Abstract
Despite its abuse potential, methylphenidate (MPH) is widely prescribed for treatment of attention deficit hyperactivity disorder (ADHD). The purpose of the present study was to examine MPH self-administration in a rat model of ADHD. Experiment 1 examined the acquisition of MPH self-administration and assessed the MPH dose-effect curve in spontaneously hypertensive rats (SHR), an inbred rat model of ADHD, Wistar Kyotos (WKY), the progenitor strain for SHR, and Sprague-Dawleys (SD), an outbred control strain. Experiment 2 replicated Experiment 1, but replaced MPH infusions with sucrose pellets. Initial acquisition of MPH self-administration was greater in SHR and SD than WKY. With extended training using an incrementing fixed ratio schedule, however, SHR and WKY did not differ in responding for MPH using the training dose (0.3 mg/kg/infusion) or other unit doses, except that SHR showed greater responding than WKY at 0.1 mg/kg/infusion. SHR also showed greater acquisition and maintenance of sucrose-reinforced responding compared to both WKY and SD. Greater initial acquisition of MPH self-administration in SHR than WKY may not be due to a strain specific difference in sensitivity to the reinforcing effect of MPH.
Keywords: methylphenidate, rat, self-administration, SHR, WKY
Introduction
Attention deficit hyperactive disorder (ADHD) is one of the most commonly diagnosed psychiatric disorders among children, affecting 3–5% of the school age population (DSM-IV; American Psychiatric Association, 2000). ADHD is characterized by a heterogeneous combination of behavioral symptoms (Sagvolden et al., 2005), including inattentiveness, impulsiveness, and/or hyperactivity that usually manifests by 7 years of age (Sagvolden and Sergeant, 1998). Although the cause is not fully understood, research suggests that ADHD results from hypofunctioning dopaminergic systems (Sagvolden and Sergeant, 1998; Volkow et. al., 2009). Interestingly, both genetic and environmental etiologies have been proposed to explain the dopamine dysfunction that gives rise to ADHD (Volkow et al., 2009). The most common treatment for ADHD involves prescription of stimulants, including methylphenidate (MPH; Challman and Lipsky, 2000; Volkow and Swanson, 2003; Wilens et al., 2003). The number of prescriptions for MPH and other stimulants increased from less than 2 million in 1991 to over 10 million in 2001 (Volkow and Swanson, 2003). MPH alone is being used to treat 5–15% of children and adolescents in the U.S. (Barbaresi et al., 2003). Although clinical trials show that MPH effectively reduces the problematic behavioral symptoms of ADHD, there has been increasing concern about the abuse potential of MPH with long-term use.
MPH has been found to be a reinforcer in humans with ADHD, and humans with and without ADHD have reported “liking” the effects of MPH and would take it again (Kollins et al., 2009). Similar to cocaine and methamphetamine, MPH increases extracellular dopamine in brain regions associated with reward, including the nucleus accumbens (Di Chiara andImperato, 1988). One study found that children diagnosed with ADHD preferred MPH over a placebo in a double-blind choice procedure (Fredericks and Kollins, 2005). Further, calls to the National Poison Data System (NPDS) regarding abuse of MPH or amphetamine originally prescribed for ADHD increased 76% in adolescents between the years of 1998 and 2005, whereas the total number of calls to the NPDS did not increase during this time period. Some of the cases that generated these calls resulted in life-threatening situations or even death due to MPH abuse (Setlik et al., 2009). Because of an increase in the number of MPH prescriptions and documented misuse, it is clear that there is a legitimate cause for concern about the abuse potential of MPH. Additionally, adults who show all the characteristics of ADHD are more likely to have used cigarettes and marijuana, and are more likely to have tried a variety of drugs compared to adults without ADHD (Faraone et al., 2007). With respect to ADHD medications in particular (MPH and amphetamine), research has found that individuals with and without ADHD abuse these medications (see Wilens et al., 2008 for a review). Investigations of the risk of future drug abuse because of a history of using stimulants to treat ADHD has yielded mixed results, with some studies showing no effect, some showing increased vulnerability, and some showing a protective effect (Biederman et al., 2008; Faraone and Upadhyaya, 2007; Kollins, 2007; Lambert and Hartsough, 1998; Wilens et al., 2003).
One way to help understand the effects of MPH in individuals with ADHD is to use an animal model under controlled conditions. Although many preclinical models have been proposed (Adriani et al., 2003; Davids et al., 2003), the most widely used animal model of ADHD is the spontaneously hypertensive rat (SHR; Pardey et al., 2009; Russell, 2007; Sagvolden et al., 2005). SHR is an inbred strain that was derived from Wistar Kyoto (WKY) rats, and was originally bred for cardiovascular research. SHR exhibit the main characteristics of clinically diagnosed ADHD, including hyperactivity, impulsiveness and inattention, compared to their normotensive WKY control strain (Sagvolden and Sergeant, 1998; Sagvolden, 2000; Russell, 2002). More specifically, compared to WKY controls, SHR are hyperactive (Amini et al., 2004; Sukhanov et al., 2004) and show greater impulsivity on a variety of tasks, including delay discounting, differential reinforcement of low rates (DRL) and a fixed consecutive number (FCN) task (Evenden and Meyerson, 1999; Fox et al., 2008; Pardey et al., 2009; Sanabria and Killeen, 2008). Although SHR are presumed to be a valid animal model of ADHD, their use in behavioral research is not without criticism. Some reports have shown that SHR do not display ADHD characteristics consistently, and that the hyperactivity- or impulsivity-decreasing effects of MPH only occur for WKY and Wistar rats (van den Bergh et al., 2006). Other reports have shown that hyperactivity in SHR is artificially inflated when compared to WKY, because WKY are hypoactive (Alsop, 2007). Despite these criticisms, the SHR strain continues to be the most commonly used animal model of ADHD (Sagvolden et al., 2005).
While there are currently no reports examining MPH reinforcement among inbred rat strains, evidence indicates that SHR respond differently to the psychostimulant effects of MPH compared to WKY. In these studies, outbred Sprague-Dawley (SD) rats are often included as a comparison because they model “normal” variability in behavior (Pardey et al., 2009). With locomotor activity, MPH produces hyperactivity in SHR, WKY and SD (Yang et al., 2003; 2006); however, the MPH-induced hyperactivity in SHR is longer in duration and amplitude than SD (Amini et al., 2004). In contrast, across repeated administration, MPH (2.5 mg/kg) produces sensitization in WKY and SD, but not SHR (Yang et al., 2003). Thus, SHR may be more sensitive to the initial psychostimulant effect of acute MPH, but not repeated MPH. To the extent that the stimulant and reinforcing effects of MPH share common mechanisms, these results suggest that SHR may be more sensitive to the initial reinforcing effect of MPH, but not the long-term reinforcing effect.
Multiple studies show evidence for MPH serving as an i.v. reinforcer in outbred Sprague-Dawley rats. One study found that MPH was as reinforcing as cocaine (Collins et al., 1984), and another study found that rats self-administering amphetamine (0.06 mg/kg/infusion) continued to lever press at the same rate when the drug was switched to MPH (0.2 or 0.4 mg/kg/infusion; Nielsen et al., 1984). More recent experiments have found that rats will self-administer a variety of doses of MPH on fixed ratio (FR) and progressive ratio (PR) schedules, will lever press more for a variety of MPH doses compared to saline, and will lever press more on an active (MPH delivery) lever than an inactive (no drug delivery) lever (Botly et al., 2008; Marusich and Bardo, 2009; Marusich et al., 2010). Additionally, non-contingent MPH does not maintain lever pressing, and MPH self-administration is subject to escalation of self-administration when rats are given daily long access to the drug (Marusich et al., 2010). Both adult (Botly et al., 2008; Marusich and Bardo, 2009; Marusich et al., 2010) and adolescent (Burton et al., 2010) outbred rats have been shown to lever press for MPH; however, no published studies have examined MPH self-administration in inbred strains. Thus, the purpose of the present experiments was to examine MPH self-administration in SHR, WKY, and SD. These strains were also compared for their responding using a non-drug (sucrose pellet) reinforcer under similar conditions.
Method
Subjects
Experiment 1 used 12 male SHR, 12 male WKY and 12 male SD rats, all approximately 70 days old (Harlan Industries, Indianapolis, IN). Experiment 2 used 12 male SD (Harlan Industries, Indianapolis, IN), 12 male SHR (Harlan Industries, Indianapolis, IN), and 12 male WKY (Charles River Laboratories Inc., Indianapolis, IN) rats, all approximately 70 days old. WKY were ordered from a vendor different from Experiment 1 due to unavailability from Harlan. Rats were experimentally and drug naïve at the beginning of the experiment. They were housed in individual, hanging plastic home cages in a colony on a 16/8 hr light/dark cycle (lights on at 06:00 h). They had free access to water in the home cage, and were restricted to a constant amount of food daily based on the average weight of rats of each strain at 70 days of age (see Table 1); SD were fed 20 g of food daily, SHR were fed 15 g of food daily, and WKY were fed 14 g of food daily. Food was delivered immediately after the session for all rats. Experimental protocols were approved by the Institutional Animal Care and Use Committee (IACUC) at the University of Kentucky, and followed the principles of laboratory animal care.
Table 1.
Mean and standard deviation of body weights for each strain, averaged across the seven beginning sessions and seven end sessions of Experiments 1 and 2. Note: weight gain from the beginning to the end of the experiments was due to both aging and the change from food restriction to free feeding (see Methods).
| Expt 1 | Expt 2 | |||||
|---|---|---|---|---|---|---|
| SHR | SD | WKY | SHR | SD | WKY | |
| Beginning | 238.6±25.2 | 280.5±15.6 | 205.6±12.9 | 246.0±16.0 | 283.4±16.8 | 234.0±15.8 |
| End | 325.1±24.8 | 372.8±14.1 | 280.5±14.9 | 357.6±9.0 | 384.4±35.1 | 300.9±9.0 |
Apparatus
Experimental sessions were conducted in standard operant conditioning chambers for rats (28 cm × 24 cm × 25 cm; ENV-001; MED Associates, St. Albans, VT) housed inside sound-attenuating chambers (ENV-018M; MED Associates). Sucrose pellets (F0042 sugar-based dustless precision pellets, 45 mg, Bio-Serv, Frenchtown, NJ) were dispensed individually from a pellet dispenser (ENV-203M-45; Med Associates) into a recessed food receptacle (5 × 5 × 3 cm) located 2 cm above the floor and centered on the front wall. Retractable levers (4.5 cm) were located 6 cm above the floor on each side of the food receptacle on the front wall, and white stimulus lights (28 V; 3 cm in diameter) were located 3 cm above each lever. Side walls were made of Plexiglas, the front and back walls were aluminum, and floors were made of metal rods. A fan located inside the sound-attenuating chamber produced noise to mask extraneous sounds. Experimental events were arranged and recorded by MED-PC software (Med-Associates) on a computer in the experimental room. The same chambers were used for all three strains.
Experiment 1
Self-administration surgical procedure
Prior to any experimental manipulations, rats were surgically implanted with a chronic indwelling jugular catheter (0.2 mm in diameter). Before the surgical procedure, rats were anesthetized with 100 mg/kg ketamine and 5 mg/kg diazepam (i.p.). One end of the catheter was inserted into the jugular vein, and the other end was attached to a metal cannula that exited the skin. The cannula was secured in a head mount adhered to the skull with dental acrylic and metal jeweler screws. Catheter patency was maintained by 0.2 ml infusions of a mixture containing 20 ml saline, 0.6 ml heparin, and 0.2 ml gentamicin administered daily after experimental sessions. Prior to daily experimental sessions, the cannula was attached to tubing within a flexible, spring covered leash (PHM-120; MED Associates) that was connected to a swivel (PHM-115; MED Associates) outside the operant chamber. This tubing exited the operant chamber and was connected to an infusion pump (PHM-100; MED Associates) located adjacent to the sound-attenuating chamber.
Acquisition of MPH self-administration
Rats were trained to press a lever for 0.3 mg/kg/infusion MPH through the method of autoshaping (Carroll and Lac, 1993). This particular dose of MPH was selected because it has been used previously to establish lever pressing for MPH in rats, and produces stable response rates (Marusich and Bardo, 2009; Marusich et al., 2010). Rats were exposed to autoshaping sessions for seven consecutive daily sessions in which the active (drug) lever was extended into the chamber on a random time 60-s schedule. Following 15 s of lever extension or a lever press, the lever retracted and an infusion (0.1 ml; 0.3 mg/kg/infusion) of MPH was delivered over 5.9 s. The infusion was paired with a 20-s timeout, signaled by the illumination of both stimulus lights, during which lever pressing had no programmed consequence. The inactive lever (no drug) was present at all times, except during timeouts. The side of the operant chamber corresponding to the active lever was counter balanced across rats. Rats earned or were non-contingently delivered 10 infusions of MPH during the first 15 min of the session. Rats then remained in the chamber for an additional 45 min with only the inactive lever present and no drug infusions available. Autoshaping sessions were followed by a subsequent daily session in which MPH (0.3 mg/kg/infusion) was available on an FR 1 for 60 min. Autoshaping and FR1 sessions were separated by 30–90 min. Rats were exposed to daily autoshaping and FR 1 sessions for seven consecutive days.
Following this, autoshaping sessions were terminated and rats were continued on access to contingent MPH (0.3 mg/kg/infusion) on an FR 1 schedule for three consecutive days, followed by three days on FR 2, three days on FR 3, three days on FR 4, and seven days on FR 5. This progression through FR 1–FR 5 was chosen because it was used previously to establish acquisition of MPH self-administration in rats (Marusich and Bardo, 2009). Throughout the experiment, doses were administered in mg per kg of body weight basis (within a 20 g body weight range), with drug concentrations adjusted to keep the infusion volume constant. Therefore, despite the weight differences between strains, rats received the same unit dose at a constant rate and volume. All FR sessions were 60 min in duration, and there was no limit to the number of MPH infusions that could be earned during the session beyond the 20-s timeouts imposed following each infusion. This phase of the experiment was conducted over the course of 26 consecutive days.
MPH dose-effect determination
During the next phase of the experiment, rats were given access to different unit doses of MPH for self-administration on an FR 5 schedule of reinforcement. Rats were tested with 0.056 mg/kg/infusion, 0.1 mg/kg/infusion, 0.56 mg/kg/infusion, and 1.0 mg/kg/infusion MPH in either ascending or descending order, with half of the rats in each strain tested in each order. These specific doses were selected because they produce differential response rates in SD (Marusich and Bardo, 2009; Marusich et al., 2010), and thus would be sensitive to potential shifts in the dose effect curves among strains based on differences in MPH reinforcement. All doses were administered at a volume of 0.1 ml/infusion. Rats were exposed to each dose for three consecutive sessions. Rats were then exposed to saline self-administration for seven consecutive sessions. This phase of the experiment was conducted over 19 consecutive days.
MPH self-administration during free feeding
Following the seventh extinction session of saline self-administration, rats were no longer food restricted, but were allowed free access to food in the home cage. Rats were then re-tested with the original training dose of MPH (0.3 mg/kg/infusion) on an FR 5 schedule of reinforcement for seven consecutive daily sessions. This manipulation was conducted to examine potential strain-dependent effects on MPH self-administration during free feeding. Previous research has been conducted with rats on food restriction (Botly et al., 2008; Marusich and Bardo, 2009; Marusich et al., 2010), and if rats continued to lever press for MPH while free feeding, this would be further evidence that MPH is a robust reinforcer for rats (cf. Carroll & Lac, 1993; de Wit & Stewart, 1981).
If a catheter malfunctioned, the rat was removed from the experiment and all data from that rat were excluded from that phase of the experiment. The three phases were defined as acquisition (autoshaping-FR 5), dose-effect determination (including saline), and self-administration during free feeding. Nine SHR, nine SD, and eight WKY completed the acquisition phase; six SHR, seven SD, and eight WKY completed the dose-effect determination phase; and five SHR, six SD, and seven WKY completed self-administration during the free feeding phase.
Experiment 2
Acquisition of lever pressing for sucrose
In this phase, rats were exposed to identical autoshaping procedures as were used for the acquisition of MPH self-administration phase in Experiment 1, except that rats did not undergo surgery and one sucrose pellet (45 mg, Bio-Serv) was delivered in place of the drug infusion. The programs used and lever press training were otherwise identical to were used in Experiment 1. Rats were exposed to autoshaping sessions for seven consecutive days, followed by training on FR 1, FR 2, FR 3, FR 4, and FR 5.
Lever pressing for sucrose during free feeding
Following the completion of lever pressing for sucrose on an FR 5 schedule of reinforcement for seven sessions, rats were given free access to food in the home cage. They were then exposed to the FR 5 schedule for another seven consecutive sessions.
Lever press extinction
In this phase, lever pressing no longer produced sucrose pellets, but the completion of five responses was still paired with stimulus light onset and a time out. The purpose of this phase was to approximate the saline substitution used in Experiment 1. Therefore, lever pressing in this phase still produced the stimulus light, but it did not provide any pellet reinforcement, similar to saline substitution in Experiment 1. Rats were exposed to this phase for seven consecutive sessions.
Drug
Methylphenidate HCl (Mallinckrodt, St Louis, MO) was prepared in sterile 0.9% NaCl (saline).
Data Analysis
Experiment 1
Mixed-factor analyses of variance (ANOVAs) were used to compare the number of active and inactive lever presses across the seven days of acquisition for all three strains, and during exposure to different FR values. Another mixed-factor ANOVA was used to compare lever pressing during food restriction and free feeding across strains lever pressing on an FR 5. During dose-effect determination, only the final two sessions of exposure to each dose were used in graphical and statistical analyses. An additional mixed factor ANOVA was used to compare the number of active lever presses for each dose of MPH (including saline) from the dose-effect determination phase across the three strains. Bonferroni adjusted pairwise comparisons were used as post-hoc tests to compare strains for all ANOVAs that produced significant results and to compare across strains at individual doses from the dose-effect. All tests were considered significant at p < .05, except when alpha levels were adjusted for Bonferroni comparisons where appropriate.
Experiment 2
Mixed-factor ANOVAs were used to compare the number of active and inactive lever presses across the seven days of acquisition for all three strains, and during exposure to different FR values. Additional mixed-factor ANOVAs were used to compare lever pressing during food restriction and free feeding phases across strains, and to compare lever pressing during free feeding on an FR 5 and during free feeding on extinction. Bonferroni adjusted pairwise comparisons were used as post-hoc tests to compare strains for all ANOVAs that produced significant results. All tests were considered significant at p < .05, except when alpha levels were adjusted for Bonferroni comparisons where appropriate.
Results
Experiment 1
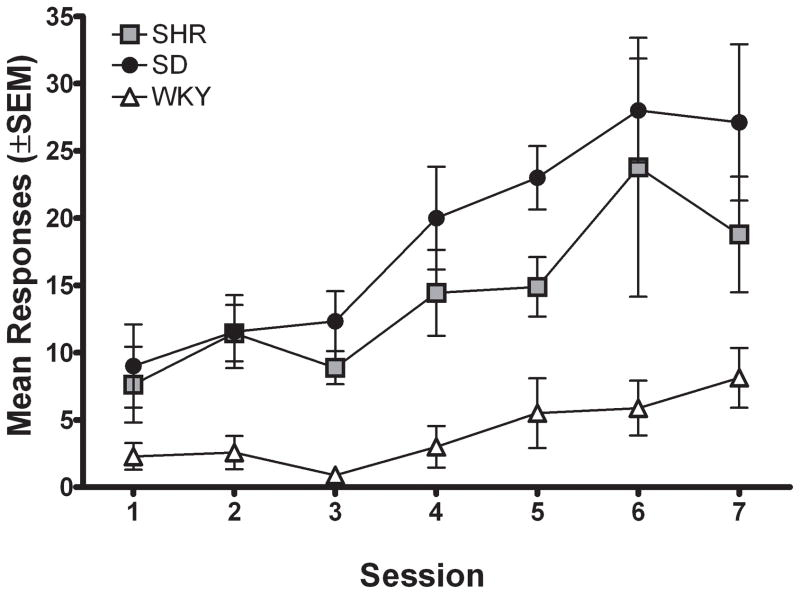
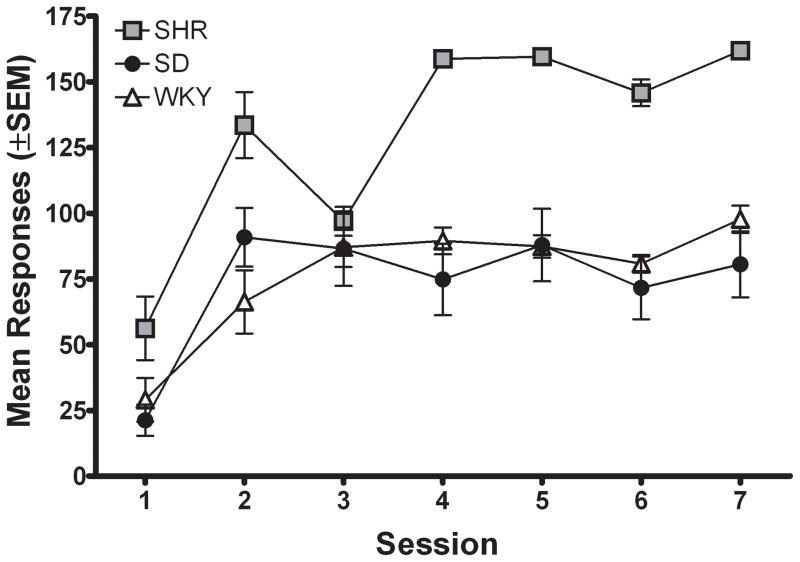
Figure 1a shows the number of active lever presses for each strain during the first seven sessions of FR 1 acquisition. Inactive lever presses did not differ between the three strains and are therefore not presented (inactive lever pressing remained low across sessions, averaging less than 10 presses per session). The number of active lever presses was significantly different across acquisition sessions [F (6, 138) = 8.74; p < .05], with rats in all strains increasing the number of active lever presses across sessions. There also was a significant main effect of strain for active lever presses during acquisition [F (2, 23) = 15.31; p < .05], indicating that the strains differed in total number of lever presses collapsed across sessions. Subsequent post hoc analyses showed that SHR and SD lever pressed significantly more than WKY [SHR v. WKY: t (117) = 5.93; p < .05; SD v. WKY: t (117) = 7.97; p < .05]; however, there was no interaction between session and strain, indicating that the pattern of responding across acquisition was not significantly different among strains.
Figure 1.
Upper panel; Number of active lever presses for the group mean of each strain plotted as a function of session during acquisition of MPH self-administration for Experiment 1. Data are plotted from the FR 1 session that followed the autoshaping session.
Lower panel; Number of active lever presses for the mean of each strain plotted as a function of FR requirement during MPH self-administration for Experiment 1.
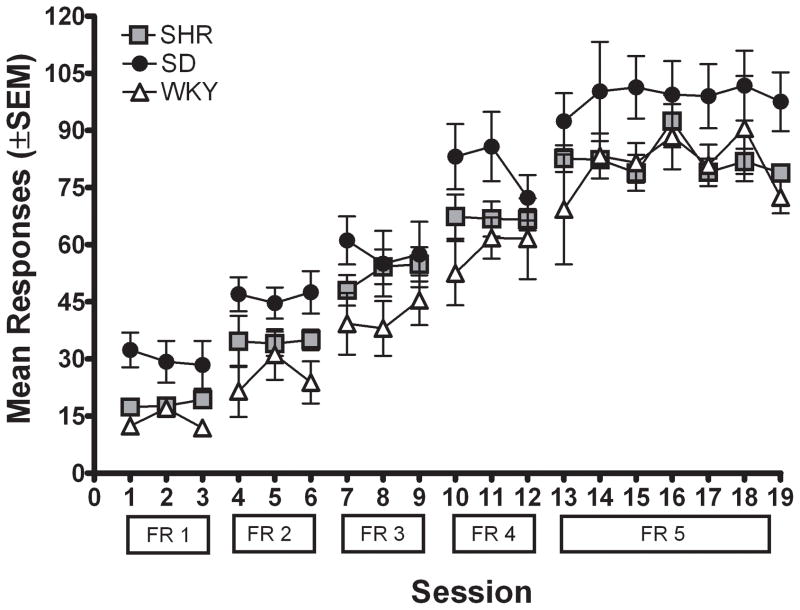
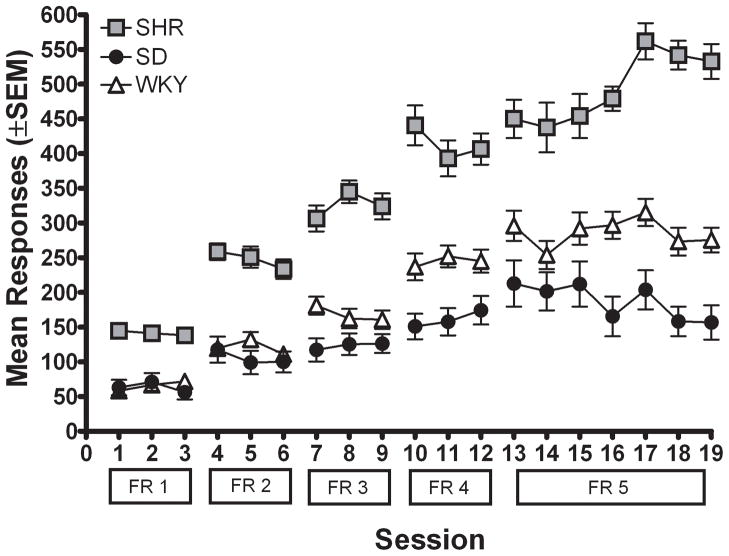
Figure 1b shows active lever presses for each strain across different FR values when rats self-administered 0.3 mg/kg/infusion MPH. Again, there were no significant differences among strains in the number of inactive lever presses, and therefore inactive lever presses are not presented. Active lever pressing increased with the higher FR requirements due to the increase in workload necessary to obtain MPH. There were significant main effects of FR value [F (4, 92) = 173.52; p < .05] and strain [F (2, 23) = 6.01; p < .05], but no significant interaction. Subsequent post hoc analyses indicated that each strain lever pressed more as the FR requirement was increased [SHR: F (4, 32) = 121.89; p < .05; WKY: F (4, 32) = 84.53; p < .05; SD: F (4, 32) = 35.58; p < .05]. Collapsed across incremental FR values, SHR and WKY did not differ significantly; however, SD lever pressed more overall than WKY during incremental FR training [t (253) = 4.77; p < .05].
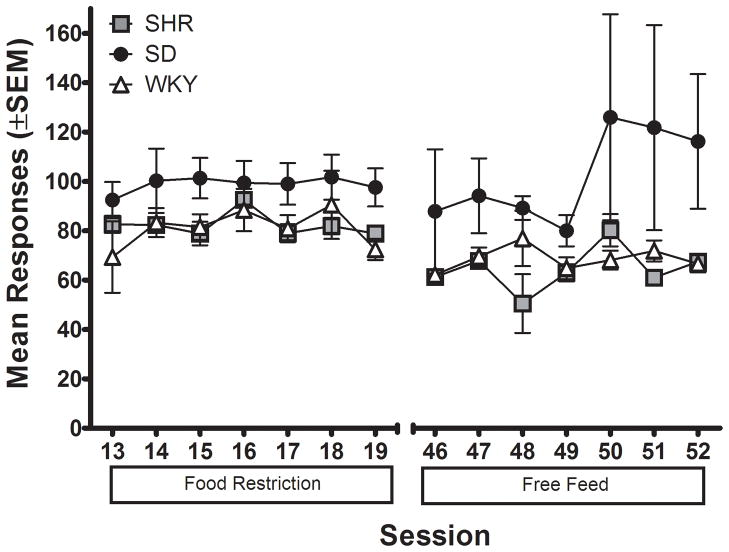
Figure 2 shows the number of active lever presses for each strain while responding on FR 5 for 0.3 mg/kg/infusion MPH during food restriction and free feeding phases. There were no significant differences in lever pressing based on feeding regimen, and there was no interaction between feeding regimen and strain. There was a significant main effect of strain [F (2, 16) = 3.81; p < .05], with SD tending to show more lever pressing than SHR and WKY; however, subsequent post hoc analyses did not reveal any significant differences among strains.
Figure 2.
Number of active lever presses for the mean of each strain plotted as a function of session during food restriction compared to during free feeding conditions.
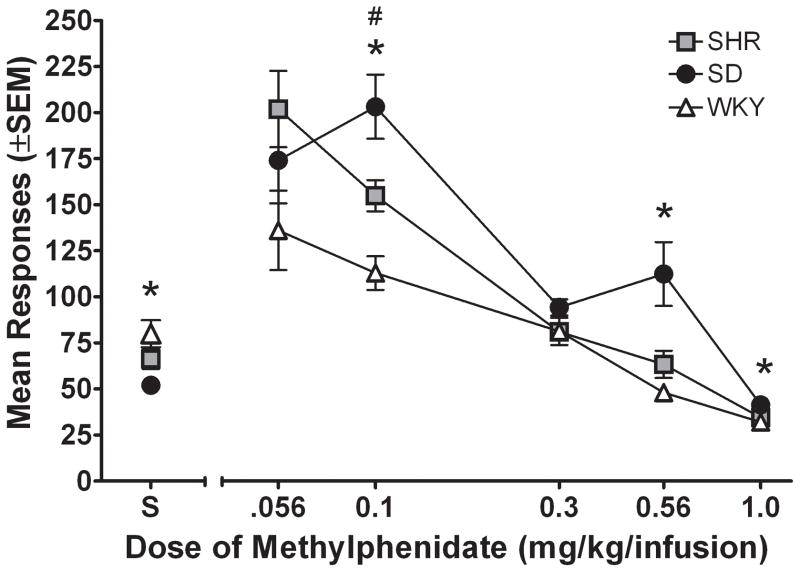
Figure 3a shows the number of active lever presses for each strain during the dose-effect determination. ANOVA showed significant main effects of dose [F (5, 90) = 80.11; p < .05] and strain [F (2, 18) = 10.64; p < .05], as well as a significant dose × strain interaction [F (10, 90) = 5.91; p < .05]. Subsequent post hoc analyses indicated that SHR lever pressed more than WKY at the 0.1 unit dose only [t (26) = 2.89; p < .05]; no significant differences were obtained between SHR and SD at any dose. Collapsed across doses, SD lever pressed significantly more than WKY [t (178) = 3.14; p < .05]. In addition, Bonferroni pairwise comparisons revealed that SD lever pressed significantly less than WKY with saline [t (26) = 3.25; p < .05], and more than WKY with MPH unit doses of 0.1 mg/kg/infusion [t (26) = 4.17; p < .05], 0.56 mg/kg/infusion [t (26) = 3.30; p < .05], and 1.0 mg/kg/infusion [t (26) = 3.61; p < .05]; the relatively small magnitude of effect observed at 1.0 mg/kg/infusion, while not obvious in the graph (Fig 3a), was statistically significant because of the low variance obtained across strains at this unit dose.
Figure 3.
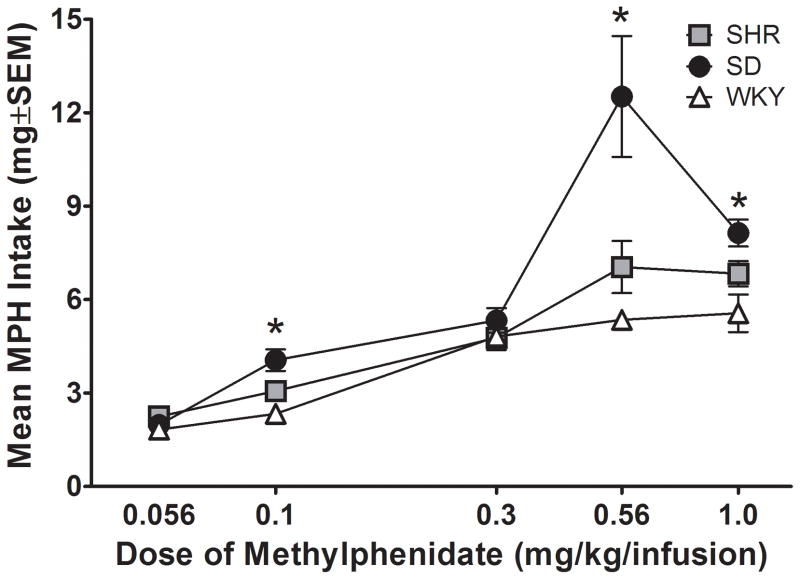
Upper panel. Number of active lever presses plotted as a function of MPH dose (log scale) for the mean of each strain for Experiment 1. “S” stands for saline vehicle. * significant difference (p < .05) between SD and WKY. # significant difference (p < .05) between SHR and WKY. Lower panel: Amount of MPH intake plotted as a function of MPH dose (log scale) for the mean of each strain for Experiment 1. “S” stands for saline vehicle. * significant difference (p < .05) between SD and WKY.
Figure 3b shows total MPH intake for each strain during the dose-effect determination. ANOVA showed significant main effects of dose [F (4, 72) = 36.91; p < .05] and strain [F (2, 18) = 7.87; p < .05], as well as a significant dose × strain interaction [F (8, 72) = 4.32; p < .05]. Collapsed across doses, SD self-administered significantly more MPH than WKY [t (148) = 3.95; p < .05]. Bonferroni pairwise comparisons for each strain at each individual dose also revealed that MPH intake was greater in SD than WKY at unit doses of 0.1 mg/kg/infusion [t (26) = 4.17; p < .05], 0.56 mg/kg/infusion [t (26) = 3.31; p < .05], and 1.0 mg/kg/infusion [t (26) = 3.48; p < .05]. No significant differences in total intake were obtained between SHR and WKY at any dose.
Experiment 2
Figure 4a shows the number of active lever presses for each strain during the first seven sessions of acquisition. Inactive lever presses did not differ among the three strains and are therefore not presented (inactive lever pressing remained low across sessions, averaging less than 10 presses per session). The number of active lever presses was significantly different across sessions of FR 1 acquisition [F (6, 198) = 47.07; p < .05], with rats in all strains increasing the number of lever presses across sessions, although the increase was primarily from the first to second session. There also was a significant main effect of strain for active lever presses during acquisition [F (2, 33) = 22.77; p < .05], with SHR lever pressing significantly more than WKY [t (166) = 8.86; p < .05] and SD [t (166) = 8.12; p < .05]. However, there was no significant interaction between session and strain, indicating that the pattern of responding across acquisition was not significantly different among strains.
Figure 4.
Figure 4a. Number of active lever presses for the group mean of each strain plotted as a function of session during acquisition of sucrose-reinforced responding for Experiment 2. Data are plotted from the FR 1 session that followed the autoshaping session.
Figure 4b. Number of active lever presses for the mean for each strain plotted as a function of FR requirement during sucrose-reinforced responding for Experiment 2.
Figure 4b shows active lever presses for each strain across incremental FR values. Again, there were no significant differences among strains in the number of inactive lever presses and therefore inactive lever presses are not presented. There were significant main effects of FR value [F (4, 132) = 279.31; p < .05] and strain [F (2, 33) = 192.93; p < .05]. There was no significant interaction between FR value and strain, indicating that the increasing pattern of lever pressing across incremental FR values was similar for all strains. Collapsed across FR value, SHR lever pressed more than WKY [t (358) = 11.76; p < .05] and SD [t (358) = 24.88; p < .05]; SD also lever pressed more than WKY [t (358) = 18.02; p < .05].
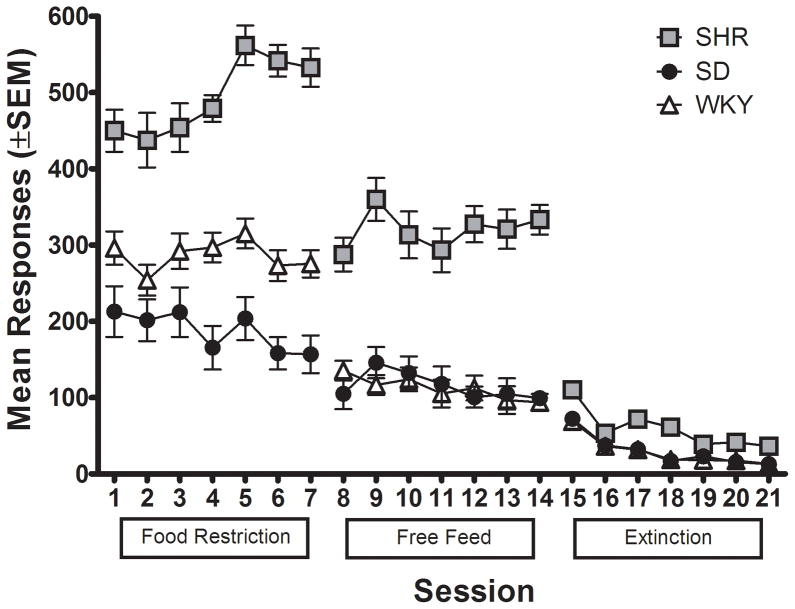
Figure 5 shows the number of active lever presses for each strain while responding on FR 5 during food restriction and free feeding, and then during lever press extinction. ANOVA comparing average lever pressing during food restriction and free feeding revealed significant main effects of feeding regimen [F (1, 33) = 105.51; p < 0.05] and strain [F (2, 33) = 130.35; p < .05], as well as a significant strain × feeding regimen interaction [F (2, 33) = 91.00; p < .05]. Post hoc analyses showed that all three strains differed from one other in overall amount of lever pressing during food restriction [SHR v. WKY: t (166) = 14.96; p < .05; SHR v. SD: t (166) = 25.45; p < .05; SD v. WKY: t (166) = 22.35; p < .05]. All three strains showed a significant decrease in lever pressing when switched from food restriction to free feeding [SHR: t (166) = 11.82; p < .05; WKY: t (166) = 17.70; p < .05; SD: t (166) = 9.54; p < .05]. During free feeding, SHR lever pressed significantly more overall than WKY [t (166) = 17.14; p < .05] and SD [t (166) = 15.53; p < .05].
Figure 5.
Number of active lever presses for the mean of each strain plotted as a function of session during food restriction, free feeding, and extinction phases with sucrose-reinforced responding for Experiment 2.
As shown in Figure 5, ANOVA comparing sucrose-reinforced lever pressing during free feeding to lever pressing during extinction showed significant main effects of contingency [F (1, 33) = 235.13; p < 0.5] and strain [F (2, 33) = 54.23; p < .05], as well as a significant strain × contingency interaction [F (2, 33) = 39.89; p < .05]. All three strains showed a significant decrease in number of responses when switched from contingent food delivery to extinction [SHR: t (166) = 19.82; p < .05; WKY: t (166) = 12.16; p < .05; SD: t (166) = 10.32; p < .05]. In addition, while SHR lever pressed significantly more than the other two strains during both the free feed [SHR v. WKY: t (166) = 17.14; p < .05; SHR v. SD: t (166) = 15.53; p < .05] and extinction phases [SHR v. WKY: t (166) = 7.49; p < .05; SHR v. SD: t (166) = 7.29; p < .05], the difference was reduced in the extinction phase.
Discussion
Experiment 1 found that SHR, WKY and SD acquired MPH self-administration, and this was achieved in each strain without previous training to lever press for food or for another drug. Switching from food restriction to free feeding had no significant effect on MPH self-administration, suggesting that MPH is a relatively robust reinforcer for all three strains. This finding contrasts with previous research showing that acquisition and maintenance of cocaine self-administration is decreased when rats are free fed (Carroll and Lac, 1993; de Wit and Stewart, 1981). More importantly, as an ADHD model, SHR acquired MPH self-administration more rapidly early in training compared to the genetic progenitor control WKY, suggesting SHR may either have a greater sensitivity to MPH reinforcement, or an enhanced ability to learn the operant response. In contrast, with extended training and with increasing FR requirements, there was no difference in MPH self-administration between SHR and WKY, indicating that any potential difference in MPH reinforcement was restricted to the early training phase.
An analysis of the data from the incremental FR phase expressed as a percent change from baseline, with baseline defined as the last day of autoshaping, showed that WKY gradually increased responding by approximately 350% over the course of the incremental FR phase, whereas SHR and SD showed an initial high rate of responding that showed little increase over the incremental FR phase (data not shown). Taken together, these results suggest that SHR may learn faster than WKY. While the exact reason behind these strain dependent differences in acquisition of responding was not identified in the present experiment, these differences were not likely attributable to differences in body weight. Although the relative differences in weight across strains remained constant throughout the experiment (SD were ~40 g heavier than SHR; SHR were ~40 g heavier than WKY; see Table 1), differences in MPH self-administration did not remain constant. Thus, the differences in weight cannot, by themselves, account for the differences in acquisition of MPH self-administration.
SHR did not differ from SD controls in lever pressing at any point in training, nor did they differ in MPH intake at any unit dose of MPH. In addition, SHR did not differ from WKY during the incremental FR phase or when the unit dose of MPH was varied between 0.056 and 1.0 mg/kg/infusion, except at one dose (0.1 mg/kg/infusion). Thus, the enhanced MPH self-administration in SHR compared to WKY early in training does not appear to survive with extended training. These results are generally consistent with previous results for locomotor activity showing that while SHR may show greater hyperactivity than WKY and SD following acute MPH (Amini et al., 2004), this difference dissipates with repeated MPH (2.5 mg/kg; Yang et al., 2003). Further, these results are consistent with previous results showing that while SHR initially acquire amphetamine self-administration more rapidly than WKY, these strains display similar rates of amphetamine self-administration with extended training across incremental FR sessions and multiple unit doses (Meyer et al., 2010).
An interesting finding of the present study was that SHR did not differ significantly from SD in MPH self-administration, whereas SHR did differ from WKY in some aspects of MPH self-administration. The two control strains, SD and WKY, differed from each other in many aspects of MPH self-administration. Previous research suggests that the differences between SHR and WKY may arise because WKY are hypoactive (Alsop, 2007). While locomotor activity was not measured in the present study, the hypoactivity of WKY could have led to lower rates of MPH self-administration. SD rats are not used as a comparison strain for SHR as commonly as WKY, so it is difficult to determine if SD and SHR would show similarities in other behavioral tasks, and if the two strains would show similar effects of MPH on behavioral tasks. Results of the present experiment also call into question the appropriate control strain for SHR. Future experiments should further compare SD to SHR in both in vivo and in vitro research to aid in answering these questions.
To the extent that the SHR model translates to humans with ADHD (Pardey et al., 2009; Russell, 2007; Sagvolden et al., 2005), these preclinical results suggest that the presence of ADHD may confer greater risk for stimulant abuse compared to non-ADHD populations based on the faster acquisition of MPH self-administration in the SHR strain. Given that humans have a limited number of exposures to stimulants, the rate at which they acquire drug use is an important factor in whether or not future drug abuse will develop. Therefore, if people with ADHD are more likely to initiate stimulant use faster following initial exposure, they may also be more likely to develop an abuse pattern.
Experiment 2 found that, similar to MPH self-administration, SHR, WKY and SD acquired sucrose-reinforced responding, and that each strain showed a similar pattern of acquisition. Switching from food restriction to free feeding decreased sucrose-reinforced responding, indicating that altering hunger motivation affected performance in all strains; however, this effect was different for each strain as indicated by the interaction in effects of feeding regimen and strain. This manipulation produced a larger change for SHR and WKY strains compared to SD. More important, SHR acquired sucrose-reinforced responding more rapidly during initial exposure compared to WKY and SD, suggesting that SHR may be more sensitive to sucrose reinforcement or may have an enhanced ability to learn the operant response. In contrast to the MPH self-administration results, however, the greater lever-pressing for sucrose in SHR rats was maintained across all sessions, including the incremental FR and free-feed phases. An interesting manipulation for future research would be to establish responding for sucrose in these strains, and then switch the reinforcer to MPH. Regardless, the present results indicate that the enhanced MPH self-administration noted in SHR during early training is not specific to MPH, but rather reflects a more general enhancement in lever-press performance.
Previous research has found that SHR acquire a task faster when the task involves varying their behavior, whereas WKY acquire faster when the task involves repeating their behavior (Mook et al., 1993; Mook and Neuringer, 1994). Learning to self-administer drug requires an initial variation in behavior until the correct behavior is emitted, and then it requires repeating that behavior. Therefore, those previous findings could account for why SHR initially acquired MPH self-administration more quickly than WKY, while the two strains did not differ in lever pressing during the later phases of the experiment.
Regardless of any strain differences, these results corroborate previous research showing that i.v. MPH is a robust reinforcer in outbred rats (Botly et al., 2008; Burton et al., 2010; Collins et al., 1984; Marusich and Bardo, 2009; Marusich et al., 2010; Nielson et al., 1984). The present experiments add to this literature by showing that rats will self-administer MPH under free feeding conditions. Additionally, this is the first demonstration that inbred rat strains (SHR and WKY) will self-administer MPH in patterns similar to outbred rats. Future research may examine whether this pattern of drug self-administration in young adulthood is altered by early life (adolescent) exposure to MPH using an ADHD rat model.
While rats in the present experiment self-administered MPH for over 30 consecutive days, there was little evidence of tolerance or sensitization. A common method to demonstrate the development of tolerance or sensitization is to re-determine the dose-effect curve following extended drug exposure, or to examine changes in responding following prolonged exposure to a single dose (Hardman et al., 1996; Rang et al., 2003). In Experiment 1, rats were trained on one dose (0.3 mg/kg/infusion MPH) under an FR 5 schedule for seven consecutive sessions (see Figure 1b), but showed no significant change in lever pressing, which suggests that tolerance did not develop. Similarly, none of the previous experiments examining MPH self-administration in rats provide any evidence for the development of tolerance or sensitization (Botly et al., 2008; Burton et al., 2010; Marusich and Bardo, 2009; Marusich et al., 2010). Nonetheless, we cannot rule out the possibility that SHR are more sensitive than WKY to MPH reinforcement early in training, but that tolerance develops to this effect, thus negating any potential strain-dependent difference.
While the present study is the first to demonstrate self-administration of MPH in an animal model of ADHD, limitations remain. One limitation is that only the descending limb of the dose-effect curve was characterized. The doses of MPH were chosen based on previous work examining MPH self-administration in SD (Marusich and Bardo, 2009; Marusich et al., 2010). Previous research found that unit doses of 0.03 and 0.056 mg/kg/infusion engendered a response rate that was on the ascending portion of the FR or progressive ratio dose effect curve, and only slightly higher than responding for saline (Marusich and Bardo, 2009; Marusich et al., 2010). There were several procedural differences among studies which do not allow any firm conclusions about why the lowest dose in the current study (0.056 mg/kg/infusion) engendered robust responding compared to saline. Future research should characterize the ascending limb of the dose-effect curve of MPH self-administration in SHR and WKY. Another limitation of the present experiment was that all rats were trained to lever press for MPH using the same unit dose of MPH (0.3 mg/kg/infusion). Previous research found that the training dose can affect the acquisition of drug self-administration (Schenk and Partridge, 2000), and thus it is possible that long-lasting differences among strains may have been observed if rats were trained to lever press using a different training dose of MPH.
Although the current study did not examine directly any neural mechanisms, other research has found that SHR have reduced electrically- and methylphenidate-evoked dopamine release in slices from prefrontal cortex, nucleus accumbens and caudate- putamen compared to WKY (Russell et al., 1995; Russell et al., 1998). Extended exposure to MPH does not ameliorate the dopamine hypofunctioning in SHR (Russell et al., 2000). Additional research using in vivo microdialysis found differences in extracellular dopamine levels between SHR and WKY in various brain regions (Carboni et al., 2003; Fujita et al., 2003), as well as greater MPH-evoked dopamine release in nucleus accumbens from SHR compared to WKY when MPH was administered acutely (Carboni et al., 2003). The enhanced release of accumbal dopamine following acute methylphendiate may play a role in the enhanced acquisition of MPH self-administration in SHR. Therefore, early exposure to MPH may be more reinforcing for SHR compared to WKY.
Finally, in addition to differences in dopamine function, we cannot rule out the possibility that greater bioavailability of MPH in SHR may have also played a role in the behavioral differences obtained in MPH self-administration. Unfortunately, there is no published information on the pharmacokinetics of MPH in SHR and WKY. With regard to the sucrose-reinforced responding experiment, however, SHR have an increased insulin response (Buchanan et al., 1992), which has been linked to the onset of obesity in this inbred strain (de Artiñano, and Castro, 2009). Therefore, SHR may lever press more for sucrose than WKY due to greater absorption of blood sugars into depot store. Additional work is needed to determine what pharmacodynamic and/or pharmacokinetic differences play a role in explaining the enhanced initial acquisition of MPH self-administration in SHR.
Acknowledgments
Research supported by NIH Grants P50 DA05312 and T32 DA007304.
The authors thank Kristin Alvers, Emily Denehy, Kate Fischer, A. Chip Meyer, and Justin Yates for assistance.
Footnotes
The authors have no conflicts of interest.
References
- Adriani W, Caprioli A, Granstrem O, Carli M, Laviola G. The spontaneously hypertensive-rat as an animal model of ADHD: evidence for impulsive and non-impulsive subpopulations. Neurosci Biobehav Rev. 2003;27:639–651. doi: 10.1016/j.neubiorev.2003.08.007. [DOI] [PubMed] [Google Scholar]
- Alsop B. Problems with spontaneously hypertensive rats (SHR) as a model of attention-deficit/hyperactivity disorder (AD/HD) J Neurosci Methods. 2007;162:42–8. doi: 10.1016/j.jneumeth.2006.12.002. [DOI] [PubMed] [Google Scholar]
- American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. 4. Washington, DC: American Psychiatric Association; 2000. [Google Scholar]
- Amini B, Yang PB, Swann AC, Dafny N. Differential locomotor responses in male rats from three strains to acute methylphenidate. Int J Neurosci. 2004;114:1063–84. doi: 10.1080/00207450490475526. [DOI] [PubMed] [Google Scholar]
- Barbaresi WJ, Katusic SK, Colligan RC, Oankratz VZ, Weber AL, Mrazek KJ, Jacobsen SJ. How common is attention-deficit/hyperactivity disorder? Incidence in a population-based birth cohort in Rochester, Minn. Arch Pediatr Adolesc Med. 2002;156:217–24. doi: 10.1001/archpedi.156.3.217. [DOI] [PubMed] [Google Scholar]
- Biederman J, Wilens T, Mick E, Spencer T, Faraone SV. Pharmacotherapy of attention-deficit/hyperactivity disorder reduces risk for substance use disorder. Pediatrics. 2008;104:20. doi: 10.1542/peds.104.2.e20. [DOI] [PubMed] [Google Scholar]
- Botly LCP, Burton CL, Rizos Z, Fletcher PJ. Characterization of methylphenidate self-administration and reinstatement in the rat. Psychopharmacology. 2008;199:55–66. doi: 10.1007/s00213-008-1093-z. [DOI] [PubMed] [Google Scholar]
- Buchanan TA, Youn JH, Campese VM, Sipos GF. Enhanced glucose tolerance in spontaneously hypertensive rats: Pancreatic beta cell hyperfunction with normal insulin sensitivity. Diabetes. 1992;41:872–8. doi: 10.2337/diab.41.7.872. [DOI] [PubMed] [Google Scholar]
- Burton CL, Nobrega JN, Fletcher PJ. The effects of adolescent methylphenidate self-administration on responding for a conditioned reward, amphetamine-induced locomotor activity, and neuronal activation. Psychopharmacology. 2010;208:455–68. doi: 10.1007/s00213-009-1745-7. [DOI] [PubMed] [Google Scholar]
- Carboni E, Silvagni A, Valentini V, Di Chiara G. Effect of amphetamine, cocaine and depolarization by high potassium on extracellular dopamine in the nucleus accumbens shell of SHR rats. An in vivo microdyalisis study. Neurosci Biobehav Rev. 2003;27:653–9. doi: 10.1016/j.neubiorev.2003.08.008. [DOI] [PubMed] [Google Scholar]
- Carroll ME, Lac ST. Autoshaping i.v. cocaine self-administration in rats: Effects of nondrug alternative reinforcers on acquisition. Psychopharmacology. 1993;110:5–12. doi: 10.1007/BF02246944. [DOI] [PubMed] [Google Scholar]
- Challman TD, Lipsky JJ. Methylphenidate: its pharmacology and uses. Mayo Clin Proc. 2000;75:711–21. doi: 10.4065/75.7.711. [DOI] [PubMed] [Google Scholar]
- Collins RJ, Weeks RJ, Cooper MM, Good PI, Russell RR. Prediction of abuse liability of drugs using IV self-administration by rats. Psychopharmacology. 1984;82:6–13. doi: 10.1007/BF00426372. [DOI] [PubMed] [Google Scholar]
- Davids E, Zhang K, Tarazi FI, Baldessarini RJ. Animal models of attention-deficit hyperactivity disorder. Brain Res Brain Res Rev. 2003;42:1–21. doi: 10.1016/s0165-0173(02)00274-6. [DOI] [PubMed] [Google Scholar]
- de Artiñano AA, Castro MM. Experimental rat models to study the metabolic syndrome. Br J Nutr. 2009;102:1246–53. doi: 10.1017/S0007114509990729. [DOI] [PubMed] [Google Scholar]
- de Wit H, Stewart J. Reinstatement of cocaine-reinforced responding in the rat. Psychopharmacology. 1981;75:134–43. doi: 10.1007/BF00432175. [DOI] [PubMed] [Google Scholar]
- Di Chiara GD, Imperato A. Drugs abused by humans preferentially increase synaptic dopamine concentrations in the mesolimbic system of freely moving rats. Proc Natl Acad Sci. 1988;85:5274–8. doi: 10.1073/pnas.85.14.5274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evenden J, Meyerson B. The behavior of spontaneously hypertensive and Wistar Kyoto rats under a paced fixed consecutive number schedule of reinforcement. Pharmacol Biochem Behav. 1999;63:71–82. doi: 10.1016/s0091-3057(98)00222-6. [DOI] [PubMed] [Google Scholar]
- Faraone SV, Upadhyaya HP. The effect of stimulant treatment for ADHD on later substance abuse and the potential for medication misuse, abuse, and diversion. J Clin Psychiatry. 2007;68:e28. doi: 10.4088/jcp.1107e28. [DOI] [PubMed] [Google Scholar]
- Faraone SV, Wilens TE, Petty C, Antshel K, Spencer T, Biederman J. Substance use among ADHD adults: implications of late onset and subthreshold diagnoses. Am J Addict. 2007;16(Suppl 1):24–32. doi: 10.1080/10550490601082767. [DOI] [PubMed] [Google Scholar]
- Fox AT, Hand DJ, Reilly MP. Impulsive choice in a rodent model of attention-deficit/hyperactivity disorder. Behav Brain Res. 2008;187:146–52. doi: 10.1016/j.bbr.2007.09.008. [DOI] [PubMed] [Google Scholar]
- Fredericks EM, Kollins SH. Assessing methylphenidate preference in ADHD patients using a choice procedure. Psychopharmacology. 2005;175:391–8. doi: 10.1007/s00213-004-1838-2. [DOI] [PubMed] [Google Scholar]
- Fujita S, Okutsu H, Yamaguchi H, Nakamura S, Adachi K, Saigusa T, Koshikawa N. Altered pre- and postsynaptic dopamine receptor functions in spontaneously hypertensive rat: an animal model of attention-deficit hyperactivity disorder. J Oral Sci. 2003;45:75–83. doi: 10.2334/josnusd.45.75. [DOI] [PubMed] [Google Scholar]
- Hardman JG, Limbird LE, Molinoff PB, Ruddon RW, Gilman AG. Goodman and Gilman’s the Pharmacological Basis of Therapeutics. New York: McGraw-Hill; 1996. [Google Scholar]
- Kollins SH. Abuse liability of medications used to treat attention-deficit/hyperactivity disorder (ADHD) Am J Addict. 2007;16(Suppl 1):35–42. doi: 10.1080/10550490601082775. [DOI] [PubMed] [Google Scholar]
- Kollins SH, English J, Robinson R, Hallyburton M, Chrisman AK. Reinforcing and subjective effects of methylphenidate in adults with and without attention deficit hyperactivity disorder (ADHD) Psychopharmacology. 2009;204:73–83. doi: 10.1007/s00213-008-1439-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lambert NM, Hartsough CS. Prospective study of tobacco smoking and substance dependencies among samples of ADHD and non-ADHD participants. J Learn Disabil. 1998;31:533–44. doi: 10.1177/002221949803100603. [DOI] [PubMed] [Google Scholar]
- Marusich JA, Bardo MT. Differences in impulsivity on a delay discounting task predict self-administration of a low unit dose of methylphenidate in rats. Behav Pharmacol. 2009;20:447–54. doi: 10.1097/FBP.0b013e328330ad6d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marusich JA, Beckmann JS, Gipson CD, Bardo MT. Methylphenidate as a reinforcer for rats: Contingent delivery and intake escalation. Exp Clin Psychopharmacol. 2010;18:257–66. doi: 10.1037/a0019814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer AC, Rahman S, Charnigo RJ, Dwoskin LP, Crabbe JC, Bardo MT. Genetics of novelty seeking, amphetamine self-administration and reinstatement using inbred rats. Genes Brain Behav. 2010;9:790–8. doi: 10.1111/j.1601-183X.2010.00616.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mook DM, Neuringer A. Different effects of amphetamine on reinforced variations versus repetitions in spontaneously hypertensive rats (SHR) Physiol Behav. 1993;56:939–44. doi: 10.1016/0031-9384(94)90327-1. [DOI] [PubMed] [Google Scholar]
- Mook DM, Jeffrey J, Neuringer A. Spontaneously hypertensive rats (SHR) readily learn to vary but not repeat instrumental responses. Behav Neural Biol. 1993;59:126–35. doi: 10.1016/0163-1047(93)90847-b. [DOI] [PubMed] [Google Scholar]
- Nielsen JA, Duda NJ, Mokler DJ, Moore KE. Self-administration of central stimulants by rats: A comparison of the effects of d-amphetamine, methylphenidate and McNeil 4612. Pharmacol Biochem Behav. 1984;20:227–32. doi: 10.1016/0091-3057(84)90247-8. [DOI] [PubMed] [Google Scholar]
- Pardey MC, Homewood J, Taylor A, Cornish JL. Re-evaluation of an animal model for ADHD using a free-operant choice task. J Neurosci Methods. 2009;176:166–71. doi: 10.1016/j.jneumeth.2008.09.009. [DOI] [PubMed] [Google Scholar]
- Rang HP, Dale MM, Ritter JM, Moore PK. Pharmacology. 5. Edinburgh: Churchill Livingstone; 2003. [Google Scholar]
- Russell VA. Hypodopaminergic and hypernoradrenergic activity in prefrontal cortex slices of an animal model for attention-deficit hyperactivity disorder--the spontaneously hypertensive rat. Behav Brain Res. 2002;130:191–6. doi: 10.1016/s0166-4328(01)00425-9. [DOI] [PubMed] [Google Scholar]
- Russell VA. Neurobiology of animal models of attention-deficit hyperactivity disorder. J Neurosci Methods. 2007;161:185–98. doi: 10.1016/j.jneumeth.2006.12.005. [DOI] [PubMed] [Google Scholar]
- Russell V, de Villiers A, Sagvolden T, Lamm M, Taljaard J. Altered dopaminergic function in the prefrontal cortex, nucleus accumbens and caudate-putamen of an animal model of attention-deficit hyperactivity disorder--the spontaneously hypertensive rat. Brain Res. 1995;676:343–51. doi: 10.1016/0006-8993(95)00135-d. [DOI] [PubMed] [Google Scholar]
- Russell V, de Villiers A, Sagvolden T, Lamm M, Taljaard J. Differences between electrically-, ritalin- and D-amphetamine-stimulated release of [3H]dopamine from brain slices suggest impaired vesicular storage of dopamine in an animal model of Attention-Deficit Hyperactivity Disorder. Behav Brain Res. 1998;94:163–71. doi: 10.1016/s0166-4328(97)00177-0. [DOI] [PubMed] [Google Scholar]
- Russell V, de Villiers A, Sagvolden T, Lamm M, Taljaard J. Methylphenidate affects striatal dopamine differently in an animal model for attention-deficit/hyperactivity disorder--the spontaneously hypertensive rat. Brain Res Bull. 2000;53:187–92. doi: 10.1016/s0361-9230(00)00324-5. [DOI] [PubMed] [Google Scholar]
- Sagvolden T. Behavioral validation of the spontaneously hypertensive rat (SHR) as an animal model of attention-deficit/hyperactivity disorder (AD/HD) Neurosci Biobehav Rev. 2000;24:31–9. doi: 10.1016/s0149-7634(99)00058-5. [DOI] [PubMed] [Google Scholar]
- Sagvolden T, Sergeant JA. Attention deficit/hyperactivity disorder–from brain dysfunctions to behavior. Behav Brain Res. 1998;94:1–10. [PubMed] [Google Scholar]
- Sanabria F, Killeen PR. Evidence for impulsivity in the Spontaneously Hypertensive Rat drawn from complementary response-withholding tasks. Behav Brain Funct. 2008;4:7. doi: 10.1186/1744-9081-4-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sagvolden T, Russell VA, Aase H, Johansen EB, Farshbaf M. Rodent models of attention-deficit/hyperactivity disorder. Biol Psychiatry. 2005;57:1239–47. doi: 10.1016/j.biopsych.2005.02.002. [DOI] [PubMed] [Google Scholar]
- Schenk S, Partridge B. Sensitization to cocaine’s reinforcing effects produced by various cocaine pretreatment regimens in rats. Pharmacol Biochem Behav. 2000;66:765–70. doi: 10.1016/s0091-3057(00)00273-2. [DOI] [PubMed] [Google Scholar]
- Setlik J, Bond GR, Ho M. Adolescent prescription ADHD medication abuse is rising along with prescriptions for these medications. Pediatrics. 2009;124:875–80. doi: 10.1542/peds.2008-0931. [DOI] [PubMed] [Google Scholar]
- Sukhanov IM, Zakharova ES, Danysz W, Bespalov AY. Effects of NMDA receptor channel blockers, MK-801 and memantine, on locomotor activity and tolerance to delay of reward in Wistar–Kyoto and spontaneously hypertensive rats. Behav Pharmacol. 2004;15:263–71. doi: 10.1097/01.fbp.0000137212.03247.f1. [DOI] [PubMed] [Google Scholar]
- van den Bergh FS, Bloemarts E, Chan JS, Groenink L, Olivier B, Oosting RS. Spontaneously hypertensive rats do not predict symptoms of attention-deficit hyperactivity disorder. Pharmacol Biochem Behav. 2006;83:380–90. doi: 10.1016/j.pbb.2006.02.018. [DOI] [PubMed] [Google Scholar]
- Volkow ND, Swanson JM. Variables that affect the clinical use and abuse of methylphenidate in the treatment of ADHD. Am J Psychiatry. 2003;160:1909–18. doi: 10.1176/appi.ajp.160.11.1909. [DOI] [PubMed] [Google Scholar]
- Volkow ND, Wang GJ, Kollins SH, Wigal TL, Newcorn JH, Telang F, et al. Evaluating dopamine reward pathway in ADHD: clinical implications. JAMA. 2009;302:1084–91. doi: 10.1001/jama.2009.1308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilens TE, Faraone SV, Biederman J, Gunawardene S. Does stimulant therapy of attention-deficit/hyperactivity disorder beget later substance abuse? A meta-analytic review of the literature. Pediatrics. 2003;111:179–85. doi: 10.1542/peds.111.1.179. [DOI] [PubMed] [Google Scholar]
- Wilens TE, Adler LA, Adams J, Sgambati S, Rotrosen J, Sawtelle R, Utzinger L, Fusillo S. Misuse and diversion of stimulants prescribed for ADHD: a systematic review of the literature. J Am Acad Child Adolesc Psychiatry. 2008;47:21–31. doi: 10.1097/chi.0b013e31815a56f1. [DOI] [PubMed] [Google Scholar]
- Yang PB, Amini B, Swann AC, Dafny N. Strain differences in the behavioral responses of male rats to chronically administered methylphenidate. Brain Res. 2003;971:139–52. doi: 10.1016/s0006-8993(02)04240-3. [DOI] [PubMed] [Google Scholar]
- Yang PB, Swann AC, Dafny N. Acute and chronic methylphenidate dose-response assessment on three adolescent male rat strains. Brain Res Bull. 2006;71:301–10. doi: 10.1016/j.brainresbull.2006.09.019. [DOI] [PMC free article] [PubMed] [Google Scholar]