Abstract
Moringa oleifera Lamarack is commonly consumed for nutritional or medicinal properties. We recently reported the isolation and structure elucidation of novel bioactive phenolic glycosides, including 4-[(2′-O-acetyl-α-l-rhamnosyloxy)benzyl]isothiocyanate (RBITC), which was found to suppress inducible nitric oxide synthase (iNOS) expression and nitric oxide production in lipopolysaccharide-stimulated RAW 264.7 mouse macrophage cells. Inhibitors of proteins such as cyclooxygenase-2 (COX-2) and iNOS are potential anti-inflammatory and cancer chemopreventive agents. The inhibitory activity of RBITC on NO production (IC50 = 0.96 ± 0.23 µM) was greater than that mediated by other well-known isothiocyanates such as sulforaphane (IC50 = 2.86 ± 0.39 µM) and benzyl isothiocyanate (IC50 = 2.08 ± 0.28 µM). RBITC inhibited expression of COX-2 and iNOS at both the protein and mRNA levels. Major upstream signaling pathways involved mitogen-activated protein kinases and nuclear factor-κB (NF-κB). RBITC inhibited phosphorylation of extracellular signal regulated kinase and stress-activated protein kinase, as well as ubiquitin-dependent degradation of inhibitor κBα (IκBα). In accordance with IκBα degradation, nuclear accumulation of NF-κB, and subsequent binding to NF-κB cis-acting element, was attenuated by treatment with RBITC. These data suggest RBITC should be included in the dietary armamentarium of isothiocyanates potentially capable of mediating anti-inflammatory or cancer chemopreventive activity.
Keywords: cancer prevention, nitric oxide, cell signaling, in vitro/cell culture, isothiocyanates
INTRODUCTION
Moringa oleifera Lamarack (Moringaceae), also known as drumstick tree, horseradish tree, or kelor tree, is widely distributed in tropical and sub-tropical areas, including sub-Himalayan regions of India, Pakistan, Bangladesh, some parts of Afghanistan, South Africa, Arabia, Philippines, Cambodia, South America, Pacific Islands, and Caribbean Islands (1,2). Various parts of M. oleifera, including young leaves, flowers, and green pods, are consumed for nutritional value. In addition, most parts of M. oleifera have been used as traditional herbal remedies for the treatment of a variety of disorders such as skin diseases, respiratory sickness, ear and dental infections, hypertension, diabetes, anemia, and cancer (1,3). Pharmacological properties include antihypertensive, diuretic, cholesterol lowering, antispasmodic, antiulcer, hepatoprotective, antibacterial, antifungal, antitumor/anticaner, antioxidant, and antihelmintic activities (1). Further, the seed of the plant is considered as an effective natural coagulant used to purify water (4), and as a possible source of biodiesel (5). Some glycosidic constituents have been identified (e.g., niazirin, niazimicin, and niazicin A) and reported to mediate antitumor or anti-inflammatory activities (6,7).
More recently, we reported several novel isothiocyanates that were discovered on the basis of inhibiting nitric oxide (NO) production in lipopolysaccharide (LPS)-stimulated RAW 264.7 mouse macrophage cells. The most potent activity was observed with 4-[(2′-O-acetyl-α-l-rhamnosyloxy)benzyl]isothiocyanate (RBITC) (8). Bearing in mind the importance of other naturally-occurring isothiocyanates in the diet, such as sulforaphane and mustard oils, we have further investigated the mode of action of RBITC, with emphasis on the inflammatory response.
Inflammation is a protective response of the body to various intrinsic or extrinsic stimuli from physical, chemical, or biological sources including physical forces, irradiation, extreme temperature, irritants, pathogens, and metabolic overload (9,10). However, deregulated and consistent inflammatory responses, i.e., chronic inflammation, can promote heart attack and stroke (11), and may contribute to some disease states including atherosclerosis, hepatitis, gastritis, neurodegenerative disease, and rheumatoid arthritis (12–14). In particular, a growing body of evidence suggests chronic inflammation can lead to cancer (15). During chronic inflammatory processes, activated macrophages are thought to play critical roles in pathological conditions via continuous and excessive generation of inflammatory mediators including cytokines, chemokines, lysozymes, proteases, growth factors, eicosanoids such as prostaglandins, and nitric oxide (NO) (16,17).
Generation of the latter two mediators of inflammation results from cyclooxygenase-2 (COX-2) and inducible nitric oxide synthase (iNOS), respectively. Moreover, since up-regulation of COX-2 or iNOS has been observed during carcinogenesis or malignancy, inhibition of these processes presents opportunities for cancer chemoprevention (18,19).
In this context, we evaluated the effect of RBITC on the expression levels of iNOS and COX-2 in LPS-induced RAW 264.7 murine macrophage cells. LPS, an endotoxin which is a component of the outer membrane of Gram-negative bacteria, can evoke innate immune responses and subsequent inflammatory responses by inducing the expression of a number of pro-inflammatory genes in macrophages (20). Transmission of the LPS signal with macrophages is initiated by binding and activation of LPS receptor complex which is comprised of Toll-like receptor4 (TLR4) and myeloid differentiation protein-2 (MD-2). Activated TLR4 consequently transfers the signal via two main downstream signaling pathways: myeloid differentiation factor 88 (MyD88)-dependent and Toll/IL-1 receptor domain-containing adapter inducing interferon-β (TRIF)-dependent pathways. Through the MyD88-dependent pathway, phosphorylation of mitogen-activated protein kinases (MAPKs) and ubiquitin-degradation of inhibitor κBα (IκBα) result in the activation of several transcription factors such as nuclear factor κB (NF-κB), activating protein-1 (AP-1), cAMP-responsive element binding protein (CREB), and CCAAT-enhancer box binding protein (C/EBP) which, in turn, promote expression of COX-2 and iNOS (8,20).
To date, various compounds have been identified as inhibitors of COX-2 and iNOS from natural sources, including resveratrol, curcumin, and epigallocatechin gallate (21). Also, naturally-occurring or synthetic isothiocyanates (ITCs) such as sulforaphane (22), 2-phenylethyl ITC (23), 8-methylsulphinyloctyl ITC (24), tetrahydrofurfuryl ITC, 3-morpholinopropyl ITC, 3,4-methyelendioxybenzyl ITC (25), and benzyl ITC (BITC) (26) are known to mediate anti-inflammatory activities in RAW 264.7 cells. Here, we report that RBITC suppresses the expression of iNOS and COX-2 expression through the inhibition of MAPKs and NF-κB signaling pathways. These data suggest the anti-inflammatory and chemoprevention potential of RBITC, a novel natural product found in the diet of human beings.
MATERIALS AND METHODS
Reagents
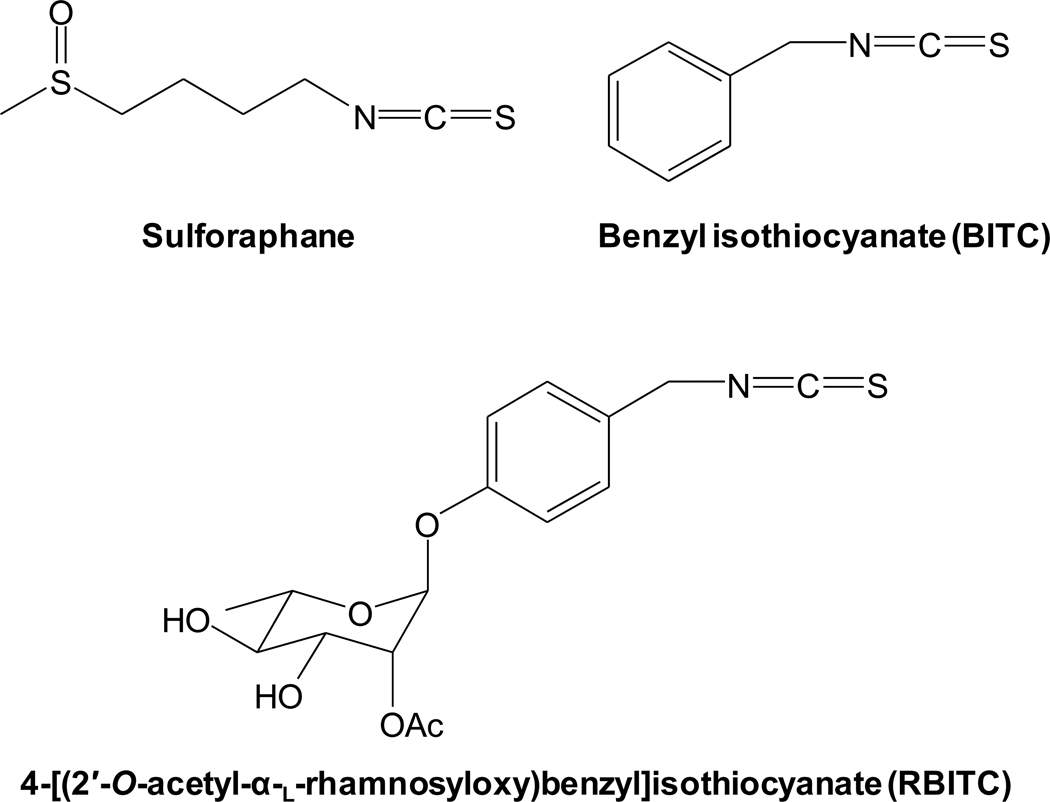
4-[(2′-O-Acetyl-α-l-rhamnosyloxy)benzyl]isothiocyanate (RBITC) (Figure 1) was isolated as previously described (8). Sulforaphane (Figure 1), benzyl isothiocyanate (BITC) (Figure 1), lipopolysaccharide (LPS), and sulforhodamine B (SRB) were purchased from Sigma-Aldrich Co. (St. Louis, MO, USA). Rabbit polyclonal anti-iNOS antibody was purchased from Abcam Inc. (Cambridge, MA, USA). Cell Lysis Buffer (10×), antibodies against β-actin, GAPDH, COX-2, phospho (p)-p44/42 MAPK (ERK1/2) (Thr202/Tyr204), ERK1/2, p-p38 MAPK (Thr180/Tyr182), p38 MAPK, p-SAPK/JNK (Thr183/Tyr185), SAPK/JNK, NF-κB p65, IκBα, p-IκB kinase (IKK) (Ser176/180), and U0126 (a selective inhibitor of MEK1/2) were purchased from Cell Signaling Technology (Beverly, MA, USA). Rabbit polyclonal antibody against lamin A/C was purchased from Santa Cruz Biotechnology (Santa Cruz, CA). Enhanced chemiluminescence (ECL) detection kit was purchased from Amersham Biosciences (Piscataway, NJ, USA). Oligonucleotide PCR primers (Table 1) and Trizol® reagent were purchased from Invitrogen™ (Carlsbad, CA, USA). RT2 First Strand Kit (C-03) was purchased from SABiosciences™ (Frederick, MD, USA). PerfeCTa™ SyBR® Green FastMix™, ROX was purchased from Quanta biosciences™ (Gaithersburg, MD, USA). IRDye® 700 infrared dye-5′-end-labelled NF-κB oligonucleotide was purchased from Integrated DNA Technologies Inc. (Coralville, IA, USA).
Figure 1.
Chemical structure of sulforaphane, benzyl isothiocyanate (BITC), and 4-[(2′-O-acetyl-α-l-rhamnosyloxy)benzyl]isothiocyanate (RBITC).
Table 1.
Sequences of mouse gene specific primers used in PCR
| Target gene | Sequences | |
|---|---|---|
| iNOS | Sense | 5′ - GGAGCGAGTTGTGGATTGTC - 3′ |
| Antisense | 5′ - GTGAGGGCTTGGCTGAGTGAG - 3′ | |
| COX-2 | Sense | 5' - GAAGTCTTTGGTCTGGTGCCTG - 3′ |
| Antisense | 5' - GTCTGCTGGTTTGGAATAGTTGC - 3′ | |
| β-Actin | Sense | 5′- GCTACAGCTTCACCACCACAG - 3′ |
| Antisense | 5′ - GGTCTTTACGGATGTCAACGTC - 3′ | |
Cell culture
RAW 264.7 mouse macrophage cells (ATCC, TIB-71) were cultured in Dulbecco's Modified Eagle’s Medium (DMEM) supplemented with 10% heat-inactivated fetal bovine serum (FBS) and antibiotics-antimycotics (100 U/ml penicillin G sodium, 100 µg/ml streptomycin sulfate and 0.25 µg/ml amphotericin B) at 37°C in 5% CO2 humidified air.
Measurement of nitric oxide (nitrite assay)
The assay was performed as described previously (8). Due to a short half-life of NO and subsequent oxidation to stable nitrite (27), nitrite levels were measured to estimate NO levels. RAW 264.7 cells were incubated in 96-well culture plates at 37°C, 5% CO2 in humidified air for 24 h. As with previous studies performed with known inhibitors of MAPKs (28,29), in the current work, cells were pretreated with test compounds prior to LPS exposure. With these experimental conditions, inhibition of enzymatic activity may be detected, as well as inhibition of the expression levels of iNOS controlled by any mechanism, including upstream NF-κB and MAPK signaling pathways. Accordingly, for a period of 15 min, cultured RAW 264.7 cells were treated with phenol red-free medium containing various concentrations of sulforaphane, BITC, or RBITC, followed by treatment with 1 µg/ml of LPS. After various periods of incubation, the amount of nitrite released in the culture media was measured with Griess reagent [1:1 mixture (v/v) of 1% sulfanilamide in 5% H3PO4 and 0.1% N-(1-naphthyl)ethylenediamine dihydrochloride solution]. Serially diluted sodium nitrite was used as a standard and absorbance was measured at 540 nm. To evaluate the cytotoxic effects of ITCs with RAW 264.7 cells under the same experimental condition, the SRB assay was performed (30).
Western blot analysis
RAW 264.7 cells were lysed using 1× cell lysis buffer according to the manufacturer’s protocol. After 5 min of incubation with lysis buffer, cells were centrifuged at 14,000 ×g for 10 min at 4°C, and the resultant supernatant as cell lysate was collected and stored at −80°C until use. After quantification of protein using the Bradford method (31), equal amounts of total protein in each cell lysate were resolved using SDS-PAGE, and electrotransferred to PVDF membranes. The membranes were incubated with 5% skimmed milk in 0.1% Tween 20 containing TBS for 1 h at room temperature to block non-specific protein binding. Then, membranes were incubated overnight at 4°C with corresponding primary antibodies in 3% skimmed milk in TBS followed by incubation with horseradish peroxidase-conjugated secondary antibodies, and visualization using an ECL detection kit according to the manufacturer’s instructions with a Geliance 1000 imager (Perkin Elmer, Inc., Waltham, MA, USA). Band densities were quantified using GeneTools software (v3.07.g, PerkinElmer).
Real time-polymerase chain reaction (RT-PCR) analysis
Total RNA was extracted from cells using Trizol® reagent according to the method of Chomczynski and Sacchi (32). Isolated RNA was dissolved in RNase-free water and the quality and quantity were measured using a NanoDrop ND-1000 spectrophotometer (Thermo Fisher Scientific Inc., Wilmington, DE, USA). Total RNA (1 µg) was incubated with genomic DNA elimination mixture at 42°C for 5 min followed by reverse transcription to cDNA under the condition of 42°C for 15 min and 95°C for 5 min using RT2 First Strand Kit (C-03) on a ABI 7300 thermocycler (Applied Biosystems Inc., Foster City, CA, USA). The cDNA amplification was performed with the corresponding primer pairs (Table 1) and 2× PerfeCTa™ SyBR® Green FastMix™, ROX (MgCl2, dNTPs, AccuFast Taq DNA polymerase, SYBR Green I dye, ROX reference dye, and stabilizer) under the following cycling conditions: after 94°C for 3 min, 50 cycles of denaturation at 94°C for 30 s, annealing at 57°C for 30 s, and elongation at 72°C for 30 s. The fluorescence signal was detected at the end of each cycle. The web-based analysis tool from SABiosciences™ using the 2−ΔΔCT method was performed to analyze the results (33).
Preparation of nuclear extracts and electrophoretic mobility shift assay (EMSA)
Nuclear extracts were prepared as previously described (34). Briefly, PBS-washed cells were incubated in EMSA lysis buffer (10 mM Tris-HCl, pH 8.0, 60 mM KCl, 1 mM EDTA, 1 mM dithiothreitol, 100 mM PMSF, and 0.1% NP-40) for 5 min on ice. Following centrifugation at 2500 rpm at 4°C for 4 min, the pellet was washed with lysis buffer without NP-40. The nuclear proteins were extracted from the pellet after 10 min incubation with nuclear extract buffer (20 mM Tris-HCl, pH 8.0, 420 mM NaCl, 1.5 mM MgCl2, 0.2 mM EDTA and 25% glycerol) and centrifugation at 14,000 rpm at 4°C for 15 min. Binding reactions were performed by incubation of 5 µg of nuclear protein extracts, 2.5 mM dithiothreitol, 1 µg of poly (dI-dC), 2.5% glycerol, 50 mM KCl, 10 mM EDTA, and 50 nM IRDye® 700 infrared dye-5′-end-labelled NF-κB oligonucleotides for 30 min at room temperature. Protein-DNA complexes were resolved by electrophoresis on 5% polyacrylamide gels in 0.5× TBE buffer at 100 V for 1 h 30 min. The gel was visualized using an Odyssey® Infrared Imaging System (LI-COR Biosciences, Lincoln, NE, USA). Binding of the unlabeled competitor DNA was also assessed.
Statistical analysis
Data were expressed as means ± standard deviation (SD). Statistical comparisons were made by use of one-way ANOVA followed by Tukey’s post-hoc test (SigmaPlot 12, Systat Software Inc.). P values less than 0.05 were considered statistically significant.
RESULTS
Inhibitory effect of sulforaphane, BITC, and RBITC on LPS-induced NO production
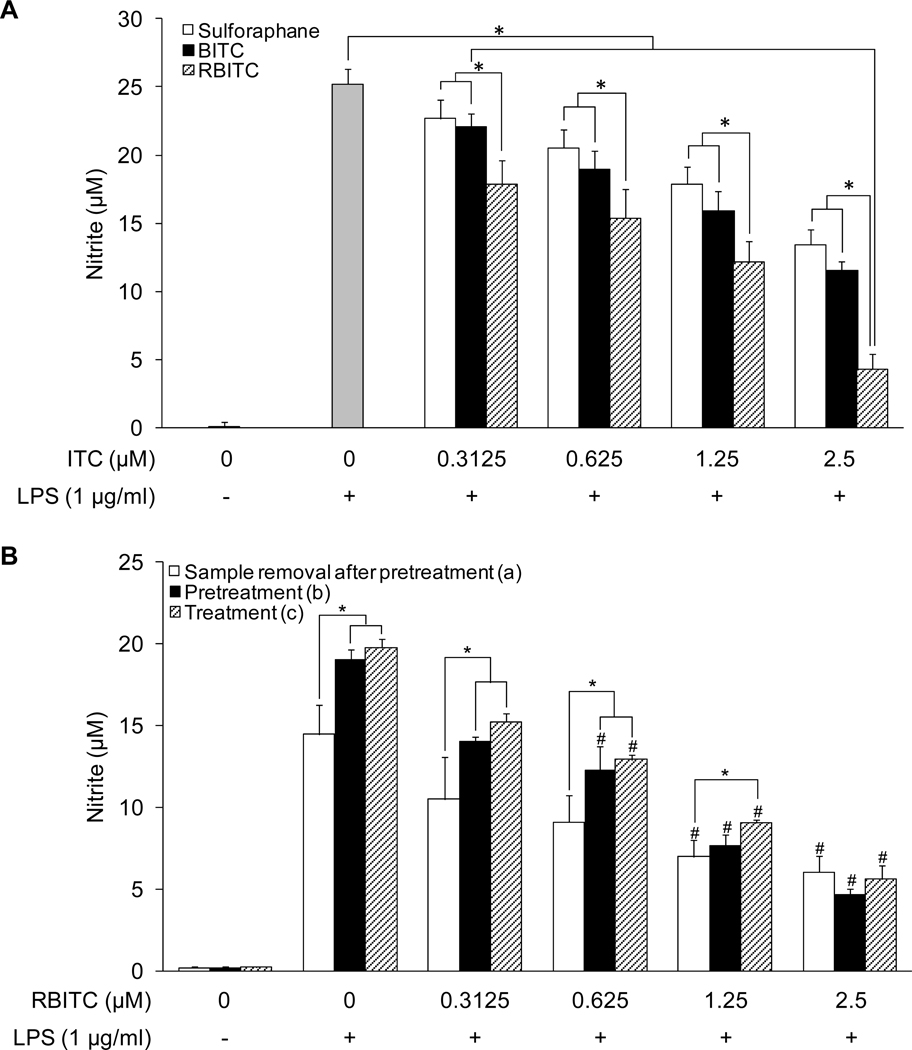
Previously, we reported the inhibitory effect of RBITC on NO production in LPS-stimulated macrophages (8). In the present study, we compared the inhibitory activity of RBITC with other well-known ITC inhibitors of nitrite production, sulforaphane and BITC. As shown in Figure 2A, untreated RAW 264.7 cells demonstrated a low basal level of nitrite in cell culture media (0.03 ± 0.40 µM), while stimulation with LPS (1 µg/ml) greatly enhanced nitrite production (25.20 ± 1.13 µM). The increased production of nitrite was attenuated by treatment with sulforaphane, BITC, and RBITC, in a concentration-dependent manner, with IC50 values of 2.86 ± 0.39, 2.08 ± 0.28, and 0.96 ± 0.23 µM, respectively. There was a significant decrease of nitrite production mediated by each of the compounds at every concentration tested, except at the lowest concentration of sulforaphane (0.3125 µM). Notably, the greatest potency of inhibition was mediated by RBITC, which was significantly more active than sulforaphane and BITC at each concentration. No reduction of cell growth was observed at any concentration tested.
Figure 2.
Concentration-dependent inhibitory effects of sulforaphane, BITC, and RBITC on LPS-induced NO production in RAW 264.7 cells. A, Cells were pretreated with the indicated concentrations (0–2.5 µM) of sulforaphane (open bars), BITC (solid bars) or RBITC (hatched bars) for 15 min, and then stimulated with LPS for 20 h. The level of nitrite produced by LPS treatment was significantly suppressed by each concentration of the test compounds. Suppression mediated by RBITC was significantly greater that sulforaphane and BITC at each concentration tested. An asterisk (*) indicates a significant difference between indicated the groups with P values less than 0.05. B, (a) Cells were pretreated with the indicated concentrations of RBITC for 15 min, washed with PBS, and then treated with LPS for 20 h; (b) Cells were pretreated with RBITC for 15 min followed by LPS treatment for 20 h; (c) Cells were treated simultaneously with RBITC and LPS for 20 h. For respective controls, cells were treated with LPS for a period of 20 h. After incubation, the concentration of nitrite in the media was determined by using the Griess reaction. Results were expressed as means ± SD (n = 6). An asterisk (*) indicates a significant difference between the indicated groups with P values less than 0.05. A number sign (#) indicates a significant difference between the groups treated with LPS only and the respective RBITC-treated groups with P values less than 0.05.
To examine the potential effect of treatment time and temporal sequence on the inhibitory potency of RBITC on nitrite production, the following experiments were performed: (a) cells were treated with RBITC for 15 min, medium was removed, cells were washed with PBS, and treated with LPS for 20 h, (b) cells were treated with RBITC for a period of 15 min followed by treatment with LPS for 20 h, or (c) cells were simultaneously treated with RBITC and LPS for 20 h. As shown in Figure 2B, in the absence of test substance, the nitrite level of control cells washed with PBS (14.46 ± 1.80 µM) appears slightly less than those not subjected to this procedure (ca. 19–20 µM). However, with each of these experimental conditions, the IC50 values [(a):1.11 µM; (b):1.07 µM; (c):1.19 µM] were similar.
Inhibitory effects of RBITC on LPS-induced protein and mRNA expression of COX-2 and iNOS
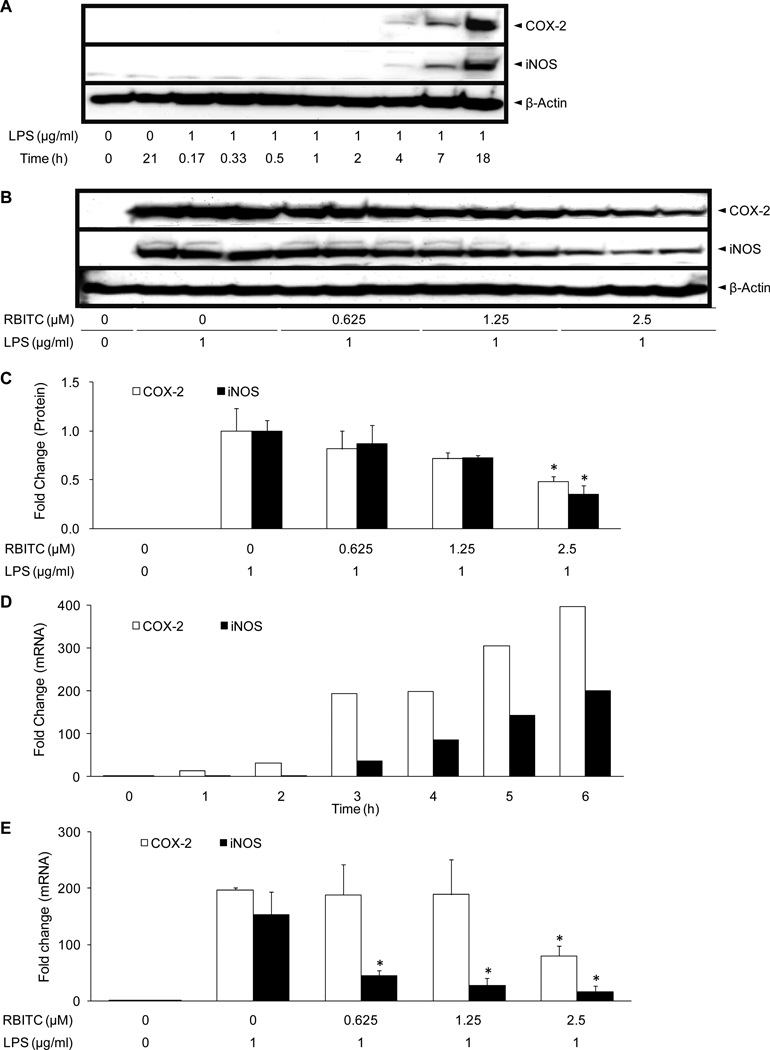
Further experiments were performed to determine the mode of inhibition of COX-2 and iNOS by RBITC. In preliminary Western blot analyses using antibodies against iNOS or COX-2, it was found that expression levels of COX-2 and iNOS in RAW 264.7 cells were negligible in the absence of LPS. When LPS was added to the culture media, levels of both COX-2 and iNOS proteins began to increase after 4 h, and intense bands were observed after 18 h of incubation (Figure 3A). RAW 264.7 cells were then treated with various concentrations of RBITC (0, 0.625, 1.25, 2.5 µM) for 15 min, followed by LPS for 18 h. As expected, in comparison with untreated cells, expression levels of COX-2 and iNOS were up-regulated by treatment with LPS (Figure 3B). Pretreatment with RBITC attenuated the expression of COX-2 and iNOS protein in a concentration-dependent manner, reaching statistical significance at a concentration of 2.5 µM (Figure 3C).
Figure 3.
Effect of RBITC on iNOS and COX-2 protein expression and RNA levels. A, Time course of COX-2 and iNOS protein expression. RAW 264.7 cells were treated with 1 µg/ml LPS for the indicated periods of time and lysed. Levels of COX-2 and iNOS protein expression were determined using Western blot analyses. B, Inhibitory effect of RBITC on COX-2 and iNOS protein expression. RAW 264.7 cells were pretreated with the indicated concentrations of RBITC (0–2.5 µM) for 15 min, and then stimulated with 1 µg/ml LPS. After an 18 h incubation period, cells were lysed, and 15 µg protein was applied on a 9% SDS-polyacrylamide gel. The levels of COX-2 and iNOS protein expression were examined by Western blot analyses using antibodies against COX-2 or iNOS. β-Actin was used as an internal standard. Triplicate determinations are shown. C, The band intensities of COX-2 (open bars) and iNOS (solid bars) were was normalized to that of the β-actin. The data are presented as fold-changes with the band intensity of LPS-treated control set at 1.0 (n = 3). D, Time-course of COX-2 and iNOS mRNA expression. RAW 264.7 cells were treated with 1 µg/ml LPS for the indicated periods of time and RNA was extracted to examine the mRNA expression levels of COX-2 (open bars) and iNOS (closed bars). E, Inhibitory effect of RBITC on COX-2 (open bars) and iNOS (closed bars) mRNA levels. RAW 264.7 cells were pretreated with the indicated concentrations of RBITC (0–2.5 µM) for 15 min, and then stimulated with 1 µg/ml LPS. After a 4 h incubation period, RNA was extracted and quantified. Total RNA (1 µg) was used in the RT-PCR reaction; primers are specified in Table 1. After normalization to the internal standard, β-actin, fold-changes relative to vehicle-treated control is presented (n = 3). An asterisk (*) indicates a significant difference with P values less than 0.05.
Next, the effect of RBITC on COX-2 and iNOS mRNA levels was investigated using RT-PCR. As shown in Figure 3C, COX-2 and iNOS were elevated after 3 h of treatment with LPS, by 37.0- and 193.3-fold, respectively. The increase continued in a time-dependent manner, such that COX-2 and iNOS mRNA levels were 396.6- and 200.8-fold greater than controls after 6 h of incubation, respectively (Figure 3D). Using a 4 h LPS treatment period, expression levels of iNOS were significantly reduced at concentrations of RBITC ≥0.625 µM; expression levels of both COX-2 and iNOS were significantly down-regulated at the highest concentration tested (2.5 µM) (Figure 3E). β-Actin mRNA was monitored as a control, and no specific changes were observed with these experimental conditions (data not shown).
Effect of RBITC on MAPK signaling pathway
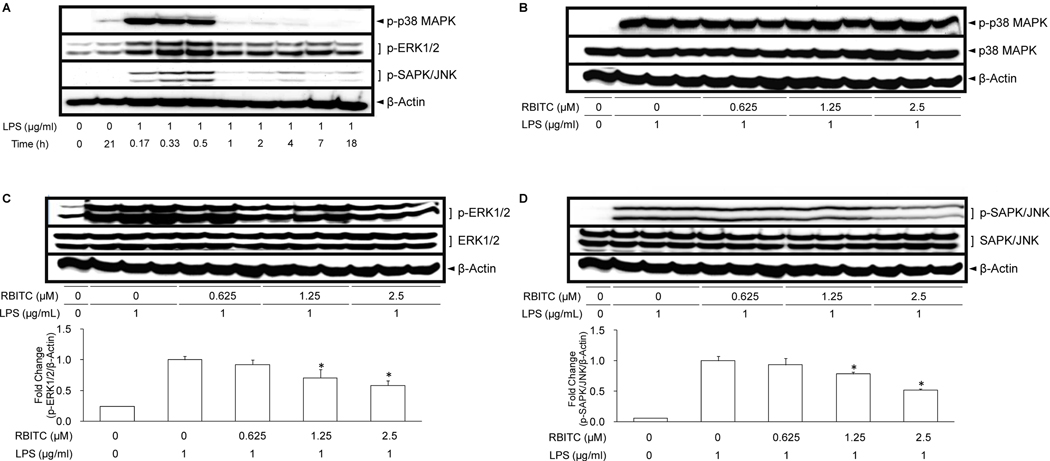
To further elucidate the molecular mechanism underlying RBITC-mediated inhibition of COX-2 and iNOS, cellular levels of upstream signaling molecules, phosphorylated and total MAPKs, were investigated. As illustrated by Western blot analyses, MAPKs showed appreciable levels of phosphorylation (p-p38 MAPK, p-ERK1/2, p-SAPK/JNK) when cells were treated with LPS from 10 to 30 min (Figure 4A). Using 30 min LPS incubation period, treatment with RBITC did not reduce the level of p-p38 MAPK (Figure 4B). However, p-ERK1/2 (Figure 4C) and p-SAPK/JNK (Figure 4D) levels were significantly suppressed by RBITC treatment at concentrations of 1.25 and 2.5 µM. No corresponding changes were observed with p38 MAPK (Figure 4B), ERK1/2 (Figure 4C), or SAPK/JNK (Figure 4D).
Figure 4.
Effect of RBITC on LPS-induced MAPKs activation in cultured RAW 264.7 cells. A, Time-course of MAPKs protein expression. RAW 264.7 cells were treated with 1 µg/ml LPS for the indicated periods of time and lysed to determine protein expression levels of MAPKs using Western blot analyses. RAW 264.7 cells were pretreated with various concentrations (0–2.5 µM) of RBITC for 15 min, and then incubated with LPS for 30 min. Total cell lysate was prepared and the levels of p-p38 MAPK, total p38 MAPK (B), p-ERK1/2, total ERK1/2 (C), and p-SAPK/JNK, SAPK/JNK (D) were analyzed by Western blotting. The band intensities of each group (n = 3) were normalized to that of the β-actin; data are presented as fold-changes. An asterisk (*) indicates a significant difference with P values less than 0.05.
Effect of RBITC on the NF-κB signaling pathway
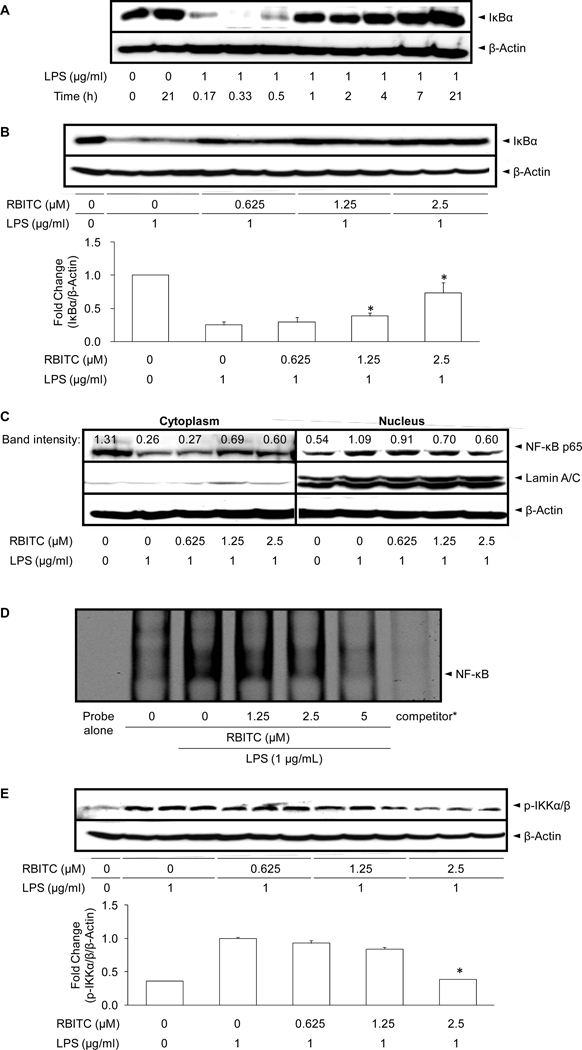
As a first step, the levels of IκBα, a molecular marker in the NF-κB signaling pathway, was examined by Western blot analysis. As shown in Figure 5A, the basal level of IκBα in resting cells was high, but LPS treatment led to IκBα degradation through the ubiquitin-proteasome pathway, with decreases observed at 10, 20, and 30 min post-incubation. This degradation process was blocked by RBITC in a concentration-dependent manner, with significant differences noted at 1.25 and 2.5 µM (Figure 5B).
Figure 5.
Effect of RBITC on LPS-induced IκBα degradation, NF-κB nuclear translocation, NF-κB DNA binding, and IKKα/β phosphorylation in cultured RAW 264.7 cells. A, Time-course of IκBα protein expression. RAW 264.7 cells were treated with 1 µg/ml LPS for the indicated periods of time and lysed to determine the protein expression levels of IκBα using Western blot analyses. B, Cells were pretreated with the indicated concentration of RBITC for 15 min followed by incubation with LPS for 15 min. Cells were then lysed and IκBα protein levels were analyzed by Western blotting. The band intensities of each group (n = 3) were normalized to that of the β-actin; the data were presented as fold-changes (lower panel). C, Cells were pretreated with the indicated concentrations of RBITC for 15 min, incubated with LPS for 1 h, and nuclear fractions were extracted and analyzed by Western blotting. Representative bands from two different experiments are illustrated. D, RAW 264.7 cells were pretreated with the indicated concentrations of RBITC for 15 min, and then stimulated with LPS for 1.5 h. Nuclear fractions were prepared as described in Materials and Methods. The NF-κB protein-DNA binding complex was resolved on 5% polyacrylamide gel and visualized with an Odyssey® Infrared Imaging System. * During the binding reaction, a concentrated NF-κB specific unlabeled consensus (10 µM, ×200) was added to verify the specific NF-κB protein-DNA interaction. E, After pretreatment with the indicated concentration of RBITC for 15 min followed by incubation with LPS for 15 min, cells were lysed and p-IKKα/β protein levels were analyzed by Western blotting. Band intensities of each p-IKKα/β group (n = 3) were normalized to that of the β-actin; data are presented as fold- changes.
IκBα sequesters NF-κB in unstimulated RAW 264.7 cells, but in LPS-induced cells, degradation of IκBα allows NF-κB to translocate to the nucleus, with subsequent activation of gene expression resulting from the interaction of NF-κB and the corresponding cis-acting element (35). Therefore, we examined the levels of NF-κB p65, the major subunit of NF-κB, in nuclear fractions, using lamin A/C as a nuclear loading control. As shown in Figure 5C, NF-κB p65 subunit was decreased in the cytoplasm and increased in nuclear extracts after 1 h of treatment with LPS. Treatment with RBITC reversed these trends in a concentration-dependent manner. Consistent with this, stimulation of the cells with 1 µg/ml LPS for 1.5 h led to an increase in the band intensities of NF-κB DNA binding. However, pretreatment with RBITC for 15 min prior to LPS treatment suppressed DNA binding activity in a concentration-dependent manner (Figure 5D). The addition of an excessive amount of specific unlabeled consensus oligonucleotide (competitor) completely blocked the band shifts with IRDye® 700 infrared dye-5'-end-labelled NF-κB, demonstrating the specificity of the protein-DNA interaction.
Finally, since IKK is one of the upstream molecules involved in the phosphorylation of IκBα and its subsequent degradation, the effect of RBITC on LPS-induced IKK activation was examined by assessing the level of phosphorylation at Ser176/180. Pretreatment with RBITC reduced IKKα/β phosphorylation in a concentration-dependent manner (Figure 5E), suggesting a relationship to RBITC-mediated inhibition of IκBα degradation.
Evaluation of RBITC on MAPK signaling inhibition and the relationship to the NF-κB signaling pathway
Previous studies have shown different types of relationships between ERK1/2 and NF-κB. For example, U0126 (a MEK inhibitor) reduced IκBα phosphorylation in TPA-induced mouse skin (36), NF-κB activation was blocked in LPS-stimulated RAW cells which expressed dominantly negative mutant MEK1 (37), and IκBα degradation was reduced by PD098059 (a MEK inhibitor) in LPS-stimulated J774 macrophages (38), while IκBα was persistently reduced after U0126 co-administration with LPS in RAW 264.7 cells (39). On the other hand, it has been reported that PD98059 had no effect on phosphorylation and degradation of IκBα in IL-1α-stimulated in rat vascular smooth muscle cells (40).
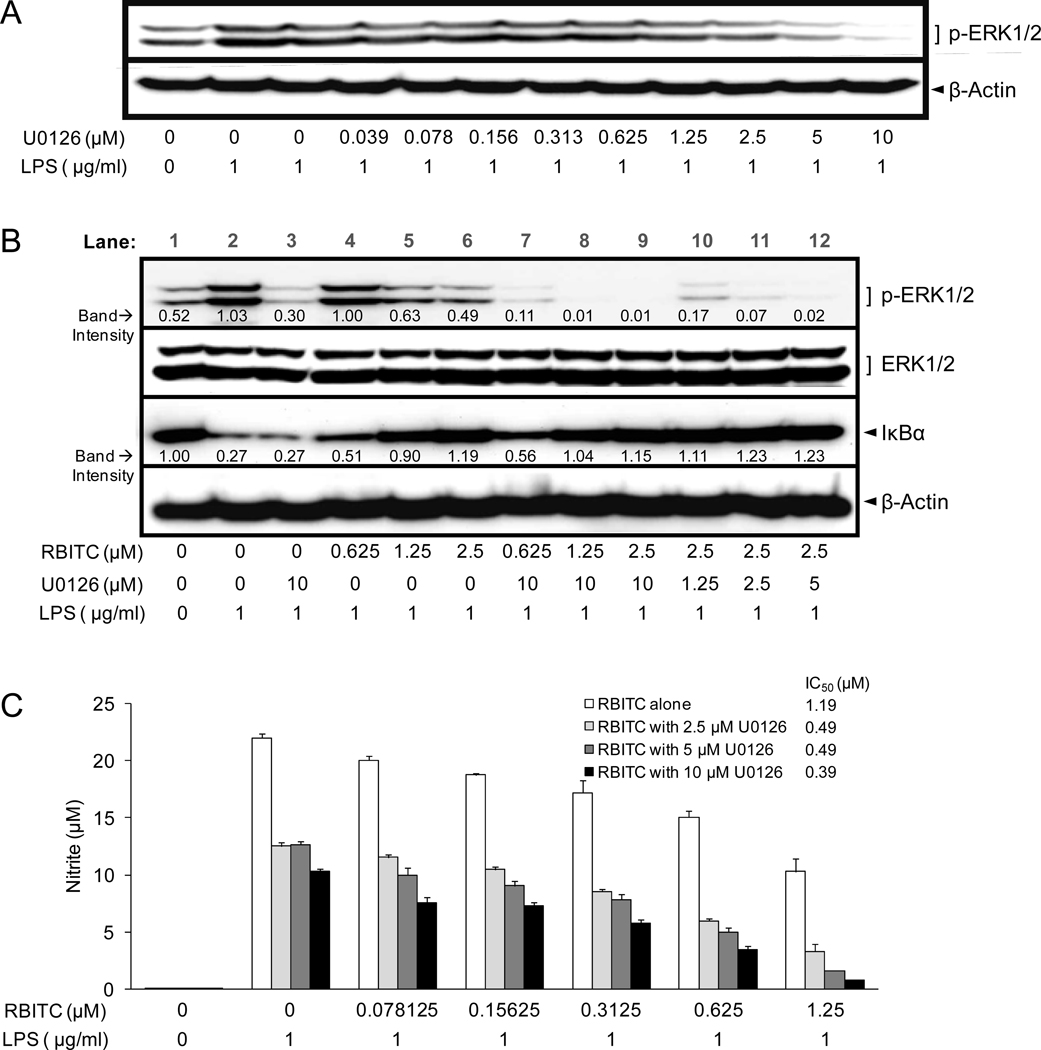
Therefore, to further investigate the affect of RBITC on the potential link between ERK1/2 and NF-κB with our experimental conditions, studies were performed with U0126, a MEK specific inhibitor known to block NO formation (41). First, as shown in Figure 6A, U0126 reduced p-ERK1/2 levels in a concentration-dependent manner. LPS-induced p-ERK1/2 levels were reduced to below those of basal level at concentrations in the range of 5–10 µM.
Figure 6.
Effect of U0126 and RBITC on LPS-treated RAW 264.7 cells. A, Effect of U0126 on p-ERK1/2 protein levels. Following pre-treatment of RAW 264.7 cells with the indicated concentrations of U0126 for 30 min, incubations were continued with LPS (1 µg/ml) for 15 min, cells were lysed, and p-ERK1/2 protein levels were analyzed by Western blotting. B, C, Effect of U0126 on the activity of RBITC. RAW 264.7 cells were pre-treated with U0126 for 30 min, followed by RBITC for an additional 15 min, and the LPS (1 µg/ml) for 15 min (B) or 20 h (C). Levels of the indicated proteins (B) and nitrite production (C) were determined as described in Materials and Methods. Results were expressed as means ± SD (n = 6).
As shown in Figure 6B, combination treatment of U0126 (10 µM) with RBITC augmented the inhibition of p-ERK1/2 (lanes 7–9), relative to treatment with U0126 (10 µM) (lane 3) or RBITC alone (lanes 4–6). RBITC (2.5 µM) enhanced the inhibitory effect of U0126 on p-ERK1/2 (lanes 6, 10–12; cf. Figure 6A). However, whereas U0126 alone did not prevent the degradation of IκBα (lane 1 versus lane 3), RBITC alone was effective in this process (lanes 4–6), as was combination treatment with RBITC and U0126 (lanes 7–9), indicating that the inhibition of MEK-ERK signaling has no direct connection to IκBα degradation in the NF-κB signaling pathway. Despite these differences in inhibitory potential, both compounds blocked nitrite production in LPS-stimulated RAW 264.7 cells (Figure 6C). RBITC was more effective (IC50 = 1.19 µM) relative to U0126 (IC50 = 8.90 µM). In combination, even greater inhibitory potential was observed, as reflected by decreased IC50 values.
DISCUSSION
There are three types of NOSs: constitutively expressed endothelial NOS (eNOS, NOS I), inducible NOS (iNOS, NOS II), and neuronal NOS (nNOS, NOS III). The common function of NOS is to catalyze the production of NO from L-arginine. NO is known to mediate diverse physiological processes in cardiovascular (42), gastrointestinal (43), nervous (44), and innate immune defense systems (45). Likewise, COXs are divided into three types, including constitutively expressed COX-1, inducible COX-2, and a splice variant of COX-1, so-called COX-3 (46). These enzymes catalyze the conversion of arachidonic acid to prostaglandins which are involved in inflammatory reactions, gastrointestinal cytoprotection, hemostatsis, thrombosis, and renal hemodynamics (47). Constitutively expressed forms of both NOS and COX are generally involved in the maintenance of homeostasis in body systems, while inducible forms such as iNOS and COX-2 are involved in pathogenesis, including cancer (48). Therefore, iNOS or COX-2 inhibitors are of value as anti-inflammatory and cancer chemopreventive agents. Accordingly, we have evaluated the effect of natural products and recently discovered RBITC as an inhibitor of NO production (8).
RBITC is an ITC derivative, and cancer chemopreventive potential has been associated with this structural class. For example, sulforaphane, a component of broccoli, cauliflower, brassicas and kale (49), has been shown to suppression azoxymethane-induced colonic aberrant crypt foci in rats (50) and adenoma formation in APCMin/+ mice (51). At the molecular level, sulforaphane shows remarkably potent induction of phase II detoxification enzymes such as glutathione S-transferase and NAD(P)H: quinone oxidoreductase (52), inhibits some cytochrome P450s such as CYP1A1 and 2B1/2 in rat hepatocytes, CYP2E1 in acetone-induced rat liver microsomes, CYP3A4 activity in human hepatocytes, regulates estrogen receptor α in human breast cancer cells, and up-regulates death receptors (49). Other studies have shown that BITC, similar in structure to RBITC, effectively inhibits carcinogenesis of the breast and lung in mouse models (53,54). BITC also inactivates CYP2A6 and 2A13 (55), and inhibits excessive superoxide generation (56).
Given these promising activities and the structural similarity of RBITC, we further investigated the mechanism of this novel substance, using LPS-induced RAW 264.7 cells as a model. Since acute and chronic inflammation share similar features (57), it may be inferred that the ability of a test compound to mediate responses with stimulated RAW 264.7 cells is of potential relevance for the reduction of chronic inflammation. Treatment with RBITC decreased NO production with greater efficacy than sulforaphane or BITC but, interestingly, the effect was persistent even following a brief incubation period followed by washing of the cells (Figure 2). Additional work is required provide an explanation for this response, but it seems likely RBITC interacts with a cellular target in an irreversible manner, perhaps through a Michael addition.
To further explore the basis of this response, upstream signal transduction molecules were examined. Inducible NOS and COX-2 are governed by the MyD88- and TRIF-dependent pathways. In the MyD88-dependent pathway, signal transduction is dependent on MAPKs and NF-κB. As currently described, RBITC affected the phosphorylation of MAPKs, including ERK1/2 and SAPK/JNK, and inhibited IκBα degradation, accompanied altered NF-κB p65 nuclear translocation and DNA binding with the complementary cis-acting element. One upstream signaling molecule of IκBα, IKKα/β, was examined. IκBα is known to be phosphorylated by the IKK complex, and subsequently ubiquitinated and degraded by the proteasome (58). RBITC inhibited the phosphorylation of IKKα/β, thus providing an indication of one mode of action.
As another possibility, previous studies have demonstrated a connection between ERK1/2 and NF-κB (36–40) as mentioned above. Since ERK1/2 phosphorylation is inhibited by RBITC, we performed additional studies to determine if this inhibitory activity could be responsible for stabilization of IκBα protein levels in this model system. Treatment of RAW 254.7 cells with the MEK-specific inhibitor U0126 reduced NO levels (IC50 = 8.9 µM), presumably by blocking the phosphorylation of ERK1/2 (Figure 6). However, since IκBα degradation was not blocked under these conditions, it appears U0126 functions by virtue of inhibiting the MEK-ERK pathway. On the other hand, RBITC did block the degradation of IκBα, but we presume this was not due to the simultaneous inhibition of ERK1/2 phosphorylation, based on the data obtained with U0126. When U0126 was co-incubated with RBITC, in addition to p-ERK1/2 generation, IκBα degradation was blocked, and greater inhibition of nitrite was observed. Previous reports have indicated that inhibition of ERK1/2 activation or IκBα degradation is related to reduced nitrite production in LPS-stimulated macrophage cells (37,59,60). Our data suggest the inhibitory effect of RBITC on nitrite production is due to multiple mechanisms, including inhibition of ERK1/2 phosphorylation and NF-κB activation.
Other natural products that appear to function in a similar manner include rosmanol, lucidone, tomatidine, and 4-methoxyhonokiol (61–64). Interesting, however, although RBITC inhibited the LPS-induced expression of both iNOS and COX-2, greater efficacy was observed with iNOS. As described above, the expression of iNOS and COX-2 can be induced by the same transcription factors. However, other transcription factors can differentially affect these processes. For instance, it has been reported that COX-2 genes are regulated by NF-κB, NF-IL6/C/EBP, PEA3, NFAT, CRE, AP-2 and SP-1 (65), while iNOS can be regulated by NF-κB, interferon regulatory factor-1, signal transducer and activator of transcription-1α, cAMP-responsive element binding protein, CCAAT-enhancer box binding protein, activating protein-1, octamer factor, T-cell factor 4, transcription factor 11/musculoaponeurotic fibrosarcoma homolog G, epidermal growth factor receptor/signal transducer and activator of transcription-3, forkhead (Drosophila) homolog rhabdomyosarcoma-like 1, nuclear receptors, glucocorticoid receptor (GR)-α, GR-β, estrogen receptor (ER)-α, ER-β, peroxisome proliferator-activated receptors, retinoic acid receptor- α, retinoid X receptor-α, constitutive androstane receptor, pregnane X receptor, Krüppel-like factor 6, and nonhistone high mobility group protein A1 (66). Thus, there are many possible explanations as to how RBITC could function in a differential manner.
Of major importance, however, RBITC is a novel isothiocyanate found in the diet of human beings. In part, it inhibits iNOS and COX-2 expression by blocking the activation of ERK1/2, SAPK/JNK, and NF-κB. Since it is likely that other mechanisms relevant to cancer chemoprevention could also be mediated by this compound, studies are ongoing. Nonetheless, based on existing data, it is reasonable to suggest that RBITC is a promising compound that could play a role in disease prevention.
ACKNOWLEDEMENTS
This work was supported by P01 CA48112 awarded by the National Cancer Institute and P20RR016467 from the National Center for Research Resources.
REFERENCES
- 1.Anwar F, Latif S, Ashraf M, Gilani AH. Moringa oleifera: A food plant with multiple medicinal uses. Phytother Res. 2007;21:17–25. doi: 10.1002/ptr.2023. [DOI] [PubMed] [Google Scholar]
- 2.Debnath S, Biswas D, Ray K, Guha D. Moringa oleifera induced potentiation of serotonin release by 5-HT(3) receptors in experimental ulcer model. Phytomedicine. 2011;18:91–95. doi: 10.1016/j.phymed.2010.06.003. [DOI] [PubMed] [Google Scholar]
- 3.Thurber MD, Fahey JW. Adoption of Moringa oleifera to combat under-nutrition viewed through the lens of the "Diffusion of Innovations" theory. Ecol Food Nutr. 2009;48:212–225. doi: 10.1080/03670240902794598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ndabigengeserea A, Narasiah KS. Quality of water treated by coagulation using Moringa oleifera seeds. Helv Chim Acta. 1998;32:781–791. [Google Scholar]
- 5.Rashid U, Anwar F, Moser BR, Knothe G. Moringa oleifera oil: A possible source of biodiesel. Bioresour Technol. 2008;99:8175–8179. doi: 10.1016/j.biortech.2008.03.066. [DOI] [PubMed] [Google Scholar]
- 6.Guevara AP, Vargas C, Sakurai H, Fujiwara Y, Hashimoto K, et al. An antitumor promoter from Moringa oleifera Lam. Mutat Res. 1999;440:181–188. doi: 10.1016/s1383-5718(99)00025-x. [DOI] [PubMed] [Google Scholar]
- 7.Francis JA, Jayaprakasam B, Olson LK, Nair MG. Insulin secretagogues from Moringa oleifera with cyclooxygenase enzyme and lipid peroxidation inhibitory activities. Helv Chem Acta. 2004;87:317–326. [Google Scholar]
- 8.Cheenpracha S, Park EJ, Yoshida WY, Barit C, Wall M, et al. Potential anti-inflammatory phenolic glycosides from the medicinal plant Moringa oleifera fruits. Bioorg Med Chem. 2010;18:6598–6602. doi: 10.1016/j.bmc.2010.03.057. [DOI] [PubMed] [Google Scholar]
- 9.Cheenpracha S, Park EJ, Rostama B, Pezzuto JM, Chang LC. Inhibition of nitric oxide (NO) production in lipopolysaccharide (LPS)-activated murine macrophage RAW 264.7 cells by the norsesterterpene peroxide, epimuqubilin A. Mar Drugs. 2010;8:429–437. doi: 10.3390/md8030429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Anderson N, Borlak J. Molecular mechanisms and therapeutic targets in steatosis and steatohepatitis. Pharmacol Rev. 2008;60:311–357. doi: 10.1124/pr.108.00001. [DOI] [PubMed] [Google Scholar]
- 11.Kanwar JR, Kanwar RK, Burrow H, Baratchi S. Recent advances on the roles of NO in cancer and chronic inflammatory disorders. Curr Med Chem. 2009;16:2373–2394. doi: 10.2174/092986709788682155. [DOI] [PubMed] [Google Scholar]
- 12.Coussens LM, Werb Z. Inflammation and cancer. Nature. 2002;420:860–867. doi: 10.1038/nature01322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chung CP, Avalos I, Raggi P, Stein CM. Atherosclerosis and inflammation: Insights from rheumatoid arthritis. Clin Rheumatol. 2007;26:1228–1233. doi: 10.1007/s10067-007-0548-7. [DOI] [PubMed] [Google Scholar]
- 14.Wyss-Coray T. Inflammation in Alzheimer disease: Driving force, bystander or beneficial response? Nature Med. 2006;12:1005–1015. doi: 10.1038/nm1484. [DOI] [PubMed] [Google Scholar]
- 15.Schottenfeld D, Beebe-Dimmer J. Chronic inflammation: A common and important factor in the pathogenesis of neoplasia. CA Cancer J Clin. 2006;56:69–83. doi: 10.3322/canjclin.56.2.69. [DOI] [PubMed] [Google Scholar]
- 16.Kopydlowski KM, Salkowski CA, Cody MJ, van Rooijen N, Major J, et al. Regulation of macrophage chemokine expression by lipopolysaccharide in vitro and in vivo. J Immunol. 1999;163:1537–1544. [PubMed] [Google Scholar]
- 17.Bingle L, Brown NJ, Lewis CE. The role of tumour-associated macrophages in tumour progression: Implications for new anticancer therapies. J Pathol. 2002;196:254–265. doi: 10.1002/path.1027. [DOI] [PubMed] [Google Scholar]
- 18.Harris RE. Cyclooxygenase-2 (cox-2) blockade in the chemoprevention of cancers of the colon, breast, prostate, lung. Inflammopharmacology. 2009;17:55–67. doi: 10.1007/s10787-009-8049-8. [DOI] [PubMed] [Google Scholar]
- 19.Xu W, Liu LZ, Loizidou M, Ahmed M, Charles IG. The role of nitric oxide in cancer. Cell Res. 2002;12:311–320. doi: 10.1038/sj.cr.7290133. [DOI] [PubMed] [Google Scholar]
- 20.Pålsson-McDermott EM, O'Neill LA. Signal transduction by the lipopolysaccharide receptor, Toll-like receptor-4. Immunology. 2004;113:153–162. doi: 10.1111/j.1365-2567.2004.01976.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Murakami A, Ohigashi H. Targeting NOX, INOS and COX-2 in inflammatory cells: Chemoprevention using food phytochemicals. Int J Cancer. 2007;121:2357–2363. doi: 10.1002/ijc.23161. [DOI] [PubMed] [Google Scholar]
- 22.Heiss E, Herhaus C, Klimo K, Bartsch H, Gerhäuser C. Nuclear factor κB is a molecular target for sulforaphane-mediated anti-inflammatory mechanisms. J Biol Chem. 2001;276:32008–32015. doi: 10.1074/jbc.M104794200. [DOI] [PubMed] [Google Scholar]
- 23.Chen YH, Dai HJ, Chang HP. Suppression of inducible nitric oxide production by indole and isothiocyanate derivatives from Brassica plants in stimulated macrophages. Planta Med. 2003;69:696–700. doi: 10.1055/s-2003-42790. [DOI] [PubMed] [Google Scholar]
- 24.Rose P, Won YK, Ong CN, Whiteman M. β-Phenylethyl and 8-methylsulphinyloctyl isothiocyanates, constituents of watercress, suppress LPS induced production of nitric oxide and prostaglandin E2 in RAW 264.7 macrophages. Nitric Oxide. 2005;12:237–243. doi: 10.1016/j.niox.2005.03.001. [DOI] [PubMed] [Google Scholar]
- 25.Prawan A, Saw CL, Khor TO, Keum YS, Yu S, et al. Anti-NF-κB and anti-inflammatory activities of synthetic isothiocyanates: Effect of chemical structures and cellular signaling. Chem Biol Interact. 2009;179:202–211. doi: 10.1016/j.cbi.2008.12.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lee YM, Seon MR, Cho HJ, Kim JS, Park JH. Benzyl isothiocyanate exhibits anti-inflammatory effects in murine macrophages and in mouse skin. J Mol Med. 2009;87:1251–1261. doi: 10.1007/s00109-009-0532-6. [DOI] [PubMed] [Google Scholar]
- 27.Ignarro LJ, Fukuto JM, Griscavage JM, Rogers NE, Byrns RE. Oxidation of nitric oxide in aqueous solution to nitrite but not nitrate: comparison with enzymatically formed nitric oxide from L-arginine. Proc Natl Acad Sci U S A. 1993;90:8103–8107. doi: 10.1073/pnas.90.17.8103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Xagorari A, Roussos C, Papapetropoulos A. Inhibition of LPS-stimulated pathways in macrophages by the flavonoid luteolin. Br J Pharmacol. 2002;136:1058–1064. doi: 10.1038/sj.bjp.0704803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Jang SI, Kim HJ, Kim YJ, Jeong SI, You YO. Tanshinone IIA inhibits LPS-induced NF-κB activation in RAW 264.7 cells: possible involvement of the NIK-IKK, ERK1/2, p38 and JNK pathways. Eur J Pharmacol. 2006;542:1–7. doi: 10.1016/j.ejphar.2006.04.044. [DOI] [PubMed] [Google Scholar]
- 30.You M, Wickramaratne DB, Silva GL, Chai H, Chagwedera TE, et al. (−)-Roemerine, an aporphine alkaloid from Annona senegalensis that reverses the multidrug-resistance phenotype with cultured cells. J Nat Prod. 1995;58:598–604. doi: 10.1021/np50118a021. [DOI] [PubMed] [Google Scholar]
- 31.Bradford MM. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976;72:248–254. doi: 10.1006/abio.1976.9999. [DOI] [PubMed] [Google Scholar]
- 32.Chomczynski P, Sacchi N. Single-step method of RNA isolation by acid guanidinium thiocyanate-phenol-chloroform extraction. Anal Biochem. 1987;162:156–159. doi: 10.1006/abio.1987.9999. [DOI] [PubMed] [Google Scholar]
- 33.Zhao L, Tao JY, Zhang SL, Jin F, Pang R, et al. n-Butanol extract from Melilotus suaveolens Ledeb affects pro- and anti-Inflammatory cytokines and mediators. Evid Based Complement Alternat Med. 2010;7:97–106. doi: 10.1093/ecam/nem165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Park HJ, Chung HJ, Min HY, Park EJ, Hong JY, et al. Inhibitory effect of DA-125, a new anthracyclin analog antitumor agent, on the invasion of human fibrosarcoma cells by down-regulating the matrix metalloproteinases. Biochem Pharmacol. 2005;71:21–31. doi: 10.1016/j.bcp.2005.10.007. [DOI] [PubMed] [Google Scholar]
- 35.Luqman S, Pezzuto JM. NFκB, A promising target for natural products in cancer chemoprevention. Phytother Res. 2010;24:949–963. doi: 10.1002/ptr.3171. [DOI] [PubMed] [Google Scholar]
- 36.Chun KS, Keum YS, Han SS, Song YS, Kim SH, et al. Curcumin inhibits phorbol ester-induced expression of cyclooxygenase-2 in mouse skin through suppression of extracellular signal-regulated kinase activity and NF-κB activation. Carcinogenesis. 2003;24:1515–1524. doi: 10.1093/carcin/bgg107. [DOI] [PubMed] [Google Scholar]
- 37.Chakravortty D, Kato Y, Sugiyama T, Koide N, Mu MM, et al. The inhibitory action of sodium arsenite on lipopolysaccharide-induced nitric oxide production in RAW 267.4 macrophage cells a role of Raf-1 in lipopolysaccharide signaling. J Immunol. 2001;166:2011–2017. doi: 10.4049/jimmunol.166.3.2011. [DOI] [PubMed] [Google Scholar]
- 38.Chen BC, Lin WW. PKC- and ERK-dependent activation of IκB kinase by lipopolysaccharide in macrophages: enhancement by P2Y receptor-mediated CaMK activation. Br J Pharmacol. 2001;134:1055–1065. doi: 10.1038/sj.bjp.0704334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Aga M, Watters JJ, Pfeiffer ZA, Wiepz GJ, Sommer JA, et al. Evidence for nucleotide receptor modulation of cross talk between MAP kinase and NF-κB signaling pathways in murine RAW 264.7 macrophages. Am J Physiol Cell Physiol. 2001;286:C923–C930. doi: 10.1152/ajpcell.00417.2003. [DOI] [PubMed] [Google Scholar]
- 40.Jiang B, Xu S, Hou X, Pimentel DR, Brecher P, et al. Temporal control of NF-κB activation by ERK differentially regulates interleukin-1β-induced gene expression. J Biol Chem. 2004;279:1323–1329. doi: 10.1074/jbc.M307521200. [DOI] [PubMed] [Google Scholar]
- 41.Suh SJ, Chung TW, Son MJ, Kim SH, Moon TC, et al. The naturally occurring biflavonoid, ochnaflavone, inhibits LPS-induced iNOS expression, which is mediated by ERK1/2 via NF-κB regulation, in RAW264.7 cells. Arch Biochem Biophys. 2006;447:136–146. doi: 10.1016/j.abb.2006.01.016. [DOI] [PubMed] [Google Scholar]
- 42.Rubio AR, Morales-Segura MA. Nitric oxide, an iceberg in cardiovascular physiology: Far beyond vessel tone control. Arch Med Res. 2004;35:1–11. doi: 10.1016/j.arcmed.2003.09.011. [DOI] [PubMed] [Google Scholar]
- 43.Wallace JL, Miller MJ. Nitric oxide in mucosal defense: A little goes a long way. Gastroenterology. 2004;119:512–520. doi: 10.1053/gast.2000.9304. [DOI] [PubMed] [Google Scholar]
- 44.Contestabile A, Ciani E. Role of nitric oxide in the regulation of neuronal proliferation, survival and differentiation. Neurochem Int. 2004;45:903–914. doi: 10.1016/j.neuint.2004.03.021. [DOI] [PubMed] [Google Scholar]
- 45.Patel RP, McAndrew J, Sellak H, White CR, Jo H, et al. Biological aspects of reactive nitrogen species. Biochim Biophys Acta. 1999;1411:385–400. doi: 10.1016/s0005-2728(99)00028-6. [DOI] [PubMed] [Google Scholar]
- 46.Hersh EV, Lally ET, Moore PA. Update on cyclooxygenase inhibitors, Has a third COX isoform entered the fray? Curr Med Res Opin. 2005;21:1217–1226. doi: 10.1185/030079905X56367. [DOI] [PubMed] [Google Scholar]
- 47.Méric JB, Rottey S, Olaussen K, Soria JC, Khayat D, et al. Cyclooxygenase-2 as a target for anticancer drug development. Crit Rev Oncol Hematol. 2006;59:51–64. doi: 10.1016/j.critrevonc.2006.01.003. [DOI] [PubMed] [Google Scholar]
- 48.Kundu JK, Surh YJ. Inflammation: Gearing the journey to cancer. Mutat Res. 2008;659:15–30. doi: 10.1016/j.mrrev.2008.03.002. [DOI] [PubMed] [Google Scholar]
- 49.Cheung KL, Kong AN. Molecular targets of dietary phenethyl isothiocyanate and sulforaphane for cancer chemoprevention. AAPS J. 2010;12:87–97. doi: 10.1208/s12248-009-9162-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Chung FL, Conaway CC, Rao CV, Reddy BS. Chemoprevention of colonic aberrant crypt foci in Fischer rats by sulforaphane and phenethyl isothiocyanate. Carcinogenesis. 2000;21:2287–2291. doi: 10.1093/carcin/21.12.2287. [DOI] [PubMed] [Google Scholar]
- 51.Hu R, Khor TO, Shen G, Jeong WS, Hebbar V, et al. Cancer chemoprevention of intestinal polyposis in ApcMin/+ mice by sulforaphane, a natural product derived from cruciferous vegetable. Carcinogenesis. 2006;27:2038–3046. doi: 10.1093/carcin/bgl049. [DOI] [PubMed] [Google Scholar]
- 52.Park EJ, Pezzuto JM. Botanicals in cancer chemoprevention. Cancer Metastasis Rev. 2002;21:231–255. doi: 10.1023/a:1021254725842. [DOI] [PubMed] [Google Scholar]
- 53.Warin R, Chambers WH, Potter DM, Singh SV. Prevention of mammary carcinogenesis in MMTV-neu mice by cruciferous vegetable constituent benzyl isothiocyanate. Cancer Res. 2009;69:9473–9480. doi: 10.1158/0008-5472.CAN-09-2960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Hecht SS, Kenney PM, Wang M, Upadhyaya P. Benzyl isothiocyanate: an effective inhibitor of polycyclic aromatic hydrocarbon tumorigenesis in A/J mouse lung. Cancer Lett. 2002;187:87–94. doi: 10.1016/s0304-3835(02)00410-x. [DOI] [PubMed] [Google Scholar]
- 55.von Weymarn LB, Chun JA, Hollenberg PF. Effects of benzyl and phenethyl isothiocyanate on P450s 2A6 and 2A13, potential for chemoprevention in smokers. Carcinogenesis. 2006;27:782–790. doi: 10.1093/carcin/bgi301. [DOI] [PubMed] [Google Scholar]
- 56.Miyoshi N, Takabayashi S, Osawa T, Nakamura Y. Benzyl isothiocyanate inhibits excessive superoxide generation in inflammatory leukocytes: Implication for prevention against inflammation-related carcinogenesis. Carcinogenesis. 2004;25:567–575. doi: 10.1093/carcin/bgh051. [DOI] [PubMed] [Google Scholar]
- 57.Feghali CA, Wright TM. Cytokines in acute and chronic inflammation. Front Biosci. 1997;2:d12–d26. doi: 10.2741/a171. [DOI] [PubMed] [Google Scholar]
- 58.Greten FR, Karin M. The IKK/NF-κB activation pathway-a target for prevention and treatment of cancer. Cancer Lett. 2004;206:193–199. doi: 10.1016/j.canlet.2003.08.029. [DOI] [PubMed] [Google Scholar]
- 59.Kim SH, Kim J, Sharma RP. Inhibition of p38 and ERK MAP kinases blocks endotoxin-induced nitric oxide production and differentially modulates cytokine expression. Pharmacol Res. 2004;49:433–439. doi: 10.1016/j.phrs.2003.11.004. [DOI] [PubMed] [Google Scholar]
- 60.Choi MS, Lee SH, Cho HS, Kim Y, Yun YP, et al. Inhibitory effect of obovatol on nitric oxide production and activation of NF-κB/MAP kinases in lipopolysaccharide-treated RAW 264.7cells. Eur J Pharmacol. 2007;556:181–189. doi: 10.1016/j.ejphar.2006.10.054. [DOI] [PubMed] [Google Scholar]
- 61.Lai CS, Lee JH, Ho CT, Liu CB, Wang JM, et al. Rosmanol potently inhibits lipopolysaccharide-induced iNOS and COX-2 expression through downregulating MAPK, NF-κB, STAT3 and C/EBP signaling pathways. J Agric Food Chem. 2009;57:10990–10998. doi: 10.1021/jf9025713. [DOI] [PubMed] [Google Scholar]
- 62.Senthil Kumar KJ, Wang SY. Lucidone inhibits iNOS and COX-2 expression in LPS-induced RAW 264.7 murine macrophage cells via NF-κB and MAPKs signaling pathways. Planta Med. 2009;75:494–500. doi: 10.1055/s-0029-1185309. [DOI] [PubMed] [Google Scholar]
- 63.Chiu FL, Lin JK. Tomatidine inhibits iNOS and COX-2 through suppression of NF-κB and JNK pathways in LPS-stimulated mouse macrophages. FEBS Lett. 2008;582:2407–2412. doi: 10.1016/j.febslet.2008.05.049. [DOI] [PubMed] [Google Scholar]
- 64.Zhou HY, Shin EM, Guo LY, Youn UJ, Bae K, et al. Anti-inflammatory activity of 4-methoxyhonokiol is a function of the inhibition of iNOS and COX-2 expression in RAW 264.7 macrophages via NF-κB, JNK and p38 MAPK inactivation. Eur J Pharmacol. 2008;586:340–349. doi: 10.1016/j.ejphar.2008.02.044. [DOI] [PubMed] [Google Scholar]
- 65.Chun KS, Surh YJ. Signal transduction pathways regulating cyclooxygenase-2 expression, potential molecular targets for chemoprevention. Biochem Pharmacol. 2004;68:1089–1100. doi: 10.1016/j.bcp.2004.05.031. [DOI] [PubMed] [Google Scholar]
- 66.Pautz A, Art J, Hahn S, Nowag S, Voss C, et al. Regulation of the expression of inducible nitric oxide synthase. Nitric Oxide. 2010;23:75–93. doi: 10.1016/j.niox.2010.04.007. [DOI] [PubMed] [Google Scholar]