Abstract
The endospore-forming bacterium Pasteuria penetrans is an obligate parasite of root-knot nematodes (Meloidogyne spp.). The primary objective of this study was to determine the effect of crop sequence on abundance of P. penetrans. The experiment was conducted from 2000 to 2008 at a field site naturally infested with both the bacterium and its host Meloidogyne arenaria and included the following crop sequences: continuous peanut (Arachis hypogaea) (P-P-P) and peanut rotated with either 2 years of corn (Zea mays) (C-C-P), 1 year each of cotton (Gossypium hirsutum) and corn (Ct-C-P), or 1 year each of corn and a vegetable (V-C-P). The vegetable was a double crop of sweet corn and eggplant (Solanum melongena). A bioassay with second-stage juveniles (J2) of M. arenaria from a greenhouse (GH) population was used to estimate endospore abundance under the different crop sequences. A greater numerical increase in endospore densities was expected in the P-P-P and V-C-P sequences than in the other sequences because both peanut and eggplant are good hosts for M. arenaria. However, endospore densities, as determined by bioassay, did not substantially increase in any of the sequences during the 9-year experiment. To determine whether the nematode population had developed resistance to the resident P. penetrans, five single egg-mass (SEM) lines from the field population of M. arenaria were tested alongside the GH population for acquisition of endospores from the field soil. Four of the five SEM lines acquired 9 to 14 spores/J2 whereas the GH population and one of the SEM lines acquired 3.5 and 1.8 spores/J2, respectively. Endospore densities estimated with the four receptive SEM lines were highest in the P-P-P plots (14-20 spores/J2), intermediate in the V-C-P plots (6-7 spores/J2), and lowest in the Ct-C-P plots (< 1 spore/J2). These results indicate that the field population of M. arenaria is heterogeneous for attachment of P. penetrans endospores. Moreover, spore densities increased under intensive cropping of hosts for M. arenaria, but the GH population of the nematode was not receptive to spore attachment. However, previously, the GH population was very receptive to spore acquisition from this field site. One explanation for this inconsistency is that the M. arenaria population in the field became resistant to the dominant subpopulation of P. penetrans that had been present, and this led to the selection of a different subpopulation of the bacterium that is incompatible with the GH population.
Keywords: Arachis hypogaea, biological control, crop rotation, peanut, root-knot nematode
The gram-positive bacterium Pasteuria penetrans is an obligate parasite of root-knot nematodes, Meloidogyne spp. Second-stage juveniles (J2) of the nematode acquire endospores of the bacterium as they move through soil. Endospores typically do not infect the juvenile until the nematode has entered the root and established a feeding site (Sayre and Wergin, 1977). The bacterium grows vegetatively in the pseuocoelom and produces endospores within the mature root-knot female. A parasitized female can contain 2 x 106 P. penetrans spores (Stirling, 1991). When the root containing infected females decomposes, endospores are released into the soil to begin the cycle again. The endospores are heat and desiccation tolerant, and can persist in the soil for several years (Chen and Dickson, 1998; Giannakou et al., 1997). At low spore densities, P. penetrans reduces nematode populations primarily by inhibiting egg production of infected females (Bird and Brisbane, 1988; Kariuki et al., 2006); however, at high spore densities, the bacterium also reduces infection of roots by J2 encumbered with spores (Ahmed and Gowen, 1991; Davies et al., 1991).
Pasteuria penetrans has a nearly world-wide distribution (Chen and Dickson, 1998; Trudgill et al., 2000) and has been associated with several Meloidogyne-suppressive soils (Bird and Brisbane, 1988; Timper et al., 2001; Weibelzahl-Fulton et al., 1996). High spore densities of the bacterium have mostly been observed in perennial and monocultured crops. Because the bacterium can only complete its lifecycle after a J2 with attached spores infects a host plant, crop rotations with non-hosts of Meloidogyne sp. have lower densities of P. penetrans spores than continuously cropped host plants (Ciancio and Queneherve, 2000; Madulu et al., 1994; Oostendorp et al., 1991; Timper et al., 2001). The presence of P. penetrans, however, does not always lead to obvious reductions in the nematode population even in the constant presence of a host for the nematode (Spaull, 1984; Verdejo-Lucas, 1992; Walker and Wachtel, 1988). In a large multi-national project, microplot and field studies were conducted to test the hypothesis that intensive cropping of Meloidogyne-susceptible crops would lead to an increase in abundance of P. penetrans spores and suppression of the nematode population (Trudgill et al., 2000). In three trials, nematode suppression was only documented where an exotic isolate of P. penetrans had been introduced to supplement an endemic population present at low background levels. Because the endemic P. penetrans in five different trials failed to increase following repeated cropping of a Meloidogyne-susceptible host, the authors speculated that the nematode populations had undergone selection for reduced attachment of spores (Tzortzakakis et al., 1996), leading to low equilibrium levels of parasitism by the endemic P. penetrans. This theory is partially supported by Stirling (1985), who tested 15 Meloidogyne populations from Australia against four P. penetrans isolates, one from the USA and three from Australia. The isolate from the USA exhibited high rates of attachment to most of the nematode populations, whereas the isolates from Australia exhibited low rates of attachment to most of the populations. However, the Australian isolates of P. penetrans attached at high rates to the nematode population from which they were isolated.
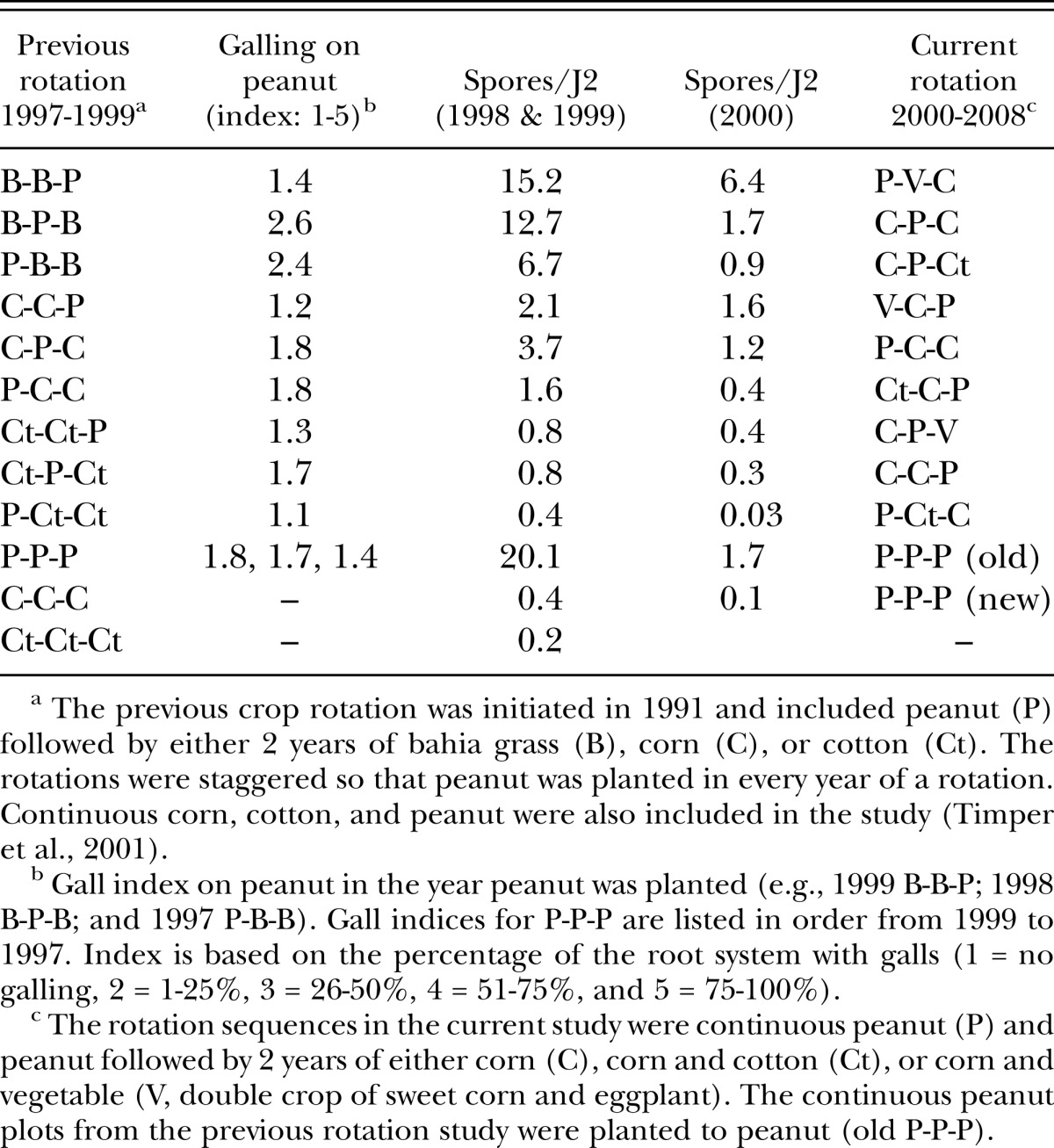
In an earlier rotation study (1991 to 1999), a bioassay was used to determine the effect of crop sequence on densities of P. penetrans spores (Timper et al., 2001). The bioassay involved adding J2 of M. arenaria from a greenhouse population into a soil-water suspension and determining the average number of spores/J2 after 24 hours. In continuous peanut (Arachis hypogaea), densities averaged 22 and 19 spores/J2 in 1998 and 1999, respectively. Gall ratings (1 to 5 scale) in the continuous peanut plots were low, oscillating around 2 (range 1.4 to 2.3) during the 9-year experiment. Spore densities were also moderately high (12 spores/J2) in a rotation of bahia grass (Paspalum notatum) and peanut. This was likely due to the presence of a host for M. arenaria being present 2 out of 3 years in the rotation cycle (peanut and weeds in first cycle of bahia grass).
The overall goal in this study was to develop a rotation system for peanut that would sustain a nematode-suppressive soil while still maintaining acceptable yields. Initially, the specific objectives were to determine: (i) whether growing two different hosts for M. arenaria in a 3-year annual rotation cycle would lead to greater densities of P. penetrans spores and nematode suppression compared to conventional rotations and (ii) the rate of endospore increase under different crop sequences. When densities of P. penetrans failed to increase even after 8 years of continuous peanut, two hypotheses were developed to explain the lack of a numerical response by the bacterium. The first hypothesis was that the nematode population had become resistant to P. penetrans either because of a species shift from M. arenaria to another Meloidogyne spp., or because of a genetic shift in the population of M. arenaria. If this hypothesis was correct, then the field population would be less receptive to attachment of spores from the field soil than the greenhouse (GH) population that was never exposed to selection pressure from P. penetrans. The second hypothesis was that the P. penetrans population in the field had changed, presumably in response to a change in the susceptibility of its host. If this second hypothesis was correct, then J2 from the field population would be more receptive to spore attachment than J2 from the GH population.
Materials and Methods
Study site.
The study was conducted on the Gibbs Farm, Tifton, Georgia. The soil was a Tifton loamy sand (fine-loamy, siliceous, thermic Plinthic Kandiudult) naturally infested with M. arenaria race 1 and P. penetrans. Meloidogyne incognita was also present at very low densities. A rotation study had been conducted at this field site from 1991 to 1999 (Timper et al., 2001) which led to large differences in P. penetrans population densities and small differences in M. arenaria population densities among the plots (Table 1). At the end of the field season in 1999, the field was leveled with eight passes of a land leveler, four across and four diagonal, to a maximum depth of ca. 15 cm.
Table 1.
Root gall ratings on peanut (Arachis hypogaea) from Meloidogyne arenaria and endospore densities of Pasteuria penetrans estimated by bioassay with second-stage juveniles (J2) in the previous and current crop rotation study.
Experimental design and treatments.
The experiment was a randomized complete-block design with four crop rotations: peanut rotated with either 2 years of corn (Zea mays) (C-C-P), 1 year each of cotton (Gossypium hirsutum) and corn (Ct-C-P), or 1 year each of vegetable and corn (V-C-P), and continuous peanut (P-P-P). The vegetable was a double crop of sweet corn and eggplant (Solanum melongena). Peanut and eggplant are good hosts for M. arenaria, whereas corn is a relatively poor host and cotton is a nonhost for the nematode (Luc et al., 2005). There were three staggered sequences of each rotation which were randomly assigned to different rotation treatments from the previous study (Table 1). Continuous peanut was assigned to the former continuous corn plots. All rotational sequences were replicated four times. The former continuous peanut plots continued to be planted with peanut but they were not considered part of the experiment.
Crop management and root-gall ratings.
Prior to planting, the soil was plowed to a depth of 20 to 25 cm and shaped into beds 1.8 m wide and 10 to 15 cm high. Plots were 7.6 m long and consisted of three beds. The crops were planted in May, except eggplant, which was transplanted following harvest of the sweet corn in August. The seeds or transplants were planted in two rows, 0.9 m apart on the bed at the following densities: peanut cv. Georgia green (19.7 seed/m), corn cv. Pioneer 3223 or 31N27 (4.6 seed/m), cotton cv. Deltapine 458 BG/RR (15.7 seed/m), sweet corn cv. Merit (4.6 seed/m), and eggplant cv. Santana (19.7 plants/m). Cultural practices, fertilization, and pest management for the peanut, corn, cotton, and vegetable crops were based on recommendations for the area (Guillebeau, 2009; Kissel and Sonon, 2008). All crops were harvested at optimum maturity. The field corn was harvested in mid September, the peanuts in mid to late September, the cotton from late October to mid November, and the eggplant from early November to early December. Immediately after the peanuts were inverted in the field, roots from 10 plants per subplot were examined for galls of M. arenaria and rated on a 1-to-5 scale based on the percentage of the root system with galls: 1 = no galls; 2 = 1% to 25%; 3 = 26% to 50%; 4 = 51% to 75%; and 5 = 76% to 100%.
Pasteuria penetrans bioassay.
To determine the density of P. penetrans spores, soil samples were collected at planting in the spring (2000 to 2004) and both at planting and at harvest in the remaining years of the experiment. The samples were a composite of 10 to 12 soil cores (2.5 cm diam. x 15 cm deep) collected from the root zone of each row within a plot. The soil was stored at 10°C for 1 to 2 months before determining the abundance of P. penetrans using a previously described bioassay (Timper et al., 2001). Briefly, following thorough mixing of the soil, a 100 cm3 subsample was added to a 250 cm3 flask along with water. The mixture was shaken, allowed to settle for 5 sec, and the soil-water suspension decanted into another flask. The assay nematodes were obtained from a GH population of M. arenaria (originally from the Gibbs Farm) by placing roots with egg masses in a mist chamber and collecting the hatched J2 3 to 4 d later. The flasks containing the soil-water suspension were inoculated with 1,500 J2 and shaken for 24 hr. The nematodes were extracted by centrifugal flotation (Jenkins, 1964) and the number of spores adhering to 30 individual J2, selected at random, was determined using an inverted microscope at 400x magnification.
Nematode suppression in soil from different crop sequences.
To determine whether P. penetrans was suppressing reproduction of M. arenaria, soil was collected in July and October 2005 from crop sequences where peanut was present in that year: P-P-P, V-C-P, Ct-C-P, and C-C-P. Approximately 5 liters of soil was collected from within the root zone (5 to 20 cm below the surface) from at least five locations. At each of the collection times, the soil was thoroughly mixed and air dried in shallow pans in a greenhouse to kill native M. arenaria. Soil from each treatment was distributed among three 10-cm square pots (700 cm3 of soil/pot) and watered. The pots were kept in the greenhouse for a week before planting two peanut cv. Georgia green seeds. The seedlings were thinned to one per pot and inoculated with 8,000 eggs of M. arenaria 2 wk after planting. Nematode eggs were extracted 8 wk after inoculation by cutting the roots from each pot into ca. 5 cm pieces, placing them in a 1 liter flask and agitating for 4 min in a 1.2% NaOCl solution (Hussey and Barker, 1973). The number of eggs per pot in the three pots was averaged prior to statistical analysis. There were four replicates per crop sequence and the experiment was conducted twice; once with soil collected midseason and again with soil collected at the end of the season.
Diversity of the Meloidogyne population.
In October 2008, a pooled soil sample from the four replicate plots of continuous peanut was planted with peanut in the greenhouse. After 4 mon, the esterase phenotype of six females was determined (Esbenshade and Triantaphyllou, 1990). In March 2009, a pooled soil sample was again collected from the four replicates of the continuous peanut plots, distributed among three 1.5 liter pots and planted with peanut. After 1 month, 12 single egg masses were picked off the roots and transferred to individual pots containing steam-sterilized soil and an eggplant seedling. After 3 months of growth, five of the single egg-mass (SEM) lines and the standard GH population of M. arenaria were used to assay soil from the following five plots for P. penetrans spores: Rep. 1 Ct-C-P, Rep. 2 V-C-P, Rep. 3 V-C-P, Rep. 2 P-P-P, and Rep. 4 P-P-P. These five soils were collected in March 2009 and were selected because they had relatively high densities of spores the previous fall. The bioassay was the same as described previously, except that prior to testing, the soil was air dried in the greenhouse to kill background Meloidogyne spp. Each soil was thoroughly mixed and subsamples added to flasks. Juveniles from the SEM lines and GH population were collected from roots placed in a mist chamber and used to assay the soils for abundance of spores. The experiment was repeated using the soil collected in March 2009.
Statistical analysis.
On each sampling date, the effect of crop sequence on the relative abundance of P. penetrans endospores was determined with Analysis of Variance (PROC GLM, v. 9; SAS Institute, Cary, NC). Fisher's Protected LSD test was used to establish differences among means (P ≤ 0.05). For the greenhouse experiment and the SEM lines, PROC GLM and Fisher's LSD was also used to determine the effect of the treatments on the number of M. arenaria eggs per pot (log transformed) and spores per J2. If there was no interaction between treatment and trial, the data for the trials were combined. The Anderson-Darling test (PROC UNIVARIATE, v. 9; SAS Institute, Carey, NC) was used to determine whether the frequency distribution of spore number/J2 differed from a normal distribution (P ≤ 0.05).
Results
Root galling.
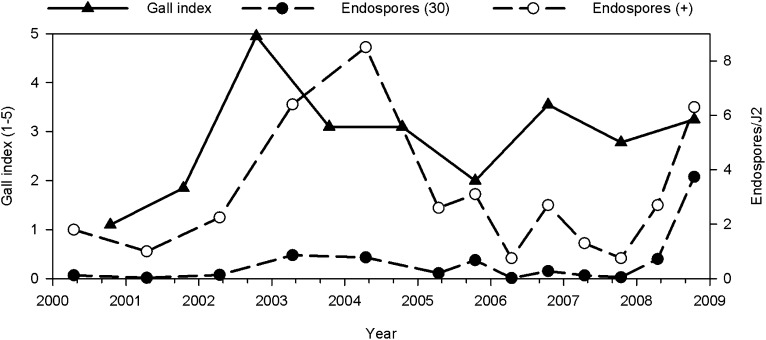
Galling of peanut roots by M. arenaria was minimal in the first year of the study, but rapidly increased to very high levels in the P-P-P plots in 2002 (Fig. 1). In subsequent years, root galling in the P-P-P plots fluctuated around an index of 3 (1 to 5 scale). In all years except 2000, root galling was lower (P < 0.0001) in peanut rotated with other crops than in continuous peanut (2.8 vs. 1.5), but there was no difference in galling among the rotations.
Fig. 1.
Root gall ratings in continuous peanut (Arachis hypogaea) from Meloidogyne arenaria and densities of Pasteuria penetrans endospores based on a bioassay with second-stage juveniles (J2) from soil samples collected in the spring and fall over a 9-year-period. The gall index is based on the percentage of the root system with galls (1 = no galling, 2 = 1-25%, 3 = 26-50%, 4 = 51-75%, and 5 = 75-100%). Endospores (30) is the average endospores/J2 from 30 randomly encountered J2 and Endospores (+) is the average endospores/J2 of only J2 with attached endospores.
Pasteuria penetrans bioassay.
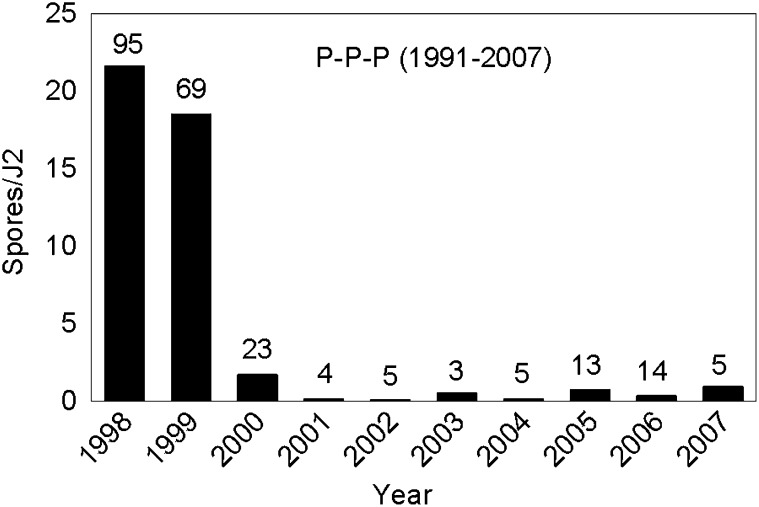
The average number of P. penetrans spores/J2 (GH population) declined from the fall of 1998 and 1999 to the spring of 2000 in all plots, but the decline was sharpest in the plots planted with continuous peanut in the previous rotation study (Table 1). The number of spores/J2 continued to decline from 2000 to 2001 in all the crop sequences (Fig. 2). In general, spore densities based on the bioassay remained low (< 4 spores/J2) relative to spore densities observed in the previous study (Table 1). This trend was also seen in the “old” P-P-P plots which had been in continuous peanut from 1991 to 2007 (Fig. 3). Densities were >15 spores/J2 in 1998 and 1999, but dropped to < 2 spores/J2 in 2000 and remained at this low level for 8 years.
Fig. 2.
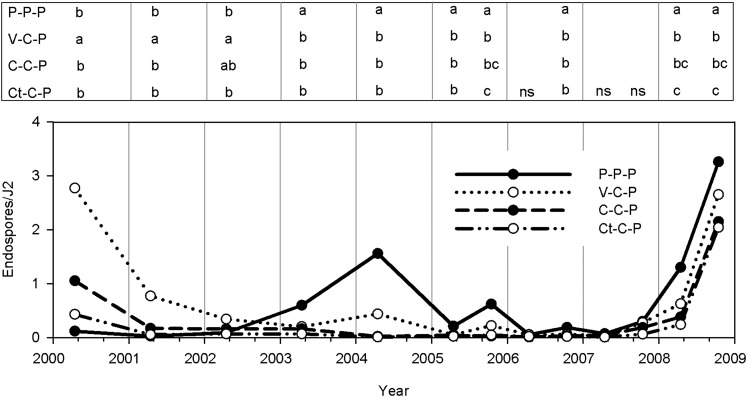
Densities of Pasteuria penetrans endospores based on a bioassay with second-stage juveniles (J2) of Meloidogyne arenaria in different crop sequences from 2000 to 2008. Soil samples for the bioassay were collected in the spring and or fall. Within each sampling time, letters in the legend indicate differences among crop sequences based on Fisher's LSD test (P ≤ 0.05). The crop sequences were continuous peanut (P) and peanut followed by 2 years of either corn (C), corn and cotton (Ct), or corn and vegetable (V, double crop of sweet corn and eggplant).
Fig. 3.
Densities of Pasteuria penetrans endospores based on a bioassay with second-stage juveniles (J2) of Meloidogyne arenaria in continuous peanut (Arachis hypogaea) from 1998 to 2007. These continuous peanut (P) plots were initiated in 1991. Numbers above bars represent the percentage of J2 with at least one attached spore.
Initially, endospore attachment to nematodes in the V-C-P rotation was greater (P ≤ 0.05) than the other rotations. However, by 2003, endospore attachment declined and was not different from the other rotations in subsequent years (Fig. 2). In the continuous peanut plots, the number of spores/J2 was initially very low, but increased in 2003 and 2004 to levels greater (P ≤ 0.05) than in plots where peanut was rotated with other crops. However, endospore attachment declined after that and no further increase was observed until 2008. In the fall of 2005 and in the spring and fall of 2008, the number of spores/J2 was highest in P-P-P and greater in V-C-P than in Ct-C-P (Fig. 2).
Nematode suppression in soil from different crop sequences.
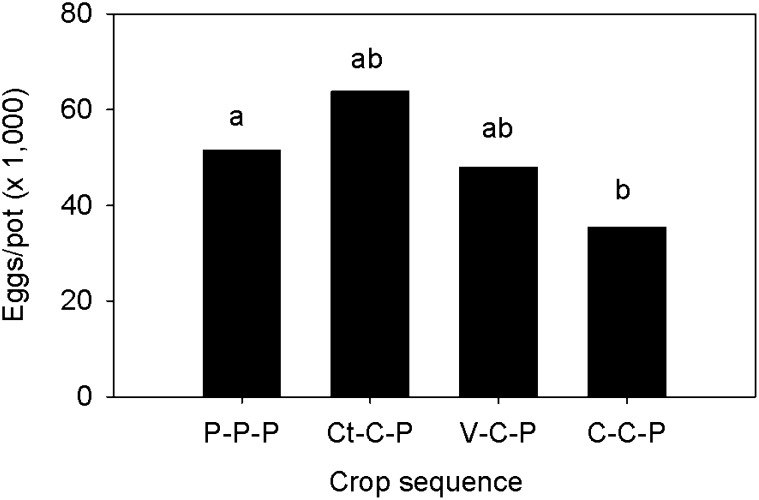
Reproduction of the GH population of M. arenaria on peanut was similar in soil from the P-P-P, Ct-C-P, and V-C-P plots (Fig. 4). Egg production was only lower (P ≤ 0.05) in soil from the C-C-P compared to the P-P-P plots. At the time the greenhouse experiment was conducted (2005), spore densities were greater in the P-P-P plots than in the rotated plots (Fig. 2).
Fig. 4.
Reproduction of a greenhouse population of Meloidogyne arenaria on peanut (Arachis hypogaea) in soil collected from the different crops sequences. Bars represent the mean of four replications and two trials (n = 8). Letters above bars indicate differences among crop sequences based on Fisher's LSD test (P ≤ 0.05) of the log-transformed data.
Diversity of the Meloidogyne population.
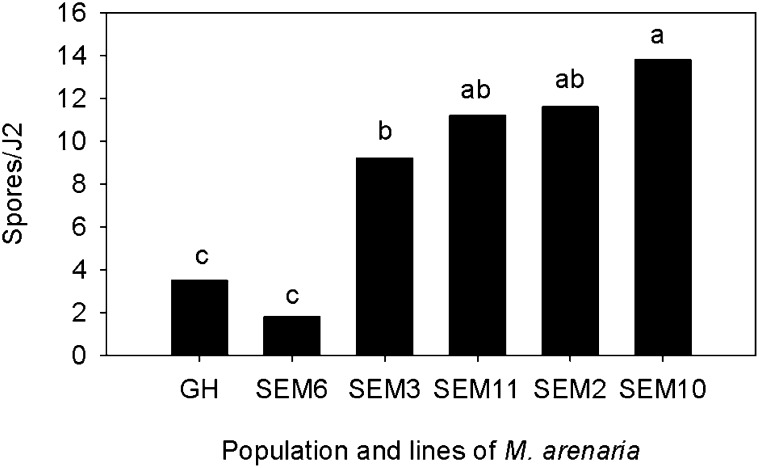
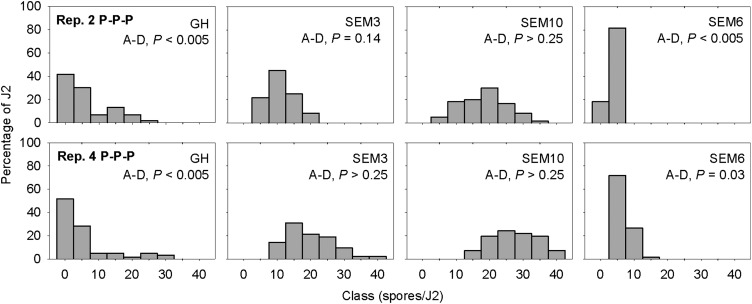
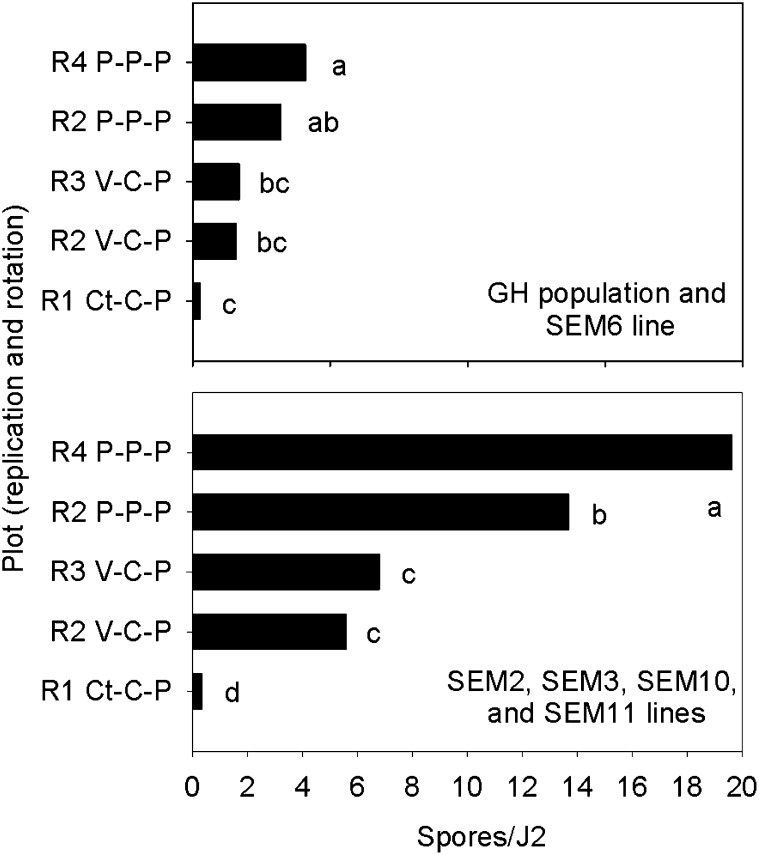
All of the Meloidogyne females examined from the continuous peanut plots at the Gibbs Farm, including the SEM6 and SEM3 lines, showed an A2 esterase phenotype typical of M. arenaria (Esbenshade and Triantaphyllou, 1990). The esterase phenotype of the other SEM lines was not determined. Four of the five SEM lines derived from M. arenaria at the Gibbs Farm acquired more spores from the P-P-P and V-C-P soils than the GH population and SEM6 line (Fig. 5). This trend was consistent among field replications and trials. Although the mean number of spores/J2 was similar for the GH population and SEM6 line, the frequency distribution of spore acquisition was different (Fig. 6). In the two replications of continuous peanut, the majority of J2 from SEM6 acquired 1 to 5 spores, whereas spore acquisition by the GH population was more variable, with 40% to 50% of J2 without spores and a few J2 with >15 spores. Spore acquisition by SEM2, SEM3, SEM10, and SEM11 followed a normal distribution (Anderson-Darling test, P > 0.05) in the continuous peanut plots (Fig. 6; data for SEM2 and SEM11 are not shown). The distribution frequencies of both the GH population and SEM6 line were skewed (Anderson-Darling test, P < 0.05). For all the SEM lines and the GH population, the number of spores/J2 was greatest in soil from the P-P-P plots, intermediate in the V-C-P plots, and lowest in the Ct-C-P plots. The amount of P. penetrans spores detected, however, was much greater when only SEM2, SEM3, SEM10, and SEM11 lines were used to assay the soils (Fig. 7).
Fig. 5.
Average number of Pasteuria penetrans endospores adhering to second-stage juveniles (J2) of Meloidogyne arenaria from a greenhouse (GH) population and from five single egg-mass lines (SEM2, SEM3, SEM6, SEM10, and SEM11) exposed to soil from the Gibbs Farm. Bars represent the mean of four plots (two continuous peanut and two vegetable-corn-peanut) and two trials (n = 8). Letters above bars indicate differences among the nematode population and lines based on Fisher's LSD test (P ≤ 0.05).
Fig. 6.
Frequency distribution of the number of endospores of Pasteuria penetrans adhering to second-stage juveniles (J2) of Meloidogyne arenaria from a greenhouse (GH) population and from three single egg-mass lines (SEM3, SEM6, and SEM10) exposed to soil from two field replicates of continuous peanut. The Anderson-Darling (A-D) test was used to determine whether spore acquisition by the different nematode sources differed from a normal distribution (P ≤ 0.05).
Fig. 7.
Average number of Pasteuria penetrans endospores adhering to second-stage juveniles (J2) of Meloidogyne arenaria from a greenhouse (GH) population and from five single egg-mass lines (SEM2, SEM3, SEM6, SEM10, and SEM11) exposed to soil from different crop sequences. Two field replicates (R) each of continuous peanut (P) and the vegetable-corn-peanut rotation (V-C-P) and one replicate of the cotton-corn-peanut rotation (Ct-C-P) were bioassayed for spore abundance. The two graphs represent different estimates of endospore abundance depending on whether non-receptive J2 (GH population and SEM6) or receptive J2 (SEM2, SEM3, SEM10, and SEM11) were used in the bioassay. Letters above bars within a graph indicate differences among crop sequences based on Fisher's LSD test (P ≤ 0.05).
Discussion
Under continuous cultivation of a host plant for Meloidogyne sp., the density of P. penetrans has been shown to rapidly increase to suppressive levels. In a field study, Melki et al. (1998) showed that an initial inoculum of 40,000 endospores/g soil resulted in <1 spore/assay J2 after one cycle of cucumber (3 mon), 2 spores/assay J2 after the second cycle, and 8 spores/assay J2 after the third cycle of cucumber. In microplots infested with M. arenaria, application of 100,000 and 10,000 spores/g soil reduced root galling of peanut by 81% and 61%, respectively, 2 years after initial application (Chen et al., 1996). After 3 years, root galling was reduced even in plots initially infested with only 1,000 and 3,000 spores/g soil (Chen and Dickson, 1998). Kariuki and Dickson (2007) used dried roots from an infested field site to transfer P. penetrans to another field site. Three years after infestation of the new field site, root galling on peanut was reduced to the same level as in plots fumigated with 1,3-dichloropropene. In the current study, the number of endospores per assay nematode did not substantially increase during 9 years of continuous peanut even though high spore densities had previously been detected at this field site using the same bioassay (Timper et al., 2001). Had native Meloidogyne J2 in the soil been examined, increased spore attachment over the 9-year study may have been observed. Bioassays were relied on exclusively to infer spore abundance because when levels of suppression are high, J2 are scarce and because the bioassay was effective in assessing relative spore densities in the previous study. It was assumed that the attachment specificity of the P. penetrans population at the Gibbs Farm would not change and that the bioassay would continue to be reliable.
Populations of P. penetrans differ in their host specificity. Endopsores of some populations readily adhere to several Meloidogyne spp., while others adhere to only a few populations within a nematode species (Channer and Gowen, 1992; Espanol et al., 1997; Stirling, 1985). Specificity of attachment appears to be at the nematode population level rather than at the species level because bacterial populations may attach to different nematode species, but not all nematode populations within a species (Stirling, 1985). Variation among spores both within and between populations of P. penetrans has been demonstrated with monoclonal antibodies (Mabs) specific to the surface of spores (Davies and Redden, 1997; Davies et al., 1994). Davies et al. (1994) demonstrated that Mabs recognized different subpopulations of spores within a single population of P. penetrans, and these different subpopulations displayed differential adherence to nematode populations. Individual J2 of Meloidogyne spp. within a population and even within single egg-mass lines are also known to be heterogeneous for spore attachment (Channer and Gowen, 1992; Davies et al., 2008). As a result of this heterogeneity within populations of P. penetrans and Meloidogyne spp., selection for both increased nematode resistance and increased attachment rates by the bacterium has been observed under laboratory and greenhouse conditions (Channer and Gowen, 1992; Tzortzakakis and Gowen, 1994; Tzortzakakis et al., 1996).
In this study, the data obtained using the single egg-mass (SEM) lines indicate that the population of M. arenaria at the Gibbs Farm is heterogeneous for spore attachment. The dominant attachment phenotype (represented by SEM2, SEM3, SEM10, and SEM11) acquired numerous spores from the soil, whereas the minor phenotype (represented by SEM6) acquired very few spores. The low percentage of J2 acquiring spores in the GH population compared to the SEM6 line suggests that the GH population (originally from the Gibbs Farm) is also heterogeneous for spore attachment (Fig 6). The SEM6 line appeared to be more resistant to attachment by the Gibbs Farm population of P. penetrans than the other SEM lines. It is not clear whether this resistance was to the entire spore population or to the dominant subpopulation of spores. In other words, SEM6 may be acquiring a different subpopulation of spores than SEM11, a situation similar to the observations of Davies et al. (1994).
There is circumstantial evidence that the population of P. penetrans at the field site has undergone a change in host preference, presumably in response to a change in the nematode population. The spore population readily adhered to the greenhouse population of M. arenaria in the past, but currently exhibits poor attachment (Timper et al., 2001). It is unlikely that the greenhouse population has changed because it is not under any selection pressure from P. penetrans. The following conceptual model is proposed to explain the observations in this study and the previous study: In the previous rotation study, a different genotype (designated Ma1) predominated in the field population of M. arenaria; this is also the dominant genotype in the greenhouse population. Ma1 was heavily parasitized by the predominant subpopulation of P. penetrans (designated Pp1), which also readily attached to the greenhouse population. Over time, a second genotype of M. arenaria (designated Ma2) that was resistant to Pp1 displaced Ma1 as the dominant genotype in the field. In the current rotation study, a subpopulation of P. penetrans (designated Pp2) that adapted to Ma2 became dominant. Such a scenario has been documented with Daphnia magna and its host-specific parasite Pasteuria ramosa, where the parasite caused a genetic shift in the host population to favor genotypes exhibiting lower rates of parasitism (Duncan and Little, 2007). In another study, populations of P. ramosa from pond sediment were shown to rapidly adapt over time to their host populations (Decaestecker et al., 2007).
Prior to initiating the current study, P. penetrans appeared to be in a stable equilibrium with M. arenaria, which kept the nematode population at very low levels for 9 years (Timper et al., 2001). This equilibrium seemed to be disrupted by moving the soil during land leveling at the end of 1999. Spore densities had been high in only 15% of the field site and leveling the land may have diluted these high densities. However, this “spore-dilution” hypothesis only explains the initial decline from 1999 to 2000, but does not explain the further decline in spore densities that occurred from 2000 to 2001 (Fig. 2). Monitoring the different genotypes of M. arenaria in the field over time and the subpopulations of P. penetrans that attach to them should clarify the complex dynamics that are occurring in this field between the nematode and its parasite.
Continuous planting of a host in soil infested with M. arenaria potentially leads to high levels of root galling (Minton et al., 1993; Timper et al., 2007). Root galling by M. arenaria in the continuous peanut plots was very high in 2002, but then declined and fluctuated around an index of 3 (1 to 5 scale) indicating that something was regulating the nematode population (Fig. 1). Based on bioassays of spore density and the greenhouse reproduction experiment in 2005, it did not appear that P. penetrans was contributing to this regulation. However, the greenhouse M. arenaria was weakly receptive to the P. penetrans spores in the soil. When only J2 with adhering spores were used to estimate spore abundance (these may have been receptive individuals from the greenhouse culture or native J2 in the soil), the density of P. penetrans seemed to correspond to increases and decreases in the nematode population (Fig. 1).
The initial objective of this study was to determine whether growing two different hosts for M. arenaria in a 3-year annual rotation cycle would lead to greater densities of P. penetrans spores and nematode suppression compared to conventional rotations. Greater spore densities were detected in the V-C-P rotation than in the Ct-C-P rotation using the bioassay (Fig. 2), but the differences were small (2.7 vs. 2.0 spores/J2). However, when the receptive clones from the field population were used to assay spore abundance, the differences between these two rotations were much greater (Fig. 7). It is likely that the spore density (6.2 spores/J2) in the V-C-P rotation was sufficient to suppress the dominant phenotype of M. arenaria. Despite the additional host plant (eggplant) in the vegetable rotation, root galling in peanut was similar among all the rotations.
A genetically heterogeneous population of Meloidogyne can complicate biological control. If selection pressure from P. penetrans is great enough, then the nematode population may shift to genotypes or species that are more resistant to the bacterium. Some populations of P. penetrans, such as the one at the Gibbs Farm, may be sufficiently diverse to adapt to these changes; however, other populations may be unable to adapt (Cetintas and Dickson, 2004; Trudgill et al., 2000). The application of mixed isolates of P. penetrans offers some protection against the development of resistant nematode populations (Channer and Gowen, 1992; Tzortzakakis and Gowen, 1994). The efficacy of this tactic will depend on the diversity of resistance genes in the nematode population and pathogenicity genes in the bacterial population. Ultimately, greater knowledge of the genetics underlying compatible and incompatible Meloidogyne-Pasteuria interactions is needed (Davies, 2009).
Footnotes
The author thanks David Clements, William Wilson, and A. Kyle Montfort for technical assistance, and Drs. Keith Davies and Richard Davis for reviewing the manuscript prior to submission.
Mention of trade names or commercial products in this publication is solely for the purpose of providing specific information and does not imply recommendation or endorsement by the U.S. Department of Agriculture.
This manuscript was edited by I.A. Zasada.
Literature Cited
- Ahmed R, Gowen SR. Studies on the infection of Meloidogyne spp. with isolates of Pasteuria penetrans. Nematologia mediterranea. 1991;19:229–233. [Google Scholar]
- Bird AF, Brisbane PG. The influence of Pasteuria penetrans in field soils on the reproduction of root-knot nematodes. Revue de Nematologie. 1988;11:75–81. [Google Scholar]
- Cetintas R, Dickson DW. Persistence and suppressiveness of Pasteuria penetrans to Meloidogyne arenaria race 1. Journal of Nematology. 2004;36:540–549. [PMC free article] [PubMed] [Google Scholar]
- Channer AGDR, Gowen SR. Selection for increased host resistance and increased pathogen specificity in the Meloidogyne - Pasteuria penetrans interaction. Fundamentals of Applied Nematology. 1992;15:331–339. [Google Scholar]
- Chen ZX, Dickson DM. Review of Pasteuria penetrans: Biology, ecology, and biological control potential. Journal of Nematology. 1998;30:313–340. [PMC free article] [PubMed] [Google Scholar]
- Chen ZX, Dickson DW, McSorley R, Mitchell DJ, Hewlett TE. Suppression of Meloidogyne arenaria race 1 by soil application of endospores of Pasteuria penetrans. Journal of Nematology. 1996;28:159–168. [PMC free article] [PubMed] [Google Scholar]
- Ciancio A, Queneherve P. Population dynamics of Meloidogyne incognita and infestation levels by Pasteuria penetrans in a naturally infested field in Martinique. Nematropica. 2000;30:77–86. [Google Scholar]
- Davies KG. Understanding the interaction between an obligate hyperparasitic bacterium, Pasteuria penetrans and its obligate plant-parasitic nematode host, Meloidogyne spp. Advances in Parasitology. 2009;68:211–245. doi: 10.1016/S0065-308X(08)00609-X. [DOI] [PubMed] [Google Scholar]
- Davies KG, Redden M. Diversity and partial characterization of putative virulence determinants in Pasteuria penetrans, the hyperparasitic bacterium of root-knot nematodes (Meloidogyne spp.) Journal of Applied Microbiology. 1997;83:227–235. doi: 10.1046/j.1365-2672.1997.00223.x. [DOI] [PubMed] [Google Scholar]
- Davies KG, Laird V, Kerry BR. The motility, development and infection of Meloidogyne incognita encumbered with spores of the obligate hyperparasite Pasteuria penetrans. Revue de Nematologie. 1991;14:611–618. [Google Scholar]
- Davies KG, Redden M, Pearson TK. Endospore heterogeneity in Pasteuria penetrans related to adhesion to plant-parasitic nematodes. Letters in Applied Microbiology. 1994;19:370–373. [Google Scholar]
- Davies KG, Rowe JA, Williamson VM. Inter- and intra-specific cuticle variation between amphimictic and parthenogenetic species of root-knot nematode (Meloidogyne spp.) as revealed by a bacterial parasite (Pasteuria penetrans) International Journal for Parasitology. 2008;38:851–859. doi: 10.1016/j.ijpara.2007.11.007. [DOI] [PubMed] [Google Scholar]
- Decaestecker E, Gaba S, Raeymaekers JAM, Stoks R, Van Kerckhoven L, Ebert D, De Meester L. Host-parasite 'Red Queen' dynamics archived in pond sediment. Nature. 2007;450:870–873. doi: 10.1038/nature06291. [DOI] [PubMed] [Google Scholar]
- Duncan AB, Little TJ. Parasite-driven genetic change in a natural population of Daphnia. Evolution. 2007;61:796–803. doi: 10.1111/j.1558-5646.2007.00072.x. [DOI] [PubMed] [Google Scholar]
- Esbenshade PR, Triantaphyllou AC. Isozyme phenotypes for the identification of Meloidogyne species. Journal of Nematology. 1990;22:10–15. [PMC free article] [PubMed] [Google Scholar]
- Espanol M, Verdejo-Lucas S, Davies KG, Kerry BR. Compatibility between Pasteuria penetrans isolates and Meloidogyne populations from Spain. Biocontrol Science and Technology. 1997;7:219–230. [Google Scholar]
- Giannakou IO, Pembroke B, Gowen SR, Davies KG. Effects of long term storage and above normal temperatures on spore adhesion of Pasteuria penetrans and infection of the root-knot nematode Meloidogyne javanica. Nematologica. 1997;43:185–192. [Google Scholar]
- Guillebeau P, editor. Cooperative Extension Service, University of Georgia: Athens, GA; 2009. Georgia pest control handbook, Special Bulletin 28. Available: www.ent.uga.edu/pmh/. Accessed 30 November 2009. [Google Scholar]
- Hussey RS, Barker KR. A comparison of methods of collecting inocula for Meloidogyne spp., including a new technique. Plant Disease Reporter. 1973;57:1025–1028. [Google Scholar]
- Jenkins WR. A rapid centrifugal-flotation technique for separating nematodes from soil. Plant Disease Reporter. 1964;48:692. [Google Scholar]
- Kariuki GM, Brito JA, Dickson DW. Effects of Pastueria penetrans endospore rate of attachment on root penetration and fecundity of Meloidogyne arenaria Race 1. Nematropica. 2006;36:261–267. [Google Scholar]
- Kariuki GM, Dickson DW. Transfer and development of Pasteuria penetrans. Journal of Nematology. 2007;39:55–61. [PMC free article] [PubMed] [Google Scholar]
- Kissel DE, Sonon L, editors. University of Georgia: Athens, GA; 2008. Soil test handbook for Georgia, Special Bulletin 62. in Cooperative Extension Service. Available: http://aesl.ces.uga.edu/publications/soil/STHandbook.pdf. Accessed 30 November 2009. [Google Scholar]
- Luc M, Sikora RA, Bridge J, editors. CABI Publishing: Wallingford; 2005. Plant parasitic nematodes in subtropical and tropical agriculture. [Google Scholar]
- Madulu JD, Trudgill DL, Phillips MS. Rotational management of Meloidogyne javanica and effects on Pasteuria penetrans and tomato and tobacco Yields. Nematologica. 1994;40:438–455. [Google Scholar]
- Melki KC, Giannakou IO, Pembroke B, Gowen SR. The cumulative build-up of Pasteuria penetrans spores in root-knot nematode infested soil and the effect of soil applied fungicides on its infectivity. Fundamental and Applied Nematology. 1998;21:679–683. [Google Scholar]
- Minton NA, Brenneman TB, Bondari K, Harrison GW. Activity of fosthiazate against Meloidogyne arenaria, Frankliniella spp., and Sclerotium rolfsii in peanut. Peanut Science. 1993;20:66–70. [Google Scholar]
- Oostendorp M, Dickson DW, Mitchell DJ. Population development of Pasteuria penetrans on Meloidogyne arenaria. Journal of Nematology. 1991;23:58–64. [PMC free article] [PubMed] [Google Scholar]
- Sayre RM, Wergin WP. Bacterial parasite of a plant nematode: morphology and ultrastructure. Journal of Bacteriology. 1977;129:1091–1101. doi: 10.1128/jb.129.2.1091-1101.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spaul VW. Observations of Bacillus penetrans infecting Meloidogyne in sugarcane fields in South Africia. Revue de Nematologie. 1984;7:277–282. [Google Scholar]
- Stirling GR. Host specificity of Pasteuria penetrans within the genus Meloidogyne. Nematologica. 1985;31:203–209. [Google Scholar]
- Stirling GR. C.A.B. International: Wallingford, England; 1991. Biological control of plant parasitic nematodes: Progress, problems and prospects. [Google Scholar]
- Timper P, Brenneman TB, Hanna WW, Wilson JP. Pearl millet as a rotation crop for peanut. Plant Health Progress. 2007 [Google Scholar]
- Timper P, Minton NA, Johnson AW, Brenneman TB, Culbreath AK, Burton GW, Baker SH, Gascho GJ. Influence of cropping systems on stem rot (Sclerotium rolfsii), Meloidogyne arenaria, and the nematode antagonist Pasteuria penetrans in peanut. Plant Disease. 2001;85:767–772. doi: 10.1094/PDIS.2001.85.7.767. [DOI] [PubMed] [Google Scholar]
- Trudgill DL, Bala G, Blok VC, Daudi A, Davies KG, Gowen SR, Fargette M, Madulu JD, Mateille T, Mwageni W, Netscher C, Phillips MS, Sawadogo A, Trivino CG, Voyoukallou E. The importance of tropical root-knot nematodes (Meloidogyne spp.) and factors affecting the utility of Pasteuria penetrans as a biocontrol agent. Nematology. 2000;2:823–845. [Google Scholar]
- Tzortzakakis EA, Gowen SR. Resistance of population of Meloidogyne spp. to parasitism by the obligate parasite Pasteuria penetrans. Nematologica. 1994;40:258–266. [Google Scholar]
- Tzortzakakis EA, Gowen SR, Goumas DE. Decreased ability of Pasteuria penetrans spores to attach to successive generations of Meloidogyne javanica. Fundamental and Applied Nematology. 1996;19:201–204. [Google Scholar]
- Verdejo-Lucas S. Seasonal population fluctuations of Meloidogyne spp. and the Pasteuria penetrans group in kiwi orchards. Plant Disease. 1992;76:1275–1279. [Google Scholar]
- Walker GE, Wachtel MF. The influence of soil solarisation and non-fumigant nematicides on infection of Meloidogyne javanica by Pasteuria penetrans. Nematologica. 1988;34:477–483. [Google Scholar]
- Weibelzahl-Fulton E, Dickson DW, Whitty EB. Suppression of Meloidogyne incognita and M. javanica by Pasteuria penetrans in field soil. Journal of Nematology. 1996;28:43–49. [PMC free article] [PubMed] [Google Scholar]