Summary
Erythrocytes infected with malaria parasites have increased permeability to diverse organic and inorganic solutes. While these permeability changes have been known for decades, the molecular basis of transport was unknown and intensively debated. CLAG3, a parasite protein previously thought to function in cytoadherence, has recently been implicated in formation of the plasmodial surface anion channel (PSAC), an unusual small conductance ion channel that mediates uptake of most solutes. Consistent with transport studies, the clag genes are conserved in all plasmodia but are absent from other genera. The encoded protein is integral to the host membrane, as also predicted by electrophysiology. An important question is whether functional channels are formed by CLAG3 alone or through interactions with other proteins. In either case, gene identification should advance our understanding of parasite biology and may lead to new therapeutics.
Introduction
The malaria parasite is a single-celled eukaryotic pathogen with a complex life cycle in its vertebrate host and its mosquito vector. To successfully progress through these developmental stages, the parasite must adapt to and survive in divergent intra- and extracellular environments.
Such adaptation usually involves specialized proteins for transmembrane transport of ions and organic solutes. These transport proteins allow the parasite to respond to changes in external ion concentrations, acquire soluble nutrients, eliminate metabolic waste products, regulate cell volume, and complete other activities through intracellular signaling. Oddly though, computational searches of the parasite genome reveal a paucity of conventional transporters and surprisingly few ion channel genes (Gardner et al., 2002). In contrast, other single-celled eukaryotes with less complex life cycles have an abundance of transport proteins (Paulsen et al., 1998). One explanation is that the malaria parasite may have evolved atypical transport proteins not detected by homology searches.
This review will focus on one such transport mechanism, the plasmodial surface anion channel (PSAC). This channel mediates the well-established increase in host erythrocyte permeability to organic and inorganic solutes after infection and appears to be an excellent target for therapeutic intervention against malaria. In light of PSAC permeability to various nutrients required for in vitro propagation of P. falciparum (Desai, 2004), this channel may function as the first step in a sequential pathway for parasite nutrient acquisition. Because host plasma has higher concentrations of key nutrients, PSAC provides a passive conduit for their uptake into erythrocyte cytosol. These solutes then encounter the parasitophorous vacuolar membrane (PVM) and can cross that barrier via a broad selectivity channel present at that membrane (Desai et al., 1993; Desai and Rosenberg, 1997). Patch-clamp suggests that the PVM channel is present at high density and that it functions as a molecular sieve for solutes up to 1200 Dal in size. This high molecular weight cutoff allows uptake not only of plasma nutrients acquired via PSAC, but also metabolic precursors like ATP manufactured in host cytosol. After passing the PVM, these solutes can be taken up at the parasite plasma membrane via specific carriers, some of which have been identified through homology searches (Woodrow et al., 1999; Carter et al., 2000).
After summarizing background literature that set the stage for mechanistic studies, I will review recent findings that support a central role for PSAC and implicate parasite clag3 genes in this activity. Because this conserved gene family lacks homology to known ion channels from other organisms, gene identification raises interesting new questions for future research.
A brief background
Increased erythrocyte permeability after infection was first described by Overman using malarious monkeys and flame photometry (Overman, 1948). Although the methods used are crude by today’s standards, this study identified changes in cation and anion distributions that correlated with parasitemia and is fully consistent with our present-day understanding. After a lull of some two decades, several groups took up the charge, producing a steady output of papers that continues to this date. These studies used classical macroscopic transport methods that included tracer flux and osmotic fragility measurements. They determined that increased permeability is restricted to infected cells and that it does not occur until many hours after host cell invasion (Homewood and Neame, 1974; Kutner et al., 1982). They identified the range of solutes with increased permeability (Ginsburg et al., 1985; Upston and Gero, 1995; Staines et al., 2000), found inhibitors of reasonable potency (Kutner et al., 1987; Kirk et al., 1993; Kirk et al., 1994), and speculated on possible physiological roles (Kutner et al., 1987; Tanabe et al., 1986; Kirk et al., 1994; Staines et al., 2001). However, there were a number of questions that could not be addressed with the available methods. Most importantly, the precise transport mechanism was unknown. Workers considered ion channels, carriers, membrane defects resulting from host cell damage due to merozoite invasion, endocytosis, and a controversial membraneous duct. Given the complexities associated with a multi-compartment system, it was also not clear whether the putative transport protein(s) localized to the host erythrocyte membrane (Kirk et al., 1994).
Patch-clamp methodologies
Patch-clamp of the infected erythrocyte membrane directly addressed the questions of mechanism and location, but then also brought to light several new questions. Before describing these findings, it is useful to review the advantages and limitations of patch-clamp methods. When compared to macroscopic transport methods, a key advantage is that patch-clamp can isolate and quantify flux through a single channel molecule in real time (Neher and Sakmann, 1976). The cell-attached patch-clamp configuration achieves this feat by limiting measurements to a small area of membrane under a recording pipette. This “patch” of membrane may correspond to less than 1/1000 of the human erythrocyte’s surface depending on the geometry of the pipette, which must be fabricated from a glass or quartz capillary immediately prior to use. Aside from the marvel of following the stochastic transitions of a single ion channel, these experiments can provide unparalleled insight into the transport mechanism. Because gating (the process of channel opening and closing) and conductance (the rate of ion flow through an open channel) are generally reproducible from one molecule to the next for each channel type, these recordings can determine the number of distinct channels active under the chosen experimental conditions. Other properties of channels such as selectivity (relative transport rates for different solutes), pharmacology, and regulation by ligands also contribute to definitive recognition of an ion channel.
Detailed mechanistic insights into how a channel recognizes and transports ions as well as testable models for conformational changes involved in gating can also be gleaned through the power of single molecule study. When combined with techniques such as site-directed mutagenesis, patch-clamp can also probe where and precisely how ligands, inhibitors, and permeating solutes interact with the channel. A remarkable example of the power of these functional studies is the extensively studied potassium channel, where detailed predictions about the dimensions of the pore and the steps in K+ ion binding and permeation were confirmed when the x-ray crystal structure was solved (Armstrong, 1998).
Other advantages of patch-clamp over traditional macroscopic approaches include the availability of multiple configurations that can probe functional aspects of the channel’s inner and outer faces, the quantification of total transport for individual cells using the whole-cell patch-clamp configuration, and the ability to examine the effects of controlled changes in membrane potential and/or ionic environment (Hamill et al., 1981).
Patch-clamp methods also have some limitations that should be explicitly recognized. An important one is that the technical details of how recordings were obtained must be critically evaluated before reliable interpretation of the data can be made. Details such as the seal resistance (a measure of how well leak currents between the pipette and the cell membrane have been excluded), whether or not recordings are affected by perfusion of the cell, digitizer sampling rate, data filtering, cell-to-cell or patch-to-patch variability, and statistical analysis are unfortunately not always reported (Levis and Rae, 1993). Considering that an hour of recording can be easily obtained and that a manuscript figure may show as little as 100 milliseconds of data, these details and statements on reproducibility are absolutely essential.
Another important limitation of patch-clamp is that some channel types may escape detection if they are inactive under the applied experimental conditions, their copy number is too low for reproducible detection, or ion flux and/or channel gating kinetics are below the instrumentation’s achievable sensitivity. While our intensive study of the erythrocyte membrane has yielded only one reproducible channel type associated with malaria parasite infection, it is important to recognize that it is not possible to definitively exclude the existence of other parasite-induced channels.
A final relevant limitation of patch-clamp that is it cannot detect transport of uncharged solutes. This has been particularly important in the study of infected erythrocytes because many relevant organic solutes are uncharged. Our approach to addressing this limitation has been to seek quantitative correlations between patch-clamp and other methods (Alkhalil et al., 2004; Lisk et al., 2006; Pillai et al., 2010).
Findings from infected cell electrophysiology
The first patch-clamp study in this field identified a single small conductance ion channel on infected cells (Desai et al., 2000). This channel, now known as the plasmodial surface anion channel (PSAC), is absent from uninfected erythrocytes. Its voltage-dependent gating in single channel recordings correlated well with inward rectifying currents in the whole-cell configuration. Noise analysis of PSAC single channel recordings also matched that of whole-cell recordings, suggesting that PSAC is the primary conductive pathway under the conditions used.
A number of studies from other groups put forward a more complicated picture of transport at the host erythrocyte membrane (Duranton et al., 2005; Verloo et al., 2004; Bouyer et al., 2006; Ginsburg and Stein, 2005). Each group, using somewhat different methods, detected two or more distinct channels. These studies reported non-overlapping collections of channel properties (gating, conductance, selectivity, and pharmacology), suggesting multiple channels and fueling debate. Each of these studies also reported similar activity on uninfected cells, either at reduced frequency or after one or more biochemical treatments. These treatments included exposure to the oxidant t-butylhydroxyperoxide or to protein kinase A with ATP and theophylline to promote protein phosphorylation with the rationale of reproducing the effects of intracellular parasite growth. Some workers also reported channel activation upon cell swelling or shrinkage. Thus, most groups favored upregulation of multiple host ion channels by the intracellular parasite; studies using erythrocytes from humans or mice carrying specific defects suggested roles for CFTR and ClC-2, respectively (Staines et al., 2007).
Addition of patch-clamp methods transformed this field from one where there was general agreement about the properties of the parasite-induced permeabilities to one where many basic features were intensively debated. This is particularly interesting in light of the high reproducibility of patch-clamp studies in other systems and the already standardized methods for data analysis. For my own personal understanding of these discrepancies and to help advance the field, I organized an NIH-funded workshop in my laboratory in 2006 (Staines et al., 2007). This workshop brought the groups together for the first time and led to candid discussions about the key unanswered questions. Although the workshop identified some technical differences in each groups’ methods that might contribute to the differing results, I remain puzzled by the remarkably divergent conclusions reached about how the parasite modifies its host cell permeability.
PSAC is a shared channel for uptake of diverse solutes
It was apparent that patch-clamp alone could not resolve these differences and advance the field; additional technological advances would be needed. This first came in the form of high-throughput screening for specific transport inhibitors. To carry out these inhibitor screens, we miniaturized a quantitative transmittance-based assay for organic solute uptake by infected erythrocytes (Wagner et al., 2003). The assay tracks infected cell osmotic lysis resulting from uptake of permeant solutes such as sorbitol. While hemoglobin release measurements had been used for many years to follow solute uptake (Ginsburg et al., 1985; Kirk et al., 1994), transmittance measurements are technically simpler, permit continuous tracking of lysis kinetics, and can be used for high-throughput screening as they do not require supernatant transfer prior to readout. These screens identified high-affinity inhibitors from several scaffolds (Pillai et al., 2010). Because these inhibitors were inactive against a battery of unrelated targets screened at the Broad Institute, they provided a specific handle to probe molecular mechanism. We determined that these compounds produced quantitatively identical inhibitory effects on uptake of sorbitol, amino acids, and organic cations; they also produced concordant block of PSAC in single-channel and whole-cell patch-clamp. We interpreted these findings as strong evidence for a single ion channel shared by most, if not all, well-studied solutes.
This evidence was not universally accepted: a number of inhibitors active against infected cell solute uptake are notoriously nonspecific. Some compounds block multiple unrelated channels and carriers and also interfere with the activity of unrelated enzymes. Although there is good precedent with other transporters, most workers were therefore skeptical about our use of correlated pharmacology. The consensus was that the only way to determine the number of distinct channels would be to identify the genes responsible for each candidate ion channel.
Identification of clag3 genes
To address this dilemma, we again turned to high-throughput screening. To gain a molecular handle that could be used for gene identification, we sought inhibitors with differing efficacies against channels from separate parasite lines. These experiments were motivated by reproducible differences in PSAC gating and pharmacology observed for channels induced by different parasite lines (Alkhalil et al., 2004; Alkhalil et al., 2009). We had also successfully generated several channel mutants through in vitro selections (Hill et al., 2007; Lisk et al., 2008; Lisk et al., 2010). These findings suggested the involvement of one or more parasite genes in host cell permeability. They also hinted at polymorphic sites on an otherwise conserved channel protein.
We used high-throughput screening to find inhibitors that interact with these presumed polymorphic sites. A library of 50,000 compounds was screened against four divergent parasite lines. Comparative analyses between these four screens revealed that about 90% of the hits were uniformly active against channels from these parasites, consistent with conserved domains involved in solute selectivity and permeation. At the same time, a fraction of compounds were specific for channels derived from one or a subset of parasites; secondary studies revealed that this differential inhibition was highly reproducible. One such isolate-specific PSAC antagonist (ISPA-28) was 800-fold more active against channels from the Dd2 parasite line than those of HB3 (Nguitragool et al., 2011). Because HB3-infected cells provide a “negative control” for host-derived transporters, ISPA-28 activity against Dd2-infected cells could no longer be dismissed as nonspecific action against multiple transporters. In addition to sorbitol, as used our transport inhibitor screens, the differential efficacy of ISPA-28 was observed in uptake studies using amino acids, an organic cation, and Cl−. Because Cl− flux was measured under patch-clamp conditions that detect only PSAC, ISPA-28 implicates this unusual ion channel as the primary mechanism for uptake of these solutes.
We chose Dd2 and HB3 for our high-throughput screens because these lines are the parents of an available genetic cross (Wellems et al., 1990). We quantified ISPA-28 efficacy in the progeny of this cross and used linkage analysis with known microsatellite markers to determine that a single locus on parasite chromosome 3 accounts for the differing effects of ISPA-28 on PSAC activity. Interestingly, none of the genes in the identified locus were homologous to known ion channels in other organisms; we were not particularly discouraged by this because genome-wide surveys had already determined that the parasite lacks conventional candidates for anion channel genes. Thus, although linkage analysis had focused in on a single locus containing only 40 genes, understanding how parasite genetic elements contribute to PSAC activity still felt like a distant goal.
To identify the responsible gene(s) from this locus, we settled on a DNA transfection approach. We chose the piggyBac transposase system as adapted to P. falciparum transfection by Balu and Adams because its high efficiency for producing stable integrants might expedite examination of our relatively large list of candidates (Balu et al., 2005). We used piggyBac to transfect Dd2 parasites with the HB3 version of 15 candidate genes. If the correct gene could be integrated and expressed, this transfection should yield a parasite carrying channels from both Dd2 and HB3; transport studies may then show an intermediate ISPA-28 affinity. While transfection with 13 genes from the mapped locus did not alter PSAC phenotype, two genes reduced ISPA-28 affinity, yielding the predicted intermediate level of transport inhibition. These genes, PFC0110w and PFC0120w, are members of the clag multigene family, named for presumed roles in cytoadherence. These two genes, also known as clag3.2 and clag3.1 respectively, are under epigenetic regulation so that individual parasites express a single copy (Cortes et al., 2007).
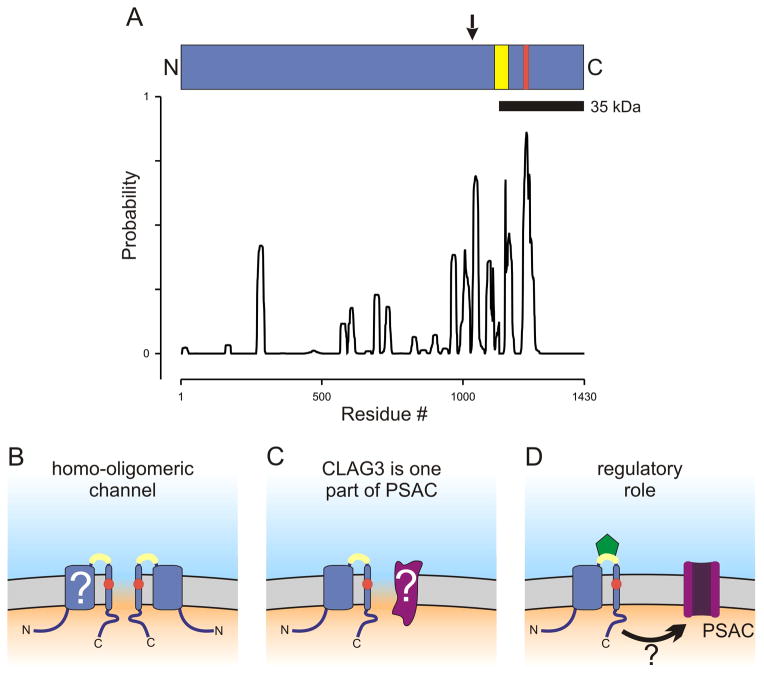
Because more complex explanations for the observed phenotype change are possible with piggyBac complementation, we also performed allelic exchange and identified HB33rec, a clone generated from the HB3 line but carrying channels with Dd2’s high ISPA-28 affinity. Because this allelic exchange yielded a chimeric gene consisting of the first ~ 70% of the HB3 gene followed by a 3′ fragment from the Dd2 PFC0120w gene (Fig. 1A, arrow above ribbon schematic), this experiment not only provided additional evidence for a role of clag3 genes but it delimited the ISPA-28 binding site to the last one-third of the protein’s primary sequence.
Fig. 1.
CLAG3 primary structure and possible contributions to PSAC formation. (A) Ribbon schematic showing the CLAG3 polypeptide aligned with a posterior probability plot for prediction of transmembrane domains, as calculated using Polyphobius. Arrow above the ribbon indicates site of recombination in the allelic exchange parasite, HB33rec. Yellow and red shading indicate variable residues and the site of a point mutation in a PSAC mutant, respectively. (B–D) Cartoons showing how erythrocyte membrane-embedded CLAG3 may contribute to PSAC formation and solute transport. The channel may be formed exclusively by CLAG3 oligomerization (panel B). The variable domain near the C-terminus is shown in yellow and is extracellular; a change in a PSAC mutant is indicated by a red circle within a predicted transmembrane segment. The transmembrane topology is not fully established (“?”). Panel C shows a heteromeric channel, where CLAG3 and an unidentified protein (purple, marked with “?”) both contribute to the channel pore. Panel D shows CLAG3 modulation of PSAC activity at a distance. ISPA-28 (green) is shown bound to the CLAG3 variable domain; how inhibitor binding is transmitted to the functional channel is unclear in this scenario (arrow with “?”).
Our biochemical studies with a specific antibody indicated that the encoded protein is integral to the host membrane, as expected by patch-clamp studies. A small variable domain within the above allelic exchange fragment is exposed at the host cell surface based on protease susceptibility studies (yellow shading on ribbon, Fig. 1A); this domain likely defines the ISPA-28 binding pocket.
Finally, DNA sequencing of clag3 genes in an available leupeptin-resistant PSAC mutant revealed a nonsynonymous mutation within a predicted transmembrane domain (A1210T; red shading on ribbon, Fig. 1A), providing additional evidence for a role of clag3 in transport at the host membrane.
What is the precise contribution of clag3?
“The difficulty the authors face is the one they started with: how do we know whether this novel protein is a Cl channel or a modifier of an intrinsic Cl conductance?”
-- insightful reviewer of (Nguitragool et al., 2011).
Maybe the most important question going forward is how exactly does the CLAG3 protein contribute to channel formation and solute transport? A role in transmembrane transport of ions and nutrients would not have been predicted by hydrophobicity analyses, which detect few if any conventional transmembrane domains in this protein. Nevertheless, biochemical studies revealed that CLAG3 is an integral membrane protein resistant to extraction by carbonate treatment (Nguitragool et al., 2011). Although informatic analyses are of limited utility in such cases, the Polyphobius algorithm may be more predictive because it incorporates information on hydrophobic regions from homologs (Kall et al., 2005). Figure 1A shows this algorithm’s prediction for the Dd2 clag3.1 product after preferential weighting for conserved hydrophobic residues identified through alignments of clag products from P. falciparum, P. yoelli, and P. chabaudi. The algorithm confidently predicts one transmembrane domain centered at residue 1224 from the start methionine; this domain contains the mutation associated with leupeptin-resistant channels (marked in red, Fig. 1A ribbon and Figs. 1B–D). This prediction is also consistent with protease susceptibility studies (Nguitragool et al., 2011), which reveal that a 35 kDa C-terminal fragment released by proteolysis is still integral to the membrane (black bar below the ribbon, Fig. 1A). Because a C-terminal FLAG epitope tag is not susceptible to extracellular protease application, this fragment must be oriented so that the protein’s C-terminus is intracellular. Our unpublished biochemical studies suggest additional transmembrane domains that may correspond to other conserved hydrophobic regions detected by Polyphobius (Fig. 1A and “?” in Fig. 1B).
So, how exactly does CLAG3 contribute to functional channels at the host erythrocyte membrane? One possibility is that CLAG3 alone forms PSAC. In this scenario, the paucity of predicted transmembrane domains suggests that 2 or more CLAG3 subunits may come together to form a stable aqueous pore (Fig. 1B), as seen with many other channels. Another possibility is that CLAG3 interacts with other parasite or host proteins to form the channel (Fig. 1C); such hetero-multimeric channels are also well known in other systems. These interacting proteins remain to be identified, but their presence is suggested by some PSAC mutants that lack mutations in CLAG3. A final possibility is that CLAG3 activates or modulates PSAC without a stable and direct involvement in channel formation (Fig. 1D). Such a contribution in trans is not excluded by our findings, but I believe it is less likely for two reasons. First, our transfection studies suggest that ISPA-28 binds directly and reversibly to CLAG3 (green symbol in graphic, Fig. 1D); this binding event produces inhibition of solute transport without measurable delay under non-physiological patch-clamp conditions. If CLAG3 is not itself a part of the channel, it is unclear how ISPA-28 binding can be rapidly transmitted to the channel without a direct interaction. Second, a CLAG3 mutation associated with altered PSAC selectivity, as described above (Nguitragool et al., 2011), also suggests a direct involvement of CLAG3: modifications of pore-lining residues are known to affect solute permeation in other channels. If CLAG3 serves only a regulatory role, more complicated explanations for how the mutation yields altered channels will need to be sought (red circle, Fig. 1D).
More questions than answers
Several additional questions are raised by our study. First, how is the CLAG3 protein trafficked and inserted in the host cell membrane? Are there contributions from host proteins as suggested by some studies? Is channel copy number at the host membrane regulated? If so, how? How does the channel achieve broad permeability to organic and inorganic solutes while maintaining stringent exclusion of Na+, as required to preserve host cell osmotic stability (Cohn et al., 2003)? What is the exact role served by the channel and can it be targeted for antimalarial development?
Identification of clag3 genes as determinants of increased erythrocyte permeability after infection is only a starting point. The addition of molecular biology and high-throughput screening methods to this already technology-rich field makes this an exciting time to be studying membrane transport in malaria parasites.
Acknowledgments
This research was funded by the Intramural Research Program of the National Institutes of Health, NIAID.
References
- Alkhalil A, Cohn JV, Wagner MA, Cabrera JS, Rajapandi T, Desai SA. Plasmodium falciparum likely encodes the principal anion channel on infected human erythrocytes. Blood. 2004;104:4279–4286. doi: 10.1182/blood-2004-05-2047. [DOI] [PubMed] [Google Scholar]
- Alkhalil A, Pillai AD, Bokhari AA, Vaidya AB, Desai SA. Complex inheritance of the plasmodial surface anion channel in a Plasmodium falciparum genetic cross. Mol Microbiol. 2009;72:459–469. doi: 10.1111/j.1365-2958.2009.06661.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Armstrong C. The vision of the pore. Science. 1998;280:56–57. doi: 10.1126/science.280.5360.56. [DOI] [PubMed] [Google Scholar]
- Balu B, Shoue DA, Fraser MJ, Jr, Adams JH. High-efficiency transformation of Plasmodium falciparum by the lepidopteran transposable element piggyBac. Proc Natl Acad Sci USA. 2005;102:16391–16396. doi: 10.1073/pnas.0504679102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bouyer G, Egee S, Thomas SL. Three types of spontaneously active anionic channels in malaria-infected human red blood cells. Blood Cells Mol Dis. 2006;36:248–254. doi: 10.1016/j.bcmd.2006.01.005. [DOI] [PubMed] [Google Scholar]
- Carter NS, Ben Mamoun C, Liu W, Silva EO, Landfear SM, Goldberg DE, et al. Isolation and functional characterization of the PfNT1 nucleoside transporter gene from Plasmodium falciparum. J Biol Chem. 2000;275:10683–10691. doi: 10.1074/jbc.275.14.10683. [DOI] [PubMed] [Google Scholar]
- Cohn JV, Alkhalil A, Wagner MA, Rajapandi T, Desai SA. Extracellular lysines on the plasmodial surface anion channel involved in Na+ exclusion. Mol Biochem Parasitol. 2003;132:27–34. doi: 10.1016/j.molbiopara.2003.08.001. [DOI] [PubMed] [Google Scholar]
- Cortes A, Carret C, Kaneko O, Yim Lim BY, Ivens A, Holder AA. Epigenetic silencing of Plasmodium falciparum genes linked to erythrocyte invasion. PLoS Pathog. 2007;3:e107. doi: 10.1371/journal.ppat.0030107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Desai SA. Targeting ion channels of Plasmodium falciparum-infected human erythrocytes for antimalarial development. Curr Drug Targets Infect Disord. 2004;4:79–86. doi: 10.2174/1568005043480934. [DOI] [PubMed] [Google Scholar]
- Desai SA, Bezrukov SM, Zimmerberg J. A voltage-dependent channel involved in nutrient uptake by red blood cells infected with the malaria parasite. Nature. 2000;406:1001–1005. doi: 10.1038/35023000. [DOI] [PubMed] [Google Scholar]
- Desai SA, Krogstad DJ, McCleskey EW. A nutrient-permeable channel on the intraerythrocytic malaria parasite. Nature. 1993;362:643–646. doi: 10.1038/362643a0. [DOI] [PubMed] [Google Scholar]
- Desai SA, Rosenberg RL. Pore size of the malaria parasite’s nutrient channel. Proc Natl Acad Sci USA. 1997;94:2045–2049. doi: 10.1073/pnas.94.5.2045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duranton C, Tanneur V, Brand V, Sandu CD, Akkaya C, Huber SM, et al. Permselectivity and pH-dependence of Plasmodium falciparum-induced anion currents in human erythrocytes. Pflugers Arch. 2005;450:335–344. doi: 10.1007/s00424-005-1415-5. [DOI] [PubMed] [Google Scholar]
- Gardner MJ, Hall N, Fung E, White O, Berriman M, Hyman RW, et al. Genome sequence of the human malaria parasite Plasmodium falciparum. Nature. 2002;419:498–511. doi: 10.1038/nature01097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ginsburg H, Kutner S, Krugliak M, Cabantchik ZI. Characterization of permeation pathways appearing in the host membrane of Plasmodium falciparum infected red blood cells. Mol Biochem Parasitol. 1985;14:313–322. doi: 10.1016/0166-6851(85)90059-3. [DOI] [PubMed] [Google Scholar]
- Ginsburg H, Stein WD. How many functional transport pathways does Plasmodium falciparum induce in the membrane of its host erythrocyte? Trends Parasitol. 2005;21:118–121. doi: 10.1016/j.pt.2005.01.004. [DOI] [PubMed] [Google Scholar]
- Hamill OP, Marty A, Neher E, Sakmann B, Sigworth FJ. Improved patch-clamp techniques for high-resolution current recording from cells and cell-free membrane patches. Pflugers Arch. 1981;391:85–100. doi: 10.1007/BF00656997. [DOI] [PubMed] [Google Scholar]
- Hill DA, Pillai AD, Nawaz F, Hayton K, Doan L, Lisk G, et al. A blasticidin S-resistant Plasmodium falciparum mutant with a defective plasmodial surface anion channel. Proc Natl Acad Sci USA. 2007;104:1063–1068. doi: 10.1073/pnas.0610353104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Homewood CA, Neame KD. Malaria and the permeability of the host erythrocyte. Nature. 1974;252:718–719. doi: 10.1038/252718a0. [DOI] [PubMed] [Google Scholar]
- Kall L, Krogh A, Sonnhammer EL. An HMM posterior decoder for sequence feature prediction that includes homology information. Bioinformatics. 2005;21(Suppl 1):i251–i257. doi: 10.1093/bioinformatics/bti1014. [DOI] [PubMed] [Google Scholar]
- Kirk K, Horner HA, Elford BC, Ellory JC, Newbold CI. Transport of diverse substrates into malaria-infected erythrocytes via a pathway showing functional characteristics of a chloride channel. J Biol Chem. 1994;269:3339–3347. [PubMed] [Google Scholar]
- Kirk K, Horner HA, Spillett DJ, Elford BC. Glibenclamide and meglitinide block the transport of low molecular weight solutes into malaria-infected erythrocytes. FEBS Lett. 1993;323:123–128. doi: 10.1016/0014-5793(93)81462-9. [DOI] [PubMed] [Google Scholar]
- Kutner S, Baruch D, Ginsburg H, Cabantchik ZI. Alterations in membrane permeability of malaria-infected human erythrocytes are related to the growth stage of the parasite. Biochim Biophys Acta. 1982;687:113–117. doi: 10.1016/0005-2736(82)90178-x. [DOI] [PubMed] [Google Scholar]
- Kutner S, Breuer WV, Ginsburg H, Cabantchik ZI. On the mode of action of phlorizin as an antimalarial agent in in vitro cultures of Plasmodium falciparum. Biochem Pharmacol. 1987;36:123–129. doi: 10.1016/0006-2952(87)90389-3. [DOI] [PubMed] [Google Scholar]
- Levis RA, Rae JL. The use of quartz patch pipettes for low noise single channel recording. Biophys J. 1993;65:1666–1677. doi: 10.1016/S0006-3495(93)81224-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lisk G, Kang M, Cohn JV, Desai SA. Specific inhibition of the plasmodial surface anion channel by dantrolene. Eukaryot Cell. 2006;5:1882–1893. doi: 10.1128/EC.00212-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lisk G, Pain M, Gluzman IY, Kambhampati S, Furuya T, Su XZ, et al. Changes in the plasmodial surface anion channel reduce leupeptin uptake and can confer drug resistance in P. falciparum-infected erythrocytes. Antimicrob Agents Chemother. 2008;52:2346–2354. doi: 10.1128/AAC.00057-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lisk G, Pain M, Sellers M, Gurnev PA, Pillai AD, Bezrukov SM, et al. Altered plasmodial surface anion channel activity and in vitro resistance to permeating antimalarial compounds. Biochim Biophys Acta. 2010;1798:1679–1688. doi: 10.1016/j.bbamem.2010.04.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neher E, Sakmann B. Single-channel currents recorded from membrane of denervated frog muscle fibres. Nature. 1976;260:799–802. doi: 10.1038/260799a0. [DOI] [PubMed] [Google Scholar]
- Nguitragool W, Bokhari AA, Pillai AD, Rayavara K, Sharma P, Turpin B, et al. Malaria parasite clag3 genes determine channel-mediated nutrient uptake by infected red blood cells. Cell. 2011;145:665–677. doi: 10.1016/j.cell.2011.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Overman RR. Reversible cellular permeability alterations in disease. In vivo studies on sodium, potassium and chloride concentrations in erythrocytes of the malarious monkey. Am J Physiol. 1948;152:113–121. doi: 10.1152/ajplegacy.1947.152.1.113. [DOI] [PubMed] [Google Scholar]
- Paulsen IT, Sliwinski MK, Nelissen B, Goffeau A, Saier MH., Jr Unified inventory of established and putative transporters encoded within the complete genome of Saccharomyces cerevisiae. FEBS Lett. 1998;430:116–125. doi: 10.1016/s0014-5793(98)00629-2. [DOI] [PubMed] [Google Scholar]
- Pillai AD, Pain M, Solomon T, Bokhari AA, Desai SA. A cell-based high-throughput screen validates the plasmodial surface anion channel as an antimalarial target. Mol Pharmacol. 2010;77:724–733. doi: 10.1124/mol.109.062711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Staines HM, Alkhalil A, Allen RJ, De Jonge HR, Derbyshire E, Egee S, et al. Electrophysiological studies of malaria parasite-infected erythrocytes: current status. Int J Parasitol. 2007;37:475–482. doi: 10.1016/j.ijpara.2006.12.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Staines HM, Ellory JC, Kirk K. Perturbation of the pump-leak balance for Na(+) and K(+) in malaria- infected erythrocytes. Am J Physiol Cell Physiol. 2001;280:C1576–C1587. doi: 10.1152/ajpcell.2001.280.6.C1576. [DOI] [PubMed] [Google Scholar]
- Staines HM, Rae C, Kirk K. Increased permeability of the malaria-infected erythrocyte to organic cations. Biochim Biophys Acta. 2000;1463:88–98. doi: 10.1016/s0005-2736(99)00187-x. [DOI] [PubMed] [Google Scholar]
- Tanabe K, Izumo A, Kageyama K. Growth of Plasmodium falciparum in sodium-enriched human erythrocytes. Am J Trop Med Hyg. 1986;35:476–478. doi: 10.4269/ajtmh.1986.35.476. [DOI] [PubMed] [Google Scholar]
- Upston JM, Gero AM. Parasite-induced permeation of nucleosides in Plasmodium falciparum malaria. Biochim Biophys Acta. 1995;1236:249–258. doi: 10.1016/0005-2736(95)00055-8. [DOI] [PubMed] [Google Scholar]
- Verloo P, Kocken CH, Van der Wel A, Tilly BC, Hogema BM, Sinaasappel M, et al. Plasmodium falciparum-activated chloride channels are defective in erythrocytes from cystic fibrosis patients. J Biol Chem. 2004;279:10316–10322. doi: 10.1074/jbc.M311540200. [DOI] [PubMed] [Google Scholar]
- Wagner MA, Andemariam B, Desai SA. A two-compartment model of osmotic lysis in Plasmodium falciparum-infected erythrocytes. Biophys J. 2003;84:116–123. doi: 10.1016/S0006-3495(03)74836-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wellems TE, Panton LJ, Gluzman IY, do Rosario VE, Gwadz RW, Walker-Jonah A, et al. Chloroquine resistance not linked to mdr-like genes in a Plasmodium falciparum cross. Nature. 1990;345:253–255. doi: 10.1038/345253a0. [DOI] [PubMed] [Google Scholar]
- Woodrow CJ, Penny JI, Krishna S. Intraerythrocytic Plasmodium falciparum expresses a high affinity facilitative hexose transporter. J Biol Chem. 1999;274:7272–7277. doi: 10.1074/jbc.274.11.7272. [DOI] [PubMed] [Google Scholar]