Abstract
Limited data exist on the effect of IVIg on anti-HLA antibodies as determined by solid phase assays. We reviewed our experience treating sensitized waitlisted kidney transplant recipients with IVIg as a method for desensitization and report our results utilizing Luminex single antigen (LSA) bead assay to quantify antibody reactivity (MFI). Fifteen patients with a cPRA>40% received 2 g/kg IVIg per month for 4 months or until transplanted. LSA testing was done before and after IVIg. Median MFI for anti-class I antibodies fell in 11 (73%), and increased in 4 (27%) patients after IVIg. Similar significant changes in MFI for anti-class II antibodies were observed in 10 patients (66%). Administration of IVIg was associated with a modest decrease in reactivity to both class I and II HLA antigens (median MFI change 493 and 1110 respectively; p<0.0001) but did not significantly alter mean cPRA (85% before IVIg vs. 80% after IVIg; p=0.1). Our data suggest a smaller effect of IVIg on HLA antibody reactivity than previously described, leading us to question how best to measure the efficacy of a desensitization protocol in current practice.
Keywords: desensitization, IVIg, kidney transplantation, PRA, sensitization
INTRODUCTION
Sensitized transplant candidates represent an increasing proportion of the patients on the deceased-donor kidney transplant waiting list [21]. Currently 18% of the patients on the waiting list have elevated panel reactive antibody (PRA) levels (10-79%) with an additional 18% considered highly sensitized (PRA >80%) [21]. The clinical consequences of sensitization include longer waiting times for deceased donor kidneys [21] and increased risk for acute rejection and shortened graft survival [1-3].
Over the past decade the development and commercialization of Luminex single antigen (LSA) bead technology has revolutionized anti-HLA antibody detection [4, 5]. Extending the information derived from PRA testing, LSA analysis delineates antibody specificity for individual HLA alleles, and through documenting signal intensity (mean fluorescence intensity, MFI), provides an estimation of antibody binding ability which is indirectly interpreted as a quantitative measure of antibody in the serum. Increasing antibody binding as measured by MFI correlates with a positive complement-dependent cytotoxicity (CDC) and flow-cytometry cross-match results and increases the risk for acute rejection [1, 6-8].
The increased recognition of the impact of antibody sensitization and the ability to define and quantify antibody reactivity has induced the transplant community to develop novel strategies for “desensitization”. The goal of these therapies is to lower antibody levels sufficiently so as to permit organ transplantation and minimize the risk of antibody mediated rejection. Most common protocols include intravenous immunoglobulin (IVIg) with or without anti-CD20 mAb (rituximab) and plasmapheresis [9-12]. Despite widespread use, little is known about the effect of these regimens on alloantibody repertoires. Reports suggested that IVIg is able to lower PRA [10] but effects on single antibodies, measured by LSA are not well characterized. We began to desensitize our sensitized patients with IVIg in 2007 and here report the observed changes in anti-HLA antibody repertoires using LSA bead technology. We found that high dose IVIg lowered HLA antibodies in the majority of patients but the intensity of the effect was highly variable and modest.
PATIENTS AND METHODS
Study patients and IVIg protocol
From January 2007 to January 2010 patients with a PRA > 40% and at the top of the kidney transplant waiting list were prospectively enrolled for desensitization with IVIg. Twenty patients received 1 g/kg of IVIg (Gamunex, Talecris Biotheraputics, Research triangle park, NC) twice a month during 2 consecutive dialysis sessions for a total of 4 months. Patients with LSA testing before and after at least one dose of IVIg were identified and included in the study (n=15). Clinical and demographic variables including self reported race, age, sex, time on dialysis, cause of end-stage renal disease, and sensitizing events were reviewed. The study was approved by the Institutional Review Board of the Mount Sinai School of Medicine.
Detection of Anti-HLA antibodies and calculated PRA
Seven patients had serum samples prospectively collected immediately before treatment session 1, 3, 5 and 7 which were used for antibody testing at a research lab within Mount Sinai. The remaining patients had antibody testing performed for clinical use (Rogosin Immunogenetics Institute, NY, NY) within 6 months of starting and completing IVIg therapy. When patients had antibody analysis performed by both labs, the Rogosin data was used (n=3). Alloantibodies were measured with LABScreen Single Antigen beads (One Lambda Inc., Canoga Park, CA) using HLA Visual Luminex IS V2.3 software (One Lambda Inc., Canoga Park, CA) at the Rogosin Immunogenetics Institute and HLA Fusion (One Lambda Inc., Canoga Park, CA) at The Mount Sinai School of Medicine. Both software programs analyze raw MFI data identically. MFI values of less than 1,000 were considered negative.
Alloantibodies were analyzed with regard to strength by Luminex MFI values, number, type and subgroup, and calculated PRA (cPRA). Since the study period took place during the implementation of unacceptable antigens in UNET, cPRA was calculated retrospectively using our current protocol. Anti-HLA A, B, DR, and DQ antibodies with a MFI > 10,000 were entered into the UNOS cPRA calculator as unacceptable antigens. To account for laboratory variation, data from The Mount Sinai School of Medicine was normalized to the results from the Rogosin Immunogenetics Institute as described below.
Isotype Analysis
IgG isotyping was performed using the One Lambda LABScreen Single Antigen beads. Serum was incubated at room temperature with beads on a filter plate, washed and then incubated with PE-labeled secondary antibody (mouse anti-human IgG1 or IgG2, Southern Biotech; Birmingham, AL). Plates were read on a BioPlex Protein Array System (BioRad, Hercules, CA) using BioPlex Manager software (BioRad, Hercules, CA) and analyzed using the HLA Fusion software (One Lambda Inc., Canoga Park, CA).
Statistical Analysis
Statistical analysis was performed using SPSS version 16 (Chicago, IL), Microsoft Exel and GraphPad Prism software. Median and mean antibody MFI values were compared using Wilcoxon signed rank test and the Mann Whitney test. P <0.05 was considered significant. If the MFI value of an antibody fell below our threshold of 1000 MFI after IVIg, we assigned it a value of 999 for statistical analysis. To account for inter-laboratory variability in MFI assays, data from the two laboratories were normalized using results from three patients tested at both labs. In brief, a mean and standard deviation (SD) was calculated for: pre IVIg class I antibody MFI values at the Rogosin lab, post IVIg class I antibody MFI values at the Rogosin lab, pre IVIg class II antibody MFI values at the Rogosin lab, and post IVIg class II antibody MFI values at the Rogosin lab. Similar calculations were performed for pre and post class I and class II antibody MFI values from the Mount Sinai (MS) lab. These values can be seen in Table S1. In patients with testing only in the Mount Sinai lab, a Z score (Z) was calculated for each antibody using the formula: Z = (antibody MFI − mean MS lab antibody MFI)/ SD MS lab. Values were then recalculated into “normalized” MFI values with the formula: normalized MFI = (Z × Rogosin SD)+Rogosin mean. An example of normalization is provided in Table S2. The normalized values were used in all statistical and graphical analyses.
RESULTS
Patient demographics and IVIg therapy
Twelve adult and 3 pediatric highly-sensitized patients were included in the study. Four patients had Luminex testing performed only at the Mount Sinai School of Medicine which were normalized to the Rogosin Immunogenetics Institute before analysis. Of the 15 study patients (Table 1), 6 (40%) were male, 5 (33%) were African-American, 14 (93%) had previous failed transplants, 12 (80%) had a transfusion history and 3 (33%) women had prior pregnancies. All patients had at least one sensitizing event. The median time on dialysis for the 12 adults was 12.5 years (IQR 4.3-20.3) and the median age at study entry was 42 years (IQR range 31-59).
Table 1.
Patient demographics and cPRA before and after IVIg.
| Pt | Age | Sex | Race | Cause of ESRD |
Transfused | Prior Transplant |
Pregnancy | cPRA pre-IVIg |
cPRA post-IVIg |
|---|---|---|---|---|---|---|---|---|---|
| 1 | 13 | F | Asian | FSGS | yes | Yes | no | 47% | 64% |
| 2 | 17 | M | AA | FSGS | yes | Yes | no | 59% | 37% |
| 3 | 52 | F | AA | GN | no | Yes | no | 100% | 99% |
| 4 | 67 | M | AA | HTN | yes | No | no | 99% | 98% |
| 5 | 59 | F | White | Congenital | yes | Yes | yes | 80% | 74% |
| 6 | 48 | F | White | HTN | yes | Yes | yes | 100% | 100% |
| 7 | 31 | M | Asian | IgA | no | Yes | no | 92% | 97% |
| 8 | 64 | F | White | Unknown | yes | Yes | no | 51% | 24% |
| 9 | 9 | M | Latino | Reflux | yes | Yes | no | 97% | 72% |
| 10 | 67 | M | White | PKD | yes | Yes | no | 98% | 98% |
| 11 | 46 | F | Asian | Unknown | yes | Yes | yes | 100% | 100% |
| 12 | 38 | F | Asian | Unknown | yes | Yes | no | 100% | 100% |
| 13 | 35 | F | Latino | Unknown | yes | Yes | no | 73% | 61% |
| 14 | 43 | M | AA | HTN | no | Yes | no | 90% | 81% |
| 15 | 51 | F | AA | SLE | yes | Yes | no | 100% | 100% |
Thirteen patients (87%) received a total dose of 8 gm/kg IVIg, one patient experienced extreme restlessness during treatments resulting in termination of therapy after 5.5 gm/kg and another patient received 4 g/kg then was transplanted. This patients single antigen testing was performed after a total of 2gms/kg IVIg. Three additional patients had testing after 6 gm/kg of IVIg because sera after completion of therapy were not available. The median time to LSA testing after IVIg for the entire cohort of patients was 33 days (IQR 30-49 days). Three patients had LSA testing performed less than 1 month after receiving IVIg. In ascending order, these patients had testing performed at 11 days, 14 days, and 22 days after the last dose of IVIg.
Effect of IVIG on anti-HLA antibodies and cPRA
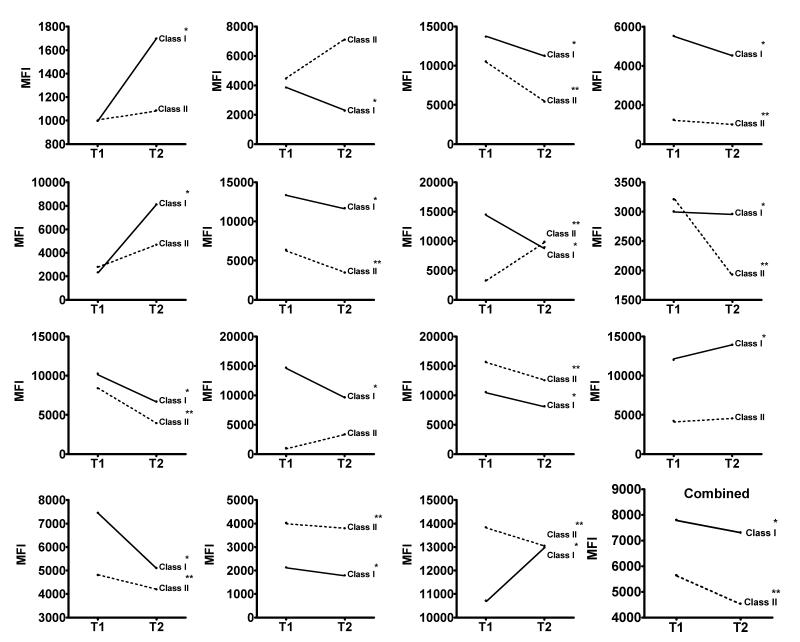
LSA-derived median anti-class I and class II antibody MFI before and after IVIg are shown in Figure 1. We observed a significant decrease in the median class I antibody MFI in 11 patients (73%) and a significant increase in 4 patients (27%). The change in median class I MFI for an individual ranged from a reduction of 5,573 MFI to an increase of 5,784 MFI . In the group of patients, the post IVIg median anti-class I antibody MFI value was 6.3% lower than the pre-IVIg median MFI value (MFI 7,804 vs. 7,311, p<0.0001). We observed significant changes in anti-class II HLA antibodies in 10 (66%), with a median change in MFI ranging from a reduction of 5,134 units to an increase of 6,569 units. Median MFI for anti-class II antibodies in our cohort of patients post IVIg were 19% lower than pretreatment values (MFI 5,648 vs. 4,538, p<0.0001). Only eight patients (53%) had a simultaneous decrease in both class I and class II antibodies.
Figure 1.
Median class I and class II antibody MFI is plotted before (T1) and after (T2) IVIg for each patient and for the entire cohort of patients (combined). * p<0.05 for class I ** p<0.05 for class II.
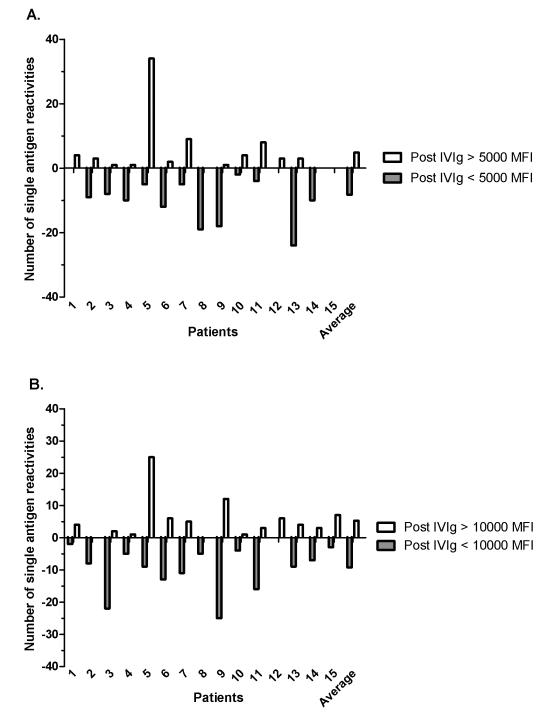
To better understand the effect of IVIg associated changes on antibodies of different strengths, we quantified the number of antibody specificities that either decreased or increased beyond 2 thresholds of 5,000 MFI and 10,000 MFI (Figure 2). For example, in Figure 2A, patient 2 had more antibodies that fell below the threshold of 5,000 MFI than antibodies that rose above the threshold of 5,000 MFI after IVIg (gray bar is longer than the white bar), resulting in a net decrease in antibodies above 5,000 MFI. We chose these thresholds because there is evidence that DSA with MFI > 5,000 is associated with antibody-mediated rejection (AMR), and unpublished data (Rogosin Immunogenetics Institute, NY, NY) indicate that DSA > 10,000 MFI results in a positive CDC cross-match, and therefore, our listing of an unacceptable antigen [7, 8]. Eight patients (53%) had a net decrease in specificities above 5,000 MFI, 6 had a net increase, and 1 patient had no change. In contrast, 11 patients (73%) had a net decrease in specificities above 10,000 MFI, while 4 had a net increase in antibodies above 10,000 MFI after IVIg therapy.
Figure 2.
The number of antibody reactivities for each patient and average number of antibody reactivities for the cohort of patients that decreased (in gray) or increased (in white) around the thresholds of (A) 5,000 MFI and (B) 10,000 MFI.
Next, cPRA was calculated by entering anti-HLA antibodies with MFI > 10,000 into the UNOS cPRA calculator. This threshold was based on a 63% correlation with a positive CDC crossmatch. Following IVIg treatment cPRA fell in 8 (53%), was unchanged in 5 (33%), and increased in 2 patients (13%) (Table 1). The change in cPRA ranged from a reduction of 27 to an increase of 17. Of the 9 patients with a baseline cPRA > 90%, only one patient had a decrease in cPRA greater than 1. Patients with a baseline cPRA ≤ 90% were more likely to have a greater than 1 point decrease in cPRA then those with a baseline cPRA >90% (Fishers exact test, p=0.01). IVIg did not not change the mean cPRA (85 vs. 80%, p=0.1).
Effect of IVIg on IgG1 and IgG2
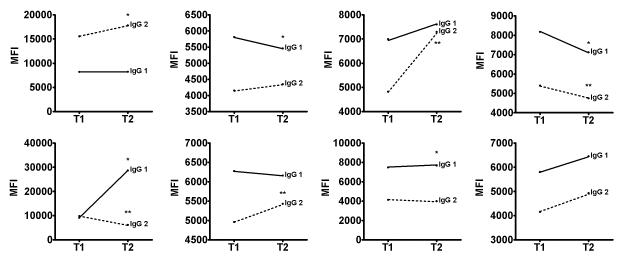
Because the effector function of a given antibody is in part determined by its isotype, we tested sera from a subset of 8 patients to determine associated changes in IgG1 and IgG2. IgG3 and IgG4 make up less than 10% of serum immunoglobulins [22] and were not analyzed. We tested sera from 7 patients before and after 6g/kg IVIg and in one patient after 2g/kg IVIg. IVIg decreased IgG1 in 2, did not change in 4 and increased in 2 patients, all of which paralleled the alterations detected using the standard assay for total IgG (Figure 3). IgG2 subtype analysis revealed post IVIg decreases in 2 patients, no change in 3 patients and significantly increased anti-HLA IgG2 in 3 patients.
Figure 3.
IgG1 and IgG2 subtypes. Median IgG1 and IgG2 antibody MFI is plotted before (T1) and after (T2) IVIg for each patient. * p<0.05 for IgG1 ** p<0.05 for IgG2.
HLA antibody patterns of transplanted patients
Five of the 15 patients (33%) received a deceased donor transplant after a median time of 3 months (range 0 to 4 months) post IVIg. Patients with a baseline cPRA < 90% were more likely to be transplanted after IVIg (Fishers exact test, p=0.002) and their median cPRA was lower (59% vs. 99%, p=0.001). Using HLA typing along with LSA testing before and after IVIg, we investigated if IVIg treatment enabled them to be transplanted, or reduced their risk of AMR as determined by a reduction in DSA levels (Table 2). Serum from all 5 patients contained persistent DSA after IVIg. One patient was found to have no DSA (patient 1) during further antibody testing at the time of transplant. Two patients had a reduction of DSA below 5,000 after IVIg.
Table 2.
Characteristics of patients transplanted after IVIg.
| Patient | Age (yrs) |
cPRA pre |
cPRA post |
Time to Transplant (months) |
Pre- treatment DSA (MFI) |
Post- treatment DSA (MFI) |
Pre- Transplant |
|---|---|---|---|---|---|---|---|
| 1 | 17 | 47% | 64% | 4 | B57 2250 | A1 1406; A23 1107; B72 2345 |
No DSA |
| 2 | 13 | 59% | 37% | 3 | A11 6369/ 2392; DR13 1199; DQ9 7424; DQ6 9052/ 3900 |
A11 3259; DR13 1128 DQ9 8517, 1326; DQ6 4027, 2009 |
Same as post treatment |
| 8 | 64 | 51% | 24% | 1 | B7 1006; DR52 3792 |
DR52 2020; DQ7 2050/1950/ 1910/ 115 |
Same as post treatment |
| 13 | 35 | 73% | 61% | <1 | A74 7926 | A74 2950 | A74 3441; Cw4 2457; Cw 5 1708 |
| 14 | 43 | 90% | 81% | 1 | DR15 4025; DQ6 19917 |
DR15 3840; DQ6 17225 |
DQ6 16441 |
DISCUSSION
This is one of few studies analyzing the effects of high dose of IVIg on alloantibodies by Luminex single antigen beads in sensitized (median PRA 97%, range 47%-100%) wait-listed kidney transplant candidates [12,14]. While MFI values of anti-HLA antibodies decreased in most patients as suggested by the literature [9, 10, 12, 13], the effect was not uniform and in fact MFIs for anti-HLA antibodies increased in some patients (Figure 1). Half of the patients had a net decrease in antibodies greater than 5000 MFI while 73% of patients had a net decrease in antibodies greater than 10,000 MFI after treatment with IVIg (Figure 2). Despite this reduction, IVIg treatment did not lower the number or strength of antibodies sufficiently to result in a decrease in mean cPRA. However, most patients had some decrease in cPRA (53%), especially patients with a baseline cPRA ≤ 90% (Table 1). IVIg did not result in patients being transplanted by virtue of a reduction of unacceptable antigens, however, pretransplant DSA MFI values were sometimes reduced (Table 2). Our data suggest that IVIg only modestly decreases HLA antibodies, an effect that patients with a lower degree of sensitization are more likely to benefit from. Though IVIg may have other properties that result in a higher transplantation rate in sensitized patients; our observations are important as more transplant centers are relying on solid phase assays for antibody analysis and using the results to create a cPRA, rather than CDC PRA which is no longer being utilized for kidney allocation. It is therefore important that the benefit of IVIg in treating sensitized patients be evaluated with current methods of antibody testing.
Our findings are similar with the previous report that observed no significant reduction of the anti-HLA antibody repertoire in 5 patients treated with 3 monthly infusions of IVIg [14]. In contrast, the NIH sponsored IGO2 trial demonstrated a significant reduction in PRA and a higher transplant rate (35% vs. 17%) in patients treated with IVIg versus placebo [9]. Interestingly, most of the fall in PRA, tested in a in a microlymphocytotoxicity assay, occurred after the first dose of IVIg. Six months post IVIg PRA returned to near baseline value but patients continued to be transplanted, suggesting that the IVIg effect is incompletely explained by the reduction in HLA antibody reactivity. The same group found a smaller change in PRA in patients transplanted after desensitization with IVIg and Rituximab when assessed by Luminex Flow PRA beads (80% vs. 67%; pre versus post desensitization) [12]. As Luminex single antigen beads are more sensitive for detecting HLA antibody than microlymphocytotoxicity and Flow PRA beads it is possible that the smaller reduction in cPRA seen in our study was directly related to the high sensitivity of the assay.
Despite the long use of IVIg in transplantation there is little data to explain its mechanism [15]. Published reports suggest that IVIg can have “immunomodulatory” effects, without altering antibody isotype or titers, such as neutralizing HLA antibodies with anti-idiotypic antibodies, modifying cell-mediated immunity, and interfering with antibody-mediated complement activation [15-19]. These effects may explain the difference in efficacy of IVIg as assessed by functional assays of PRA, such as CDC PRA, versus LSA beads which detect IgG antibodies against an array of recombinant class I and class II HLA molecules. LSA beads may differ in allele and antigen density despite expressing the same serologic HLA molecule. In addition LSA beads detect both complement binding and non-complement binding antibodies. In contrast, CDC PRA is determined by complement fixing antibodies dependent on antibody affinity, titer and antigen density on actual donor cells [4, 5]. If complement activation and fixation is being affected by IVIg, there may be a decrease in CDC PRA but no change in single antibody reactivity as measured by Luminex beads. Therefore IVIg may result in fewer positive crossmatches resulting in higher transplant rates in sensitized patients without affecting the quantity of HLA antibodies.
We acknowledge limitations of our study. Prior studies administered 2 grams of IVIg over one session rather than two. It is unlikely that this changed the efficacy of IVIg since the half-life of IVIg is 3 weeks longer than our dosing interval [20]. Five patients had antibody testing before completing the full course of IVIg, however the results in this subgroup was similar to the rest of the patients. We performed antibody analysis in two separate laboratories; yet neither lab revealed large decreases in antibody MFI making it unlikely that a particular lab biased our results. In addition it is possible that the small changes in antibody MFI would have occurred regardless of the treatment with IVIg or that IVIg may have a direct affect on the LSA bead assay. The addition of a control group of patients not treated with IVIg may have helped clarify these points, however, we only perform annual single antigen testing of our patients unless they undergo a sensitizing event or desensitization making control data unavailable within the required timeframe. The relationship between MFI and antibody strength is also not linear, therefore it is possible to have a reduction in antibody strength not appreciated by MFI changes in undiluted serum. Regardless, the strength of antibody binding (MFI) remained significantly above our clinical threshold to transplant which is our main observation – that IVIg did not make a clinically meaningful reduction in HLA antibody strength when measured by single antigen beads. Finally, it is possible that IVIg attenuates HLA-antibody mediated injury without changing antibody levels or binding properties.
In conclusion after treating our sensitized patients with IVIg we observed a modest decrease of anti-HLA antibody intensity but no change in cPRA. A subgroup of sensitized patients with baseline cPRA ≤ 90% were more likely to have a reduction in cPRA and be transplanted after IVIg. Further studies are needed to confirm our findings and determine how best to measure the efficacy of a desensitization protocol.
Supplementary Material
ACKNOWLEDGEMENTS
V.N., P.S.H, B.S., E.A. and D.S. participated in writing of the article and data analysis.
P.S.H. was awarded an NIH U01AI63594 grant used for assay development.
R.K. participated in patient selection. D.S. is a recipient of a fellowship award from the American Society of Transplantation
R.F., Z.E., V.S., R.D, S.A., S.L., B.M. and J.S.B. participated in manuscript review.
References
- 1.Amico P, et al. Clinical relevance of pretransplant donor-specific HLA antibodies detected by single-antigen flow-beads. Transplantation. 2009;87(11):1681–8. doi: 10.1097/TP.0b013e3181a5e034. [DOI] [PubMed] [Google Scholar]
- 2.Riethmuller S, et al. Donor-specific antibody levels and three generations of crossmatches to predict antibody-mediated rejection in kidney transplantation. Transplantation. 90(2):160–7. doi: 10.1097/tp.0b013e3181e36e08. [DOI] [PubMed] [Google Scholar]
- 3.Opelz G. Non-HLA transplantation immunity revealed by lymphocytotoxic antibodies. Lancet. 2005;365(9470):1570–6. doi: 10.1016/S0140-6736(05)66458-6. [DOI] [PubMed] [Google Scholar]
- 4.Tait BD, et al. Review article: Luminex technology for HLA antibody detection in organ transplantation. Nephrology (Carlton) 2009;14(2):247–54. doi: 10.1111/j.1440-1797.2008.01074.x. [DOI] [PubMed] [Google Scholar]
- 5.Tinckam K. Histocompatibility methods. Transplant Rev (Orlando) 2009;23(2):80–93. doi: 10.1016/j.trre.2009.01.001. [DOI] [PubMed] [Google Scholar]
- 6.Lefaucheur C, et al. Clinical relevance of preformed HLA donor-specific antibodies in kidney transplantation. Am J Transplant. 2008;8(2):324–31. doi: 10.1111/j.1600-6143.2007.02072.x. [DOI] [PubMed] [Google Scholar]
- 7.Akalin E, et al. Addition of plasmapheresis decreases the incidence of acute antibody-mediated rejection in sensitized patients with strong donor-specific antibodies. Clin J Am Soc Nephrol. 2008;3(4):1160–7. doi: 10.2215/CJN.05321107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zachary AA, et al. Using real data for a virtual crossmatch. Hum Immunol. 2009;70(8):574–9. doi: 10.1016/j.humimm.2009.06.007. [DOI] [PubMed] [Google Scholar]
- 9.Jordan SC, et al. Evaluation of intravenous immunoglobulin as an agent to lower allosensitization and improve transplantation in highly sensitized adult patients with end-stage renal disease: report of the NIH IG02 trial. J Am Soc Nephrol. 2004;15(12):3256–62. doi: 10.1097/01.ASN.0000145878.92906.9F. [DOI] [PubMed] [Google Scholar]
- 10.Vo AA, et al. Rituximab and intravenous immune globulin for desensitization during renal transplantation. N Engl J Med. 2008;359(3):242–51. doi: 10.1056/NEJMoa0707894. [DOI] [PubMed] [Google Scholar]
- 11.Stegall MD, et al. A comparison of plasmapheresis versus high-dose IVIG desensitization in renal allograft recipients with high levels of donor specific alloantibody. Am J Transplant. 2006;6(2):346–51. doi: 10.1111/j.1600-6143.2005.01178.x. [DOI] [PubMed] [Google Scholar]
- 12.Vo AA, et al. Use of intravenous immune globulin and rituximab for desensitization of highly HLA-sensitized patients awaiting kidney transplantation. Transplantation. 89(9):1095–102. doi: 10.1097/TP.0b013e3181d21e7f. [DOI] [PubMed] [Google Scholar]
- 13.Reinsmoen NL, et al. Acceptable donor-specific antibody levels allowing for successful deceased and living donor kidney transplantation after desensitization therapy. Transplantation. 2008;86(6):820–5. doi: 10.1097/TP.0b013e3181856f98. [DOI] [PubMed] [Google Scholar]
- 14.Ferrari-Lacraz S, et al. Anti-HLA antibody repertoire after IVIg infusion in highly sensitized patients waiting for kidney transplantation. Swiss Med Wkly. 2006;136(43-44):696–702. doi: 10.4414/smw.2006.11517. [DOI] [PubMed] [Google Scholar]
- 15.Jordan SC, Toyoda M, Vo AA. Intravenous immunoglobulin a natural regulator of immunity and inflammation. Transplantation. 2009;88(1):1–6. doi: 10.1097/TP.0b013e3181a9e89a. [DOI] [PubMed] [Google Scholar]
- 16.Wassmuth R, et al. Differential inhibitory effects of intravenous immunoglobulin preparations on HLA-alloantibodies in vitro. Transplantation. 2001;71(10):1436–42. doi: 10.1097/00007890-200105270-00014. [DOI] [PubMed] [Google Scholar]
- 17.Arumugam TV, et al. Intravenous immunoglobulin (IVIG) protects the brain against experimental stroke by preventing complement-mediated neuronal cell death. Proc Natl Acad Sci U S A. 2007;104(35):14104–9. doi: 10.1073/pnas.0700506104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Glotz D, et al. Suppression of HLA-specific alloantibodies by high-dose intravenous immunoglobulins (IVIg) A potential tool for transplantation of immunized patients. Transplantation. 1993;56(2):335–7. doi: 10.1097/00007890-199308000-00015. [DOI] [PubMed] [Google Scholar]
- 19.Basta M. Ambivalent effect of immunoglobulins on the complement system: activation versus inhibition. Mol Immunol. 2008;45(16):4073–9. doi: 10.1016/j.molimm.2008.07.012. [DOI] [PubMed] [Google Scholar]
- 20.Bonilla FA. Pharmacokinetics of immunoglobulin administered via intravenous or subcutaneous routes. Immunol Allergy Clin North Am. 2008;28(4):803–19. ix. doi: 10.1016/j.iac.2008.06.006. [DOI] [PubMed] [Google Scholar]
- 21. [accessed April 4th 2011];OPTN/SRTR Annual Report: Transplant Data 1999 - 2008. www.srtr.org.
- 22.Murphy Kenneth M., Travers Paul, Walport Mark. Janeway’s Immunobiology. Seventh edition Garland Science Publishing; 2008. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.