Various treatment options are available for hepatocellular carcinoma (HCC); however, the overwhelming majority of them fail to extend patient survival, especially in cases of advanced disease. Although a number of different genetic lesions have been linked to HCC, the expression of oncogenic microRNAs (miRNAs), such as miR-221 and miR-222, are consistently found elevated in HCC tumor specimens,1,2 suggesting that silencing of these miRNAs might slow or stop tumor growth. In a recent study, Park et al. show that systemic administration of a chemically modified oligonucleotide that binds to and sequesters miR-221 is efficacious in HCC.3 Their approach identified a preferred chemical modification on the oligonucleotide that allowed its accumulation in hepatocytes, which led to therapeutic silencing of miR-221, reduced cellular proliferation, and most importantly increased survival of an orthotopic mouse model of HCC. Although this approach has yet to be tried in human patients, it appears likely that treatments involving miRNA overexpression or silencing, such as these, will translate efficiently.
HCC is the fifth most prevalent cancer worldwide and the third most common cause of cancer-related deaths.4 Molecular markers of the disease include genetic instability, reexpression of telomerase reverse transcriptase, loss of cell-cycle checkpoint control, and inhibition of apoptotic signaling pathways, such as those mediated by p53. In addition, loss of heterozygosity of the insulin-like growth factor 2 receptor is common. Perhaps somewhat unexpectedly, post-translational activation of oncogenic proteins is heterogeneous in HCC, and therefore not very useful as a diagnostic or a general treatment target. In contrast, miR-221 and miR-222, which target cyclin-dependent kinase inhibitors, such as p27Kip1 and p57Kip2, drive sustained cell-cycle progression in HCC.5,6 They also suppress the tumor suppressor PTEN and other inhibitors of the AKT signaling pathway, thereby contributing to enhanced HCC cell migration.7
miRNAs are a class of small, untranslated, RNA polymerase-II transcribed genes that post-transcriptionally regulate the translation of messenger RNAs (mRNAs).8 The ability of a miRNA to regulate a gene stems from a 6-nucleotide seed region in the miRNA that typically interacts with the 3′ untranslated region of a mRNA, so as to prevent translation or induce degradation of the mRNA. Due to their small size, which contributes to their pleiotropic role in targeting a large number of critical protein-coding genes, misregulation of a single miRNA can give rise to multiple adverse cellular effects. Aberrant miRNA levels have been implicated in promoting and maintaining various disease states including cancer. In some cases, overexpression of single miRNA is sufficient to drive tumorigenesis, although its silencing leads to tumor regression9. Based on these findings, and on recent human tumor miRNA profiling studies that have confirmed altered levels of some key miRNAs, it is becoming increasingly attractive to target these master gene-regulators in vivo with cancer therapeutics.
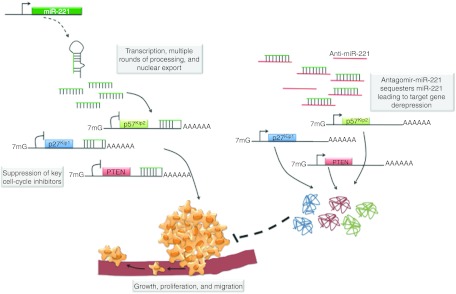
One mechanism to silence oncogenic miRNAs (oncomiRs), such as miR-221, takes advantage of their affinity for complementary sequences. Introducing a small molecule that has a higher affinity for the miRNA than that target mRNA or one that is in greater abundance than the target gene, can lead to miRNA sequestration (Figure 1). Although simple in practice, a great deal of research design and development has gone into creating these chemically modified miRNA complementary molecules, termed antagomirs10. Of the many challenges in antagomir design, off-target induced toxicity, tissue distribution, and pharmacokinetics are some of the most important. Each of these points was addressed by Park and colleagues,3 who synthesized a total of ten antagomirs with various chemical modifications and tested their efficacy at inhibiting cellular proliferation in culture and ultimately their capacity to localize to the liver and decrease HCC in vivo. One of the most promising antagomir chemistries identified in the cell culture work was 2′-O-methyl, phosphorothioate. The phosphorothioate substitutes sulfur for one of the nonbridging oxygens in the nucleotide. This modification decreases the likelihood of endo- and exonuclease degradation and also aids the oligonucleotide in crossing the lipid bilayer. A 2′-O-methyl addition to each of the individual nucleotides is also a nuclease protective modification. Although the chemistry of the aforementioned antagomir had the strongest effect in culture, a cholesterol-tagged version showed better pharmacokinetics in vivo, giving rise to a 50-fold enrichment in maximum liver concentration over the untagged oligonucleotide. The cholesterol-tagged anti-miR-221 reduced miR-221 levels by 80–90% leading to elevations in miR-221 targets. Following systemic delivery of the cholesterol-tagged anti-miR-221 to orthotopic tumors, the G1 cell-cycle checkpoint inhibitors p27Kip1 and p57Kip2 were elevated approximately threefold. Increased p27Kip1 and p57Kip2 most likely contributed to loss of the cellular proliferation marker, Ki-67, and to reductions in tumor-volume doubling times. PTEN levels were also increased in cholesterol-tagged anti-miR-221 treated tumors. PTEN is a negative regulator of AKT signaling and therefore its elevation is also most likely central to the decreased growth of the treated tumors. Finally, the lack of hepatocyte toxicity in these mice was also confirmed following treatment.
Figure 1.
Therapeutic use of antagomir-miR-221. When overexpressed in cancers such as HCC, miR-221 targets and suppresses the expression of tumor-suppressive target proteins, such as p27Kip1, p57Kip2, and PTEN. Decreased levels of these cell-cycle regulators promote continued cell division, a hallmark of cancer cells. Introducing an oligonucleotide with complementarity to miR-221 sequesters the latter and leads to derepression of the target genes. This restoration of p27Kip1, p57Kip2, and PTEN protein resumes normal cell-cycle control, and contributes to reduced cellular proliferation and perhaps tumor regression. HCC, hepatocellular carcinoma.
These results are quite promising; although the tumors still progressed, these mice were only treated for 2 weeks with little to no increase in the tumor volume during the treatment period. It is encouraging to envisage that altered dosing schedules might improve the overall outcome in these animals. Because toxicity was not an issue with the current dose and treatment schedule, it is definitely within reason to propose additional dosing, increased dosing, or even more attractively, combinatorial approaches to therapy. For example, sorafenib is one of the only approved targeted therapies for HCC that is associated with increased overall survival, although the increase is only about 2–3 months11. Therefore, combining cholesterol-tagged anti-miR-221 with current chemotherapies such as sorafenib warrants exploration.
Certainly since the biology of miRNAs has unfolded, the therapeutic potential of these molecules has exploded8. The field is in on an upward trajectory to identify and confirm the best candidate miRNAs to silence, or in the case of tumor-suppressive miRNAs, to reintroduce. In the case of HCC, miR-221 represents a valid target and cholesterol-tagged antagomirs undoubtedly accumulate in the liver representing a major step towards targeted delivery. The challenge of the field is to continue to design and test the targeting ability of different chemical moieties on antagomirs and miRNAs such that they can be delivered adequately to the tissue/tumor of interest. As better delivery chemistries are developed and the biology of miRNAs continues to be unraveled, the potential for transitioning these antagomirs into a clinical setting for many cancers becomes a very achievable possibility.
References
- Braconi C, Henry JC, Kogure T, Schmittgen T., and, Patel T. The role of microRNAs in human liver cancers. Semin Oncol. 2011;38:752–763. doi: 10.1053/j.seminoncol.2011.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pineau P, Volinia S, McJunkin K, Marchio A, Battiston C, Terris B.et al. (2010miR-221 overexpression contributes to liver tumorigenesis Proc Natl Acad Sci USA 107264–269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park JK, Kogure T, Nuovo GJ, Jiang J, He L, Kim JH.et al. (2011miR-221 silencing blocks hepatocellular carcinoma and promotes survival Cancer Res 717608–7616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- El-Serag HB. Hepatocellular carcinoma. N Engl J Med. 2011;365:1118–1127. doi: 10.1056/NEJMra1001683. [DOI] [PubMed] [Google Scholar]
- le Sage C, Nagel R, Egan DA, Schrier M, Mesman E, Mangiola A.et al. (2007Regulation of the p27(Kip1) tumor suppressor by miR-221 and miR-222 promotes cancer cell proliferation EMBO J 263699–3708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fornari F, Gramantieri L, Ferracin M, Veronese A, Sabbioni S, Calin GA.et al. (2008MiR-221 controls CDKN1C/p57 and CDKN1B/p27 expression in human hepatocellular carcinoma Oncogene 275651–5661. [DOI] [PubMed] [Google Scholar]
- Garofalo M, Di Leva G, Romano G, Nuovo G, Suh SS, Ngankeu A.et al. (2009miR-221&222 regulate TRAIL resistance and enhance tumorigenicity through PTEN and TIMP3 downregulation Cancer Cell 16498–509. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Kasinski AL., and, Slack FJ. Epigenetics and genetics. MicroRNAs en route to the clinic: progress in validating and targeting microRNAs for cancer therapy. Nat Rev Cancer. 2011;11:849–864. doi: 10.1038/nrc3166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Medina PP, Nolde M., and, Slack FJ. OncomiR addiction in an in vivo model of microRNA-21-induced pre-B-cell lymphoma. Nature. 2010;467:86–90. doi: 10.1038/nature09284. [DOI] [PubMed] [Google Scholar]
- Krützfeldt J, Rajewsky N, Braich R, Rajeev KG, Tuschl T, Manoharan M.et al. (2005Silencing of microRNAs in vivo with ‘antagomirs' Nature 438685–689. [DOI] [PubMed] [Google Scholar]
- Llovet JM, Ricci S, Mazzaferro V, Hilgard P, Gane E, Blanc JF.et al. (2008Sorafenib in advanced hepatocellular carcinoma N Engl J Med 359378–390. [DOI] [PubMed] [Google Scholar]