Abstract
The root cause of preeclampsia is the placenta. Preeclampsia begins to abate with the delivery of the placenta and can occur in the absence of a fetus but with the presence of trophoblast tissue with hydatidiform moles. In view of this, study of the placenta should provide insight into the pathophysiology of preeclampsia. In this presentation we examine placental pathological and pathophysiological changes with preeclampsia and fetal growth restriction (FGR). It would seem that this comparison should be illuminating as both conditions are associated with similarly abnormal placentation yet only in preeclampsia is there a maternal pathophysiological syndrome. Similar insights about early and late onset preeclampsia should also be provided by such information.
We report that the placental abnormalities in preeclampsia are what would be predicted in a setting of reduced perfusion and oxidative stress. However, the differences from FGR are inconsistent. The most striking differences between the two conditions are found in areas that have been the least studied. There are differences between the placental findings in early and late onset preeclampsia but whether these are qualitative, indicating different diseases, or simply quantitative differences within the same disease is difficult to determine.
We attempt to decipher the true differences, seek an explanation for the disparate results and provide recommendations that we hope may help resolve these issues in future studies.
Keywords: Preeclampsia, Fetal Growth Restriction, Placenta, Pathology, Morphology, Pathophysiology
Introduction
The placenta has long been recognized as the necessary component for the genesis of preeclampsia. Hydatidiform moles with abundant trophoblast but no fetus are associated with an excess incidence of preeclampsia.[1] The unique feature of the placenta proposed to result in preeclampsia is its exposure to reduced placental perfusion. This is supported by clinical, pathological and experimental findings.[2] Preeclampsia is more common in diseases that feature microvascular disease (e.g. hypertension, diabetes mellitus), and in obstetric disorders with large placentas (e.g. hydatidiform moles, multiple gestations).[3] The latter association is felt secondary to the inability of even a normal placental blood supply to adequately perfuse the very large placental mass, thus resulting in a relative hypoperfusion. A common pathological feature of preeclampsia is the failure of the maternal arteries supplying the placenta to undergo the physiological adaptations of normal pregnancy that facilitate adequate placental perfusion.[4] Finally, although animal models of preeclampsia are far from perfect, strategies to reduce placental perfusion result in a preeclampsia like syndrome in several species.[5, 6]
We and others have proposed a “Two Stage Model” to explain the relationship of the placenta to the maternal syndrome.[7] This hypothesis proposes that the placenta in response to reduced perfusion produces material(s) that act upon the mother to bring about the clinical findings of preeclampsia. The placenta as the key component of this hypothesis should provide clues about preeclampsia and its genesis. Especially important insights should arise from placental differences between preeclampsia and fetal growth restriction (FGR) without preeclampsia. In both of these conditions there is reduced placental perfusion but only in preeclampsia is there a maternal syndrome. For this reason, throughout this presentation we will where possible compare features of the placenta in these two conditions. Many questions must be answered. What is it about abnormal perfusion that results in preeclampsia? The Two Stage Model proposes placental abnormalities that result from reduced perfusion generated materials that produce maternal disease. What are the important abnormalities of placental function leading to the production of these factors? What is released into the maternal circulation to cause preeclampsia? Furthermore is this one or several agents, and is it the same in all cases of preeclampsia? Finally, it has been suggested that preeclampsia with and without growth restriction or of early or late onset could be different disorders. Is this supported by placental findings?
Abnormal implantation and vascular remodeling
Although not strictly placental, the changes in the vessels supplying the intervillus space are considered to be the key feature in the genesis of preeclampsia. The embryo originally implants in maternal decidua and is not supplied directly by any modifications of maternal vessels. Until about 10 weeks gestation the embryo exists in a low oxygen environment with nutrients provided by endometrial glands. At about 10 weeks, maternal vessels begin to perfuse the embryonic placenta as the intervillus space is vascularized.[8] Placental cytotrophoblast respond to the initial low oxygen environment with proliferation and to the subsequent increased oxygen with reduced proliferation and differentiation to an invasive phenotype.[9] The maternal vessels are invaded by the trophoblast and dramatic modification of the vessels occurs. The spiral arteries, which are initially fairly standard small muscular arteries, dilate strikingly at the decidual end of the vessel. The dilated portions of the vessels lose their endothelium, smooth muscle and inner elastic lamina. This modification extends to the inner third of the myometrium resulting in the terminal portion of the spiral arteries becoming modified to flaccid large diameter tubes unable to constrict in response to humoral or neural signals.[10] These changes do not occur normally in preeclampsia. Some vessels undergo modest remodeling in their decidual segments but this change never extends to the myometrium and some vessels do not remodel.[11–15] For many years the failure to increase the diameter of the terminal spiral artery received greatest attention. The diameter of this section of the spiral artery increases several fold and the impact of this change on flow has been emphasized. However, recent modeling of these changes indicates that dilating only the terminal portion of the vessel as occurs with the remodeling of spiral arteries in normal pregnancy will have little effect on flow.[16] Analogous to the effect of placing a funnel on the end of a garden hose, the flow will not increase with the fourth power of the radius (Poiseuille’s law) but rather may double. Graham Burton has proposed that perhaps a more influential change is the depth of the vascular modification.[16] He points out that in the spiral arteries located at about the inner third of the myometrium in nonpregnant women there is a condensation of vascular smooth muscle proposed as important to stop blood flow at the time of menses. This smooth muscle is lost in normal pregnancy but persists in preeclampsia. The result, he posits, is spiral arteries that remain contractile in the preeclamptic placental vascular bed with the potential for a perfusion/reperfusion scenario with the subsequent generation of oxidative stress. He proposes the main consequence of the terminal vascular dilation is to reduce the rate of flow that would be greatly increased with the striking increase in uterine perfusion that accompanies normal pregnancy. Although this abnormal finding is described in the setting of preeclampsia, it is thought that an identical change occurs in FGR[12] without preeclampsia and in about one third of cases of preterm birth.[17]. Another characteristic change in the untransformed spiral arteries in preeclampsia and FGR is “acute atherosis”. This change results in vessels occluded by fibrinoid and surrounded by foam cells. Acute atherosis has been compared to the vascular lesion of atherosclerosis[18] and also is claimed to be similar to changes in vessels of tissues undergoing rejection.[19] Atherosis is found in disorders other than preeclampsia and FGR and can be seen in decidual vessels beyond site of placental implantation.[20]
Morphological changes in the placenta with preeclampsia and FGR
Gross pathological changes are most common with severe preeclampsia occurring preterm.[21]. The characteristic placental changes of preeclampsia would be predicted to be those associated with placental ischemia. Consistent with this prediction, the placenta in preterm preeclampsia is small with several types of infarction. The gross placental changes with severe preeclampsia and FGR are quite similar.
Interestingly, if placental weight is examined as a centile for gestational age, infants with placentas less than the 30th centile were smaller in preeclamptic pregnancies, while those with the placental weight greater than the 90th centile were larger.[22] Thus, there are at least some gross differences in placentas from preeclampsia and FGR. Moreover, the shape of the placenta in preeclampsia may also be characteristic. In a large (n = 6410) series of placental measurements performed in Finland between 1934 and 1944 the placenta was more “oblong”.[23] Rather than the circular shape usually present in pregnancy there was a greater discrepancy between the shortest and longest diameter in preeclampsia. Burton has proposed that such an abnormal placental shape is associated with reduced endovascular invasion. [8] The hypothesis proposes that with normal trophoblastic invasion the maternal spiral arteries are “plugged” by trophoblast for a period of time sufficient to allow for vascular remodeling to reduce the velocity of flow into the intervillus space. Normally, invasion is most robust in the center of the placenta and less so moving towards the periphery. In the peripheral areas with reduced invasion and plugging, villi are damaged. This is by mechanical effects as well as by excess oxidative stress with increased oxygenation due to lesser trophoblast invasion and plugging of maternal vessels in the periphery. The resulting villus necrosis leads to the formation of a circular placenta with surrounding chorion laevae. With more generalized reduced plugging because of shallow invasion the regression of the placenta is not circumferential and unusual shapes result. Interestingly, this hypothesis also predicts a thicker placenta in response to damage to cell columns and villi by the high velocity blood flow from untransformed spiral arteries. Consistent with this hypothesis, a thicker placenta was also more prevalent in the placentas from preeclamptic pregnancies. Unfortunately, there was no statement in the presentation regarding placental shape and thickness in isolated FGR.[23]
Histological changes in the placenta with preeclampsia and with FGR are also those of reduced perfusion. These can be found in term but more commonly in preterm preeclampsia as well as with FGR and include accelerated villus branching, large and numerous syncytial knots, and small sclerotic villi.[21] It is suggested that most of these findings are related to low oxygenation secondary to reduced perfusion. However, with the hypothesis forwarded by Burton, hypoxia may not be a major feature of the abnormal vascular remodeling but rather the generation of reactive oxygen species.[16] Syncytial knots, for example, can be induced in vitro by either hypoxia or oxidative stress.[24]
Quantitative assessment of placental morphology
Thus, standard histological examinations do not appear to differentiate the abnormal placental findings in preeclampsia and FGR without preeclampsia. Nonetheless, the qualitative assessment of villus vascularity has led to the conclusion that preeclampsia is associated with increased branching of villi that could result in increased surface area for exchange.[25] This would be predicted as an adaptive response to reduced oxygen delivery supporting the hypothesis that hypoxia resulting from reduced oxygen delivery increases branching morphogenesis.[26]
The morphological examination of the placenta has been extended to include quantitative assessments using stereological techniques. However, this approach does not seem to be support increased villus branching in preeclampsia. In a review of the available literature it was concluded that terminal villus volume, terminal villus surface area and terminal capillary surface were similar in preeclampsia without accompanying FGR to findings in normal pregnancy. In preeclampsia with a FGR infant the findings were quite similar to findings with FGR without preeclampsia.[27] In both of these settings terminal villus volume, terminal villus surface area and terminal capillary surface were reduced compared to normal pregnancy.
One study reported differences between preeclampsia and FGR.[28] Syncytiotrophoblast to total villus area was reduced in preeclampsia without FGR but not with FGR with or without preeclampsia. However, even in this study these abnormal groups appear more similar to each other than similar to the control. (control=22±3%, preeclampsia 13±3%, preeclampsia + FGR 14±3%, FGR 16±4%; mean ± standard error of the mean).
The certainty that the quantitative data does not support the preexisting concept of an increase in syncytial surface areas due to increased villous branching must be taken cautiously. These are labor-intensive analyses done on a limited numbers of subjects. In fact, it appears the same 10 preeclamptic women have been assessed for different quantitative morphological measurements in at least 3 studies.[28–30] Also, with the limited numbers of patients that have been studied it is likely that women with severe and mild preeclampsia and preeclampsia of early and late onset were combined and the findings in these two groups are quite different. This is demonstrated in investigation by Egbors who studied placentas from 20 women with preeclampsia of early or late onset with and without FGR. In this study, in preeclampsia in the absence of FGR there were few differences from normal pregnancy.[31] In preeclampsia with FGR the villus volume and terminal villus surface area were reduced compared to placentas from normal pregnancies but were not different than FGR alone. In another publication by the same author with apparently the same subjects, he divided the preeclamptic pregnancies into early onset (≥34 weeks) and late onset.[32] He found that as reported previously the placenta from pregnancies with FGR, whether complicated by preeclampsia or not were very different. For some measurements the preeclampsia with FGR, placental findings were worse than FGR alone but the degree of growth restriction was also greater in this group. The major new finding was that whereas there was no difference between term preeclampsia without FGR placentas and placentas of term controls, there were differences in the early onset preeclampsia without FGR placentas compared to placentas from preterm controls. Thus, terminal villi volume and terminal villi surface area were reduced in placentas from early onset preeclamptic pregnancies without FGR compared to gestationally age matched control pregnancies. This study raises another problem present in such studies. The early onset control pregnancies are not normal pregnancies but rather pregnancies from preterm births.
In summary, as powerful as is the technique of quantifying key placental morphological features that could influence nutrient and gas exchange, the results currently available are compromised by small numbers, poor definitions and poor controls. With this disclaimer, the consensus of findings is that FGR with and without preeclampsia manifest a similar reduced surface area for exchange. The placentas from preeclamptic pregnancies delivering at term are similar to controls and placentas from preterm preeclamptic pregnancies even without FGR resembles FGR.
Pathophysiological Markers of Placental Dysfunction
Thus, the question as to whether placental morphological differences, qualitative or quantitative, can explain the maternal manifestations of preeclampsia that are not present in FGR is an equivocal no. Are there differences in pathophysiological markers of placental dysfunction?
Inflammation and oxidative stress are proposed as important components of the pathophysiology of preeclampsia. Can differences in these two phenomena explain the systemic findings of preeclampsia that are absent in FGR? Oxidative stress is almost universally demonstrated in the placenta of preeclamptic pregnancies and somewhat less consistently in FGR without preeclampsia.[33–36] Burton has proposed that the reduced uterine perfusion characteristic of preeclampsia and FGR may be a spectrum. Modest reductions of placental perfusion engender endoplasmic reticular stress. Endoplasmic stress with consequent reduced protein synthesis results in a small placenta and baby with FGR. As perfusion is reduced more severely oxidative stress ensues with consequent maternal systemic findings of preeclampsia.[36] The occurrence of oxidative stress in some studies of FGR is not supportive of this hypothesis, although as cited, oxidative stress markers in the placenta with FGR are less prevalent and less severe. Further, many studies of placental oxidative stress are done with placentas delivered after labor that clearly confounds the findings since labor itself induces placental oxidative stress.[36] However, as appealing as is this hypothesis of quantitative differences in oxidative stress in preeclampsia and FGR the story is likely more complex. A systemic marker of oxidative stress, reduced plasma ascorbate is associated with reduced placental perfusion as assessed by Doppler velocimetry of uterine arteries independent of pregnancy outcome. Oxidative stress by this criterion is present in women with abnormal Doppler velocimetry whether they subsequently have preeclampsia, FGR or normal pregnancy outcomes.[37]
Normal pregnancy is associated with increased inflammatory activation, which is accentuated in preeclampsia.[38–41] Morphological changes in the placenta support increased inflammation,[42, 43] as do systemic markers of inflammation.[42, 44] Although there are few, if any, direct comparisons of inflammatory changes of serum markers of inflammation in preeclampsia and FGR it is clear that inflammation is increased in most studies of FGR without preeclampsia.[45, 46]
Although there is evidence that the trophoblast surface is exposed to increased oxygen concentrations with severe FGR because of reduced oxygen extraction,[25] the consequences of reduced extraction is reduced oxygen delivery to the placenta and fetus.[36] Examination of molecules sensitive to reduced oxygenation suggests differences in FGR and preeclampsia. Analyses, largely by Conrad, and Rajakumar with our group, [47] have indicated that protein concentration for HIF-1 and HIF-2 alpha, the hypoxia responses elements, as well as proteins downstream from HIF-1 and HIF2 alpha, Flt and s-Flt are increased in placentas from preeclamptic women with or without FGR. By contrast, in placentas from FGR infants (< 2.4 centile) whose mothers did not have preeclampsia, HIF-1 alpha and HIF-2 alpha were not higher nor were proteins downstream of these transcription factors greater than in placentas from normal pregnancies. This, despite the fact, that the concentrations of mRNA for HIF-1alpha and HIF-2 alpha were not different in placentas from infants (with or without FGR) of preeclamptic women and of women with FGR without preeclampsia.[48] In another study investigators modified gene expression by exposing early pregnancy trophoblast to hypoxia in vitro and found that genes they identified as activated by hypoxia were increased in late pregnancy placentas from preeclamptic but not FGR pregnancies.[49]. However in an older study HIF-2 alpha but not HIF-1 alpha was increased in FGR without preeclampsia.[50] Other than this last study it would seem there is a striking difference in hypoxia or the response of the placenta to hypoxia in preeclampsia and FGR.
The results with circulating concentrations of putative placentally produced proteins modified by hypoxia are less clear. Circulating s-Flt-1, one of the downstream markers of HIF activation, is increased in preeclampsia, particularly early onset [51] and severe preeclampsia[52] and in early[53] and late onset[54] FGR pregnancies without hypertension. However, in keeping with the lack of difference in placental s-Flt with late onset FGR, our group found no differences in circulating s-Flt in FGR (3.4 centile) near term.[55] In another study with normal pregnant controls, concentrations measured in preeclampsia with and without FGR were not different from each other but were significantly higher than FGR without hypertension[56]. However, other studies report increased s-Flt or reduced PlGF in FGR placentas[57] or maternal blood compared with controls.[53, 58]
Release of placental components into maternal circulation
Almost twenty years ago the Oxford group proposed that microparticles released from placental syncytium, syncytiotrophoblast microparticles (STBM), might be involved in the pathogenesis of preeclampsia.[59] They demonstrated that STBM were increased with preeclampsia with more present in severe preeclampsia but interestingly there was no increase with FGR suggesting a role for these particles in the maternal preeclampsia syndrome.[60] Originally considered as a pathophysiological component of preeclampsia, it has become increasingly evident that circulating microparticles are far more than pathological. Microparticles circulate from many tissues and in pregnancy SBTM are only about 1.5 to 3% percent of circulating microparticles with the majority (97–99%) coming from platelets. Further, included with these shed portions of cells are smaller particles (nanoparticles) that include endosomes, particles with a specific pathway of synthesis and release. Endosomes contain RNA and DNA and appear to provide another method of intercellular communication. It is also speculated that STBM provide a means of immunological communication between mother and fetus. Although most attention has been directed at quantitative differences in STBM with an increase in preeclampsia, the functionality of these particles suggests that qualitative differences are also worthy of study.[61] Qualitative differences could reflect the “toxicity” by modifications to syncytiotrophoblast membranes with the hypoxia, oxidative and mechanical stress but could also include failure of these particles to induce appropriate responses.
The mechanism by which SBTM contribute to the pathophysiology of preeclampsia has been studied extensively.[62] Original in vitro studies suggested a direct effect upon endothelium to reduce proliferation and disrupt endothelial monolayers.[59] Particles prepared from normal and preeclamptic placentas had similar effects. The endothelial dysfunction of preeclampsia in response to these particles was proposed to be quantitative with more STBM in preeclampsia than normal pregnancies.[60] Subsequently, increased attention has been directed at the inflammatory effects of STBM. STBM prepared by placental perfusion (but not by mechanical means) stimulate inflammatory cytokine release from PBMC’s. Monocytes have STBM bound to them in vivo associated with activation.[44]. Other in vitro studies have demonstrated the ability of STBM prepared from placentas or isolated from blood of preeclamptic women to affect coagulation[62] or modify vascular responses.[63] It is important to bear in mind the limitations of these studies, as the activity of prepared STBM varies with preparation (e.g. mechanically or by placental perfusion) and the study of microparticles isolated from blood is confounded by binding of the microparticles to blood products or modifications of their activity by the isolation techniques.[62] Nonetheless, the multiple functions demonstrated and potential of these particles makes them interesting candidates for the placental genesis of the maternal preeclampsia syndrome.
Consistent with this idea of placental syncytial shedding, preeclampsia is associated with high circulating concentrations of cell-free fetal DNA (ffDNA) in maternal plasma[64, 65]. Once again the situation is not clear with FGR[64, 66, 67]. In one study the concentration of fetal DNA was significantly higher in subjects with preeclampsia than with isolated FGR or normal control subjects; while the concentration in the latter two groups were similar[67]. In addition, higher concentrations of ffDNA were associated with preeclampsia severity.[64]
Maternal fDNA is also increased in overt preeclampsia and is felt to be an indicator of maternal tissue injury.[68] Although the increase of fetal and maternal cell free DNA have been primarily considered as markers of disease it is also relevant that the metabolism of DNA generates vasoactive molecules such as adenosine, ADP and ATP.[69] Furthermore, with the placental hypoxia posited to be associated with preeclampsia, ATP is released and its breakdown increased.[70] Supporting this is the observation that adenosine is higher in maternal circulation from preeclamptic compared with normal pregnancies, and the highest concentration is present in severe preeclampsia.[71] This difference has not been reported with FGR. Interestingly the implications of the altered concentration of the vasoactive materials, ATP and adenosine, has received little attention. Suggesting that these might be relevant for the regulation of maternal blood flow, reduced expression of A2A adenosine receptor have been found in microvascular placental endothelial cells[72] while the expression of all adenosine receptors, A1, A2A, A2B and A3, was increased in placentas from preeclamptic but not FGR pregnancies.[73]
Apoptosis and necrosis
The observation that STBM are increased in the circulation of pregnancies complicated by preeclampsia but not FGR without preeclampsia suggests structural placental differences in the two conditions.[60] The source of these microparticles has been proposed to be due to apoptosis, necrosis and felt by some to represent the shedding of syncytial knots.[74] There is evidence of increased apoptosis as well as necrosis in preeclampsia but the concept that the origin of STBM is solely or primarily syncytial knots has been questioned.[75]
Although apoptosis is increased in placentas from FGR and preeclampsia,[76, 77] necrosis is felt to be more a characteristic of preeclampsia.[78] Apoptosis as the major source for STBM has been disputed by Burton.[75] He proposes that the release of STBM is due to the increased velocity of blood entering the intervillus space with failed spiral artery remodeling. He posits that this leads to the release of placental sprouts and more importantly necrotic trophoblast into the maternal circulation. Necrotic tissues unlike apoptotic tissue induce an inflammatory response when taken up by endothelial cells and thus would contribute to the pathophysiology of preeclampsia.[79, 80] Implicit in this observation is that somehow the placenta, despite similar exposure to the results of abnormal spiral artery remodeling, is more damaged and is more likely to contain necrotic material with preeclampsia than with FGR. Huppertz has proposed that the excess of STBM in preeclampsia is due to a very early defect in syncytiotrophoblast development that is not present with FGR, emphasizing that the failed remodeling of the spiral arteries in both conditions does not explain the differential release of STBM.[78] The abnormal differentiation of syncytiotrophoblast leads to an excess of necrosis and release of material into the maternal circulation. Since syncytiotrophoblast differentiation in this model is not abnormal in FGR there is no excess STBM release in this condition. Although this speculation seems logical, and explains the dichotomy of STBM it will be difficult to test.
Genomics and proteomics
Genomic and proteomic strategies take a “discovery science” approach to the understanding of altered placental function in preeclampsia.[81–85] Unencumbered by hypotheses these strategies assess differences in gene or protein expression between preeclampsia and other pregnancies. Differential gene and protein expression and modification should then be useful guides for hypothesis generation.[86] Unfortunately, despite this promise, most genomic studies have been performed on placentas obtained at term, sometimes without concern for exposure to labor and findings are not consistent. In one study, for example, expression of genes shown to be up-regulated by hypoxia in placental tissue from early pregnancy was increased in preeclamptic pregnancies compared to placentas from FGR pregnancies with abnormal umbilical artery Doppler determinations.[49] In another study using microarray no differences were present with the two diagnoses.[87]
Of much more relevance would be the expression of genes related to trophoblast invasion and angiogenesis at the time these events are occurring (10–20 weeks gestation) during pregnancy. In keeping with this recommendation, samples remaining after chorionic villus sampling at 10–12 weeks of gestation, were examined by microarray in women who later developed preeclampsia (n=4) or had normal pregnancy outcome (n=8).[88] There were 36 genes differentially expressed in preeclamptic women (five up-regulated and 31 down-regulated). Bioinformatic pathway analysis indicated that the differentially expressed genes were in two signaling networks 1) network: cancer, respiratory disease, and cellular movement and 2) network: inflammatory disease, cellular movement, and hematological system development and function. MMP-12 was the only gene shared between networks 1 and 2[89]. These data and other similar data obtained at a time in pregnancy when what is considered the root cause of preeclampsia is occurring should provide insights and perhaps useful predictors for preeclampsia.[90, 91] It might also be feasible to use these markers to contribute to the understanding of early mechanisms in preeclampsia. Unfortunately we found no study including placental samples from FGR obtained in early pregnancy, thus the question of similarities or differences between FGR and preeclampsia cannot be addressed.
Protein expression, the step beyond gene expression, is also beginning to be assessed in preeclampsia. Proteomic approaches such as microarray with or without mass spectrometry; or specific protein analyses of post-translational modification such as nitration or nitrosylation, or examining specific subsets of proteins with metabolomics have been used with several types of biological samples from preeclamptic pregnancies. Most of these studies have had as their goal to establish predictors, but useful pathophysiological information has also emerged. Focusing on placental tissue, at least 12 proteins were differentially expressed in preeclamptic compared to normal pregnancy. These differentially expressed proteins included signal transduction and molecular chaperon proteins[92]. In another study utilizing the same approach, at least 11 proteins, which were associated with anti-oxidant activities and altered expression of stress-response proteins were different in preeclampsia[93]. Interestingly, one study examined placental samples from normal pregnancies, early onset preeclampsia, late-onset preeclampsia and preeclampsia associated with FGR. Twelve proteins associated with immune response (i.e., Th1 and mainly Th2), were increased in samples from preeclampsia or FGR in relationship to normal pregnancies. There also appeared to be a different protein pattern with the different disease expression[94]. In another study 11 proteins related with oxidative stress protection were reduced in cells from preeclamptic compared to normal pregnancies.[95] Unfortunately in the reported studies differential expression of the same proteins is not consistently identified; samples are frequently collected after labor while early and late onset preeclampsia are compared without considering the role of differential gestational age on protein expression in the placenta.
Numerous studies of urine, amniotic fluid and serum/plasma have used genomic and proteomic approaches largely assessing prediction. One study took the analysis a step further testing the relevance to placental function. “Finger print” analysis of urine samples by proteomic profiling with mass spectrometry identified 13 peaks that correlated with the severity of preeclampsia.[96]. The authors sought the identity of the proteins to obtain pathophysiological insights (and simplify the identification of the protein predictors). They identified multiple forms of the serine protease inhibitors SERPINA1 and albumin. The SERPINA1 was expressed more in the placentas from preeclamptic women. The multiple protein forms suggested misfolding of proteins. This process is characteristic of endoplasmic reticulum stress that has been suggested as etiological relevant to preeclampsia and FGR.[36] The combination of discovery science and the generation of testable hypotheses with the data generated should hold real promise for increasing the understanding of preeclampsia
Subsets of preeclampsia
It is likely that preeclampsia is not a single disease. The clinical presentations and the wide scatter of virtually all laboratory assessments in the disorder, support this concept. Further, the striking differences in recurrence rate, frequency of later life cardiovascular disease and the likelihood of FGR with early but not late onset preeclampsia, suggest these are different disorders.[97, 98] Might the morphology of the placenta or the placental bed provide further support for subsets of preeclampsia? There does seem to be greater morphological evidence of hypoxia/oxidative stress with consequent infarctions and small placental size in early onset preeclampsia.[21] It is difficult to determine if these are qualitative differences or merely a matter of degree of exposure or perhaps a differential response (adaptation?) to reduced oxygen and nutrient availability. It is disturbing that in recent years the fact that uterine Doppler abnormalities are more likely to precede early than late onset preeclampsia and the increased risk of FGR primarily with early onset preeclampsia have led to the conclusion, largely unsupported by objective data, that only early onset but not late onset preeclampsia is associated with reduced placental perfusion.[78] The definitive proof that this is the case rather than for example a different response to reduced perfusion would come from placental bed biopsy studies. There are very few data in this area. In reviewing the available literature it is difficult to separate results from term and preterm preeclampsia. In most cases mean values of gestational age, which are in all cases less than term, is the only information presented. We identified two papers in which early and late onset preeclampsia could be differentiated. In a study by Moldenhauer, failed remodeling of basal plate spiral arteries was present more often in term preeclampsia than in normal pregnancy but failed remodeling was even more common in preterm preeclampsia.[99] This is likely an underestimate of the differences in remodeling since the most consistent difference between preeclampsia and normal pregnancy is found in the myometrial (not present in basal plate) rather than decidual segments of the spiral arteries.[100] In another study, myometrial spiral artery invasion was absent in 6 of 12 mild preeclamptics with a mean gestational age of 37 weeks and in 15 of 20 severe preeclamptics with a mean gestational age of 34 weeks.[101] Similarly, spiral artery vascular remodeling was abnormal not only in all 21 cases of preeclampsia with FGR but also in 8 of 9 cases of preeclampsia without FGR.[102] In a study of myometrial spiral artery remodeling, it was absent in 1 of 26 normal pregnancies, 7 of 11 preeclamptics with FGR, and 6 of 22 preeclamptics without FGR.[103]. In a study with a similar endpoint, remodeling was absent in preeclampsia with FGR in 2 of 2 cases and 6 of 6 cases or preeclampsia without FGR.[104] Thus, although the data are sparse it does not appear that failed remodeling of spiral arteries is a feature of only preterm preeclampsia or only preeclampsia with FGR. There may be a difference in the degree of failed remodeling, being more extensive with FGR and preterm but the phenomenon is more common in all forms of preeclampsia compared to normal pregnancy.
Conclusions
Review of the pathology and pathophysiology of the placenta, the organ known to be the proximate cause of preeclampsia, provides some interesting answers but leaves several important questions unanswered. The pathological changes of preeclampsia provide evidence of reduced oxygen delivery and exposure to oxidative and likely endoplasmic reticular stress. The materials released from the placenta into the maternal circulation are compatible with these observations. Some or all of these materials likely serve to link the abnormal placenta to the maternal syndrome (although the disparity of results within and between studies raises the question if this is the same linkage in all subjects). The clinical subtypes of preeclampsia, early onset, late onset and associated or not associated with FGR, present certain differences of placental findings. Nonetheless, it is difficult to determine if these indicate that the subsets are different diseases or are simply quantitatively different. One of the most strikingly different presentations of preeclampsia is the contrast clinically and epidemiologically between early and late onset preeclampsia. Although placentas in term preeclampsia without FGR are only minimally different from control placentas, early onset preeclampsia even without FGR is quite different than the placentas from their “control” (spontaneous preterm birth) placentas (and from term preeclampsia). These findings support that preeclampsia of early and late onset may be more than quantitatively different and provide weak support for the concept that late onset preeclampsia is a maternal rather than a placental disease. However, the concept that the placenta is normally perfused in late onset preeclampsia is not supported by placental bed findings. Failed remodeling of the spiral arteries is present in a percentage of preeclampsia of all varieties, late, early, with and without FGR. The difference in vascular remodeling appears more quantitative than qualitative in these forms of preeclampsia.
The major quandary is the lack of clear and consistent differences between the placenta from preeclamptic pregnancies and pregnancies with FGR without preeclampsia. Despite the presence of a maternal syndrome caused by the placenta in preeclampsia and the absence of this syndrome with FGR, the pathological placental differences do not provide great insights. In almost every case, if several studies are available, some indicate that changes are present only in preeclampsia and not FGR while others of almost equal number find little differences between the two conditions. It is probably not coincidental that the clearest differences come from studies that have not been extensively replicated. The work of Rajakumar et. al. [47, 48]clearly demonstrates that HIF protein and products of HIF activation are increased in the placenta from preeclampsia but not FGR. This finding raises the possibility that the placenta in FGR is either exposed to a lesser degree of oxidative stress and low oxygen delivery than it is in preeclampsia or that it does not respond to this insult. However, there are numerous studies of the concentration of placental products of hypoxia in the maternal circulation (e.g. leptin, s-Flt etc.), which do or do not indicate differences between the two conditions. Similarly, the quantitation of STBM indicates higher circulating concentrations with preeclampsia but not FGR. Thus far, there are few observations to confirm or refute this finding. There is also the interesting observation of placental/fetal size differences between preeclampsia and FGR with small preeclamptic infants having a smaller fetal/placental ratio than FGR infants from non-preeclamptic pregnancies, but again this has not been replicated. Virtually every morphological or physiological observation comparing FGR and preeclampsia that has been subjected to extensive study arrives at a similar conclusion. FGR with and without preeclampsia are quite similar and preeclampsia without FGR is subtly different from normal.
What is the explanation for this overlap between FGR and preeclampsia? Some portion of the explanation relates to flawed experimental design. In some studies it is not clear whether FGR includes FGR with preeclampsia. In others, the subjects called FGR without preeclampsia, seem more like preeclamptics than normals with, for example, blood pressure, which although not diagnostically elevated, are higher than controls. Also in some placental studies sufficient attention is not directed to whether a sample was taken before or after labor, which can markedly change evidence of hypoxia or hypoxia reperfusion. Another technical issue is how areas to be investigated in the placenta are chosen. Usually, microscopic analyses are representative of a small part of the placenta. Attempts to compare several randomly selected areas reduce bias but still represent only a small proportion of the “universe” of possible pathological findings.
However, this is unlikely the explanation for all of the overlap in placental findings. It is likely that this comes from the real overlap of the two conditions and the fact that there may be multiple routes to preeclampsia and to FGR. Both conditions also suffer from the problem of non-specific findings that arbitrarily divide a continuous outcome. In preeclampsia the selection of blood pressure and proteinuria is purely historical. These were the first two changes identified in women with what at the time was considered a pregnancy specific seizure disorder, eclampsia. They are not specific or sensitive[105] and are not actual direct indicators of the most important pathophysiological changes in preeclampsia. Further the division of 140/90 as blood pressure indicating preeclampsia (while 139/89 does not) is obviously arbitrary. Likewise, even if one takes the conceptual diagnosis of FGR, the failure of the infant to exercise its growth potential, it is unlikely to be due to a single etiology. Most published studies present findings due to failed spiral artery remodeling with reduced nutrient delivery but most FGR worldwide is due to reduced nutrient intake. Is it possible that even in developed countries nutritional abnormalities can be a part of the genesis in some subjects? Attempts to standardize the diagnosis of FGR include the use of abnormal umbilical artery velocimetry to clarify the diagnosis. Interestingly, in a study of infants less than the 5th centile, with middle cerebral artery redistribution half of the infants with normal umbilical artery Doppler had oligohydramnios, head circumference > than the 95th centile and were delivered by C-section for intolerance of labor.[106] This would seem to suggest different forms of FGR even when abnormal spiral artery remodeling rather than reduced nutrient intake is the usual explanation for FGR. The studies from our group stand out as very different than those from other investigators. In our studies we find little difference between SGA infants defined as less than the fifth centile and normally grown infants. The findings of Rajakumar et. al. and and the lack of evidence of circulating markers of hypoxia in our other studies have led us to propose a blunted fetal response to reduced oxygen and nutrient availability in FGR without preeclampsia. These findings are not confirmed in the studies by others of infants with the same degree of FGR and especially when accompanied by abnormal uterine artery velocimetry and with early FGR delivering prematurely. (e.g. [58]) Our subjects are taken from a prospective collection of “normal” subjects. SGA was not recognized in many of these subjects until delivery, which was frequently at term and rarely accompanied by Doppler assessment. Another differentiation that has received little attention is fetal sex. In epidemiological and animal studies it appears that male and female fetuses respond differently to reduced nutrient availability and the maternal response may also be influenced by fetal sex.[107–109] Also, the definition of SGA is completely arbitrary and depending on the cut off selected will include a greater or smaller number of constitutionally small infants. With these considerations it is not surprising that laboratory or pathological findings do not clearly differentiate the two conditions.
In summary, the participation of the placenta in the pathophysiology of preeclampsia is clear; however, to maximize information gained from study of the placenta we must direct meticulous attention to several issues of analysis and diagnosis.
Recommendations
It seems incontrovertible that the placenta will provide insights into preeclampsia and just as likely that the imprecision of clinical diagnoses will hinder the ability to interpret the findings. In order to maximize the impact of future studies, several points should be considered. In studies of preeclampsia diagnostic precision is mandatory. Criteria used for diagnosis should be included in the presentation. A table should be included that presents the values for these criteria satisfying the diagnosis as well as parity, gestational age at delivery and birth weight centile of offspring. The table should also include data indicating the severity of the preeclampsia (platelet count, liver function and evidence of hemolysis). Sufficient data should be presented about the normal controls to guarantee to the reader that they are normal.
Placental samples should ideally be obtained from pregnancies that have not labored, especially when oxidative stress or hypoxia markers are part of the research. Failing this, information about labor or not laboring before the time of collection should be presented. In identifying controls for placentas from preeclamptic pregnancies delivered preterm, it should be recognized that placentas collected from women delivering preterm are not “normal” placentas. In such studies term control placentas should also be compared to preterm pregnancies. Investigators should be alert for the rare placenta collected preterm from pregnancies without labor or other disease. The samples from preterm labor patients should be carefully defined based on the clinical presentation and pathological evidence of infection or lack of infection.
Currently there are several quantitative morphological studies in which the same samples have been compared in several different ways. If samples are used for several investigations in several publications this information should be presented. This is important to keep findings in different studies in perspective but also to allow the combination of extensive findings in an individual subject. Fetal growth restriction is potentially a valuable control for preeclampsia. Simplistically, findings present in preeclampsia but not FGR may provide insight into the maternal syndrome. As stated, to date it has not been possible to ascertain due to the overlap in the two conditions. Whether this difficulty can be overcome is difficult to determine but it is worth attempting to carefully characterize FGR to aid in this attempt. Maternal BMI should be presented as a crude evidence of nutrient sufficiency. The results of umbilical, and if available, uterine artery Doppler velocimetry should be presented and if not available should be so stated. To increase the likelihood of SGA indicating FGR, birth weight centiles of less than at most the 5th centile should be considered as diagnostic. However, whatever the cutoff, mean birth weight centile in the SGA group should be presented rather than or in addition to “less than nth centile”.
We also recommend continuing with the analysis of the biological activity of the products released by the placenta, which can be a clue to understanding placental response or adaptation. It will also be extremely beneficial to apply state-of-the-art technology in placental analyses such as “omic” and quantitative morphological assessments to the study of the placenta. However, such studies should include individuals with expertise in the relevant clinical areas (e.g. preeclampsia, FGR etc.). Sophisticated analytical strategies and expertise in these approaches cannot substitute for expertise relating to the disorders being studied.
Attention to these considerations we believe will provide the optimum opportunity to fully utilize the power of placental analysis to unravel these (and the other) “great obstetric syndromes”.[110]
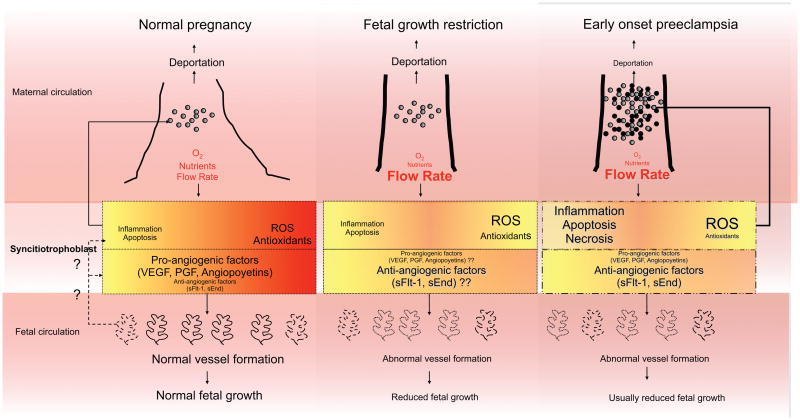
Figure 1. A Schematic Representation of Human Placentation.
Based on the available data it appears that the abnormal modification of the maternal vessels supplying the intervillus space is the same in preeclampsia and fetal growth restriction (FGR) without preeclampsia. The placental changes are similar in early onset preeclampsia and FGR (particularly when early onset preeclampsia is accompanied by FGR). The consequences of the reduced nutrient and oxygen delivery and the increased flow of blood delivered to the intervillus space are similar in FGR and preeclampsia but not identical. In FGR, placental inflammation is increased, as is apoptosis. These changes are also present in preeclampsia but may be more severe and are also accompanied by necrosis that is not as prevalent in FGR. Oxidative stress, as indicated by increased reactive oxygen species (ROS) and reduced antioxidants is present in the periphery of the placenta where maternal blood supply is reduced compared with the central region, in either normal or pathological conditions. The degree of oxidative stress is more in FGR than normal pregnancy and even greater in preeclampsia. Data on the response to reduced oxygen in FGR is not consistent (indicated by ??). Hypoxia and the response to hypoxia may be less in FGR than preeclampsia. In response to apoptosis, increased pressure and necrosis, fragments of syncitiotrophoblast are released into the maternal circulation, which are both increased in quantity, and qualitatively different in preeclampsia (necrotic particles indicated by black circles are greater with preeclampsia) compared with normal pregnancy or FGR. It is postulated (?? and dotted line) that apoptosis, or inflammatory and proangiogenic factors from syncitiotrophoblast in normal and pathological conditions regulate placental vascularization. In both FGR and preeclampsia the fetal vasculature responds to reduce the villous surface area. Whether this is quantitatively and qualitatively different in the two conditions is not established, but only in preeclampsia has spotty placental vascular development resulting in an abnormally shaped placenta been reported. The final fetal result is by definition reduced fetal growth in FGR but this is not universally present even with early onset preeclampsia.
Acknowledgments
This research was supported by NIH P01 HD30367, FONDECYT 1100684 and CONICYT Anillo ACT73, Chile
Role of the funding sources
The funding sources referenced above had no involvement in the writing or preparation of this manuscript or in the decision to submit for publication.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Lain KY, Roberts JM. Contemporary concepts of the pathogenesis and management of preeclampsia.[comment] Journal of the American Medical Association. 2002;287(24):3183–6. doi: 10.1001/jama.287.24.3183. [DOI] [PubMed] [Google Scholar]
- 2.Roberts JM, Gammill HS. Preeclampsia: recent insights. Hypertension. 2005 Dec;46(6):1243–9. doi: 10.1161/01.HYP.0000188408.49896.c5. [DOI] [PubMed] [Google Scholar]
- 3.Roberts J. Pregnancy related hypertension. In: Creasy R, Resnik R, Iams JD, editors. Maternal-Fetal Medicine: Principles and Practice. 6. Philadelphia: Saunders Elsevier; 2009. pp. 650–88. [Google Scholar]
- 4.Brosens IA, Robertson WB, Dixon HG. The role of the spiral arteries in the pathogenesis of preeclampsia. In: Wynn R, editor. Obstetrics and Gynecology Annual. 1979. pp. 177–91. [PubMed] [Google Scholar]
- 5.Granger J, Alexander B, Abram S, Reckelhoff J, Wilson J, Rinewalt N. Chronic reductions in uterine perfusion pressure in the pregnant rat produces hypertension and reduces pressure- natriuresis. Hypertension. 2001 Mar;37(3):1013. [Google Scholar]
- 6.Podjarny E, Baylis C, Losonczy G. Animal models of preeclampsia. Seminars in Perinatology. 1999 Feb;23(1):2–13. doi: 10.1016/s0146-0005(99)80055-x. [DOI] [PubMed] [Google Scholar]
- 7.Roberts J. Pre-eclampsia a two-stage disorder: what is the linkage? Are there directed fetal/placental signals? In: FL, MB, editors. Pre-eclampsia: Etiology and Clinical Practice. New York: Cambridge University Press; 2007. pp. 183–94. [Google Scholar]
- 8.Burton GJ, Jauniaux E, Charnock-Jones DS. The influence of the intrauterine environment on human placental development. [Review] [77 refs] International Journal of Developmental Biology. 2010;54(2–3):303–12. doi: 10.1387/ijdb.082764gb. [DOI] [PubMed] [Google Scholar]
- 9.Zhou Y, Genbacev O, Damsky CH, Fisher SJ. Oxygen regulates human cytotrophoblast differentiation and invasion - implications for endovascular invasion in normal pregnancy and in pre-eclampsia. Journal of Reproductive Immunology. 1998;39(1–2):197–213. doi: 10.1016/s0165-0378(98)00022-9. [DOI] [PubMed] [Google Scholar]
- 10.Pijnenborg R, Vercruysse L, Hanssens A. The uterine spiral arteries in human pregnancy: Facts and controversies. Placenta. 2006 Sep-Oct;27(9–10):939–58. doi: 10.1016/j.placenta.2005.12.006. [DOI] [PubMed] [Google Scholar]
- 11.Lyall F. The human placental bed revisited. Placenta. 2002;23(8–9):555–62. doi: 10.1053/plac.2002.0850. [DOI] [PubMed] [Google Scholar]
- 12.Khong TY, De Wolf F, Robertson WB, Brosens I. Inadequate maternal vascular response to placentation in pregnancies complicated by pre-eclampsia and by small-for-gestational age infants. British Journal of Obstetrics & Gynaecology. 1986 Oct;93(10):1049–59. doi: 10.1111/j.1471-0528.1986.tb07830.x. [DOI] [PubMed] [Google Scholar]
- 13.Robertson WB, Brosens IA, Dixon HG. Placental bed vessels. American Journal of Obstetrics and Gynecology. 1973;117(2):294–5. doi: 10.1016/0002-9378(73)90655-8. [DOI] [PubMed] [Google Scholar]
- 14.Brosens IA, Robertson WB, Dixon HG. The role of the spiral arteries in the pathogenesis of pre-eclampsia. Journal of Pathology. 1970 Aug;101(4):Pvi. [PubMed] [Google Scholar]
- 15.Robertson WB, Brosens I, Dixon HG. The pathological response of the vessels of the placental bed to hypertensive pregnancy. Journal of Pathology & Bacteriology. 1967 Apr;93(2):581–92. doi: 10.1002/path.1700930219. [DOI] [PubMed] [Google Scholar]
- 16.Burton GJ, Woods AW, Jauniaux E, Kingdom JCP. Rheological and physiological consequences of conversion of the maternal spiral arteries for uteroplacental blood flow during human pregnancy. Placenta. 2009 Jun;30(6):473–82. doi: 10.1016/j.placenta.2009.02.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Arias F, Rodriquez L, Rayne SC, Kraus FT. Maternal placental vasculopathy and infection: two distinct subgroups among patients with preterm labor and preterm ruptured membranes. Am J Obstet Gynecol. 1993 Feb;168(2):585–91. doi: 10.1016/0002-9378(93)90499-9. [DOI] [PubMed] [Google Scholar]
- 18.Meekins JW, Pijnenborg R, Hanssens M, McFadyen IR, Van Asshe A. A study of placental bed spiral arteries and trophoblast invastion in normal and severe pre-eclamptic pregnancies. Br J Obstet Gynaecol. 1994:669–74. doi: 10.1111/j.1471-0528.1994.tb13182.x. [DOI] [PubMed] [Google Scholar]
- 19.Kitzmiller JL, Watt N, Driscoll SG. Decidual arteriopathy in hypertension and diabetes in pregnancy: immunofluorescent studies. American Journal of Obstetrics & Gynecology. 1981;141(7):773–9. doi: 10.1016/0002-9378(81)90703-1. [DOI] [PubMed] [Google Scholar]
- 20.Zhang P, Schmidt M, Cook L. Maternal vasculopathy and histologic diagnosis of preeclampsia: poor correlation of histologic changes and clinical manifestation. American Journal of Obstetrics & Gynecology. 2006 Apr;194(4):1050–6. doi: 10.1016/j.ajog.2005.10.196. [DOI] [PubMed] [Google Scholar]
- 21.Roberts DJ, Post MD. The placenta in pre-eclampsia and intrauterine growth restriction. [Review] [65 refs] Journal of Clinical Pathology. 2008;61(12):1254–60. doi: 10.1136/jcp.2008.055236. [DOI] [PubMed] [Google Scholar]
- 22.Dahlstrom B, Romundstad P, Oian P, Vatten LJ, Eskild A. Placenta weight in pre-eclampsia. Acta Obstetricia et Gynecologica Scandinavica. 2008;87(6):608–11. doi: 10.1080/00016340802056178. [DOI] [PubMed] [Google Scholar]
- 23.Kajantie E, Thornburg KL, Eriksson JG, Osmond C, Barker DJ. In preeclampsia, the placenta grows slowly along its minor axis. International Journal of Developmental Biology. 2010;54(2–3):469–73. doi: 10.1387/ijdb.082833ek. [DOI] [PubMed] [Google Scholar]
- 24.Heazell AEP, Moll SJ, Jones CJP, Baker PN, Crocker IP. Formation of syncytial knots is increased by hyperoxia, hypoxia and reactive oxygen species. Placenta. 2007 Apr;28:S33–S40. doi: 10.1016/j.placenta.2006.10.007. [DOI] [PubMed] [Google Scholar]
- 25.Kingdom J, Huppertz B, Seaward G, Kaufmann P. Development of the placental villous tree and its consequences for fetal growth. [Review] [89 refs] European Journal of Obstetrics, Gynecology, & Reproductive Biology. 2000;92(1):35–43. doi: 10.1016/s0301-2115(00)00423-1. [DOI] [PubMed] [Google Scholar]
- 26.Kingdom JC, Kaufmann P. Oxygen and placental villous development: origins of fetal hypoxia. [Review] [94 refs] Placenta. 1997;18(8):613–21. doi: 10.1016/s0143-4004(97)90000-x. [DOI] [PubMed] [Google Scholar]
- 27.Mayhew TM. A stereological perspective on placental morphology in normal and complicated pregnancies. [Review] [97 refs] Journal of Anatomy. 2009;215(1):77–90. doi: 10.1111/j.1469-7580.2008.00994.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Daayana S, Baker P, Crocker I. An image analysis technique for the investigation of variations in placental morphology in pregnancies complicated by preeclampsia with and without intrauterine growth restriction. Journal of the Society for Gynecologic Investigation. 2004;11(8):545–52. doi: 10.1016/j.jsgi.2004.06.009. [DOI] [PubMed] [Google Scholar]
- 29.Mayhew TM, Manwani R, Ohadike C, Wijesekara J, Baker PN. The placenta in pre-eclampsia and intrauterine growth restriction: studies on exchange surface areas, diffusion distances and villous membrane diffusive conductances. Placenta. 2007 Feb-Mar;28(2–3):233–8. doi: 10.1016/j.placenta.2006.02.011. [DOI] [PubMed] [Google Scholar]
- 30.Mayhew TM, Ohadike C, Baker PN, Crocker IP, Mitchell C, Ong SS. Stereological investigation of placental morphology in pregnancies complicated by pre-eclampsia with and without intrauterine growth restriction. Placenta. 2003 Feb-Mar;24(2–3):219–26. doi: 10.1053/plac.2002.0900. [DOI] [PubMed] [Google Scholar]
- 31.Egbor M, Ansari T, Morris N, Green CJ, Sibbons PD. Pre-eclampsia and fetal growth restriction: how morphometrically different is the placenta? Placenta. 2006 Jun-Jul;27(6–7):727–34. doi: 10.1016/j.placenta.2005.06.002. [DOI] [PubMed] [Google Scholar]
- 32.Egbor M, Ansari T, Morris N, Green CJ, Sibbons PD. Morphometric placental villous and vascular abnormalities in early- and late-onset pre-eclampsia with and without fetal growth restriction. BJOG: An International Journal of Obstetrics & Gynaecology. 2006 May;113(5):580–9. doi: 10.1111/j.1471-0528.2006.00882.x. [DOI] [PubMed] [Google Scholar]
- 33.Takagi Y, Nikaido T, Toki T, Kita N, Kanai M, Ashida T, et al. Levels of oxidative stress and redox-related molecules in the placenta in preeclampsia and fetal growth restriction. Virchows Arch. 2004 Jan;444(1):49–55. doi: 10.1007/s00428-003-0903-2. [DOI] [PubMed] [Google Scholar]
- 34.Myatt L, Cui XL. Oxidative stress in the placenta. Histochemistry and Cell Biology. 2004 Oct;122(4):369–82. doi: 10.1007/s00418-004-0677-x. [DOI] [PubMed] [Google Scholar]
- 35.Hnat MD, Meadows JW, Brockman DE, Pitzer B, Lyall F, Myatt L. Heat shock protein-70 and 4-hydroxy-2-nonenal adducts in human placental villous tissue of normotensive, preeclamptic and intrauterine growth restricted pregnancies. American Journal of Obstetrics & Gynecology. 2005 Sep;193(3 Pt 1):836–40. doi: 10.1016/j.ajog.2005.01.059. [DOI] [PubMed] [Google Scholar]
- 36.Burton GJ, Yung HW, Cindrova-Davies T, Charnock-Jones DS. Placental Endoplasmic Reticulum Stress and Oxidative Stress in the Pathophysiology of Unexplained Intrauterine Growth Restriction and Early Onset Preeclampsia. Placenta. 2009 Mar;30:S43–S8. doi: 10.1016/j.placenta.2008.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Stepan H, Heihoff-Klose A, Faber R. Reduced antioxidant capacity in second-trimester pregnancies with pathological uterine perfusion. Ultrasound Obstet Gynecol. 2004 Jun;23(6):579–83. doi: 10.1002/uog.1045. [DOI] [PubMed] [Google Scholar]
- 38.Romero R, Gotsch F, Pineles B, Kusanovic JP. Inflammation in pregnancy: Its roles in reproductive physiology, obstetrical complications, and fetal injury. Nutrition Reviews. 2007 Dec;65(12):S194–S202. doi: 10.1111/j.1753-4887.2007.tb00362.x. [DOI] [PubMed] [Google Scholar]
- 39.Sargent IL, Germain SJ, Sacks GP, Kumar S, Redman CW. Trophoblast deportation and the maternal inflammatory response in pre-eclampsia. J Reprod Immunol. 2003 Aug;59(2):153–60. doi: 10.1016/s0165-0378(03)00044-5. [DOI] [PubMed] [Google Scholar]
- 40.Roberts JM, Lain KY. Recent Insights into the pathogenesis of pre-eclampsia. Placenta. 2002;23(5):359–72. doi: 10.1053/plac.2002.0819. [DOI] [PubMed] [Google Scholar]
- 41.Redman CW, Sacks GP, Sargent IL. Preeclampsia: an excessive maternal inflammatory response to pregnancy. American Journal of Obstetrics & Gynecology. 1999;180(2 Pt 1):499–506. doi: 10.1016/s0002-9378(99)70239-5. [DOI] [PubMed] [Google Scholar]
- 42.Redman CWG, Sargent IL. Placental Stress and Pre-eclampsia: A Revised View. Placenta. 2009 Mar;30:S38–S42. doi: 10.1016/j.placenta.2008.11.021. [DOI] [PubMed] [Google Scholar]
- 43.Gandley RE, Rohland J, Zhou Y, Shibata E, Harger GF, Rajakumar A, et al. Increased myeloperoxidase in the placenta and circulation of women with preeclampsia. Hypertension. 2008 Aug;52(2):387–93. doi: 10.1161/HYPERTENSIONAHA.107.107532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Germain SJ, Sacks GP, Sooranna SR, Sargent IL, Redman CW. Systemic inflammatory priming in normal pregnancy and preeclampsia: the role of circulating syncytiotrophoblast microparticles. Journal of immunology. 2007 May 1;178(9):5949–56. doi: 10.4049/jimmunol.178.9.5949. [DOI] [PubMed] [Google Scholar]
- 45.Georgiou HM, Thio YS, Russell C, Permezel M, Heng YJ, Lee S, et al. Association between maternal serum cytokine profiles at 7–10 weeks’ gestation and birthweight in small for gestational age infants. American Journal of Obstetrics and Gynecology. 2011 May;204(5) doi: 10.1016/j.ajog.2010.12.005. [DOI] [PubMed] [Google Scholar]
- 46.Boutsikou T, Mastorakos G, Kyriakakou M, Margeli A, Hassiakos D, Papassotiriou I, et al. Circulating Levels of Inflammatory Markers in Intrauterine Growth Restriction. Mediators of Inflammation. 2010 doi: 10.1155/2010/790605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Rajakumar A, Whitelock KA, Weissfeld LA, Daftary AR, Markovic N, Conrad KP. Selective overexpression of the hypoxia-inducible transcription factor, HIF-2alpha, in placentas from women with preeclampsia. Biology of Reproduction. 2001;64(2):499–506. doi: 10.1093/biolreprod/64.2.499. [DOI] [PubMed] [Google Scholar]
- 48.Rajakumar A, Jeyabalan A, Markovic N, Ness R, Gilmour C, Conrad KP. Placental HIF-1 alpha, HIF-2 alpha, membrane and soluble VEGF receptor-1 proteins are not increased in normotensive pregnancies complicated by late-onset intrauterine growth restriction. American Journal of Physiology - Regulatory Integrative & Comparative Physiology. 2007 Aug;293(2):R766–74. doi: 10.1152/ajpregu.00097.2007. [DOI] [PubMed] [Google Scholar]
- 49.Vaiman D, Mondon F, Garces-Duran A, Mignot T-M, Robert B, Rebourcet R, et al. Hypoxia-activated genes from early placenta are elevated in preeclampsia, but not in Intra-Uterine Growth Retardation. BMC Genomics. 2005;6:111. doi: 10.1186/1471-2164-6-111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Dai S-Y, Kanenishi K, Ueno M, Sakamoto H, Hata T. Hypoxia-inducible factor-2alpha is involved in enhanced apoptosis in the placenta from pregnancies with fetal growth restriction. Pathology International. 2004 Nov;54(11):843–9. doi: 10.1111/j.1440-1827.2004.01750.x. [DOI] [PubMed] [Google Scholar]
- 51.Levine RJ, Maynard SE, Qian C, Lim KH, England LJ, Yu KF, et al. Circulating angiogenic factors and the risk of preeclampsia.[see comment] New England Journal of Medicine. 2004 Feb 12;350(7):672–83. doi: 10.1056/NEJMoa031884. [DOI] [PubMed] [Google Scholar]
- 52.Powers RW, Roberts JM, Cooper KM, Gallaher MJ, Frank MP, Harger GF, et al. Maternal serum soluble fms-like tyrosine kinase 1 concentrations are not increased in early pregnancy and decrease more slowly postpartum in women who develop preeclampsia. American Journal of Obstetrics & Gynecology. 2005 Jul;193(1):185–91. doi: 10.1016/j.ajog.2004.11.038. [DOI] [PubMed] [Google Scholar]
- 53.Wallner W, Sengenberger R, Strick R, Strissel PL, Meurer B, Beckmann MW, et al. Angiogenic growth factors in maternal and fetal serum in pregnancies complicated by intrauterine growth restriction. Clinical Science. 2007 Jan;112(1):51–7. doi: 10.1042/CS20060161. [DOI] [PubMed] [Google Scholar]
- 54.Asvold BO, Vatten LJ, Romundstad PR, Jenum PA, Karumanchi SA, Eskild A. Angiogenic Factors in Maternal Circulation and the Risk of Severe Fetal Growth Restriction. American Journal of Epidemiology. 2011 Mar;173(6):630–9. doi: 10.1093/aje/kwq373. [DOI] [PubMed] [Google Scholar]
- 55.Shibata E, Rajakumar A, Powers RW, Larkin RW, Gilmour C, Bodnar LM, et al. Soluble fms-like tyrosine kinase 1 is increased in preeclampsia but not in normotensive pregnancies with small-for-gestational-age neonates: relationship to circulating placental growth factor. Journal of Clinical Endocrinology & Metabolism. 2005 Aug;90(8):4895–903. doi: 10.1210/jc.2004-1955. [DOI] [PubMed] [Google Scholar]
- 56.Schlembach D, Wallner W, Sengenberger R, Stiegler E, Mortl M, Beckmann MW, et al. Angiogenic growth factor levels in maternal and fetal blood: correlation with Doppler ultrasound parameters in pregnancies complicated by pre-eclampsia and intrauterine growth restriction. Ultrasound Obstet Gynecol. 2007 Apr;29(4):407–13. doi: 10.1002/uog.3930. [DOI] [PubMed] [Google Scholar]
- 57.Nevo O, Many A, Xu J, Kingdom J, Piccoli E, Zamudio S, et al. Placental expression of soluble fms-like tyrosine kinase 1 is increased in singletons and twin pregnancies with intrauterine growth restriction. Journal of Clinical Endocrinology & Metabolism. 2008 Jan;93(1):285–92. doi: 10.1210/jc.2007-1042. [DOI] [PubMed] [Google Scholar]
- 58.Crispi F, Dominguez C, Llurba E, Martin-Gallan P, Cabero L, Gratacos E. Placental angiogenic growth factors and uterine artery Doppler findings for characterization of different subsets in preeclampsia and in isolated intrauterine growth restriction. 2006. [DOI] [PubMed] [Google Scholar]
- 59.Smarason AK, Sargent IL, Starkey PM, Redman CW. The effect of placental syncytiotrophoblast microvillous membranes from normal and pre-eclamptic women on the growth of endothelial cells in vitro. Br J Obstet Gynaecol. 1993 Oct;100(10):943–9. doi: 10.1111/j.1471-0528.1993.tb15114.x. [DOI] [PubMed] [Google Scholar]
- 60.Goswami D, Tannetta DS, Magee LA, Fuchisawa A, Redman CWG, Sargent IL, et al. Excess syncytiotrophoblast microparticle shedding is a feature of early-onset pre-eclampsia, but not normotensive intrauterine growth restriction. Placenta. 2006 Jan;27(1):56–61. doi: 10.1016/j.placenta.2004.11.007. [DOI] [PubMed] [Google Scholar]
- 61.Lok CAR, Van Der Post JAM, Sargent IL, Hau CM, Sturk A, Boer K, et al. Changes in microparticle numbers and cellular origin during pregnancy and preeclampsia. Hypertension in Pregnancy. 2008;27(4):344–60. doi: 10.1080/10641950801955733. [DOI] [PubMed] [Google Scholar]
- 62.van der Post JA, Lok CA, Boer K, Sturk A, Sargent IL, Nieuwland R. The functions of microparticles in pre-eclampsia. Seminars in thrombosis and hemostasis. 2011 Mar;37(2):146–52. doi: 10.1055/s-0030-1270342. [DOI] [PubMed] [Google Scholar]
- 63.Meziani F, Tesse A, David E, Martinez MC, Wangesteen R, Schneider F, et al. Shed membrane particles from preeclamptic women generate vascular wall inflammation and blunt vascular contractility. American Journal Of Pathology. 2006 Oct;169(4):1473–83. doi: 10.2353/ajpath.2006.051304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Sekizawa A, Farina A, Sugito Y, Matsuoka R, Iwasaki M, Saito H, et al. Proteinuria and hypertension are independent factors affecting fetal DNA values: a retrospective analysis of affected and unaffected patients. Clin Chem. 2004 Jan;50(1):221–4. doi: 10.1373/clinchem.2003.023259. [DOI] [PubMed] [Google Scholar]
- 65.Alberry MS, Maddocks DG, Hadi MA, Metawi H, Hunt LP, Abdel-Fattah SA, et al. Quantification of cell free fetal DNA in maternal plasma in normal pregnancies and in pregnancies with placental dysfunction. Am J Obstet Gynecol. 2009 Jan;200(1):98, e1–6. doi: 10.1016/j.ajog.2008.07.063. [DOI] [PubMed] [Google Scholar]
- 66.Al Nakib M, Desbriere R, Bonello N, Bretelle F, Boubli L, Gabert J, et al. Total and Fetal Cell-Free DNA Analysis in Maternal Blood as Markers of Placental Insufficiency in Intrauterine Growth Restriction. Fetal Diagnosis and Therapy. 2009;26(1):24–8. doi: 10.1159/000236355. [DOI] [PubMed] [Google Scholar]
- 67.Sekizawa A, Jimbo M, Saito H, Iwasaki M, Matsuoka R, Okai T, et al. Cell-free fetal DNA in the plasma of pregnant women with severe fetal growth restriction. American Journal of Obstetrics and Gynecology. 2003 Feb;188(2):480–4. doi: 10.1067/mob.2003.27. [DOI] [PubMed] [Google Scholar]
- 68.Zhong XY, Laivuori H, Livingston JC, Ylikorkala O, Sibai BM, Holzgreve W, et al. Elevation of both maternal and fetal extracellular circulating deoxyribonucleic acid concentrations in the plasma of pregnant women with preeclampsia. American Journal of Obstetrics and Gynecology. 2001 Feb;184(3):414–9. doi: 10.1067/mob.2001.109594. [DOI] [PubMed] [Google Scholar]
- 69.Volonte C, D’Ambrosi N. Membrane compartments and purinergic signalling: the purinome, a complex interplay among ligands, degrading enzymes, receptors and transporters. Febs J. 2009 Jan;276(2):318–29. doi: 10.1111/j.1742-4658.2008.06793.x. [DOI] [PubMed] [Google Scholar]
- 70.Malek A, Miller RK, Mattison DR, Ceckler T, Panigel M, di Sant’Agnese PA, et al. Continuous measurement of ATP by 31P-NMR in term human dually perfused placenta in vitro: response to ischemia. Journal of Applied Physiology. 1995 May;78(5):1778–86. doi: 10.1152/jappl.1995.78.5.1778. [DOI] [PubMed] [Google Scholar]
- 71.Yoneyama Y, Suzuki S, Sawa R, Yoneyama K, Power GG, Araki T. Increased plasma adenosine concentrations and the severity of preeclampsia. Obstet Gynecol. 2002 Dec;100(6):1266–70. doi: 10.1016/s0029-7844(02)02247-0. [DOI] [PubMed] [Google Scholar]
- 72.Escudero C, Casanello P, Sobrevia L. Human equilibrative nucleoside transporters 1 and 2 may be differentially modulated by A2B adenosine receptors in placenta microvascular endothelial cells from pre-eclampsia. Placenta. 2008 Sep;29(9):816–25. doi: 10.1016/j.placenta.2008.06.014. [DOI] [PubMed] [Google Scholar]
- 73.von Versen-Hoynck F, Rajakumar A, Bainbridge SA, Gallaher MJ, Roberts JM, Powers RW. Human placental adenosine receptor expression is elevated in preeclampsia and hypoxia increases expression of the A2A receptor. Placenta. 2009 May;30(5):434–42. doi: 10.1016/j.placenta.2009.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Huppertz B, Kingdom J, Caniggia I, Desoye G, Black S, Korr H, et al. Hypoxia favours necrotic versus apoptotic shedding of placental syncytiotrophoblast into the maternal circulation. Placenta. 2003 Feb-Mar;24(2–3):181–90. doi: 10.1053/plac.2002.0903. [DOI] [PubMed] [Google Scholar]
- 75.Burton GJ, Jones CJP. Syncytial Knots, Sprouts, Apoptosis, and Trophoblast Deportation from the Human Placenta. Taiwanese Journal of Obstetrics & Gynecology. 2009 Mar;48(1):28–37. doi: 10.1016/S1028-4559(09)60032-2. [DOI] [PubMed] [Google Scholar]
- 76.Mendilcioglu I, Karaveli S, Erdogan G, Simsek M, Taskin O. Apoptosis and expression of Bcl-2, Bax, p53, caspase-3, and Fas, Fas ligand in placentas complicated by preeclampsia. Clinical and Experimental Obstetrics & Gynecology. 2011;38(1):38–42. [PubMed] [Google Scholar]
- 77.Heazell AEP, Sharp AN, Baker PN, Crocker IP. Intra-uterine growth restriction is associated with increased apoptosis and altered expression of proteins in the p53 pathway in villous trophoblast. Apoptosis. 2011 Feb;16(2):135–44. doi: 10.1007/s10495-010-0551-3. [DOI] [PubMed] [Google Scholar]
- 78.Huppertz B. Placental origins of preeclampsia: challenging the current hypothesis. Hypertension. 2008 Apr;51(4):970–5. doi: 10.1161/HYPERTENSIONAHA.107.107607. [DOI] [PubMed] [Google Scholar]
- 79.Chen Q, Ding JX, Liu B, Stone P, Feng YJ, Chamley L. Spreading endothelial cell dysfunction in response to necrotic trophoblasts. Soluble factors released from endothelial cells that have phagocytosed necrotic shed trophoblasts reduce the proliferation of additional endothelial cells. Placenta. 2010 Nov;31(11):976–81. doi: 10.1016/j.placenta.2010.08.013. [DOI] [PubMed] [Google Scholar]
- 80.Chen Q, Chen L, Liu B, Vialli C, Stone P, Ching L-M, et al. The role of autocrine TGFbeta1 in endothelial cell activation induced by phagocytosis of necrotic trophoblasts: a possible role in the pathogenesis of pre-eclampsia. J Pathol. 2010;221(1):87–95. doi: 10.1002/path.2690. [DOI] [PubMed] [Google Scholar]
- 81.Kang JH, Song H, Yoon JA, Park DY, Kim SH, Lee KJ, et al. Preeclampsia leads to dysregulation of various signaling pathways in placenta. Journal of Hypertension. 2011;29(5):928–36. doi: 10.1097/HJH.0b013e328344a82c. [DOI] [PubMed] [Google Scholar]
- 82.Lee GSR, Joe YS, Kim SJ, Shin JC. Cytokine-related genes and oxidation-related genes detected in preeclamptic placentas. Archives of Gynecology & Obstetrics. 2010;282(4):363–9. doi: 10.1007/s00404-009-1222-x. [DOI] [PubMed] [Google Scholar]
- 83.Sitras V, Paulssen RH, Gronaas H, Leirvik J, Hanssen TA, Vartun A, et al. Differential placental gene expression in severe preeclampsia. Placenta. 2009;30(5):424–33. doi: 10.1016/j.placenta.2009.01.012. [DOI] [PubMed] [Google Scholar]
- 84.Enquobahrie DA, Meller M, Rice K, Psaty BM, Siscovick DS, Williams MA. Differential placental gene expression in preeclampsia. American Journal of Obstetrics & Gynecology. 2008 Nov;199(5):566.e1–11. doi: 10.1016/j.ajog.2008.04.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Nishizawa H, Pryor-Koishi K, Kato T, Kowa H, Kurahashi H, Udagawa Y. Microarray analysis of differentially expressed fetal genes in placental tissue derived from early and late onset severe pre-eclampsia. Placenta. 2006;28(5–6):487–97. doi: 10.1016/j.placenta.2006.05.010. [DOI] [PubMed] [Google Scholar]
- 86.Weinstein JN. ‘Omic’ and hypothesis-driven research in the molecular pharmacology of cancer. Current opinion in pharmacology. 2002 Aug;2(4):361–5. doi: 10.1016/s1471-4892(02)00185-6. [DOI] [PubMed] [Google Scholar]
- 87.Toft JH, Lian IA, Tarca AL, Erez O, Espinoza J, Eide IP, et al. Whole-genome microarray and targeted analysis of angiogenesis-regulating gene expression (ENG, FLT1, VEGF, PlGF) in placentas from pre-eclamptic and small-for-gestational-age pregnancies. J Matern Fetal Neona. 2008 Apr;21(4):267–73. doi: 10.1080/14767050801924118. [DOI] [PubMed] [Google Scholar]
- 88.Founds SA, Conley YP, Lyons-Weiler JF, Jeyabalan A, Hogge WA, Conrad KR. Altered Global Gene Expression in First Trimester Placentas of Women Destined to Develop Preeclampsia. Placenta. 2009 Jan;30(1):15–24. doi: 10.1016/j.placenta.2008.09.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Founds SA, Conley YP, Lyons-Weiler JF, Jeyabalan A, Hogge WA, Conrad KP. Altered global gene expression in first trimester placentas of women destined to develop preeclampsia. Placenta. 2009 Jan;30(1):15–24. doi: 10.1016/j.placenta.2008.09.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Apps R, Sharkey A, Gardner L, Male V, Trotter M, Miller N, et al. Genome-wide expression profile of first trimester villous and extravillous human trophoblast cells. Placenta. 2011 Jan;32(1):33–43. doi: 10.1016/j.placenta.2010.10.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Farina A, Zucchini C, De Sanctis P, Morano D, Sekizawa A, Purwosunu Y, et al. Gene expression in chorionic villous samples at 11 weeks of gestation in women who develop pre-eclampsia later in pregnancy: implications for screening. Prenatal Diagnosis. 2011 Feb;31(2):181–5. doi: 10.1002/pd.2675. [DOI] [PubMed] [Google Scholar]
- 92.Shin JK, Baek JC, Kang MY, Park JK, Lee SA, Lee JH, et al. Proteomic analysis reveals an elevated expression of heat shock protein 27 in preeclamptic placentas. Gynecologic and obstetric investigation. 2011;71(3):151–7. doi: 10.1159/000315162. [DOI] [PubMed] [Google Scholar]
- 93.Gharesi-Fard B, Zolghadri J, Kamali-Sarvestani E. Proteome differences of placenta between pre-eclampsia and normal pregnancy. Placenta. 2010 Feb;31(2):121–5. doi: 10.1016/j.placenta.2009.11.004. [DOI] [PubMed] [Google Scholar]
- 94.Dexlin-Mellby L, Sandstrom A, Centlow M, Nygren S, Hansson SR, Borrebaeck CA, et al. Tissue proteome profiling of preeclamptic placenta using recombinant antibody microarrays. Proteomics Clin Appl. 2010 Nov;4(10–11):794–807. doi: 10.1002/prca.201000001. [DOI] [PubMed] [Google Scholar]
- 95.Johnstone ED, Sawicki G, Guilbert L, Winkler-Lowen B, Cadete VJ, Morrish DW. Differential proteomic analysis of highly purified placental cytotrophoblasts in preeclampsia demonstrates a state of increased oxidative stress and reduced cytotrophoblast antioxidant defense. Proteomics. 2011 Jul 28; doi: 10.1002/pmic.201000505. [DOI] [PubMed] [Google Scholar]
- 96.Buhimschi IA, Zhao G, Funai EF, Harris N, Sasson IE, Bernstein IM, et al. Proteomic profiling of urine identifies specific fragments of SERPINA1 and albumin as biomarkers of preeclampsia. Am J Obstet Gynecol. 2008 Nov;199(5):551, e1–16. doi: 10.1016/j.ajog.2008.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Roberts JM, Hubel CA. The Two Stage Model of Preeclampsia: Variations on the Theme. Placenta. 2009 Mar;30:S32–S7. doi: 10.1016/j.placenta.2008.11.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.von Dadelszen P, Magee LA, Roberts JM. Subclassification of preeclampsia. Hypertension in Pregnancy. 2003;22(2):143–8. doi: 10.1081/PRG-120021060. [DOI] [PubMed] [Google Scholar]
- 99.Moldenhauer JS, Stanek J, Warshak C, Khoury J, Sibai B. The frequency and severity of placental findings in women with preeclampsia are gestational age dependent. American Journal of Obstetrics & Gynecology. 2003 Oct;189(4):1173–7. doi: 10.1067/s0002-9378(03)00576-3. [DOI] [PubMed] [Google Scholar]
- 100.Meekins JW, Pijnenborg R, Hanssens M, McFadyen IR, Van Assche FA. Immunohistochemical Identification of Placental Bed Biopsies and the Implications for the Inclusion of Specimens When Studying the Spiral Artery Response to Pregnancy. Hypertension in Pregnancy. 1994:61–70. [Google Scholar]
- 101.Guzin K, Tomruk S, Tuncay YA, Naki M, Sezginsoy S, Zemheri E, et al. The relation of increased uterine artery blood flow resistance and impaired trophoblast invasion in pre-eclamptic pregnancies. Archives of Gynecology & Obstetrics. 2005 Oct;272(4):283–8. doi: 10.1007/s00404-005-0005-2. [DOI] [PubMed] [Google Scholar]
- 102.Gerretsen G, Huisjes HJ, Elema JD. Morphological changes of the spiral arteries in the placental bed in relation to pre-eclampsia and fetal growth retardation. British Journal of Obstetrics & Gynaecology. 1981;88(9):876–81. doi: 10.1111/j.1471-0528.1981.tb02222.x. [DOI] [PubMed] [Google Scholar]
- 103.Sheppard BL, Bonnar J. An ultrastructural study of utero-placental spiral arteries in hypertensive and normotensive pregnancy and fetal growth retardation. Br J Obstet Gynaecol. 1981;88:695–705. doi: 10.1111/j.1471-0528.1981.tb01268.x. [DOI] [PubMed] [Google Scholar]
- 104.Hanssens M, Pijnenborg R, Keirse MJ, Vercruysse L, Verbist L, Van Assche FA. Renin-like immunoreactivity in uterus and placenta from normotensive and hypertensive pregnancies. European Journal of Obstetrics, Gynecology, & Reproductive Biology. 1998 Dec;81(2):177–84. doi: 10.1016/s0301-2115(98)00187-0. [DOI] [PubMed] [Google Scholar]
- 105.Zhang J, Klebanoff MA, Roberts JM. Prediction of adverse outcomes by common definitions of hypertension in pregnancy. Obstetrics & Gynecology. 2001;97(2):261–7. doi: 10.1016/s0029-7844(00)01125-x. [DOI] [PubMed] [Google Scholar]
- 106.Hershkovitz R, Kingdom JC, Geary M, Rodeck CH. Fetal cerebral blood flow redistribution in late gestation: identification of compromise in small fetuses with normal umbilical artery Doppler. Ultrasound in Obstetrics & Gynecology. 2000 Mar;15(3):209–12. doi: 10.1046/j.1469-0705.2000.00079.x. [DOI] [PubMed] [Google Scholar]
- 107.Clifton VL. Sexually dimorphic effects of maternal asthma during pregnancy on placental glucocorticoid metabolism and fetal growth. Cell & Tissue Research. 2005 Oct;322(1):63–71. doi: 10.1007/s00441-005-1117-5. [DOI] [PubMed] [Google Scholar]
- 108.Clifton VL, Murphy VE. Maternal asthma as a model for examining fetal sex-specific effects on maternal physiology and placental mechanisms that regulate human fetal growth. Placenta. 2004 Apr;25( Suppl A):S45–52. doi: 10.1016/j.placenta.2004.01.004. [DOI] [PubMed] [Google Scholar]
- 109.Murphy VE, Gibson PG, Giles WB, Zakar T, Smith R, Bisits AM, et al. Maternal asthma is associated with reduced female fetal growth. American Journal of Respiratory & Critical Care Medicine. 2003 Dec 1;168(11):1317–23. doi: 10.1164/rccm.200303-374OC. [DOI] [PubMed] [Google Scholar]
- 110.Brosens I, Pijnenborg R, Vercruysse L, Romero R. The “Great Obstetrical Syndromes” are associated with disorders of deep placentation. American Journal of Obstetrics & Gynecology. 2011 Mar;204(3):193–201. doi: 10.1016/j.ajog.2010.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]