There are currently two major competing pharmaceutical strategies to inhibit the expression of specific genes in cells, animals, and also recently in humans. One is small interfering RNA (siRNA)-mediated cleavage of mRNA by the RNA interference mechanism, and the other is antisense oligonucleotide (ASO)-triggered mRNA degradation and miRNA silencing.1,2,3,4 Both systems represent promising new targets for drug candidates for human diseases due to their broad applicability, high efficiency, and specificity. However, both targeted delivery of macromolecular drugs such as siRNA or ASO to the site of disease and subsequent intracellular targeting need to be improved before the clinical potential of these molecules can be realized. In the December 2011 issue of Molecular Therapy, Moschos et al. investigate the potential of delivering three different Cy5-labeled oligonucleotides, locked nucleic acid (LNA)-modified ASO (LNA-ASO) with either a phosphodiester (PO-LNA), or phosphorothioate (PS) backbone (PS-LNA) and 2′-O-methyl modified siRNA, to the lungs of mice.5 Unfortunately, none of these reagents led to knockdown of the target mRNA or protein in any of the cell types investigated. However, the authors observed that the PS-LNA accumulated in the liver and kidney at a concentration that, at least in case of the liver, was therapeutically relevant, suggesting noninvasive pulmonary delivery as a possible route for administration of oligonucleotide therapeutics to the liver and kidney.
Human diseases are often associated with deregulation of gene expression, either as a primary cause or as an outcome of other events. Disease symptoms can, in many instances, be alleviated by inhibiting gene expression through the delivery of oligonucleotides. Various methods, including include nanocarrier delivery,6 chemical modification,7 and conjugation8 have been used to facilitate the delivery of oligonucleotides. Traditionally, systemic delivery of oligonucleotide drugs has been performed by intravenous injection of either naked molecules or within delivery systems, but nonspecific accumulation in various tissues has favored local delivery routes such as to the lungs so as to ensure direct access to the target site.
Delivery to the lung will be key to moving RNA interference and antisense technology into the clinic. The lungs represent the site of entry and intracellular establishment of many airborne pathogens including viruses (e.g., influenza, SARS, RSV, and common cold) and bacteria (e.g., Streptococcus pneumonia, Mycoplasma pneumonia, Neisseria meningitides, and Mycobacterium tuberculosis) and is a frequent site of tumor development, genetic diseases (e.g., cystic fibrosis), and immunological disorders (e.g., bronchitis, asthma, lung fibrosis, and chronic obstructive pulmonary disease). However, the translocation of oligonucleotide-based drugs across the pulmonary mucosal epithelia is hampered by several biological barriers. These include the physical barriers of the overlying mucus layer and the tight packing of epithelial cells combined with the sweeping movement of apical cilia that removes luminal material away from the mucosal surface.9 In addition, both naked and formulated siRNA and ASO are vulnerable to recognition and subsequent destruction by alveolar macrophages that are part of the mononuclear phagocyte system. After reaching the target cells, the oligonucleotides must transfer across the cell membrane, escape hydrolytic destruction in the endosomes, and interact with the mRNA target before translation.
In the new study, Moschos et al. administered naked oligonucleotides by the intratracheal route to luciferase-expressing mice and studied cell-type uptake and activity of the oligonucleotides using a tissue disruption and cell sorting method involving fractionation of pulmonary cell types.5 Disappointingly, the initial result showed no knockdown effect in any of the lung cell types with any of the tested oligonucleotides. However, investigation of the biodistribution of the dye-labeled material and mass spectrometric analysis provided a more detailed picture of oligonucleotides deposition in lung tissue and subsequent systemic transfer into other tissues. The PO-LNA and siRNA levels decreased over 24 hours (with the siRNA virtually absent after 1 hour) with rapid renal clearance evident after only 15 minutes. In contrast, the thiol-containing PS-LNA was retained as discreet, particulate structures within macrophages and to a lesser extent in alveolar and bronchial epithelia. After 24 hours, accumulation of PS-LNA was also observed in submucosal membranes and endothelial cells, but surprisingly no knockdown of the target mRNA or protein could be detected in any of the cell types. Despite this negative finding, the study yielded another surprising and potentially useful result for the PS-LNA construct. The PS-LNA accumulated in the liver and kidney at concentrations that, at least in case of the liver, was therapeutically relevant, demonstrated by an efficient knockdown of the liver specific apolipoprotein BmRNA. Notably, the potency was comparable to the result obtained when the same amount of PS-LNA was injected via the intravenous route. This is a highly interesting observation that promotes noninvasive pulmonary delivery as a realistic route for administration of oligonucleotide therapeutics to the liver and kidney.
The lack of functional activity of naked ASO and siRNA in the lung contradicts many other reports conducted in animals10,11,12 and humans.13 The discrepancies may be due to differences in the experimental set up, but another explanation, to which Moschos et al.5 allude, is the problem of nonspecific cellular effects induced mainly by Toll-like receptor type 3 and 7 signaling by double- and single-stranded oligonucleotides, respectively.14,15,16 Different oligonucleotide sequences, structures, and patterns of chemical modifications in the various constructs applied could account for the different results observed between the different studies.
The results presented in this article and others17 also highlight the importance of combining LNA-ASO or siRNA with delivery reagents to increase the efficiency of gene knockdown in pulmonary tissue. Successful knockdown experiments in lung tissue has been reported using lipids18 and polyplexes,19 which may be attributed to higher stability, increased residence time in the lung, improved cell membrane penetration, and escape from the endosomal compartment. There is a necessity, however, to use materials that limit adverse immunological response and possible cellular damage. Optimization of the delivery system and oligonucleotide design are important considerations that need to be addressed in order to enable the clinical application of pulmonary nucleic acid–based therapeutics.
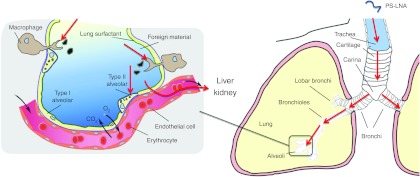
Figure 1.
Intratracheal delivery and macrophage capture of PS-LNA in the mouse lung based on the results obtained by Moschos et al.5 PS-LNA accumulates within macrophages and alveolar cells which further facilitated systemic migration to the kidney and liver. LNA, locked nucleic acid; PS, phosphorothioate.
References
- de Fougerolles A, Vornlocher HP, Maraganore J., and, Lieberman J. Interfering with disease: a progress report on siRNA-based therapeutics. Nat Rev Drug Discov. 2007;6:443–453. doi: 10.1038/nrd2310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davidson BL., and, McCray PB., Jr Current prospects for RNA interference-based therapies. Nat Rev Genet. 2011;12:329–340. doi: 10.1038/nrg2968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lennox KA., and, Behlke MA. Chemical modification and design of anti-miRNA oligonucleotides. Gene Ther. 2011;18:1111–1120. doi: 10.1038/gt.2011.100. [DOI] [PubMed] [Google Scholar]
- Goodchild J. Therapeutic oligonucleotides. Methods Mol Biol. 2011;764:1–15. doi: 10.1007/978-1-61779-188-8_1. [DOI] [PubMed] [Google Scholar]
- Moschos SA, Frick M, Taylor B, Turnpenny P, Graves H, Spink KG.et al. (2011Uptake, efficacy, and systemic distribution of naked, inhaled short interfering RNA (siRNA) and locked nucleic acid (LNA) antisense Mol Ther 192163–2168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howard KA., and, Kjems J. Polycation-based nanoparticle delivery for improved RNA interference therapeutics. Expert Opin Biol Ther. 2007;7:1811–1822. doi: 10.1517/14712598.7.12.1811. [DOI] [PubMed] [Google Scholar]
- Bramsen JB., and, Kjems J. Chemical modification of small interfering RNA. Methods Mol Biol. 2011;721:77–103. doi: 10.1007/978-1-61779-037-9_5. [DOI] [PubMed] [Google Scholar]
- Soutschek J, Akinc A, Bramlage B, Charisse K, Constien R, Donoghue M.et al. (2004Therapeutic silencing of an endogenous gene by systemic administration of modified siRNAs Nature 432173–178. [DOI] [PubMed] [Google Scholar]
- Merkel OM., and, Kissel T.2011Nonviral pulmonary delivery of siRNA Acc Chem Resepub ahead of print). [DOI] [PubMed]
- Bitko V, Musiyenko A, Shulyayeva O., and, Barik S. Inhibition of respiratory viruses by nasally administered siRNA. Nat Med. 2005;11:50–55. doi: 10.1038/nm1164. [DOI] [PubMed] [Google Scholar]
- Alvarez R, Elbashir S, Borland T, Toudjarska I, Hadwiger P, John M.et al. (2009RNA interference-mediated silencing of the respiratory syncytial virus nucleocapsid defines a potent antiviral strategy Antimicrob Agents Chemother 533952–3962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ripple MJ, You D, Honnegowda S, Giaimo JD, Sewell AB, Becnel DM.et al. (2010Immunomodulation with IL-4R alpha antisense oligonucleotide prevents respiratory syncytial virus-mediated pulmonary disease J Immunol 1854804–4811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeVincenzo J, Lambkin-Williams R, Wilkinson T, Cehelsky J, Nochur S, Walsh E.et al. (2010A randomized, double-blind, placebo-controlled study of an RNAi-based therapy directed against respiratory syncytial virus Proc Natl Acad Sci USA 1078800–8805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kleinman ME, Yamada K, Takeda A, Chandrasekaran V, Nozaki M, Baffi JZ.et al. (2008Sequence- and target-independent angiogenesis suppression by siRNA via TLR3 Nature 452591–597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robbins M, Judge A, Ambegia E, Choi C, Yaworski E, Palmer L.et al. (2008Misinterpreting the therapeutic effects of small interfering RNA caused by immune stimulation Hum Gene Ther 19991–999. [DOI] [PubMed] [Google Scholar]
- Hornung V, Guenthner-Biller M, Bourquin C, Ablasser A, Schlee M, Uematsu S.et al. (2005Sequence-specific potent induction of IFN-alpha by short interfering RNA in plasmacytoid dendritic cells through TLR7 Nat Med 11263–270. [DOI] [PubMed] [Google Scholar]
- Glud SZ, Bramsen JB, Dagnaes-Hansen F, Wengel J, Howard KA, Nyengaard JR.et al. (2009Naked siLNA-mediated gene silencing of lung bronchoepithelium EGFP expression after intravenous administration Oligonucleotides 19163–168. [DOI] [PubMed] [Google Scholar]
- Tompkins SM, Lo CY, Tumpey TM., and, Epstein SL. Protection against lethal influenza virus challenge by RNA interference in vivo. Proc Natl Acad Sci USA. 2004;101:8682–8686. doi: 10.1073/pnas.0402630101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howard KA. Delivery of RNA interference therapeutics using polycation-based nanoparticles. Adv Drug Deliv Rev. 2009;61:710–720. doi: 10.1016/j.addr.2009.04.001. [DOI] [PubMed] [Google Scholar]