Abstract
We evaluated data from gastroenteritis outbreaks in Oregon to assess sensitivity of stool testing for norovirus and determine number of specimens needed to confirm norovirus as the cause. Norovirus can be readily confirmed if 3–6 specimens are collected any time <7 days after onset of diarrhea and for almost that long after symptoms resolve.
Keywords: Norovirus, outbreaks, stool specimens, diarrhea, viruses, dispatch
One goal of any outbreak investigation is to identify the causative pathogen (1). In a recent analysis of foodborne disease outbreaks in the United States during 2006, only 49% had a confirmed causative pathogen (2). A review of foodborne disease outbreaks investigated in the 10-site Foodborne Disease Active Surveillance Network during 1998–1999 found that an etiologic agent was identified for only 29% (3). The major limitation in identifying etiologic agents was a lack of specimens; no stool specimens were collected in two thirds of the unconfirmed outbreaks.
In Oregon, outbreaks of illness are reportable to public health authorities, who investigate to determine the causative pathogen and means of transmission and to implement control measures accordingly. Obtaining stool specimens within 3 days of onset has been recommended (4), but carrying out this recommendation is frequently not feasible.
Since 1999, specimens from case-patients in outbreaks of acute gastroenteritis have been tested for norovirus at the Oregon State Public Health Laboratory. Noroviruses are a group of related, nonenveloped, single-stranded RNA viruses that cause acute gastroenteritis in humans. We reviewed Oregon data to assess the sensitivity of stool testing for norovirus at different times after illness onset and to determine the number of specimens needed to ensure a high probability of confirming a norovirus outbreak.
The Study
We reviewed all outbreaks of acute gastroenteritis reported in Oregon from August 23, 1999, through January 31, 2007, for which norovirus was the suspected cause on the basis of incubation period, symptoms, and duration of illness. As part of the outbreak investigations, demographic, clinical and exposure information was gathered, and specimens were solicited with the goal (often unmet) of obtaining at least 3 specimens per cluster. Public health investigators were exhorted to collect specimens “as soon as possible” after a cluster was identified. From 1999 until November 2005, specimens were tested by conventional reverse transcription PCR (RT-PCR) by using consensus primers for norovirus (5). From November 2005 onward, specimens were tested by real-time RT-PCR by using primers to a highly conserved region in the open reading frame 1–2 junction (6,7). Laboratory results were linked to epidemiologic and clinical information ascertained during the outbreak investigation. We defined a confirmed norovirus outbreak as a cluster of compatible illnesses for which norovirus sequences were identified from specimens of at least 2 case-patients (8).
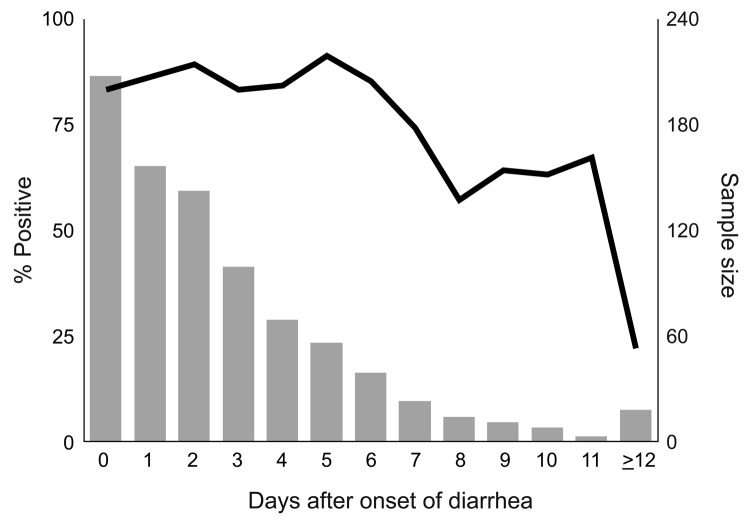
From August 23, 1999, through January 31, 2007, a total of 486 (59%) of 824 reported outbreaks of acute gastroenteritis in Oregon were suspected to be caused by norovirus. Norovirus was confirmed as the cause of 355 outbreaks; of these reports, 275 (77%) provided analyzable data. The outbreaks were associated with the following settings: 151 (55%) with nursing homes, long-term-care facilities, or assisted-living centers; 41 (15%) with restaurants or delicatessens; 10 (4%) with schools; 11 (4%) with hospitals; 8 (3%) with private homes; 6 (2%) with camps; 4 (1%) with correction facilities; and 44 (16%) with other settings. From these 275 outbreaks, 1,117 specimens were submitted to the laboratory with a median of 4 (range 2–13) per outbreak; 888 (79%) tested positive for norovirus. Of the 1,117 specimens, dates of diarrhea onset and stool collection were available for 845 (76%). Of those, 698 (83%) tested positive for norovirus. Figure 1 depicts the percentage of specimens that tested positive, by time since diarrhea onset.
Figure 1.
Percentage of specimens positive for norovirus (line), by days after onset of diarrhea, Oregon, USA, August 1999–January 2007.
Of the 1,117 specimens sent to the laboratory, dates on diarrhea resolution and stool collection were available for 360 (32%) in 153 outbreaks. Of those, 302 (84%) tested positive for norovirus. No association was found between PCR positivity and patient sex (relative risk for male patients 1.06; p = 0.07) or age.
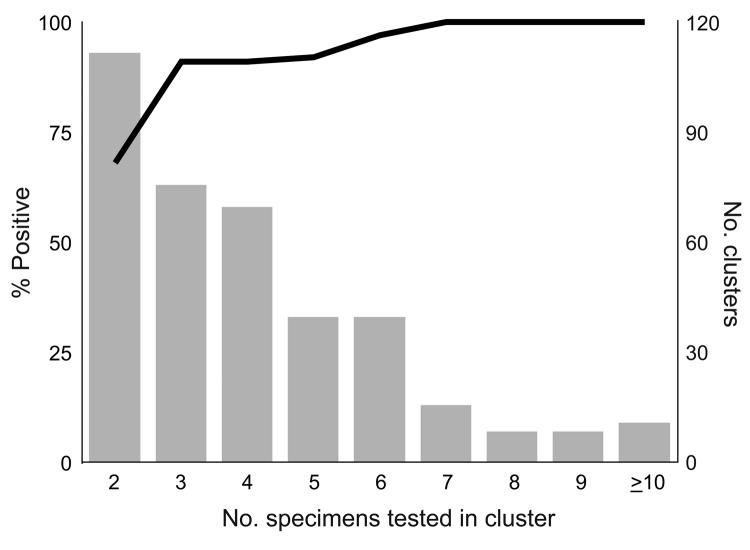
To evaluate the number of stool specimens needed to confirm norovirus as the etiologic agent of an outbreak (suspected to have been caused by norovirus), we analyzed 377 such outbreaks from the same period. A total of 1,532 specimens from these outbreaks were tested, and norovirus was identified in 1,134 (74%). Figure 2 shows likelihood of an outbreak being confirmed as caused by norovirus as a function of the number of specimens tested.
Figure 2.
Percentage of outbreaks confirmed as norovirus (line), by number of specimens tested, Oregon, USA, August 1999–January 2007.
Conclusions
Our data indicate that 3 specimens were sufficient to confirm 91% of norovirus outbreaks, and when 6 specimens were submitted, 97% were confirmed. Testing >7 specimens added nothing to the sensitivity. Therefore, we recommend that to confirm that norovirus is the etiologic agent of an outbreak, at least 3, but not more than 6, specimens should be collected. Norovirus can be readily confirmed as the cause of an outbreak of acute gastroenteritis if specimens are collected at any time during the first 7 days after onset of diarrhea and (though period is somewhat more variable) for almost that long after symptoms resolve. No apparent decline in sensitivity occurs for specimens collected up to 6 days after onset of diarrhea. Sensitivity drops during days 7–14, but remains substantial for up to 2 weeks. The common excuse that “I’ve already gotten better” can be safely ignored. Several studies have examined the duration of norovirus excretion and found that the average period of shedding is ≈28 days (range 13–56 days), well past the resolution of symptoms (9,10). Infectivity cannot be inferred from these findings, however.
These data have several limitations. First, at nursing homes, the responsibility for data collection was often delegated to staff, and no attempt was made to validate the data they submitted. Second, data were incomplete for many cases. Third, few specimens were collected >14 days after diarrhea onset or >7 days after diarrhea cessation, yielding wider confidence intervals around late-sample point estimates. Lastly, a real-time RT-PCR is now being used by the Oregon State Public Health Laboratory (and many others); this assay is up to 10,000× more sensitive than the conventional RT-PCR (11), and the number of specimens needed to confirm norovirus outbreaks may decrease as the acceptable time range for collection increases. We noted a trend toward higher positivity rates with the real-time RT-PCR, so fewer specimens might be required; norovirus was confirmed in 100% of outbreaks with >5 specimens tested by this method. Despite these limitations, we believe that this information will be helpful to outbreak investigators and to researchers studying sporadic cases of acute gastroenteritis. Taking into account these findings, in turn, will lead to a more accurate picture of the incidence of norovirus infections.
Acknowledgments
We thank the many local and state public health officials who investigated these outbreaks, as well as the participating staff at the many private institutions involved.
Biography
Mrs Plantenga was a research analyst at the Department of Human Services in Portland, Oregon, when this research was conducted. Her interests include public health surveillance and outbreak investigations.
Suggested citation for this article: Plantenga MS, Shiferaw B, Keene WE, Biggs C, Terry JM, Grenz L. Speciman collection and confirmation of norovirus outbreaks. Emerg Infect Dis [serial on the Internet]. 2011 Aug [date cited]. http://dx.doi.org/10.3201/eid1708.101815
Preliminary report presented at the 2004 International Conference on Emerging Infectious Diseases, February 29–March 3, 2004, Atlanta, Georgia, USA (abstract no. 244).
References
- 1.Centers for Disease Control and Prevention. Principles of epidemiology (self-study course SS3030), 2nd ed. Washington: American Public Health Association; 1992:353–7. [Google Scholar]
- 2.Centers for Disease Control and Prevention. Surveillance for foodborne disease outbreaks—United Sates, 2006. MMWR Morb Mortal Wkly Rep. 2009;58:609–15. [PubMed] [Google Scholar]
- 3.Jones TF, Imhoff B, Samuel M, Mshar P, McCombs KG, Hawkins M, et al. Limitations to successful investigations and reporting of foodborne outbreaks: an analysis of foodborne disease outbreaks in FoodNet catchment areas, 1998–1999. Clin Infect Dis. 2004;38(Suppl 3):S297–302. 10.1086/381599 [DOI] [PubMed] [Google Scholar]
- 4.Centers for Disease Control and Prevention. “Norwalk-like viruses”: public health consequences and outbreak management. MMWR Morb Mortal Wkly Rep. 2001;50(RR-9):11. [PubMed] [Google Scholar]
- 5.Ando T, Monroe SS, Gentsch JR, Jin Q, Lewis DC, Glass RI. Detection and differentiation of antigenically distinct small round-structured viruses (Norwalk-like viruses) by reverse transcription-PCR and Southern hybridization. J Clin Microbiol. 1995;33:64–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kageyama T, Kojima S, Shinohara M, Uchida K, Fukushi S, Hoshino FB, et al. Broadly reactive and highly sensitive assay for Norwalk-like viruses based on real-time quantitative reverse transcription-PCR. J Clin Microbiol. 2003;41:1548–57. 10.1128/JCM.41.4.1548-1557.2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Trujillo AA, McCaustland KA, Zheng DP, Hadley LA, Vaughn G, Adams SM, et al. Use of TaqMan real-time reverse transcription-PCR for rapid detection, quantification, and typing of norovirus. J Clin Microbiol. 2006;44:1405–12. 10.1128/JCM.44.4.1405-1412.2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Centers for Disease Control and Prevention. Outbreak Response Team overview [cited 2009 Dec 10]. http://www.cdc.gov/foodborneoutbreaks/overview_cont.htm
- 9.Tu ET, Bull RA, Kim M, McIver CJ, Heron L, Rawlinson WD, et al. Norovirus excretion in an aged-care setting. J Clin Microbiol. 2008;46:2119–21. 10.1128/JCM.02198-07 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Atmar RL, Opekun AR, Gilger MA, Estes MK, Crawford SE, Neill FH, et al. Norwalk virus shedding after experimental human infection. Emerg Infect Dis. 2008;14:1553–7. 10.3201/eid1410.080117 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Pang X, Lee B, Chui L, Preiksaitis JK, Monroe SS. Evaluation and validation of real-time reverse transcription-PCR assay using the LightCycler system for detection and quantitation of norovirus. J Clin Microbiol. 2004;42:4679–85. 10.1128/JCM.42.10.4679-4685.2004 [DOI] [PMC free article] [PubMed] [Google Scholar]