Abstract
How tumor-infiltrating lymphocytes (TILs) that are tumor-specific but functionally tolerant persist in the antigen-expressing tumor tissue is largely unknown. We have previously developed a modified TRansgenic Adenocarcinoma of the Mouse Prostate (TRAMP) model where prostate cancer cells express the T-cell epitope SIYRYYGL (SIY) recognized by CD8 T cells expressing the 2C T-cell receptor (TCR) (referred to as TRP-SIY mice). In TRP-SIY mice, activated 2C T cells rapidly become tolerant following infiltration into the prostate tumor. In this study, we show that tolerant 2C T cells persist in the prostate tumor of TRP-SIY mice by proliferating slowly in a tumor-dependent, but antigen-, interleukin (IL)-7- and IL-15-independent manner. We also show that disappearance of 2C T cells from the lymphoid organs of TRP-SIY mice are due to antigen-induced T-cell contraction rather than altered trafficking or generalized T-cell depletion in the mice. Finally, we show that clonal T cells unreactive to SIY are equally capable of persisting in the prostate tumor. These findings suggest that while functional tolerance of TILs is induced by antigen, persistence of tolerant TILs in the tumor tissue is mediated by a novel mechanism: slow proliferation independent of antigen and homeostatic cytokines. These results also allow CD8 T-cell survival in the tumor environment to be compared with T-cell survival in chronic infection.
Keywords: antigen, cytokines, tolerance, tumor-associated factors, tumor-infiltrating lymphocytes
Introduction
Tumor-infiltrating lymphocytes (TILs) are frequently found in human tumors, for example, of the skin, lung, prostate, pancreas, ovaries and brain.1, 2, 3, 4, 5 Among the TILs, studies have focused on cytolytic CD8 T cells as many of these cells have been found to react to tumor-derived self-epitopes. For example, CD8 T cells from human melanomas are shown to recognize peptides derived from tyrosinase, the MAGE family of proteins and mutated β-catenin.6 However, CD8 TILs from human tumors are often functionally inactivated, failing to express interferon-γ after in vitro stimulation.7, 8 Similarly, CD8 TILs from human prostate cancer patients did not proliferate following stimulation through the T-cell receptor (TCR).9
The use of TCR-transgenic CD8 T cells specific for tumor antigens in mice has unequivocally demonstrated functional tolerance of TILs. In an autochthonous tumor model of TRansgenic Adenocarcinoma of the Mouse Prostate (TRAMP),10 Anderson et al.11 showed that adoptively transferred naive CD8 T cells expressing a transgenic TCR specific for an epitope derived from the large T antigen undergo an abortive proliferative response in the prostate-draining lymph nodes (PDLNs). The persisting transgenic CD8 T cells in the prostate tumor tissue failed to degranulate and secrete interferon-γ or granzyme following stimulation with antigenic peptide in vitro.11 Even if the transferred T cells were activated by peptide-pulsed dendritic cells in the recipient mice, those persisting in the prostate tumor gradually lost antigen reactivity. In the same TRAMP model, we have previously introduced a nominal T-cell epitope SIYRYYGL (SIY) that is recognized by CD8 T cells expressing the 2C TCR (referred to as TRP-SIY mice).12 Upon adoptive transfer of CD8 2C T cells into TRP-SIY mice and intranasal infection with influenza virus WSN-SIY that expresses the SIY epitope,13 2C T cells were robustly activated in the lung draining lymph nodes. Following proliferation and acquisition of effector functions, the activated 2C cells infiltrated prostate tumor tissue but rapidly became tolerized as indicated by their expression of programmed death-1 (PD-1) and loss of effector functions, including expression of interferon-γ or tumor necrosis factor-α, killing antigen-loaded target cells and proliferation to SIY stimulation.12 We have further demonstrated that intraprostatic injection of SIY-loaded bone marrow-derived dendritic cells can delay tolerance induction of infiltrating 2C T cells and reactivate already tolerized 2C T cells in the prostate tumor tissue.14 These data are consistent with reports that reactivated TILs have had some success in clinical trials.15, 16 Together, this and other evidence in both human and mice indicates that tumor-reactive CD8 TILs are functionally tolerant, but can be reactivated to target tumor cells.
During T-cell development, autoreactive T cells are either deleted by negative selection in the thymus or become tolerant in the peripheral lymphoid organs where they encounter self-antigen. These tolerant T cells are eventually deleted by exhaustion due to constant stimulation by self-antigen. Similar to tolerant T cells in the lymphoid organs, the tolerant tumor-reactive TILs are also constantly exposed to antigen in the tumor tissue. However, despite their tolerant state and exposure to antigen, tumor-reactive CD8 TILs persist in tumor tissues. In humans, CD8 TILs are found in both early- and late-stage tumors,4, 17 although it is unknown whether the same TILs persist throughout the different stages of tumor development. In mice, adoptive transfer of clonal CD8 T cells that can be uniquely identified has unequivocally demonstrated the persistence of tumor-specific CD8 TILs.12, 18, 19 In the TRP-SIY model, 2C T cells can persist in the prostate tumor tissue in a tolerant state for at least 4 months following initial transfer and activation.12 Similarly, transferred TCR-transgenic T cells also persist in the tumor tissues of other mouse tumor models.18, 19 Thus, it is puzzling how the tolerant TILs persist in the tumors.
Many studies have investigated the mechanisms of naïve and memory T-cell survival. Survival of naïve CD8 T cells requires TCR interaction with self-peptide–MHC complexes and interleukin (IL)-7.20, 21, 22, 23, 24 Because naive T cells do not proliferate under physiological conditions, their stable maintenance (homeostasis) is largely through replenishment by the newly generated T cells from the thymus. In contrast, survival of memory CD8 T cells does not require TCR–self-peptide–MHC interaction. To maintain their homeostasis, memory CD8 T cells undergo slow proliferation in response to IL-7 and IL-15.22, 25, 26, 27, 28 Of greater relevance to the persistence of tolerant TILs is the persistence of antigen-specific CD8 T cells following chronic infections by viruses such as lymphocytic choriomeningitis virus (LCMV), hepatitis B and C viruses.29 Survival in these situations where chronic exposure to antigen leads to T-cell exhaustion (rather than lack of costimulation leading to tolerance) is generally driven by rapid antigen-induced proliferation.30 To our knowledge, there has been no study of how TILs survive in the tumor environment.
In this study, we have assessed the role of antigen, IL-7, IL-15, tumor-associated factors and cell trafficking in the persistence of CD8 T cells in the prostate tumors of TRAMP and TRP-SIY mice. Our results show that like antigen-specific CD8 T cells in chronic infection, tolerant tumor-reactive CD8 T cells do not respond to IL-7 and IL-15. However, unlike antigen-specific CD8 T cells in chronic infection, persistence of tolerant tumor-reactive CD8 T cells in the prostate tumor is antigen-independent and likely mediated by slow proliferation of resident T cells in response to tumor-associated factors.
Materials and methods
Mice and viruses
2C TCR, congenic Thy1.1+ 2C TCR and OT-I TCR-transgenic mice on RAG1−/− and B6 backgrounds were maintained at the Massachusetts Institute of Technology (MIT) Animal Care Facility. TRAMP, TRP-SIY, B6 and B6-SIY mice were also maintained in the MIT facility and used as recipients at 3–5 months of age. The B6-SIY strain is the same as the PB-SIY strain previously published.12 Recombinant WSN (H1N1) influenza virus with either SIYRYYGL (SIY) or SIINFEKL (SIIN) peptide engineered into the neuraminidase stalk31, 32 was grown on Madine–Darby canine kidney cells. For infection, mice were anesthetized with 2.5% avertin and intranasally infected with 100 plaque forming units of WSN-SIY or WSN-SIIN influenza virus in 50 µl of phosphate-buffered saline (PBS). All research with mice was performed in compliance with the institutional guidelines.
Antibodies and reagents
Anti-mouse monoclonal antibodies, CD16/32 (Fc blocker), streptavidin-APC, vβ5-FITC, vα2-PE, CD127 (IL-7Rα)-FITC clone A7R34, CD122 (IL-2/15Rβ)-FITC clone TM-β1, PD-1-PE clone J43, CD62L-PE, CCR7-PE, CD8α clone 53-6.7-PerCP-Cy5.5/APC/PE/FITC and CD90.1 (Thy1.1)-APC/FITC were purchased from BioLegend (San Diego, CA, USA), BD Biosciences (San Jose, CA, USA) and eBioscience (San Diego, CA, USA). 1B2 monoclonal antibody, specific for the 2C TCR, was purified from hybridoma and biotinylated in our lab. Pierce Chemical 4′,6-diamidino-2-phenylindole hydrochloride and propidium iodide were purchased from VWR (West Chester, PA, USA) and Sigma-Aldrich (St Louis, MO, USA), respectively.
Lymphocyte isolation and transfer
Lymph nodes and spleens were gently mashed between rough surfaces of two microscope slides immersed in RPMI 1640 media containing 5% fetal bovine serum and 10 mM HEPES buffer solution (RPMI complete) to release lymphocytes. Cell suspensions were filtered through an 80 µm nylon mesh (Sefar). Red blood cells in splenocytes suspension were lysed with 144 mM ammonium chloride and 17 mM Tris-HCl, pH7.4 solution. To extract cells from the lung, tissues were ground through a cell strainer and digested with 2 mg/ml collagenase A solution at 37 °C for 1 h, vortexing at 15–20 min intervals. Tissue debris was removed by Percoll centrifugation, followed by red blood cell lysis. Prostate lobes were harvested by microdissection33 and digested with 1 mg/ml collagenase A at 37 °C, for about 45 min, vortexing at 15–20 min intervals. Digested tissues were diluted with RPMI complete, gently mashed and filtered. The viable cells for each tissue specimen was counted using a hemacytometer and trypan blue exclusion. For adoptive transfer, cells from lymph nodes and spleen of 2C RAG1−/− mice were injected retroorbitally (1×106–2×106 2C cells in 100 µl HBSS) into infected mice that were still under anesthesia. A relatively large number of 2C T cells were adoptively transferred into recipient mice for two reasons. First, the large number of activated 2C T cells generated following influenza virus infection facilitates quantification of persisting 2C cells in the prostate tumor tissue over a long period of time. Second, compared to transferring 500 or 10 000 2C cells, 2C cell activation and development into memory T cells are not significantly affected by transferring 1×106–2×106 2C T cells.34
For memory 2C cell transfer, B6 mice were transferred with Thy1.1+ 2C T cells and infected intranasally with WSN-SIY virus. Thirty dpi, 2C cells were purified from spleen using the magnetic CD8α+ T cell isolation kit (Miltenyi Biotec, Inc., Auburg, CA). A portion of the enriched cell suspension was analyzed by flow cytometry to determine the frequency of Thy1.1+ CD8+ 2C T cells. The cells were injected into TRAMP or TRP-SIY mice as above (5×105 Thy1.1+ CD8+ 2C cells per recipient).
Flow cytometry
Cells were stained in FACS buffer (PBS with 1% bovine serum albumin) and 0.1% sodium azide) on ice. Anti-mouse CD16/32 (Fc blocker) was added to the cell suspension for 10 min on ice prior to adding the primary biotinylated antibody. Following washing, the cell suspension was incubated with the secondary and fluorophore-conjugated antibodies. The cells were washed and resuspended in 50-200 µl of 4′,6-diamidino-2-phenylindole hydrochloride or propidium iodide (1 µg/ml) solution except where indicated. For CCR7-PE (eBioscience) staining, cells were incubated in a 37 °C water bath following the manufacturer's instructions. Samples were analyzed using a LSRII or FACSCalibur flow cytometer (BD Biosciences). Further data analysis was carried out using FlowJo software (Tree Star, Inc., Ashland, OR, USA).
5'-bromo-2'-deoxyuridine (BrdU) proliferation assays
Thirty days after infection and retroorbital 2C T-cell transfer, recipient mice were fed with 0.8 mg/ml BrdU in their drinking water kept in opaque bottles. The BrdU water was changed daily for up to 16 days. For short-term (2 days) BrdU labeling, mice were injected intraperitoneally (i.p.) with 2 mg BrdU in 200 µl (BD Biosciences). 2C T cells that incorporated BrdU were determined by flow cytometry using a BrdU Flow Kit (BD Biosciences). Antibody volumes suggested by the manufacturer were adjusted to 100 µl for each sample (1∶100 dilution). Data analysis was carried out using FlowJo software and Microsoft Excel spreadsheet functions. The percentage of 2C T cells that incorporated BrdU in each tissue was calculated as follows: (BrdU+Thy1.1+CD8+ cell/Total Thy1.1+CD8+ cells)×100%.
Phosphorylated Stat5 (pStat5) intracellular staining
Freshly isolated cell suspensions of prostates were stained with anti-mouse CD16/32, Thy1.1-APC (or biotinylated 1B2 followed by streptavidin-APC) and CD8α-FITC antibodies. Then cells were stimulated with IL-7 (20 ng/ml) or IL-15 (40 ng/ml) prepared in prewarmed RPMI complete by incubating them in a 37 °C water bath for 30 min. Controls did not receive any cytokine. After stimulation, the cells were fixed with 1 ml of 0.37% of paraformaldehyde at 37 °C for 10 min, washed with 1× PBS and then permeabilized with cold 90% methanol on ice for 30 min. Cells were washed with wash buffer (PBS containing 5 g/L bovine serum albumin) and then stained with pStat5 antibody (Cell Signaling Technology, Beverly, MA, USA) following the manufacturer's instruction. After incubation at room temperature for 30 min, cells were washed and stained with either the secondary goat anti-rabbit IgG-PE or the isotype control antibody for 30 min at room temperature. The cells were washed before flow cytometry analysis.
RNA extraction and quantitative real-time PCR
The RNeasy Plus Mini and the RNeasy Fibrous Tissue Mini Kits (QIAGEN, Valencia, CA, USA) were used for RNA extraction from bone marrows and prostates respectively according to the manufacturer's instruction. Extracted RNA was quantified using a Nanodrop spectrophotometer and reverse transcribed into cDNA (RT product) using the Taqman Reverse Transcription Reagents Kit (Applied Biosystems, Foster City, CA, USA). Samples were stored at −20 °C until quantitative real-time PCR analysis. Quantitative real-time PCR was performed in duplicates of 20 µl per well. Each well contained 100 ng (based on original RNA concentration) RT product and a cocktail of 1 µl of primer (IL-7 or IL-15 or GAPDH), DNase-RNase-free water and 2× Taqman Universal PCR Master Mix. An ABI 7500 real-time PCR system (Applied Biosystems) was used with the following conditions: 2 min at 50 °C, 10 min at 95 °C followed by 40 cycles of 15 s at 95 °C, and 1 min at 60 °C. Relative expression levels were calculated using Microsoft Excel.
Statistical analyses
All P values were calculated using the unpaired two-tailed equal variance Student's t-tests.
Results
Tumor-specific CD8 T cells persist in the prostate but disappear from the spleen of TRP-SIY mice
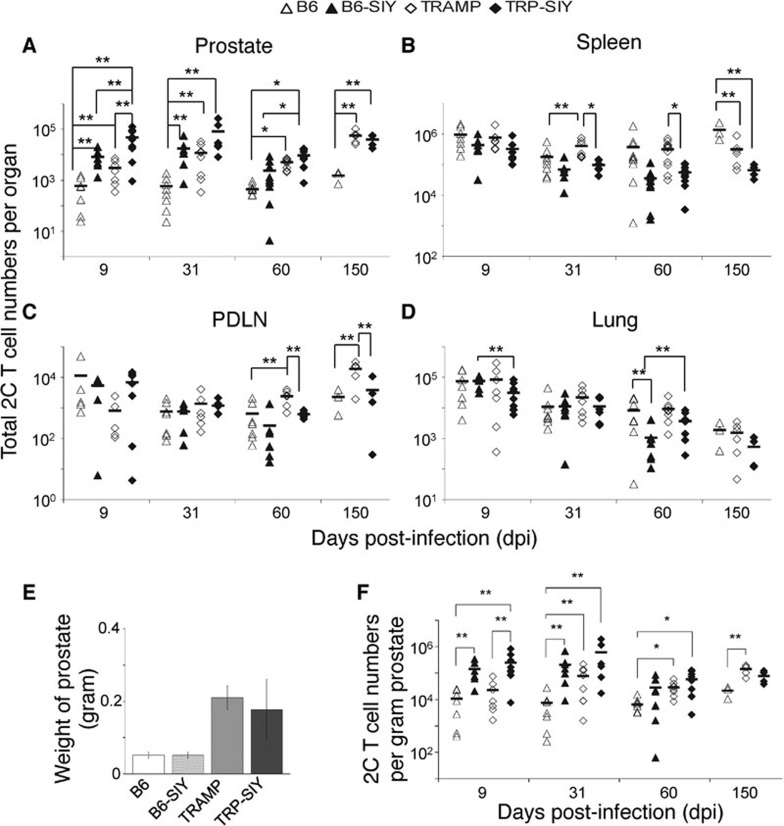
To determine the persistence of SIY-specific 2C T cells in different tissues over time, C57BL/6 (B6), B6 mice expressing the SIY transgene (B6-SIY), TRAMP and TRP-SIY mice were adoptively transferred with 2C cells and infected with WSN-SIY virus intranasally. Different days post-infection (dpi), the numbers of 2C cells in the prostate, spleen, PDLN and the lung were quantified by cell counting and flow cytometry analysis (Figure 1a–d). Overall, a larger number of 2C cells were recovered from the prostates of TRP-SIY mice than the prostates of B6 mice (Figure 1a). However, the prostates of tumor-bearing TRAMP and TRP-SIY mice were three times the size of those from age-matched B6 and B6-SIY mice (Figure 1e), we therefore normalized the number of 2C cells by the weight of the prostate to account for the differing masses of tissue samples (Figure 1f). At 9 dpi, the number of 2C cells per gram of prostate was increased by the presence of antigen, likely due to further proliferation of effector 2C cells following encounter with SIY in the prostate.30 By 31 dpi tumor-specific effects begin to play a role and the number of 2C cells per gram of prostate was similar between TRAMP and TRP-SIY mice. This similarity persisted at 60 and 150 dpi. However, at 31 and 60 dpi, the number of 2C cells per gram of prostate in B6-SIY mice resembled that in tumor mice, not that in B6 controls. Therefore, while 2C cells persisted independent of antigen in TRAMP mice, 2C cell persistence in TRP-SIY mice could be either antigen-dependent (as in the B6-SIY mice) or independent as in the TRAMP mice.
Figure 1.
Spatial and temporal distribution of 2C T cells. Naive 2C cells were transferred into B6, B6-SIY, TRAMP and TRP-SIY mice and infected with WSN-SIY virus. At the indicated dpi, single-cell suspensions were prepared from prostate, spleen, PDLN and lung. Cells were counted and assayed for the presence of 2C cells by staining with antibodies specific for 2C TCR and CD8 followed by flow cytometry. The 2C cell number in each tissue was calculated by multiplying the total cell numbers by the frequency of 2C (CD8+ and 2C TCR+) cells in the tissue. (a–d) The total 2C cell numbers in different tissues at the indicated dpi pooled from at least three separate experiments for each time point. (e) Comparison of average prostate weights of B6, B6-SIY, TRAMP and TRP-SIY mice between 5 and 7 months of age. The prostate lobes were dissected from the urogenital system and weighed. The error bars are standard deviations (n≥5–7 per group). (f) The number of 2C cells per gram of prostate. The number was calculated by normalizing the number of total 2C cells (from (a)) to the weight of the prostate for each type of mouse. Each symbol represents one mouse. The lines indicate the average 2C cell numbers (n=4–9 per group). *P<0.01, **P≤0.05 are indicated. B6, C57BL/6; dpi, days post-infection; PDLN, prostate draining lymph node; SIY, SIYRYYGL; TCR, T-cell receptor; TRAMP, TRansgenic Adenocarcinoma of the Mouse Prostate; TRP-SIY, TRAMP mice expressing SIY antigen in prostate tumor tissue; WSN-SIY, influenza A/WSN virus expressing the SIY epitope.
In the spleen, the number of 2C cells at 9 dpi was similar among B6, B6-SIY, TRAMP and TRP-SIY mice (Figure 1b). By day 31, a more pronounced contraction of 2C cells can be seen in the spleens of mice expressing SIY (B6 vs. B6-SIY and TRAMP vs. TRP-SIY) and this difference persists to 60 and 150 dpi between TRAMP and TRP-SIY mice. Fewer 2C cells were also detected in the PDLN of TRP-SIY mice than TRAMP mice at 60 and 150 dpi (Figure 1c). In the lung, the number of persisting 2C cells decreased continuously from 9 to 150 dpi in all four types of mice, consistent with a previous report.13 Together, these results show an enhanced antigen-dependent 2C cell contraction in lymphoid organs, especially in the spleen, following an influenza virus-induced response in TRP-SIY mice, but not in the prostate, the site of ongoing antigen expression.
T-cell persistence in the prostate is tumor-dependent, but contraction of 2C cells from the spleen is antigen-specific in TRP-SIY
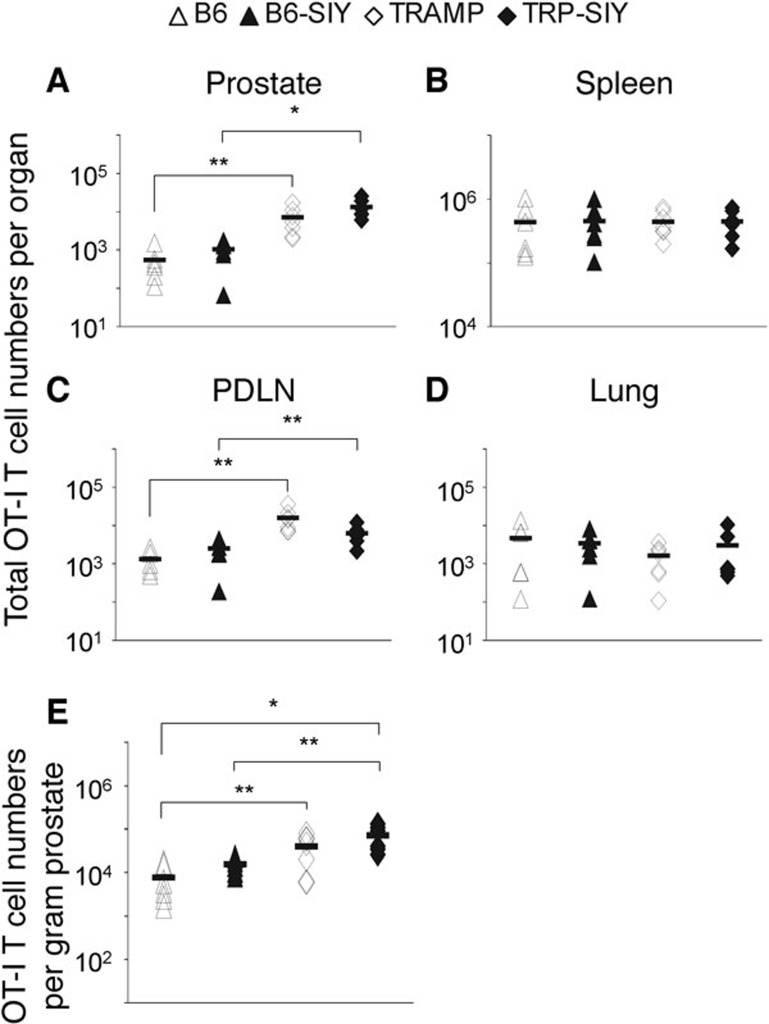
To further examine antigen-independent 2C cell persistence in the prostate and distinguish the effect of antigen (SIY) and tumor, we measured the distribution of another clonotypic CD8 T cell, OT-I, which is specific for SIINFEKL (SIIN), an antigen absent from all the mice. B6, B6-SIY, TRAMP and TRP-SIY mice were adoptively transferred with naive OT-I T cells and infected with the WSN virus expressing SIIN (WSN-SIIN). Sixty dpi, OT-I cells were quantified in the prostate, spleen, PDLN and the lung (Figure 2a–d). Significantly more OT-I cells were recovered from the prostate of TRP-SIY than B6-SIY mice and TRAMP than B6 mice (Figure 2a) even after normalization to the weight of the prostate (Figure 2e). More OT-I cells were also detected in the PDLN of TRP-SIY than B6-SIY mice and TRAMP than B6 mice (Figure 2c). However, there was no difference in the number of OT-I cells in the prostate between TRAMP and TRP-SIY mice or between B6 and B6-SIY mice with or without normalization. Similarly, there was no difference in the number of OT-I cells in the spleen or the lung among the four types of mice (Figure 2b and c). These results confirm that the accumulation of OT-I (and 2C) cells in the prostate tumor tissue of TRAMP and TRP-SIY mice is tumor-dependent but antigen-independent, whereas depletion of 2C cells from the spleen of TRP-SIY mice is antigen-specific.
Figure 2.
Tumor-dependent versus antigen-dependent distribution of T cells in the prostate and spleen of TRP-SIY mice. Naive OT-I cells were transferred into B6, B6-SIY, TRAMP and TRP-SIY mice followed by WSN-SIIN infection. Sixty dpi, OT-I cells were quantified in the prostate, spleen, PDLN and lung as in Figure 1, except that cells were stained for vα2, vβ5 and CD8. (a–d) The total OT-I cell numbers in different tissues pooled from at least three separate experiments. (e) The number of OT-I cells per gram of prostate. Each symbol represents one mouse. The lines indicate the average OT-I cell numbers (n=5–6 per group). *P<0.01, **P≤0.05. B6, C57BL/6; dpi, days post-infection; PDLN, prostate draining lymph nodes; SIY, SIYRYYGL; TRAMP, TRansgenic Adenocarcinoma of the Mouse Prostate; TRP-SIY, TRAMP mice expressing SIY antigen in prostate tumor tissue; WSN-SIIN, influenza A/WSN virus expressing the SIINFEKL epitope.
Memory 2C cells from spleen of B6 mice do not preferentially traffic to the prostate of TRP-SIY mice
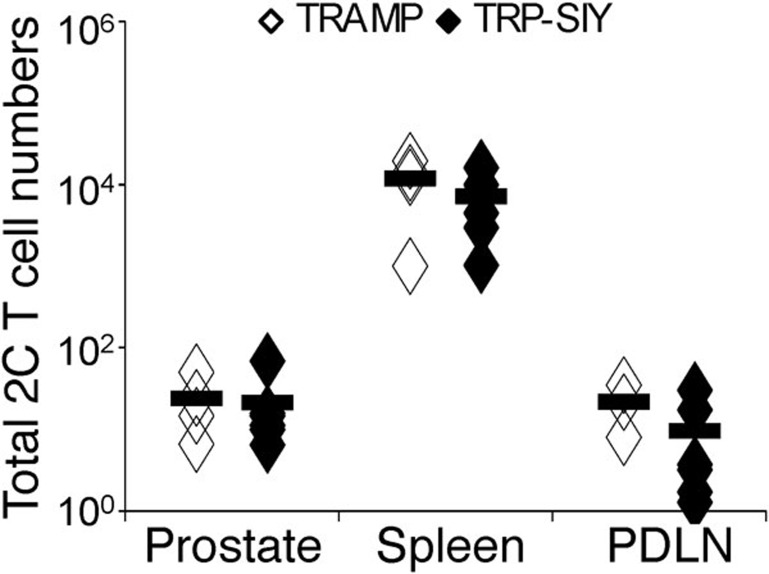
Persistence of CD8 T cells in a given organ, such as the prostate of TRP-SIY mice, is determined by cell trafficking into and out of that organ, as well as the proliferation and death of resident CD8 T cells in the organ. As there are approximately fivefold more 2C cells in the spleen than in the prostate of TRP-SIY mice at 31 and 60 dpi, preferential homing of 2C cells to the prostate from other tissues such as the spleen could account for the observed persistence of 2C cells in the prostate tumor tissue. To investigate this possibility, we transferred Thy1.1+ memory 2C cells purified from spleens of WSN-SIY-infected B6 mice into TRP-SIY and TRAMP mice, which were on the Thy1.2+ background. Thy1.1+ 2C cells were quantified in the prostate, spleen and PDLN 1.5 days after transfer. Majority of the transferred memory 2C cells were detected in the spleen with much smaller numbers (>100-fold fewer) in the prostate and PDLN (Figure 3). Critically, there was no difference in the corresponding organs between TRP-SIY and TRAMP recipients, suggesting that antigen does not cause preferential trafficking to the prostate and therefore cannot explain the persistence of tolerant 2C cells in the TRP-SIY prostate.
Figure 3.
Memory 2C cells from spleen of B6 mice do not preferentially traffic to the prostate of TRP-SIY mice. Memory 2C (Thy1.1+CD8+) cells were purified from spleen of WSN-SIY-infected B6 (Thy1.2) mice (30 dpi) and ∼5×105 cells were transferred into TRAMP and TRP-SIY (Thy1.2) recipient mice. One and half days later, transferred 2C cells (Thy1.1+CD8+) were quantified by cell counting and flow cytometry in the prostate, spleen and PDLN. The total 2C cell numbers are shown in each organ. Each symbol represents one mouse (n=4–6 per group, pooled from two separate experiments). Solid lines indicate the averages. B6, C57BL/6; dpi, days post-infection; PDLN, prostate draining lymph nodes; SIY, SIYRYYGL; TRAMP, TRansgenic Adenocarcinoma of the Mouse Prostate; TRP-SIY, TRAMP mice expressing SIY antigen in prostate tumor tissue; WSN-SIY, influenza A/WSN virus expressing the SIY epitope.
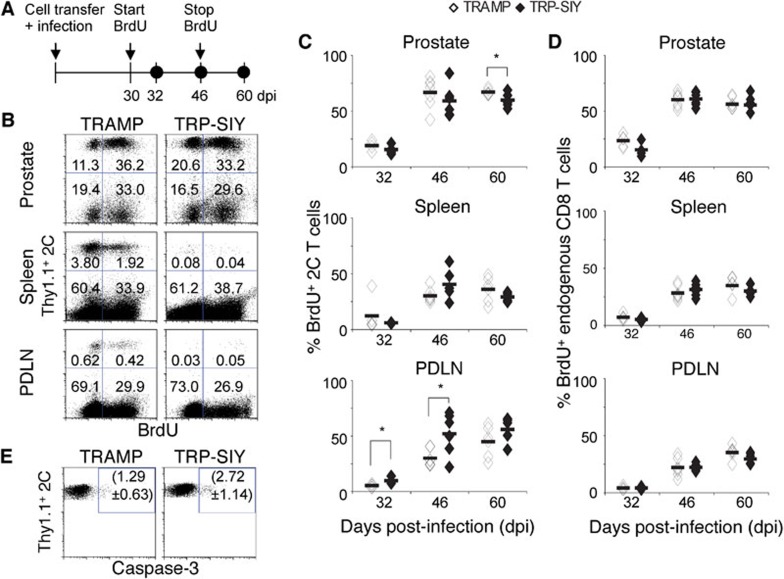
Resident tolerant 2C cells undergo slow proliferation in prostate tumor tissue of both TRAMP and TRP-SIY mice
Next, we measured proliferation of resident 2C cells in the prostate, PDLN and spleen of TRAMP and TRP-SIY mice by 5′-bromo-2′-deoxyruridine (BrdU) labeling (Figure 4a). Thirty days post-transfer of 2C cells and WSN-SIY infection, TRAMP and TRP-SIY mice were given BrdU. When BrdU was given for 1.5 and 16 days (i.e., 32 and 46 dpi, respectively), similar percentages of 2C cells (about 20% and 60%, respectively) incorporated BrdU in the prostates of TRAMP and TRP-SIY mice (Figure 4b and c). When BrdU was given for 16 days followed by 14 days' chase (i.e., 60 dpi) without BrdU, a lower percentage of BrdU+ 2C cells was detected in the prostate of TRP-SIY than TRAMP mice. This difference may correspond to death of BrdU+ 2C cells in TRP-SIY prostate. Consistently, a higher percentage of 2C cells was stained positive for caspase-3 in the prostate of TRP-SIY than TRAMP mice (Figure 4e), indicating a higher rate of apoptosis. Considering that division of one in two cells (50% division) gives rise to three cells, two of which are BrdU+ (66%), ∼60% BrdU labeling of 2C cells in 16 days would suggest that approximately 50% of 2C cells have divided in the prostate during this period. This once per month rate of division is similar to the slow homeostatic proliferation of memory CD8 T cells in mice.35 Furthermore, the same rate of BrdU incorporation in the prostate of TRAMP and TRP-SIY mice would suggest that the slow proliferation is independent of SIY antigen. Supporting this notion, endogenous presumably polyclonal CD8 T cells show similar rates of proliferation in the prostate of TRAMP and TRP-SIY mice (Figure 4b and d).
Figure 4.
Tolerant 2C cells undergo slow proliferation in the prostate tumor tissue independent of presence of SIY. (a) TRAMP and TRP-SIY mice were transferred with Thy1.1+ 2C cells and infected with WSN-SIY as described in Figure 1. At 30 dpi, mice were either injected with 2 mg BrdU i.p. for analysis 1.5 days later, i.e., the 32 dpi data point, or given water containing 0.8 mg/ml BrdU for 16 days (the 46 and 60 dpi data points). (b) Cells from prostates of recipient mice were analyzed 46 dpi for Thy1.1, CD8 and BrdU by flow cytometry. Representative Thy1.1 versus BrdU staining profiles are shown for CD8+ cells from prostate, spleen and PDLN of TRAMP and TRP-SIY mice. The numbers indicate percentages of BrdU− and BrdU+ 2C (Thy1.1+CD8+) cells or endogenous CD8 T cells. (c, d) The percentages of BrdU+ 2C (c) and endogenous CD8 (d) T cells at the indicated time points pooled from at least two separate experiments. Each symbol represents one mouse. Solid lines indicate the average percentage (n=4–6 per group). P<0.05. (e) Staining of cleaved caspase-3 in 2C cells in the prostate of TRAMP and TRP-SIY mice. Single-cell suspensions from prostates of TRAMP and TRP-SIY mice (85 dpi) were stained for Thy1.1, CD8 and caspase-3. Representative Thy1.1 versus caspase-3 staining profiles are shown for Thy1.1+CD8+ 2C cells. The numbers (average±standard deviation) indicate the percentage of Thy1.1+caspase-3+ cells in the gated region (n≥4 per group). The P value comparing five TRP-SIY and four TRAMP mice pooled from two separate experiments is 0.05. BrdU, 5′-bromo-2′-deoxyruridine; dpi, days post-infection; i.p., intraperitoneally; PDLN, prostate draining lymph nodes; SIY, SIYRYYGL; TRAMP, TRansgenic Adenocarcinoma of the Mouse Prostate; TRP-SIY, TRAMP mice expressing SIY antigen in prostate tumor tissue; WSN-SIY, influenza A/WSN virus expressing the SIY epitope.
Compared to the prostate, lower percentages of 2C cells incorporated BrdU in the PDLN following 1.5 and 16 days of labeling (Figure 4b and c). However, the percentage of BrdU+ 2C cells was significantly higher in TRP-SIY than TRAMP mice (9.8% vs. 5.2% at 1.5 days and 52% vs. 30% at 16 days of labeling). In the spleen, no significant difference in BrdU incorporation was detected in 2C cells or endogenous CD8 T cells between TRAMP and TRP-SIY mice. Together, these results suggest that tolerant 2C cells undergo slow proliferation in the prostate of TRP-SIY mice independent of SIY antigen.
Slow proliferation of tolerant T cells in the prostate tumor tissue is not driven by IL-7 and IL-15
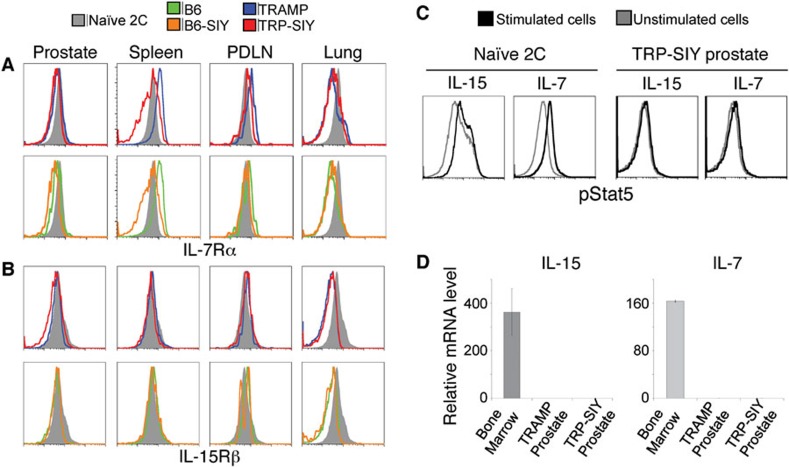
Next, we investigated whether the slow proliferation of tolerant 2C cells in the prostate tumor tissue is driven by IL-7 and IL-15. We measured the expression of IL-7Rα and IL-15Rβ receptor subunits on persisting 2C cells in B6, B6-SIY, TRAMP and TRP-SIY mice (60 dpi). Compared to naive 2C cells, 2C cells from prostates of all four types of mice expressed a lower level of IL-7Rα (Figure 5a). The level of IL-7Rα was even lower on 2C cells from the lung, consistent with a previous report.13 However, there was no apparent difference among the four types of mice. In the spleen, while the level of IL-7Rα was significantly higher on 2C cells from B6 and TRAMP mice, and some of the 2C cells from B6-SIY and TRP-SIY mice had a much reduced level of IL-7Rα, suggesting an antigen-dependent downregulation. The levels of IL-15Rβ on persisting 2C cells from prostate, spleen and PDLN of all four types of mice were similar to those on naive 2C cells, except that it was significantly lower on 2C cells from the lung (Figure 5b). We also investigated whether persisting 2C cells in the prostate of TRP-SIY mice respond to IL-7 and IL-15 by measuring phosphorylation of Stat5. Following either IL-7 or IL-15 treatment, naive 2C cells upregulated pStat5, whereas 2C cells from TRP-SIY prostate did not (Figure 5c). In addition, no IL-15 and IL-7 transcripts were detected in TRP-SIY and TRAMP prostates (Figure 5d), consistent with a recent report of a loss of IL-7 in neoplastic glands from prostate cancer patients.36 These results suggest that the slow proliferation of tolerant 2C cells in the prostate tumor tissue of TRP-SIY mice is unlikely to be driven by homeostatic cytokines IL-7 and IL-15.
Figure 5.
Tolerant 2C cells do not respond to IL-15 or IL-7. (a, b) Comparison of IL-7Rα and IL-15Rβ in 2C T cells from prostate, spleen, PDLN and lung of B6, B6-SIY, TRAMP and TRP-SIY mice. Sixty dpi, cells from the indicated tissues of B6, B6-SIY, TRAMP and TRP-SIY mice were stained for 2C TCR, CD8 and IL-15Rβ or IL-7Rα followed by flow cytometry analysis. Histograms show representative IL-7Rα or IL-15Rβ expression of CD8+ 2C T cells from at least two separate experiments. Naive 2C T cells from spleen were analyzed at the same time for comparison. (c) Tolerant 2C T cells do not respond to IL-15 and IL-7. Cells from TRP-SIY prostates (∼47 dpi) were analyzed for phosphorylated Stat5 (pStat5) as described in the section on ‘Materials and methods'. Histograms show pStat5 level of Thy1.1+CD8+ 2C cells. Naive 2C T-cell response to IL-7 and IL-15 are shown as control. (d) IL-15 and IL-7 transcripts are not detected in prostate tumor tissue. RNA was extracted from prostates of TRP-SIY and TRAMP mice and the transcript levels of IL-7, IL-15 and GAPDH were determined using quantitative real-time PCR. RNA from the bone marrow of B6 mice was used as control. The IL-7 and IL-15 levels were normalized to GAPDH levels of the same tissue. The relative expression levels of IL-7 and IL-15 in different tissues are shown. Error bars represent the standard deviation from duplicate (B6) or triplicate (TRAMP and TRP-SIY) samples. B6, C57BL/6; dpi, days post-infection; PDLN, prostate draining lymph nodes; SIY, SIYRYYGL; TRAMP, TRansgenic Adenocarcinoma of the Mouse Prostate; TRP-SIY, TRAMP mice expressing SIY antigen in prostate tumor tissue.
Discussion
In this study, we investigated factors that contribute to the persistence of tolerant tumor-reactive CD8 T cells in the tumor tissue using a mouse model of prostate cancer. Persistence of CD8 T cells in a given organ is determined by cell trafficking into and out of that organ, as well as the proliferation and death of resident CD8 T cells in the organ. We found that tolerant tumor-reactive 2C T cells persisted in the prostate tumor tissue of TRP-SIY mice, but they were depleted from the spleens of the same mice in an antigen-dependent manner. Because the prostate is a relatively minor reservoir of 2C cells compared to the spleen, antigen-dependent trafficking may explain the consistent levels of tolerant 2C cells in the TRP-SIY prostates. However, memory 2C cells from spleen of B6 mice did not preferentially migrate into the prostate tumor of TRP-SIY mice following adoptive transfer. In addition, 2C T cells also persist in the prostate tumor of TRAMP mice where they are not depleted from the spleen. Finally, non-antigen-specific OT-I T cells also persist in the prostate tumor of TRP-SIY mice and they are not depleted from the spleen. Although we could not fully exclude the possibility of cell migration as a mechanism contributing to the persistence of tolerant 2C T cells in the prostate tumor of TRP-SIY mice, this mechanism is unlikely to be a major factor.
Egress of tolerant 2C T cells from the prostate tumor could also affect their persistence. Although we did not specifically examine this issue, circumstantial evidence suggest that tolerant 2C cells do not migrate out of the prostate tumor tissue significantly. T cells migrate out of tissues to draining lymph node through lymphatics. The tolerant 2C cells expressed only a modest level of CCR7 and virtually no CD62L (Supplementary Figure 1), suggesting that they are unlikely to migrate37, 38 from the prostate tumor tissue to draining lymph nodes. The lack of egress of tolerant 2C cells could also contribute to the persistence of 2C cells in the prostate tumor.
Our results suggest that the slow in situ proliferation of tolerant tumor-reactive 2C T cells is likely to be the main mechanism of their persistence in the prostate tumor tissue. BrdU labeling showed that resident tolerant 2C cells proliferated slowly in the prostate tumor, on the order of once per month as memory CD8 T cells. The proliferation is the fastest in the prostate tumor tissue when compared to that in the spleen and PDLN of the same mice. The slow proliferation of tolerant 2C cells in the prostate tumor tissue is not driven by IL-7 and IL-15, two major cytokines known to support maintenance of memory CD8 T cells,23, 25, 26, 39, 40 as they did not respond to these cytokines in vitro and there is no local source of these cytokines in the prostate tumor tissue (Figure 5d).36 Furthermore, the slow proliferation also occurs in the prostate tumor tissue of TRAMP mice in the absence of SIY, suggesting that it is not driven by antigen in TRP-SIY prostate. Together with the observations that 2C T cells also persist in the prostate tumor tissue of TRAMP mice and that OT-I T cells persist in the prostate tumor tissue of TRP-SIY mice, these results suggest that the persistence of tolerant tumor-reactive 2C cells in the prostate tumor tissue of TRP-SIY mice is largely a tumor-dependent phenomenon. Identification of the tumor-associated factors that drive the slow proliferation of tolerant tumor-reactive T cells would help to elucidate the molecular basis underlying the persistence of TILs.
The tumor-dependent, but antigen- and IL-7/IL-15-independent, slow proliferation of tolerant CD8 T cells in the prostate tumor tissue is a novel mechanism for mediating the persistence of antigen-specific tolerant T cells. This mechanism is distinct from peripheral T-cell tolerance where newly differentiated T cells from thymus become tolerant when they encounter self-antigen in the periphery and are eventually deleted from the repertoire.41 Many studies have documented the gradual disappearance of antigen-specific T cells in antigen-transgenic mice. For example, in InsHA-transgenic mice, antigen-specific CD8 T cells proliferated locally and were eventually deleted from the draining lymph nodes.42 Our findings also distinguish the persistence of TILs from persistence of CD8 T cells in chronic infection. In chronic LCMV infection, antigen-specific CD8 T cells also gradually disappear from spleen,43 while some tolerant or exhausted antigen-specific T cells persist.44 Recently, Shin et al.30 reported that antigen-specific CD8 T cells in mice with chronic LCMV infection persist as a result of extensive proliferation in response to the antigen. These persisting CD8 T cells are non-functional44 and do not respond to IL-7 or IL-15.30 While our results also show that tolerized 2C TILs persist independent of IL-15 and IL-7 in TRP-SIY mice, the persistence is also antigen-independent and lacks the rapid proliferation that drives exhausted CD8 T-cell survival in chronic LCMV infection. Taken together, our results indicate that persistence of tolerant TILs in the tolerizing tumor environment is mediated by a new mechanism driven by yet-to-be-defined tumor-associated factors.
Finally, the results reported here also shed light on the nature of TILs. The persistence of non-tumor-specific T cells in the tumor, such as 2C cells in the prostate of TRAMP mice or OT-I cells in the prostate of TRAMP and TRP-SIY mice, suggests that not all TILs are tumor-reactive. As only activated T cells are capable of trafficking into peripheral tissues, we propose that the repertoire of TILs reflects the antigenic stimulation history of the tumor-bearing animal, a large fraction of which is likely not tumor-specific. This helps to explain the wide variation of frequencies of tumor-responsive cells reported in the literature, as well as the variation in the success of studies to reactivate TILs for immunotherapy.
Acknowledgments
We thank Dr Paul Matsudaira for input and support for the project and Dr Herman N. Eisen and Dr Eileen M. Higham for carefully reviewing this manuscript, as well as Eileen M. Higham, Oezcan Talay, Ilya Leskov, Kristyn Ferber, Maria F. Fragoso, Carol McKinley and Camille Jusino for reagents and technical assistance. This work was supported by grants from the National Institutes of Health (F31-AI080286 to MO and CA100875 to JC), UNCF-Merck Graduate Research and NSF Graduate Research Fellowships (to MO) and Singapore-MIT Alliance and Koch Research Fund (to JC).
Footnotes
Supplementary information accompanies the paper on Cellular & Molecular Immunology's website(http://www.nature.com/cmi/).
Supplementary Information
References
- Husby G, Hoagland PM, Strickland RG, Williams RC., Jr Tissue T and B cell infiltration of primary and metastatic cancer. J Clin Invest. 1976;57:1471–1482. doi: 10.1172/JCI108417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- von Hanwehr RI, Hofman FM, Taylor CR, Apuzzo ML. Mononuclear lymphoid populations infiltrating the microenvironment of primary CNS tumors. Characterization of cell subsets with monoclonal antibodies. J Neurosurg. 1984;60:1138–1147. doi: 10.3171/jns.1984.60.6.1138. [DOI] [PubMed] [Google Scholar]
- Miescher S, Whiteside TL, Carrel S, von Fliedner V. Functional properties of tumor-infiltrating and blood lymphocytes in patients with solid tumors: effects of tumor cells and their supernatants on proliferative responses of lymphocytes. J Immunol. 1986;136:1899–1907. [PubMed] [Google Scholar]
- Whitford P, Mallon EA, George WD, Campbell AM. Flow cytometric analysis of tumour infiltrating lymphocytes in breast cancer. Br J Cancer. 1990;62:971–975. doi: 10.1038/bjc.1990.419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ademmer K, Ebert M, Muller-Ostermeyer F, Friess H, Buchler MW, Schubert W, et al. Effector T lymphocyte subsets in human pancreatic cancer: detection of CD8+CD18+ cells and CD8+CD103+ cells by multi-epitope imaging. Clin Exp Immunol. 1998;112:21–26. doi: 10.1046/j.1365-2249.1998.00546.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boon T, Coulie PG, van den Eynde BJ, van der Bruggen P. Human T cell responses against melanoma. Annu Rev Immunol. 2006;24:175–208. doi: 10.1146/annurev.immunol.24.021605.090733. [DOI] [PubMed] [Google Scholar]
- Zippelius A, Batard P, Rubio-Godoy V, Bioley G, Lienard D, Lejeune F, et al. Effector function of human tumor-specific CD8 T cells in melanoma lesions: a state of local functional tolerance. Cancer Res. 2004;64:2865–2873. doi: 10.1158/0008-5472.can-03-3066. [DOI] [PubMed] [Google Scholar]
- Harlin H, Kuna TV, Peterson AC, Meng Y, Gajewski TF. Tumor progression despite massive influx of activated CD8+ T cells in a patient with malignant melanoma ascites. Cancer Immunol Immunother. 2006;55:1185–1197. doi: 10.1007/s00262-005-0118-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bronte V, Kasic T, Gri G, Gallana K, Borsellino G, Marigo I, et al. Boosting antitumor responses of T lymphocytes infiltrating human prostate cancers. J Exp Med. 2005;201:1257–1268. doi: 10.1084/jem.20042028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenberg NM, DeMayo F, Finegold MJ, Medina D, Tilley WD, Aspinall JO, et al. Prostate cancer in a transgenic mouse. Proc Natl Acad Sci USA. 1995;92:3439–3443. doi: 10.1073/pnas.92.8.3439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson MJ, Shafer-Weaver K, Greenberg NM, Hurwitz AA. Tolerization of tumor-specific T cells despite efficient initial priming in a primary murine model of prostate cancer. J Immunol. 2007;178:1268–1276. doi: 10.4049/jimmunol.178.3.1268. [DOI] [PubMed] [Google Scholar]
- Bai A, Higham E, Eisen HN, Wittrup KD, Chen J. Rapid tolerization of virus-activated tumor-specific CD8+ T cells in prostate tumors of TRAMP mice. Proc Natl Acad Sci USA. 2008;105:13003–13008. doi: 10.1073/pnas.0805599105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen CH, Ge Q, Talay O, Eisen HN, Garcia-Sastre A, Chen J. Loss of IL-7R and IL-15R expression is associated with disappearance of memory T cells in respiratory tract following influenza infection. J Immunol. 2008;180:171–178. doi: 10.4049/jimmunol.180.1.171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Higham EM, Shen CH, Wittrup KD, Chen J. Cutting edge: delay and reversal of T cell tolerance by intratumoral injection of antigen-loaded dendritic cells in an autochthonous tumor model. J Immunol. 2010;184:5954–5958. doi: 10.4049/jimmunol.1000265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dudley ME, Wunderlich JR, Yang JC, Sherry RM, Topalian SL, Restifo NP, et al. Adoptive cell transfer therapy following non-myeloablative but lymphodepleting chemotherapy for the treatment of patients with refractory metastatic melanoma. J Clin Oncol. 2005;23:2346–2357. doi: 10.1200/JCO.2005.00.240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dudley ME, Yang JC, Sherry R, Hughes MS, Royal R, Kammula U, et al. Adoptive cell therapy for patients with metastatic melanoma: evaluation of intensive myeloablative chemoradiation preparative regimens. J Clin Oncol. 2008;26:5233–5239. doi: 10.1200/JCO.2008.16.5449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kondratiev S, Sabo E, Yakirevich E, Lavie O, Resnick MB. Intratumoral CD8+ T lymphocytes as a prognostic factor of survival in endometrial carcinoma. Clin Cancer Res. 2004;10:4450–4456. doi: 10.1158/1078-0432.CCR-0732-3. [DOI] [PubMed] [Google Scholar]
- Thomas DL, Kim M, Bowerman NA, Narayanan S, Kranz DM, Schreiber H, et al. Recurrence of intracranial tumors following adoptive T cell therapy can be prevented by direct and indirect killing aided by high levels of tumor antigen cross-presented on stromal cells. J Immunol. 2009;183:1828–1837. doi: 10.4049/jimmunol.0802322. [DOI] [PubMed] [Google Scholar]
- Ohlen C, Kalos M, Cheng LE, Shur AC, Hong DJ, Carson BD, et al. CD8+ T cell tolerance to a tumor-associated antigen is maintained at the level of expansion rather than effector function. J Exp Med. 2002;195:1407–1418. doi: 10.1084/jem.20011063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanchot C, Lemonnier FA, Perarnau B, Freitas AA, Rocha B. Differential requirements for survival and proliferation of CD8 naive or memory T cells. Science. 1997;276:2057–2062. doi: 10.1126/science.276.5321.2057. [DOI] [PubMed] [Google Scholar]
- Nesic D, Vukmanovic S. MHC class I is required for peripheral accumulation of CD8+ thymic emigrants. J Immunol. 1998;160:3705–3712. [PubMed] [Google Scholar]
- Murali-Krishna K, Lau LL, Sambhara S, Lemonnier F, Altman J, Ahmed R. Persistence of memory CD8 T cells in MHC class I-deficient mice. Science. 1999;286:1377–1381. doi: 10.1126/science.286.5443.1377. [DOI] [PubMed] [Google Scholar]
- Schluns KS, Kieper WC, Jameson SC, Lefrancois L. Interleukin-7 mediates the homeostasis of naive and memory CD8 T cells in vivo. . Nat Immunol. 2000;1:426–432. doi: 10.1038/80868. [DOI] [PubMed] [Google Scholar]
- Tan JT, Dudl E, LeRoy E, Murray R, Sprent J, Weinberg KI, et al. IL-7 is critical for homeostatic proliferation and survival of naive T cells. Proc Natl Acad Sci USA. 2001;98:8732–8737. doi: 10.1073/pnas.161126098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lodolce JP, Boone DL, Chai S, Swain RE, Dassopoulos T, Trettin S, et al. IL-15 receptor maintains lymphoid homeostasis by supporting lymphocyte homing and proliferation. Immunity. 1998;9:669–676. doi: 10.1016/s1074-7613(00)80664-0. [DOI] [PubMed] [Google Scholar]
- Kennedy MK, Glaccum M, Brown SN, Butz EA, Viney JL, Embers M, et al. Reversible defects in natural killer and memory CD8 T cell lineages in interleukin 15-deficient mice. J Exp Med. 2000;191:771–780. doi: 10.1084/jem.191.5.771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang X, Sun S, Hwang I, Tough DF, Sprent J. Potent and selective stimulation of memory-phenotype CD8+ T cells in vivo by IL-15. Immunity. 1998;8:591–599. doi: 10.1016/s1074-7613(00)80564-6. [DOI] [PubMed] [Google Scholar]
- Ku CC, Murakami M, Sakamoto A, Kappler J, Marrack P. Control of homeostasis of CD8+ memory T cells by opposing cytokines. Science. 2000;288:675–678. doi: 10.1126/science.288.5466.675. [DOI] [PubMed] [Google Scholar]
- Virgin HW, Wherry EJ, Ahmed R. Redefining chronic viral infection. Cell. 2009;138:30–50. doi: 10.1016/j.cell.2009.06.036. [DOI] [PubMed] [Google Scholar]
- Shin H, Blackburn SD, Blattman JN, Wherry EJ. Viral antigen and extensive division maintain virus-specific CD8 T cells during chronic infection. J Exp Med. 2007;204:941–949. doi: 10.1084/jem.20061937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fodor E, Devenish L, Engelhardt OG, Palese P, Brownlee GG, Garcia-Sastre A. Rescue of influenza A virus from recombinant DNA. J Virol. 1999;73:9679–9682. doi: 10.1128/jvi.73.11.9679-9682.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stevenson PG, Efstathiou S, Doherty PC, Lehner PJ. Inhibition of MHC class I-restricted antigen presentation by gamma 2-herpesviruses. Proc Natl Acad Sci USA. 2000;97:8455–8460. doi: 10.1073/pnas.150240097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hurwitz AA, Foster BA, Allison JP, Greenberg NM, Kwon ED. New York; John Wiley & Sons, Inc; 2001. The TRAMP Mouse as a Model for Prostate Cancer. Current Protocols in Immunology; pp. 20.5.1–20.5.23. [DOI] [PubMed] [Google Scholar]
- Shen CH, Talay O, Mahajan VS, Leskov IB, Eisen HN, Chen J. Antigen-bearing dendritic cells regulate the diverse pattern of memory CD8 T-cell development in different tissues. Proc Natl Acad Sci USA. 2010;107:22587–22592. doi: 10.1073/pnas.1016350108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wherry EJ, Ahmed R. Memory CD8 T-cell differentiation during viral infection. J Virol. 2004;78:5535–5545. doi: 10.1128/JVI.78.11.5535-5545.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Carlo E, D'Antuono T, Pompa P, Giuliani R, Rosini S, Stuppia L, et al. The lack of epithelial interleukin-7 and BAFF/BLyS gene expression in prostate cancer as a possible mechanism of tumor escape from immunosurveillance. Clin Cancer Res. 2009;15:2979–2987. doi: 10.1158/1078-0432.CCR-08-1951. [DOI] [PubMed] [Google Scholar]
- Bromley SK, Thomas SY, Luster AD. Chemokine receptor CCR7 guides T cell exit from peripheral tissues and entry into afferent lymphatics. Nat Immunol. 2005;6:895–901. doi: 10.1038/ni1240. [DOI] [PubMed] [Google Scholar]
- Gallatin WM, Weissman IL, Butcher EC. A cell-surface molecule involved in organ-specific homing of lymphocytes. 1983. J Immunol. 2006;177:5–9. [PubMed] [Google Scholar]
- Becker TC, Wherry EJ, Boone D, Murali-Krishna K, Antia R, Ma A, et al. Interleukin 15 is required for proliferative renewal of virus-specific memory CD8 T cells. J Exp Med. 2002;195:1541–1548. doi: 10.1084/jem.20020369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schluns KS, Williams K, Ma A, Zheng XX, Lefrancois L. Cutting edge: requirement for IL-15 in the generation of primary and memory antigen-specific CD8 T cells. J Immunol. 2002;168:4827–4831. doi: 10.4049/jimmunol.168.10.4827. [DOI] [PubMed] [Google Scholar]
- Kurts C, Kosaka H, Carbone FR, Miller JF, Heath WR. Class I-restricted cross-presentation of exogenous self-antigens leads to deletion of autoreactive CD8+ T cells. J Exp Med. 1997;186:239–245. doi: 10.1084/jem.186.2.239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hernandez J, Aung S, Redmond WL, Sherman LA. Phenotypic and functional analysis of CD8+ T cells undergoing peripheral deletion in response to cross-presentation of self-antigen. J Exp Med. 2001;194:707–717. doi: 10.1084/jem.194.6.707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wherry EJ, Blattman JN, Murali-Krishna K, van der Most R, Ahmed R. Viral persistence alters CD8 T-cell immunodominance and tissue distribution and results in distinct stages of functional impairment. J Virol. 2003;77:4911–4927. doi: 10.1128/JVI.77.8.4911-4927.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zajac AJ, Blattman JN, Murali-Krishna K, Sourdive DJ, Suresh M, Altman JD, et al. Viral immune evasion due to persistence of activated T cells without effector function. J Exp Med. 1998;188:2205–2213. doi: 10.1084/jem.188.12.2205. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.