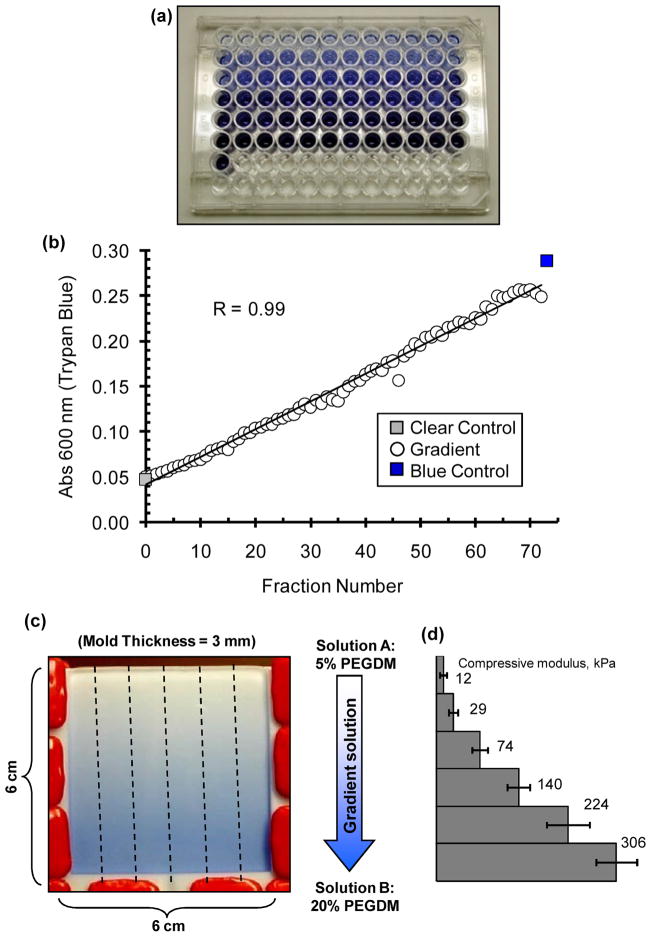
Trypan blue can be added to the stock chamber solution in the gradient maker to visualize and confirm gradient formation. (a) A few drops of Trypan blue (0.01% by mass) was added to the 20% PEGDM pre-polymer solution in the stock chamber (with 5% PEGDM in the mixing chamber). In order to check linearity of the gradient maker effluent, the effluent was collected drop-wise in a 96-well plate. (b) Plot of absorbance at 600 nm measured for the different fractions collected in the 96-well plate in (a). (c) Image of the mold, as observed through the glass slide, filled with the output of the gradient maker wherein. Dashed lines indicate how the cured slab of gel (6 cm × 6 cm × 3 mm) is cut with a razor into six gradient scaffolds of 6 cm × 1 cm × 3 mm each. (d) Plot of compressive elastic moduli measured at different positions along the gradient hydrogel.
2.6. Testing the Gradient Maker
It is advisable to test the effluent of the gradient maker to verify that the output from the gradient maker generates the desired profile. This helps to troubleshoot inconsistent gradients formation, in particular poor mixing in the gradient maker and poor mold design. A soluble dye, such as Trypan blue is suitable for this purpose.
Assemble the fabrication platform by connecting the gradient maker and the peristaltic pump. Do not connect the tube from the outlet of the pump to the mold.
Add pre-polymer solutions described in Sec 3.4 (20% PEGDM by mass in stock chamber and 5% PEGDM by mass in mixing chamber) with the addition of two drops of Trypan blue (0.01% by mass) to the solution B in the stock chamber.
Collect the effluent in a 96-well plate with 2 drops in each well.
Measure absorbance (600 nm) of the solution using a plate reader to generate a composition profile for the different fractions.
In Fig. (3), we present results from an experiment where Trypan blue was added to the solution in the stock chamber. Plot of the effluent is linear in composition and compares favorably with the composition of the solutions added in the two chambers of the gradient maker (Fig. 3b). If the effluent of the gradient maker is found to be sufficiently linear, the design of the mold can be tested using a similar approach. Fill the mold as described above with solution B (in the stock chamber) containing Trypan blue. The Trypan blue gradient in should be visible through the glass slide of the mold, as shown Fig. (3c). It is important to note that the solution in the stock chamber must have a higher density than the solution in the mixing chamber in order to fabricate a gradient when the solution is pumped into the mold. Therefore, the higher density 20% PEGDM solution must be added to the stock chamber and the lower density 5% PEGDM must be added to the mixing chamber. See Table 1 for a list of common problems for making gradients and how to troubleshoot them.
Table 1.
Trouble-Shooting Common Problems
| Problem | Explanation | Potential Solutions |
|---|---|---|
| Poor gradient formation |
|
|
| Difficult to remove the gel from the mold |
|
|
| Gels do not cure |
|
|
2.7. Cell Encapsulation in Gradient Hydrogels
To encapsulate cells in hydrogels, follow the procedure described above except follow aseptic cell-culture technique in a laminar flow hood. All equipment must be sterilized in ethylene oxide (Anprolene AN74i, Anderson Products) before use including everything shown in Fig. (2), the stir plate, the peristaltic pump and the red clips for the mold. Sterilize the pre-polymer solutions with a 0.22 μm syringe filter and then suspend the cells in the solutions. In recent work we encapsulated MC3T3-E1 cells in gradient hydrogels [14]. Cells are expanded in standard culture flasks in growth media (α-modification of minimum essential medium containing 10 volume% fetal bovine serum, FBS and 0.06% kanamycin sulfate) prior to encapsulation using standard culture techniques. Cells in the solutions should then be loaded into the gradient maker to make the gradient hydrogel libraries as described above. After curing gels with cells, they can be cut into strips (as shown in Fig. 3c) and cultured in rectangular 4-well plates for various time points.
2.8. Mechanical Characterization of Gradient Hydrogels
This section describes the procedure to measure the elastic modulus and the swelling ratio at the different positions along the gradient. Previously we found that gradient gels prepared as described above spanned a 30-fold range in compressive modulus from ≈ 10 kPa to ≈ 300 kPa [14].
Incubate the hydrogels in PBS for at least 1 d to attain equilibrium swelling prior to measuring elastic modulus.
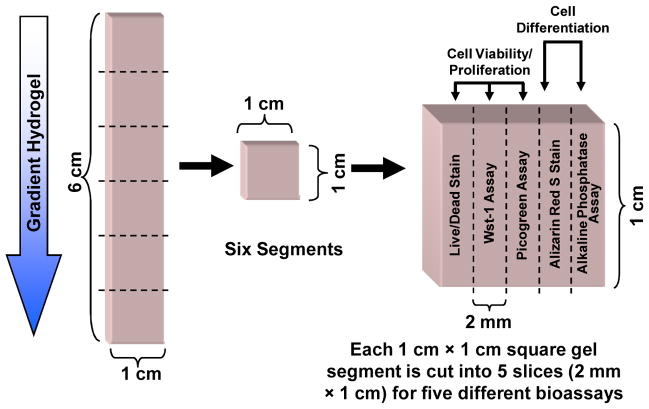
Using a sharp razor, cut each gradient into six segments (1 cm × 1 cm × 3 mm) along the direction of the gradient (Fig. 4).
Punch a circular disk of 8 mm diameter from each segment.
Measure the mass on a balance.
Subject the gel disk to unconfined uniaxial compressive load at constant strain rate of 0.01 mm/s at room temperature (EnduraTEC).
The load-displacement data can be analyzed to generate a stress-strain plot. The slope of the linear fit to a low strain (< 5%) is a measure of the compressive elastic modulus of the gel.
Dry the gel under vacuum to remove water. Measure the mass of the dried gel.
Swelling ratio is calculated as the ratio of the masses of the hydrated and the dried gels.
Fig. 4.
Experimental design for mechanical and biological characterization of gradient samples. Each gradient sample (6 cm × 1 cm × 3 mm) is cut in to six segments (1 cm × 1 cm × 3 mm). To assay the cell state in each gel segment, the segments are cut into five equal sections (1 cm × 2 mm × 3 mm) for use in the five assays indicated.
2.9. Measurement of the Cellular Response in the Gradients
We have cultured MC3T3-E1 murine osteoblasts (2.5 × 106 cells/mL, Riken Cell Bank) in the hydrogel gradients and used the following assays to characterize their response to modulus differences [14]. A number of commercially-available assays and stains can be adapted to measure cell viability/proliferation and cell differentiation. Here we describe a scheme and associated procedures for five bioassays: three to determine cell viability/number and two to evaluate osteogenic differentiation. We also discuss X-ray microcomputed tomography for 3D quantification of mineral deposition.
Using a sharp razor cut each gradient into six segments (1 cm × 1 cm × 3 mm) along the direction of the gradient (Fig. 4), as in Sec 3.4.
Each segment is further cut in to five smaller sections (1 cm × 2 mm × 3 mm) parallel to the direction of the gradient (Fig. 4). Each gel section can then be used for up to five bioassays as described below.
Viability staining
A commercially-available vitality stains are used to measure cell viability (Fig. 5a, b). The live/dead stain is a vital fluorescence double-stain based on membrane integrity and intracellular esterase activity and is used for a semi-quantitative assessment of viability.
Fig. 5.
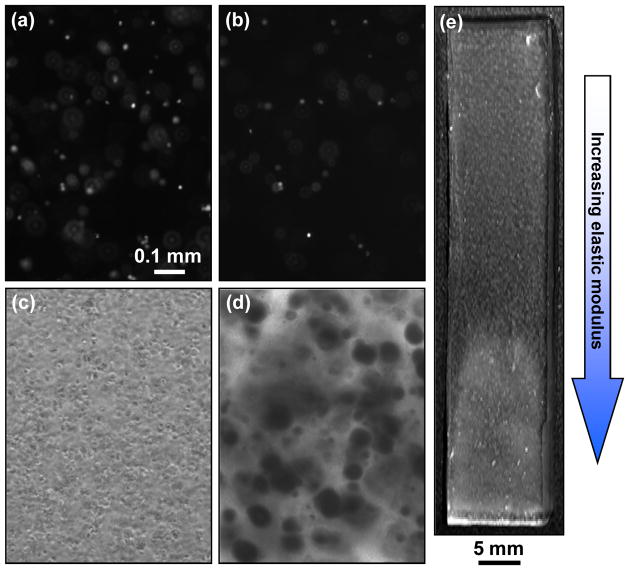
Images of cell response after encapsulation within hydrogel gradients. (a, b) Epi-fluorescence images of cells stained with the live (a) and the dead (b) stains at the soft end (≈ 10 kPa) of the gradient 1 d after encapsulation. (c) Phase contrast images of cells in soft end (≈ 10 kPa) of hydrogel gradients after 1 d. Spherical objects are cells. (d) Phase contrast micrographs of mineral deposits at the stiff end (≈ 300 kPa) of the gradient stained with Alizarin red S after 42 d. Dark spots are indicative of mineral deposits. Scale bar in (a) applies to (a–d) and equals 0.1 mm. (e) Photograph of gradient hydrogel after 42 d culture. Note that the hydrogel appears white at the stiff end (high modulus) due to the presence of large amounts of mineral deposits. The mineralization occurs in normal medium without osteogenic differentiation supplements.
Incubate gel sections at 37°C in PBS containing 2 μmol/L calcein AM and 2 μmol/Lethidium homodimer-1 (Invitrogen) for 30 min. Minimize exposure to light to prevent photobleaching.
Image gels using an epi-fluorescence microscope. For each field of view record two images in the green and red channels corresponding to the live and dead images, respectively.
Count the number of cells in the red and the green channels for each field of view.
Viability is calculated as the ratio of green cells to the sum of red and green cells.
Metabolic activity assay
Wst-1 assay measures the metabolic activity (dehydrogenase activity) indicative of viable cell numbers.
Prepare a Wst-1 working solution {Tyrode’s-Hepes buffer containing 45 μmol/L Wst-1 [2-(4-iodo-phenyl)-3-(4-nitrophenyl)-5-(2,4-disulfophenyl)-2H-tetrazolium, monosodium salt] and 2 μmol/L 1-methoxy-5-methylphenazinium methylsulfate}.
Incubate intact gel sections in 24-well plates in 1 mL of Wst-1 working solution for 3h at 37°C.
Transfer a 0.2 mL aliquot of the reacted solution to a 96-well plate and measure absorbance at 450nm.
Note that there is no standard reference available for this assay and the measurements are, therefore, semi-quantitative.
DNA content assay
Picogreen dsDNA Quantitation Kit is used to quantify the DNA, a measure of cell number. Gel sections must be homogenized for the assay to extract DNA.
Homogenize the gel sections in a small glass dounce tissue homogenizer in TE buffer (available in the kit; TE stands for Tris-EDTA; Tris is tris(hydroxymethyl) aminomethane; EDTA is ethylenediaminetetraacetic acid) containing 0.3% by mass SDS and 0.8 mg/mL Proteinase K.
Incubate samples overnight at 37°C.
Centrifuge and transfer 0.1 mL of the supernatant to a 96-well plate with 0.1mL of Picogreen dye solution.
In the same plate, prepare a standard curve of solutions of known DNA concentration according to manufacturer protocols.
Incubate in the dark at room temperature for 5min and measure fluorescence intensity on a microplate reader using 488nm and 525nm as excitation and emission wavelengths, respectively.
Prepare a standard plot from the solutions of known DNA concentration and calculate the amount of DNA in the samples.
Alkaline phosphatase expression assay
Alkaline phosphatase is an early marker of osteogenic differentiation and can be quantitatively measured using the Alkaline Phosphatase Detection Reagent.
Incubate intact gel sections in a 24-well plate with 0.5mL of 1% by mass Nonidet P-40 detergent solution for 4 h at 37°C.
Transfer 0.1mL of the reacted solution to a 48-well plate with 0.5mL of the reconstituted alkaline phosphatase reagent (17mmol/L p-nitrophenylphosphate and 4mmol/L magnesium acetate).
Setup a standard well for calibrating activity using control human serum solution of known alkaline phosphatase activity containing 1% Nonidet P-40.
Also, prepare a blank solution containing only 1% Nonidet P-40 as reference for background.
Incubate at room temperature for 3h.
Measure absorbance at 405 nm in the 48-well plate using a microplate reader.
Determine the alkaline phosphatase expression in the samples using the serum control as a reference.
The data must be normalized to cell numbers (DNA content determined using the Picogreen assay) to accurately measure the level of cell differentiation in the gel.
Alizarin red S staining for mineral deposits
Formation of calcium containing mineral deposits is indicative of late stage osteogenic differentiation and can be stained using the Alizarin red S dye (Fig. 5c, d).
Fix the encapsulated cells in the intact gel sections with 1 mL formalin for 24h.
Replace the formalin solution with 1 mL water and incubate for 24h.
Remove the water solution and incubate gel sections in 1 mL of 1% by mass alizarin red S solution in water (pH adjusted to 4.3) for 30 min at room temperature.
Rinse the gel twice in fresh water.
Image the gel on an optical microscope using phase contrast.
Note that the gels are transparent and mineral deposits typically appear as dark spots in micrographs of the gel sections (Fig. 5c, d). Gels will turn visibly cloudy and then white and opaque as MC3T3-E1 osteoblasts deposit more minerals over time. Gels can be imaged by standard photography to visualize graded mineralization (Fig. 5e).
Quantification of mineral deposits using X-ray microcomputed tomography
X-ray micro-computed tomography (μCT) is a useful technique that can be used to examine and quantify the distribution of minerals deposited by osteoblasts within hydrogel scaffolds.
Fix encapsulated cells in the intact gradient hydrogels in 10 mL formalin for 24h. Replace the formalin solution with 10 mL water and incubate for 24h.
Image fixed sample in the μCT [Scanco μCT 40, 55 kVp, 145 μA, 15 μm voxel size (isotropic resolution), 0.3 s integration, 325 slices, sigma 1.2, support 2, threshold 95].
For analysis and image reconstruction, select a contour region to cover the cross-section of the imaged gel.
The threshold for analysis can be selected by scanning and analyzing a non-mineralized gradient hydrogel. Voxel intensity histograms can be created from scans of the mineralized and non-mineralized gels. Previously we chose a threshold value that eliminated 99% of the signal from control gradients (in this case non-mineralized gradients are the background) so that the remaining signal used for generating 3D reconstructions only contained 1% of the signal from background.
2.9. Statistical Guidelines
For all measurements, physical characterization and bioassays, data are taken from multiple specimens (n ≥ 3) and presented as mean ± standard deviation (S.D.). One-way analysis of variance (ANOVA) with Tukey’s test must be applied to determine significant differences (p < 0.05) in measurements along the different positions of the gradient hydrogels.
3. RESULTS AND DISCUSSION
PEG hydrogels are non-toxic and well suited for use as tissue scaffolds in vivo and for cell culture over long time periods in vitro. In recent work we cultured cells up to 77 d [14] whereas others have encapsulated chondrocytes in PEG gels and demonstrated their biocompatibility in vivo [17]. PEGDM hydrogels with gradients in elastic modulus will be generated using the CHT platform described above. The modulus will increase steadily with increase in the fraction of solid (PEGDM) dissolved in the pre-polymer solution whereas the swelling ratio is expected to decrease concomitantly. Cellular response to changes in scaffold modulus can be studied in within a single hydrogel sample. The bioassays described enable screening of the effect of a 30-fold range of gel modulus on cell response within a single specimen.
It is being increasingly recognized that material properties can be used to direct biological responses in a manner similar to chemistry and biomolecules. Physical properties such as elastic modulus, pore size, topography, roughness, particle size, etc. have been shown to influence cellular behavior [23]. Modulus of tissue scaffolds is particularly interesting since it is well recognized that cells sense and respond to changes in the elasticity of their environments, both in vivo and in vitro [18–20]. Such cell-material interactions will be critical in tissue engineering and can be leveraged to maximize tissue regeneration. CHT platforms offer a route to systematically investigate cell-material interactions to identify optimal material properties [3]. Here we described a method to fabricate continuous gradients hydrogel scaffolds that enable screening of cellular response to a wide range of scaffold properties within a single specimen. We applied this technique to screen viability and differentiation of encapsulated osteoblasts in hydrogel gradients spanning 30-fold change in compressive elastic modulus (≈ 10 kPa to ≈ 300 kPa) [14]. We demonstrated that increased modulus can be used to induce osteogenic differentiation and mineralization (at 225 kPa and higher) even in the absence of biomolecules.
It is possible that the gels could hinder nutrient diffusion. However, neutron scattering indicates that the correlation lengths of 10%, 20% and 30% PEGDM gels are similar (11 nm, 11 nm and 9 nm, respectively) suggesting minimal differences in diffusion conditions with increasing modulus and percent PEGDM [24]. We observed that the viability of the encapsulated MC3T3-E1 cells dropped with increase in modulus [14]. However, in unpublished work, we observed that viability primary human bone marrow stromal cells increased with modulus. Thus there is not a consistent effect of modulus on cell viability, further suggesting that changing gel modulus had only minor effects on diffusion.
The modulus range of the hydrogel gradients can be tailored through the choice of the pre-polymer solutions (mass % PEGDM). In unpublished results, we have observed that using 5% PEGDM and 30% PEGDM solutions yielded a gradient gel with range of 48 kPa to 689 kPa in compressive modulus, whereas gels prepared from 5% and 15% solutions yielded gradients spanning 4 kPa to 155 kPa. The physical dimensions of the gradient can also be adjusted by changing the mold dimensions.
The CHT platform described here can be used to fabricate hydrogels with gradients in modulus. In our recent work, we have used osteoblasts as model towards tissue engineering of bone [14]. Other cells besides the MC3T3-E1 osteoblasts can be encapsulated in the gel gradients and we have successfully encapsulated primary human bone marrow stromal cells in unpublished results. We did not observe a significant increase in modulus of the gel after mineralization with MC3T3-E1 cells [14]. However, use of osteogenic supplements to enhance differentiation or using other types of cells that deposit larger volume of minerals may lead to significant increases in gel modulus with time.
The gradient hydrogel technique presented here can be used to make gradients of any two components that can be loaded into the gradient maker. For instance, gels with gradients in biomolecules can be fabricated by loading reactive-functionalized (acrylate, for instance) peptides, proteins or growth factors into one of the chambers of the gradient maker.
4. CONCLUSIONS
In summary, we have developed a simple and inexpensive CHT platform that can be used to screen 3D hydrogel scaffolds that requires use of only a few commonly-available laboratory supplies. Gradient hydrogels can be used to systematically screen the effect of hydrogel properties, such as elastic modulus, on encapsulated cells.
Acknowledgments
We gratefully acknowledge technical inputs from Sheng Lin-Gibson, William E. Wallace, Kathy Flynn, Ed Parry, Kathryn L. Beers, Sapun H. Parekh, Young J. Lee and Marcus T. Cicerone at NIST. This work was performed while K.C. held a Research Associateship Award from the National Research Council of the National Academy of Sciences in the NIH-NIBIB/NIST Joint Postdoctoral Program (National Institutes of Health-National Institute of Biomedical Imaging and Bioengineering/National Institute of Standards and Technology). This work was supported by NIST, NIH-NIBIB R21 EB006497-01 and the Intramural Program of NIH-NIDCR (National Institute of Dental and Craniofacial Research).
The “standard deviation” (S.D.) is the same as the “combined standard uncertainty of the mean” for the purposes of this work. The content is solely the responsibility of the authors and does not necessarily represent the official views of NIH, NIBIB, NIDCR or NIST. This article, a contribution of NIST and NIH, is not subject to US copyright. Certain equipment and instruments or materials are identified in the paper to adequately specify the experimental details. Such identification does not imply recommendation by NIST, nor does it imply the materials are necessarily the best available for the purpose.
Footnotes
CONFLICT OF INTEREST
The authors have no conflict of interests to declare.
References
- 1.Lysaght MJ, Jaklenec A, Deweerd E. Great expectations: Private sector activity in tissue engineering, regenerative medicine, and stem cell therapeutics. Tissue Eng Part A. 2008;14(2):305–315. doi: 10.1089/tea.2007.0267. [DOI] [PubMed] [Google Scholar]
- 2.Inglese J, Johnson RL, Simeonov A, Xia M, Zheng W, Austin CP, Auld DS. High-throughput screening assays for the identification of chemical probes. Nat Chem Biol. 2007;3(8):466–479. doi: 10.1038/nchembio.2007.17. [DOI] [PubMed] [Google Scholar]
- 3.Simon CG, Yang Y, Thomas V, Dorsey SM, Morgan AW. Cell interactions with biomaterials gradients and arrays. Comb Chem High Throughput Screen. 2009;12(6):544–553. doi: 10.2174/138620709788681961. [DOI] [PubMed] [Google Scholar]
- 4.Meredith JC, Sormana JL, Keselowsky BG, Garcia AJ, Tona A, Karim A, Amis EJ. Combinatorial characterization of cell interactions with polymer surfaces. J Biomed Mater Res Part A. 2003;66(3):483–490. doi: 10.1002/jbm.a.10004. [DOI] [PubMed] [Google Scholar]
- 5.Anderson DG, Levenberg S, Langer R. Nanoliter-scale synthesis of arrayed biomaterials and application to human embryonic stem cells. Nat Biotechnol. 2004;22(7):863–866. doi: 10.1038/nbt981. [DOI] [PubMed] [Google Scholar]
- 6.Flaim CJ, Chien S, Bhatia SN. An extracellular matrix microarray for probing cellular differentiation. Nat Methods. 2005;2(2):119–125. doi: 10.1038/nmeth736. [DOI] [PubMed] [Google Scholar]
- 7.Gallant ND, Lavery KA, Amis EJ, Becker ML. Universal gradient substrates for “click” biofunctionalization. Adv Mater. 2007;19:965–969. [Google Scholar]
- 8.DeLong SA, Gobin AS, West JL. Covalent immobilization of RGDS on hydrogel surfaces to direct cell alignment and migration. J Control Release. 2005;109(1–3):139–148. doi: 10.1016/j.jconrel.2005.09.020. [DOI] [PubMed] [Google Scholar]
- 9.Yang J, Rose FRAJ, Gadegaard N, Alexander MR. A high-throughput assay of cell-surface interactions using topographical and chemical gradients. Adv Mater. 2009;21(3):300–304. [Google Scholar]
- 10.Lin NJ, Lin-Gibson S. Osteoblast response to dimethacrylate composites varying in composition, conversion and roughness using a combinatorial approach. Biomaterials. 2009;30(27):4480–4487. doi: 10.1016/j.biomaterials.2009.05.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Yamada KM, Cukierman E. Modeling tissue morphogenesis and cancer in 3D. Cell. 2007;130(4):601–610. doi: 10.1016/j.cell.2007.08.006. [DOI] [PubMed] [Google Scholar]
- 12.Yang Y, Bolikal D, Becker ML, Kohn J, Zeiger D, Simon CG., Jr Combinatorial polymer scaffold libraries for screening cell-biomaterial interactions in 3D. Adv Mater. 2008;20(11):2037–2043. [Google Scholar]
- 13.Simon CG, Jr, Stephens JS, Dorsey SM, Becker ML. Fabrication of combinatorial polymer scaffold libraries. Rev Sci Instrum. 2007:78072207. doi: 10.1063/1.2755761. [DOI] [PubMed] [Google Scholar]
- 14.Chatterjee K, Lin-Gibson S, Wallace WE, Parekh SH, Lee YJ, Cicerone MT, Simon CG. Combinatorial screening of material-induced osteogenic differentiation in a 3D tissue scaffold. Biomaterials. 2010;31:5051–5062. doi: 10.1016/j.biomaterials.2010.03.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cushing MC, Anseth KS. Material science: Hydrogel cell cultures. Science. 2007;316(5828):1133–1134. doi: 10.1126/science.1140171. [DOI] [PubMed] [Google Scholar]
- 16.Lin-Gibson S, Bencherif S, Cooper JA, Wetzel SJ, Antonucci JM, Vogel BM, Horkay F, Washburn NR. Synthesis and characterization of PEG dimethacrylates and their hydrogels. Biomacromolecules. 2004;5(4):1280–1287. doi: 10.1021/bm0498777. [DOI] [PubMed] [Google Scholar]
- 17.Elisseeff J, Anseth KS, Sims D, McIntosh W, Randolph M, Yaremchuk M, Langer R. Transdermal photopolymerization of poly (ethylene oxide)-based injectable hydrogels for tissue-engineered cartilage. Plast Reconstruct Surg. 1999;104(4):1014–1022. doi: 10.1097/00006534-199909040-00017. [DOI] [PubMed] [Google Scholar]
- 18.Levental I, Georges PC, Janmey PA. Soft biological materials and their impact on cell function. Soft Matter. 2007;3(3):299–306. doi: 10.1039/b610522j. [DOI] [PubMed] [Google Scholar]
- 19.Engler AJ, Sen S, Sweeney HL, Discher DE. Matrix elasticity directs stem cell lineage specification. Cell. 2006;126(4):677–689. doi: 10.1016/j.cell.2006.06.044. [DOI] [PubMed] [Google Scholar]
- 20.Saha K, Keung AJ, Irwin EF, Li Y, Little L, Schaffer DV, Healy KE. Substrate modulus directs neural stem cell behavior. Biophys J. 2008;95(9):4426–4438. doi: 10.1529/biophysj.108.132217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kraehenbuehl TP, Zammaretti P, Van der Vlies AJ, Schoenmakers RG, Lutolf MP, Jaconi ME, Hubbell JA. Three-dimensional extracellular matrix-directed cardioprogenitor differentiation: Systematic modulation of a synthetic cell-responsive PEG-hydrogel. Biomaterials. 2008;29(18):2757–2766. doi: 10.1016/j.biomaterials.2008.03.016. [DOI] [PubMed] [Google Scholar]
- 22.Peyton SR, Kim PD, Ghajar CM, Seliktar D, Putnam AJ. The effects of matrix stiffness and RhoA on the phenotypic plasticity of smooth muscle cells in a 3-D biosynthetic hydrogel system. Biomaterials. 2008;29(17):2597–2607. doi: 10.1016/j.biomaterials.2008.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mitragotri S, Lahann J. Physical approaches to biomaterial design. Nat Mater. 2009;8(1):15–23. doi: 10.1038/nmat2344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lin-Gibson S, Jones RL, Horkay F, Washburn NR. Structure- property relationships of photopolymerizable poly(ethylene glycol) dimethacrylate hydrogels. Macromolecules. 2005;38(7):2897–2902. [Google Scholar]