Abstract
In aquatic systems, prey animals associate predation risk with cues that originate either from the predator or from injured conspecifics. Sources and benefits of these cues have received considerable attention in river, lake, and pond ecosystems but are less well understood in small container ecosystems that can hold less than a liter of water. Mosquitoes Aedes triseriatus (Say) and Aedes albopictus (Skuse) encounter predatory Corethrella appendiculata (Grabham) and Toxorhynchites rutilus (Coquillett) in small containers and show antipredatory behavioral responses. We investigated the sources of the predation cues to which these prey larvae respond. We tested whether Ae. albopictus larvae show behavioral responses to cues emanating from the predator or from damage to prey caused by the act of predation. We also tested whether Ae. triseriatus respond to cues present in fluid or solid residues from predator activity. Ae. albopictus showed behavioral modifications only in response to waterborne cues from a feeding predator and not to cues from a starving predator, indicating that Ae. albopictus respond to cues created by the act of predation, which could include substances derived from damaged prey or substances in predator feces. Ae. triseriatus showed behavioral responses to solid residues from predation but not to fluid without those solids, indicating that the cues to which they respond originate in predator feces or uneaten prey body parts. Our results suggest that cues in this system may be primarily chemicals that are detected upon contact with solid residues that are products of the feeding processes of these predators.
Keywords: predation risk, container community, antipredatory behavior
In aquatic systems, prey from many taxa modify behavior in response to cues to predation risk and these modifications can reduce an individual’s risk from predation (Sih and Moore 1993, Hughes et al. 1994, Chivers and Smith 1998, Wisenden 2000, Hamilton and Heithaus 2001, Laurila et al. 2004). The cues by which aquatic prey perceive the risk of predation are often chemical and can originate with the predator itself (Chivers and Smith 1998, Wisenden 2000, Tollrian and Heibl 2004, Gyssels and Stoks 2006, Ferris and Rudolf 2007) or can be created by the act of predation, including alarm cues that arise when prey are damaged (Chivers and Smith 1998, Relyea 2001), and dietary cues that originate only after consumed prey are digested (Chivers and Smith 1998). Understanding the source and nature of the cues to predation is central for understanding the proximate mechanisms of adaptive behavior, and may help in understanding specificity of predator detection mechanisms, the potential costs of antipredatory behavior, and ultimately the evolutionary origin of prey responses to predators. Apart from direct predation, costs due to nonlethal effects can be important in structuring communities and hence studies focusing on nonlethal effects have increased (Ripple and Beschta 2004, Trussell et al. 2004). As one practical example of the value of understanding the origins of cues to predation risk, knowing sources of cues can be useful when designing experiments in which investigators simulate predator cues to induce behavioral response as a means of testing hypotheses about the costs (e.g., reduced foraging) of prey’s responses to the predator (Werner et al. 1983, Werner 1991, Van Buskirk and Arioli 2002, Van Buskirk et al. 2002, Schoeppner and Relyea 2005) and the nonlethal effects of predators (Preisser et al. 2005).
Several studies have reported prey antipredatory behavior in response to alarm cues. Mayfly nymphs (Huryn and Chivers 1999), caddisfly larvae (Gall and Brodie 2009), and amphipods (Wisenden et al. 1999) reduce their activity in the presence of cues from injured conspecifics. The intertidal snail Littorina scutulata (Gould) shows a graded response with increase in predation risk perceived from predator alone, injured conspecifics alone, or a combination of both cues (Keppel and Scrosati 2004). Newts, Notopthalamus viridiscens (Rafinesque), increase their frequency of low-risk behaviors in the presence of macerated conspecifics (Rohr and Madison 2001). Mechanisms of detection of predation risk are best understood for ostariophysian fishes (reviewed by Wisenden 2000, Chivers and Smith 1998, Chivers et al. 2007). Alarm substances from damage to epidermis play a major role in this process and these fishes show behavioral responses to lab synthesized hypoxan-thine-3-N-oxide (Brown et al. 2000). These alarm chemicals are species-specific (Brown et al. 1995a) and can be released from the act of predation (Wisenden 2008) or be present in the feces of the predators that fed on conspecifics (Brown et al. 1995b). A single conditioning treatment with a combination of predator stimuli (visual or odor) from a novel predator and injured conspecifics is sufficient for fathead minnows to recognize the novel predator and their taxonomic siblings as potential predators and to show avoidance behaviors (Ferrari et al. 2008). Studies described above all relate to large, heterogeneous aquatic systems (e.g., rivers, lakes, and ponds), but predation and the nature of cues are less well understood in small confined systems like containers. Rainwater collects in small (often <1 liter) containers such as depressions in trees, cemetery vases, and abandoned tires, and these are inhabited by communities of aquatic insects which are dominated by mosquitoes. Such small aquatic habitats limit prey ability to avoid subhabitats where predation risk is high; hence, we expect prey in such habitats to respond to cues to predation risk by modifying behavior or life history. Although the presence of responses to predation risk cues are known in container systems (Juliano and Gravel 2002; Kesavaraju and Juliano 2004, 2008; Kesavaraju et al. 2007a,b, 2008), the sources and type of those cues are poorly investigated.
Prey Mosquitoes
Aedes albopictus (Skuse) is an invasive mosquito, introduced into North America from Asia in the mid-1980s (Hawley et al. 1987), that has become abundant in the southeastern United States (Juliano and Lounibos 2005). Larvae inhabit natural (tree holes) and artificial container habitats (e.g., water filled tires and cemetery vases), co-occurring with the native mosquito Aedes triseriatus (Say), the predatory midge Corethrella appendiculata (Grabham), and the predatory mosquito Toxorhynchites rutilus (Coquillett) (Griswold and Lounibos 2005). C. appendiculata and Tx. rutilus are sit and wait predators and use mechanoreceptors to capture their prey and typically make most captures at the bottom of containers (Juliano and Reminger 1992, Kesavaraju et al. 2007a). Prey larvae that are more active at the bottom are at a higher risk of capture than are those that are motionless at the surface (Juliano and Reminger 1992, Kesavaraju et al. 2007a).
Responses to Corethrella appendiculata
Second instars of both Ae. albopictus and Ae. triseriatus are highly vulnerable to predation by fourthinstar C. appendiculata, and both species reduce activity at the bottom of containers in the presence of water-borne cues from C. appendiculata preying on Aedes larvae (Kesavaraju et al. 2007a). These changes reduce the risk of predation (Kesavaraju et al. 2007a). For Ae. albopictus, the degree of behavioral change is less than that shown by Ae. triseriatus, so that Ae. albopictus larvae are more vulnerable to C. appendiculata predation (Kesavaraju et al. 2007a). Where C. appendiculata abundances are low Ae. albopictus dominates, and where C. appendiculata abundances are high, these species coexist (Kesavaraju et al. 2008).
The waterborne cues to which Ae. albopictus and Ae. triseriatus larvae respond could come from the act of predation or from the predator itself, independent of the act of predation (Lima and Dill 1990, Lima 1998). Both species modify behavior in water that had held C. appendiculata feeding on Aedes larvae for 5 d, but both altered behavior less when a living C. appendiculata was added to the container only minutes before the trial (Kesavaraju et al. 2007a). These results suggest that cues either emanate from predation, or emanate from the predator itself, but must accumulate for 5 d to be effective.
Responses to Tx. rutilus
Fourth instars of Ae. triseriatus reduce their activity at the bottom of the container in the presence of waterborne predation risk cues from Tx. rutilus (Kesavaraju and Juliano 2004). Ae. triseriatus larvae show the same behavioral response to Tx. rutilus feeding on conspecifics or on Ae. albopictus, indicating that the cues are not species specific (Kesavaraju and Juliano 2004). In contrast to the small response of Ae. albopictus to cues from C. appendiculata (Kesavaraju et al. 2007a), they show no significant response to Tx. rutilus predation risk cues (Kesavaraju and Juliano 2004). Behavioral responses of Ae. triseriatus to Tx. rutilus predation risk cues decrease as the concentration of cue-laced water and suspended solids decreases via dilution with distilled water, suggesting a graded, threat sensitive response to the abundance or activity of predators (Kesavaraju et al. 2007b). The source of the cues to which Ae. triseriatus respond is not clear. Ae. triseriatus showed no significant behavioral response to feeding Tx. rutilus isolated in a cage (Hechtel and Juliano 1997), suggesting that solid components (e.g., predator feces) that were retained in the cage may be the source of the cue.
To understand the mechanisms by which these mosquitoes detect their predators, we tested the hypotheses 1) Ae. albopictus larvae respond to waterborne cues from either C. appendiculata itself or from con-specifics injured during the act of predation and 2) Ae. triseriatus larvae either respond to dissolved water-borne cues or to cues in the solid residues of Tx. rutilus predation.
Materials and Methods
Origin of Larvae
Ae. albopictus were F1 progeny from a colony collected initially as larvae from tree holes (Indrio Road, Fort Pierce, FL). Both C. appendiculata and Tx. rutilus are common at this site (S.A.J., personal observations). Ae. triseriatus were F1 progeny of individuals collected initially as larvae from tree holes at Parklands Merwin Reserve near Lexington, IL. Tx. rutilus are rare at this site but occur sporadically in tree holes (Juliano 1989). Thus, larvae in both experiments were descendents of field-collected individuals that probably encountered the test predator in each experiment. Both Aedes were propagated via weekly blood feeding. C. appendiculata and Tx. rutilus larvae were from laboratory colonies maintained at the Florida Medical Entomology Laboratory, Vero Beach, FL. Both predator colonies were established from larvae that were collected in Florida.
Experiment 1. Response of Ae. albopictus to Different Types of Cues From C. appendiculata
Behavior of Ae. albopictus second-instar larvae was recorded in water treated in four ways: control, predator with prey, predator alone, and deionized water (blank). All treatments were prepared for 5 d in 10-ml polystyrene cups with 10 ml of deionized water. Control was prepared by holding 10 second-instar Ae. albopictus. Predator with prey was prepared by holding 10 second-instar Ae. albopictus with three fourth-instar C. appendiculata. Dead, eaten, and pupated larvae were replaced daily. Predator alone was prepared by holding three C. appendiculata fourth instars without food. Finally, the blank treatment consisted of 10 ml of water held without addition of larvae. Each treatment was replicated 24 times, for a total of 96 replicated units.
Second-instar Ae. albopictus were used as test larvae for recording behavior. The test larvae were hatched and held with 5 ml of water in 15-ml vials and fed with 1 ml of liver powder suspension (LPS), which was prepared by stirring 0.3 g of liver powder in a 1,000-ml beaker with 1,000 ml of water on a stir plate and transferred using an Eppendorf pipette (Juliano and Gravel 2002, Kesavaraju and Juliano 2004). A single feeding was sufficient for Ae. albopictus to develop to the second instar.
Test larvae were starved for 24 h in 10-ml cups with 10 ml of water before being transferred to treatment cups for behavior recording. Before test larvae were transferred into the treatment cups, all predator and prey treatment larvae were removed from the treatment cups, leaving behind only any waterborne cues (e.g., uneaten body parts, feces, dissolved chemicals) emanating from the treatments. One second-instar Ae. albopictus larva was placed in treatment water in each container and their behavior was recorded on a computer in MPEG2 format by using a Panasonic video camera and zoom lens (WV-D5100 and WV-LZ14/15, respectively) and a Winfast XP 2000 PCI card (Leadtek Research Inc., www.leadtek.com) for 15 min. A video clip contained four cups with all treatments represented in each clip.
Behaviors were classified into activities and positions (Juliano and Reminger 1992). Activities were as follows: 1) browsing: mouthparts in contact with the container surfaces; 2) filtering: moving in the water column via feeding movements of the mouthparts; 3) thrashing: moving with vigorous lateral flexion of the body; and 4) resting: not exhibiting any previous activities. Positions were as follows: 1) surface: siphon in contact with water surface; 2) wall: within 1 mm of the sides; 3) bottom: within 1 mm of the bottom; and 4) middle: >1 mm from the sides, bottom, and surface.
Activity and position of the test larvae were recorded every 30 s for 15 min (Kesavaraju and Juliano 2008) upon playback of the video clips. Behaviors were then converted to proportions (total number of observations per replicate, 30) for each replicate. The number of behavioral variables was reduced with principal component analysis (PCA). Because past work on these species (Juliano and Gravel 2002; Kesavaraju and Juliano 2004, 2008; Kesavaraju et al. 2007a, 2008) has shown that the primary response of these mosquitoes to predation cues is an increase in the frequency of resting at the surface at the expense of foraging moving below the surface, we focus our analysis on a variable that quantifies this shift in behavior, which in these analyses was always the principal component 1 (PC1) (see Results). Thus, we analyzed PC1 via one-way analysis of variance (ANOVA) (PROC GLM, SAS 9.1, SAS Institute, Cary, NC) (Kesavaraju and Juliano 2004). We used Tukey–Kramer multiple comparisons among treatment least-squares means for pairwise comparisons.
Experiment 2. Nature of Tx. rutilus Predation Risk Cues
Behavior of fourth-instar Ae. triseriatus were recorded in water treated four ways: control fluid, control solid, predation fluid, and predation solid, all prepared for 5 d in 50-ml disposable cups with 50 ml of deionized water. Predation water was prepared by feeding a fourth-instar Tx. rutilus with 10 fourth-instar Ae. triseriatus and control water by holding 10 fourth-instar Ae. triseriatus larvae. Pupated and dead larvae were replaced daily. After 5 d, both the prey and the predator were removed from the cups leaving behind the only any waterborne cues (e.g., uneaten body parts, feces, and dissolved cues). The control and predation water treatments were then filtered with a Whatman filter paper (grade 2, size 12.5 cm) placed in a funnel on a conical flask that was connected to a vacuum pump. The solid matter retained on the filter paper was washed into a clean 50-ml disposable cup with deionized water and then the volume was brought up to 50-ml with deionized water. The filtrate from the conical flask was transferred to 50-ml disposable cups. Predation and control treatments were replicated 35 times each and because each predation cup and control cup yielded two treatments (predation solid, predation fluid, control solid, control fluid), the total number of replicated units was 140.
Fourth-instar Ae. triseriatus were used as test larvae for recording behavior. The test larvae were hatched and held in 5 ml of water in 15-ml vials and fed 1 ml of LPS as described in experiment 1 every 2 d. Test larvae were starved for 24 h in 50-ml cups with 50 ml of water before being transferred for behavior recording. One fourth-instar Ae. triseriatus was placed in treatment water in each container and their behavior was recorded using the same Panasonic videocamera and an S-VHS video cassette recorder for 30 min (Kesavaraju and Juliano 2004). A video clip contained six cups and all treatments were represented on each clip. The treatment waters were filtered and the filtrates transferred to the cups 15–30 min before video recording.
The behaviors described in experiment 1 (activity and position) were recorded every minute for 30 min. Behaviors were converted to proportions, variables were reduced with PCA, and our focal variable PC1 was analyzed with by two-way ANOVA (PROC GLM, SAS 9.1) with treatment (control, predation), cues (solid, fluid), and interaction as independent variables. A significant interaction between treatment and cues would indicate that differences in larval behavior were dependent on both treatment and type of cue and provide evidence of a differential response to either dissolved cues in the fluid or contact cues in the solid components. We used Tukey–Kramer multiple comparisons among treatment least-squares means for pairwise comparisons.
Results
Experiment 1
PCA reduced the response variables to three uncorrelated PCs, with eigenvalues >1 accounting for 87% of total variation (Table 1). A greater positive score on PC1 indicated that larvae spent more time resting at the surface, and a negative score indicated more time browsing at the wall. Greater positive scores on PC2 indicated more time spent in thrashing in the middle, and a negative score indicated more time spent in other behaviors. Greater positive score on PC3 indicated more time spent filtering at the bottom, and a negative score indicated more time spent at the wall (Table 1).
Table 1.
Rotated factor patterns for the behavioral responses of A. albopictus in different types of cues
| Variable | PC1 (50%) | PC2 (24%) | PC3 (13%) |
|---|---|---|---|
| Resting | 98 | −6 | 5 |
| Browsing | −91 | −32 | −14 |
| Thrashing | 0 | 98 | −12 |
| Filtering | 17 | 7 | 85 |
| Surface | 98 | −6 | 5 |
| Wall | −77 | −31 | −40 |
| Middle | 15 | 93 | 23 |
| Bottom | −60 | −5 | 48 |
| Interpretation | Resting, surface vs. browsing, wall, bottom | Thrashing, middle vs. other | Filtering, bottom vs. wall |
Percent variation explained by each PC is given within parentheses. Values >40 are in bold.
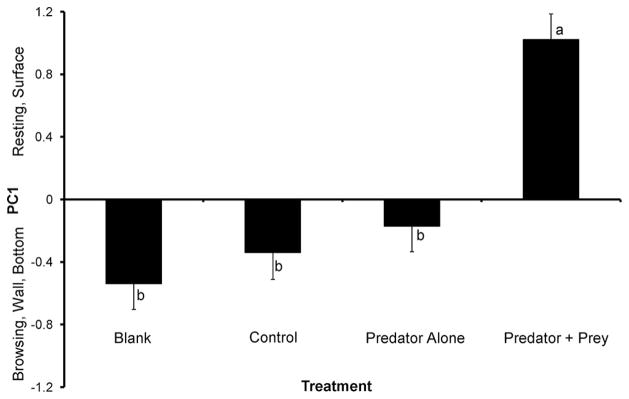
Treatments (blank, control, predator alone, predator with prey) differed significantly for PC1 (F = 18.41; df = 3, 90; P < 0.0001). Multiple comparisons revealed that there were no significant differences among blank, control, and predator alone treatments, but that all three treatments were significantly different from predator with prey (Fig. 1). Ae. albopictus reduced movement and spent more time resting at the surface in water that had held feeding predators compared with the other three treatments (Fig. 1).
Fig. 1.
Experiment 1: Ae. albopictus behavioral response on PC1 (mean ± SE) to different types of cues. Means associated with the same letters are not significantly different from each other.
Experiment 2
Similar to experiment 1, there were three PCs with eigenvalues >1 (Table 2), accounting for 86% of the variation in behavior. A greater positive score on PC1 indicated that larvae spent more time browsing at the wall, and a negative score indicated more time spent resting at the surface. Signs on PC scores are arbitrary; hence, PC1 from the two experiments describes a similar behavioral axis. A greater positive score on PC2 indicated that larvae spent more time thrashing at the bottom, and a negative score indicated more time spent resting at the surface. A greater positive score on PC3 indicated that the larva spent more time filtering in the middle, and a negative score indicated more time spent in other behaviors (Table 2).
Table 2.
Rotated factor patterns for comparing the behavioral responses of fourth-instar A. triseriatus between fluid and solid cues
| Variable | PC1 (46%) | PC2 (26%) | PC3 (13%) |
|---|---|---|---|
| Resting | −84 | −45 | −9 |
| Browsing | 90 | 29 | −23 |
| Thrashing | −8 | 78 | 11 |
| Filtering | −7 | −6 | 95 |
| Surface | −63 | −69 | −15 |
| Wall | 88 | −21 | −19 |
| Middle | −12 | 15 | 94 |
| Bottom | 33 | 83 | −12 |
| Interpretation | Resting, surface vs. browsing, wall | Resting, surface vs. thrashing, bottom | Filtering, middle vs. other |
Percent variation explained by each PC is given within parentheses. Values higher than 40 are in bold.
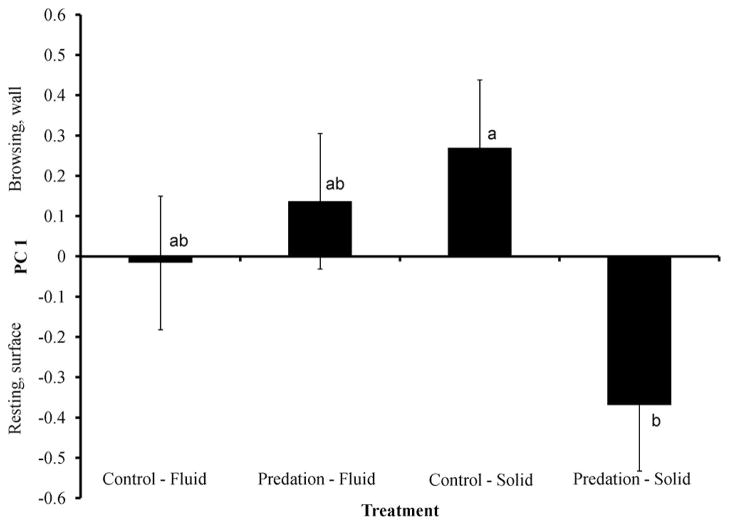
The interaction between cues (fluid and solid) and treatment (control and predation) was significant for PC1 (F =5.65; df =1, 135; P =0.0188). Multiple comparisons revealed that control solid and predation solid were significantly different from each other (P =0.0364), but there were no differences between any other treatment pairs, indicating that Ae. triseriatus responded primarily to cues present in the solid material (Fig. 2).
Fig. 2.
Experiment 2: differences in behavioral PC1 (mean ± SE) of Ae. triseriatus between fluid and solid cues of predation risk from Tx. rutilus.
Discussion
Ae. albopictus reduced movement and increased resting at the surface of the containers when predators were feeding on other Ae. albopictus, and their behavior differed significantly from all treatments without feeding predators (Fig. 1). These results suggest that Ae. albopictus larvae respond only to the cues that are created by the act of predation. Several arthropods respond to damaged conspecifics (Huryn and Chivers 1999, Wisenden et al. 1999, Gall and Brodie 2009); our data are consistent with a similar pattern of response in Ae. albopictus. Alternatively, Ae. albopictus may respond to the excrement of C. appendiculata because the larvae significantly altered their behavior in response to feeding C. appendiculata but not in response to nonfeeding C. appendiculata. Because C. appendiculata larvae were in the predator alone treatments for 5 d, it is likely that they excreted in the cups, albeit in low levels compared with those C. appendiculata that were actively feeding. Although not significantly different from the controls, the mean for PC1 for Ae. albopictus larvae in the predator alone treatment indicated slightly greater frequency of resting at the surface (Fig. 1), which may have been a result of the limited amount of feces produced by the nonfeeding C. appendiculata. Our results indicate that Ae. albopictus larvae show no significant response to waterborne predation risk cues emanating from nonfeeding C. appendiculata and thus indicate that the principal cue to predation risk is derived in some way from the act of predation.
Previous experiments show that both Ae. albopictus and Ae. triseriatus reduce their activity at the bottom of the containers in response to the physical presence of C. appendiculata (Kesavaraju et al. 2007a). The present experiment did not test for effects of the physical presence, but together with these previous results, suggests that these Aedes may use multiple cues (chemical, visual, tactile) to evaluate predation risk.
Ae. triseriatus increased low risk behaviors (resting in the surface) in water containing filtered solids from predation compared with either solid residues or filtered residues from living Ae. triseriatus. Thus, it seems that solid residues from Tx. rutilus predation are the main source of cues to predation perceived by Ae. triseriatus (Fig. 2). Although most of the literature on cues to predation in freshwater systems emphasizes dissolved chemical cues (Chivers and Smith 1998, Wisenden 2000), there are other examples of prey responses to solid residues. Fat-head minnows showed antipredatory behavior in the presence of feces from northern pike that had fed on conspecifics (Brown et al. 1995a,b, 1996). Brown et al. (1995a) prepared the stimulus by collecting, filtering, and freezing the feces of the predator and argued that the chemical alarm pheromone in the feces gets released slowly when they are resuspended in water. Brown et al. (1996) showed that behavior of their predator was attuned to the perception by prey of chemical alarm pheromones in the feces, because northern pike defecated away from their foraging area.
Although some cues may leach from solid residues into solution, it is unlikely that the observed response to filtered solids resulted from small amounts of soluble chemical cues that might have leached from solids during the filtering process. Kesavaraju et al. (2007b) prepared Tx. rutilus predation water by feeding Tx. rutilus with Ae. triseriatus in treatments similar to those in the present experiment and tested the behavior of Ae. triseriatus at different dilutions from the original concentration. Behavior of Ae. triseriatus in response to low levels of predation risk cues was not significantly different from no-predation control. The strong responses we observed in the present experiment to solid material suggest that contact with solid residues (e.g., while Aedes larvae are foraging) provides the cues to the presence of a predation threat. If these behavioral responses were induced by leached, dissolved chemicals from the feces, we also would have expected the predator fluid treatment to have yielded a significant change in behavior of Ae. triseriatus; it did not (Fig. 2). Kesavaraju and Juliano (2004) showed that fourth-instar Ae. triseriatus increased their low-risk behaviors in both predation water from Tx. rutilus feeding on conspecifics or on Ae. albopictus, indicating that solid residues from a variety of victims of predation can serve as predation risk cues.
Hechtel and Juliano (1997) held feeding fourth-instar Tx. rutilus in a small cage inside cups and recorded no increase in low-risk behavior of Ae. triseriatus outside of the cage. Our current study indicates that lack of behavioral response from Ae. triseriatus observed by Hechtel and Juliano (1997) probably arose because the solid cues were retained within the cage with Tx. rutilus. Although in experiment 1 the response of Ae. albopictus was tested in the presence of a different predator (C. appendiculata), the lack of response in predator only water is also consistent with the hypothesis that Ae. albopictus, like Ae. triseriatus, perceives risk of predation via solid residues created by the act of predation. We cannot at this point determine whether those cues arise from damage to the victim at the time of attack (e.g., bits of uneaten victim), or from the products of digestion of the victim that are in predator feces, or from some combination of those sources (Wisenden 2000, Schoppner and Relyea 2005).
Antipredator responses of prey have been typically studied by recording the behavior of prey in water that had caged feeding predators (e.g., Relyea 2000) or in water that had held a feeding predator (e.g., Kesavaraju and Juliano 2004), or by combining visual cues with water that had held a nonfeeding predator (e.g., Chivers et al. 2001). Many of these studies indicate that these predation risk cues are waterborne chemical and visual cues (e.g., Chivers et al. 2001). The results described in this study are, to our knowledge, are among the first to show that the filtered solid material from a feeding predator also can serve as predation risk cues. Some aquatic prey such as mosquitoes show antipredator responses only in the presence of cues that are created by actual predation on conspecifics and not to the cues from a nonfeeding predator (Ferrari et al. 2007b, this study). Antipredatory responses are costly because reduced activity also means reduced foraging opportunities that favor threat sensitive responses to predation risk (Ferrari et al. 2007b, Kesavaraju et al. 2007b). Because these Aedes occur in small aquatic habitats, dissolved cues from the predator itself may be pervasive, and responses to such cues too costly. Cues derived from the act of predation provide a more specific indication of an immediate threat, and thus may be more efficient cues, particularly in such small water volumes. Solid residues of predation also may provide more specific information than would dissolved chemical cues, because solid residues encountered on the bottom by a forager may provide information about the spatial distribution of predation risk.
Predation risk cues in aquatic systems degrade if not replenished by additional predation, and prey may alter their responses depending on the degradation level (Ferrari et al. 2007a). Furthermore, pupation of predators removes the source of new predation cues. Modulating the degree of behavioral response to correspond with cue amount, as it changes with natural degradation of solid cues created by predation, would help these Aedes limit costly antipredatory behaviors to times when those responses are most advantageous.
Acknowledgments
We thank M. Mathews for help with the experiments. This research was supported by National Institutes of Health National Institute of Allergy and Infectious Disease grant R01 AI-44793 (Illinois State University subcontract) and National Science Foundation grant DEB 0507015) (Illinois State University Institutional Animal Care and Use Committee protocol 01-2005).
References Cited
- Brown GE, Adrian JC, Smyth E, Leet H, Brennan S. Ostariophysan alarm pheromones: laboratory and field tests of the functional significance of nitrogen oxides. J Chem Ecol. 2000;26:139–154. [Google Scholar]
- Brown GE, Chivers DP, Smith RJF. Fat-head minnows avoid conspecific and heterospecific alarm pheromones in the feces of northern pike. J Fish Biol. 1995a;47:387–393. [Google Scholar]
- Brown GE, Chivers DP, Smith RJF. Localized defecation by pike: a response to labeling by cyprinid alarm pheromone. Behav Ecol Sociobiol. 1995b;36:105–110. [Google Scholar]
- Brown GE, Chivers DP, Smith RJF. Effects of diet on localized defecation by northern pike, Esox lucius. J Chem Ecol. 1996;22:467–475. doi: 10.1007/BF02033649. [DOI] [PubMed] [Google Scholar]
- Chivers DP, Smith RJF. Chemical alarm signalling in aquatic predator/prey systems: a review and prospectus. Ecoscience. 1998;5:338–352. [Google Scholar]
- Chivers DP, Mirza RS, Bryer PJ, Kiesecker JM. Threat-sensitive predator avoidance by slimy sculpins: understanding the importance of visual versus chemical information. Can J Zool. 2001;79:867–873. [Google Scholar]
- Chivers DP, Wisenden BD, Hindman CJ, Michalak T, Kusch RC, Kaminskyj SW, Jack KL, Ferrari MCO, Pollock RJ, Halbgewachs CF, et al. Epidermal ‘alarm substance’ cells of fishes maintained by non-alarm functions: possible defence against pathogens, parasites and UVB radiation. Proc R Soc B. 2007;274:2611–2619. doi: 10.1098/rspb.2007.0709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferrari MCO, Messier F, Chivers DP. Degradation of chemical alarm cues under natural conditions: risk assessment by larval woodfrogs. Chemoecology. 2007a;17:263–266. [Google Scholar]
- Ferrari MCO, Messier F, Chivers DP. Variable predation risk and the dynamic nature of mosquito antipredator responses to chemical alarm cues. Chemoecology. 2007b;17:223–229. [Google Scholar]
- Ferrari MCO, Messier F, Chivers DP, Messier O. Can prey exhibit threat-sensitive generalization of predator recognition? Extending the predator recognition continuum hypothesis. Proc R Soc B. 2008;275:1811–1816. doi: 10.1098/rspb.2008.0305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferris G, V, Rudolf W. Responses of larval dragonflies to conspecific and heterospecific predator cues. Ecol Entomol. 2007;32:283–288. [Google Scholar]
- Gall BG, Brodie ED., Jr Behavioral avoidance of injured conspecific and predatory chemical stimuli by larvae of the aquatic caddisfly Hesperophylax occidentalis C. J Zool. 2009;87:1009–1015. [Google Scholar]
- Griswold MW, Lounibos LP. Does differential predation permit invasive and native mosquito larvae to coexist in Florida? Ecol Entomol. 2005;30:122–127. doi: 10.1111/j.0307-6946.2005.00671.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gyssels F, Stoks R. Behavioral responses to fish kairomones and autotomy in a damselfly. J Ethol. 2006;24:79–83. [Google Scholar]
- Hamilton IM, Heithaus MR. The effects of temporal variation in predation risk on antipredator behaviour: an empirical test using marine snails. Proc R Soc B. 2001;268:2585–2588. doi: 10.1098/rspb.2001.1857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hawley WA, Reiter P, Copeland RS, Pumpuni CB, Craig CB., Jr Aedes albopictus in North America: probable introduction in used tires from northern Asia. Science (Wash, DC) 1987;236:1114–1116. doi: 10.1126/science.3576225. [DOI] [PubMed] [Google Scholar]
- Hechtel LJ, Juliano SA. Effects of a predator on prey metamorphosis: plastic responses by prey or selective mortality? Ecology. 1997;78:838–851. [Google Scholar]
- Hughes JJ, Ward D, Perrin MR. Predation risk and competition affect habitat selection and activity of Namib desert gerbils. Ecology. 1994;75:1397–1405. [Google Scholar]
- Huryn AD, Chivers DP. Contrasting behavioral responses by detritivorous and predatory mayflies to chemicals released by injured conspecifics and their predators. J Chem Ecol. 1999;25:2729–2740. [Google Scholar]
- Juliano SA. Geographic variation in vulnerability to predation and starvation in larval treehole mosquitoes. Oikos. 1989;56:99–108. [Google Scholar]
- Juliano SA, Reminger L. The relationship between vulnerability to predation and behavior of larval tree-hole mosquitoes: geographic and ontogenetic differences. Oikos. 1992;63:465–467. [Google Scholar]
- Juliano SA, Gravel ME. Predation and the evolution of prey behavior: an experiment with tree hole mosquitoes. Behav Ecol. 2002;13:301–311. [Google Scholar]
- Juliano SA, Lounibos LP. Ecology of invasive mosquitoes: effects on resident species and on human health. Ecol Lett. 2005;8:558–574. doi: 10.1111/j.1461-0248.2005.00755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kesavaraju B, Juliano SA. Differential behavioral responses to water-borne cues to predation in two container-dwelling mosquitoes. Ann Entomol Soc Am. 2004;97:194–201. doi: 10.1603/0013-8746(2004)097[0194:dbrtwc]2.0.co;2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kesavaraju B, Alto BW, Lounibos LP, Juliano SA. Behavioural responses of larval container mosquitoes to a size-selective predator. Ecol Entomol. 2007a;32:262–272. doi: 10.1111/j.1365-2311.2006.00846.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kesavaraju B, Damal K, Juliano SA. Threat-sensitive behavioral responses to concentrations of water-borne cues from predation. Ethology. 2007b;113:199–206. doi: 10.1111/j.1439-0310.2006.01317.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kesavaraju B, Damal K, Juliano SA. Do natural container habitats impede invader dominance? Predator-mediated coexistence of invasive and native container-dwelling mosquitoes. Oecologia. 2008;155:631–639. doi: 10.1007/s00442-007-0935-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kesavaraju B, Juliano SA. Behavioral responses of Aedes albopictus to a predator are correlated with size-dependent risk of predation. Ann Entomol Soc Am. 2008;101:1150–1153. doi: 10.1603/0013-8746-101.6.1150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keppel E, Scrosati R. Chemically mediated avoidance of Hemigrapsus nudus (Crustacea) by Littorina scutulata (Gastropoda): effects of species coexistence and variable cues. Anim Behav. 2004;68:915–920. [Google Scholar]
- Laurila A, Jarvi-Laturi M, Pakkasmaa S, Merila J. Temporal variation in predation risk: stage-dependency, graded responses and fitness costs in tadpole antipredator defences. Oikos. 2004;107:90–99. [Google Scholar]
- Lima SL. Stress and decision making under the risk of predation: recent developments from behavioral, reproductive, and ecological perspectives. Adv Stud Behav. 1998;27:215–290. [Google Scholar]
- Lima SL, Dill LM. Behavioral decisions made under the risk of predation: a review and prospectus. Can J Zool. 1990;68:619–640. [Google Scholar]
- Preisser EL, Bolnick DI, Benard MF. Scared to death? Behavioral effects dominate predator–prey interactions. Ecology. 2005;86:501–509. [Google Scholar]
- Relyea RA. Trait-mediated indirect effects in larval anurans: reversing competition with the threat of predation. Ecology. 2000;81:2278–2289. [Google Scholar]
- Relyea RA. Morphological and behavioral plasticity of larval anurans in response to different predators. Ecology. 2001;82:523–540. [Google Scholar]
- Relyea RA, Werner EE. Quantifying the relation between predator-induced behavior and growth performance in larval anurans. Ecology. 1999;80:2117–2124. [Google Scholar]
- Ripple WJ, Beschta RL. Wolves and the ecology of fear: can predation risk structure ecosystems? Bioscience. 2004;54:755–766. [Google Scholar]
- Rohr JR, Madison DM. A chemically mediated trade-off between predation risk and mate search in newts. Anim Behav. 2001;62:863–869. [Google Scholar]
- Schoeppner NM, Relyea RA. Damage, digestion, and defense: the roles of alarm cues and kairomones for inducing prey defenses. Ecol Lett. 2005;8:505–512. doi: 10.1111/j.1461-0248.2005.00744.x. [DOI] [PubMed] [Google Scholar]
- Sih A, Moore RD. Delayed hatching of salamander eggs in response to enhanced larval predation risk. Am Nat. 1993;142:947–960. doi: 10.1086/285583. [DOI] [PubMed] [Google Scholar]
- Tollrian R, Heibl C. Phenotypic plasticity in pigmentation in Daphnia induced by UV radiation and fish kairomones. Funct Ecol. 2004;18:497–502. [Google Scholar]
- Trussell GC, Ewanchuk PJ, Bertness MD, Silliman BR. Trophic cascades in rocky shore tide pools: distinguishing lethal and nonlethal effects. Oecologia. 2004;139:427–432. doi: 10.1007/s00442-004-1512-8. [DOI] [PubMed] [Google Scholar]
- Van Buskirk J, Arioli M. Dosage response of an induced defense: how sensitive are tadpoles to predation risk? Ecology. 2002;83:1580–1585. [Google Scholar]
- Van Buskirk J, Muller C, Portmann A, Surbeck M. A test of the risk allocation hypothesis: tadpole responses to temporal change in predation risk. Behav Ecol. 2002;13:526–530. [Google Scholar]
- Werner EE. Nonlethal effects of a predator on competitive interactions between two anuran larvae. Ecology. 1991;72:1709–1720. [Google Scholar]
- Werner EE, Gilliam JF, Hall DJ, Mittelbach GG. An experimental test of the effects of predation risk on habitat use in fish. Ecology. 1983;64:1540–1548. [Google Scholar]
- Wisenden BD. Olfactory assessment of predation risk in the aquatic environment. Proc R Soc B. 2000;355:1205–1208. doi: 10.1098/rstb.2000.0668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wisenden BD. Active space of chemical alarm cue in natural fish populations. Behaviour. 2008;145:391–407. [Google Scholar]
- Wisenden BD, Cline A, Sparkes TC. Survival benefit to antipredator behavior in the amphipod Gammarus minus (Crustacea: Amphipoda) in response to injury-released chemical cues from conspecifics and heterospecifics. Ethology. 1999;105:407–414. [Google Scholar]