Abstract
Animal approach-avoidance conflict paradigms have been used extensively to characterize effects of anxiolytic agents and probe neural circuitry related to anxiety. However, there are few behavioral approaches to measure conflict in human populations, limiting the translation of findings from animal conflict tasks to human clinical research. We developed a novel approach-avoidance conflict (AAC) paradigm involving situations in which the same decision is associated with “reward” (points) and “punishment” (negative affective stimuli). The AAC task was completed by 95 young adults (56 female) with varying levels of self-reported trait anxiety. As expected, conflict-related approach behavior correlated with self-reported motivation to approach reward and avoid punishment and greater reward level increased approach behavior. Additionally, females exhibited less approach behavior than males. Anxiety Sensitivity Index (Physical subscale) scores related negatively to approach behavior for males, while Behavioral Activation Scale (BAS, Fun Seeking subscale) scores related positively to approach behavior for females. Results support the utility of the AAC task as a behavioral test that has strong reverse translational features. Findings indicate that approach drives and anxiety sensitivity may be important in determining conflict behavior for females and males respectively. The approach-avoidance conflict task offers a novel, translational measure to probe neural systems underlying conflict behavior, motivational processes, and anxiety disorders.
Keywords: Conflict, Reward, Anxiety, Avoidance, Decision making, Translational research
1. INTRODUCTION
Approach-avoidance conflict is an important concept for characterizing reward- and punishment- related animal and human behavior. Schneirla [1] described approach behavior as “adjustments such as food-getting, shelter-getting, and mating” and withdrawal or avoidance behavior as “adjustments such as defense, huddling, flight, and other protective reactions. ” Thus, approach behavior is instigated in the presence of rewards or stimuli that further ensure the integrity of the individual, whereas avoidance behavior is often related to impending or experienced punishments, i.e. situations that threaten the integrity of the individual [2–4]. Conflict situations occur when a person or animal is faced with opposing drives, or incentives to act, that are incompatible with one another [5–7]. More specifically, approach-avoidance conflict arises when the same action is associated with both reward and punishment. Approach-avoidance conflict poses a unique challenge for comparing the value of available options because individuals must integrate information concerning the value of potential rewards and punishments and the likelihood and magnitude of those potential outcomes [8–10].
Balance between opposing approach and avoidance drives is thought to be important for successful navigation of our environment, as avoidance protects from primary and secondary threats such as prey, pain, or reprimand, while approach helps obtain primary and secondary rewards such as food, money, or social support [11, 12]. Imbalance of approach and avoidance drives have been theorized to result in less optimal decision making and, in the extreme, to the development or expression of psychopathology [8, 11–15]. In particular, excessive avoidance behavior has been implicated as a cardinal symptom of anxiety disorders, is thought to be an underlying mechanism maintaining anxiety, and is a primary target of current psychotherapeutic treatments [16–23]. Notably, avoidance becomes most problematic and distressing when maintained in situations in which doing so involves the sacrifice of potential reward. For example, an individual may avoid crowded places due to fears of having a panic attack, but this may only become distressing when this avoidance prevents him from spending time with family and friends. Clinically-significant avoidance can therefore be considered a decision to sacrifice potential rewards in order to avoid potential negative outcomes, i.e., avoidance in response to approach-avoidance conflict situations.
Animal models of approach-avoidance conflict have been used extensively as models of anxiety and for characterizing anxiolytic agents [5, 24]. Although there are different versions of animal conflict paradigms (e.g., Vogel, Geller-Seifter), the basic model involves the same behavior being associated with both reward (e.g., water or food) and punishment (e.g., mild electric shock). This creates a conflict between approaching the reward and avoiding the feared stimulus. In most cases, the ability of pharmacologic agents to increase approach behavior in animal conflict corresponds to efficacy in decreasing human clinical anxiety [5, 24]. For example, acute administration of benzodiazepines (e.g., alprazolam, diazepam) has been shown to reliably increase punished responding [5, 25, 26]. Additionally, temporary lesions of the ventromedial prefrontal cortex or central amygdala—brain regions implicated in the neural circuitry of anxiety—have been shown to modulate animal behavior during conflict [27–29]. A recent study also reported female rodents to exhibit less approach behavior during the Vogel conflict test than males [30], which is consistent with human research reporting females to exhibit greater levels of avoidance behavior and to have a higher prevalence of anxiety disorders [31–34]. Approach-avoidance conflict therefore represents a valuable construct for understanding the neurobiology of anxiety and the mechanisms of anxiolytic treatments.
In comparison to animal research, there are few empirical behavioral approaches to measure conflict in humans. Instead, investigators typically rely on subjective rating scales to quantify the strength of reward and punishment-related drives, such as the theorized behavioral activation (BAS) and behavioral inhibition (BIS) systems proposed by Gray [3, 4, 6]. Human behavioral paradigms focused on risk taking, value-based decision making, or learning in response to punishment- or reward-based feedback, also undoubtedly relate to approach and avoidance constructs [35–45]. These paradigms, however, are often heavily focused on approach processing, as the “punishment” often involves losing a reward (e.g., money or points). Therefore, these tasks may not tap directly into the constructs measured via animal models, where the “punishment” is more directly threatening or painful. They also may not model the affective “risk” or punishment (e.g., emotional triggers) that is often the motivation for avoidance in anxiety disorders. More recently, Rinck and Becker [46] developed the approach avoidance test (AAT), a modified implicit association task measuring reaction times when individuals are asked to pull towards or push away a joystick in response to anxiety-provoking stimuli. The AAT, as well as measures of affective attentional bias have provided important insights into automatic tendencies associated with anxiety [46–49]. However, strong approach motivations could potentially override automatic tendencies to avoid and vice versa. A behavioral measure tapping into how approach and avoidance motivations influence decisional outcomes could provide an important complement to this research and could help to bridge the gap between human and animal behavioral models of approach-avoidance conflict.
The current study utilizes a reverse translational approach, i.e., starts with the basic behavioral model of approach-avoidance conflict in animals and attempts to translate this model into testable behavior in humans. The novel approach-avoidance conflict (AAC) paradigm presented here involves situations in which the same decision or behavior is associated with both a reward and an affective “punishment”. The development of a behavioral paradigm to measure approach-avoidance conflict is a critical first step towards its use in quantifying abnormal approach-avoidance in clinical populations and to examine the effect of interventions. The current study tested this paradigm in a population of young adults with varying levels of trait anxiety to enable not only characterization of task effects in male and female young adults but also to investigate the relationship between task performance and measures of behavioral inhibition and activation, trait anxiety, and anxiety sensitivity. It was hypothesized that 1) increasing reward levels during conflict situations would increase approach behavior, 2) approach behavior would positively relate to self-report measures concerning the behavioral activation system and negatively relate to measures concerning anxiety and the behavioral inhibition system and 3) males would exhibit greater levels of approach behavior than females.
2. METHODS AND MATERIALS
2.1. Participants
This study was approved by University of California, San Diego and San Diego State University human research protection programs. Approximately 1000 undergraduates were prescreened using the Spielberger State-Trait Anxiety Inventory (STAI-T) [50] and the Brief Symptom Inventory – Anxiety subscale (BSI-18-A) [51]. Individuals who scored in the high (upper 15th percentile) or normal (40th–60th percentile) range on these measures were invited to participate in the current study. A total of 95 subjects participated, including 56 females and 39 males (mean age=19.43 (SD=1.77); mean education=12.82 years (SD=0.99)).
2.2. Task Procedures
All subjects completed the computer-based approach-avoidance conflict (ACC) task, which was programmed using Adobe Flash Professional CS5©. Subjects were trained on the AAC, completed three practice trials, and were shown an example of the final feedback screen, in order to ensure full understanding of instructions and the potential outcomes associated with the task.
For each trial of the AAC, participants are shown a runway with pictures on each side to represent two potential outcomes (see Figure 1). Each potential outcome includes an affective stimulus and a certain level of reward points. The valence of the affective stimulus is represented via a sun indicating a positive affective stimulus or a cloud indicating a negative affective stimulus. Level of reward points is represented by the red in the rectangles (more red = more points). The subject is to move an avatar on the runway (by pressing keys on a keyboard) to indicate their relative preference for each potential outcome. The location of the avatar at the end of the decision phase corresponds to the probability of each of the two outcomes occurring. If the subject moves the avatar to the middle of the runway, there is a 50% chance of each outcome; if he moves all the way to one side, there is a 90% chance of the nearest outcome and a 10% chance of the further outcome; and so on. Subjects therefore control the likelihood of the two outcomes but are unable to determine one or the other outcome for certain. The initial starting position of the avatar (at each of the nine possible positions, ranging from −4 to +4) is counterbalanced to enable characterization regarding the influence of effort on behavior.
Figure 1. Decisional conditions included within the approach-avoidance conflict (AAC) paradigm.
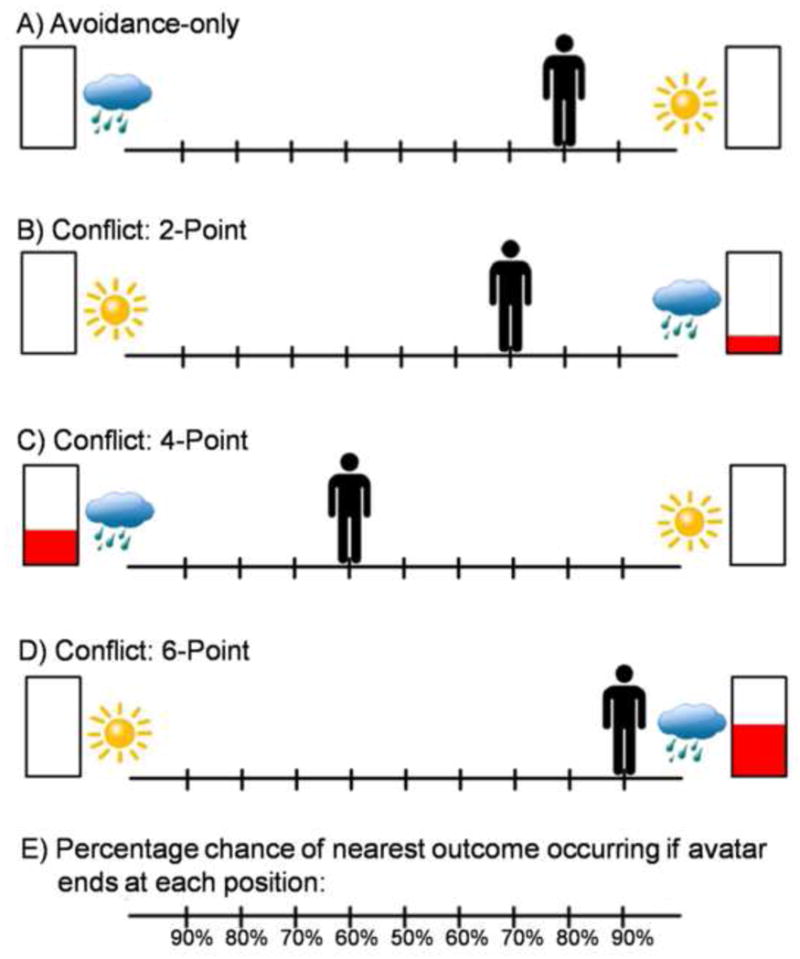
Avoidance-only conditions (Part A) involve no point-reward incentives but only the possibility of viewing a negative (indicated by a cloud) or positive (indicated by a sun) affective stimulus. During conflict conditions (Parts B–D), reward points (2, 4, or 6 point levels) are given only for the outcomes associated with a negative affective stimulus while the competing choice includes no points but a positive affective stimulus. The avatar starts out at different locations on the runway, counterbalanced so there were two trials for each position within each of the condition types. The subject is asked to move the avatar (by pressing arrow keys on a keyboard) to a position that accurately reflects their preference between the two potential outcomes. The position in which they move the avatar determines the relative probability of each of the two outcomes occurring (Part E; 10/90%, 20/80%, 30/70%, 40/60%, 50/50% and vice versa probabilities, corresponding to the nine potential avatar positions ranging from −4 to +4). Therefore, if they move their avatar to the middle, there is a 50% chance of each outcome occurring; if they moved all the way to one side, there is a 90% chance of the nearest outcome occurring, but still a 10% chance of the furthest outcome occurring, and so on.
The affective stimuli used in the AAC paradigm include image and sound combinations (International Affective Picture System (IAPS) [52]; International Affective Digitized Sounds (IADS) [53] and other freely available audio files). The “reward” includes 0, 2, 4, or 6 points presented along with a trumpet sound. (See supplementary material for further description and examples of affective stimuli used in the AAC task.) There are two types of trials in the AAC task as shown in Figure 1: (1) ‘Avoidance-only’, in which 0 points are offered for both outcomes. For these conditions, there is no explicit motivation to approach the negative affective outcome. (2) Three levels of ‘Conflict’ in which 2, 4, or 6 points are offered for the outcome involving a negative affective stimulus while 0 points are offered for the outcome involving a positive affective stimulus. These conditions are designed to produce approach-avoidance conflict, as the same behavior is associated both with reward and punishment. There were a total of 72 trials included in the AAC task, with 18 trials of each trial type (avoidance-only, and three levels of conflict). At the end of the task, a screen appears displaying total points received and an award ribbon (“1st place” ribbon for >150 points, “2nd place” ribbon for 100–150 points, and so on). Notably, the points do not translate into monetary reward. The timing of each AAC trial is displayed in Figure 2.
Figure 2. Sequence of screens presented during one trial of the approach-avoidance conflict (AAC) task.
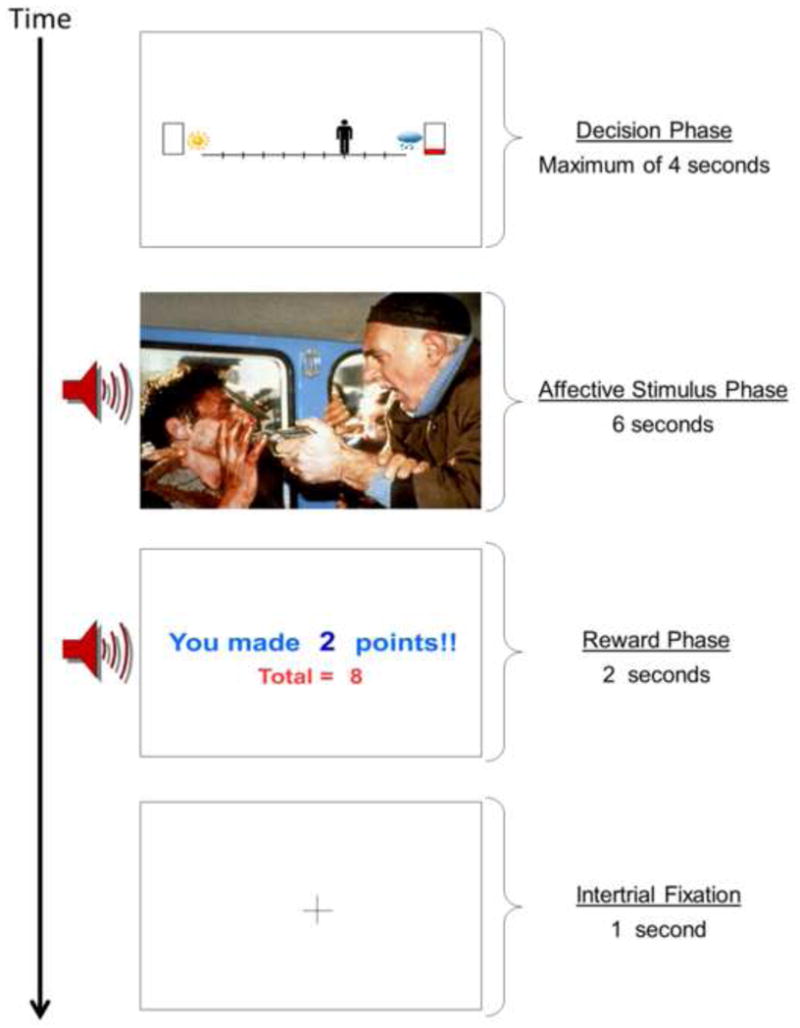
A decisional phase is first presented for a maximum of 4 seconds. The affective stimulus phase consists of either a negative or positive affective image (from the International Affective Picture System [IAPS]) [52] and a matched affective sound (from free source websites such as freesound.org and the International Affective Digitized Sounds [IADS]) [53]. The affective stimulus phase lasts a total of 6 seconds. The reward phase consists of a screen displaying points earned on the current trial as well as the total points collected thus far on the task in combination with a reward-related trumpet sound. The reward phase lasts a total of 2 seconds. An intertrial fixation of 1 second is displayed to allow the subject to prepare for the next trial. The AAC task consists of 18 trials of each condition type (displayed in Figure 1), for a total of 72 trials.
The main dependent variables for the AAC included the following. 1) Approach behavior, modeled in two ways: a) mean approach behavior, or the avatar’s end position on the runway in relation to the negative affective outcome (range of −4 to +4), averaged across trials, and b) change in approach behavior with increasing reward, modeled by calculating the slope for approach behavior from 2-point to 4-point to 6-point conflict ((6pt−2pt)+(4pt−2pt)). 2) Response time for initial button press, averaged across trials. As a secondary outcome measure, we also included area of the slope for the avatar start position to model the influence of effort on approach behavior during conflict. Subjects also completed pre- and post- task visual analogue scale (VAS; 10 cm) ratings of how pleasant and unpleasant they felt and a post-task questionnaire where they rated on a 1–7 Likert scale 1) how upsetting the task was, 2) how difficult it was for them to make decisions during the task, 3) how motivated they were to get reward points, 4) how motivated they were to avoid negative affective stimuli, 5) how enjoyable they found the positive pictures, and 6) how anxious or uncomfortable they felt in response to the negative pictures. These scales were included to assess the effect of the task on affective state and confirm that behavioral measures of performance related to subjective ratings of motivation.
2.3 Self-Report Measures
Subjects completed the following within the same session as the AAC: 1) State Trait Anxiety Inventory-Trait (STAI-T) [50], consisting of a total score. 2) Anxiety Sensitivity Index (ASI) [54, 55], consisting of total, Physical, Psychological, and Social scores. 3) Behavioral Inhibition/Activation Scale (BIS/BAS) [6], consisting of four subscales: BAS Drive, Fun Seeking, and Reward Responsiveness, and BIS (behavioral inhibition or punishment sensitivity).
2.4 Data Analysis
The selection procedure produced a sample with continuous and normally distributed scores on the STAI-T (Shapiro-Wilk test W(95)=.976, p=.080). However, AAC task performance was not normally distributed (see Supplementary Figures 1–3). Therefore, nonparametric tests were used for all analyses. Previous research suggests females report higher levels of fear and avoidance, and have a higher prevalence of anxiety disorders than males [32–34]. Therefore, we examined gender differences regarding self-report measures and AAC behavior. Due to there being significant differences between genders, the following analyses were conducted separately for males and females.
To characterize differences in behavior between task conditions and pre- and post-task VAS ratings, we used Friedman’s test for nonparametric repeated measures comparisons. For variables exhibiting significant effects, post-hoc tests were conducted using Wilcoxon signed ranks test to further characterize differences. Spearman’s rho correlations were used to investigate relationships between behavioral measures during conflict and self-report measures and post-task questionnaire ratings. Due to the fact that there were 4 behavioral outcome measures (approach behavior, change in approach behavior with increasing reward, response time, and effort), results with these measures were considered significant at p<.013 (.05/4).
Similarly, given there were 8 self-report outcome measures (two VAS scales, 6 post-task questionnaire items), results with these measures were considered significant at p<.006 (.05/8). Post-hoc tests were conducted using Fisher’s Z transformation to examine whether correlations within males and females were significantly different from one another and were considered significant at p<.05.
3. RESULTS
3.1 Task Effects
Confirming the hypothesis that level of reward and conflict influence human behavior, approach behavior significantly differed between task conditions for both males (χ2(3)=57.828, p<0.001) and females (χ2(3)=67.465, p<0.001). Post-hoc tests revealed that approach behavior significantly increased for each increase in point reward for females (Avoidance-only vs. 2-point: Z=−5.18, p<.001; 2-point vs. 4-point: Z=−3.79, p<.001; 4-point vs. 6-point: Z=−3.82, p<.001) and males (Avoidance-only vs. 2-point: Z=−5.21, p<.001; 2-point vs. 4-point: Z=−3.05, p=.002; 4-point vs. 6-point: Z=−3.68, p<.001). Approach behavior is displayed in Figure 3.
Figure 3. Approach behavior during the approach-avoidance conflict (AAC) task.
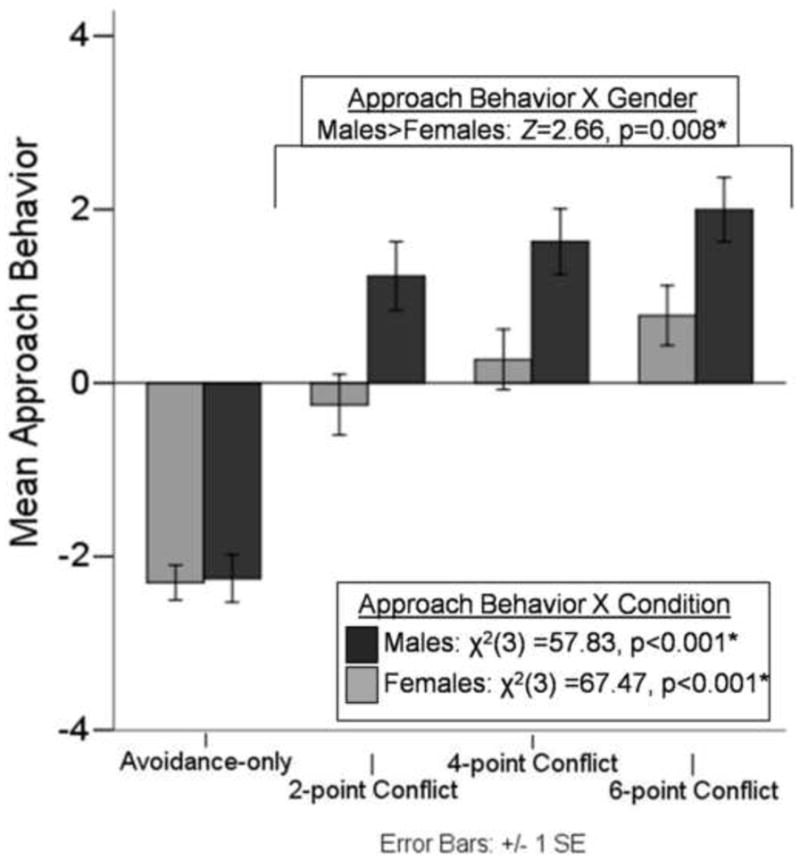
Approach behavior significantly increased with each successive condition (Avoidance-only < 2-point Conflict < 4-point Conflict < 6-point Conflict) for both males (χ2(3)=57.828, p< 0.001) and females (χ2(3)=67.465, p< 0.001). Males exhibited greater levels of approach behavior during conflict than females (Z=2.66, p=0.008).
Response time differed between conditions for males (χ2(3)=12.262, p=.007), but this effect was nonsignificant for females (χ2(3)=9.150, p=.027). Post-hoc tests for males revealed slower response times during avoidance-only compared to 4-point (Z=−2.85, p=.004) and 6-point (Z=−2.93, p=.003) conflict conditions, with a non-significant difference between avoidance-only and the 2-point conflict conditions (Z=−2.07, p=.039) and no difference between conflict conditions (2-point vs 4-point: Z=−.60, p=.548; 4-point vs. 6-point: Z=−1.24, p=.214).
Conflict approach behavior significantly related to the avatar’s start position for both males (χ2(8)=38.84, p<.001) and females (χ2(8)=66.58, p<.001) indicating that the end position was more likely to be nearer to the start position of the avatar. This suggests that the amount of effort required on an individual trial influenced subjects’ behavior. The AAC task was developed in a way that allows for the explicit assessment regarding effects of effort on behavior, with the start position of the avatar being counterbalanced across trials. However, to investigate the primary aim of the study, i.e., the influence of conflict and reward level on approach behavior (regardless of the effort required for each individual trial), we averaged across counterbalanced trials. The rest of the analyses therefore focus on mean end position of the avatar unless otherwise noted.
For both males and females, ratings of unpleasantness increased (males: Z=−4.41, p<0.001; females: Z=−5.72, p<.001) while ratings of pleasantness decreased (males: Z=−4.52, p<.001; females: Z=−5.48, p<.001), from pre- to post- task. See Table 1 for scores on post-task questionnaire ratings and Table 2 for self-report measures.
Table 1.
Post-task questionnaire ratings and correlations with behavior during conflict conditions of the approach-avoidance conflict (AAC) task.
| Item | Min | Max | Mean | SD | Correlations with AAC behavior
|
|||
|---|---|---|---|---|---|---|---|---|
| approach behavior | response time | |||||||
| rho | p | rho | p | |||||
| “Overall, this task was upsetting” | ||||||||
| Male | 1.00 | 7.00 | 3.54 | 1.63 | −.393 | .016 | −.188 | .265 |
| Female | 2.00 | 7.00 | 4.38 | 1.46 | .123 | .383 | −.113 | .427 |
| “I often found it difficult to decide which outcome I wanted” | ||||||||
| Male | 1.00 | 7.00 | 2.59 | 1.85 | −.231 | .169 | .276 | .099 |
| Female | 1.00 | 7.00 | 2.88 | 1.84 | .108 | .448 | .366 | .008* |
| “I was motivated to get reward points on this task” | ||||||||
| Male | 1.00 | 7.00 | 4.73 | 1.97 | .739 | .001** | −.253 | .131 |
| Female | 1.00 | 7.00 | 3.56 | 1.96 | .702 | .001** | .302 | .029 |
| “I was motivated to avoid the negative pictures/sounds on this task” | ||||||||
| Male | 1.00 | 7.00 | 3.22 | 2.06 | −.633 | .001** | .309 | .062 |
| Female | 1.00 | 7.00 | 4.12 | 1.90 | −.684 | .001** | −.236 | .093 |
| “I found the positive pictures enjoyable” | ||||||||
| Male | 1.00 | 7.00 | 4.24 | 1.69 | −.004 | .984 | .118 | .487 |
| Female | 1.00 | 7.00 | 4.10 | 1.56 | .030 | .835 | −.083 | .561 |
| “The negative pictures made me feel anxious or uncomfortable” | ||||||||
| Male | 1.00 | 7.00 | 4.43 | 1.59 | −.488 | .002* | .392 | .016 |
| Female | 1.00 | 7.00 | 5.17 | 1.67 | −.130 | .358 | −.046 | .746 |
Notes: Results are from Spearman’s nonparametric correlation analyses.
Significant at p<.01,
p<.001.
Abbreviations: AAC = approach avoidance conflict task; SD = standard deviation.
Table 2.
Self-report measures and correlations with behavior during conflict conditions of the approach-avoidance conflict (AAC) task.
| Measure | Min | Max | Mean | SD | Correlations with AAC behavior
|
|||
|---|---|---|---|---|---|---|---|---|
| approach behavior | response time | |||||||
| rho | p | rho | p | |||||
| STAI-Trait | ||||||||
| Male | 24.00 | 64.00 | 42.67 | 9.69 | −.170 | .300 | .141 | .392 |
| Female | 25.00 | 69.00 | 41.89 | 10.31 | .228 | .091 | .019 | .892 |
| ASI Total | ||||||||
| Male | 1.00 | 37.00 | 15.67 | 9.05 | −.329 | .041 | .431 | .006* |
| Female | 2.00 | 52.00 | 18.45 | 11.44 | .074 | .587 | .122 | .370 |
| ASI Physical | ||||||||
| Male | 0.00 | 25.00 | 7.36 | 6.49 | −.407 | .010* | .451 | .004* |
| Female | 0.00 | 33.00 | 9.91 | 7.71 | .054 | .692 | .112 | .412 |
| ASI Psychological | ||||||||
| Male | 0.00 | 10.00 | 3.31 | 2.92 | −.223 | .173 | .417 | .008* |
| Female | 0.00 | 14.00 | 3.32 | 3.94 | .050 | .716 | .072 | .599 |
| ASI Social | ||||||||
| Male | 1.00 | 8.00 | 5.00 | 1.76 | .141 | .391 | −.106 | .522 |
| Female | 2.00 | 8.00 | 5.21 | 1.72 | .062 | .650 | .151 | .266 |
| BIS | ||||||||
| Male | 11.00 | 26.00 | 19.33 | 3.70 | −.225 | .118 | .377 | .018 |
| Female | 10.00 | 28.00 | 20.89 | 3.30 | .206 | .128 | .140 | .304 |
| BAS Reward | ||||||||
| Male | 11.00 | 20.00 | 16.96 | 2.06 | −.010 | .951 | .119 | .471 |
| Female | 9.37 | 21.00 | 17.02 | 2.07 | .055 | .685 | −.148 | .277 |
| BAS Drive | ||||||||
| Male | 7.00 | 16.00 | 11.74 | 2.37 | .130 | .432 | .149 | .365 |
| Female | 7.00 | 16.00 | 11.46 | 2.01 | .259 | .054 | −.057 | .677 |
| BAS Fun Seeking | ||||||||
| Male | 5.00 | 16.00 | 12.41 | 2.36 | −.113 | .493 | .038 | .818 |
| Female | 9.00 | 16.00 | 12.18 | 1.67 | .385 | .003* | .194 | .153 |
Notes: Results are from Spearman’s nonparametric correlation analyses.
Significant at p<.01.
Abbreviations: STAI = State Trait Anxiety Inventory; ASI = Anxiety Sensitivity Index; BIS = Behavioral Inhibition Scale; BAS = Behavioral Activation Scale; AAC = approach avoidance conflict task; SD = standard deviation.
3.2 Gender Effects on Task Behavior
Consistent with previous research suggesting females report higher levels of avoidance behavior, females in the current study exhibited less approach behavior during conflict than males (Z=−2.66, p=.008; See Figure 3). Gender was unrelated to response time (Z=−.386, p=.700), or change in approach behavior with increasing reward (Z=−.276, p=.782) during conflict. Moreover, gender was unrelated to approach behavior (Z=−.099, p=.921) or response time (rho=−.870, p=.384) during avoidance-only trials—suggesting the gender effect was specific to conflict conditions.
There were non-significant trends for females to report the task to be more upsetting (Z=−2.57, p=.010), to be less motivated by the reward (Z=−2.70, p=.007), to be more motivated to avoid the negative affective stimuli (Z=−2.06, p=.039), and to feel more anxious in response to the negative stimuli (Z=−2.10, p=.036). Gender was unrelated to change in VAS ratings (pre-post) of pleasantness (Z=−.104, p=.917) and unpleasantness (Z=−.712, p=.476), and reports of how difficult it was to make decisions (Z=−.84, p=.403), and how enjoyable they reported the positive pictures to be (Z=−.718, p=.472). Gender was unrelated to scores on self-report measures, including STAI-T (Z=−.454, p=.650), ASI total (Z=−1.508, p=.131), ASI psychological (Z=−.861, p=.389), ASI physical (Z=−1.508, p=.131), ASI social (Z=−.495, p=.620), BAS reward (Z=−.096, p=.924), BAS Drive (Z=−1.214, p=.225), BAS Fun (Z=−1.040, p=.298), and BIS (Z=−2.12, p=.034).
3.3 Relationship between conflict task behavior and self-report measures
Approach Behavior
For both males and females, approach behavior during conflict significantly related to self-reported motivation to obtain reward (females: rho=.70, p<.001; males: rho=.74, p<.001) and avoid negative affective stimuli (females: rho=−.68, p<.001; males: rho=−.63, p<.001) in the expected directions (see Figure 4 and Table 1). Additionally, for males only, self-report of how anxious they felt in response to the negative pictures was negatively related to approach behavior during conflict (males: rho=−.488, p=.002; females: rho=−.130, p=.358; difference between male and females correlations: Z =1.83, two-tailed p=.067). Conflict approach behavior also showed the expected negative correlation with anxiety sensitivity (Physical subscale) for males only (see Figure 5). For females, conflict approach behavior was associated with higher scores on the BAS Fun Seeking subscale (see Figure 6 and Table 2). Using the Fisher’s Z transformation to compare the correlation coefficients, it was determined that the difference between females and males in regards to the correlation between approach behavior and ASI Physical was significant (Z =−2.22, two-tailed p=.026), though the difference in correlations between approach behavior and BAS Fun Seeking did not reach significance (Z =1.33, two-tailed p=.184). Approach behavior was unrelated to measures of trait anxiety (STAI-T) or inhibition (BIS) for either males or females.
Figure 4. Scatterplot displaying relationship between approach behavior on the approach avoidance conflict task (AAC) and ratings of how motivated the individual was to obtain reward.
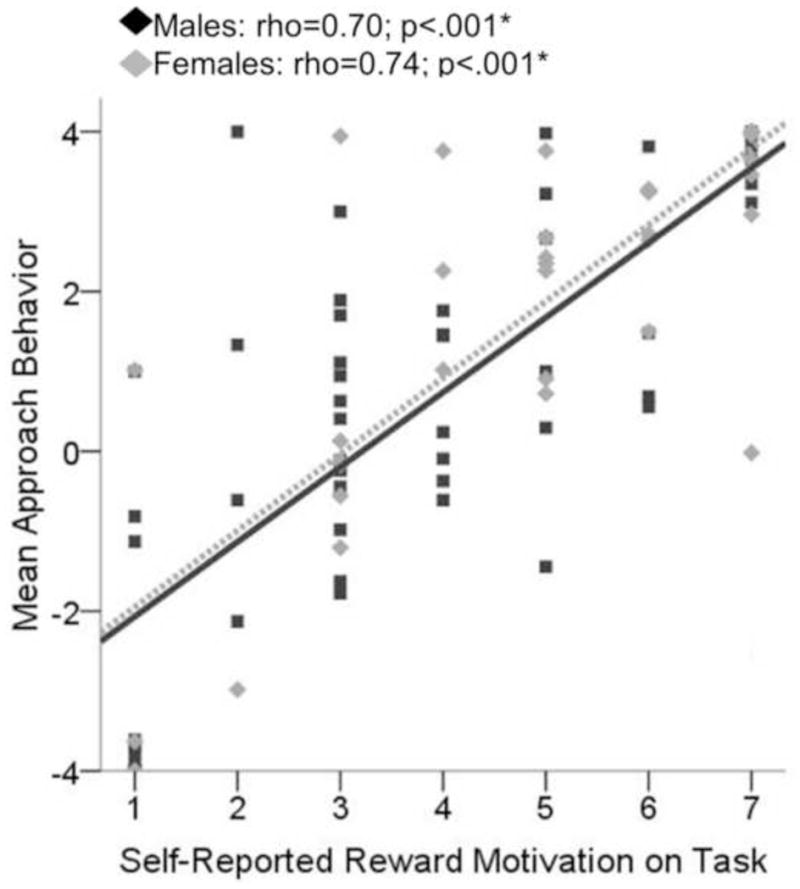
Approach behavior during conflict conditions of the AAC task was significantly related to individual’s post-task questionnaire ratings of how motivated they were to seek reward during the task (males: rho=0.70; p<.001*; females: rho=0.74; p<.001).
Figure 5. Scatterplot displaying relationship between approach behavior on the approach avoidance conflict task (AAC) and scores on the Anxiety Sensitivity Index (ASI) Physical subscale.
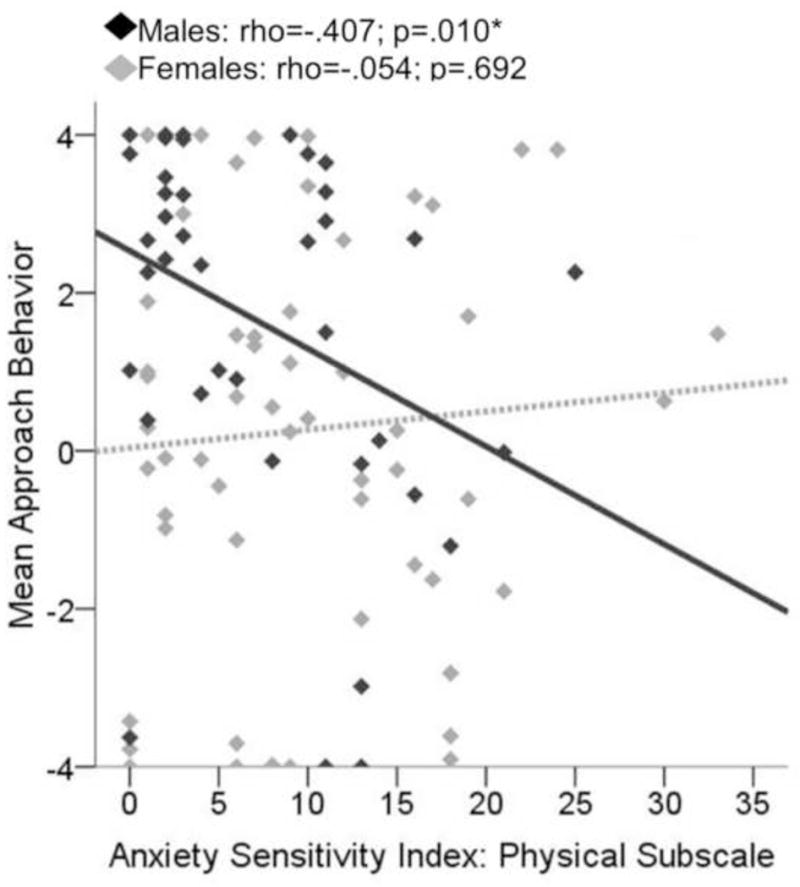
Approach behavior for males was positively related to scores on the Anxiety Sensitivity Index (ASI) Physical subscale during conflict conditions of the approach-avoidance conflict (AAC) task (rho=−.407; p=.010), while this relationship was nonsignificant for females (rho=−.054; p=.692).
Figure 6. Scatterplot displaying relationship between approach behavior on the approach avoidance conflict task (AAC) and scores on the Behavioral Activation Scale (BAS) Fun-Seeking subscale.
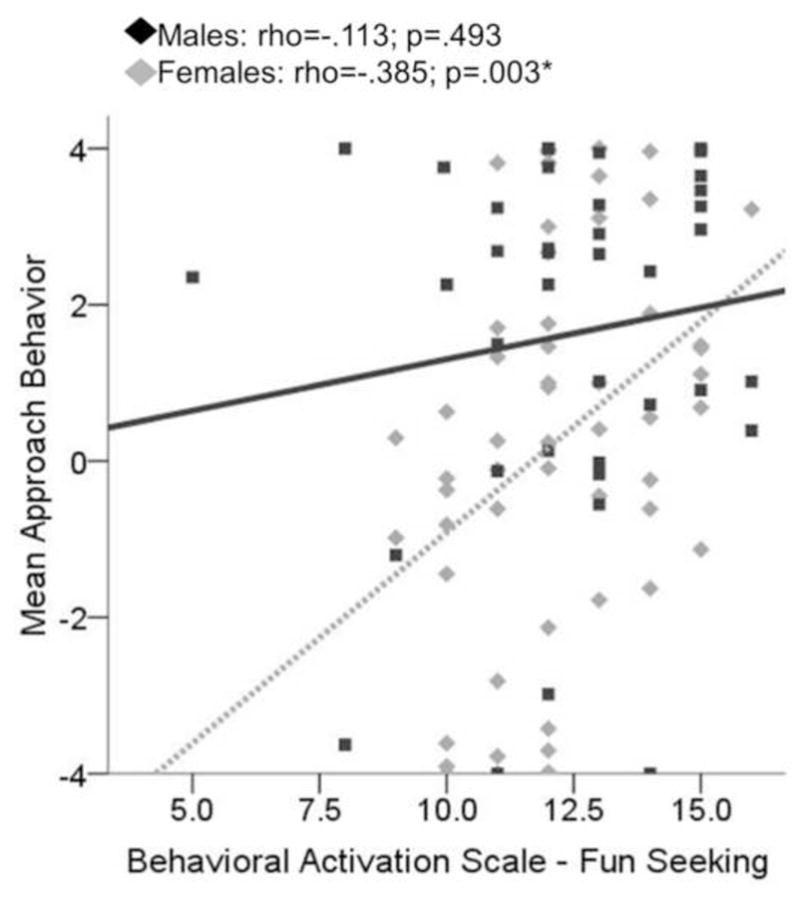
Approach behavior for females was positively related to scores on the BAS Fun Seeking subscale during conflict conditions of the approach-avoidance conflict (AAC) task (rho=.385; p=.003), while this relationship was nonsignificant for males (rho=−.113; p=.493).
Response Time
For females, there was a relationship between conflict response time and self-report of how difficult it was to make decisions on the task (see Table 1; difference in correlation between males and females: Z=0.46, p=.648). For males, conflict response time correlated positively with scores on the ASI psychological subscale, physical subscale, and total score (see Table 2). The difference between males and females in regards to correlations between response time and ASI psychological, physical, and total subscales were at a trend level (ASI psychological: Z=1.70, two-tailed p=.091; ASI physical: Z=1.70, two-tailed p=.089; ASI total: Z=1.54, two-tailed p=.124).
4. DISCUSSION
This study presented results from a novel behavioral paradigm measuring approach-avoidance conflict (AAC) decision making in human populations. The study yielded four main results. First, conflict approach behavior correlated with self-reported motivation to approach reward and avoid punishment during the task and greater reward induced more approach behavior. Second, males exhibited greater levels of approach behavior than females during conflict. Additionally, Anxiety Sensitivity Index (Physical subscale) scores, and self-report ratings of how anxiety-provoking the negative pictures were, related negatively to approach behavior for males, while Behavioral Activation Scale (BAS, Fun Seeking subscale) scores related positively to approach behavior for females. These results support the potential utility of a simple computerized approach-avoidance conflict paradigm in quantitatively assessing the degree of conflict in clinical populations and to help characterize the effects of interventions for anxiety disorders.
Importantly, approach behavior during conflict conditions of the AAC task showed a) a strong positive relationship to self-report of how motivated subjects were to obtain reward and b) a strong negative relationship to self-report of how motivated they were to avoid the negative affective stimulus. These findings were true for both males and females and lend support for the paradigm in measuring approach and avoidance motivated behavior. Response time during conflict related to self-report of how difficult it was to make decisions on the task (though only met significance for females), suggesting this may be a proxy measure for amount of conflict experienced in the decision-making process.
In animal conflict paradigms, the duration in which the animal goes without water or food and the relative “value” of the reward influence approach behavior [56–59]. The AAC paradigm offers different levels of reward points during conflict. Results indicate that as reward level increased, participants exhibited greater levels of approach behavior. This finding provides some evidence of similarity between the current human conflict paradigm and animal conflict paradigms and provides initial support for the use of the AAC in characterizing reward-modulated behavior.
Human research suggests females report greater levels anxiety and are more likely to be diagnosed with anxiety disorders than males [32–34]. There is also some evidence that whereas females and males may experience similar levels of certain anxiety symptoms (i.e., panic attacks), females may report greater levels of avoidance behavior in particular [32]. It is therefore interesting that on the AAC task, females exhibited less approach behavior during conflict than males. This gender effect on behavior was found despite there being no differences between genders in regards to scores on self-report measures (e.g., STAI-T, ASI, BAS/BIS) in this sample. Although there was a trend for females to report greater anxiety/distress in response to the negative affective stimuli, these ratings were not related to approach behavior for females (but instead seemed to relate more to behavior for males). Thus, it is possible that the increased avoidance behavior observed for females may not simply be due to increased anxiety experienced in response to negative affective stimuli. The gender effect observed for the approach-avoidance conflict task may be a cross-species effect, as a recent study found that female rats exhibited less approach behavior than male rats on the Vogel conflict test [30].
However, gender effects have not been consistent within or across animal (e.g., fear conditioning, potentiated startle) and human models of anxiety (e.g., fear conditioning, attentional bias, autonomic reactivity tasks) [32, 60–65]. Future research should investigate the consistency of the gender effect identified for approach-avoidance conflict. The stage of the estrous cycle and hormonal levels reportedly relate to behavior on animal models of anxiety and have been suggested to play a role in human anxiety symptoms [66–70]. The potential relationship between hormonal levels, estrous cycle, and approach-avoidance behavior in human populations could be investigated using the AAC paradigm. Considering that gender may have subtle effects on anxiolytic treatment response in both animals [30] and human populations [71], it would also be important for future research to investigate whether gender effects on approach-avoidance conflict relate to treatment outcome in anxiety disorders.
For males, approach behavior and response time on the AAC task related negatively to scores on the Anxiety Sensitivity Index (ASI; Physical and Psychological subscales). This suggests that, at least for males, increased sensitivity to anxiety-related symptoms is associated with increased avoidance behavior and response time during conflict. The ASI, particularly the physical subscale, has been shown particularly relevant for panic disorder, though may also play an important role in other anxiety disorders, including agoraphobia, posttraumatic stress disorder (PTSD) and generalized anxiety disorder [72]. The results identified for males in the current study can therefore be taken as indication that the presented AAC task may be more relevant to these disorders (e.g., as compared to social anxiety, which relates strongest the social component of the ASI). Conflict approach behavior for females was positively related to self-report on subscales of the Behavioral Activation Scale (BAS), which was designed to measure the strength of approach drives [6, 73–75]. This suggests the reward system may be important for determining approach-avoidance decisions for certain populations. Given that avoidance is a core symptom of most anxiety disorders, it may be useful for future research to probe the relative importance of the reward or approach system in driving avoidance behavior for the various types of anxiety. Thus far, there has been a relative dearth of research on reward processing and reward-dependent behavior for any of the anxiety disorders. However, recent research has shown posttraumatic stress disorder may be associated with decreased motivations for reward and difficulties learning optimal response patterns during reward processing [76–78]. If, in fact, the strength of the reward system has a strong influence on approach-avoidance behavior, this would have important implications for anxiety treatments. Current treatments often aim to decrease negative emotions, such as anxiety or fear responses to triggering stimuli [17, 18, 20–22]. Paradigms such as the AAC task may also be beneficial in determining the relative importance of also targeting the reward system (e.g., through motivational enhancement strategies or dopaminergic agents).
The strength of the correlation coefficients between AAC conflict behavior and self-report measures (e.g., BAS, ASI) were in the moderate range. This is similar to what has been reported in previous studies concerning correlations between self-report measures and behavioral and psychophysiological measures [46, 79–81]. These moderate correlations suggest we are measuring a construct that is related, yet distinct, from that measured via self-report. Behavioral paradigms may therefore be a valuable complement to self-report when working towards a full and integrated understanding of anxiety and related behaviors.
The types of stimuli used in the presented AAC paradigm (points as “reward”, emotional image and sound combinations as “punishment”) were chosen to maintain a balance between approach and avoidance related drives and to ensure ease of translating the paradigm to various research environments, including functional neuroimaging). Male subjects’ ratings concerning motivations to avoid affective stimuli (Mean: 3.22, on a scale of 1–7) were slightly lower than ratings concerning motivations to seek reward (Mean: 4.73), while the opposite was true for females (Mean for motivation to avoid: 4.12; Mean for motivation to seek reward: 3.56). The current format of the AAC task may be useful for probing variability in reward vs. avoidance drives and associated conflict behavior between different populations, including not only gender but also different anxiety disorders. However, it is also possible that the AAC task would need to be modified for the specific population being studied in order to be sensitive to the types of conflict situations experienced by those individuals. For example, one could include threat of public speaking as the affective “punishment” for social anxiety disorder, trauma-related images for PTSD, or disgust-related pictures for obsessive-compulsive disorder (OCD). We feel that it will be important for future research to establish whether the current AAC paradigm or versions modified to be population- or disorder-specific are most predictive of other measures of avoidance and/or treatment outcome.
The presented AAC paradigm could be used in conjunction with fMRI to identify brain systems involved in conflict decision-making. Previous animal and human research primarily implicates various areas of the prefrontal cortex (PFC; e.g., anterior cingulate, orbitofrontal cortex, dorsolateral PFC) and striatal regions (e.g., caudate, nucleus accumbens) in value-based decision-making [10, 82–84], while the medial PFC, insula, and amygdala regions have been primarily implicated in fear processing [85–87]. It could be theorized that both of these systems would be engaged during approach-avoidance conflict and that an imbalance in these systems could relate to over-reliance on either approach or avoidance behaviors [8]. Additionally, the AAC task provides measurable behavior which could potentially be modified by administration of pharmacologic agents targeting various neural systems (e.g., dopaminergic versus serotonergic agents). Such research could a) help to characterize the involvement of these neural systems in determining approach-avoidance conflict behavior and b) determine the potential of this paradigm in characterizing and predicting effects of anxiolytics.
There are several limitations of this study. First, participants were recruited from a young adult population and generalizability of findings to other cohorts is unknown. Given that the population was subclinical, any theories postulated concerning implications for anxiety disorders must remain speculative. Also, interpretation of findings could have been enhanced by assessing women’s estrous cycle stage as well as by including measures of psychophysiological responses (e.g., skin conductance, heart rate). The current study represents an initial step in characterizing this novel AAC task and its potential in research on anxiety traits and disorders. Future studies are needed to examine test-retest reliability and construct validity of the AAC through comparisons with other approach-avoidance and decision-making tasks.
5. CONCLUSIONS
The current study presented a novel paradigm for investigating approach-avoidance conflict behavior in human populations. The task elicited behavior that 1) has face validity with animal paradigms (e.g., increasing reward value produced greater approach behavior) and 2) appears sensitive to human approach and avoidance-related drives (e.g., approach behavior correlated highly with self-report of task-related motivations). Females exhibited less approach behavior during conflict conditions than males, which could be important for understanding the increased prevalence of anxiety disorders in women. Results suggest anxiety sensitivity may influence conflict behavior for males while approach drives may be important for determining conflict behavior for females. Future research could investigate the usefulness of the approach-avoidance conflict (AAC) paradigm for characterizing anxiolytic treatment effects and for investigating neural systems involved in conflict decision-making and anxiety disorders.
Supplementary Material
Research Highlights.
Approach avoidance conflict tests are used extensively to model anxiety in animals.
We present a novel approach-avoidance conflict paradigm for use in human research.
Females exhibited less approach behavior during conflict than males on the task.
Anxiety level influenced behavior for males while reward drives influenced behavior for females.
This novel conflict task provides a translational model for use in human anxiety research.
Acknowledgments
This study was funded by the National Institute of Mental Health (MH64122), the Veterans Administration Mental Illness Research and Education Clinical Center (MIRECC) Postdoctoral Fellowship, and the VA San Diego Center for Excellence in Stress and Mental Health (CESAMH).
Abbreviations
- AAC
approach-avoidance conflict
- BAS
behavioral activation system or scale
- BIS
behavioral inhibition system or scale
- AAT
approach avoidance test
- STAI-T
Spielberger State-Trait Anxiety Inventory
- IAPS
International Affective Picture System
- IADS
International Affective Digitized Sounds
- VAS
Visual analogue scales
- ASI
Anxiety Sensitivity Index
- PFC
prefrontal cortex
Footnotes
FINANCIAL DISCLOSURES
Robin Aupperle, Ph.D., Sarah Sullivan, B.A., and Andrew Melrose report no potential conflicts of interest related to this project. Dr. Aupperle reports receiving salary from the VA San Diego Healthcare System and University of California – San Diego. Ms. Sullivan reports receiving salary from the University of California – San Diego. Mr. Melrose reports receiving salary from the University of California – San Diego. Dr. Paulus reports having received salary or research funding from the VA San Diego Healthcare System and the University of California – San Diego, the Department of Veterans Affairs, National Institute of Mental Health, GlaxoSmithKline, and Hoffmann-La Roche. Dr. Stein reports receiving salary from the VA San Diego Healthcare System and the University of California – San Diego and research funding from Department of Veterans Affairs, US Department of Defense, and the National Institute of Mental Health. He also receives compensation for his work as an editor with Up-to-Date, and Wiley (Depression and Anxiety).
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
Robin L. Aupperle, Email: raupperle@ucsd.edu.
Sarah Sullivan, Email: s2sullivan@ucsd.edu.
Andrew J. Melrose, Email: amelrose@ucsd.edu.
Martin P. Paulus, Email: mpaulus@ucsd.edu.
Murray B. Stein, Email: mstein@ucsd.edu.
References
- 1.Schneirla T. An evolutionary and developmental theory of biphasic processes underlying approach and withdrawal. Lincoln: University of Nebraska Press; 1959. [DOI] [PubMed] [Google Scholar]
- 2.Lang PJ, Bradley MM, Cuthbert BN. Emotion, motivation, and anxiety: brain mechanisms and psychophysiology. Biological Psychiatry. 1998;44:1248–63. doi: 10.1016/s0006-3223(98)00275-3. [DOI] [PubMed] [Google Scholar]
- 3.Gray JA, McNaughton N. The neuropsychology of anxiety: An enquiry into the functions of the septo-hippocampal system. 2. Oxford, England: Oxford University Press; 2000. [Google Scholar]
- 4.Gray JA. A critique of Eysenck’s theory of personality. In: Eysenck HJ, editor. A model for personality. Berlin: Springer; 1981. pp. 246–76. [Google Scholar]
- 5.Millan MJ. The neurobiology and control of anxious states. Progress in Neurobiology. 2003;70:83–244. doi: 10.1016/s0301-0082(03)00087-x. [DOI] [PubMed] [Google Scholar]
- 6.Carver CS, White TL. Behavioral Inhibition, Behavioral Activation, and Affective Responses to Impending Reward and Punishment. Journal of Personality and Social Psychology. 1994;67:319–33. [Google Scholar]
- 7.Brown JS. Principles of intrapersonal conflict. Journal of Conflict Resolution. 1957;1:135–54. [Google Scholar]
- 8.Aupperle RL, Paulus MP. Neural systems underlying approach and avoidance in anxiety disorders. Dialogues in Clinical Neuroscience. 2010;12:305–19. doi: 10.31887/DCNS.2010.12.4/raupperle. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Quartz SR. Reason, emotion and decision-making: risk and reward computation with feeling. Trends in Cognitive Sciences. 2009;13:209–15. doi: 10.1016/j.tics.2009.02.003. [DOI] [PubMed] [Google Scholar]
- 10.Rolls ET, Grabenhorst F. The orbitofrontal cortex and beyond: from affect to decision-making. Progress in Neurobiology. 2008;86:216–44. doi: 10.1016/j.pneurobio.2008.09.001. [DOI] [PubMed] [Google Scholar]
- 11.McNaughton N, Corr PJ. A two-dimensional neuropsychology of defense: fear/anxiety and defensive distance. Neuroscience and Biobehavioral Reviews. 2004;28:285–305. doi: 10.1016/j.neubiorev.2004.03.005. [DOI] [PubMed] [Google Scholar]
- 12.Williams LM, Gordon E. Dynamic organization of the emotional brain: responsivity, stability, and instability. Neuroscientist. 2007;13:349–70. doi: 10.1177/10738584070130040801. [DOI] [PubMed] [Google Scholar]
- 13.Cloninger CR. A unified biosocial theory of personality and its role in the development of anxiety states. Psychiatric Developments. 1986;4:167–226. [PubMed] [Google Scholar]
- 14.Depue RA, Iacono WG. Neurobehavioral aspects of affective disorders. Annual Review of Psychology. 1989;40:457–92. doi: 10.1146/annurev.ps.40.020189.002325. [DOI] [PubMed] [Google Scholar]
- 15.Urosevic S, Abramson LY, Harmon-Jones E, Alloy LB. Dysregulation of the behavioral approach system (BAS) in bipolar spectrum disorders: review of theory and evidence. Clinical Psychology Review. 2008;28:1188–205. doi: 10.1016/j.cpr.2008.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.American Psychiatric Association. Disagnostic and statistical manual of mental disorders DSM-IV-TR. 4. Washington D.C: American Psychiatric Association; 2000. [Google Scholar]
- 17.Foa EB. Psychosocial therapy for posttraumatic stress disorder. The Journal of Clinical Psychiatry. 2006;67 (Suppl 2):40–5. [PubMed] [Google Scholar]
- 18.Foa EB, Kozak MJ. Emotional processing of fear: exposure to corrective information. Psychological Bulletin. 1986;99:20–35. [PubMed] [Google Scholar]
- 19.Mennin DS, Heimberg RG, Turk CL, Fresco DM. Preliminary evidence for an emotion dysregulation model of generalized anxiety disorder. Behaviour Research and Therapy. 2005;43:1281–310. doi: 10.1016/j.brat.2004.08.008. [DOI] [PubMed] [Google Scholar]
- 20.Barlow DH. Anxiety and its disorders: The nature and treatment of anxiety and panic. New York: Guilford Press; 2002. [Google Scholar]
- 21.Barlow DH, Craske MG. Mastery of your anxiety and panic II. Albany, NY: Graywind Publications Inc; 1994. [Google Scholar]
- 22.Rapee RM, Heimberg RG. A cognitive-behavioral model of anxiety in social phobia. Behaviour Research and Therapy. 1997;35:741–56. doi: 10.1016/s0005-7967(97)00022-3. [DOI] [PubMed] [Google Scholar]
- 23.Stein MB, Paulus MP. Imbalance of approach and avoidance: the yin and yang of anxiety disorders. Biological Psychiatry. 2009;66:1072–4. doi: 10.1016/j.biopsych.2009.09.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Millan MJ, Brocco M. The Vogel conflict test: procedural aspects, gamma-aminobutyric acid, glutamate and monoamines. European Journal of Pharmacology. 2003;463:67–96. doi: 10.1016/s0014-2999(03)01275-5. [DOI] [PubMed] [Google Scholar]
- 25.Kilts CD, Commissaris RL, Rech RH. Comparison of anti-conflict drug effects in three experimental animal models of anxiety. Psychopharmacology (Berl) 1981;74:290–6. doi: 10.1007/BF00427112. [DOI] [PubMed] [Google Scholar]
- 26.Kuribara H, Asahi T. Assessment of the anxiolytic and amnesic effects of three benzodiazepines, diazepam, alprazolam and triazolam, by conflict and non-matching to sample tests in mice. Nihon Shinkei Seishin Yakurigaku Zasshi. 1997;17:1–6. [PubMed] [Google Scholar]
- 27.Resstel LB, Souza RF, Guimaraes FS. Anxiolytic-like effects induced by medial prefrontal cortex inhibition in rats submitted to the Vogel conflict test. Physiology & Behavior. 2008;93:200–5. doi: 10.1016/j.physbeh.2007.08.009. [DOI] [PubMed] [Google Scholar]
- 28.Moller C, Wiklund L, Sommer W, Thorsell A, Heilig M. Decreased experimental anxiety and voluntary ethanol consumption in rats following central but not basolateral amygdala lesions. Brain Research. 1997;760:94–101. doi: 10.1016/s0006-8993(97)00308-9. [DOI] [PubMed] [Google Scholar]
- 29.Yamashita K, Kataoka Y, Shibata K, Ozaki T, Miyazaki A, Kagoshima M, et al. Neuroanatomical substrates regulating rat conflict behavior evidenced by brain lesioning. Neuroscience Letters. 1989;104:195–200. doi: 10.1016/0304-3940(89)90354-6. [DOI] [PubMed] [Google Scholar]
- 30.Basso AM, Gallagher KB, Mikusa JP, Rueter LE. Vogel conflict test: sex differences and pharmacological validation of the model. Behavioural brain research. 2011;218:174–83. doi: 10.1016/j.bbr.2010.11.041. [DOI] [PubMed] [Google Scholar]
- 31.McLean CP, Asnaani A, Litz BT, Hofmann SG. Gender differences in anxiety disorders: Prevalence, course of illness, comorbidity and burden of illness. J Psychiatr Res. 2011 doi: 10.1016/j.jpsychires.2011.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.McLean CP, Anderson ER. Brave men and timid women? A review of the gender differences in fear and anxiety. Clinical Psychology Review. 2009;29:496–505. doi: 10.1016/j.cpr.2009.05.003. [DOI] [PubMed] [Google Scholar]
- 33.McLean CP, Hope DA. Subjective anxiety and behavioral avoidance: Gender, gender role, and perceived confirmability of self-report. Journal of Anxiety Disorders. 2010;24:494–502. doi: 10.1016/j.janxdis.2010.03.006. [DOI] [PubMed] [Google Scholar]
- 34.Seedat S, Scott KM, Angermeyer MC, Berglund P, Bromet EJ, Brugha TS, et al. Cross-national associations between gender and mental disorders in the World Health Organization World Mental Health Surveys. Archives of General Psychiatry. 2009;66:785–95. doi: 10.1001/archgenpsychiatry.2009.36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lejuez CW, Read JP, Kahler CW, Richards JB, Ramsey SE, Stuart GL, et al. Evaluation of a behavioral measure of risk taking: The Balloon Analogue Risk Task (BART) Applied. 2002;8:75–84. doi: 10.1037//1076-898x.8.2.75. [DOI] [PubMed] [Google Scholar]
- 36.Daughters SB, Reynolds EK, MacPherson L, Kahler CW, Danielson CK, Zvolensky M, et al. Distress tolerance and early adolescent externalizing and internalizing symptoms: the moderating role of gender and ethnicity. Behav Res Ther. 2009;47:198–205. doi: 10.1016/j.brat.2008.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Newman JP, Patterson CM, Kosson DS. Response perseveration in psychopaths. J Abnorm Psychol. 1987;96:145–8. doi: 10.1037//0021-843x.96.2.145. [DOI] [PubMed] [Google Scholar]
- 38.Vorhold V. The neuronal substrate of risky choice: an insight into the contributions of neuroimaging to the understanding of theories on decision making under risk. Annals of the New York Academy of Sciences. 2008;1128:41–52. doi: 10.1196/annals.1399.006. [DOI] [PubMed] [Google Scholar]
- 39.Bechara A. Neurobiology of decision-making: risk and reward. Seminars in Clinical Neuropsychiatry. 2001;6:205–16. doi: 10.1053/scnp.2001.22927. [DOI] [PubMed] [Google Scholar]
- 40.Mohr PN, Biele G, Heekeren HR. Neural processing of risk. The Journal of Neuroscience. 2010;30:6613–9. doi: 10.1523/JNEUROSCI.0003-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Rangel A, Camerer C, Montague PR. A framework for studying the neurobiology of value-based decision making. Nature Reviews Neuroscience. 2008;9:545–56. doi: 10.1038/nrn2357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Lorian CN, Grisham JR. The safety bias: Risk-avoidance and social anxiety pathology. Behaviour Change. 2010;27:29–41. [Google Scholar]
- 43.Maner JK, Richey A, Cromer K, Mallot M, Lejuez CW, Joiner TE, et al. Dispositional anxiety and risk-avoidant decision-making. Personality and Individual Differences. 2007;42:665–75. [Google Scholar]
- 44.Miu AC, Heilman RM, Houser D. Anxiety impairs decision-making: psychophysiological evidence from an Iowa Gambling Task. Biological Psychiatry. 2008;77:353–8. doi: 10.1016/j.biopsycho.2007.11.010. [DOI] [PubMed] [Google Scholar]
- 45.Werner NS, Duschek S, Schandry R. Relationships between affective states and decision-making. International Journal of Psychophysiology. 2009;74:259–65. doi: 10.1016/j.ijpsycho.2009.09.010. [DOI] [PubMed] [Google Scholar]
- 46.Rinck M, Becker ES. Approach and avoidance in fear of spiders. Journal of Behavior Therapy and Experimental Psychiatry. 2007;38:105–20. doi: 10.1016/j.jbtep.2006.10.001. [DOI] [PubMed] [Google Scholar]
- 47.Bar-Haim Y, Lamy D, Pergamin L, Bakermans-Kranenburg MJ, van Ijzendoorn MH. Threat-related attentional bias in anxious and nonanxious individuals: a meta-analytic study. Psychological Bulletin. 2007;133:1–24. doi: 10.1037/0033-2909.133.1.1. [DOI] [PubMed] [Google Scholar]
- 48.Cisler JM, Koster EH. Mechanisms of attentional biases towards threat in anxiety disorders: An integrative review. Clinical Psychology Review. 2010;30:203–16. doi: 10.1016/j.cpr.2009.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Heuer K, Rinck M, Becker ES. Avoidance of emotional facial expressions in social anxiety: The Approach-Avoidance Task. Behaviour Research and Therapy. 2007;45:2990–3001. doi: 10.1016/j.brat.2007.08.010. [DOI] [PubMed] [Google Scholar]
- 50.Spielberger CD, Gorsuch RL, Lushene PR, Vagg PR, Jacobs AG. Manual for the State-Trait Anxiety Inventory (Form Y) Palo Alto: Consulting Psychologists Press, Inc; 1983. [Google Scholar]
- 51.Derogatis LR. Brief Symptom Inventory-18. Minneapolis, MN: NCS Assessments; 2001. [Google Scholar]
- 52.Lang PJ, Bradley MM, Cuthbert BN. International affective picture system (IAPS): Affective ratings of pictures and instruction manual, Technical Report A-8. Gainesville, FL: University of Florida; 2008. [Google Scholar]
- 53.Bradley MM, Lang PJ. Tech Rep No B-2. Gainesville, FL: The Center for Research in Psychophysiology, University of Florida; 1999. International affective digitized sounds (IADS): Stimuli, instruction manual, and affective ratings. [Google Scholar]
- 54.Peterson RA, Heilbronner RL. The Anxiety Sensitivity Index: Construct validity and factor analytic structure. Journal of Anxiety Disorders. 1987;1:117–21. [Google Scholar]
- 55.Peterson RA, Reiss RJ. Anxiety Sensitivity Index Manual. 2. Worthington, OH: International Diagnostic Systems; 1992. [Google Scholar]
- 56.Choi JS, Kim JJ. Amygdala regulates risk of predation in rats foraging in a dynamic fear environment. Proceedings of the National Academy of Sciences of the United States of America. 2010;107:21773–7. doi: 10.1073/pnas.1010079108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Cabanac M. Influence of food and water deprivation on the behavior of the white rat foraging in a hostile environment. Physiology & Behavior. 1985;35:701–9. doi: 10.1016/0031-9384(85)90400-7. [DOI] [PubMed] [Google Scholar]
- 58.Haddon JE, Killcross S. Both motivational and training factors affect response conflict choice performance in rats. Neural Networks. 2006;19:1192–202. doi: 10.1016/j.neunet.2006.04.004. [DOI] [PubMed] [Google Scholar]
- 59.Brown JS. Gradients of approach and avoidance responses and their relation to level of motivation. Journal of Comparative and Physiological Psychology. 1948;41:450–65. doi: 10.1037/h0055463. [DOI] [PubMed] [Google Scholar]
- 60.Dalla C, Shors TJ. Sex differences in learning processes of classical and operant conditioning. Physiology & Behavior. 2009;97:229–38. doi: 10.1016/j.physbeh.2009.02.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Johnston AL, File SE. Sex differences in animal tests of anxiety. Physiology & Behavior. 1991;49:245–50. doi: 10.1016/0031-9384(91)90039-q. [DOI] [PubMed] [Google Scholar]
- 62.Blanchard DC, Shepherd JK, De Padua Carobrez A, Blanchard RJ. Sex effects in defensive behavior: baseline differences and drug interactions. Neuroscience and Biobehavioral Reviews. 1991;15:461–8. doi: 10.1016/s0149-7634(05)80132-0. [DOI] [PubMed] [Google Scholar]
- 63.Zimmerberg B, Farley MJ. Sex differences in anxiety behavior in rats: role of gonadal hormones. Physiology & Behavior. 1993;54:1119–24. doi: 10.1016/0031-9384(93)90335-d. [DOI] [PubMed] [Google Scholar]
- 64.Sass SM, Heller W, Stewart JL, Silton RL, Edgar JC, Fisher JE, et al. Time course of attentional bias in anxiety: emotion and gender specificity. Psychophysiology. 2010;47:247–59. doi: 10.1111/j.1469-8986.2009.00926.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Kajantie E, Phillips DI. The effects of sex and hormonal status on the physiological response to acute psychosocial stress. Psychoneuroendocrinology. 2006;31:151–78. doi: 10.1016/j.psyneuen.2005.07.002. [DOI] [PubMed] [Google Scholar]
- 66.Milad MR, Igoe SA, Lebron-Milad K, Novales JE. Estrous cycle phase and gonadal hormones influence conditioned fear extinction. Neuroscience. 2009;164:887–95. doi: 10.1016/j.neuroscience.2009.09.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Childs E, Dlugos A, De Wit H. Cardiovascular, hormonal, and emotional responses to the TSST in relation to sex and menstrual cycle phase. Psychophysiology. 2010;47:550–9. doi: 10.1111/j.1469-8986.2009.00961.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Marcondes FK, Miguel KJ, Melo LL, Spadari-Bratfisch RC. Estrous cycle influences the response of female rats in the elevated plus-maze test. Physiology & Behavior. 2001;74:435–40. doi: 10.1016/s0031-9384(01)00593-5. [DOI] [PubMed] [Google Scholar]
- 69.Le Melledo JM, Baker G. Role of progesterone and other neuroactive steroids in anxiety disorders. Expert Review of Neurotherapeutics. 2004;4:851–60. doi: 10.1586/14737175.4.5.851. [DOI] [PubMed] [Google Scholar]
- 70.Palanza P. Animal models of anxiety and depression: how are females different? Neuroscience and Biobehavioral Reviews. 2001;25:219–33. doi: 10.1016/s0149-7634(01)00010-0. [DOI] [PubMed] [Google Scholar]
- 71.Serretti A, Chiesa A, Calati R, Perna G, Bellodi L, De Ronchi D. Common genetic, clinical, demographic and psychosocial predictors of response to pharmacotherapy in mood and anxiety disorders. International Clinical Psychopharmacology. 2009;24:1–18. doi: 10.1097/YIC.0b013e32831db2d7. [DOI] [PubMed] [Google Scholar]
- 72.Naragon-Gainey K. Meta-analysis of the relations of anxiety sensitivity to the depressive and anxiety disorders. Psychol Bull. 2010;136:128–50. doi: 10.1037/a0018055. [DOI] [PubMed] [Google Scholar]
- 73.Campbell-Sills L, Liverant GI, Brown TA. Psychometric evaluation of the behavioral inhibition/behavioral activation scales in a large sample of outpatients with anxiety and mood disorders. Psychological Assessment. 2004;16:244–54. doi: 10.1037/1040-3590.16.3.244. [DOI] [PubMed] [Google Scholar]
- 74.Gomez R, Gomez A, Cooper A. Neuroticism and extraversion as predictors of negative and positive emotional information processing: Comparing Eysenck’s, Gray’s and Newman’s theories. European Journal of Personality. 2002;16:333–50. [Google Scholar]
- 75.Jorm AF, Christensen H, Henderson AS, Jacomb PA, Korten E, Rodgers B. Using the BIS/BAS scales to measure behavioral inhibition and behavioral activation: Factor structure, validity and norms in a large community sample. Personality and Individual Differences. 1999;25:785–800. [Google Scholar]
- 76.Hopper JW, Pitman RK, Su Z, Heyman GM, Lasko NB, Macklin ML, et al. Probing reward function in posttraumatic stress disorder: expectancy and satisfaction with monetary gains and losses. Journal of Psychiatric Research. 2008;42:802–7. doi: 10.1016/j.jpsychires.2007.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Elman I, Ariely D, Mazar N, Aharon I, Lasko NB, Macklin ML, et al. Probing reward function in post-traumatic stress disorder with beautiful facial images. Psychiatry Research. 2005;135:179–83. doi: 10.1016/j.psychres.2005.04.002. [DOI] [PubMed] [Google Scholar]
- 78.Sailer U, Robinson S, Fischmeister FP, Konig D, Oppenauer C, Lueger-Schuster B, et al. Altered reward processing in the nucleus accumbens and mesial prefrontal cortex of patients with posttraumatic stress disorder. Neuropsychologia. 2008;46:2836–44. doi: 10.1016/j.neuropsychologia.2008.05.022. [DOI] [PubMed] [Google Scholar]
- 79.Vaidyanathan U, Patrick CJ, Bernat EM. Startle reflex potentiation during aversive picture viewing as an indicator of trait fear. Psychophysiology. 2009;46:75–85. doi: 10.1111/j.1469-8986.2008.00751.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Hettema JM, Annas P, Neale MC, Fredrikson M, Kendler KS. The genetic covariation between fear conditioning and self-report fears. Biological Psychiatry. 2008;63:587–93. doi: 10.1016/j.biopsych.2007.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Leue A, Beauducel A. A meta-analysis of reinforcement sensitivity theory: on performance parameters in reinforcement tasks. Personality and Social Psychology Review. 2008;12:353–69. doi: 10.1177/1088868308316891. [DOI] [PubMed] [Google Scholar]
- 82.Haber SN, Knutson B. The reward circuit: linking primate anatomy and human imaging. Neuropsychopharmacology. 2010;35:4–26. doi: 10.1038/npp.2009.129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Wise RA. Dopamine and reward: the anhedonia hypothesis 30 years on. Neurotoxicity Research. 2008;14:169–83. doi: 10.1007/BF03033808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Rangel A, Hare T. Neural computations associated with goal-directed choice. Current Opinion in Neurobiology. 2010;20:262–70. doi: 10.1016/j.conb.2010.03.001. [DOI] [PubMed] [Google Scholar]
- 85.Shin LM, Liberzon I. The neurocircuitry of fear, stress, and anxiety disorders. Neuropsychopharmacology. 2010;35:169–91. doi: 10.1038/npp.2009.83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Quirk GJ, Mueller D. Neural mechanisms of extinction learning and retrieval. Neuropsychopharmacology. 2008;33:56–72. doi: 10.1038/sj.npp.1301555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.LeDoux JE. Emotion circuits in the brain. Annual Review of Neuroscience. 2000;23:155–84. doi: 10.1146/annurev.neuro.23.1.155. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


