Abstract
This article reviews attempts to characterize the mental operations mediated by left inferior prefrontal cortex, especially the anterior and inferior portion of the gyrus, with the functional neuroimaging techniques of positron emission tomography and functional magnetic resonance imaging. Activations in this region occur during semantic, relative to nonsemantic, tasks for the generation of words to semantic cues or the classification of words or pictures into semantic categories. This activation appears in the right prefrontal cortex of people known to be atypically right-hemisphere dominant for language. In this region, activations are associated with meaningful encoding that leads to superior explicit memory for stimuli and deactivations with implicit semantic memory (repetition priming) for words and pictures. New findings are reported showing that patients with global amnesia show deactivations in the same region associated with repetition priming, that activation in this region reflects selection of a response from among numerous relative to few alternatives, and that activations in a portion of this region are associated specifically with semantic relative to phonological processing. It is hypothesized that activations in left inferior prefrontal cortex reflect a domain-specific semantic working memory capacity that is invoked more for semantic than nonsemantic analyses regardless of stimulus modality, more for initial than for repeated semantic analysis of a word or picture, more when a response must be selected from among many than few legitimate alternatives, and that yields superior later explicit memory for experiences.
Mapping the cortical geography of human cognition with functional neuroimaging techniques, such as PET or fMRI is an exciting but perilous endeavor. It is exciting because these techniques permit a systematic exploration of the functional neural architecture of such valued cognitive abilities as language and memory. Heretofore, this exploration occurred unsystematically through the analysis of the consequences of accidental lesions upon language and memory that resulted in aphasia or amnesia, respectively. Although lesion evidence still provides important constraints upon the interpretation of functional imaging studies (1), the opportunity to programmatically study the healthy human brain promises a new breadth and depth in our understanding of the neural basis of language and memory.
This venture is perilous, however, because the brain mapping of human cognition has few facts to guide the formation of hypotheses or the interpretation of activations. This paucity of knowledge may be compared with the elegant functional mapping of the initial stages of human vision (2–4). These studies exploit anatomical knowledge of the visual system to focus on occipital cortex, take advantage of relatively well understood spatial principles of retinotopy, and often parametrically manipulate objective stimulus dimensions such as size, motion, color, and spatial frequency. In contrast, cognitive studies typically examine mental operations that involve multiple brain regions, lack spatial definition, and compute abstract representations of stimuli with unknown dimensional scales. Consequently, cognitive functional neuroimaging studies often yield unexpected findings, but the precise meaning of those findings is resistant to precise characterization.
The present article reviews attempts to characterize the mental operations mediated by left inferior prefrontal cortex, especially the anterior and ventral (inferior) portion of the gyrus. This area was somewhat unexpectedly identified as being involved in the analysis of word meaning (semantics). Since then, it has also been implicated in many aspects of language and memory.
Left Prefrontal Cortex: Semantic Activations.
Petersen et al. (5) provided the first functional neuroimaging evidence implicating left prefrontal regions in the semantic analysis of words. In a positron emission tomography (PET) study, they compared activation when participants generated a verb (e.g., “eat”) to a presented noun (e.g., “cake”) or merely read the noun. The two conditions involved similar visual input and motor output but differed in the greater semantic analysis required to generate a verb than to read a presented noun. Generation relative to reading resulted in three main activations in the left inferior frontal gyrus, the cingulate, and the right cerebellum. The cerebellar activation occurred in a location different than that associated with simply reading a word, so these findings supported the view that the cerebellum plays a role in cognition beyond that of the motor control involved in articulation.
Subsequent PET and functional magnetic resonance imaging (fMRI) studies have found similar left prefrontal activations associated with semantic relative to nonsemantic analysis of words. For example, one fMRI study found greater left prefrontal activations when participants made semantic judgments about whether words referred to concrete (“table”) or abstract (“truth”) entities than when they made nonsemantic judgments about whether words appeared in uppercase letters (“CHAIR”) or lowercase letters (“love”) (6). The perceptual input (visual words), output (two-choice response), and stimuli (words) were constant across the two encoding conditions, so the left prefrontal activation reflected only the difference between the semantic and nonsemantic requirements of the two conditions. Similarly, a PET study found greater left prefrontal activations when participants decided whether words referred to living or nonliving entities than when the words contained a particular letter (7). The fact that activations occurred in the same left prefrontal region for verb generation and for two-choice judgments involving mostly nouns indicates that the activations reflect the semantic requirements common across these diverse tasks rather than generation of verbs per se. Thus, left prefrontal activation occurs in a range of semantic tasks.
One limitation of these studies is that left prefrontal activation occurred in conditions that were always more difficult than the comparison condition, where difficulty is operationalized as response time to a stimulus. It takes substantially longer to generate a verb to a presented noun than to read the noun, to decide whether a word is abstract or concrete in meaning than whether it is uppercase or lowercase in appearance (6), or to decide whether a word is living or nonliving in meaning than whether it contains a particular letter (7). Thus, semantic analysis and question difficulty were correlated (i.e., confounded) in all three studies making it impossible to determine whether the left prefrontal activation reflected semantic analysis per se or simply extended processing of words.
In an attempt to disambiguate these two potential bases of left prefrontal activations, one fMRI study (8) added a third condition with minimal semantic processing but even more extended processing than the semantic task. Thus, as before, activation was compared between a difficult semantic task (abstract/concrete classifications) and an easy nonsemantic task (uppercase/lowercase classifications). In a second scan, activation was compared between the same semantic task and a nonsemantic task in which participants had to decide whether the first and last letters of a word were ascending (“car”) or descending (“table”) alphabetically. Critically, the nonsemantic ascending/descending task was more difficult (as measured by time to respond) than the semantic abstract/concrete task. Left prefrontal activations, however, were almost identical in the two scans with greater activation for the semantic task than the less difficult (case) and more difficult (alphabetic) tasks. Another fMRI study compared two tasks that were matched in difficulty (response time): judging whether pairs of words were related in meaning or whether rows of asterisks had the same or different colors. Again, there was left prefrontal activation for the semantic task relative to the nonsemantic color task (9). Thus, left prefrontal activation reflects semantic processing rather than task difficulty. More precisely, left prefrontal activation may be correlated with the specific semantic difficulty of a task.
In all of the above studies, left prefrontal activations occurred for conditions in which participants made intentional or overt semantic analyses. Other studies have compared conditions in which no semantic analysis was required but where the stimuli in different conditions had greater or lesser (usually no) semantic content. In one such study, participants simply viewed words (that had meaning) or visually similar but meaningless stimuli such as pronounceable nonwords, unpronounceable letter strings, or false-font strings (10). In the absence of any semantic task, greater activation for the meaningful stimuli could only be due to incidental or covert analyses of meaning. Indeed, only the words yielded activation relative to a fixation baseline, and this activation was almost identical in location to that observed for the intentional generation of verbs. Similar findings are noted for silent viewing of words versus false-font strings, with the added finding that the duration of stimulus presentation can influence the magnitude of the left prefrontal response (for reasons not yet understood) (11).
In the passive viewing conditions of the above two studies, participants may have been performing some sort of voluntary semantic task when the stimuli allowed. This possibility was eliminated in another PET study in which participants had to detect letter features, specifically if a letter string contained a letter with an ascending feature (e.g., “b” and “d” but not “a” or “g”) (12). There was left prefrontal activation for feature detection in words relative to false fonts, to unpronounceable consonant strings, and to pronounceable nonwords. The activations relative to false fonts and consonant strings occurred in the left inferior frontal gyrus, the locus of the semantic activations described above. The activation relative to pronounceable nonwords occurred in the left middle frontal gyrus. It is unclear at present whether these anatomic distinctions reflect truly separate processes, measurement variability, or both.
All of the above studies employed visual presentation of verbal stimuli. The same left prefrontal and right cerebellar areas, however, are activated when participants generate verbs to visual or auditory nouns (5) or make semantic judgments for words or line drawings (13). Thus, the left prefrontal activations seem to reflect psychological processes that are involved in semantic analyses across many tasks, that operate across verbal, pictorial, visual, and auditory modalities, and that are engaged both intentionally and incidentally.
Left Prefrontal Cortex: Relations to Episodic Memory and Language.
Tulving (14) noted that conditions yielding greater left prefrontal activations are often the same conditions that yield superior memory for words and other stimuli. It is well known that later memory for words is superior when encoded at study for meaning (semantic or deep encoding) than for appearance (perceptual or shallow encoding) (15). Indeed, the semantic encoding conditions that provoked left prefrontal activation also yielded superior later memory for words (6–8). Memory for words is also superior when participants generate words relative to when they merely read words (16), and this principle applies specifically to generating verbs versus reading nouns (14). Thus, the left prefrontal activations seem to reflect processes that are important for enhancing memory for materials encountered in particular episodes.
The fundamental linguistic nature of these left prefrontal processes is made evident in a study of epileptic patients in whom the lateralization of language dominance had been established definitively through invasive Wada evaluation (17). Seven such patients performed semantic (abstract/concrete) and nonsemantic (uppercase/lowercase) tasks. The four patients who were left-hemisphere dominant for language displayed the usual left prefrontal activation for the semantic relative to the nonsemantic task. The three patients who were right-hemisphere dominant for language, however, displayed right prefrontal activations. Thus, the lateralization of activation was in accord with that for language dominance in each of the seven patients.
These results are of interest for several reasons. First, they demonstrate the intimate relation between semantics and language. Second, they validate the accuracy of fMRI activation on an individual-by-individual basis, at least for such a major feature of brain organization as the laterality of language dominance. Wada testing remains the gold standard for establishing the laterality of language dominance, but it has a number of drawbacks. Because Wada testing is invasive, it can be associated with morbidity. It is often unpleasant and sometimes distressing for patients, which can result in some ambiguity in determining the basis of language performance deficits. It is expensive. With further development, fMRI may replace Wada testing as a less dangerous, more pleasant, and less expensive method that cannot only determine which hemisphere is dominant for language but also which part of the hemisphere is critical for language. Further, whereas Wada testing is only justified in cases of potential surgery, fMRI can be used to examine the role of hemispheric dominance in many disorders of language such as dyslexia or stuttering. In regard to psychological issues, these results suggest that the importance of left prefrontal processes in episodic memory may reside in the power of language to encode memories meaningfully.
Left Prefrontal Cortex: Relations to Implicit Memory.
The above discussion of left prefrontal cortex and memory refers specifically to declarative (18) or explicit (19) memory for experience measured by direct tests of recall and recognition. Declarative or explicit memory depends upon the integrity of medial–temporal and diencephalic structures that when damaged bilaterally result in global amnesia, a specific, severe, and pervasive deficit in explicit memory for facts and events encountered after the onset of the amnesia.
In contrast, implicit memory is measured by experience-induced changes in performance on indirect tests that make no reference to that experience. A well studied kind of implicit memory is repetition priming. In a typical repetition priming study, participants are exposed to words or pictures in a study phase. They then perform a test-phase task with the same or related stimuli as well as baseline (novel) stimuli. Repetition priming refers to the difference in performance, measured in speed or accuracy, with studied and baseline stimuli; that difference reflects implicit memory acquired in the study phase. Quite strikingly, amnesic patients show normal implicit memory on many repetition priming tasks (reviewed in ref. 1). Thus, repetition priming is mediated by different memory systems, which are intact in amnesia, than those that support declarative memory, which are injured in amnesia.
A fundamental distinction in repetition priming is that between perceptual priming, which reflects stimulus form, and semantic or conceptual priming, which reflects stimulus meaning (20, 21). Lesion studies indicate that visual perceptual priming depends on occipital cortices (22–24). Concordant PET studies have reported occipital deactivations associated with visual priming (25–27) such that there was a decrease in activation for studied (primed) items relative to baseline items. The interpretation of the deactivations is that prior study leads to more efficient processing of primed relative to baseline items. This greater efficiency is associated with decreased neural computational demands and, therefore, decreased activation.
To examine the neural basis of semantic repetition priming, we asked participants to make abstract/concrete judgments for words (6). In alternate blocks, words appeared for the first (initial) or second (repeated) time. Behaviorally, participants showed repetition priming by making judgments more quickly for repeated than for initial word presentations. The priming was word-specific because response times slowed to baseline levels whenever new words were initially shown. The repetition priming was accompanied by decreased activity in left prefrontal cortex for repeated relative to initial word presentations. Thus, as with visual priming in occipital cortex, semantic priming in frontal cortex was associated with a facilitation in processing and a reduction in activation. A similar pattern of faster responding and reduced left prefrontal activation was found also for repeated verb generations (28).
Because the same participants took part in the two experiments that examined semantic versus nonsemantic analysis of words and semantic repetition priming, we were able to examine on a person-by-person basis whether the same brain region that showed activation for semantic encoding also showed reduced activation for semantic priming. Indeed, there was a positive 0.70 correlation between pixels that showed increased activation for semantic analysis and decreased activation for semantic priming (6). These results show that repetition priming in a given domain, in this case semantics, reflects experience-induced plasticity in the same neural network that subserves encoding or initial processing in that domain. Further, this learning mechanism seems to be as broadly tuned as is semantic analysis because semantic priming for different questions (abstract/concrete and living/nonliving) and for different modalities (words and pictures) results in a similar locus of reduced left prefrontal activation (29). Thus, semantic experience may constantly update or educate the organization of semantic knowledge to reflect what ideas and meanings are more frequently or more rarely encountered. Frequently encountered meanings may come to be represented more strongly than rarely encountered meanings because they represent more useful semantic knowledge. Semantic priming may reflect the plastic processes by which long-term semantic knowledge is pruned constantly to accurately reflect ongoing experience.
An alternative to this view of repetition priming as purposive plasticity is that it simply reflects habituation to a repeated stimulus. According to habituation, it is not the repeated semantic analysis of a word that results in reduced activation but merely the repeated presentation of a stimulus regardless of what analysis is undertaken. To select between these alternative interpretations, participants were shown alternate blocks of new and repeated words while constantly performing a nonsemantic task of judging whether words appeared in uppercase or lowercase (8). There was no change in left prefrontal activation between new and repeated words. Thus, the reduction for repeated words occurred due to repeated semantic analysis rather than mere habituation.
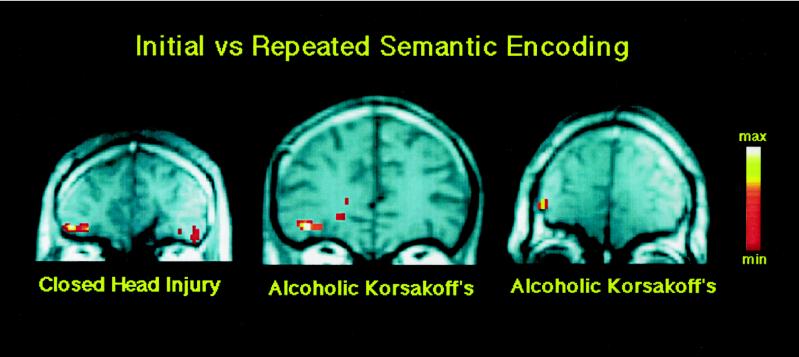
It is difficult to be certain that the left-frontal deactivation associated with semantic priming reflects implicit rather than explicit memory for the initial presentation of words. Three facts, however, favor the implicit nature of the left prefrontal deactivation. First, explicit memory retrieval is typically associated with right-frontal activation rather than left-frontal deactivation (14). Second, amnesic patients show intact verb generation priming despite impaired memory for the nouns for which they are generating verbs (30), and verb generation priming is associated with left-frontal deactivation (28). Third, amnesic patients with impaired explicit, but intact implicit, memory abilities also show a left prefrontal deactivation for repeated words when making abstract/concrete judgments (Fig. 1).
Figure 1.
Coronal view of prefrontal cortex in three amnesic patients with two different etiologies of amnesia. The slices, from left to right, are 39, 35, and 42 mm anterior to the anterior commissure. Individual activations are overlaid on T1-weighted anatomic sections. Each patient shows greater activation in the left inferior prefrontal cortex (corresponding to Brodmann area 47) for the initial relative to the repeated semantic processing of words. The individual activations correspond to the anterior extent of regions that show semantic activation in Fig. 3. The right side of the brain is depicted on the right side of Figs. 1–3.
A remaining question is whether the reduction in left prefrontal activation reflects neural plasticity at that location, in another location, or both. Repetition priming is almost invariably manifested by faster processing for repeated than novel (baseline) items. Shorter processing epochs could result in reduced activations for the entire neural network involved in performing a priming task. Thus, plasticity underlying priming could occur locally in one component of the network but drive reduced processing times and activations globally throughout that network, including areas where no plasticity occurred during initial processing of a stimulus. This issue of interpretation may be called the problem of proximal plasticity; does a memory-related change in activation reflect proximal or distal neuronal plasticity in an adaptive network?
Left Prefrontal Cortex: Selection in Semantic Search.
A surprising finding in the original observation of left prefrontal activation for semantic analysis was a similar activation in the right cerebellum (5). Further, when people repeatedly generated verbs to nouns, both left-frontal and right-cerebellar activations decreased (28). Thus, left-frontal and right-cerebellar activations increased or decreased in tandem across conditions. The idea that left-prefrontal and right-cerebellar regions are components of an interactive network is consistent with clinical reports of cerebellar hypometabolism occurring after contralateral frontal lobe lesions (i.e., crossed cerebellar diaschisis) (31–33) and frontal hypometabolism subsequent to cerebellar damage (34). Accordingly, cerebellar damaged patients often exhibit signs of frontal lobe damage, as measured by tests of initiation/perseveration and verbal fluency (35), planning (36, 37), agrammatism (38), and associative learning (39–41). These functional and clinical interactions between frontal and cerebellar regions occur via anatomical pathways that connect the cerebellum with prefrontal cortex (42).
Although functional activations in frontal cortex are correlated with those of the contralateral cerebellum, it seems unlikely that these two anatomically disparate brain regions are performing the identical computations. We performed a study aimed at dissociating left-frontal and right-cerebellar activations to elucidate the distinctive functions of the two regions. We hypothesized that left-frontal activation reflects how much knowledge is retrieved in task performance, including selection of the appropriate information, whereas right-cerebellar activation reflects the sustained search for this knowledge. We therefore compared activations when participants completed three-letter word stems that had either many possible completions (e.g., “sta”) or few possible completions (e.g., “psa”) into the first word that came to mind. When participants complete stems with many possible answers (e.g., “stall,” “star,” “stamp,” “stallion,” “stand,” “stalk,” “staff,” “stampede,” “start,” “state”), they may retrieve many words before selecting one response. When participants complete stems with few possible answers, the problem they face is less one of selection and more one of sustaining a search until they find a legitimate response. Indeed, participants completed stems with many completions 16% more often and 84 ms more quickly than stems with few completions.
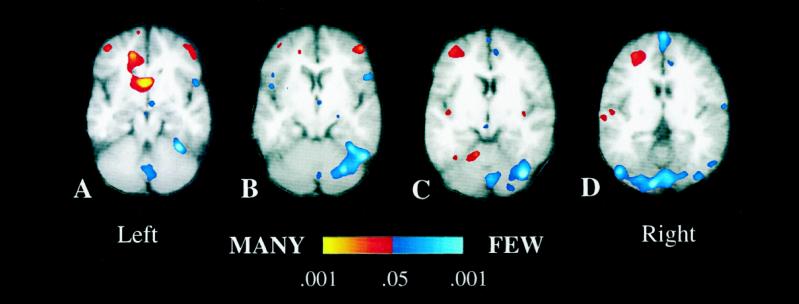
The differential demands of the two kinds of stem completion yielded a double dissociation between left-frontal and right-cerebellar regions that in prior studies have shown associated activations. Left-frontal regions were more active when subjects completed stems with many than with few possible completions, whereas right-cerebellar regions were more active when subjects completed stems with few than with many completions. (Fig. 2). These results do not challenge the many prior findings indicating that left-frontal and right-cerebellar regions regularly interact in verbal performance but rather indicate that these two regions make distinctive contributions to that interaction and provide some clues about the nature of those unique contributions. Specifically, the present study indicates that left-frontal activations reflect selection of a verbal response, whereas cerebellar activations reflect sustained search for a verbal response.
Figure 2.
Averaged fMRI activation over six subjects depicted on averaged T1-weighted oblique axial sections (obtained at a 25-degree angle from the AC-PC line). (Left to Right) The sections depict increasingly superior planes that are 6 mm thick and separated by a 1-mm gap. Regions depicted in red–yellow represent areas that exhibited increased activation when subjects completed stems that had many, relative to few, completions (MANY condition), whereas regions in blue–light blue represent areas that exhibited increases during completion of stems with few, relative to many, completions (FEW condition). Major areas of increased activation during the MANY condition include the left middle frontal gyrus (Brodmann area 9/10, C and D), the right middle frontal gyrus (Brodmann area 10, A and B), the left cingulate gyrus (Brodmann areas 24/32, A), the left caudate nucleus (A), the left postcentral gyrus (Brodmann area 43, D), and the left anterior quandragular lobule of the cerebellum (C). Regions of increased activation during the FEW condition include several portions of the cerebellar vermis (Larsell’s lobules VI, VII, and VIII; A, C, and D), right cerebellar hemisphere (HVI and superior HVIIA, B and C), the right inferior frontal gyrus (Brodmann area 47, not depicted in figure), along the midline in the superior frontal gyrus (Brodmann area 8, D), and bilaterally in the fusiform gyrus (Brodmann area 37/19 and right Brodmann area 37, B–D).
The interpretation that left-frontal activations reflect response selection is based on the assumption that completing a stem with many responses that come easily to mind requires a selection of the one response to be reported. If many responses come easily to mind, however, it is also the case that more semantic knowledge associated with those responses may be invoked than in conditions where few responses are considered. In most verbal tasks, amount and selection are correlated, with more selection required as more knowledge is retrieved. In the present study, stems with many completions may have accessed many words and required selection of one completion. Verb generation requires more semantic information than noun reading because more information is required to generate an appropriate verb than to read a presented noun. Verb generation also involves more selection than noun reading because there are always more alternative verbs to select among (“eat,” “slice,” “bake”) than the one presented noun (“cake”) (5). Thus, a common principle seems to emerge across different tasks that invoke different left-frontal activations: greater left-frontal activation occurs for the task that involves more knowledge and more selection of an appropriate response from that knowledge. At present, it is unknown whether amount and selection of information are inevitably intertwined or whether those two processing dimensions can be dissociated.
The specific location of left-frontal activation for stems with many than with few completions was in the left middle frontal gyrus rather than the inferior frontal gyrus that was activated in semantic tasks of verb generation or word classification. It may be that the left middle frontal gyrus is especially involved in lexical rather than semantic retrieval processes because participants had to retrieve a word on the basis of a three-letter stem rather than a semantic cue. A left middle frontal locus of activation was also noted when participants detected letter features in words relative to pronounceable nonwords (12). The stem-completion and letter-detection tasks may both require the selection of salient information and the suppression of irrelevant information about other completions or the meaning of the letter string.
Left Prefrontal Cortex: Distinguishing Semantic and Phonological Processes.
When reading visually presented words, people are thought to engage multiple processes, including orthographic processes involved in visual analysis of letter and words forms, phonological processes involved in phonemic analysis of words, and semantic processes involved in conceptual analysis of words. There is little, if any, evidence that orthographic processes activate left prefrontal cortex (e.g., the absence of such activation for nonword letter strings versus fixation) (10). There is, however, considerable evidence that phonological tasks provoke left prefrontal activation. Thus, there is activation in the left inferior frontal gyrus for phonological relative to orthographic discrimination tasks for visually and auditorily presented words (43), phonetic relative to pitch judgments for auditorily presented syllables (44), and phonetic monitoring of nonwords relative to pitch monitoring for tones (45). There is enough separation among some of these activations that they may reveal several distinct subregions of left prefrontal processing of phonological information. The maxima of all of these activations, however, occur more posteriorly, near Broca’s area, in left inferior frontal cortex than those obtained in semantic tasks. Such anatomic distinctions support the possibility of separate specialization for phonological and semantic analysis of words within the left prefrontal region.
Other functional neuroimaging studies, however, provide different views of the organization of left prefrontal language functions. A PET study found selective left prefrontal activations for semantic relative to phonetic tasks but not for phonetic relative to semantic tasks (45). These findings suggest that phonological processes are always engaged as a component of semantic processes but that separable semantic processes need not be engaged for phonological tasks. This view is concordant with the above studies in so far as indicating an anatomic separation between phonological and semantic processes.
Such anatomic separation in the left prefrontal areas was not found in two studies that compared semantic and phonological generation tasks. An fMRI study that compared silent generation of category exemplars and silent generation of rhymes found greater bilateral frontal activation for the semantic than for the phonological task (46). The same frontal regions showed some activation for the phonological task, but there was no evidence for any frontal area specialized for phonological processing. In direct contrast, a PET study reported left prefrontal activations for semantic (generation of synonyms) and phonological (generation of a rhymes) tasks relative to reading words but no difference in that region between the semantic and phonological tasks (47). This study indicated that the same left prefrontal areas are involved in phonologically and semantically guided word retrieval. If semantic processes are engaged incidentally for meaningful words, however, it is possible that the rhyme generation tasks, in which auditory words were presented as cues, engaged both phonological and semantic processes.
One strategy for distinguishing between phonological and semantic processes is to use pronounceable nonwords that have phonological status (they can be read aloud) but no semantic status (they lack meaning). An fMRI study compared nonword rhyme judgments and semantic category judgments to a baseline of line orientation judgments and found left prefrontal activations for both the phonological and semantic tasks but no difference between those tasks in that region (48). Although pronounceable nonwords seem to offer an ideal method for separating phonological and semantic processes, there are aspects of performance with nonwords that may complicate the interpretation of activations. For example, when deciding how to pronounce a nonword, participants may use knowledge of similar real words (e.g., ref. 49). To the extent that such knowledge was used with nonwords, the difference in mental operations involved in rhyme and semantic judgments would be minimized.
The results reviewed above have points of convergence and divergence. The major convergent point is that all studies involving semantic judgments yielded left prefrontal activations. The major divergent point concerns whether semantic and phonological activations are separable or not separable within left prefrontal cortex. Further knowledge of what psychological processes are engaged in rhyme and nonword tasks may be important for resolving the apparent disagreements among these studies.
We have performed an fMRI study aimed at asking whether there is any left prefrontal region that is associated specifically with semantic relative to phonological processes. Four scans comprised the following direct comparisons: semantic (abstract/concrete) versus perceptual (uppercase/lowercase) judgments for words, phonological (deciding whether a word had two syllables or not) versus perceptual judgments for words, semantic versus phonological judgments for words, and phonological versus perceptual judgments for pronounceable nonwords. Behaviorally, participants made the case judgments most quickly (about 500 ms), the semantic and phonological judgments more slowly but quite similarly (about 830 ms), and the phonological judgments for nonwords most slowly (about 900 ms). These results illustrate that the semantic status of real words influenced phonological judgments. Interestingly, the semantic status of real words also had a small influence upon the perceptual judgments, with participants being 30 ms faster to make case judgments for nonwords than for words. It may be that although meaning was irrelevant to the case task, participants automatically diverted some of their attention to the meaning present in real words. These results are consistent with literature that reveals many interactions between semantic, phonological, and perceptual processes in word analyses (e.g., ref. 50). The complexity of these interactions may be the reason that a simple story has not emerged about the relation between semantic and phonological activations in left prefrontal cortex.
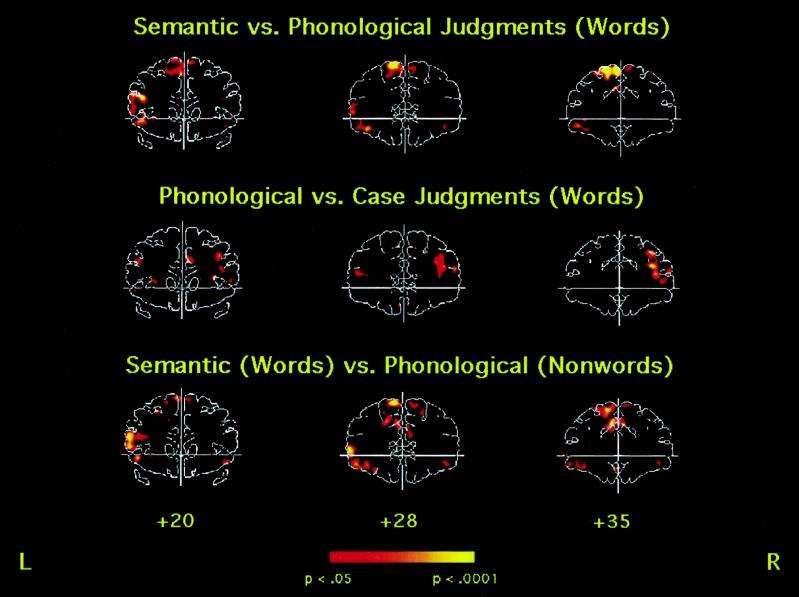
The results of the present study, however, were consistent in finding a region of the left inferior gyrus that was activated specifically by semantic analysis (Fig. 3). This was evident in the direct comparison of semantic versus phonological analysis of words. The same region was not activated in the phonological versus perceptual analysis of either words or nonwords. The phonological versus perceptual analysis of nonwords did activate a more posterior left prefrontal region. Because the individual scans employed common tasks, corroborative within-participant comparisons could also be made across scans. Thus, comparison of the semantic/perceptual and phonological/perceptual scans permitted a second, independent measure of the difference between semantic and phonological activations for words. In accord with the direct comparison, a region of the left inferior frontal gyrus was more activated for semantic than for phonological analysis of words (Fig. 3). Because the nonword syllable task required the longest time for response and yielded greater activation than the word syllable task, we also compared the semantic/perceptual scan with the phonological/perceptual scan with nonwords. Although the phonological demands of the syllable task with nonwords were considerable, there was still greater activation for the semantic task in the left inferior gyrus (but greater activation for the nonword phonological task in more dorsal prefrontal regions) (Fig. 3). These results support the conclusion that there are separable loci of semantic and phonological processing in left prefrontal cortex but do not explain why some studies failed to find such differentiation (47, 48).
Figure 3.
Averaged fMRI activation in the prefrontal cortex over eight subjects for three coronal slice locations (20, 28, and 35 mm anterior to the anterior commissure). (Top) Areas that were more active for semantic processing (abstract/concrete judgment) than for phonological processing (syllable judgment). (Middle) Regions that were more active for phonological processing than for perceptual processing (letter case judgment). (Bottom) Areas that were activated for semantic processing of words compared with phonological processing of pronounceable nonwords. Left inferior frontal cortex (Brodmann areas 45 and 47) is located just above and below the left end of the horizontal line. Activation in left inferior cortex is prominent when semantic judgments are made (Top and Bottom) but not when phonological judgments are made (Middle).
Left Prefrontal Cortex:
What Does It Do? This article has focused on functional neuroimaging analyses of psychological processing in one brain region, the left prefrontal cortex. It has not reviewed a wealth of related evidence about language (semantic, phonological, and orthographic) or memory processes in other brain regions, except for a brief consideration of right cerebellar activation. We have chosen this somewhat atypical approach because it emphasizes a goal of the functional neuroimaging of cognition that is not often emphasized, namely the attempt to define the large-scale functional units of cognition. Studies often report multiple activations associated with particular tasks (or more precisely task comparisons) and then interpret the significance of each activation in terms of what process may plausibly be mediated by that brain region. In principle, that same process and brain region ought to be invoked by a broad range of tasks that require the same sort of mental operation.
We have proposed that the left prefrontal activations of the kind reviewed above signify a domain-specific process of semantic working memory (6, 8, 29). This proposal builds upon the idea from Goldman-Rakic (51) that prefrontal cortex mediates domain-specific working memory representations that guide mental action in the absence of external, perceptual cues. Domain-specific working memory activations have been reported in right prefrontal cortex for spatial working memory tasks (52) and in posterior left prefrontal cortex for phonological working memory tasks (53). Similarly, it may be hypothesized that left inferior prefrontal cortex is activated to the extent that semantic information must be held temporarily in working memory (in mind) to answer a particular semantic question.
Thus, more semantic information must be held in mind to generate a verb than to read a noun or to answer a question about the meaning of a word (abstract/concrete or living/nonliving) than about the sound of a word (rhyme or syllable judgments) or the appearance of a word (uppercase/lowercase). More semantic information (or the same semantic information but for a shorter time) must be held in mind to make an initial than a repeated semantic judgment about a word or picture. More semantic (or lexical) information must be held in mind when a person must select one of many appropriate answers than when one must search carefully for even one appropriate answer. In all of these conditions, left prefrontal activation occurs in conditions that require a greater amount, a longer duration, or more selection of semantic knowledge held in working memory. Further, the more people think about the meaning of a word, or other stimuli, the better they later remember that stimulus (superior episodic encoding). Thus, the semantic working hypothesis can account for most relevant findings in a unified fashion.
In psychological research on cognition, it is common for different researchers to focus on language, on working memory, on episodic memory, or on implicit memory. The brain and mind, however, need not be organized in the same way that researchers divide cognitive domains. Indeed, one promise of functional neuroimaging is to reveal the natural organization of the brain and mind. Although there is a great deal yet to be understood about the mental operations mediated by the left prefrontal cortex, including how many distinct but adjacent operations occur in that region, it seems already that those operations may be the same whether they are considered in the context of language, working memory, episodic memory, or implicit memory. The left prefrontal cortex thus serves as a crossroads between meaning in language and memory.
Acknowledgments
We thank Matthew Prull and Mark Won for assistance in preparing this manuscript. The research reported herein and the writing of this article was supported by National Institutes of Health Grants NIA AG12995, NIA AG09466, NIMH MN53673, NIAAA AA10723, and NINDS NS09628 and the McDonnell-Pew Program in Cognitive Neuroscience.
ABBREVIATIONS
- fMRI
functional magnetic resonance imaging
- PET
positron emission tomography
References
- 1.Gabrieli, J. D. E. (1998) Annu. Rev. Psychol. 49, in press. [DOI] [PubMed]
- 2.Engel S A, Rumelhart D E, Wandell B A, Lee A T, Glover G H, Chichilnisky E J, Shadlen M N. Nature (London) 1994;369:525. doi: 10.1038/369525a0. [DOI] [PubMed] [Google Scholar]
- 3.Engel S A, Zhang X, Wandell B. Nature. 1997;388:68–71. doi: 10.1038/40398. [DOI] [PubMed] [Google Scholar]
- 4.Tootell R B H, Dale A M, Sereno M I, Malach R. Trends Neurosci. 1996;19:481–489. doi: 10.1016/S0166-2236(96)10053-9. [DOI] [PubMed] [Google Scholar]
- 5.Petersen S E, Fox P T, Posner M I, Mintun M, Raichle M E. Nature (London) 1988;331:585–589. doi: 10.1038/331585a0. [DOI] [PubMed] [Google Scholar]
- 6.Gabrieli J D E, Desmond J E, Demb J B, Wagner A D. Psychol Sci. 1996;7:278–283. [Google Scholar]
- 7.Kapur S, Rose R, Liddle P F, Zipursky R B, Brown G M, Stuss D, Houle S, Tulving E. NeuroReport. 1994;5:2193–2196. doi: 10.1097/00001756-199410270-00051. [DOI] [PubMed] [Google Scholar]
- 8.Demb J B, Desmond J E, Wagner A D, Vaidya C J, Glover G H, Gabrieli J D E. J Neurosci. 1995;15:5870–5878. doi: 10.1523/JNEUROSCI.15-09-05870.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Spitzer M, Kwong K K, Kennedy W, Rosen B R, Belliveau J W. NeuroReport. 1995;6:2109–2112. doi: 10.1097/00001756-199511000-00003. [DOI] [PubMed] [Google Scholar]
- 10.Petersen S E, Fox P T, Snyder A Z, Raichle M E. Science. 1990;249:1041–1044. doi: 10.1126/science.2396097. [DOI] [PubMed] [Google Scholar]
- 11.Price C J, Wise R J, Watson J D, Patterson K, Howard D, Frackowiak R S. Brain. 1994;117:1255–1269. doi: 10.1093/brain/117.6.1255. [DOI] [PubMed] [Google Scholar]
- 12.Price C J, Wise R J, Frackowiak R S. Cereb Cortex. 1996;6:62–70. doi: 10.1093/cercor/6.1.62. [DOI] [PubMed] [Google Scholar]
- 13.Vandenberghe R, Price C, Wise R, Josephs O, Frackawiak R S J. Nature (London) 1996;383:254–256. doi: 10.1038/383254a0. [DOI] [PubMed] [Google Scholar]
- 14.Tulving E, Kapur S, Craik F I M, Moscovitch M, Houle S. Proc Natl Acad Sci USA. 1994;91:2016–2020. doi: 10.1073/pnas.91.6.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Craik F I M, Lockhart R S. J Verb Learn Verb Behav. 1972;11:671–684. [Google Scholar]
- 16.Slamecka N J, Graf P. J Exp Psychol Hum Learn Mem. 1978;4:592–604. [Google Scholar]
- 17.Desmond J E, Sum J M, Wagner A D, Demb J B, Shear P K, Glover G H, Gabrieli J D E, Morrell M J. Brain. 1995;118:1411–1419. doi: 10.1093/brain/118.6.1411. [DOI] [PubMed] [Google Scholar]
- 18.Cohen N J, Squire L R. Science. 1980;210:207–210. doi: 10.1126/science.7414331. [DOI] [PubMed] [Google Scholar]
- 19.Graf P, Schater D L. J Exp Psychol Learn Mem Cognit. 1985;11:501–518. doi: 10.1037//0278-7393.11.3.501. [DOI] [PubMed] [Google Scholar]
- 20.Blaxton T A. J Exp Psychol Learn Mem Cognit. 1989;15:657–668. [Google Scholar]
- 21.Roediger H L, III, Weldon M S, Challis B. In: Varieties of Memory and Consciousness: Essays in Honor of Endel Tulving. Roediger H L III, Craik F I M, editors. Hillsdale, NJ: Erlbaum; 1989. pp. 3–42. [Google Scholar]
- 22.Fleischman D A, Gabrieli J D E, Reminger S, Rinaldi J. Neuropsychology. 1995;9:187–197. [Google Scholar]
- 23.Gabrieli J D E, Fleischman D A, Keane M A, Reminger S L, Morrell F. Psychol Sci. 1995;6:76–82. [Google Scholar]
- 24.Keane M, Gabrieli J D E, Mapstone H C, Johnson K A, Corkin S. Brain. 1995;118:1129–1148. doi: 10.1093/brain/118.5.1129. [DOI] [PubMed] [Google Scholar]
- 25.Buckner R L, Petersen S E, Ojemann J G, Miezin F M, Squire L R, Raichle M E. J Neurosci. 1995;15:12–29. doi: 10.1523/JNEUROSCI.15-01-00012.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Schacter D L, Reiman E, Curran T, Yun L S, Bandy D, McDermott K B, Roediger H L I. Neuron. 1996;17:267–274. doi: 10.1016/s0896-6273(00)80158-0. [DOI] [PubMed] [Google Scholar]
- 27.Squire L R, Ojemann J G, Miezin F M, Petersen S E, Videen T O, Raichle M E. Proc Natl Acad Sci USA. 1992;89:1837–1841. doi: 10.1073/pnas.89.5.1837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Raichle M E, Fiez J A, Videen T O, Macleod A M K, Pardo J V, Fox P T, Petersen S E. Cereb Cortex. 1994;4:8–26. doi: 10.1093/cercor/4.1.8. [DOI] [PubMed] [Google Scholar]
- 29.Wagner, A. D., Desmond, J. E., Demb, J. B., Glover, G. H. & Gabrieli, J. D. E. J. Cognit. Neurosci., in press.
- 30.Seger C A, Rabin L A, Zarella M, Gabrieli J D E. Neuropsychologia. 1997;35:1069–1074. doi: 10.1016/s0028-3932(97)00041-9. [DOI] [PubMed] [Google Scholar]
- 31.Fulham M J, Brooks R A, Hallett M, Di Chiro G. Neurology. 1992;42:2267–2273. doi: 10.1212/wnl.42.12.2267. [DOI] [PubMed] [Google Scholar]
- 32.Tanaka M, Kondo S, Hirai S, Ishiguro K, Ishihara T, Morimatsu M. J Neurol Neurosurg Psychiatry. 1992;55:121–125. doi: 10.1136/jnnp.55.2.121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Miura H, Nagata K, Hirata Y, Satoh Y, Watahiki Y, Hatazawa J. J Neuroimaging. 1994;4:91–96. doi: 10.1111/jon19944291. [DOI] [PubMed] [Google Scholar]
- 34.Boni S, Valle G, Cioffi R P, Bonetti M G, Perrone E, Tofani A, Maini C L. Nucl Med Commun. 1992;13:824–831. doi: 10.1097/00006231-199211000-00009. [DOI] [PubMed] [Google Scholar]
- 35.Appollonio I M, Grafman J, Schwartz V, Massaquoi S, Hallett M. Neurology. 1993;43:1536–1544. doi: 10.1212/wnl.43.8.1536. [DOI] [PubMed] [Google Scholar]
- 36.Botez M I, Botez T, Elie R, Attig E. Ital J Neurol Sci. 1989;10:291–300. doi: 10.1007/BF02333774. [DOI] [PubMed] [Google Scholar]
- 37.Grafman J, Litvan I, Massaquoi S, Stewart M, Sirigu A, Hallett M. Neurology. 1992;42:1493–1496. doi: 10.1212/wnl.42.8.1493. [DOI] [PubMed] [Google Scholar]
- 38.Silveri M C, Leggio M G, Molinari M. Neurology. 1994;44:2047–2050. doi: 10.1212/wnl.44.11.2047. [DOI] [PubMed] [Google Scholar]
- 39.Bracke-Tolkmitt R, Linden A, Canavan A G, Rockstroh B, et al. Behav Neurosci. 1989;103:442–446. [Google Scholar]
- 40.Canavan A G M, Springelmeyer R, Diener H C, Homberg V. Behav Neurosci. 1994;108:475–485. doi: 10.1037//0735-7044.108.3.475. [DOI] [PubMed] [Google Scholar]
- 41.Tucker J, Harding A E, Jahanshahi M, Nixon P D, Rushworth M, Quinn N P, Thompson P D, Passingham R E. Behav Neurosci. 1996;110:1229–1234. doi: 10.1037//0735-7044.110.6.1229. [DOI] [PubMed] [Google Scholar]
- 42.Middleton F A, Strick P L. Science. 1994;266:458–461. doi: 10.1126/science.7939688. [DOI] [PubMed] [Google Scholar]
- 43.Fiez J A, Tallal P, Raichle M E, Miezin F M, Katz W F, Petersen S E. J Cognit Neurosci. 1995;7:357–375. doi: 10.1162/jocn.1995.7.3.357. [DOI] [PubMed] [Google Scholar]
- 44.Zatorre R J, Evans A C, Meyer E, Gjedde A. Nature (London) 1992;256:846–849. doi: 10.1126/science.1589767. [DOI] [PubMed] [Google Scholar]
- 45.Demonet J F, Chollet F, Ramsay S, Cardebat D, Nespoulous J L, Wise R, Rascol A, Frackowiak R. Brain. 1992;115:1753–1768. doi: 10.1093/brain/115.6.1753. [DOI] [PubMed] [Google Scholar]
- 46.Shaywitz B A, Pugh K R, Constable R T, Shaywitz S E, Bronen R A, Fulbright R K, Shankweiler D P, Katz L, Fletcher J M, Skudlarski P, Gore J C. Hum Brain Mapp. 1995;2:149–158. [Google Scholar]
- 47.Klein D, Milner B, Zatorre R J, Meyer E, Evans A C. Proc Natl Acad Sci USA. 1995;92:2899–2903. doi: 10.1073/pnas.92.7.2899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Pugh K R, Shaywitz B A, Shaywitz S E, Constable R T, Skudlarski P, Fulbright R K, Bronen R A, Shankweiler D P, Katz L, Fletcher J M, Gore J C. Brain. 1996;119:1221–1238. doi: 10.1093/brain/119.4.1221. [DOI] [PubMed] [Google Scholar]
- 49.Rugg M D, Nagy M E. Mem Cognit. 1987;15:473–481. doi: 10.3758/bf03198381. [DOI] [PubMed] [Google Scholar]
- 50.Van Orden G C, Johnston J C, Hale B L. J Exp Psychol Learn Mem Cognit. 1988;14:371–386. doi: 10.1037//0278-7393.14.3.371. [DOI] [PubMed] [Google Scholar]
- 51.Goldman-Rakic P S. In: Handbook of Physiology, Section 1: The Nervous System, Higher Functions of the Brain, Part 1. Plum F, Mountcastle V, editors. V. Washington, DC: Am. Physiol. Soc.; 1987. pp. 373–417. [Google Scholar]
- 52.Jonides J, Smith E E, Koeppe R A, Awh E, Minoshima S, Mintun M A. Nature (London) 1993;363:623–625. doi: 10.1038/363623a0. [DOI] [PubMed] [Google Scholar]
- 53.Paulesu E, Frith C D, Frackowiak R S J. Nature (London) 1993;362:342–345. doi: 10.1038/362342a0. [DOI] [PubMed] [Google Scholar]