Abstract
The antibiotic 2,4-diacetylphloroglucinol (DAPG) is produced by some isolates of the beneficial bacterium Pseudomonas fluorescens. DAPG is toxic to many organisms, and crop yield increases have been reported after application of DAPG-producing P. fluorescens. This study was conducted to determine whether DAPG is toxic to selected nematodes. The plant-parasitic nematodes Heterodera glycines, Meloidogyne incognita, Pratylenchus scribneri and Xiphinema americanum, and the bacterial-feeding nematodes Caenorhabditis elegans, Pristionchus pacificus, and Rhabditis rainai, were immersed in concentrations ranging from 0 to 100 μg/ml DAPG. Egg hatch and viability of juveniles and adults were determined. DAPG was toxic to X. americanum adults, with an LD50 of 8.3 μg/ml DAPG. DAPG decreased M. incognita egg hatch, but stimulated C. elegans hatch during the first hours of incubation. Viability of M. incognita J2 and of C. elegans J1 and adults was not affected. There were no observed effects on the other nematodes. The study indicated that DAPG is not toxic to all nematodes, and did not affect the tested species of beneficial bacterial-feeding nematodes. Augmentation of DAPG-producing P. fluorescens populations for nematode biocontrol could be targeted to specific nematode species known to be affected by this compound and by other antibiotics produced by the bacteria, or these bacteria could be used for other possible effects, such as induced plant resistance.
Keywords: biological control, Caenorhabditis elegans, Heterodera glycines, management, Meloidogyne incognita, Pratylenchus scribneri, Pristionchus pacificus, Pseudomonas fluorescens, Rhabditis rainai, Xiphinema americanum
Production of the antibiotic 2,4-diacetylphloroglucinol (DAPG) contributes to biological control activity of many beneficial strains of the bacterium Pseudomonas fluorescens (McSpadden Gardener, 2007; Weller, 2007). DAPG is active against numerous organisms, including plants, fungi, viruses, bacteria, and nematodes, and production or increased production of DAPG has been associated with enhanced activity against plant pathogens (Keel et al., 1992; Maurhofer et al., 1992; Mazzola et al., 1995; Cronin et al., 1997; Delany et al., 2001; Dwivedi and Johri, 2003; Siddiqui and Shaukat, 2003a; 2003b; 2004a). In addition, DAPG-producing P. fluorescens can induce plant resistance against pathogens (Iavicoli et al., 2003; Siddiqui and Shaukat, 2003b; 2004b; Weller et al., 2004; Van Loon and Bakker, 2005; Bakker et al., 2007). Consequently, application of DAPG-producing pseudomonads can result in increased crop yields (McSpadden Gardener et al., 2006a; 2006b).
In studies with plant-parasitic nematodes, synthetic DAPG increased egg hatch of the potato cyst nematode Globodera rostochiensis but decreased mobility of hatched juveniles (Cronin et al., 1997). A DAPG-producing strain of P. fluorescens, and a strain with restored ability to produce DAPG, (a complemented derivative of a DAPG-negative mutant), increased egg hatch and decreased juvenile mobility in vitro and in the soil. A DAPG-negative mutant did not have these effects (Cronin et al., 1997). In other studies, culture filtrates from the DAPG-producing P. fluorescens isolate CHA0 and its DAPG-overproducing derivative strain were toxic to juveniles of the root-knot nematodes Meloidogyne javanica and Meloidogyne incognita, and also reduced egg hatch (Siddiqui and Shaukat, 2003b; 2004a). An antibiotics-deficient strain had the same activity as a culture broth control (Siddiqui and Shaukat, 2003b; 2004a). Additionally, in vitro experiments with culture filtrate from a DAPG-overproducing strain of P. fluorescens demonstrated that it caused greater mortality to M. incognita juveniles than did the wild-type strain CHA0 (Siddiqui and Shaukat, 2004c). On plant roots, CHA0 and the DAPG-overproducing strain suppressed M. incognita gall formation on brinjal, mungbean, soybean and tomato, while a derivative strain that was antibiotic-deficient did not demonstrate such activity (Siddiqui and Shaukat, 2003a). CHA0 and the over-producing strain were also more effective in reducing M. javanica gall numbers on tomato than the deficient strain (Siddiqui and Shaukat, 2003b). However, later studies indicated that production of hydrogen cyanide by CHA0 was a primary factor in inhibiting M. javanica egg hatch and killing juveniles (Siddiqui et al., 2006). Since effects of DAPG on nematodes have usually been studied with the presence of other bacterial metabolites, except for the research on G. rostochiensis (Cronin et al., 1997), further research would determine whether suppression of nematodes by DAPG-producing P. fluorescens is due to nematotoxicity of DAPG.
The primary goal of this research was to determine whether direct application of DAPG to various nematode species would result in either toxic or stimulatory effects. Two groups of nematodes were selected for the study: plant-parasitic nematodes and bacterial-feeding nematodes. If DAPG is toxic to plant-parasitic nematodes, then it may also have an adverse effect on nontarget, beneficial bacterial-feeding nematodes. The seven nematode species tested were the four plant parasites Heterodera glycines (soybean cyst nematode), Meloidogyne incognita (root-knot nematode), Pratylenchus scribneri (lesion nematode) and Xiphinema americanum (dagger nematode) and the three bacterial-feeding nematodes Caenorhabditis elegans, Pristionchus pacificus and Rhabditis rainai. Objectives included: A) determining whether DAPG was toxic to nematodes from different taxa and trophic groups; B) determining if effects of DAPG changed over time in nematodes with short life spans; C) documenting effects of DAPG on various life stages, including eggs, juveniles and adults; and 4) generating in vitro dose-response curves for adversely affected nematodes and determining lethal doses required to cause death in 50% of the population (LD50).
Materials and Methods
Nematode Cultures: Xiphinema americanum cultures are maintained in the Pennsylvania State University Fruit Research and Extension Center; all other cultures are maintained in the Beltsville USDA ARS Nematology Laboratory.
Heterodera glycines (HG-type 7, isolate NL-1-RH) and Meloidogyne incognita (race 1, isolate NL4) were originally isolated in Maryland. Both species were grown on plants in greenhouse pots; Heterodera glycines on soybean (Glycine max) cv. ‘Essex’ and M. incognita on pepper (Capsicum annuum) ‘PA-136’, and then used for assays following procedures similar to those already described (Meyer et al., 2004; 2006). Eggs were refrigerated overnight and used the day after collection. Second-stage juveniles (J2) were allowed to hatch from eggs for 3 d and were collected after passage through Spectra/Mesh® nylon filter (Spectrum Laboratories, Inc., Rancho Dominguez, CA); 25 μm for M. incognita, 30 μm for H. glycines.
Pratylenchus scribneri (isolate NL13), originally isolated from Ohio, was maintained in axenic root explant cultures of corn (Zea mays) cv. ‘IoChief’ on Gamborg's B-5 Medium with Minimal Organics (Caisson Laboratories, Inc., North Logan, UT) in Petri dishes in a growth chamber. Juveniles and adults were obtained by cutting pieces of roots and agar from root explant cultures, swirling the pieces briefly in sterile water and pouring the mixture into nested sieves (numbers 60/200/500/635, opening diam. = 250/75/25/20 μm, respectively). Adult females were collected on the #200 sieve (any juveniles retained on this sieve were not counted in the adult assays) and juveniles were collected in a beaker under the #635 sieve.
Xiphinema americanum, which was originally isolated from an apple orchard in Pennsylvania, was grown in the greenhouse on sudangrass (Sorghum sudanense) cv. ‘Piper’, collected from Baermann funnels, rinsed in sterile water and used immediately (procedures in Zasada et al., 2005).
The C. elegans wild-type strain (var. Bristol-N2, isolate 257) was obtained from the CGC (C. elegans Genetics Center), and had originally been isolated from mushroom compost near Bristol, England. The nematode was grown in Caenorhabditis briggsae maintenance medium (CbMM) similar to that described in Chitwood and Feldlaufer (1990), supplemented with hemoglobin and cholesterol but without Tween or silicone antifoam c emulsion. Ingredients were filtered for sterilization, not purified to remove contaminating sterols. Additionally, the hemoglobin was suspended in 0.001 M KOH (potassium hydroxide) solution and then filtered into the basal mix for a final concentration of 0.0001 M KOH in the completed medium. Protocol 5 from Stiernagle (1999) was used to obtain eggs from gravid adults, except that C. elegans in liquid media was centrifuged 1 min at 1300 x g to form a pellet. Protocol 7 from Stiernagle (1999) was followed to obtain synchronous cultures of first-stage juveniles (J1) from the eggs of gravid adults, and eggs were collected by centrifugation. Adults for assays were collected on 53 μm diam. Nitex nylon mesh (Sefar America Inc., Depew, NY); J1 were collected by centrifugation.
Pristionchus pacificus (PS312) was originally isolated from soil in California (Sommer et al., 1996), and Rhabditis rainai (LKC20) from Formosan termites (Coptotermes formosanus) in Louisiana (Carta and Osbrink, 2005). Both species were maintained on the bacterium Escherichia coli OP50 on Nematode Growth Medium (NGM) (Stiernagle, 1999) in culture plates. Plates of mixed-stage nematodes on which the bacterial food source had been mostly consumed were rinsed with sterile distilled water, and the nematodes were collected on sieves; 41 μm diam. Nitex mesh was used for adults, and 30 μm diam. Nitex mesh for eggs. Both life stages were then further rinsed with water, and eggs were surface-sterilized in 0.75% NaOCl:4 M NaOH (3:2) and rinsed again in sterile distilled water. Eggs were also obtained by collecting adults on 41 μm diam. Nitex mesh, and then digesting the adult females with 0.6% NaOCl for 3 min and collecting the eggs on 30 μm diam. Nitex mesh.
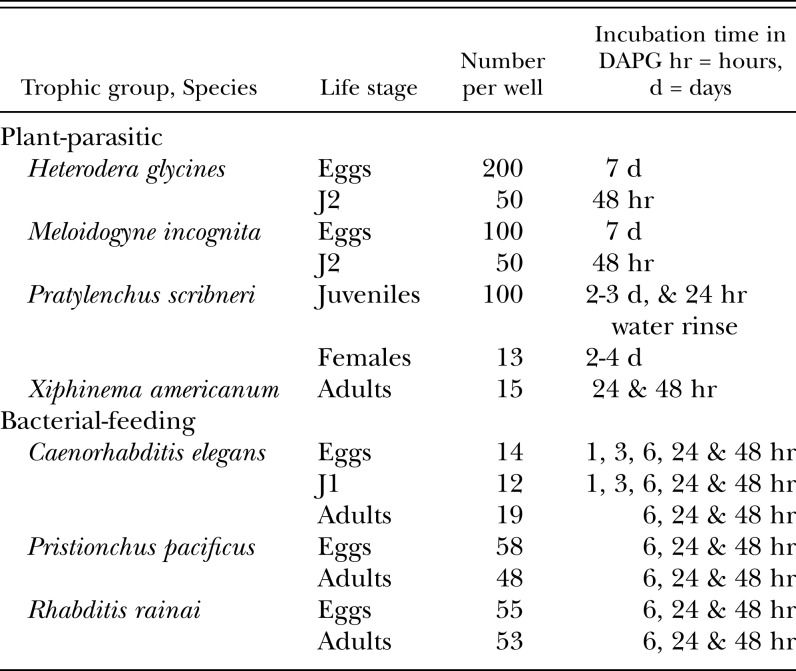
DAPG Assays: Assays were conducted in 24- or 96-well culture plates. Life stages, nematode numbers and incubation times were dependent on the life cycle and availability of life stages of each species (Table 1). All nematodes except X. americanum were assayed in aqueous DAPG prepared at the following concentrations: 0 μg/ml DAPG (water control), 1, 10, 25, 50, 75 and 100 μg/ml DAPG. Meloidogyne incognita was also assayed with DAPG dissolved in aqueous methanol as a solvent. This was done by preparing a stock solution of 11,100 μg DAPG/ml in 50% methanol, and then diluting the stock solution with water to 1, 10 and 100 μg/ml DAPG. Controls included 0% methanol (water control) and aqueous methanol concentrations equivalent to those found in the DAPG treatments: 0.0045%, 0.045%, and 0.45% methanol, respectively. Five replicate wells were used per trial, and each assay was conducted in a minimum of two trials for every nematode species/nematode life stage/DAPG concentration/incubation time combination. Nematodes were incubated in the DAPG treatments in the dark at ca. 25 C or at ambient laboratory temperature.
Table 1.
Nematode taxon, life stage assayed, approximate number of nematodes per well, and incubation times in DAPG.
Xiphinema americanum was treated with aqueous DAPG concentrations of 0, 2, 4, 6, 8, 10, 12, 14, 16, 18, 20, 25, 50, and 75 μg/ml, with four replicate wells per treatment in each trial. Nematodes were moved to a counting dish and inactive adults probed to determine if they were viable. A minimum of two trials per treatment were conducted for concentrations under 20 μg DAPG/ml, and one to two trials per treatment for higher concentrations.
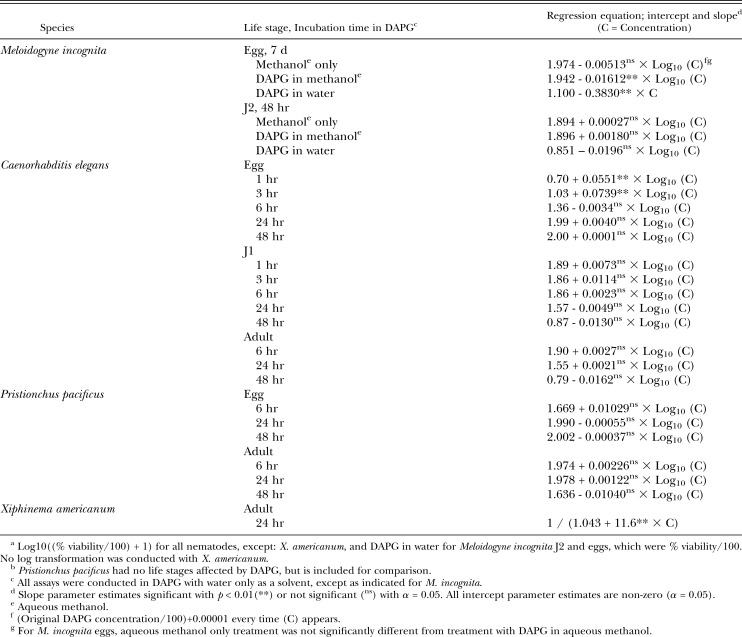
Statistical Methods: The relationship between percentage of viable eggs, juveniles or adults and the DAPG concentration was examined for each of 84 trials observed on 37 combinations of nematode species, nematode life stage, and length of time in DAPG (exposure time). The model Log10(%Viable+1) = b0 + b1·Log10(μg/ml DAPG+0.00001) provided a consistently good fit for the data for each trial, based on statistically non-zero parameters b0 and b1 and random scatter of the model's residuals. A 3-factor, nested-effects analysis of covariance model was conducted with dependent variable Log10(%Viable+1), covariate Log10(μg/ml DAPG+0.00001), and trial as experimental unit (i.e., replicate) using SAS® 9.2 Proc GLIMMIX1. Significant differences (α = 0.05) in the model intercept among nematode species and among exposure times within some nematode species × life stage were identified using the Extended Shaffer-Royen2 (ESR) multiple comparisons method by specifying ADJUST=SIMULATE and STEPDOWN options in the LSMEANS1 statement. Significant differences (α = 0.05) in model slope among the nematode species were identified by specifying estimate statements to generate the seven nematode species slope estimates and their pairwise differences to obtain letter groupings using the PDMIX8003 SAS macro. For M. incognita, percent viability of eggs and of J2, at 7 and 2 d exposure (respectively), were each compared between DAPG applications at 1, 10, and 100 μg/ml DAPG vs. percent viability in DAPG dissolved in methanol via a nested analysis of covariance. All estimates back-transformed to the original percent viable scale were bias-adjusted using the multiplier exp[0.5·variance(residual)] obtained from the analysis of covariance.
Results
Based on the results of the assays with M. incognita (as described below), water only was used as a DAPG solvent for all further tests.
Meloidogyne incognita: For this nematode (eggs and J2), the effects of DAPG dissolved in aqueous methanol were compared with the effects of DAPG in water only as a solvent. As with all other assays, water alone was used as a control. Aqueous methanol without DAPG was also used as a control in the M. incognita assays. The pH readings for DAPG in aqueous methanol ranged from 4.05 to 7.16; pH readings for DAPG in water ranged from 5.28 to 7.65. In water controls (0% methanol and 0 μg/ml DAPG) egg hatch was ca. 99.8%; J2 viability was 77.7-88.8%.
Meloidogyne incognita eggs in aqueous methanol: Aqueous methanol without DAPG was tested at three percentages: 0.0045, 0.045 and 0.45%. These were equivalent to the methanol concentrations in the 1, 10 and 100 μg/ml DAPG in aqueous methanol treatments, respectively. Without DAPG, 91.2%-93.3% of eggs hatched in the methanol treatments, which was a significant decrease in egg hatch compared to water controls. The effect did not vary among the methanol concentrations (Fig. 1).
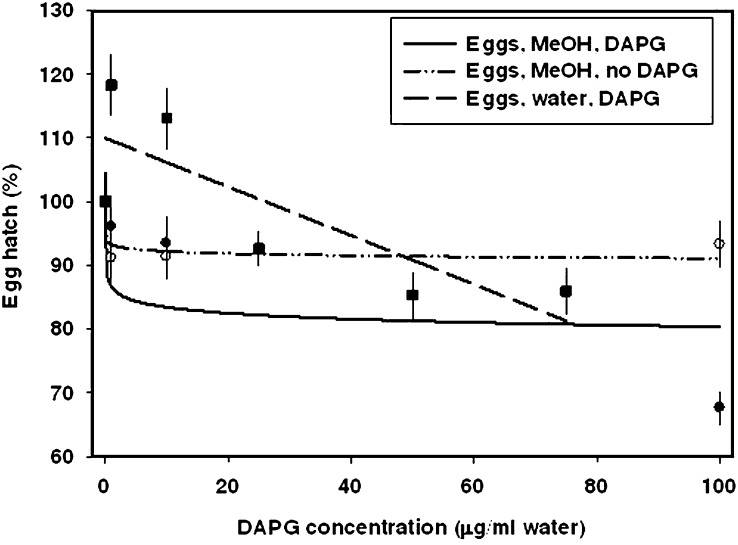
Fig. 1.
Dose-response curves for percentage Meloidogyne incognita egg hatch in microwell assays following incubation in various concentrations of DAPG. Observed means are indicated by symbols with standard error bars. Treatments included aqueous methanol without DAPG, DAPG in aqueous methanol, and DAPG in water only. Egg hatch was recorded after an incubation time of 7 d. The mean number of eggs per well was based on the estimated number in an aliquot, so hatch could be greater than 100% if the mean number of eggs per well was greater than the estimated number. The dose response curve for eggs in aqueous methanol without DAPG represents the methanol concentrations used for the corresponding treatments with DAPG in aqueous methanol.
Meloidogyne incognita eggs in DAPG with aqueous methanol as a solvent: This treatment resulted in a significant decrease in egg hatch, with the most marked effect at low concentrations below ca. 10 μg/ml DAPG (Fig. 1, Table 2). The percentage of hatched eggs continued to decrease somewhat at higher DAPG concentrations; hatch was ca. 85% at 75 μg/ml DAPG (based on model in Fig. 1), and was recorded as 67.6% in 100 μg/ml DAPG in 0.45% methanol. Fig. 1 does not indicate this low percentage hatch at 100 μg/ml because the model was not generated by drawing a line directly through the mean hatch recorded at each DAPG concentration. There was not a significant difference between treatment with DAPG in aqueous methanol and treatment with aqueous methanol.
Table 2.
Regression model equationsa for nematodes with a life stage affected by DAPG, and for Pristionchus pacificus.b
Meloidogyne incognita eggs in DAPG with water as a solvent: When water alone was used as a solvent for DAPG, effects were significantly different from all other treatments. There was a constant decrease in egg hatch as DAPG concentration increased, with hatch decreasing to 85.9% at 75 μg/ml DAPG. The regression equation was therefore not the same as that generated by incubating eggs in DAPG with aqueous methanol as a solvent (Fig. 1, Table 2).
Meloidogyne incognita J2 in all treatments: Unlike M. incognita eggs, J2 did not respond to DAPG treatment. Incubation in aqueous methanol, DAPG in aqueous methanol, or DAPG in water did not significantly affect J2 viability (Table 2).
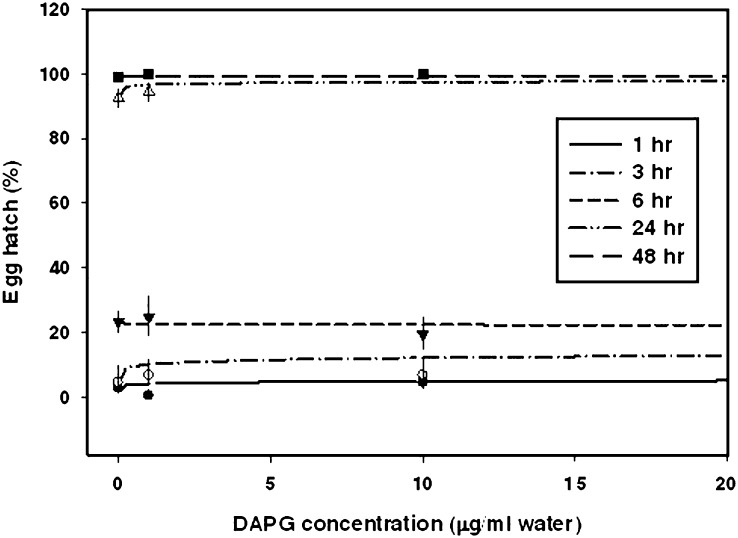
Caenorhabditis elegans: Egg hatch was stimulated by 1 and 3 hr incubation time in DAPG (Fig. 2, Table 2). For example, at 1 hr, egg hatch in 0, 10 and 75 μg/ml DAPG was ca. 2.8, 4.5 and 9.1%, respectively. At longer incubation times, egg hatch in water controls increased, with 23.4 and 98.8% hatch after 6 and 48 hr incubation, respectively. No stimulatory effect of DAPG on C. elegans egg hatch was observed at 6, 24 or 48 hr, when egg hatch in water and in DAPG treatments was higher than percentage egg hatch at shorter incubation times.
Fig. 2.
Dose-response curves for percentage Caenorhabditis elegans egg hatch in microwell assays following incubation in various concentrations of DAPG in water. Observed means are indicated by symbols with standard error bars. Egg hatch was recorded at multiple incubation times.
In water controls, C. elegans J1 were most active in the first 6 hr, with a mean recorded percent viability of 62.9% to 88.3% (depending on the incubation time in water). After 24 and 48 hr in the water controls, percentage viable J1 decreased to 38.7 and 8.2%, respectively. The percentage of viable adults in water controls was also highest with the shorter incubation times; recorded means were 77.8, 35.5 and 6.1% after 6, 24 and 48 hr in water, respectively. Incubation in DAPG gave similar results to incubation in the water controls; DAPG did not affect percentage of viable C. elegans J1 or adults (Table 2). This was similar to the effect of DAPG on M. incognita J2.
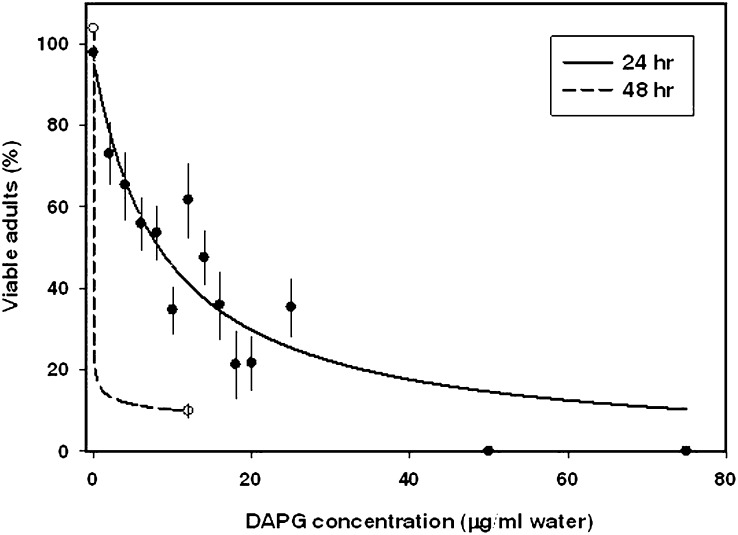
Xiphinema americanum: DAPG was toxic to adults of X. americanum (Fig. 3, Table 2). The regression line indicated a substantial and significant decrease in viability upon exposure to low concentrations of DAPG. After 24 hr incubation, observed adult viability averaged 97.8% in water controls, 73% in 2 μg/ml DAPG, and 0% in 50 μg/ml DAPG (indicated by circles on the x axis in Fig. 3). The toxic effect was even more pronounced after 2 d in DAPG (Fig. 3). Xiphinema americanum was the only nematode sensitive enough to DAPG to allow for calculation of a lethal dose that resulted in 50% loss of viability of the population. The LD50 varied among trials, ranging from 2.9 to 10.1 μg/ml DAPG. The mean LD50 for X. americanum was calculated as 8.3 μg/ml DAPG (95% confidence interval of 4.69% to 11.82%).
Fig. 3.
Dose-response curves for percentage viable Xiphinema americanum adults in microwell assays following incubation in various concentrations of DAPG in water. Observed means are indicated by symbols with standard error bars. Adult viability was recorded at two incubation times.
Heterodera glycines eggs and J2, P. scribneri juveniles and adults, P. pacificus eggs and adults, and R. rainai eggs and adults: None of the tested life stages of these species were significantly affected by DAPG. Regression model equations are shown only for P. pacificus (Table 2), as an example. The mean Pristionchus pacificus egg hatch in water was 41.4% after 6 hr (higher than that recorded for C. elegans at the same time), increasing to an average of 96.8% and 99.7% after 24 and 48 hr, respectively. Adult P. pacificus viability in water was also higher than that recorded for C. elegans, averaging 90.8% and 92.2% at 6 hr and 1 d, and decreasing to 46.9% at 2 d. Regression equations demonstrated no significant effect of DAPG (Table 2).
Discussion
Reactions of nematodes to DAPG varied with taxon and life stage tested. DAPG stimulated or inhibited egg hatch of two nematode species (C. elegans and M. incognita, respectively), and was toxic to adults of another species (X. americanum). However, viability of M. incognita J2 and of C. elegans J1 was not affected by DAPG. In addition, C. elegans egg hatch was only stimulated by DAPG when the nematodes were freshly immersed and had not yet attained the high levels of egg hatch that naturally occurred as incubation time increased. In the other four nematode species, H. glycines, P. scribneri, P. pacificus and R. rainai, neither egg hatch nor viability of juveniles or adults was affected by DAPG. The results of these studies demonstrate that DAPG is not toxic to most of the nematode species tested, and that DAPG could be active against one life stage of a species but not against another life stage of the same species. It is notable that the only adverse effects observed occurred with plant-parasitic nematodes. Further studies would indicate whether the particularly strong toxic effect against X. americanum occurs with closely related nematodes.
The only other study on direct effects of synthetic DAPG on nematode hatch and activity was conducted with G. rostochiensis (Cronin et al., 1997). In that study, synthetic DAPG was dissolved in 99% methanol (1000 μM DAPG), and then the stock solution was serially diluted in water to concentrations of 1μM to 1 mM DAPG. For egg hatch assays, these were then further diluted by adding 100 μl of each DAPG concentration to 20 ml buffer (Cronin et al., 1997). This would be equivalent to a range of 1.1 ng/ml (5 nM) to 1.1 μg/ml DAPG (5 μM). Our lowest tested concentration was 1 μg/ml DAPG (= 4.8 μM), dissolved in water only as the solvent for all assays except those with M. incognita. In the G. rostochiensis study, juvenile activity was measured with nematodes in Ringer's solution exposed to 0 to 1 mM DAPG for 48 hr (Cronin et al., 1997). Globodera rostochiensis egg hatch increased with increasing concentration of DAPG, while the percentage of active J2 decreased with increasing DAPG (Cronin et al., 1997). In their experiments, the methanol alone did not affect egg hatch. In our study, methanol alone did not affect M. incognita J2 viability, but M. incognita egg hatch did decrease in methanol. We also included a cyst nematode, H. glycines, in our study, and it showed no response to DAPG in water. Our study and that of Cronin indicate that taxonomic identities might not serve as predictors of DAPG response.
In our assay, application of DAPG in aqueous methanol as a solvent suppressed M. incognita egg hatch, but results were not significantly different from those recorded with methanol alone. Because methanol had an independent effect on the nematode eggs, and use of water alone would more closely replicate soil conditions, water was used as the only solvent in the rest of the assays. It is also notable that pH was not a factor in activity in these assays, as the measured pH ranges should not have affected egg hatch or nematode viability (Meyer et al., 2004).
Although a possible mode of action of DAPG against nematodes has not been studied, research with microbes has indicated that the compound can cause an increase in cell permeability and alteration in membranes, and that DAPG can interfere with function of mitochondria (de Souza et al., 2003; Goldberg et al., 2008). Since DAPG might have effects that do not result in reduced egg hatch or nematode viability, a small, unreplicated trial was conducted with M. incognita J2 that had been treated with all tested concentrations of DAPG. The J2 were placed onto tomato (cv. Rutgers) grown in axenic root explant culture. Some J2 from all tested DAPG concentrations were able to find and infect roots, and then produce galls and egg masses (unpublished). It did not appear that sublethal doses of DAPG were causing long-term effects that would disrupt the nematode life cycle. Other bacterial metabolites, such as HCN or pyoluteorin, may play a more major role in nematotoxicity, as suggested by Siddiqui et al. (2005; 2006), or act in synergy with DAPG. In addition, the effects of DAPG on plant roots may affect the environment of the infection court, thereby affecting infectivity and/or disease development. For example, DAPG may induce host plant resistance pathways (Iavicoli et al., 2002), and it was also shown that 50 μM concentrations of DAPG significantly altered tomato root morphology (Brazelton et al., 2008).
The results of this study indicate that application of DAPG-producing P. fluorescens for nematode management by DAPG nematotoxicity would have to be targeted to specific nematode species that had been shown to be sensitive to the compound. DAPG produced by P. fluorescens might be more effective against some plant-parasitic nematodes by causing induced resistance in host plants. It is also likely that some or even many nontarget, beneficial nematodes would not be adversely affected by DAPG production in the soil.
Footnotes
Thanks are extended to Paula Crowley, Sharon Ochs, Maria Hult (USDA ARS Nematology Laboratory) and Phyllis Price (Pennsylvania State University Fruit Research and Extension Center) for assistance in the laboratory, and to P. Crowley for preparation of final graphs. Mention of trade names or commercial products in this publication is solely for the purpose of providing specific information and does not imply recommendation or endorsement by the U.S. Department of Agriculture.
This paper was edited by Kris N. Lambert.
Literature Cited
- Bakker PAHM, Pieterse CMJ, van Loon LC. Induced systemic resistance by fluorescent Pseudomonas spp. Phytopathology. 2007;97:239–243. doi: 10.1094/PHYTO-97-2-0239. [DOI] [PubMed] [Google Scholar]
- Brazelton JN, Pfeufer EE, Sweat TA, McSpadden Gardener BB, Coenen C. 2,4-Diacetylphloroglucinol alters plant root development. Molecular Plant-Microbe Interactions. 2008;21:1349–1358. doi: 10.1094/MPMI-21-10-1349. [DOI] [PubMed] [Google Scholar]
- Carta LK, Osbrink W. Rhabditis rainai n. sp. (Nematoda: Rhabditida) associated with the Formosan subterranean termite, Coptotermes formosanus (Isoptera: Rhinotermitidae) Nematology. 2005;7:863–879. [Google Scholar]
- Chitwood DJ, Feldlaufer MF. Ecdysteroids in axenically propagated Caenorhabditis elegans and culture medium. Journal of Nematology. 1990;22:598–607. [PMC free article] [PubMed] [Google Scholar]
- Cronin D, Moënne-Loccoz Y, Fenton A, Dunne C, Dowling DN, O'Gara F. Role of 2,4-diacetylphloroglucinol in the interactions of the biocontrol pseudomonad strain F113 with the potato cyst nematode Globodera rostochiensis. Applied and Environmental Microbiology. 1997;63:1357–1361. doi: 10.1128/aem.63.4.1357-1361.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delany IR, Walsh UF, Ross I, Fenton AM, Corkery DM, O'Gara F. Enhancing the biocontrol efficacy of Pseudomonas fluorescens F113 by altering the regulation and production of 2,4-diacetylphloroglucinol. Plant and Soil. 2001;232:195–205. [Google Scholar]
- de Souza JT, Arnould C, Deulvot C, Lemanceau P, Gianinazzi-Pearson V, Raaijmakers JM. Effect of 2,4-diacetylphloroglucinol on Pythium: Cellular responses and variation in sensitivity among propagules and species. Phytopathology. 2003;93:966–975. doi: 10.1094/PHYTO.2003.93.8.966. [DOI] [PubMed] [Google Scholar]
- Dwivedi D, Johri BN. Antifungals from fluorescent pseudomonads: biosynthesis and regulation. Current Science. 2003;85:1693–1703. [Google Scholar]
- Goldberg JB, Hancock REW, Parales RE, Loper J, Cornelis P. Pseudomonas 2007. Journal of Bacteriology. 2008;190:2649–2662. doi: 10.1128/JB.01950-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iavicoli A, Boutet E, Buchala A, Métraux JP. Induced systemic resistance in Arabidopsis thaliana in response to root inoculation with Pseudomonas fluorescens CHA0. Molecular Plant-Microbe Interactions. 2003;16:851–858. doi: 10.1094/MPMI.2003.16.10.851. [DOI] [PubMed] [Google Scholar]
- Keel C, Schnider U, Maurhofer M, Voisard C, Laville J, Burger U, Wirthner P, Haas D, Défago G. Suppression of root diseases by Pseudomonas fluorescens CHA0: Importance of the bacterial secondary metabolite 2,4-diacetylphloroglucinol. Molecular Plant-Microbe Interactions. 1992;5:4–13. [Google Scholar]
- Maurhofer M, Keel C, Schnider U, Voisard C, Haas D, Défago G. Influence of enhanced antibiotic production in Pseudomonas fluorescens strain CHA0 on its disease suppressive capacity. Phytopathology. 1992;82:190–195. [Google Scholar]
- Mazzola M, Fujimoto DK, Thomashow LS, Cook RJ. Variation in sensitivity of Gaeumannomyces graminis to antibiotics produced by fluorescent Pseudomonas spp. and effect on biological control of take-all of wheat. Applied and Environmental Microbiology. 1995;61:2554–2559. doi: 10.1128/aem.61.7.2554-2559.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McSpadden Gardener BB. Diversity and ecology of biocontrol Pseudomonas in agricultural systems. Phytopathology. 2007;97:221–226. doi: 10.1094/PHYTO-97-2-0221. [DOI] [PubMed] [Google Scholar]
- McSpadden Gardener B, Benitez MS, Camp A, Zumpetta C. Evaluation of a seed treatment containing a phlD+ strain of Pseudomonas fluorescens on organic soybeans, 2005. Biological and Cultural Tests for Control of Plant Diseases Report. 2006a;21:FC046. [Google Scholar]
- McSpadden Gardener B, Kroon van Diest C, Beuerlein J. Evaluation of biological seed treatments containing phlD+ strains of Pseudomonas fluorescens on soybeans grown in Ohio, 2005. Biological and Cultural Tests for Control of Plant Diseases Report. 2006b;21:FC045. [Google Scholar]
- Meyer SLF, Huettel RN, Liu XZ, Humber RA, Juba J, Nitao JK. Activity of fungal culture filtrates against soybean cyst nematode and root-knot nematode egg hatch and juvenile motility. Nematology. 2004;6:23–32. [Google Scholar]
- Meyer SLF, Zasada IA, Roberts DP, Vinyard BT, Lakshman DK, Lee J-K, Chitwood DJ, Carta LK. Plantago lanceolata and Plantago rugelii extracts are toxic to Meloidogyne incognita but not to certain microbes. Journal of Nematology. 2006;38:333–338. [PMC free article] [PubMed] [Google Scholar]
- Siddiqui IA, Haas D, Heeb S. Extracellular protease of Pseudomonas fluorescens CHA0, a biocontrol factor with activity against the root-knot nematode Meloidogyne incognita. Applied and Environmental Microbiology. 2005;71:5646–5649. doi: 10.1128/AEM.71.9.5646-5649.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siddiqui IA, Shaukat SS. Plant species, host age and host genotype effects on Meloidogyne incognita biocontrol by Pseudomonas fluorescens strain CHA0 and its genetically-modified derivatives. Journal of Phytopathology. 2003a;151:231–238. [Google Scholar]
- Siddiqui IA, Shaukat SS. Suppression of root-knot disease by Pseudomonas fluorescens CHA0 in tomato: Importance of bacterial secondary metabolite, 2,4-diacetylphloroglucinol. Soil Biology and Biochemistry. 2003b;35:1615–1623. [Google Scholar]
- Siddiqui IA, Shaukat SS. Suppression of Meloidogyne incognita by Pseudomonas fluorescens strain CHA0 and its genetically-modified derivatives: II. The influence of sodium chloride. Nematologia Mediterranea. 2004a;32:127–130. [Google Scholar]
- Siddiqui IA, Shaukat SS. Systemic resistance in tomato induced by biocontrol bacteria against the root-knot nematode, Meloidogyne javanica is independent of salicylic acid production. Journal of Phytopathology. 2004b;152:48–54. [Google Scholar]
- Siddiqui IA, Shaukat SS. Trichoderma harzianum enhances the production of nematicidal compounds in vitro and improves biocontrol of Meloidogyne javanica by Pseudomonas fluorescens in tomato. Letters in Applied Microbiology. 2004c;38:169–175. doi: 10.1111/j.1472-765x.2003.01481.x. [DOI] [PubMed] [Google Scholar]
- Siddiqui IA, Shaukat SS, Sheikh IH, Khan A. Role of cyanide production by Pseudomonas fluorescens CHA0 in the suppression of root-knot nematode, Meloidogyne javanica in tomato. World Journal of Microbiology and Biotechnology. 2006;22:641–650. [Google Scholar]
- Sommer RJ, Carta LK, Kim S-Y, Sternberg PW. Morphological, genetic and molecular description of Pristionchus pacificus sp. n. (Nematoda: Neodiplogastridae) Fundamental and applied Nematology. 1996;19:511–521. [Google Scholar]
- Stiernagle T. Maintenance of C. elegans. In: Hope IA, editor. C. elegans: A practical approach. New York: Oxford University Press; 1999. pp. 51–68. [Google Scholar]
- Van Loon LC, Bakker PAHM. Induced systemic resistance as a mechanism of disease suppression by rhizobacteria. In: Siddiqui ZA, editor. PGPR: Biocontrol and biofertilization. Dordrecht, The Netherlands: Springer; 2005. pp. 39–66. [Google Scholar]
- Weller DM. Pseudomonas biocontrol agents of soilborne pathogens: Looking back over 30 years. Phytopathology. 2007;97:250–256. doi: 10.1094/PHYTO-97-2-0250. [DOI] [PubMed] [Google Scholar]
- Weller DM, Van Pelt JA, Mavrodi DV, Pieterse CMJ, Bakker PAHM, Van Loon LC. Induced systemic resistance (ISR) in Arabidopsis against Pseudomonas syringae pv. tomato by 2,4-diacetylphloroglucinol (DAPG)-producing Pseudomonas fluorescens. Phytopathology. 2004;94(suppl.):S108. doi: 10.1094/PHYTO-08-11-0222. (Abstr.). [DOI] [PubMed] [Google Scholar]
- Zasada IA, Meyer SLF, Halbrendt JM, Rice C. Activity of hydroxamic acids from Secale cereale against the plant-parasitic nematodes Meloidogyne incognita and Xiphinema americanum. Phytopathology. 2005;95:1116–1121. doi: 10.1094/PHYTO-95-1116. [DOI] [PubMed] [Google Scholar]