Abstract
The CAPRISA004 trial demonstrated reduction of sexual HIV-1 acquisition in women using a vaginal microbicide containing tenofovir. A better understanding of the consequences of antiretroviral-containing microbicides for immune responses in individuals with intercurrent HIV-1 infection is needed for future trials combining the use of microbicides with HIV-1 vaccines. Investigation of immune responses in women who acquired HIV-1 whilst using tenofovir gel showed significantly higher (p=0.01) Gag-specific IFNγ+ CD4+ T-cell responses. The use of tenofovir containing gel around the time of infection can modulate HIV-1 immunity, and these immunological changes need to be considered in future trials combining vaccines and microbicides.
Keywords: HIV-1, vaginal microbicide, tenofovir, HIV-1-specific CD4+ T cell help
Introduction
A recent randomized controlled trial undertaken by the Centre for the AIDS Programme of Research in South Africa (CAPRISA) reported that a vaginal microbicide gel containing 1% tenofovir reduced the risk of HIV-1 infection in women by 39% 1. While confirmation of these findings in additional studies are needed and similar studies are currently underway, these results will have significant implication for the design of future prevention trials, including vaccine trials in which both vaccinees and placebo recipients might receive prophylactic antiretroviral drugs in gel or oral formulation 2. A better understanding of the consequences of anti-retroviral containing microbicides for immune responses in individuals with intercurrent HIV-1 infection is therefore critical to take these immunological changes into consideration in the design of future trials combining the use of microbicides with HIV-1 vaccines 2. In the present study we investigated innate and adaptive immune responses during primary HIV-1 infection in women who acquired HIV-1 whilst using either tenofovir gel or placebo in the CAPRISA004 trial.
Materials and Methods
Study population
Cyropreserved PBMCs were collected from sexually active, HIV-1 clade C infected 18- to 40-year-old women in urban and rural KwaZulu-Natal, South Africa enrolled in CAPRISA 004 1. The eligibility and exclusion criteria for the parent trial have been previously reported 1. Participants who acquired HIV stopped using study gel on confirmation of HIV infection, as per the trial protocol 1. From the total of 98 intercurrent HIV infections that occurred, 36 randomly selected HIV-1 infected female adults exposed either to tenofovir gel (n= 17) or placebo (n= 19) were selected for this sub-study. The study was approved by the UKZN and MGH Biomedical Research Ethics Committee,Family Health International Protection of Human Subjects Committee, and the South African Medicines Control Council with each subject giving informed consent for participation.
Characterization of phenotype and function of innate and adaptive immune cells using multi-parameter flow cytometry
The phenotypic characteristics of Natural Killer (NK) cells and myeloid Dendritic Cells (mDCs) were assessed by multiparameter flow cytometry using cyropreserved PBMC collected within 3 months of HIV-1 infection. Following gating on lineage negative (CD3neg, CD19neg, CD56neg) lymphocytes, anti-CD11c was used to identify mDCs, as described 3-4. Antibodies directed against HLA-DR, CD83, and CD86 were used to study the activation and maturation stage of mDCs directly ex-vivo. NK cells were defined as CD3negCD56/CD16+ cells, and antibodies directed against HLA-DR, CD38 and CD69 were used to study the activation status of NK cells, as described (5).
Intracellular measurement of IFN-γ, TNF-α and IL-2 production by CD4+ and CD8+ T-cells was undertaken as described 6-7. Cyropreserved PBMCs were stimulated for 6hrs at 37°C; 5% CO2 with Gag and Nef peptide pools spanning the HIV-1 clade C consensus sequence (final concentration 2μg/ml per peptide) in the presence of brefeldin A. Negative controls with PBMCs and media alone and positive controls stimulated with phytohemaggultinin (PHA) were included in the assays. Between 500,000-1,000,000 events were acquired on a LSRII flow cytometer and data analysis was performed using FlowJo version 8.8.2 (TreeStar, Inc.). Boolean gating was used to create a full array of the 8 possible response patterns when testing 3 functions, and data were further analyzed using SPICE 5 and PESTLE software programmes (kindly provided by Drs. Roederer and Nozzi, NIAID, NIH).
Statistical analyses
Statistical analyses and graphical presentation was done using Graphpad Prism 5 (Graphpad). Results are given as averages with standard deviations. Paired two-tailed Student t tests were used to test statistical significance. Differences after comparisons were considered statistically significant if p <0.05.
Results
Characteristics of study subjects
Cyropreserved PBMCs collected from 36 sexually active, HIV-1-infected women in urban and rural KwaZulu-Natal enrolled into the CAPRISA 004 trial1 were randomly selected and studied. Investigators were blinded regarding the study group for the immunological analysis. Seventeen of these women were part of the tenofovir gel arm and 19 of the placebo arm. The demographic, immunological and virological characteristics of the study cohort are presented in Table 1. Women in the two arms did not differ in the days since infection, and HIV-1 viral loads and CD4+ T cell counts at presentation or analysis, and did also not differ in the distribution of low, intermediate, and high adherers.
Table 1.
Characteristics of study subjects
| Tenofovir | Placebo | |
|---|---|---|
| n | 17 | 19 |
| Age | 24 (19-37) | 23 (19-31) |
| Days post infection | 85 (20–345) | 64 (15-481) |
| Initial CD4+ T cell count | 454 (182-955) | 556 (240-1036) |
| CD4+ T cell count post infection | 464 (197-955) | 510 (240-1036) |
| Initial viral log (log) | 4.66 (2.60-6.53) | 4.42 (2.60-5.85) |
| Viral load post infection (log) | 4.96 (2.60-5.86) | 4.51 (2.60-5.51) |
Average and Range is shown
Lack of differences in innate immune cells between the study arms at 3 months post infection
We initially examined the frequencies and activation status of NK cells and mDCs between women in the tenofovir or placebo arms. The average frequencies of NK cells did not differ between the two arms (average NK cells 4.7% +/− 3.8% vs. 5.5% +/− 4.6%). Furthermore, the average percentage of NK cells expressing HLA-DR (12% +/− 6.3% vs. 12.9% +/− 9.2%), CD38 (68.9% +/− 25.3% vs. 67.1% +/− 28.6%), or CD69 (10.3% +/−10.9% vs. 10.7% +/− 7.1%) did not differ between the women that received tenofovir compared to those who received placebo (p>0.05 for all comparisons). Similarly, the average percentage of mDCs (5.7% +/− 3.2% vs. 5.9% +/− 1.9%), as well as their activation status (HLA-DR: 19.8% +/− 19.5% vs. 17.5% +/− 11.3%; CD83: 6.8% +/− 9.5% vs. 6.7% +/− 4.2%, and CD86: 19.3% +/− 21.4% vs. 11.5% +/− 11.7%; p > 0.05 for all comparisons) did not differ between tenofovir and placebo gel recipients. Taken together, these data demonstrate that frequencies and activation status of cells of the innate immune system were not significantly impacted by the use of tenofovir gel in women with breakthrough HIV-1 infection approximately 3 months following infection.
Preservation of HIV-1-specific IFNγ CD4+ T cells during primary infection
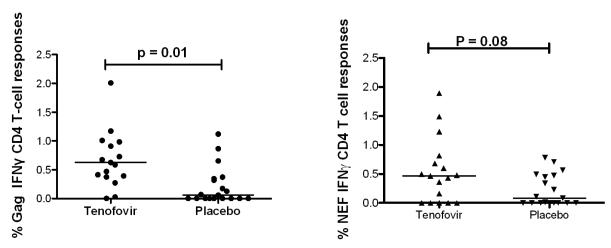
Previous studies have shown that HIV-1 Gag and Nef are targeted by HIV-1-specific T cells during primary infection, and that virus-specific CD4+ T cell responses are rapidly lost following infections 8. We compared HIV-1 clade C Gag- and Nef-specific CD4+ and CD8+ T cell activity between women that received tenofovir gel or placebo recipients. As shown in Figure 1, Gag-specific IFN-γ+ CD4+ T cell responses were significantly higher in the women in the tenofovir gel arm compared to placebo (p = 0.01). No correlation was observed between days post-infection and Gag-specific IFN-γ+ CD4+ T cell responses (R = −0.2; p = 0.3). HIV-1 Nef-specific CD4+ T cell responses also trended to be higher in women in the tenofovir gel arm compared to placebo recipients (p = 0.08, figure 1). No significant differences were observed in the percentage of Gag- and Nef-specific CD4+ T cells producing TNF-α or IL-2 between the two groups (data not shown).
Figure 1.
Higher HIV-1 Gag- and Nef-specific CD4+ T cell responses in Tenofovir recipients. The dot plot represents the median % IFNγ+ CD4+ T cells in response to HIV-1 Gag (left panel) and Nef (right panel) in women enrolled in the tenofovir arm (n = 17) compared to women that received placebo (n = 19).
Gag-specific and Nef-specific IFN-γ+ CD8+ T cell responses were also higher in the tenofovir arm compared to placebo though those differences did not reach statistical significance (Gag: average 1.1% IFN-γ+ CD8+ T cells +/− 1.0% in tenofovir arm vs. 0.7% +/− 0.7% in placebo, p = 0.33; Nef: average 1.1% IFN-γ+ CD4+ T cells +/− 1.4% in tenofovir arm vs. 0.35% +/− 0.5% in placebo, p = 0.35). Furthermore, Gag and Nef-specific CD8+ T cells IL-2 and TNF-α production did not differ significantly between the two groups (data not shown). We also assessed the polyfunctionality of the CD4+ and CD8+ T cells by the simultaneous quantification of three functions (IL-2, IFN-γ and TNF-α production) in response to Gag and Nef stimulation and observed no significant differences in the polyfunctionality of the CD4+ and CD8+ T cells in the tenofovir arm compared to placebo (p > 0.05). Overall, a vast majority of HIV-1-specific T cells were monofunctional (>90%) in both the tenofovir gel and placebo group. Taken together, these data demonstrate that the HIV-1-specific CD8+ T cell responses in women with breakthrough infection during tenofovir microbicide use did not significantly differ from those that used placebo, but that Gag-specific IFNγ+ CD4+ T cells responses were significantly higher in HIV-1-infected women randomized to the tenofovir gel arm.
Discussion
The use of a tenofovir-containing vaginal microbicide gel for the prevention of HIV-1 infection in sexually active, HIV-1-uninfected adult women in KwaZulu-Natal, South Africa showed a significant reduction of HIV-1 infection rates by 39% in the primary intent-to-treat analysis 1. Here, we examined the innate and adaptive immune responses in women with breakthrough HIV-1 infection that either used tenofovir microbicide gel or were part of the placebo arm. The frequencies and activation status of principal effector cells of the innate immune response, including NK cells and mDCs, assessed within 3 months of HIV-1 infection were not affected by the use of the tenofovir gel. Previous studies have demonstrated a significant expansion of NK cells in infected individuals during the early phase of HIV-1 infection, and a subsequent contraction of NK cell and mDC populations 9-11. We can therefore not exclude that NK cell and mDC frequencies in the initial three months of infection might have been affected by the use of the tenofovir gel, and that these changes in innate effector cells might have subsequently contributed to the observed functional differences in HIV-1-specific CD4+ T cells 12.
HIV-1-specific T cells are considered critical for the control of HIV-1 replication and disease progression 8, 13-15. While HIV-1-specific CD8+ T cell responses to both Gag and Nef peptides did not significantly differ between the groups, HIV-1 Gag-specific IFN-γ+ CD4+ T cell responses were significantly higher in women in the tenofovir gel arm compared to the placebo arm. HIV-1-specific CD4+ T cell responses are rapidly lost following acute infection in the presence of continuing HIV-1 replication, and this is in contrast to HIV-1-specific CD8+ T cell frequencies, which tend to increase over at least the first year of HIV-1 infection with continues exposure to antigen 16-20. Previous studies have shown that CD4+ T cells, and in particular gut-associated CD4+ T cells are severely depleted within the first few days/weeks of infection 21 and that HIV-1 preferentially infects HIV-1-specific CD4+ T cells 22. The presence of an antiretroviral agent, such as tenofovir, during this crucial period might protect CD4+ T cells from deletion, allowing for a preservation of HIV-1-specific IFN-γ CD4+ T cells. The observation that women receiving tenofovir gel maintained higher Gag-specific CD4+ T cell responses despite the reported lack of differences in viral load set-point between tenofovir gel and placebo recipients 1 was however unexpected, and the long-term clinical benefit of this preservation, and its consequences for HIV-1-specific immune function, will require further investigation.
Taken together, our studies in a subset of women who experienced breakthrough HIV-1 infection despite randomization to tenofovir gel usage demonstrate no significant alteration in the frequencies and activation status of key innate immune cells and HIV-1-specific CD8+ T cell responses three months following infection. However, tenofovir gel applied vaginally around the time of HIV-1 transmission might protect HIV-1-specific CD4+ T cell responses in infected individuals. This study demonstrates for the first time that the use of vaginal microbicides containing antiretroviral drugs can modulate HIV-1-specific immunity in individuals with breakthrough infection. These consequences of microbicide use for immune responses in individuals that acquire HIV-1 have to be considered in the design of future trials that will combine microbicides with HIV-1 vaccines.
Acknowledgments
MWM was supported by the National Institutes of Health Office of the Director, Fogarty International Center, through the International Clinical Research Fellows Program at Vanderbilt (R24 TW007988). These studies were also supported through the KwaZulu-Natal Research Institute for TB and HIV (K-RITH) and by a grant from the Doris Duke Charitable Foundation to MA. The TRAPS (Tenofovir gel Research for AIDS Prevention Science) Program is funded in part by CONRAD subproject agreement PPA-09-046, under a Cooperative Agreement (#GPO-A-00-08-00005-00) with the United States Agency for International Development (USAID). The CAPRISA 004 trial was funded by USAID and LIFELab, a biotechnology agency of the South African Government’s Department of Science and Technology.
Footnotes
Previous presentations: The results from this study were presented in an oral presentation at the AIDS Vaccine 2011 meeting in Bangkok, Thailand
Conflict of Interest and Financial support: QAK and SSAK are co-inventors with investigators from Gilead Sciences on two pending patents on tenofovir gel. SSAK once received $2500 for serving on an Advisory Panel on PrEP for Merck. None of the other authors reported a conflict of interest in the manuscript
This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Abdool Karim Q, Abdool Karim SS, Frohlich JA, et al. Effectiveness and safety of tenofovir gel, an antiretroviral microbicide, for the prevention of HIV infection in women. Science. Sep 3;329(5996):1168–1174. doi: 10.1126/science.1193748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Excler JL, Rida W, Priddy FH, et al. AIDS Vaccines and Pre-Exposure Prophylaxis: Is Synergy Possible? AIDS Res Hum Retroviruses. Nov 3; doi: 10.1089/aid.2010.0206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Meier A, Chang JJ, Chan ES, et al. Sex differences in the Toll-like receptor-mediated response of plasmacytoid dendritic cells to HIV-1. Nat Med. 2009 Aug;15(8):955–959. doi: 10.1038/nm.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Meier A, Fisher A, Sidhu HK, et al. Rapid loss of dendritic cell and monocyte responses to TLR ligands following venipuncture. J Immunol Methods. 2008 Dec 31;339(2):132–140. doi: 10.1016/j.jim.2008.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Alter G, Suscovich TJ, Teigen N, et al. Single-stranded RNA derived from HIV-1 serves as a potent activator of NK cells. J Immunol. 2007 Jun 15;178(12):7658–7666. doi: 10.4049/jimmunol.178.12.7658. [DOI] [PubMed] [Google Scholar]
- 6.Mkhwanazi N, Thobakgale CF, van der Stok M, et al. Immunodominant HIV-1-specific HLA-B- and HLA-C-restricted CD8+ T cells do not differ in polyfunctionality. Virology. Sep 30;405(2):483–491. doi: 10.1016/j.virol.2010.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Thobakgale CF, Ramduth D, Reddy S, et al. Human immunodeficiency virus-specific CD8+ T-cell activity is detectable from birth in the majority of in utero-infected infants. J Virol. 2007 Dec;81(23):12775–12784. doi: 10.1128/JVI.00624-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Rosenberg ES, Altfeld M, Poon SH, et al. Immune control of HIV-1 after early treatment of acute infection. Nature. 2000 Sep 28;407(6803):523–526. doi: 10.1038/35035103. [DOI] [PubMed] [Google Scholar]
- 9.Alter G, Teigen N, Ahern R, et al. Evolution of innate and adaptive effector cell functions during acute HIV-1 infection. J Infect Dis. 2007 May 15;195(10):1452–1460. doi: 10.1086/513878. [DOI] [PubMed] [Google Scholar]
- 10.Alter G, Teigen N, Davis BT, et al. Sequential deregulation of NK cell subset distribution and function starting in acute HIV-1 infection. Blood. 2005 Nov 15;106(10):3366–3369. doi: 10.1182/blood-2005-03-1100. [DOI] [PubMed] [Google Scholar]
- 11.Sabado RL, O’Brien M, Subedi A, et al. Evidence of dysregulation of dendritic cells in primary HIV infection. Blood. Aug 6; doi: 10.1182/blood-2010-03-273763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Pacanowski J, Kahi S, Baillet M, et al. Reduced blood CD123+ (lymphoid) and CD11c+ (myeloid) dendritic cell numbers in primary HIV-1 infection. Blood. 2001 Nov 15;98(10):3016–3021. doi: 10.1182/blood.v98.10.3016. [DOI] [PubMed] [Google Scholar]
- 13.Deeks SG, Walker BD. The immune response to AIDS virus infection: good, bad, or both? J Clin Invest. 2004 Mar;113(6):808–810. doi: 10.1172/JCI21318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Virgin HW, Walker BD. Immunology and the elusive AIDS vaccine. Nature. Mar 11;464(7286):224–231. doi: 10.1038/nature08898. [DOI] [PubMed] [Google Scholar]
- 15.Gloster SE, Newton P, Cornforth D, et al. Association of strong virus-specific CD4 T cell responses with efficient natural control of primary HIV-1 infection. AIDS. 2004 Mar 26;18(5):749–755. doi: 10.1097/00002030-200403260-00005. [DOI] [PubMed] [Google Scholar]
- 16.Altfeld M, Rosenberg ES, Shankarappa R, et al. Cellular immune responses and viral diversity in individuals treated during acute and early HIV-1 infection. J Exp Med. 2001 Jan 15;193(2):169–180. doi: 10.1084/jem.193.2.169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lichterfeld M, Kaufmann DE, Yu XG, et al. Loss of HIV-1-specific CD8+ T cell proliferation after acute HIV-1 infection and restoration by vaccine-induced HIV-1-specific CD4+ T cells. J Exp Med. 2004 Sep 20;200(6):701–712. doi: 10.1084/jem.20041270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Oxenius A, Price DA, Trkola A, et al. Loss of viral control in early HIV-1 infection is temporally associated with sequential escape from CD8+ T cell responses and decrease in HIV-1-specific CD4+ and CD8+ T cell frequencies. J Infect Dis. 2004 Aug 15;190(4):713–721. doi: 10.1086/422760. [DOI] [PubMed] [Google Scholar]
- 19.Streeck H, Jolin JS, Qi Y, et al. Human immunodeficiency virus type 1-specific CD8+ T-cell responses during primary infection are major determinants of the viral set point and loss of CD4+ T cells. J Virol. 2009 Aug;83(15):7641–7648. doi: 10.1128/JVI.00182-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Streeck H, Nixon DF. T cell immunity in acute HIV-1 infection. J Infect Dis. Oct 15;202(Suppl 2):S302–308. doi: 10.1086/655652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Brenchley JM, Schacker TW, Ruff LE, et al. CD4+ T cell depletion during all stages of HIV disease occurs predominantly in the gastrointestinal tract. J Exp Med. 2004 Sep 20;200(6):749–759. doi: 10.1084/jem.20040874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Douek DC, Brenchley JM, Betts MR, et al. HIV preferentially infects HIV-specific CD4+ T cells. Nature. 2002 May 2;417(6884):95–98. doi: 10.1038/417095a. [DOI] [PubMed] [Google Scholar]