Abstract
Purpose
The purpose of our study was to validate diffusion-weighted MRI (DWI) before and after superparamagnetic iron oxide (SPIO) injection for assessment of hepatic metastases.
Materials and Methods
Eighty-six hepatic metastases (size range, 0.3-4.7 cm; mean, 1.5 cm) verified pathologically or by follow-up imaging studies in 22 consecutive patients (17 men and 5 women; 44-83 years; mean age, 60 years) during a 13-month period were enrolled. Hepatic MRI, including DWI (b-factors=50, 400, 800 s/mm2) with breath-holding technique of single-shot spin-echo echo-planar imaging (TR/TE=1000/69 ms, average=2) before and after SPIO administration, were retrospectively reviewed by two independent radiologists with a 5-point scale confidence score for each hepatic lesion on pre-contrast DWI (pre-DWI), SPIO-enhanced DWI (SPIO-DWI), and SPIO-enhanced T2*-weighted imaging (SPIO-T2*wI).
Results
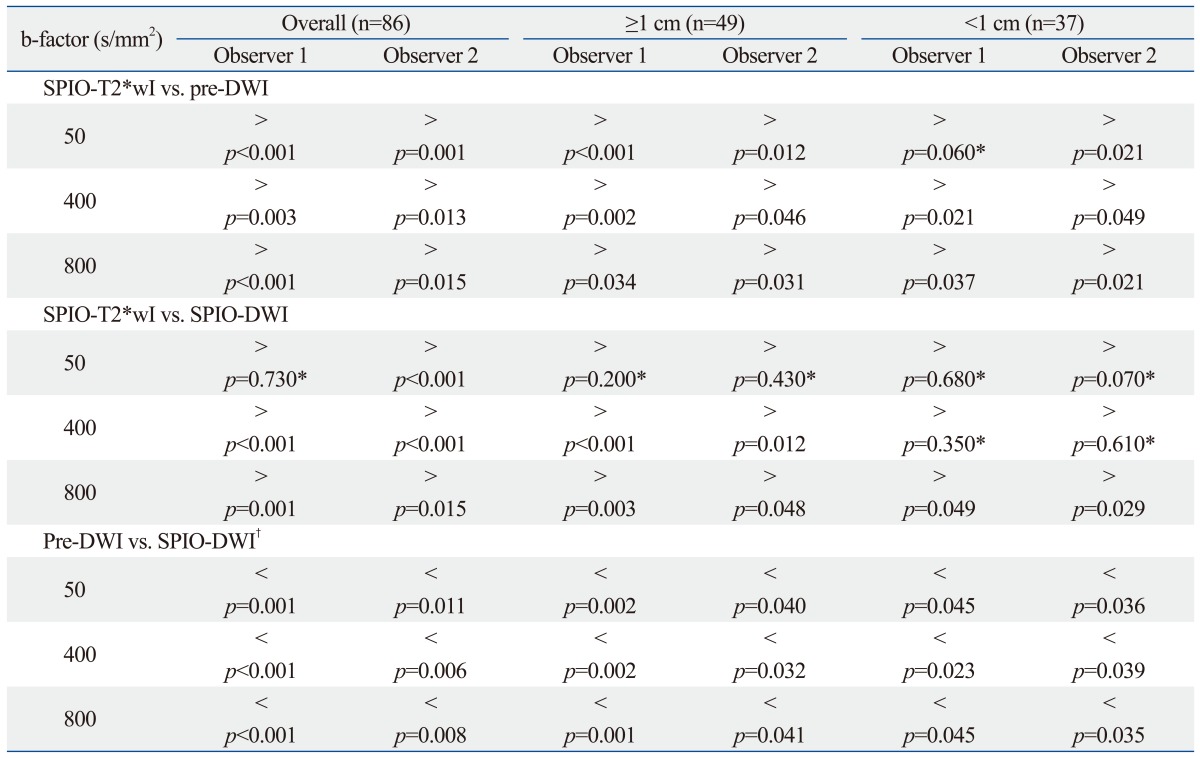
For all lesions, SPIO-T2*wI showed significantly higher confidence score in the diagnosis of hepatic metastases than pre-contrast or SPIO-DWI regardless of the size of b-factors (p<0.05) with only one exception; using b-factor=50 s/mm2, the score of SPIO-T2*wI was still higher than SPIO-DWI but there was no statistical significance given by observer 1 (p=0.730). For the subcentimeter lesions (n=37), SPIO-T2*wI showed the highest score, and using b-factor=50 or 400 s/mm2 SPIO-DWI showed similar confidence scores to SPIO-T2*wI by both observers (p>0.05). Pre-DWI using b-factor=50 sec/mm2 was also comparable with SPIO-T2*wI by observer 1 (p=0.060).
Conclusion
Pre-DWI has a limited value for the assessment of hepatic metastases, however, the repetition of DWI after SPIO injection using small b-factors could complement SPIO-T2*wI, especially for subcentimeter lesions.
Keywords: Liver, metastasis, diffusion magnetic resonance imaging, superparamagnetic iron oxide (SPIO)
INTRODUCTION
For patients with extrahepatic malignancies, especially metastases from colorectal cancer, it has been established that complete resection of these lesions is beneficial to the patient's prognosis and survival, and imaging assessment of disease extent is crucial for treatment planning.1,2 Among the various imaging techniques using computed tomography or magnetic resonance imaging (MRI), superparamagnetic iron oxide (SPIO)-enhanced MRI has been regarded as one of the most reliable methods for the detection of hepatic metastases.3 Utilizing recent advances in MR technology such as parallel imaging with the use of a phased-array multicoil, diffusion-weighted MRI (DWI) has been successfully applied in the assessment of focal hepatic lesions.4,5
Recently, some researchers have suggested that hepatic metastases tend to show higher signal intensities on DWI compared with fast spin-echo T2-weighted imaging (T2wI), and DWI is superior to the conventional T2wI in the detection of hepatic metastases.6 Despite a previous investigation by Nasu, et al.,7 which showed that DWI is comparable to SPIO-enhanced T2wI in the assessment of hepatic metastases, there are several unsolved problems in the clinical applications of DWI for examining focal liver lesions.8 One problem is that some metastatic liver tumors are invisible on DWI due to apparent diffusion coefficient (ADC) values similar to that of normal liver parenchyma.9 Another is the difficulty of standardization of the optimal b-factors in different machines made by various venders. Thirdly, it is common to have image degradation due to inherent motion artifacts in the left lobe of the liver.
As for SPIO-enhanced MRI, there is a preliminary study with a limited number of patients that DWI performed after SPIO injection could improve the recognition of malignant focal lesions by increasing the lesion-to-liver contrast which depends on the signal drop of background parenchyma with normal phagocytic function of Kupffer cells after SPIO injection compared with conventional pre-contrast DWI (pre-DWI).10 The purpose of our present study was to validate DWI with the use of various b-factors before and after SPIO injection for MRI assessment of hepatic metastases and compare them with the conventional SPIO-enhanced T2*-weighted imaging (SPIO-T2*wI).
MATERIALS AND METHODS
Patient group
Approval for this study was obtained from the institutional review board at our hospital, which waived the requirement for informed consent from individual patients. During a 13-month period from March 2007 to March 2008, 299 consecutive patients underwent hepatic MRI with DWI, gadolinium-enhanced dynamic imaging, and subsequent SPIO-enhanced MRI for the assessment of focal liver lesions in our institution. To be enrolled as subjects in this study, patients should have one or more metastatic lesions in the liver from extrahepatic malignancy and the lesions should be verified pathologically or by follow-up imaging studies. Electronically stored clinical records and imaging interpretation notes obtained from all 299 patients were reviewed by a study coordinator, and a total of 22 patients (17 men and 5 women; 44-83 years; mean age, 60 years) were finally selected. Among 86 lesions found in the subjected patients. 21 lesions were confirmed by pathology from operation or biopsy (n=21) or follow-up imaging studies (n=65). At follow-up imaging (range to 5 to 13 months), a lesion was deemed malignant if it showed 20% or greater in size. The primary cancer sites of these patients were as follows: 13 colorectal cancers, 3 gastric cancers, 2 breast cancers, 2 renal cell carcinomas, 1 pancreatic cancer, and 1 nasopharyngeal cancer. There was only 1 lesion in nine patients, 2 nodules in three patients, 3 nodules in one patient, 5 nodules in three patients, and six patients had 6, 7, 8, 9, 10 or 13 nodules in each patient.
MRI techniques
MRI was performed using a 1.5-T system (Magnetom Avanto; Siemens, Erlangen, Germany) equipped with high-performance gradients (maximum amplitude 45 mT/m) and a six-element phased-array surface coil.
The routine MRI protocol of our institute consisted of spectral fat suppression, T2-weighted, half-Fourier acquisition, single-shot turbo spin-echo images, T1-weighted double-echo chemical shift gradient-echo (GRE) sequence, and dynamic contrast-enhanced imaging, using a 3D GRE sequence in the axial plane consisting of one pre-contrast series, followed by three successive post-contrast series (early arterial, late arterial, and portal phase imaging), and by 5-min delayed phase imaging after administration of a bolus injection of gadopentetate dimeglumine (0.1 mmol/kg of Magnevist; Bayer HealthCare, Berlin, Germany). After the completion of dynamic imaging, SPIO (8 µmol iron/kg of Resovist; Bayer HealthCare, Berlin, Germany) was intravenously injected as the second contrast agent for the SPIO-enhanced T2-weighted imaging and T2*wI. After 10 minutes, free-breathing navigator-triggered T2wI was obtained using turbo spin-echo sequence (TR 3000-6000 ms, TE 60 ms, flip angle 150°, echo train length 13, bandwidth 260 kHz, matrix 384×193, FOV 273×379 mm, slice thickness 6 mm, interslice gap 1.2 mm, slices 30), followed by a breath-hold 2D GRE sequence (TR 196 ms, TE 10 ms, flip angle 30°, matrix 320×168, slice thickness 6 mm, interslice gap 1.2 mm) for SPIO-T2*wI.
DWI was performed using a single-shot spin-echo echo-planar imaging sequence that combined two diffusion (motion-probing) gradients before and after 180° pulse along all three directions - the section-select, phase-encoding, and frequency-encoding directions - and data acquisition with an echo-planar imaging (EPI) readout by applying three different b-factors of 50, 400, and 800 s/mm2. Other technical parameters were: TR 1000 ms, TE 69 ms, matrix 128×192, FOV 308×379 mm, slices 27 (9 slices for each b-factor), thickness 6 mm, interslice gap 30%, average 2, bandwidth 1735 Hz/pixel, and acquisition time 22 s. Parallel imaging algorithms (GRAPPA) with an acceleration factor of 2 were added to reduce acquisition time. Spectral fat saturation was employed systematically to suppress chemical-shift artifacts. The sequence was obtained within a single breath-holding period for upper half of the liver and another single breath-holding period for lower half of the liver, and a total of 18 slices were acquired for each b-factor. Two different DWI sets (pre- and post-SPIO) were obtained with the same imaging parameters. Pre-DWI was performed before the dynamic imaging, and SPIO-enhanced DWI (SPIO-DWI) was performed just before the acquisition of SPIO-enhanced T2 and T2*wI. All scans were sent to picture archiving and communication system (PACS) for interpretation on PACS workstations.
Image analyses
For the lesions studied, lesion localization and size measurements were performed by the study coordinator using electronic arrows and calipers on various images. The electronic arrows were digitally saved, and the location and size of each lesion were recorded on a data sheet with the image number for the future analyses. Pre-contrast T1-weighted spoiled GRE images were used for the localization and size measurement of each lesion if possible. The SPIO-enhanced T2wI or the portal phase images of dynamic 3D GRE were secondarily or thirdly used for the same purpose. For the lesions exclusively defined on the SPIO-T2*wI (n=2) or SPIO-DWI (n=6), localization and size measurements were performed on those images alone.
Two observers (10 years and 2 years of experience in abdominal imaging, respectively) independently reviewed seven different imaging sets consisted of SPIO-T2*wI, three pre-DWI with different b-factors (50, 400, 800 s/mm2) and three SPIO-DWI with different b-factors (50, 400, 800 s/mm2), and scored the individual arrow-indicated lesions according to the 5-point confidence scale (1, no distinguishable abnormal signals from background parenchyma; 2, poorly defined abnormal signals but not well distinguished from background parenchyma; 3, poorly defined but well distinguished from background parenchyma; 4, well defined with marginal blurring; 5, well defined with sharp margin).
Statistical analyses
According to the longest dimension, each lesion was categorized into one of the two groups (1 cm or greater, less than 1 cm) and separately analyzed. Lesion location was also divided into two categories (right versus left hepatic lobes) and separately analyzed. For convenience of statistical analysis, caudate lobe lesions were included in right lobe lesions in the present study. By using Wilcoxon signed rank test, the confidence scores of each lesion in pre-DWI vs. SPIO-DWI, pre-DWI vs. SPIO-T2* weighted images, and SPIO-DWI vs. SPIO-T2*-weighted images were compared to each other. SPSS version 15.0 (SPSS Inc., Chicago, IL, USA) was used for statistical analysis, and a p value of less than 0.05 was considered statistically significant. Interobserver variability was assessed for lesion scoring (0.00-0.20, indicated slight agreement; 0.21-0.40, fair agreement; 0.41-0.60, moderate agreement; 0.61-0.80, substantial agreement; 0.81-1.00, almost perfect agreement) using Cohen Kappa tests.
RESULTS
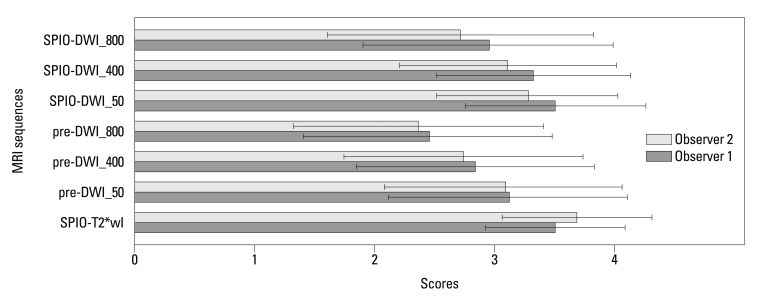
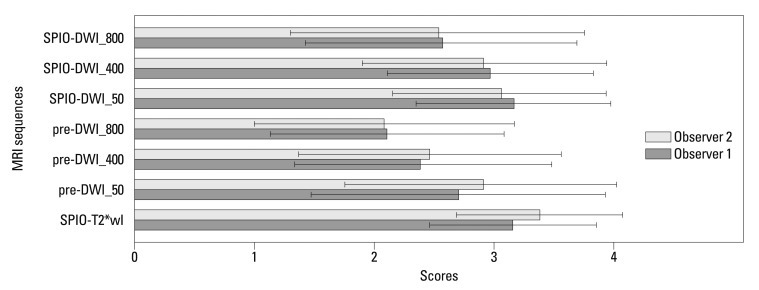
For all 86 lesions (0.3-4.7 cm; mean, 1.5 cm) studied, the mean confidence scores on each of the seven image sequences were calculated and compared with each other (Fig. 1). The mean confidence score of SPIO-T2*wI was always higher than those of pre-contrast or SPIO-DWI, regardless of the b-factors, as rated by the two observers; all mean confidence score of SPIO-T2*wI was statistically significant (p<0.05), except SPIO-DWI using a b-factor of 50 s/mm2 scored by observer 1 (p=0.730) (Table 1) (Fig. 2). Compared with pre-DWI, the mean confidence scores of SPIO-DWI were always higher using the same b-factors (p<0.05).
Fig. 1.
Mean confidence scores with standard deviations of each MRI sequence for overall lesions. SPIO-T2*wI, SPIO-enhanced T2*wI; Pre-DWI_50, pre-contrast DWI with a b-factor of 50 s/mm2; pre-DWI_400, pre-contrast DWI with a b-factor of 400 s/mm2; pre-DWI_800, pre-contrast DWI with a b-factor of 800 s/mm2; SPIO-DWI_50, SPIO-enhanced DWI with a b-factor of 50 s/mm2; SPIO-DWI_400, SPIO-enhanced DWI with a b-factor of 400 s/mm2; SPIO-DWI_800, SPIO-enhanced DWI with a b-factor of 800 s/mm2. SPIO, superparamagnetic iron oxide; DWI, diffusion-weighted MRI; MRI, magnetic resonance imaging; T2*wI, T2* weighted imaging.
Table 1.
Comparison of Mean Confidence Scores according to Lesion Size on Seven Different Sequences
T2*wI, T2*-weighted imaging; DWI, diffusion-weighted MR imaging; SPIO, superparamagnetic iron oxide.
The direction of inequality signs means the relative superiority between two sequences in comparison for the mean confidence score accompanied with p values.
*A p value >0.05 was not considered statistically significant.
†For comparison of pre-DWI with SPIO-DWI, only the corresponding sequence images using same b-factor were compared each other.
Fig. 2.
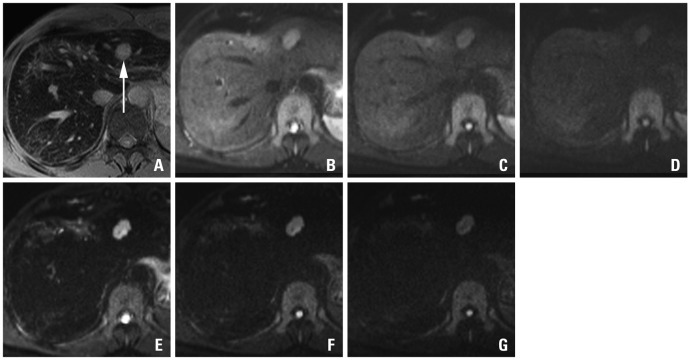
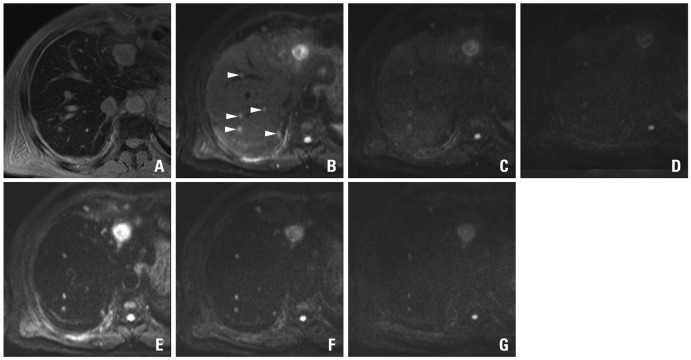
A 48-year-old man with sigmoid colon cancer. SPIO-enhanced T2*-weighted image (A) shows a metastatic lesion (arrow) in the lateral segment. On the pre-contrast DWIs using b-factor (s/mm2) of 50 (B), 400 (C) and 800 (D), relative signal intensities of the lesions are gradually decreased with the increase of b-factors. On the SPIO-enhanced DWIs using b-factor (s/mm2) of 50 (E), 400 (F) and 800 (G), the lesions are more conspicuously seen, especially on the images using smaller b-factors. With an increase of b-factors, marginal blurring of the lesion looks more severe. SPIO, superparamagnetic iron oxide; DWI, diffusion-weighted MRI.
For the lesions greater than or equal to 1 cm (n=49), the mean confidence score of SPIO-T2*wI was always higher than those of pre- or SPIO-DWI irrespective of the different b-factors; all mean confidence scores of SPIO-T2*wI were statistically significantly higher (p<0.05) than other sequences except SPIO-DWI using a b-factor of 50 s/mm2 (observer 1, p=0.200; observer 2, p=0.430) (Fig. 3). When the confidence scores were compared to each other between the pre-contrast and SPIO-DWI with corresponding b-factors, SPIO-DWI always showed higher scores regardless of the different b-factors (p<0.05).
Fig. 3.
Mean confidence scores with standard deviations of each MRI sequence for 1 cm or larger lesions. SPIO-T2*wI, SPIO-enhanced T2*wI; Pre-DWI_50, pre-contrast DWI with a b-factor of 50 s/mm2; pre-DWI_400, pre-contrast DWI with a b-factor of 400 s/mm2; pre-DWI_800, pre-contrast DWI with a b-factor of 800 s/mm2; SPIO-DWI_50, SPIO-enhanced DWI with a b-factor of 50 s/mm2; SPIO-DWI_400, SPIO-enhanced DWI with a b-factor of 400 s/mm2; SPIO-DWI_800, SPIO-enhanced DWI with a b-factor of 800 s/mm2. SPIO, superparamagnetic iron oxide; DWI, diffusion-weighted MRI; MRI, magnetic resonance imaging; T2*wI, T2* weighted imaging.
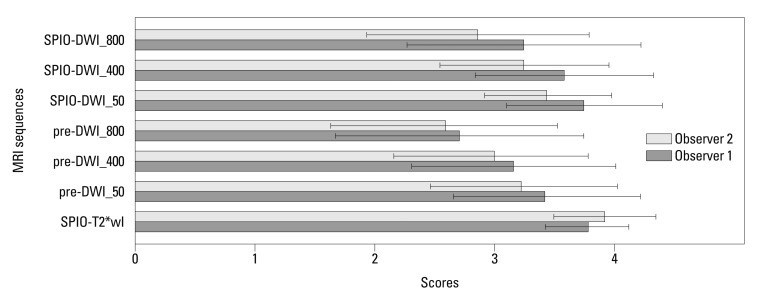
For lesions less than 1 cm (n=37), SPIO-T2*wI showed the highest mean qualitative score and SPIO-DWI (b-factors of 50 and 400 s/mm2) showed confidence scores similar to SPIO-T2*wI (observer 1, p=0.680 and 0.350, respectively; observer 2, p=0.070 and 0.610, respectively). Pre-DWI using a b-factor of 50 s/mm2 was also comparable with SPIO-T2*wI as rated by observer 1 (p=0.060). Compared with pre-DWI, the mean confidence scores of SPIO-DWI using the corresponding b-factors were always greater, even for the subcentimeter lesions (p<0.05) (Figs. 4, 5 and 6).
Fig. 4.
Mean confidence scores with standard deviations of each MRI sequence for lesions smaller than 1 cm. SPIO-T2*wI, SPIO-enhanced T2*wI; Pre-DWI_50, pre-contrast DWI with a b-factor of 50 s/mm2; pre-DWI_400, pre-contrast DWI with a b-factor of 400 s/mm2; pre-DWI_800, pre-contrast DWI with a b-factor of 800 s/mm2; SPIO-DWI_50, SPIO-enhanced DWI with a b-factor of 50 s/mm2; SPIO-DWI_400, SPIO-enhanced DWI with a b-factor of 400 s/mm2; SPIO-DWI_800, SPIO-enhanced DWI with a b-factor of 800 s/mm2. SPIO, superparamagnetic iron oxide; DWI, diffusion-weighted MRI; MRI, magnetic resonance imaging; T*wI, T2* weighted imaging.
Fig. 5.
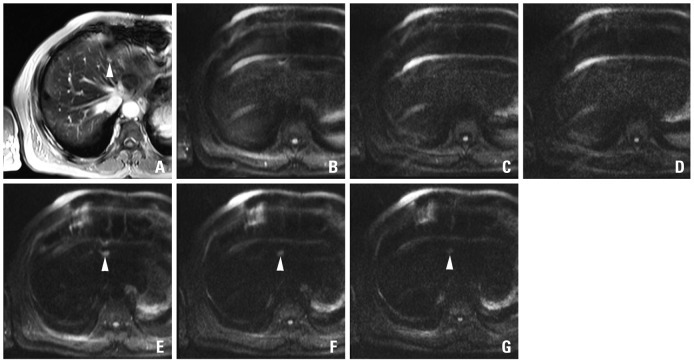
A 69-year-old man with colon cancer and numerous metastases throughout the liver including the largest one in the left hemiliver. On the SPIO-enhanced T2*-weighted image (A), metastatic lesions are not well distinguished from the background liver due to small lesion size and masking effect of the intrahepatic vasculature with high signal intensity. On the pre-contrast DWIs using b-factor (s/mm2) of 50 (B), at least 5 metastases (arrowheads) in a single level of right hemiliver are well delineated from the background liver due to 'black-blood' effect. The confidence is worsened on the images using higher b-factors of 400 (C) or 800 (D) with low signal intensity and marginal blurring of the lesions. On the SPIO-enhanced DWIs using b-factor (s/mm2) of 50 (E) or 400 (F), all five lesions are conspicuously seen, compared with poor marginal definition on the image using the b-factor of 800 (G). Compared with pre-contrast DWI, SPIO-enhanced DWI using corresponding b-factors shows consistently higher lesion-to-liver contrasts. SPIO, superparamagnetic iron oxide; DWI, diffusion-weighted MRI.
Fig. 6.
A 69-year-old man with multiple hepatic metastases in the left hemiliver. A small metastatatic lesion (arrowhead) on SPIO-enhanced T2*-weighted image (A) is not well distinguished from the background liver due to small size and the masking effect of the intrahepatic vasculature. On the pre-contrast DWIs using b-factor (s/mm2) of 50 (B), 400 (C) and 800 (D), there is severe motion artifact and no focal lesion is defined. On the SPIO-enhanced DWIs using b-factor (s/mm2) of 50 (E), 400 (F) and 800 (G), the lesion (arrowhead) is well delineated due to the increased lesion-to-liver contrast in spite of the motion artifact. SPIO, superparamagnetic iron oxide; DWI, diffusion-weighted MRI.
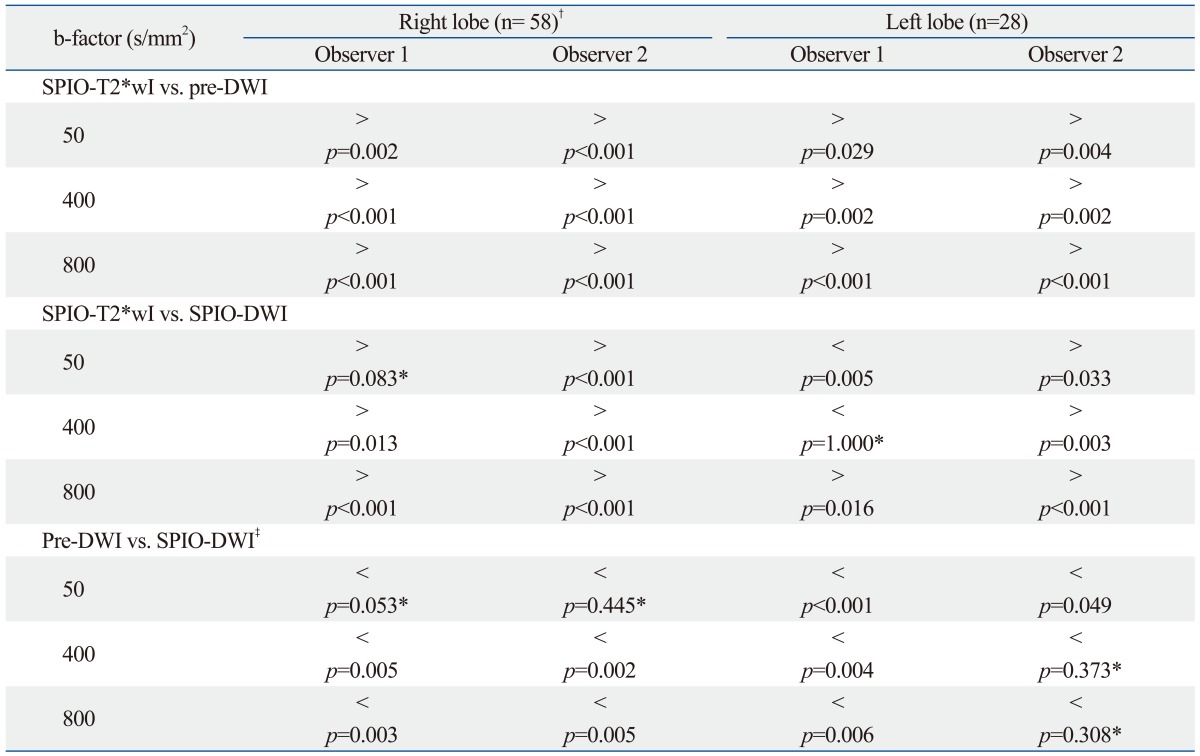
There were 58 lesions (mean size±standard deviation, 1.3±1.0 cm) in the right hepatic lobe including two caudate lobe lesions and 28 lesions (mean size±standard deviation, 1.9±1.3 cm) in the left hepatic lobe. Even in the left hepatic lobe lesions, SPIO-T2*wI had highest confidence score compared with pre-DWI or SPIO-DWI in most cases except for the comparison with SPIO-DWI by observer 1 who showed higher confidence score of SPIO-DWI with (b-factors of 50 s/mm2, p<0.001) or without statistical significance (b-factors of 400 s/mm2, p=1.000) (Table 2). Like the right lobe lesions, left lobe lesions also showed the superiority of SPIO-DWI compared to pre-DWI with (all b-factors by observer 1 and b-factor of 50 s/mm2 by observer 2, p<0.05) or without statistical significance (b-factors of 400 and 800 s/mm2 by observer 2, p>0.3) (Table 2).
Table 2.
Comparison of Mean Confidence Scores according to Lesion Location on Seven Different Sequences
T2*wI, T2*-weighted imaging; DWI, diffusion-weighted MR imaging; SPIO, superparamagnetic iron oxide.
The direction of inequality signs means the relative superiority between two sequences in comparison for the mean confidence score accompanied with p values.
*A p value >0.05 was not considered statistically significant.
†Including two caudate lobe lesions.
‡For comparison of pre-DWI with SPIO-DWI, only the corresponding sequence images using same b-factor were compared each other.
The linear-weighted kappa values for interobserver agreement in the confidence scoring showed moderate or substantial agreements for all MRI sequences (SPIO-T2*wI, 0.50; SPIO-DWI with a b-factor of 50 s/mm2, 0.47; SPIO-DWI with a b-factor of 400 s/mm2, 0.51; SPIO-DWI with a b-factor of 800 s/mm2, 0.52; pre-DWI with a b-factor of 50 s/mm2, 0.63; pre-DWI with a b-factor of 400 s/mm2, 0.55; pre-DWI using a b-factor of 800 s/mm2, 0.52).
DISCUSSION
When dealing with contrast-enhanced hepatic MRI, SPIO reduces the signal intensity of normal liver parenchyma in a manner dependent on the phagocytic effect of Kupffer cells, resulting in an increase of lesion-to-liver contrast in cases of hepatic malignancy and, therefore, SPIO-enhanced MRI has been thought to be one of the best imaging tools in the assessment of hepatic metastases.11-13 DWI creates image contrast by the difference in the random, thermally-agitated diffusion of water molecules in different tissues and has recently been included in the routine protocol of hepatic MRI with recent technical advances in reducing image distortion and increasing the signal-to-noise ratio.14
According to some recent investigations, DWI provids better sensitivity and comparable ability of lesion characterization, compared to the conventional breath-hold T2wI, in the assessment of focal hepatic lesions.6 In a comparative study between SPIO-enhanced imaging and DWI, Nasu, et al.7 showed that the combined interpretation of DWI, fast T2wI, and dual-echo gradient T1-weighted images yielded better accuracy than SPIO-enhanced T2wI in the evaluation of hepatic metastases, and suggested the potential of DWI to replace SPIO-enhanced T2wI in the preoperative evaluation of hepatic metastases.
Meanwhile, Naganawa, et al.10 applied DWI along with high b-factors (600 and 1000 s/mm2) before and after the administration of SPIO during hepatic MRI for the assessment of focal liver lesions in 5 patients with hepatic metastases, and concluded that SPIO-DWI could enhance the recognition of hepatic metastases. In their study, the number of lesions detected on SPIO-DWI was larger than other imaging sequences including SPIO-T2, T2* and pre-DWI, however, none of the lesions was verified pathologically or by follow-up studies.10 In the present study, we attempted to explore whether pre-DWI would be sufficient to replace SPIO-T2*wI and whether SPIO-DWI would be helpful in improving the lesion-to-liver contrast, depending on the lesion size and various b-factors that are available, with the use of a number of verified lesions of hepatic metastases.
In the present study, SPIO-T2*wI showed significantly better confidence scores than pre-DWI, regardless of the lesion size or the different b-factors, and only SPIO-DWI showed comparable confidence levels when the small b-factor (50 s/mm2) was used. Based on the present results, pre-DWI would not be sufficient to replace SPIO-T2*wI due to limited spatial resolution and image artifacts in addition to inherently unpredictable lesion-to-liver contrast of various hepatic metastases. With smaller b-factors, diffusion effect is not so distinctively reflected on the images, especially for the solid lesions, and the imaging characteristics of DWI were similar to those of T2wI except for the black blood effect.15 In this situation, injection of SPIO would increase the lesion-to-liver contrast in DWI for some lesions which could be missed on T2wI or DWI with low b-factors, resulting in the increase of lesion conspicuity that would be comparable with the SPIO-T2*wI in the present study. In a recent study by Coenegrachts, et al.16 comparing SPIO-enhanced T2wI with pre-contrast and SPIO-DWI for various benign and malignant lesions, lesion visibility of hepatic metastases from colorectal cancers was comparable between the pre-contrast and SPIO-DWI because of increased lesion-to-liver contrast after injection of SPIO despite degradation of overall image quality due to the size of the b-factors. In the present study, the inferiority of pre-DWI might be related to inhomogeneous group of extrahepatic primary lesion sites, which could be less conspicuous on the precontrast T2wI or DWI using a small b-factor than with the SPIO-enhanced images.
For smaller (<1 cm) lesions, however, pre-DWI with a low b-factor (50 s/mm2) in addition to the SPIO-DWI with relatively low b-factors (50 and 400 s/mm2) showed quality scores comparable to SPIO-T2*wI in the present study. Due to the fact that small lesions can be obscured by vascular structures on SPIO-T2*wI, pre-DWI could possess a comparable ability in the detection of small lesions despite the inherent limitations mentioned above (Fig. 4). For SPIO-DWI in the detection of subcentimeter lesions, Kiryu, et al.17 also concluded that SPIO-DWI is able to recognize subcentimeter hepatic metastases because of suppression effect of vascular signals, masking the small lesions on SPIO-enhanced T2wI. Even though the previous study by Kiryu, et al.17 used the PROPELLER T2 fast spin echo sequence instead of the spin-echo EPI used in the present study, our results are similar to theirs. Although not analyzed in this study, the quality of SPIO-DWI can also be limited by increased blooming effect, the susceptibility artifact from iron particles, which is worsened by increasing the b-factors.18 Despite diminution in the size of lesions by the blooming effect, however, the b-factor of 400 s/mm2 showed comparable confidence scores with increased lesion-to-liver contrast of T2*wI after SPIO injection.
This study has several limitations. First of all, due to retrospective nature of this study, pathologic proof was not possible for all lesions. Secondly, this study focused on the comparative conspicuity of each metastatic lesion from various primary malignancies on the imaging sequences studied, and the sensitivity or the specificity of each sequence was not calculated. Thirdly, there was a high potential of unpredictable image degradation for the left lobe lesions related to pulsation artifacts.7 However, even in the left hepatic lobe lesions, the mean confidence score of SPIO-DWI was significantly higher than pre-DWI, especially on the images, using small b-factor in the present study. Fourthly, according to inherent limitation of breath-holding technique for DWI, the number of excitation was limited and TR was too short to avoid T1 effect, thus resulting in lowering the signal-to-noise ratio and lesion conspicuity, especially on pre-DWI. Increasing the number of excitation and lengthening of TR would improve the quality of pre-DWI by using free-breathing data acquisition techniques which was not performed during our study period. Lastly, due to limited number of patients, separate analysis according to the nature of primary extrahepatic lesions was not possible and ADC value was not measured for each lesion.
In conclusion, compared with SPIO-T2*wI, pre-DWI could not reach a comparable confidence score for the detection of hepatic lesions, except subcentimeter lesions, on pre-DWI with a small b-factor (50 s/mm2). Despite the limitations mentioned above, using breath-holding technique, pre-DWI is insufficient to replace SPIO-enhanced imaging for hepatic metastases from various primary origins. Depending on the increased confidence score, additional DWI after SPIO injection could complement SPIO-T2*wI in the assessment of small hepatic metastases.
Footnotes
The authors have no financial conflicts of interest.
References
- 1.Ward J. New MR techniques for the detection of liver metastases. Cancer Imaging. 2006;6:33–42. doi: 10.1102/1470-7330.2006.0007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Penna C, Nordlinger B. Colorectal metastasis (liver and lung) Surg Clin North Am. 2002;82:1075–1090. doi: 10.1016/s0039-6109(02)00051-8. [DOI] [PubMed] [Google Scholar]
- 3.Robinson PJ. Imaging liver metastases: current limitations and future prospects. Br J Radiol. 2000;73:234–241. doi: 10.1259/bjr.73.867.10817037. [DOI] [PubMed] [Google Scholar]
- 4.Oner AY, Celik H, Oktar SO, Tali T. Single breath-hold diffusion-weighted MRI of the liver with parallel imaging: initial experience. Clin Radiol. 2006;61:959–965. doi: 10.1016/j.crad.2006.06.014. [DOI] [PubMed] [Google Scholar]
- 5.Erturk SM, Ichikawa T, Sano K, Motosugi U, Sou H, Araki T. Diffusion-weighted magnetic resonance imaging for characterization of focal liver masses: impact of parallel imaging (SENSE) and b value. J Comput Assist Tomogr. 2008;32:865–871. doi: 10.1097/RCT.0b013e3181591cf2. [DOI] [PubMed] [Google Scholar]
- 6.Parikh T, Drew SJ, Lee VS, Wong S, Hecht EM, Babb JS, et al. Focal liver lesion detection and characterization with diffusion-weighted MR imaging: comparison with standard breath-hold T2-weighted imaging. Radiology. 2008;246:812–822. doi: 10.1148/radiol.2463070432. [DOI] [PubMed] [Google Scholar]
- 7.Nasu K, Kuroki Y, Nawano S, Kuroki S, Tsukamoto T, Yamamoto S, et al. Hepatic metastases: diffusion-weighted sensitivity-encoding versus SPIO-enhanced MR imaging. Radiology. 2006;239:122–130. doi: 10.1148/radiol.2383041384. [DOI] [PubMed] [Google Scholar]
- 8.Müller MF, Prasad PV, Siewert B, Edelman RR. [The in-vivo diffusion measurements of the liver, kidneys, spleen and m. erector with an echo-planar imaging system in normal subjects] Rofo. 1994;161:233–236. doi: 10.1055/s-2008-1032527. [DOI] [PubMed] [Google Scholar]
- 9.Ichikawa T, Haradome H, Hachiya J, Nitatori T, Araki T. Diffusion-weighted MR imaging with a single-shot echoplanar sequence: detection and characterization of focal hepatic lesions. AJR Am J Roentgenol. 1998;170:397–402. doi: 10.2214/ajr.170.2.9456953. [DOI] [PubMed] [Google Scholar]
- 10.Naganawa S, Sato C, Nakamura T, Kumada H, Ishigaki T, Miura S, et al. Diffusion-weighted images of the liver: comparison of tumor detection before and after contrast enhancement with superparamagnetic iron oxide. J Magn Reson Imaging. 2005;21:836–840. doi: 10.1002/jmri.20346. [DOI] [PubMed] [Google Scholar]
- 11.Senéterre E, Taourel P, Bouvier Y, Pradel J, Van Beers B, Daures JP, et al. Detection of hepatic metastases: ferumoxides-enhanced MR imaging versus unenhanced MR imaging and CT during arterial portography. Radiology. 1996;200:785–792. doi: 10.1148/radiology.200.3.8756932. [DOI] [PubMed] [Google Scholar]
- 12.Vogl TJ, Schwarz W, Blume S, Pietsch M, Shamsi K, Franz M, et al. Preoperative evaluation of malignant liver tumors: comparison of unenhanced and SPIO (Resovist)-enhanced MR imaging with biphasic CTAP and intraoperative US. Eur Radiol. 2003;13:262–272. doi: 10.1007/s00330-002-1677-7. [DOI] [PubMed] [Google Scholar]
- 13.Strotzer M, Gmeinwieser J, Schmidt J, Fellner C, Seitz J, Albrich H, et al. Diagnosis of liver metastases from colorectal adenocarcinoma. Comparison of spiral-CTAP combined with intravenous contrast-enhanced spiral-CT and SPIO-enhanced MR combined with plain MR imaging. Acta Radiol. 1997;38:986–992. doi: 10.1080/02841859709172115. [DOI] [PubMed] [Google Scholar]
- 14.Taouli B, Koh DM. Diffusion-weighted MR imaging of the liver. Radiology. 2010;254:47–66. doi: 10.1148/radiol.09090021. [DOI] [PubMed] [Google Scholar]
- 15.Hussain SM, De Becker J, Hop WC, Dwarkasing S, Wielopolski PA. Can a single-shot black-blood T2-weighted spin-echo echo-planar imaging sequence with sensitivity encoding replace the respiratory-triggered turbo spin-echo sequence for the liver? An optimization and feasibility study. J Magn Reson Imaging. 2005;21:219–229. doi: 10.1002/jmri.20269. [DOI] [PubMed] [Google Scholar]
- 16.Coenegrachts K, Matos C, ter Beek L, Metens T, Haspeslagh M, Bipat S, et al. Focal liver lesion detection and characterization: comparison of non-contrast enhanced and SPIO-enhanced diffusion-weighted single-shot spin echo echo planar and turbo spin echo T2-weighted imaging. Eur J Radiol. 2009;72:432–439. doi: 10.1016/j.ejrad.2008.09.005. [DOI] [PubMed] [Google Scholar]
- 17.Kiryu S, Watanabe M, Kabasawa H, Akahane M, Aoki S, Ohtomo K. Evaluation of super paramagnetic iron oxide-enhanced diffusion-weighted PROPELLER T2-fast spin echo magnetic resonance imaging: preliminary experience. J Comput Assist Tomogr. 2006;30:197–200. doi: 10.1097/00004728-200603000-00005. [DOI] [PubMed] [Google Scholar]
- 18.Liang L, Korogi Y, Sugahara T, Shigematsu Y, Okuda T, Ikushima I, et al. Detection of intracranial hemorrhage with susceptibility-weighted MR sequences. AJNR Am J Neuroradiol. 1999;20:1527–1534. [PMC free article] [PubMed] [Google Scholar]