Abstract
Purpose
We recently demonstrated the morphology of the anococcygeal ligament. As the anococcygeal ligament and raphe are often confused, the concept of the anococcygeal raphe needs to be re-examined from the perspective of fetal development, as well as in terms of adult morphology.
Materials and Methods
We examined the horizontal sections of 15 fetuses as well as adult histology. From cadavers, we obtained an almost cubic tissue mass containing the dorsal wall of the anorectum, the coccyx and the covering skin. Most sections were stained with hematoxylin and eosin or Masson-trichrome solution.
Results
The adult ligament contained both smooth and striated muscle fibers. A similar band-like structure was seen in fetuses, containing: 1) smooth muscle fibers originating from the longitudinal muscle coat of the anal canal and 2) striated muscle fibers from the external anal sphincter (EAS). However, in fetuses, the levator ani muscle did not attach to either the band or the coccyx. Along and around the anococcygeal ligament, we did not find any aponeurotic tissue with transversely oriented fibers connecting bilateral levator ani slings. Instead, in adults, a fibrous tissue mass was located at a gap between bilateral levator ani slings; this site corresponded to the dorsal side of the ligament and the EAS in the immediately deep side of the natal skin cleft.
Conclusion
We hypothesize that a classically described raphe corresponds to the specific subcutaneous tissue on the superficial or dorsal side of the anococcygeal ligament.
Keywords: Anal canal, rectum, smooth muscle, embryology, anatomy, histology
INTRODUCTION
We recently demonstrated the adult human morphology of the anococcygeal ligament along with its surgical relevance.1 Using semiserial sagittal sections of 20 human cadavers, we measured the mean length of the anococcygeal ligament (mean, 17 mm; range, 8-23 mm) as well as the mean thickness (mean, 2.7 mm; range, 1.5-4.5 mm). In contrast to the collagen-rich presacral fascia, the anococcygeal ligament was abundant in smooth muscles and elastic fibers. Kinugasa, et al. also described the distance between the coccyx and the dorsomedial margin of the levator ani, which ranged from 8 mm to 25 mm.1 Thus, on the inferior side of the coccyx, connective tissue such as the anococcygeal raphe2,3 is likely to connect bilateral levator muscle slings. Actually, the anococcygeal ligament has been considered to co-exist with the anococcygeal raphe.4 Thus, both the ligament and raphe seem to play roles in connecting bilateral levator slings. According to Kinugasa, et al.,1 the thick ventral layer of the anococcygeal ligament joined the conjoint longitudinal muscle layer of the anal canal (i.e., the anal longitudinal muscle), while the thin dorsal layer merged with midsagittal amorphous tissue to support the external anal sphincter (EAS). However, questions remain as to whether this midsagittal amorphous tissue corresponds to the anorectal raphe.
Although many studies had been conducted on the fetal development of the human levator ani muscle and EAS,5-14 the anococcygeal ligament and/or raphe seem to have been outside the focus of those investigations. Niikura, et al.15 recently reported on a fibrous tissue connecting the human fetal coccyx and anus. However, that midsagittal structure does not seem to correspond to the anococcygeal raphe because, simply, the levator ani does not attach to it. They hypothesized that the coccygeal attachment of the levator ani is derived from another muscle, the sacrococcygeus anterior. Thus, the concept of the raphe needs to be re-examined from the perspective of fetal development, as well as in terms of adult morphology. Consequently, the aim of this study was to clarify the morphology of the anococcygeal raphe by describing a basic rule and variations of anococcygeal midsagittal connective tissues in human adults and fetuses.
MATERIALS AND METHODS
The study was performed in accordance with the provisions of the Declaration of Helsinki 1995 (as revised in Edinburgh 2000).
Study using adult cadavers
We examined the paraffin-embedded histology of 30 elderly adults (20 males, 10 females; mean age at death, 85 years) without macroscopic tumor in the abdomen and pelvis. All these cadavers had been donated to Tokyo Dental College for anatomical education and research, and had been fixed with injection of 10 L of formalin solution (10% w/w in water) from the femoral artery at least 6 months before dissection. Twenty of the 30 specimens (8 males, 12 females; all sagittal sections) had also been included in our recent study of surgical anatomy.1 The use of these donated cadavers for anatomical research did not require ethics committee approval.
From the cadavers, we obtained an almost cubic tissue mass (5×5×5 cm at minimum) containing the dorsal wall of the anorectum, the coccyx and the covering skin. Decalcification was performed over more than 4 weeks at room temperature using Plank-Rychlo solution (AlCl2/6H20, 7.0 w/v%; HCl, 3.6; HCOOH, 4.6; WAKO, Tokyo, Japan). After routine processing for paraffin-embedded histological examination, 10-µm-thick sections were prepared at intervals of 1 mm. Twenty cadavers were used for sagittal sections, while 10 cadavers were used for transverse sections. One tissue block (1 specimen) included around 10 sagittal or 30 transverse sections including the coccyx and anus. These sections were stained with hematoxylin and eosin (HE) or Masson-trichrome solution for collagen fibers and smooth muscle. Due to the severe condition of decalcification, immunohistochemistry was unsuccessful.
Study using human fetal specimens
We examined the paraffin-embedded histology of 15 fetuses at 12-20 weeks of gestation (4 fetuses each at 12 and 15 weeks; 7 fetuses at 20 weeks). Specimens were classified into three clear groups according to cranio-rump length: 1) 72, 75, 80 and 85 mm (12 weeks); 2) 105, 107, 110 and 115 mm (15 weeks); and 3) 170, 180, 183 and 190 mm (20 weeks). Sections from the 8 specimens at 12 and 15 weeks were horizontal with a thickness of 5 µm, but another 7 specimens (all 20 weeks) were cut almost tangentially along the plane including the anus and coccyx (i.e., tilted horizontal sections) with a thickness of 8 µm. Sections were prepared at intervals of 20 µm (12 and 15 weeks) or 100 µm (20 weeks). Most sections were stained using HE or Masson-trichrome, while some were used for immunohistochemistry (see below).
With the agreement of the families concerned, these specimens were donated to the Department of Anatomy at Chonbuk National University in Korea, and use of these samples for research was approved by the university ethics committee. All fetuses were obtained by induced abortions. After abortion, each mother was personally informed by an obstetrician about the possibility of fetal donation for research, but no attempt was made to encourage donation. Donated fetuses were fixed with 10% w/w formalin solution for more than 3 months. No pathological findings were evident in the abdominopelvic viscera included in the present series of sections. We have attempted to describe the morphology commonly present in each group.
The primary antibodies used were: 1) rabbit polyclonal anti-human alpha smooth muscle actin (dilution, 1:100; Dako Cytomation, Kyoto, Japan) and 2) mouse monoclonal anti-human desmin (dilution, 1:50; Dako). Dako anti-smooth muscle actin antibody was used to cross-react with non-lymphatic vascular structures.16,17 Pretreatment with an autoclave was not conducted because of the fragile nature of the fetal tissues. The secondary antibody (Dako Chem Mate Envison Kit; Dako) was labelled with horseradish peroxidase (HRP), and antigen-antibody reactions were detected via an HRP-catalyzed reaction with diaminobenzidine. Counterstaining with hematoxylin was performed on the same samples.
RESULTS
Observations in elderly specimens
At the midsagittal area on the dorsal side of the anal canal, the anococcygeal ligament was identified as a thick connective tissue band irrespective of whether the EAS was well-developed (Fig. 1A) or poorly developed (Fig. 1B). The ligament consistently divided into 2 layers: a thick ventral layer joined the conjoint longitudinal muscle layer of the anal canal (i.e., the anal longitudinal muscle); while a thin dorsal layer merged with the EAS. The anococcygeal ligament contained smooth muscle in the superior part and striated muscle in the inferior part (Figs. 1, 2 and 3). On the immediately inferior side of the coccyx, a relatively dense fibrous tissue (midsagittal amorphous tissue in Kinugasa, et al.1) was discriminated from the other subcutaneous tissue. The thick periosteum of the coccyx was continuous with the dorsal fibrous tissue mass.
Fig. 1.
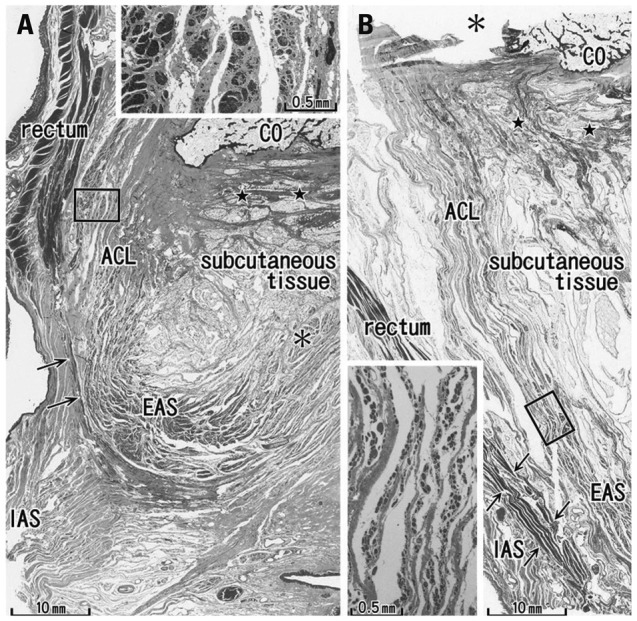
Sagittal sections of the miadsagittal area including the coccyx (CO) and anococcygeal ligament. Masson trichrome staining. (A) 96 years old male, displays a thick external anal sphincter (EAS) in the 30 mm inferior side of the CO. (B) 84 years old female, exhibits a thin EAS 40 mm below the CO. The anococcygeal ligament (ACL) merges with the EAS as well as the longitudinal anal muscle (arrows) between the EAS and internal anal sphincter (IAS). Inserts in panels A and B are higher magnification views of a square in panel A or B, respectively. Insert in panel A shows smooth muscles in the ACL, while an inset in panel B striated muscles distributing in the inferior end of the ACL. Note a fibrous tissue (stars) in the dorsal side of the ligament. Asterisk in panel A (or in the insert in panel B) indicates the dorsosuperior reflection of the well developed EAS (or an artificial damage during the histological procedure).
Fig. 2.
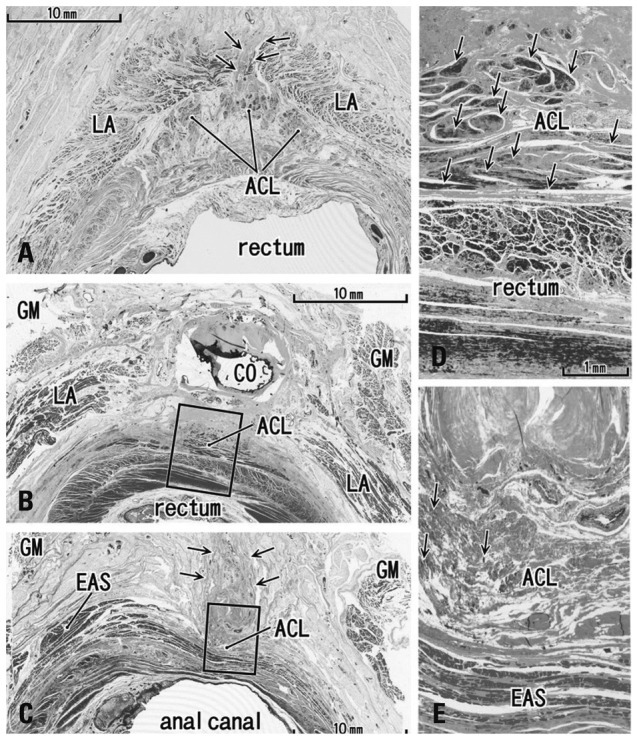
Transverse sections of the anal canal dorsal wall. (A) 86 years old female, 10 mm inferior side of the coccyx (CO), displays a small fibrous tissue mass (arrows) at a gap between the bilateral levator slings (LA). This gap was the smallest in specimens examined (3 mm). (B) 92 years old male, inferior end of the CO and the most superior part of the anococcygeal ligament (ACL). (C) Same specimen as panel B. Panel B corresponds to the, whereas panel C contains the inferior end of the ligament at a level 20 mm inferior side of the CO. In panel C, note a fibrous tissue mass (arrows) in the dorsal side of the external anal sphincter (EAS) and the ligament. (D) A higher magnification view of a square in panel B. (E) A higher magnification view of a square in panel C. Arrows in panels D and E show smooth muscles (panel D) or striated muscles (panel E) in the anococcygeal ligament, respectively. GM, gluteus maximus muscle.
Fig. 3.
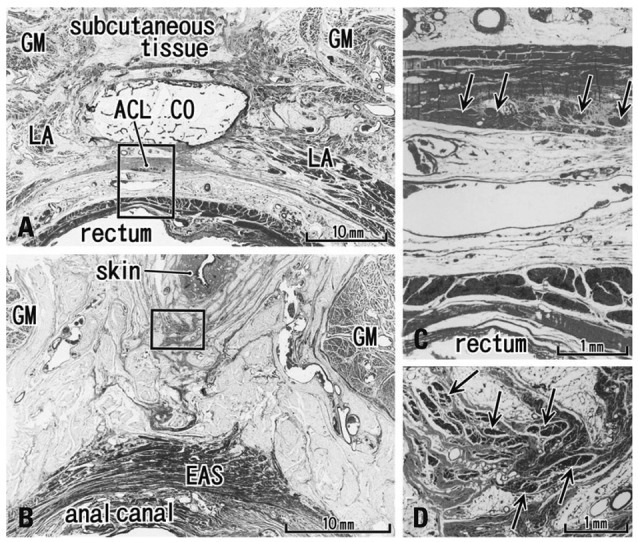
Transverse sections of the anal canal and natal skin cleft (85 years old male). (A) A level of the inferior end of the coccyx (CO) contains the most superior part of the anococcygeal ligament (ACL). (B) A level 40 mm inferior side of the CO. A fibrous tissue extends between the natal cleft (skin) and the external anal sphincter (EAS). (C) A higher magnification view of a square in panel A. Arrows show smooth muscles in the ligament. (D) A higher magnification view of a square in panel B. Arrows show striated muscles in the subcutaneous fibrous tissue. LA, levator ani muscle; GM, gluteus maximus muscle.
In transverse sections of the anal canal, the dorsal fibrous tissue was a round or oval-shaped mass of collagen fibers, located on the dorsal or superficial side of the EAS and occupying the subcutaneous tissue on the immediately deep or ventral side of the natal skin cleft (Figs. 2 and 3). Although this tissue was located in a gap between bilateral levator muscle slings, only 2 of 10 specimens among the transverse sections showed such a typical topohistology (Fig. 2A), due to poorly developed levators in the other 8 specimens. The gap ranged from 3 mm to 60 mm in width, and Fig. 2A portrays the smallest case (Fig. 2A). We did not find any aponeurotic tissue with transversely oriented fibers connecting bilateral levator slings. Instead, in the 8 specimens, the fibrous tissue mass (maximum width, 5-10 mm) was located and extended along the supero-inferior axis between the bilateral gluteus maximus muscles (Figs. 2B, C and 3B). A gap between the gluteus muscles ranged from 18-40 mm at the level of the inferior end of the coccyx. The EAS did not connect with the fibrous tissue mass, but instead with the anococcygeal ligament (Fig. 2C). In 3 of 10 specimens (Fig. 3D), the dorsal fibrous tissue mass contained striated muscles continuous with the dorsosuperior reflection of the well-developed EAS as seen in Fig. 1A.
Observations in fetal specimens
In all 8 specimens at 12 and 15 weeks, the smooth muscle layer of the anorectum showed positive immunohistochemical findings for smooth muscle actin. In the midsagittal area between the anal canal and coccyx, a connective tissue band was consistently seen in all fetuses. In 2 of the 4 specimens at 12 weeks and in 3 of the 4 at 15 weeks (Fig. 4A and B), the connective tissue band contained smooth muscle fibers continuous with the longitudinal muscle coat of the anal canal. Notably, the EAS included desmin-positive striated muscle along the dorsal smooth muscle band (Fig. 4C). At 12 and 15 weeks, the coccygeus muscle was identified as a pair of large muscles in the immediately lateral and anal side of the coccyx, as we recently described.15 In addition, at 12 and 15 weeks, a very thin, loose subcutaneous tissue was present on the dorsal side of the coccyx (not shown in figs).
Fig. 4.
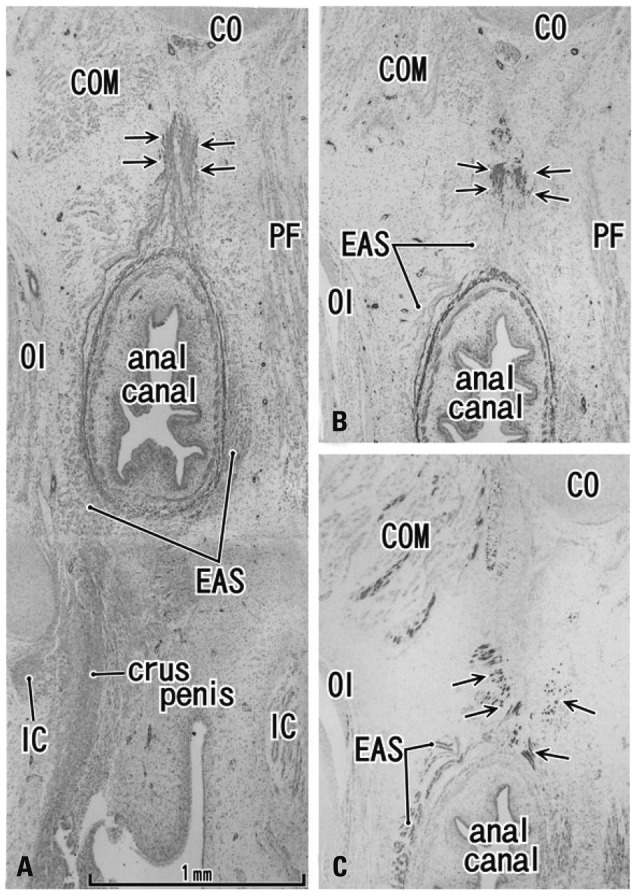
Horizontal sections of the anal canal in a 12-week fetus. (A) Immunohistochemistry for smooth muscle actin (SMA). 0.2 mm superior side of panel C. A dorsal midsagittal structure (arrows) is positive for SMA and extending from the longitudinal smooth muscle coat of the anal canal toward the coccyx (CO). (B) Immunohistochemistry for SMA. The dorsal extension (arrows) of the external anal sphincter (EAS). (C) Immunohistochemistry for desmin (a striated muscle marker) of a section near panel B. All panels are prepared at the same magnification (scale bar in panel A). COM, coccygeus muscle; IC, ishchiocavernosus muscle; OI, obturator internus muscle. PF, pelvic fascia.
Immunoreactivity was weak in 7 specimens at 20 weeks, possibly because of the long duration of preservation. In HE staining for the 20-week specimens, no specific structure connecting the coccyx and anal canal was identified (Fig. 5). However, 3 of the 7 specimens showed that a connective tissue mass was present on the dorsal side of the coccyx (i.e., in the subcutaneous tissue; Fig. 5C and D). Another connective tissue was consistently originated from the coccyx inferiorly (Fig. 5C and D). However, at all stages examined, the levator ani muscle did not attach to the coccyx, but reached to an area far ventral and superior to the coccyx. The midsagittal connective tissue band or mass thus did not play a role in the raphe connecting bilateral levator muscle slings.
Fig. 5.
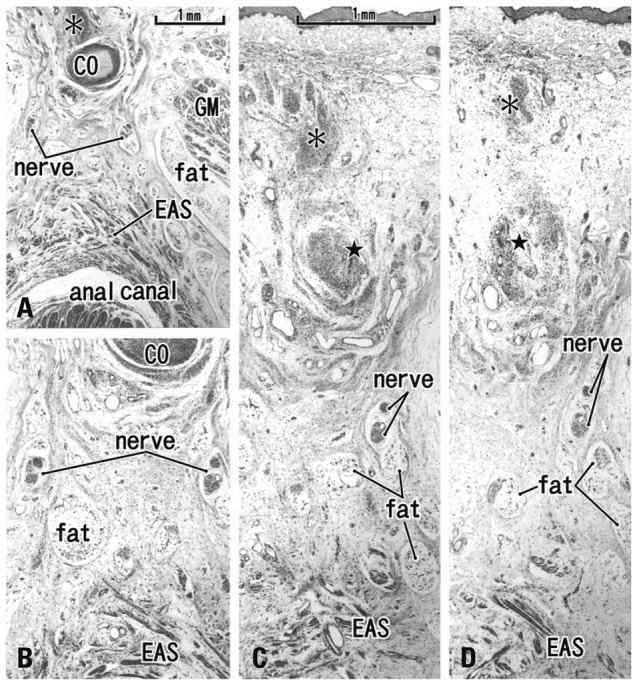
Tilted horizontal sections of the anal canal dorsal wall in a 20-week fetus. HE staining. (A) The most superior side of the figure includes the inferior end of the coccyx (CO). Lower magnification than other panels while panels B, C and D are at the same magnification (B) 0.2 mm inferior to the panel A. (C) 0.2 mm inferior to the panel B. (D) The most inferior side of the figure and 0.2 mm inferior to the panel C. The levator ani muscle is located out side of the figure (much more superior and lateral). A connective tissue mass (asterisk) is present in the dorsal side of the CO. In the inferior side of the CO (panels C and D), another connective tissue mass (star) is seen connecting to the CO. Panels C and D include the back skin in the upper side of each panel. Fatty tissues (fat) start development in the ventral side of the gluteus maximus muscle (GM). In this specimen, there is no specific structure connecting between the external anal sphincter (EAS) and the inferior elongation of the coccyx (star).
DISCUSSION
Irrespective of whether the levator ani muscle reached the midsagittal area or not, a fibrous issue extending along the supero-inferior axis between the coccyx and EAS was consistently observed. This seems to correspond to the anococcygeal ligament rather than the raphe, because the term "raphe" should be used for a structure connecting bilateral levator muscle slings. The raphe is thus most likely to run transversely, as seen in the linea alba between the bilateral abdominal rectus sheathes. Our observations thus seem to be consistent with descriptions by Ayoub,18,19 who described that all muscle fibers of the EAS retain skeletal attachments to the coccyx via the anococcygeal ligament. Fig. 6A displays one of the classical concepts of the ligament and raphe, as described by Toldt,4 in which the raphe is located along the internal or ventral side of the ligament. In contrast, according to the present histology, we hypothesize that the raphe is a specific subcutaneous tissue on the superficial or dorsal side of the anococcygeal ligament (Fig. 6B). This restricted, subcutaneous structure is quite different from the classical concept, but the function as a raphe seems to be the same (see below).
Fig. 6.
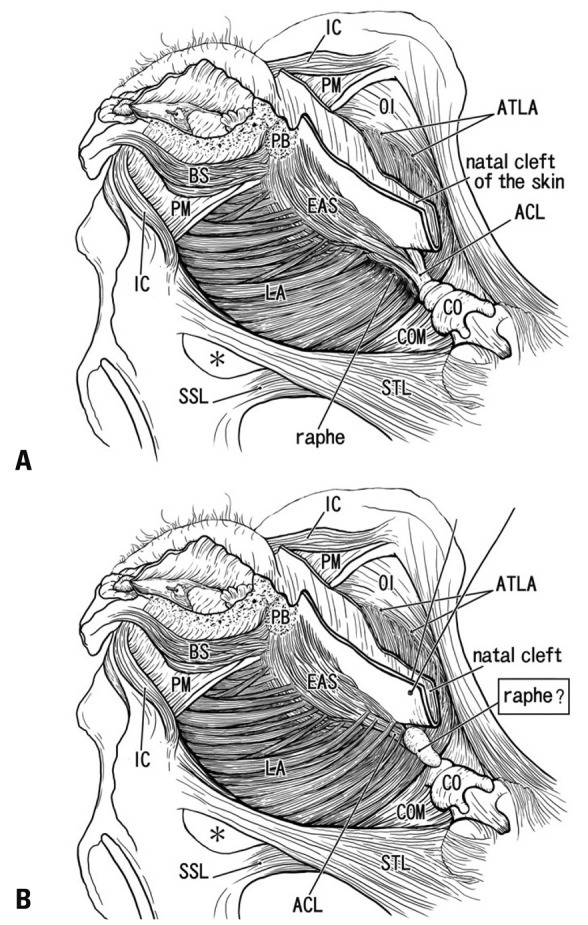
Anococcygeal raphe: a classical concept and our identification according to the present histology. (A) A classical view of the anococcygeal raphe (raphe) in dorsal views of the female perineum. (B) Our identification according to the present study. Muscles are not drawn in a dorsal part of the ischorectal fossa (asterisk). In the diagram, the ventral part of the female perineum is drawn according to our recent studies.20,30 ACL, anococcygeal ligament; ATLA, arcus tendineus for the levator ani; BS, bulbospongiosus muscle; CO, coccyx; COM, coccygeus muscle; EAS, external anal sphincter; IC, ishchiocavernosus muscle; LA, levator ani; OI, obturator internus muscle; PB, perneal body; PM, perineal membrane; SSL, sacrospinous ligament; STL, sacrotuberous ligament.
In fetuses, the longitudinal muscle coat of the anal canal gave off smooth muscle fibers into a dorsal band-like tissue, which connected the anal canal with the coccyx, i.e., the primitive anococcygeal ligament. Likewise, also on the ventral side of the anus, similar smooth muscle tissue was seen connecting with a ventral connective tissue mass or the primitive perineal body.20,21 The longitudinal muscle coat thus plays a critical role in connective tissue development around the anal canal. The dorsally extending smooth muscle seems to be maintained as smooth muscle in the superior part of the adult anococcygeal ligament. The EAS also gave off striated muscle into the fetal dorsal band-like tissue - we paid attention to the fact that fetal striated muscle morphology resembled dorsosuperior reflection of the EAS in adults. The parts of the fetal striated muscle derived from the EAS seem to be retained in the adult subcutaneous fibrous tissue, i.e., our identified anococcygeal raphe. We found a specific subcutaneous tissue mass on the dorsal side of the coccyx at 20 weeks. However, neither the levator slings nor EMS was found near the coccyx, consistent with the findings of Niikura, et al.15 Thus, depending on later development of these striated muscles, our identified raphe seemed to develop as a raphe-like structure in the final fetal stage or after birth under the influence of muscle functions.
Henrich22 described the anococcygeal ligament as inserting on the dorsal side of the dorsal end of the levator ani. Between "his ligament" and the external pelvic fascia (superficial fascia), he found a fatty tissue termed Courtney's space. This ligament thus seems to correspond to our identified raphe. Courtney23 was a rectal surgeon who described the raphe clearly in his line-drawings. Notably, the subcutaneously located raphe received the most dorsal muscle fibers of the EAS. This dorsal part of the EAS, in the well-developed cases as shown in Fig. 1A, provides a superior reflection toward the coccyx.24 Thus, rather than functioning as a raphe between bilateral levator ani slings, we speculate that this part plays a critical role in coordinating between the contraction and superior shift of the EAS for smooth defecation. Shafik25,26 considered that the width of the raphe changes depending on anal sphincter function. We do not deny his hypothesis because in the elderly individuals bilateral levator slings were not tightly connected.
Rectal surgeons are familiar with Waldeyer's description that, at the level of anorectal junction or just above the levator ani sling, a fascia connects the rectum and presacral parietal fascia (reviewed by García-Armengol, et al.27). A similar concept is also found for the so-called rectococcygeus muscle.28 However, we suspect that this concept likely represents a bias toward the notion that the coccyx should also connect with the anorectum (rather than the EAS) by a connective tissue structure. The coccyx is connected with the EAS by the anococcygeal ligament, and also by the presently described subcutaneous structure, i.e., our identified raphe. In addition, the term "anococcygeus muscle" may be based on bias from the comparative anatomy of other mammals,29 although striated muscle fibers were contained in both the anococcygeal ligament and our identified raphe.
Study limitations
A major limitation of this study was the small sample size, particularly for elderly specimens because of the possible variations in degeneration of the pelvic floor. The sample size may have been too small to allow for suitable insights into left/right differences or sex differences. Whether the levator ani attaches to the raphe seems to be one of the major reasons for evaluating raphe function. In fetal and elderly specimens, the levator did not often attach to our identified raphe.
Footnotes
The authors have no financial conflicts of interest.
References
- 1.Kinugasa Y, Arakawa T, Abe S, Ohtsuka A, Suzuki D, Murakami G, et al. Anatomical reevaluation of the anococcygeal ligament and its surgical relevance. Dis Colon Rectum. 2011;54:232–237. doi: 10.1007/DCR.0b013e318202388f. [DOI] [PubMed] [Google Scholar]
- 2.Bogduk N. Issues in anatomy: the external anal sphincter revisited. Aust N Z J Surg. 1996;66:626–629. doi: 10.1111/j.1445-2197.1996.tb00834.x. [DOI] [PubMed] [Google Scholar]
- 3.Borley NR. Anal canal. In: Standring S, editor. Gray's Anatomy. 40 ed. London: Elsevier Churchill Linvingstone; 2008. pp. 1155–1160. [Google Scholar]
- 4.Toldt BvC. Atlas of Human Anatomy for Students and Surgeons. Berlin: Urban & Schwarzenberg; 1903. [Google Scholar]
- 5.Gräfenberg E. Development of the human pelvic musculature. Anat Hefte. 1904;72:429–494. [Google Scholar]
- 6.Bardeen RC. Development and variation of the musculature of the inferior extremity and the neighboring regions of the trunk in man. Am J Anat. 1907;6:332–336. [Google Scholar]
- 7.Power RM. Embryological development of the levator ani muscle. Am J Obstet Gynecol. 1948;55:367–381. doi: 10.1016/s0002-9378(15)32955-0. [DOI] [PubMed] [Google Scholar]
- 8.Tichý M. The development and organization of the sphincter ani externus and the adjacent part of the levator ani muscle in man. Folia Morphol (Praha) 1984;32:113–120. [PubMed] [Google Scholar]
- 9.Fritsch H, Fröhlich B. Development of the levator ani muscle in human fetuses. Early Hum Dev. 1994;37:15–25. doi: 10.1016/0378-3782(94)90143-0. [DOI] [PubMed] [Google Scholar]
- 10.Fröber R, Krebs U, Haas A, Fischer MS, Schier F, Linss W. Three-dimensional reconstruction of the anal striated musculature in a human fetus. Cells Tissues Organs. 2001;169:152–157. doi: 10.1159/000047873. [DOI] [PubMed] [Google Scholar]
- 11.Schier F, Krebs U, Fröber R, Haas A. Three-dimensional reconstruction of the anorectal continence organ in a 14-week-old fetus. J Pediatr Surg. 2002;37:912–915. doi: 10.1053/jpsu.2002.32910. [DOI] [PubMed] [Google Scholar]
- 12.Koch WF, Marani E. Early development of the human pelvic diaphragm. Adv Anat Embryol Cell Biol. 2007;192:1–111. [PubMed] [Google Scholar]
- 13.Fritsch H. Developmental changes in the retrorectal region of the human fetus. Anat Embryol (Berl) 1988;177:513–522. doi: 10.1007/BF00305138. [DOI] [PubMed] [Google Scholar]
- 14.Levi AC, Borghi F, Garavoglia M. Development of the anal canal muscles. Dis Colon Rectum. 1991;34:262–266. doi: 10.1007/BF02090167. [DOI] [PubMed] [Google Scholar]
- 15.Niikura H, Jin ZW, Cho BH, Murakami G, Yaegashi N, Lee JK, et al. Human fetal anatomy of the coccygeal attachments of the levator ani muscle. Clin Anat. 2010;23:566–574. doi: 10.1002/ca.20983. [DOI] [PubMed] [Google Scholar]
- 16.Hayashi S, Murakami G, Ohtsuka A, Itoh M, Nakano T, Fukuzawa Y. Connective tissue configuration in the human liver hilar region with special reference to the liver capsule and vascular sheath. J Hepatobiliary Pancreat Surg. 2008;15:640–647. doi: 10.1007/s00534-008-1336-8. [DOI] [PubMed] [Google Scholar]
- 17.Miyake N, Hayashi S, Kawase T, Cho BH, Murakami G, Fujimiya M, et al. Fetal anatomy of the human carotid sheath and structures in and around it. Anat Rec (Hoboken) 2010;293:438–445. doi: 10.1002/ar.21089. [DOI] [PubMed] [Google Scholar]
- 18.Ayoub SF. The anterior fibres of the levator ani muscle in man. J Anat. 1979;128(Pt 3):571–580. [PMC free article] [PubMed] [Google Scholar]
- 19.Ayoub SF. Anatomy of the external anal sphincter in man. Acta Anat (Basel) 1979;105:25–36. doi: 10.1159/000145103. [DOI] [PubMed] [Google Scholar]
- 20.Kato M, Matsubara A, Murakami G, Abe S, Ide Y, Sato I, et al. Female perineal membrane: a study using pelvic floor semiserial sections from elderly nulliparous and multiparous women. Int Urogynecol J Pelvic Floor Dysfunct. 2008;19:1663–1670. doi: 10.1007/s00192-008-0701-0. [DOI] [PubMed] [Google Scholar]
- 21.Arakawa T, Hayashi S, Kinugasa Y, Murakami G, Fujimiya M. Development of the external anal sphincter with special reference to intergender difference: observations of mid-term fetuses (15-30 weeks of gestation) Okajimas Folia Anat Jpn. 2010;87:49–58. doi: 10.2535/ofaj.87.49. [DOI] [PubMed] [Google Scholar]
- 22.Henrich M. Clinical topography of the proctodeum. Acta Anat (Basel) 1980;106:161–170. doi: 10.1159/000145178. [DOI] [PubMed] [Google Scholar]
- 23.Courtney H. Anatomy of the pelvic diaphragm and anorectal musculature as related to sphincter preservation in anorectal surgery. Am J Surg. 1950;79:155–173. doi: 10.1016/0002-9610(50)90208-x. [DOI] [PubMed] [Google Scholar]
- 24.Arakawa T, Murakami G, Nakajima F, Matsubara A, Ohtsuka A, Goto T, et al. Morphologies of the interfaces between the levator ani muscle and pelvic viscera, with special reference to muscle insertion into the anorectum in elderly Japanese. Anat Sci Int. 2004;79:72–81. doi: 10.1111/j.1447-073x.2004.00069.x. [DOI] [PubMed] [Google Scholar]
- 25.Shafik A. New concept of the anatomy of the anal sphincter mechanism and the physiology of defecation. II. Anatomy of the levator ani muscle with special reference to puborectalis. Invest Urol. 1975;13:175–182. [PubMed] [Google Scholar]
- 26.Shafik A. Levator ani muscle: new physioanatomical aspects and role in the micturition mechanism. World J Urol. 1999;17:266–273. doi: 10.1007/s003450050144. [DOI] [PubMed] [Google Scholar]
- 27.García-Armengol J, García-Botello S, Martinez-Soriano F, Roig JV, Lledó S. Review of the anatomic concepts in relation to the retrorectal space and endopelvic fascia: waldeyer's fascia and the rectosacral fascia. Colorectal Dis. 2008;10:298–302. doi: 10.1111/j.1463-1318.2007.01472.x. [DOI] [PubMed] [Google Scholar]
- 28.McKirdy HC. Anatomy and function of the anal longitudinal muscle. Br J Surg. 1993;80:262. doi: 10.1002/bjs.1800800252. [DOI] [PubMed] [Google Scholar]
- 29.Olson L, Alund M. Quinacrine-binding nerves: presence in the mouse ano-coccygeus muscle, disappearance after muscle transsection. Med Biol. 1979;57:182–186. [PubMed] [Google Scholar]
- 30.Hirata E, Koyama M, Murakami G, Ohtsuka A, Abe S, Ide Y, et al. Comparative histological study of levels 1-3 supportive tissues using pelvic floor semiserial sections from elderly nulliparous and multiparous women. J Obstet Gynaecol Res. 2011;37:13–23. doi: 10.1111/j.1447-0756.2010.01298.x. [DOI] [PubMed] [Google Scholar]


