Abstract
To determine whether unchlorinated bore water in northern Australia contained Burkholderia pseudomallei organisms, we sampled 55 bores; 18 (33%) were culture positive. Multilocus sequence typing identified 15 sequence types. The B. pseudomallei sequence type from 1 water sample matched a clinical isolate from a resident with melioidosis on the same property.
Keywords: Burkholderia pseudomallei, bacteria, melioidosis, bore water, Northern Australia, dispatch
Burkholderia pseudomallei is an environmental bacterium that causes melioidosis (1), a disease that is endemic throughout much of southeastern Asia and tropical northern Australia and sporadically occurs in other regions (2). Most infection is thought to result from percutaneous inoculation, but inhalation, aspiration, and ingestion of soil or water containing B. pseudomallei bacteria are the most recognized routes of infection. Outbreaks of melioidosis in Australia after exposure to contaminated water have been described. An outbreak of 159 cases in intensive piggeries (hog lots, a type of factory farm that specializes in raising pigs up to slaughter weight) in Queensland was attributed to contamination of the water supply (3), and a clonal outbreak in pigs on a small farm outside Darwin, Northern Territory was linked to B. pseudomallei cultured from the farm’s bore water (4). Two clonal clusters of human melioidosis have also been found in remote indigenous communities in northern Australia where molecular typing of recovered bacteria traced the source of infection to a contaminated community water supply. Fatalities occurred in both outbreaks. In 1 outbreak, the water supply was not chlorinated (5); in the other, the chlorination system was not adequately maintained (6).
Bore water can be contaminated with B. pseudomallei in our region (4,7). We surveyed a series of bores to ascertain how commonly such contamination occurs and whether B. pseudomallei is transient or persistent in positive bores. We then compared the genetic diversity of B. pseudomallei strains recovered from bores with strains from human melioidosis cases and other environmental strains from the region.
The Study
Darwin, capital of the Northern Territory, Australia, is a coastal tropical city at 12°S. It has 2 distinct seasons: a hot monsoonal wet season from October through May and a dry season with very little, if any, rain from June through September. The city has a population of ≈100,000. Outside the city are many rural blocks of land 1–20 acres in size. Most have a family house, with cultivated gardens or native bush; domestic animals; and sometimes small numbers of farm animals, such as goats, pigs, and chickens. Horticultural activities include planting of mangoes, Asian vegetables, and watermelons. Most residents use unchlorinated groundwater provided by deep bores that tap into the underlying aquifers. We estimate that >3,000 such bores are in the rural areas and provide unchlorinated water to as many as 10,000 persons. Each year, 25–50 human cases of melioidosis occur in the Northern Territory; 50% occur in Darwin residents and 10%–15% occur in those living in rural areas surrounding Darwin (M. Mayo et al., unpub. data). Melioidosis also occurs in domestic and farm animals in the region.
We sampled bore water from 55 blocks in the Darwin rural region. All blocks were within a 30-km radius of Darwin, and all used unchlorinated bore water for domestic and irrigation purposes. Water samples were collected from the bore head (initial outlet of groundwater at source), water storage tank, and other water exit points (taps, hoses). For each sample, 1 liter of water was filtered through 0.22-µm filters (Millipore Corporation, Bedford, MA, USA). Filters were then cultured separately in Ashdown broth (Oxoid, Melbourne, Victoria, Australia) and tryptone soy broth (Oxoid) with gentamicin 10 mg/mL. Broth was plated onto Ashdown agar (Oxoid) on days 2, 7, and 14. Bacterial colonies suggestive of B. pseudomallei by morphologic appearance on Ashdowns agar were confirmed by Gram stain, oxidase test, agglutination with B. pseudomallei antiserum, and a specific PCR targeting B. pseudomallei type III secretion system (8). Confirmed B. pseudomallei bacteria were cultured on chocolate agar (Oxoid), and DNA was extracted by using a DNeasy tissue kit (QIAGEN, Hilden, Germany). Multilocus sequence typing (MLST) of bacterial DNA determined the sequence type (ST) for each isolate (9), allowing comparison with STs in the global MLST dataset (http://bpseudomallei.mlst.net).
B. pseudomallei was cultured from 18 (33%) of 55 water samples; 16 (36%) of 45 blocks tested during the wet season were positive, and 2 (20%) of 10 blocks tested during the dry season were positive. From 18 initial isolates, 9 distinct STs were identified; ST266 was found at 4 separate sites and ST109 at 3 (Table). Nine of the 18 positive sites were resampled 3 times during a 2-year period. In 5 (56%) of 9 sites, B. pseudomallei was recovered at least 1 additional time, and 3 sites were positive on 4 sampling occasions. STs of isolates from repeat sampling showed up to 3 different STs at the same location at the same time. At 1 site, the same ST (ST325) was present in all 4 samplings during the 2 years. Nevertheless, at each of the 5 sites with repeat positive cultures, including this site, an ST different from the original ST was recovered, even if the original ST was still present (Table).
Table. Sampling, culture, and MLST results from initial and repeat sampling of rural unchlorinated domestic water supplies, Northern Territory, Australia*.
| Site no. | 1st sampling | 2nd sampling | 3rd sampling | 4th sampling |
|---|---|---|---|---|
| 1 | 109 | Negative | Negative† | Negative |
| 2 | 266 | 558, 326, 559 | 326, 559† | 109 |
| 3 | 325 | 325 | 325, 328† | 325† |
| 4 | 109 | Negative | 334† | Negative |
| 5 | 320 | – | – | – |
| 6 | 326 | Negative | Negative† | Negative† |
| 7 | 109 | 109 | 121† | 109 |
| 8 | 132 | – | – | – |
| 9 | 325 | Negative | Negative† | Negative |
| 10 | 266 | Negative | Negative† | Negative |
| 11 | 266 | – | – | – |
| 12 | 330† | – | – | – |
| 13 | 333† | 333, 243† | Negative | Negative |
| 14 | 132 | – | – | – |
| 15 | 266 | – | – | – |
| 16 | 132 | – | – | – |
| 17 | 109 | – | – | – |
| 18 | 131 | – | – | – |
*MLST, multilocus sequence typing; –, not resampled. †Indicates sampling during the dry season (June–September); 2nd–4th samplings were conducted during a 2-year period.
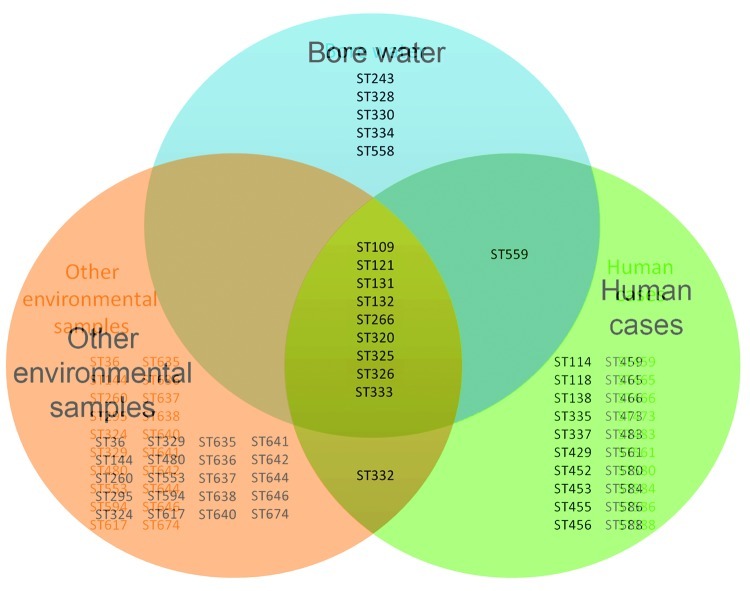
During the sampling period, a total of 15 distinct STs were recovered from water samples; of these, 10 were found in B. pseudomallei isolates collected from humans with melioidosis in the rural area (Figure), including 2 STs from fatal cases (ST109 and ST132). Of the 5 other STs, ST243 and ST334 occurred in humans in urban Darwin, and ST328 was recovered from a goat with fatal melioidosis. Although we do not have data on bacterial load in these positive water sources, the strain recovered from bore water at 1 location (ST131) was an identical ST to the B. pseudomallei isolate recovered from the sputum of a resident of that property who had nonfatal melioidosis pneumonia.
Figure.
Venn diagram of sequence types (STs) determined by multilocus sequence typing found in Burkholderia pseudomallei strains from bore water (n = 15 STs), human cases (n = 31 STs), and other environmental samples (n = 30 STs) from the rural region of Darwin, Northern Territory, Australia.
Surveys of B. pseudomallei across northern Australia have shown a large genetic diversity among strains but distinct regional separations on MLST (10). Although the overall diversity of B. pseudomallei within Australia is considered greater than that seen in southeastern Asia (11), consistent with Australian B. pseudomallei lineages being ancestral to those elsewhere, environmental studies from Thailand have also shown enormous diversity in STs within a small geographic location (12). What remains unclear from these studies is whether differential virulence exists among environmental strains of B. pseudomallei and whether only a proportion of those isolates recovered from the environment have the potential to cause clinical disease (13).
Therefore, although STs found in this study were also represented in humans with melioidosis, the actual public health implications of the findings require further elucidation. Other variables require further investigation to determine the implications of our findings. These include bacterial load and differential bacterial virulence potential among the B. pseudomallei strains in water supplies. Additional considerations would be to quantify the infection risk potential from exposure to culture-positive water through ingestion or after aspiration or inhalation of droplets or aerosols containing B. pseudomallei during, for instance, showering.
Conclusions
B. pseudomallei is common in unchlorinated domestic bore water supplies in the rural region of Darwin, Northern Territory, Australia. Initially, 33% of sites tested were positive for this bacterium, and more than half of these sites on at least 1 occasion were positive again when resampled. MLST showed a great diversity of STs, with persistence and variation in ST found on repeat sampling. STs often matched those found in humans with melioidosis from the same region. B. pseudomallei ST found in the sputum of 1 case-patient with melioidosis was a direct match to the ST of B. pseudomallei cultured from the bore water on the property on which this case-patient lived.
Acknowledgments
This work was supported by grants from the National Health and Medical Research Council of Australia, the Northern Territory Research and Innovation Fund, and the Wellcome Trust, UK.
Biography
Mr Mayo is manager of the Menzies School of Health Research melioidosis program. His areas of expertise include developing improved methods for isolation and identification of B. pseudomallei from environmental samples.
Footnotes
Suggested citation for this article: Mayo M, Kaestli M, Harrington G, Cheng AC, Ward L, Karp D, et al. Burkholderia pseudomallei in unchlorinated domestic bore water, tropical northern Australia. Emerg Infect Dis [serial on the Internet]. 2011 Jul [date cited]. http://dx.doi.org/10.3201/eid1707.100614
References
- 1.White NJ. Melioidosis. Lancet. 2003;361:1715–22. 10.1016/S0140-6736(03)13374-0 [DOI] [PubMed] [Google Scholar]
- 2.Cheng AC, Currie BJ. Melioidosis: epidemiology, pathophysiology, and management. Clin Microbiol Rev. 2005;18:383–416. 10.1128/CMR.18.2.383-416.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ketterer PJ, Webster WR, Shield J, Arthur RJ, Blackall PJ, Thomas AD. Melioidosis in intensive piggeries in south eastern Queensland. Aust Vet J. 1986;63:146–9. 10.1111/j.1751-0813.1986.tb02953.x [DOI] [PubMed] [Google Scholar]
- 4.Millan JM, Mayo M, Gal D, Janmaat A, Currie BJ. Clinical variation in melioidosis in pigs with clonal infection following possible environmental contamination from bore water. Vet J. 2007;174:200–2. 10.1016/j.tvjl.2006.05.006 [DOI] [PubMed] [Google Scholar]
- 5.Currie BJ, Mayo M, Anstey NM, Donohoe P, Haase A, Kemp DJ. A cluster of melioidosis cases from an endemic region is clonal and is linked to the water supply using molecular typing of Burkholderia pseudomallei isolates. Am J Trop Med Hyg. 2001;65:177–9. [DOI] [PubMed] [Google Scholar]
- 6.Inglis TJ, Garrow SC, Henderson M, Clair A, Sampson J, O’Reilly L, et al. Burkholderia pseudomallei traced to water treatment plant in Australia. Emerg Infect Dis. 2000;6:56–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Inglis TJ, Foster NF, Gal D, Powell K, Mayo M, Norton R, et al. Preliminary report on the northern Australian melioidosis environmental surveillance project. Epidemiol Infect. 2004;132:813–20. 10.1017/S0950268804002663 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Novak RT, Glass MB, Gee JE, Gal D, Mayo MJ, Norton R, et al. Development and evaluation of a real-time PCR assay targeting the type III secretion system of Burkholderia pseudomallei. J Clin Microbiol. 2006;44:85–90. 10.1128/JCM.44.1.85-90.2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Godoy D, Randle G, Simpson AJ, Aanensen DM, Pitt TL, Kinoshita R, et al. Multilocus sequence typing and evolutionary relationships among the causative agents of melioidosis and glanders, Burkholderia pseudomallei and Burkholderia mallei. J Clin Microbiol. 2003;41:2068–79. 10.1128/JCM.41.5.2068-2079.2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cheng AC, Ward L, Godoy D, Norton R, Mayo M, Gal D, et al. Genetic diversity of Burkholderia pseudomallei isolates in Australia. J Clin Microbiol. 2008;46:249–54. 10.1128/JCM.01725-07 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Pearson T, Giffard P, Beckstrom-Sternberg S, Auerbach R, Hornstra H, Taunyok A, et al. Phylogeographic reconstruction of a bacterial species with high levels of lateral gene transfer. BMC Biol. 2009;7:78. 10.1186/1741-7007-7-78 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wuthiekanun V, Limmathurotsakul D, Chantratita N, Feil EJ, Day NP, Peacock SJ. Burkholderia pseudomallei is genetically diverse in agricultural land in northeast Thailand. PLoS Negl Trop Dis. 2009;3:e496. 10.1371/journal.pntd.0000496 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Currie BJ. Advances and remaining uncertainties in the epidemiology of Burkholderia pseudomallei and melioidosis. Trans R Soc Trop Med Hyg. 2008;102:225–7. 10.1016/j.trstmh.2007.11.005 [DOI] [PubMed] [Google Scholar]