Abstract
Gastrointestinal stromal tumor (GIST) has become a model for targeted therapy in cancer. The vast majority of GISTs contain an activating mutation in either the KIT or platelet-derived growth factor A (PDGFRA) gene. GIST is highly responsive to several selective tyrosine kinase inhibitors. In fact, this cancer has been converted to a chronic disease in some patients. Considerable progress has been made recently in our understanding of the natural history and molecular biology of GIST, risk stratification and drug resistance. Despite the efficacy of targeted therapy, though, surgery remains the only curative primary treatment and cures more than 50% of GIST patients who present with localized disease. Adjuvant therapy with imatinib prolongs recurrence-free survival and may improve overall survival. Combined or sequential use of tyrosine kinase inhibitors with other agents following tumor molecular subtyping is an attractive next step in the management of GIST.
Keywords: Cancer, sarcoma, tyrosine kinase, imatinib, sunitinib, adjuvant therapy
INTRODUCTION
Gastrointestinal stromal tumor (GIST) is a mesenchymal (non-epithelial) tumor of the gastrointestinal tract. Although this tumor is relatively uncommon and was not well-known in the past, GIST is now the focus of intense research, in particular since novel tyrosine kinase inhibitors (TKIs) have revolutionized the outcome of GIST patients, and transformed this notoriously chemotherapy-resistant sarcoma into a model disease of modern targeted cancer therapy. GIST was the first human solid tumor in which small molecule TKIs were highly effective and prompted the development and testing of small molecule TKIs in other types of human cancer. The progress made in the understanding and management of GIST in the last decade may have been greater than in any other type of human cancer during this time period.
GIST was recognized as a discrete tumor entity in the 1980's.(1, 2) These tumors were formerly called leiomyomas, leiomyosarcomas and leiomyoblastomas of the gastrointestinal tract until they were found to have clinical, histopathological and molecular biological features that differentiated them from other soft tissue tumors. In 1998, Seiichi Hirota and colleagues made the landmark discovery that the majority of GISTs harbor an activating mutation in the KIT onco-gene.(3) KIT encodes the KIT receptor tyrosine kinase, which is the receptor for stem-cell factor (SCF). Binding of SCF to KIT induces KIT dimerization and activation.(4) Detection of KIT expression by immunohistochemistry and KIT mutations by DNA sequencing became practical tools in discriminating GIST from other soft tissue tumors. Only two years after the discovery of activating KIT mutations in GIST, the first GIST patient was treated with imatinib, a small molecule oral inhibitor of KIT. The favorable results(5) were then extended to a larger patient cohort and GIST became the first solid tumor type found to respond frequently to a small molecule TKI.(6)
CLINICAL FEATURES OF GIST
GISTs can arise at any site of the gastrointestinal tract from the esophagus to the rectum, the most common site of origin being the stomach (55%) followed by the small intestine (35%) and rectum (5%). Esophageal and colonic GISTs are rare. GIST rarely (<5%) arises outside of the gastrointestinal tract within the abdominal cavity (extra-gastrointestinal GISTs or “E-GISTs”). GIST occurs at any age, but is rare in children. The median age at presentation is approximately 63 years.
Tumor bleeding commonly leads to the diagnosis. Hemorrhage may be sudden resulting in abrupt stomach pain, dizziness, fainting and a low hemoglobin concentration and may even necessitate emergency surgery. Alternatively, insidious bleeding may occur into the abdominal cavity or the gastrointestinal tract producing anemia. GISTs may cause abdominal pain or discomfort. Bowel obstruction, polyuria or jaundice are rare. Sometimes, no symptoms are present at the time when a palpable tumor is discovered. The median size of GIST at diagnosis is approximately five to seven centimeters in diameter, but they may be as large as 30 to 40 cm. GISTs probably arise from the interstitial cells of Cajal (ICC) or their precursors, the pacemaker cells responsible for autonomous movements of the gastrointestinal tract. GISTs can give rise to metastases in the liver and peritoneal cavity, but they rarely involve the lungs.(7)
The annual incidence of GIST is approximately 10 cases per million as determined by population-based studies.(8–10) GIST is the most common sarcoma of the gastrointestinal tract and one of the most common types of soft tissue sarcoma.
MOLECULAR FEATURES
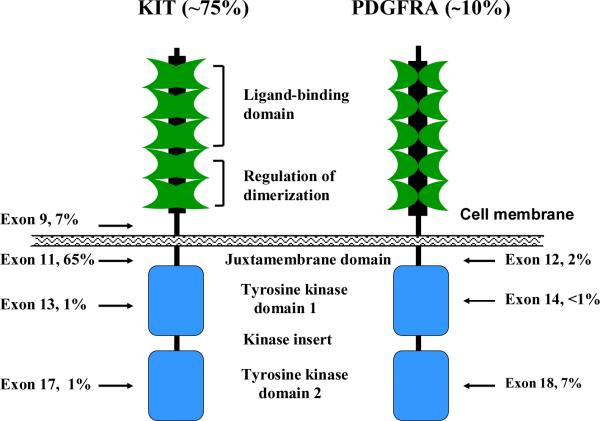
Molecular classification is critical to understanding modern GIST management. Approximately 75% of GISTs harbor a mutation in KIT. Curiously, KIT mutations occur virtually always in one of four out of the 21 exons of the gene (Figure 1). Most mutations occur in KIT exon 11, which encodes for the intracellular juxtamembrane part of the protein, sometimes in exon 9, and rarely in the intracellular kinase portion of the protein in exon 13 or 17. Exon 11 mutations can be deletions or insertions of variable length or their combinations, or point mutations. Exon 9 mutations tend to be duplications of codons 502 and 503. In approximately 10% of GISTs, the KIT gene is normal but there is a mutation in the platelet-derived growth factor receptor alpha (PDGFRA) gene.(11) PDGFRα is a type III receptor tyrosine kinase like KIT, and functions as the receptor of several platelet-derived growth factor (PDGF) isoforms.(12) Activating PDGFRA mutations have been detected in gene exons 12, 14 and 18, which correspond to KIT exons 11, 13 and 17, respectively.
Figure 1.
Schematic structures of KIT and platelet-derived growth factor receptor (PDGFR). The percentages indicate the frequency of mutations detected in each exon of the gene that encodes for the protein.
The type of KIT and PDGFRA mutations has clinical relevance. The majority of GISTs with a PDGFRA mutation occur in the stomach and may stain poorly by immunohistochemistry for KIT protein, whereas GISTs with a KIT exon 9 mutation usually arise outside of the stomach in the gastrointestinal tract (Table 1). GISTs with a KIT exon 11 deletion mutation affecting codons 557 and/or 558, and those with a KIT exon 9 mutation tend to be associated with unfavorable clinical outcome.(13, 14)
Table 1.
Classification of GISTs
| Type of GIST | Incidence | Mutated gene | Tumor types | Clinical features | Imatinib sensitivity |
|---|---|---|---|---|---|
| Sporadic GIST | |||||
| KIT mutation | |||||
| Exon 9 | ~7% | KIT | GIST | Most non-gastric | Yes. 800 mg/day recommended. |
| Exon 11 | ~65% | KIT | GIST | Gastric or non-gastric | Yes |
| Exon 13 | ~1% | KIT | GIST | Variable | |
| Exon 17 | ~0.5% | KIT | GIST | Variable | |
| PDGFRA mutation | |||||
| Exon 12 | ~1.5% | PDGFRA | GIST | Most gastric | Yes |
| Exon 14 | ~0.1% | PDGFRA | GIST | Yes | |
| Exon 18 | ~7% | PDGFRA | GIST | Most gastric | D842V insensitive. Most others sensitive. |
| Wild type (wt) | ~10% | KIT wt, PDGFRA wt; sometimes BRAF, SDHA, SDHB or SDHC mutation | GIST | Gastric or non-gastric | Variable |
| Familial GIST | Very rare | KIT, rarely PDGFRA | GIST | Often multiple, small bowel GISTs; low mitotic counts. | Yes |
| Syndromic GIST | |||||
| Carney triad | Very rare | Unknown | GIST, paraganglioma, pulmonary chondroma | Usually female, age <30. | No? |
| Carney-Stratakis | Rare | SDHB, SDHC, SDHD | Usually GIST, paraganglioma | Gastric GISTs, wt, may be familial. | Probably not |
| Neurofibromatosis-1 | Rare | NF1 | Glioma, MPNST, GIST | Often multiple small bowel GISTs, wt. | Probably not |
Abbreviations: GIST, gastrointestinal stromal tumor; MPNST, malignant peripheral nerve sheath tumor; PDGFRA, platelet-derived growth factor receptor A gene; SDHA, SDHB, SDHC and SDHD, genes that encode for succinate dehydrogenase subunits A, B, C and D, respectively; wt, wild type.
MICRO-GIST
The prevalence of GIST in the general population is much higher than the incidence of clinical GISTs, because most very small GISTs are often never diagnosed and only rarely become clinically significant. Micro-GISTs, one centimeter or smaller in diameter, were found in up to one third of the normal middle-aged and elderly population in meticulous studies based on autopsy tissue or tissue removed at surgery.(15, 16) Curiously, a substantial proportion of micro-GISTs already contain an activating KIT mutation or sometimes a PDGFRA mutation, suggesting that other molecular alterations are necessary for tumor progression before a GIST becomes clinically significant.
GIST DIAGNOSIS AND RISK STRATIFICATION
The microscopic morphology of GIST varies. The spindle cell histological variant is the most common (70%) and corresponds to tumors formerly often considered as leiomyosarcomas, whereas the epithelioid or round cell variants (30%) were often previously classified as leiomyoblastoma. GISTs have a characteristic profile of protein expression by immunohistochemistry. Approximately 95% of GISTs stain strongly for the CD117 antigen, which is an epitope of the KIT receptor tyrosine kinase, and equally many for DOG-1 (a chloride channel protein).(17, 18) GISTs are only rarely immunopositive for desmin (an intermediate filament protein typical of muscle) or S100 (a neural/schwann cell marker). Mutation analysis of KIT and PDGFRA is necessary to confirm the diagnosis in equivocal cases.
Since the malignant potential of GIST varies from virtually benign to highly aggressive, estimation of the risk of recurrence after surgery is of importance. Several risk stratification schemes are available.(19–22) Although many prognostic factors have been identified, only tumor size, tumor site, tumor mitotic activity and presence of tumor rupture are generally accepted for use in risk stratification. Gastric GISTs are associated with better outcome than non-gastic GISTs.(20) Patients whose tumor ruptures into the abdominal cavity either spontaneously or at surgery have a very high risk of tumor recurrence, probably exceeding 80%.(23, 24) Although mutation type appears to be related to outcome, it has not yet been integrated into the risk classification schemes.
Tumor mitotic count is probably the most important single prognostic factor, but also problematic, because mitosis counting is somewhat subjective, depends on the size of the field of view of the microscope, and has never been standardized. Also, some of the risk stratification schemes use only one cut-off value for the mitotic count, which leads to abrupt chances in the predicted outcome around the cut-off point. Despite these limitations, the most commonly used risk stratification schemes appear to work reasonably well in different GIST populations.(21) The high-risk category, which defines the most important target group for adjuvant systemic therapy, usually includes GIST patients whose tumor is larger than five centimeters in diameter and contains over five mitoses/50 high power fields (HPFs). Non-gastric GISTs larger than five centimeters in diameter are also considered high risk, as are tumors smaller than five centimeters with a mitotic count exceeding 5/50 HPFs.
PRINCIPLES OF SURGERY
Surgery is the standard treatment for primary GIST without metastasis. Over 50% of GIST patients are probably cured by surgery alone. The tumor is removed en bloc with its pseudocapsule and a margin of normal tissue. The optimal width of the normal tissue margin has not been determined, and this may vary based on the location of the tumor. In cases where contiguous organs are involved, en bloc resection of the tumor and the involved organs may be required. Patients who have complete tumor resection (R0) have more favorable overall survival than patients treated with less radical surgery, although this may in part be due to the more aggressive biological features of GISTs that cannot be totally removed at surgery. Negative microscopic margins should always be obtained if feasible. Tumor rupture at surgery should be avoided as it is associated with poor outcome and an increased risk for development of perito-neal metastases.(23, 24) Lymph node metastases are uncommon (1%), and regional lymph node resection is almost never required.(25) Laparoscopic removal of small (<6–8 cm) GISTs appears to be safe.
Neoadjuvant (i.e., preoperative) use of imatinib to shrink the tumor should be considered when resection may require total gastrectomy, pancreaticoduodenectomy or an abdominoperineal resection or if the tumor is particularly large.(26) Neoadjuvant systemic therapy followed by surgery with or without adjuvant therapy has never been compared with immediate surgery followed by adjuvant systemic therapy in a randomized trial, and, therefore, the relative merits and harms of each approach remain inadequately defined.
The value of surgery for metastatic GIST is unproven. Resection of residual responsive disease following TKI therapy or disease that has become refractory to TKI therapy has not been tested in a randomized trial. Because patients with the smallest tumor burden had the most durable response to imatinib,(27) there is a possibility that complete or partial surgical removal of metastases might delay the development of resistance to imatinib in responding tumors. A randomized European Organization for Research and Treatment of Cancer trial (identifier NCT00956072), comparing imatinib with or without surgery in the treatment of GIST patients whose disease was responding to imatinib, was designed to test this important hypothesis, but the study was halted due to poor accrual. We suspect that tumor debulking surgery is a reasonable option for selected patients with low-volume metastatic disease and for those who have symptomatic metastases. It is generally agreed that surgery does not play a role for patients with multiple sites of progressing disease.(28, 29)
ADVANCED GIST TRANSFORMS TO CHRONIC DISEASE
Imatinib Mesylate
GIST responds poorly to almost all conventional chemotherapy agents used in the treatment of soft tissue sarcomas.(25) In accordance with such clinical data, GISTs express P-glycoprotein and multidrug resistance protein-1 (MDR-1) more frequently than leiomyosarcomas.(30) Imatinib mesylate was the first agent identified that had substantial activity in GIST,(5, 6) and is now considered the standard first-line treatment for advanced GIST. Imatinib inhibits the tyrosine kinases KIT, PDGFRs, ABL, BCR-ABL, ARG and c-FMS, competes with ATP in binding to the intracellular kinase domain of KIT, and prevents the kinase from transferring phosphate from ATP to tyrosine residues of the substrates and thus KIT down-stream signalling. The standard dose of imatinib, 400 mg once daily orally, is generally well tolerated. The common adverse effects are infrequently severe and include periorbital edema, muscle cramps, diarrhea and anemia.
Most (65% to 70%) GIST patients respond to imatinib and another 15% to 20% obtain disease stabilization.(6, 27, 31) Patients with advanced GIST who respond to imatinib and those who have disease stabilization have similar median times to GIST progression (approximately two years) and survival times (median of approximately five years), suggesting that most disease stabilizations are in fact responses. Approximately 15% of all GISTs are primarily resistant to imatinib and thus the tumor continues to grow.
By computed tomography, responding GISTs typically transform from dense lesions into cyst-like structures, which at histological examination show hyaline degeneration with few scattered surviving GIST cells.(5) The lesion size alone may not be a reliable metric when assessing GIST response to TKIs, since the number of cancer cells within a lesion may be greatly decreased even though the lesion size remains unaltered or even increases at imaging due to fluid accumulation. In practice, it is important to identify those patients who do not respond to imatinib. Progressing GIST lesions remain dense and grow in size, and new detectable lesions may appear either within a pre-existing responding lesion or at a new site. Positron emission tomography (PET) with 18-fluoro-deoxyglucose as the tracer may sometimes be helpful in making a distinction between tumor response and progression.(32)
The type of KIT and PDGFRA mutation is predictive of response to imatinib. Therefore, mutation analysis is often recommended when imatinib therapy is considered. Patients whose GIST harbors a KIT exon 11 mutation frequently respond to imatinib and have a long response duration compared to patients with a KIT exon 9 mutation or with no detectable mutation.(33) The standard dose of imatinib in the treatment of advanced GIST is 400 mg daily based on randomized studies that compared the 400 mg dose either to 600 mg q.d. or to 400 mg b.i.d.(6, 31, 34) These trials found no benefit from a higher than 400 mg daily dose except in the subgroup of patients with a KIT exon 9 mutation, who had a longer time to disease progression when treated with 800 mg/day.(35) The most frequent PDGFRA mutation D842V that occurs in approximately 5% of metastatic GISTs is considered insensitive to imatinib.(33) Some sporadic wild-type GISTs and GISTs that occur as part of a tumor syndrome harbor mutation in one of the genes that encode for succinate dehydrogenase,(36, 37) or in BRAF(38). Such GISTs are unlikely to respond to imatinib (Table 1).
Continuous administration of imatinib at an adequate dose is required for optimal control of advanced GIST. In the French BRF-14 trial, patients who were responding to imatinib were assigned either to continue imatinib or to interrupt imatinib at one, three or five years from the start of therapy. The results obtained after one and three years of imatinib administration show that most patients in the interruption group had GIST progression within approximately two years after stopping imatinib, but they responded to imatinib rechallenge, and no difference in overall survival was found between the imatinib continuation and interruption groups.(39, 40) Based on these results, continuous administration of imatinib is considered standard. In another study carried out in the metastatic setting, patients within the lowest quartile (<1100 ng/mL) of imatinib trough levels had the shortest time to disease progression,(41) suggesting that the standard daily dose of 400 mg to 600 mg may be insufficient in some patients.
Acquired Resistance
Most patients with advanced GIST who respond to imatinib will eventually have disease progression. The median time to progression is approximately 2 years from treatment initiation. The most frequent cause of acquired imatinib resistance is a second mutation in KIT that prevents imatinib from binding to its target. The primary mutation that existed prior to initiation of treatment is virtually always found in a biopsy taken from a lesion that grows during imatinib treatment, but in addition, a second, apparently acquired, mutation is also present. The second mutation most frequently occurs either in KIT exon 13 or exon 17, which encode for the intracellular split kinase domain 1 and the kinase activation loop, respectively. Both sites are infrequently mutated in the absence of imatinib therapy (Figure 1).(42)
It is currently not known whether drug resistance-inducing mutations are present in a few GIST cells already at the time of TKI treatment initiation, being selected out by treatment, or whether the resistance mutations arise in the persisting GIST cells during imatinib treatment. Studies where multiple biopsies were taken from patients who had GIST that was resistant to one or more TKIs showed that different metastases within a patient may harbor different second mutations, and sometimes different mutations conferring drug resistance were found even in a single metastatic deposit.(43, 44) The number of secondary KIT mutations detected may depend on the number of tissue biopsies examined,(43) and, therefore, mutation analysis appears to have limited or no value in directing selection of further systemic therapies at the time when GIST progresses on imatinib. The reasons for the increasing mutational heterogeneity with time on imatinib remain speculative. KIT ligand (SCF) levels are known to increase during imatinib treatment.(45)
Imatinib-Resistant Disease
Sunitinib is currently the second-line systemic treatment for patients whose disease no longer responds to imatinib or who do not tolerate imatinib. In a randomized study, patients scheduled to receive sunitinib 50 mg orally q.d. for 4 weeks followed by a rest period of 2 weeks had a median time to progression of 6.3 months compared to 1.4 months in the subset of patients who were assigned to placebo.(46) Although the response rate to sunitinib was low (7%), 58% of patients had durable stable disease. Besides sunitinib, several other TKIs have activity in advanced GIST including sorafenib, vatalanib, nilotinib and masitinib,(47–49) and still others are currently being tested (regorafenib) or planned to be tested in the treatment of advanced GIST.
ADJUVANT TREATMENT
Randomized Studies
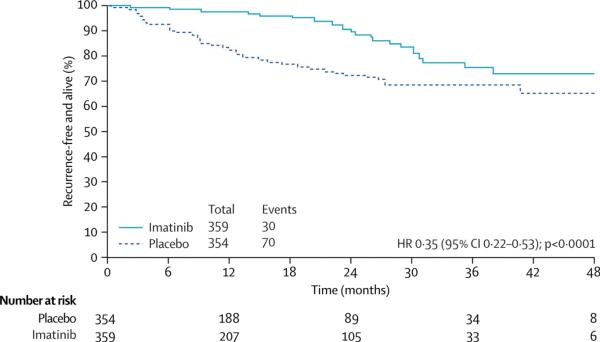
Despite complete excision of the primary tumor, many patients eventually develop tumor recurrence in either the liver or abdominal cavity. Adjuvant systemic therapy is being evaluated in three large adjuvant trials, but to date, results from only the American College of Surgeons Oncology Group trial Z9001 are available.(50) In this study 713 patients with operable GIST, three centimeters or larger in diameter, were assigned to receive either imatinib 400 mg daily or placebo for 12 months after surgery. Patients assigned to placebo were allowed to cross over to receive imatinib following tumor recurrence. Accrual to the study was stopped early, since a planned interim analysis found imatinib to prolong progression-free survival highly significantly (p < 0.0001) compared with placebo. One-year recurrence-free survival was 98% in the imatinib group and 83% in the placebo group, producing a hazard ratio of 0.33 (Figure 2). At the time of the study analysis, no difference in overall survival was observed between the groups possibly due to patient cross-over and the short follow-up time (median, 19.7 months). Adjuvant imatinib was well-tolerated, and the rate of serious adverse events was low. Both the U.S. Food and Drug Administration (FDA) and the European Medicines Agency (EMEA) approved adjuvant imatinib based on the Z9001 results.
Figure 2.
Recurrence-free survival in the American College of Surgeons Oncology Group (ACOSOG) trial Z9001. Produced with the permission of the publisher.
Mutation analysis of KIT and PDGFRA from the tumor tissue should be considered when adjuvant treatment with imatinib is initiated. An analysis of the Z9001 trial found that patients who have a KIT exon 11 mutation derive the most benefit from adjuvant imatinib, whereas patients who have confirmed wild-type GIST and those whose tumor harbors PDGFRA exon 18 substitution mutation D842V may not benefit, but the size of the latter subgroups was small.(51) Patients with a KIT exon 9 mutation had similar recurrence-free survival with or without 12 months of adjuvant imatinib, but this finding was based on a small number of patients and the imatinib dose 400 mg/day administered may not have been high enough or administered long enough.(35)
Many patients assigned to the 12-month imatinib arm in the Z9001 trial had GIST recurrence within the first two years that followed discontinuation of imatinib, suggesting that the duration of imatinib administration may not have been long enough. The Scandinavian Sarcoma Group (SSG) trial XVIII (identifier NCT00116935) is currently comparing 12 to 36 months of adjuvant imatinib in GIST patients who have operable, KIT-immunopositive GIST with a high estimated risk of recurrence. The European Organization for Research and Treatment of Cancer (EORTC) sponsored trial 62024 (identifier NCT00103168) compares two years of imatinib to observation in a patient population with operable, KIT-immunopositive GIST with an intermediate or high estimated risk of GIST recurrence. A nonrandomized, Phase II trial (NCT00867113) is addressing five years of imatinib as adjuvant treatment of GIST.
Tyrosine Kinase Inhibition and Cure of GIST
TKI administration generally does not cure advanced GIST and whether it is curative in the adjuvant setting is unknown. Complete clinical responses are infrequently achieved with TKIs in advanced GIST, and microscopic examination of the responding lesions usually reveals persisting GIST cells that express KIT. The BRF-14 trial demonstrated that responding GIST patients usually progress relatively rapidly when imatinib administration is interrupted following a few years of imatinib administration, and the Z9001 trial found that many GISTs recur soon after completion of the 12-month adjuvant treatment. Taken together, these data suggest that imatinib usually does not eradicate all GIST cells, but a fraction of cancer cells survives selective kinase inhibition and is the likely source of cancer recurrence.
Putative GIST stem cells, characterized by KITlowCD44+CD34+, were recently isolated from the postnatal murine stomach.(52) These clonogenic cells are capable of self-renewal and differentiation into ICCs, and imatinib did not influence their proliferation rate in vitro. In an in vivo model, imatinib decreased the numbers of mature (KIT+CD44+CD34−) ICCs, but not the KITlow stem cells. According to the stem cell hypothesis, interruption of TKI treatment either in advanced disease or in the adjuvant setting is associated with repopulation of KIT-expressing GIST cells from the unaffected KITlow stem cell population. If this hypothesis is correct, cure of GIST with imatinib or other TKIs is unlikely until therapy succeeds in also eradicating the stem cells.(53)
Although imatinib is not curative when administered as a single agent in the majority of patients with advanced GIST, it is still unclear whether it might lead to cure in some cases. The longest responses achieved with imatinib have now lasted over 10 years, and the hypothesis that a small proportion of patients with advanced GIST can be cured with imatinib thus cannot be refuted. In CML, interruption of imatinib in a patient population whose leukemia is in complete molecular remission during imatinib treatment has not resulted in CML recurrence in all patients, although the follow-up time is still short.(54) If drug resistance mutations arise by chance over time, early administration of imatinib might prolong survival even though the therapy is not curative, since it takes a longer time for such a mutation to surface when the tumor mass is small and presumably there are fewer pre-existing imatinib-resistant cells.
SUMMARY AND FUTURE DIRECTIONS
Tyrosine kinase inhibition has emerged as a highly effective method to treat GIST. Activating KIT and PDGFRA mutations are important drivers of the malignant process, and can be effectively inhibited with selective TKIs. Secondary mutations that confer drug resistance and hinder imatinib and other TKIs from binding to their targets have been identified as a common cause for treatment failure. Yet, with the help of TKIs, GIST patients may now live many years even with advanced disease, and have limited adverse effects from the treatment.
Further research will involve testing of novel TKIs as single agents and combinations with other agents, such as inhibitors of phosphatidylinositol 3-kinases (PI3K), the mammalian target of rapamycin (mTOR) and the mitogen-activated protein kinase (MAPK) pathway. Sequential administration of drugs that may control different populations of GIST cells may be well-suited for chronic therapy, and might prolong the time to disease progression. Combination therapies with conventional chemotherapeutic agents and different forms of radiation therapy remain incompletely studied. Immunotherapy has proven effective in a murine model of GIST (DeMatteo, unpublished data).
The key to the next wave of advances in the management of GIST may, however, be dependent on advances in molecular biology. Since many micro-GISTs harbor an activating KIT mutation and, yet, the great majority of these lesions will never grow into clinically significant cancerous tumors, it is likely that important and possibly therapeutically exploitable molecular aberrations have not yet been discovered. Studies on the whole genome and the exome sequencing may be important in revealing such molecular defects, particularly when coupled with mRNA and micro-RNA expression studies.
ABBREVIATIONS
- GIST
gastrointestinal stromal tumor
- TKI
tyrosine kinase inhibitor
- SCF
stem cell factor
- ICC
interstitial cell of Cajal
- PDGFRα
platelet-derived growth factor receptor-alpha
- PDGFRA
platelet-derived growth factor receptor A gene
- PDGF
platelet-derived growth factor
LITERATURE CITED
- 1.Mazur MT, Clark HB. Gastric stromal tumors. Reappraisal of histogenesis. Am. J. Surg. Pathol. 1983;7:507–19. doi: 10.1097/00000478-198309000-00001. [DOI] [PubMed] [Google Scholar]
- 2.Miettinen M. Gastrointestinal stromal tumors. An immunohistochemical study of cellular differentiation. Am. J. Clin. Pathol. 1988;89:601–10. doi: 10.1093/ajcp/89.5.601. [DOI] [PubMed] [Google Scholar]
- 3.Hirota S, Isozaki K, Moriyama Y, et al. Gain-of-function mutations of c-kit in human gastrointestinal stromal tumors. Science. 1998;279:577–80. doi: 10.1126/science.279.5350.577. [DOI] [PubMed] [Google Scholar]
- 4.Broudy VC. Stem cell factor and hematopoiesis. Blood. 1997;90:1345–64. [PubMed] [Google Scholar]
- 5.Joensuu H, Roberts PJ, Sarlomo-Rikala M, et al. Effect of the tyrosine kinase inhibitor STI571 in a patient with a metastatic gastrointestinal stromal tumor. N. Engl. J. Med. 2001;344:1052–6. doi: 10.1056/NEJM200104053441404. [DOI] [PubMed] [Google Scholar]
- 6.Demetri GD, von Mehren M, Blanke CD, et al. Efficacy and safety of imatinib mesylate in advanced gastrointestinal stromal tumors. N. Engl. J. Med. 2002;347:472–80. doi: 10.1056/NEJMoa020461. [DOI] [PubMed] [Google Scholar]
- 7.Joensuu H, Fletcher C. Dimitrijevic S, et al. 2002. Management of malignant gastrointestinal stromal tumours. Lancet Oncol. 3:655–64. doi: 10.1016/s1470-2045(02)00899-9. [DOI] [PubMed] [Google Scholar]
- 8.Mucciarini C, Rossi G, Bertolini F, et al. Incidence and clinicopathologic features of gastrointestinal stromal tumors. A population-based study. BMC Cancer. 2007;7:230. doi: 10.1186/1471-2407-7-230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Nilsson B, Bumming P, Meis-Kindblom JM, et al. Gastrointestinal stromal tumors: the incidence, prevalence, clinical course, and prognostication in the preimatinib mesylate era--a population-based study in western Sweden. Cancer. 2005;103:821–9. doi: 10.1002/cncr.20862. [DOI] [PubMed] [Google Scholar]
- 10.Steigen SE, Eide TJ. Trends in incidence and survival of mesenchymal neoplasm of the digestive tract within a defined population of northern Norway. APMIS. 2006;114:192–200. doi: 10.1111/j.1600-0463.2006.apm_261.x. [DOI] [PubMed] [Google Scholar]
- 11.Heinrich MC, Corless CL, Duensing A, et al. PDGFRA activating mutations in gastrointestinal stromal tumors. Science. 2003;299:708–10. doi: 10.1126/science.1079666. [DOI] [PubMed] [Google Scholar]
- 12.Pietras K, Sjoblom T, Rubin K, et al. PDGF receptors as cancer drug targets. Cancer Cell. 2003;3:439–43. doi: 10.1016/s1535-6108(03)00089-8. [DOI] [PubMed] [Google Scholar]
- 13.Martin J, Poveda A, Llombart-Bosch A, et al. Deletions affecting codons 557-558 of the c-KIT gene indicate a poor prognosis in patients with completely resected gastrointestinal stromal tumors: a study by the Spanish Group for Sarcoma Research (GEIS) J. Clin. Oncol. 2005;23:6190–8. doi: 10.1200/JCO.2005.19.554. [DOI] [PubMed] [Google Scholar]
- 14.Dematteo RP, Gold JS, Saran L, et al. Tumor mitotic rate, size, and location independently predict recurrence after resection of primary gastrointestinal stromal tumor (GIST) Cancer. 2008;112:608–15. doi: 10.1002/cncr.23199. [DOI] [PubMed] [Google Scholar]
- 15.Kawanowa K, Sakuma Y, Sakurai S, et al. High incidence of microscopic gastrointestinal stromal tumors in the stomach. Hum. Pathol. 2006;37:1527–35. doi: 10.1016/j.humpath.2006.07.002. [DOI] [PubMed] [Google Scholar]
- 16.Agaimy A, Wunsch PH, Hofstaedter F, et al. Minute gastric sclerosing stromal tumors (GIST tumorlets) are common in adults and frequently show c-KIT mutations. Am. J. Surg. Pathol. 2007;31:113–20. doi: 10.1097/01.pas.0000213307.05811.f0. [DOI] [PubMed] [Google Scholar]
- 17.Miettinen M, Wang ZF, Lasota J. DOG1 antibody in the differential diagnosis of gastrointestinal stromal tumors: a study of 1840 cases. Am. J. Surg. Pathol. 2009;33:1401–8. doi: 10.1097/PAS.0b013e3181a90e1a. [DOI] [PubMed] [Google Scholar]
- 18.Novelli M, Rossi S, Rodriguez-Justo M, et al. DOG1 and CD117 are the antibodies of choice in the diagnosis of gastrointestinal stromal tumours. Histopathology. 2010;57:259–70. doi: 10.1111/j.1365-2559.2010.03624.x. [DOI] [PubMed] [Google Scholar]
- 19.Fletcher CD, Berman JJ, Corless C, et al. Diagnosis of gastrointestinal stromal tumors: A consensus approach. Hum. Pathol. 2002;33:459–65. doi: 10.1053/hupa.2002.123545. [DOI] [PubMed] [Google Scholar]
- 20.Miettinen M, Lasota J. Gastrointestinal stromal tumors: pathology and prognosis at different sites. Semin. Diagn. Pathol. 2006;23:70–83. doi: 10.1053/j.semdp.2006.09.001. [DOI] [PubMed] [Google Scholar]
- 21.Joensuu H. Risk stratification of patients diagnosed with gastrointestinal stromal tumor. Hum. Pathol. 2008;39:1411–9. doi: 10.1016/j.humpath.2008.06.025. [DOI] [PubMed] [Google Scholar]
- 22.Gold JS, Gonen M, Gutierrez A, et al. Development and validation of a prognostic nomogram for recurrence-free survival after complete surgical resection of localised primary gastrointestinal stromal tumour: a retrospective analysis. Lancet Oncol. 2009;10:1045–52. doi: 10.1016/S1470-2045(09)70242-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ng EH, Pollock RE, Munsell MF, et al. Prognostic factors influencing survival in gastrointestinal leiomyosarcomas. Implications for surgical management and staging. Ann. Surg. 1992;215:68–77. doi: 10.1097/00000658-199201000-00010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hohenberger P, Ronellenfitsch U, Oladeji O, et al. Pattern of recurrence in patients with ruptured primary gastrointestinal stromal tumour. Br. J. Surg. 2010;97:1854–9. doi: 10.1002/bjs.7222. [DOI] [PubMed] [Google Scholar]
- 25.Dematteo RP, Heinrich MC, El-Rifai WM, Demetri G. Clinical management of gastrointestinal stromal tumors: before and after STI-571. Hum. Pathol. 2002;33:466–77. doi: 10.1053/hupa.2002.124122. [DOI] [PubMed] [Google Scholar]
- 26.Eisenberg BL, Harris J, Blanke CD, et al. Phase II trial of neoadjuvant/adjuvant imatinib mesylate (IM) for advanced primary and metastatic/recurrent operable gastrointestinal stromal tumor (GIST): early results of RTOG 0132/ACRIN 6665. J. Surg. Oncol. 2009;99:42–7. doi: 10.1002/jso.21160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Blanke CD, Demetri GD, von Mehren M, et al. Long-term results from a randomized phase II trial of standard- versus higher-dose imatinib mesylate for patients with unresectable or metastatic gastrointestinal stromal tumors expressing KIT. J. Clin. Oncol. 2008;26:620–5. doi: 10.1200/JCO.2007.13.4403. [DOI] [PubMed] [Google Scholar]
- 28.Raut CP, Posner M, Desai J, et al. Surgical management of advanced gastrointestinal stromal tumors after treatment with targeted systemic therapy using kinase inhibitors. J. Clin. Oncol. 2006;24:2325–31. doi: 10.1200/JCO.2005.05.3439. [DOI] [PubMed] [Google Scholar]
- 29.DeMatteo RP, Maki RG, Singer S, et al. Results of tyrosine kinase inhibitor therapy followed by surgical resection for metastatic gastrointestinal stromal tumor. Ann. Surg. 2007;245:347–52. doi: 10.1097/01.sla.0000236630.93587.59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Plaat BE, Hollema H, Molenaar WM, et al. Soft tissue leiomyosarcomas and malignant gastrointestinal stromal tumors: differences in clinical outcome and expression of multidrug resistance proteins. J. Clin. Oncol. 2000;18:3211–20. doi: 10.1200/JCO.2000.18.18.3211. [DOI] [PubMed] [Google Scholar]
- 31.Blanke CD, Rankin C, Demetri GD, et al. Phase III randomized, intergroup trial assessing imatinib mesylate at two dose levels in patients with unresectable or metastatic gastrointestinal stromal tumors expressing the kit receptor tyrosine kinase: S0033. J. Clin. Oncol. 2008;26:626–32. doi: 10.1200/JCO.2007.13.4452. [DOI] [PubMed] [Google Scholar]
- 32.Choi H, Charnsangavej C, Faria SC, et al. Correlation of computed tomography and positron emission tomography in patients with metastatic gastrointestinal stromal tumor treated at a single institution with imatinib mesylate: proposal of new computed tomography response criteria. J. Clin. Oncol. 2007;25:1753–9. doi: 10.1200/JCO.2006.07.3049. [DOI] [PubMed] [Google Scholar]
- 33.Heinrich MC, Corless CL, Demetri GD, et al. Kinase mutations and imatinib response in patients with metastatic gastrointestinal stromal tumor. J. Clin. Oncol. 2003;21:4342–9. doi: 10.1200/JCO.2003.04.190. [DOI] [PubMed] [Google Scholar]
- 34.Verweij J, Casali PG, Zalcberg J, et al. Progression-free survival in gastrointestinal stromal tumours with high-dose imatinib: randomised trial. Lancet. 2004;364:1127–34. doi: 10.1016/S0140-6736(04)17098-0. [DOI] [PubMed] [Google Scholar]
- 35.Debiec-Rychter M, Sciot R, Le Cesne A, et al. KIT mutations and dose selection for imatinib in patients with advanced gastrointestinal stromal tumours. Eur. J. Cancer. 2006;42:1093–103. doi: 10.1016/j.ejca.2006.01.030. [DOI] [PubMed] [Google Scholar]
- 36.Janeway KA, Kim SY, Lodish M, et al. Defects in succinate dehydrogenase in gastrointestinal stromal tumors lacking KIT and PDGFRA mutations. Proc. Natl. Acad. Sci. U S A. 2011;108:314–8. doi: 10.1073/pnas.1009199108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Pantaleo MA, Astolfi A, Indio V, et al. SDHA Loss-of-Function Mutations in KITPDGFRA Wild-Type Gastrointestinal Stromal Tumors Identified by Massively Parallel Sequencing. J. Natl. Cancer Inst. 2011 Apr;19 doi: 10.1093/jnci/djr130. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 38.Agaram NP, Wong GC, Guo T, et al. Novel V600E BRAF mutations in imatinib-naive and imatinib-resistant gastrointestinal stromal tumors. Genes Chromosomes Cancer. 2008;47:853–9. doi: 10.1002/gcc.20589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Blay JY, Le Cesne A, Ray-Coquard I, et al. Prospective multicentric randomized phase III study of imatinib in patients with advanced gastrointestinal stromal tumors comparing interruption versus continuation of treatment beyond 1 year: the French Sarcoma Group. J. Clin. Oncol. 2007;25:1107–13. doi: 10.1200/JCO.2006.09.0183. [DOI] [PubMed] [Google Scholar]
- 40.Le Cesne A, Ray-Coquard I, Bui BN, et al. Discontinuation of imatinib in patients with advanced gastrointestinal stromal tumours after 3 years of treatment: an open-label multicentre randomised phase 3 trial. Lancet Onco.l. 2010;11:942–9. doi: 10.1016/S1470-2045(10)70222-9. [DOI] [PubMed] [Google Scholar]
- 41.Demetri GD, Wang Y, Wehrle E, et al. Imatinib plasma levels are correlated with clinical benefit in patients with unresectable/metastatic gastrointestinal stromal tumors. J. Clin. Oncol. 2009;27:3141–7. doi: 10.1200/JCO.2008.20.4818. [DOI] [PubMed] [Google Scholar]
- 42.Heinrich MC, Corless CL, Blanke CD, et al. Molecular correlates of imatinib resistance in gastrointestinal stromal tumors. J. Clin. Oncol. 2006;24:4764–74. doi: 10.1200/JCO.2006.06.2265. [DOI] [PubMed] [Google Scholar]
- 43.Wardelmann E, Merkelbach-Bruse S, Pauls K, et al. Polyclonal evolution of multiple secondary KIT mutations in gastrointestinal stromal tumors under treatment with imatinib mesylate. Clin. Cancer Res. 2006;12:1743–9. doi: 10.1158/1078-0432.CCR-05-1211. [DOI] [PubMed] [Google Scholar]
- 44.Liegl B, Kepten I, Le C, et al. Heterogeneity of kinase inhibitor resistance mechanisms in GIST. J. Pathol. 2008;216:64–74. doi: 10.1002/path. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Bono P, Krause A, von Mehren M, et al. Serum KIT and KIT ligand levels in patients with gastrointestinal stromal tumors treated with imatinib. Blood. 2004;103:2929–35. doi: 10.1182/blood-2003-10-3443. [DOI] [PubMed] [Google Scholar]
- 46.Demetri GD, van Oosterom AT, Garrett CR, et al. Efficacy and safety of sunitinib in patients with advanced gastrointestinal stromal tumour after failure of imatinib: a randomised controlled trial. Lancet. 2006;368:1329–38. doi: 10.1016/S0140-6736(06)69446-4. [DOI] [PubMed] [Google Scholar]
- 47.Joensuu H, De Braud F, Coco P, et al. Phase II, open-label study of PTK787/ZK222584 for the treatment of metastatic gastrointestinal stromal tumors resistant to imatinib mesylate. Ann. Oncol. 2008;19:173–7. doi: 10.1093/annonc/mdm419. [DOI] [PubMed] [Google Scholar]
- 48.Montemurro M, Schoffski P, Reichardt P, et al. Nilotinib in the treatment of advanced gastrointestinal stromal tumours resistant to both imatinib and sunitinib. Eur. J. Cancer. 2009;45:2293–7. doi: 10.1016/j.ejca.2009.04.030. [DOI] [PubMed] [Google Scholar]
- 49.Le Cesne A, Blay JY, Bui BN, et al. Phase II study of oral masitinib mesilate in imatinib-naive patients with locally advanced or metastatic gastro-intestinal stromal tumour (GIST) Eur. J. Cancer. 2010;46:1344–51. doi: 10.1016/j.ejca.2010.02.014. [DOI] [PubMed] [Google Scholar]
- 50.DeMatteo RP, Ballman KV, Antonescu CR, et al. Adjuvant imatinib mesylate after resection of localised, primary gastrointestinal stromal tumour: a randomised, doubleblind, placebo-controlled trial. Lancet. 2009;373:1097–104. doi: 10.1016/S0140-6736(09)60500-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Corless C, Ballman KV, Antonescu CR, et al. Relation of tumor pathologic and molecular features to outcome after surgical resection of localized gastrointestinal stromal tumor (GIST): Results of the intergroup phase III trial ACOSOG Z9001. J. Clin. Oncol. 2010;28:15s. [Google Scholar]
- 52.Bardsley MR, Horvath VJ, Asuzu DT, et al. Kitlow stem cells cause resistance to Kit/platelet-derived growth factor alpha inhibitors in murine gastrointestinal stromal tumors. Gastroenterology. 2010;139:942–52. doi: 10.1053/j.gastro.2010.05.083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Heinrich MC. Imatinib treatment of metastatic GIST: don't stop (believing) Lancet Oncol. 2010;11:910–1. doi: 10.1016/S1470-2045(10)70225-4. [DOI] [PubMed] [Google Scholar]
- 54.Mahon FX, Rea D, Guilhot J, et al. Discontinuation of imatinib in patients with chronic myeloid leukaemia who have maintained complete molecular remission for at least 2 years: the prospective, multicentre Stop Imatinib (STIM) trial. Lancet Oncol. 2010;11:1029–35. doi: 10.1016/S1470-2045(10)70233-3. [DOI] [PubMed] [Google Scholar]