Abstract
Objectives
We examined whether MDCT improves the ability to define peri-infarct zone (PIZ) heterogeneity relative to MRI.
Background
The PIZ as characterized by delayed enhanced (de) MRI identifies patients susceptible to ventricular arrhythmias and predicts outcome after myocardial infarction (MI).
Methods
Fifteen mini-pigs underwent coronary artery occlusion followed by reperfusion. MDCT and MRI were performed on the same day approximately 6 months after MI induction followed by animal sacrifice and ex-vivo MRI (n=5). Signal density threshold algorithms were applied to MRI and MDCT data sets reconstructed at various slice thicknesses (1–8mm) to define the PIZ and quantify partial volume effects.
Results
De-MDCT reconstructed at 8mm slice thickness demonstrated excellent correlation of infarct size with post mortem pathology (r2=0.97; p<0.0001) and MRI (r2=0.92; p<0.0001). De-MDCT and de-MRI were able to detect a PIZ in all animals, which correlates to a mixture of viable and non-viable myocytes at the PIZ by histology. The ex-vivo de-MRI PIZ volume decreased with slice thickness from 0.9±0.2cc at 8mm to 0.2±0.1cc at 1mm (p=0.01). PIZ volume/mass by de-MDCT increased with decreasing slice thickness due to declining partial volume averaging in the PIZ, but was susceptible to increased image noise.
Conclusion
De-MDCT provides a more detailed assessment of the PIZ in chronic MI and is less susceptible to partial volume effects than MRI. This increased resolution best reflects the extent of tissue mixture by histopathology and has the potential to further enhance the ability to define the substrate of malignant arrhythmia in ischemic heart disease non-invasively.
Keywords: MDCT, delayed enhancement, peri-infarct zone, MRI
Introduction
The success of myocardial protection after myocardial infarction (MI) has led to an ever-increasing number of patients with advanced heart disease and chronic heart failure (1) where questions of viability and risk stratification are of crucial importance. Clinical assessment of myocardial viability is performed by single photon emission computed tomography (SPECT), positron emission tomography (PET), stress echocardiography or magnetic resonance imaging (MRI) (2–5). Evaluation of infarct size and morphology with delayed contrast-enhanced (de) magnetic resonance imaging MRI has been well validated and is currently considered the clinical ‘ gold standard’ for viability assessment (6–8). Recently, de-MRI was used to assess heterogeneity of the peri-infarct zone (PIZ) which was shown to identify patients susceptible to ventricular arrhythmias (9) and predict post-MI mortality (10).
The use of cardiac multi-detector computed tomography (MDCT) has been expanded beyond assessment of coronary atherosclerotic burden (11–13) to include myocardial viability by delayed contrast enhancement (de) in a similar fashion as MRI (14–16) as well as myocardial perfusion assessment (17, 18). Limited spatial resolution of in-vivo MRI in the z or axial direction (slice thickness) and the resulting partial volume effect at the infarct border is regarded a limiting factor for the assessment of nonviable myocardial tissue by MRI and is likely partly responsible for the presence and appearance of the PIZ documented by MRI (7). With its high isotropic resolution, MDCT has the potential to greatly decrease partial volume effects and accurately characterize the PIZ. However, the high z-axis spatial resolution of MDCT comes at the expense of higher image noise which may preclude accurate assessment of the PIZ. The effect of slice thickness and partial volume effects on infarct size and infarct heterogeneity relative to MRI has not been systematically studied for de-MDCT imaging. Accordingly, the purpose of this study was twofold: 1) compare de-MRI and de-MDCT assessment of the PIZ in a chronic six-month occlusion/reperfusion model of healed MI and 2) characterize the effect of slice thickness (partial volume averaging) on de-MDCT and de-MRI infarct and PIZ measurements.
Methods
Animal Model
All animal studies were approved by the Johns Hopkins University Institutional Animal Care and Use Committee and comply with the “Guide for the Care and Use of Laboratory Animals” (NIH Publication no. 80–23, revised 1985). Female Göttingen minipigs were purchased from Marshall BioResources (North Rose, NY).
Animals with chronic infarcts were created as previously described in detail (19). Briefly, MI was induced by engaging the left anterior descending coronary artery (LAD) with an 8F hockey stick catheter under fluoroscopic guidance. Then, a 0.014″ angioplasty guide wire was inserted into the LAD, and a 2.5 × 12mm Maverick balloon (Boston Scientific, Natick, MA) was inflated to 4 atmospheres just distal to the second diagonal branch of the LAD. After 120 minutes, occlusion of the vessel was terminated by deflating the balloon, and restoration of flow in the LAD was confirmed by angiography.
Animals progressed to heart failure (Table 1), and all MDCT and MR imaging studies were performed in random order on the same day 191±4 days after MI induction (Table 1). A total of 15 animals were studied. One animal died immediately after the MDCT scan before MRI data could be acquired. Pathology at 2 mm slice thickness was achieved in 12 animals. In a subset of 5 animals, ex-vivo MR images were obtained after the last in-vivo experiment.
Table 1.
Animals Characteristics (n=14)
| Mean | SD | SEM | Lower 95% CI of mean | Upper 95% CI of mean | ||
|---|---|---|---|---|---|---|
| BW | (kg) | 43.7 | 7.2 | 1.9 | 39.5 | 47.9 |
| AGE | (months) | 19.3 | 2.9 | 0.8 | 17.7 | 21.0 |
| (days) | 580 | 86 | 23 | 530 | 629 | |
| LVSV | (ml) | 18.6 | 6.0 | 1.6 | 15.2 | 22.1 |
| LVEF | (%) | 33.5 | 8.4 | 2.3 | 28.7 | 38.4 |
| LVEDV | (ml) | 56.6 | 13.5 | 3.6 | 48.8 | 64.4 |
| LVESV | (ml) | 37.0 | 10.2 | 2.7 | 31.1 | 42.9 |
| FOLLOW-UP | (months) | 6.4 | 0.5 | 0.1 | 6.1 | 6.7 |
| (days) | 191 | 15 | 4 | 182 | 200 |
SD = Standard Deviation; SEM = Standard Error of the mean; CI = Confidence Interval; BW = body weight; LVSV = left ventricular stroke volume; LVEF = left ventricular ejection fraction; LVEDV = left ventricular endiastolic volume; LVESV = left ventricular endsystolic volume; FOLLOW-UP= time from infarct to imaging studies
Multi-Detector Computed Tomography (MDCT)
Each animal was scanned, with electrocardiographic monitoring, using a 0.5 mm × 64-detector scanner (AquilionTM64 -Toshiba Medical Systems Corporation, Otawara, Japan). Animals received intravenous metoprolol (2–5 mg) and/or amiodarone (50 to150 mg) to achieve a heart rate <100 beats per minute (bpm) for delayed enhancement studies. Following scout acquisition and slice prescription, a 150-ml bolus of iodixanol (Visipaque™320 mg iodine/ml – Amersham Health, Amersham, UK) was injected intravenously (0.91±0.04 × 10−3 mg iodine/kg bodyweight). During MDCT acquisition respiration was suspended and imaging was performed using a retrospectively gated MDCT protocol with the following parameters: gantry rotation time = 400 ms, temporal resolution 212±5 ms, detector collimation = 0.5mm × 64 (isotropic voxels = 0.5 × 0.5 × 0.5 mm3 − 13 linepairs/cm), helical pitch = variable depending on heart rate (range: 6.4–6.8), tube voltage = 120 kV, tube current = 400 mA). Delayed enhancement images were acquired during first pass, 5, 10, and 15 minutes after contrast delivery.
Raw data were reconstructed as contiguous 0.5mm slice thickness by an adaptive multi-segment reconstruction algorithm(20). Images were reconstructed at 80% of the R to R interval using a standard kernel (FC43). ECG editing to account for arrhythmias was performed when necessary. Multi-planar reformation in the short-axis of the heart was implemented to achieve 8, 4, 2 and 1mm slice thickness to isolate effects of partial volume and signal to noise ratio.
Magnetic Resonance Imaging (MRI)
In vivo MRI images were acquired using a 1.5 T MR scanner (CV/i, GE Medical Systems, Waukesha, WI) 24 weeks after MI. Global LV function was assessed using a steady-state free precession pulse sequence (21). A total of eight to ten contiguous short–axis slices were prescribed to cover the entire LV, from base to apex. Image parameters were the following: TR/TE= 4.2 ms and 1.9 ms; Flip angle= 45°; 256×160 matrix; 8 mm slice thickness/no gap; 125 kHZ; 28 cm FOV and 1 NSA.
Delayed contrast enhanced images were acquired 15 minutes following intravenous injection of Gd-DTPA (0.2 mmol/kg body weight, Magnevist, Berlex, Wayne, NJ), using an ECG-gated, breath-hold, interleaved, inversion recovery, FGRE pulse sequence. De-MRI images were acquired using the same slice prescription used for short axis cine-images. Imaging parameters were: echo time/repetition time 3.3/7.3ms; flip angle= 25°, bandwidth, 31.2 kHZ; field of view, 280 mm, 256 × 196 matrix; 1.3 × 1.3 × 8.0 mm3, 8 mm slice thickness/no gap. Inversion recovery time (≈200 ms) was manually adjusted as needed to null the normal myocardium (22).
Ex-vivo MR imaging was conducted in a subset of 5 animals to visualize infarct morphology at sub-millimeter resolution. After intravenous administration of heparin 5000 IU and 0.20 mmol/kg Gd-DTPA, the animals were euthanized, and the hearts were removed and filled with vinyl polysiloxane to assure diastolic arrest. The heart was scanned in a 1.5-T Sonata MR scanner (Siemens Medical Solutions, Erlangen, Germany) with a 3D gradient echo sequence. The imaging parameters were as follows: echo time/repetition time, 4.02/9.7 ms; flip angle, 20°; bandwidth, ±130 Hz/pixel; field of view, 100 mm; image matrix, 256×256; spatial resolution, 0.39 × 0.39 × 0.39 mm3. Images were then averaged to achieve multiple slice thicknesses (1mm, 2mm, 4 mm, and 8 mm) for assessment of partial volume effects on the PIZ and infarct core.
Imaging Analysis
Cine MRI images, de-MDCT, de -MR images were analyzed using a custom research software package (Cine Tool, GE Medical Systems, Waukesha, WI). To evaluate resting left ventricular (LV) function endo- and epicardial borders of the LV were defined each in the end-diastolic and end-systolic frame in contiguous slices and end-diastolic left ventricular volume (LVEDV), end-systolic left ventricular volume (LVESV), left ventricular stroke volume (LVSV), and left ventricular ejection fraction (LVEF) were calculated (Table 1). Infarct size and volumes in de-MDCT, de-MR images and were defined based on 3 standard deviation of the signal intensity from the remote mean (non-infarct) myocardium for the core infarct and 2 standard deviation of the signal intensity from the remote mean for the PIZ as described previously (23).
Signal to noise ratios (SNR) were calculated for MDCT and MRI by dividing the mean signal intensity in the infarct area with the standard deviation (SD) of signal intensity outside of the thorax. The SD of the air measurements reflects the degree of image noise. Mean signal intensity and SD were obtained by measuring air/noise outside the body or in the lung field. Image contrast-to-noise ratios (CNR) were calculated by using the following equation: (mean signal intensity of the infarct - mean signal intensity of the remote region)/SD of noise).
Pathology and Histology
Animals were immediately sacrificed following the imaging study. Hearts were arrested in diastole by slow retrograde infusion of an ice cold solution of 4 mmol/L potassium chlorid (KCl) and 4% paraformaldehyde dissolved in PBS. The porcine hearts were excised, filled with dental rubber to avoid uneven tissue shrinkage, and were post-fixed in 4% paraformaldehyde overnight at 4°C. For post-mortem infarct size assessment the fixed porcine hearts were sectioned from apex to base at 2 mm slice thickness with a commercial available meat slicer. Myocardial slices were weight, and the apical and basal aspect of each slice was digitally photographed. The infarct scar and areas of viable myocardium were manually traced using imaging analysis software (Sigma Scan® Pro5, Systat Software Inc., San Jose, CA). The infarct mass was calculated according to slice weight and infarct size was expressed as percentage of LV mass excluding the papillary muscle.
Fixed tissue samples from the peri-infarct area were embedded in paraffin, 5 μm sections were cut and stained with a Masson’s trichrome protocol. Light microscopy images were obtained on a Zeiss Axiophot microscope (Carl Zeiss NTS GmbH, Oberkochen, Germany).
Electron microscopy
Small tissue samples including both, chronic infarct scar and viable myocardium were dissected from the porcine hearts after perfusion with ice-cold PBS solution. For transmission electron microscopy (TEM) and scanning electron microscopy (SEM) the cardiac tissue was fixed in 2% glutaraldehyde in 0.1 M cacodylate pH 7.4 with 3% sucrose and 3mM CaCl2 overnight at 4°C. TEM sample were post-fixed with 1% osmium tetroxide reduced in potassium ferrocyanide for 1 hour at 4°C Tissue was incubated in 0.15% tannic acid for 1 minute to enhance the visualization of collagen fibers, and placed in 2 % uranyl acetate for 1 hour at room temperature. After fixation the tissue was stained en bloc with a 2% aqueous solution of uranyl acetate and dehydrated in graded ethanol and embedded. Ultra-thin sections (70–90 nm) were cut and stained with uranyl acetate and lead citrate. Samples were viewed and photographed on a Hitachi 7600 TEM (Hitachi High Technologies America, Inc.; Gaithersburg, MD) SEM sample were fixed and dried with liquid CO2, mounted onto SEM stubs and sputter coated with 20 nm gold palladium. SEM images were obtained on a LEO1530 field emission scanning electron microscope (Carl Zeiss NTS GmbH, Oberkochen, Germany).
Statistical Analysis
All data are presented as mean ± standard error of the mean unless otherwise stated. For infarct size and infarct volume/mass comparison, Pearson correlation and linear regression analysis were used to compare MDCT versus post mortem pathology, and MRI. Results were confirmed by Bland-Altman analysis and agreement expressed as mean ± Standard deviation (SD) difference between methods at 95% Confidence Intervals (CI). MDCT and MRI data at 8 mm slice thickness (Table 2), were evaluated with paired Student’s t-test. These analyses were performed in MedCalc. (MedCalc Software®, Mariakerke, Belgium).
Table 2.
Comparison of MDCT and MRI at 8mm Slice Thickness (n=14)
| MDCT | MRI | p-value | |
|---|---|---|---|
| Infarct [%] LV mass (%) | 18.5 ± 1.5 | 25.0 ± 1.8 | 0.01 |
| Total infarct mass (gram) | 7.6 ± 0.7 | 10.4 ± 0.9 | 0.02 |
| Core infarct mass (gram) | 7.0 ± 0.6 | 10.1 ± 0.9 | 0.01 |
| Peri infarct mass (gram) | 0.6 ± 0.1 | 0.3 ± 0.1 | 0.01 |
| LVED mass (gram) | 39.9 ± 1.6 | 41.7 ± 1.6 | 0.46 |
Since the study involved repeated measurements of individual animals, alinear mixed model (Verbeke and Molenberghs, 2000) was used for analysis of the data depending on slice thickness. The model included fixed effects for imaging modality and/or slice thickness, as well as their interaction when both were included in the model, and a random animal effect. Post-hoc comparisons were made among adjusted means using Wald tests. The linear mixed model analyses were performed in SAS (SAS/STAT® Software Cary, North Carolina).
In all analysis p-values <0.05 were considered significant.
Results
MDCT Viability Imaging
First pass de-MDCT imaging demonstrated homogenous myocardial enhancement of the remote myocardium and hypodense regions in the LAD territory. Post-infarct MDCT imaging at 5, 10 and 15 minutes after contrast injection identified collagenous myocardial scar that was characterized by well delineated hyperdense regions. Density analysis of the left ventricular blood pool, infarct region, and remote myocardium at all time points confirmed that optimal image quality (highest CNR) occurred at 10 min post contrast and thus all de-MDCT images were analyzed at this time point.
De-MDCT versus de-MRI and pathology
The morphology and distribution of chronic infarct scar assessed by de-MDCT correlated well with gross pathology, ex-vivo and in-vivo MRI (Figure 1). Infarct mass was 4.3±0.6 gram evaluated by gross pathology. Total infarct size as percent of LV mass by MDCT at 8 mm correlated well with in-vivo MRI (r2=0.92; p< 0.0001) and post mortem pathology (r2=0.97; p< 0.0001) with a 8.2% mean underestimation by MDCT versus MRI and a qualitative trend toward overestimation by MDCT versus pathology with mean difference −8.2%. Matching slice thickness of 2mm for de-MDCT images and post mortem pathology, the overestimation of de-MDCT decreased to a mean difference −7.3%.
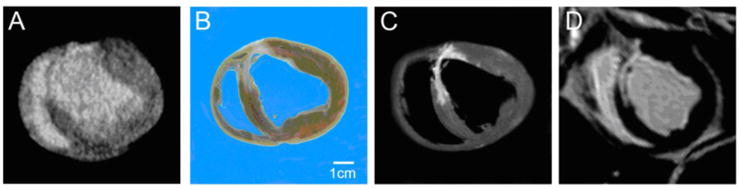
Figure 1. Comparison of infarct morphology by MDCT with post mortem pathology and MRI.
Example images in short axis orientation for (A) de-MDCT at 2mm, (B) post mortem pathology at 2mm, (C) ex-vivo and (D) in-vivo MRI at 4mm slice thickness show a similar location and distribution pattern of the chronic collagenous scar in the anterior-septal LV wall.
Peri-Infarct Zone Imaging
A PIZ could be measured for both de-MDCT and de-MRI in all animals at all slice thicknesses (Table 2 and Figure 2). We used standard histology TEM and SEM as a reference to investigate the tissue composition of the PIZ and to clarify the imaged substrate. Gross pathology and Masson’s trichrome staining revealed trans-mural infarct scars with densely packed collagen fibers (Figure 2C and F) in all animals. Infarct scar and viable myocardium were clearly delineated in all chronic MI (Figure 2G–I) and islands of viable myocytes could be detected by Masson’s trichrome staining at the borderzone of the infarcts (Figure 2C and F). All myocardial infarcts showed this intermingling of viable and non-viable tissue at the borderzone of the infarct scar.
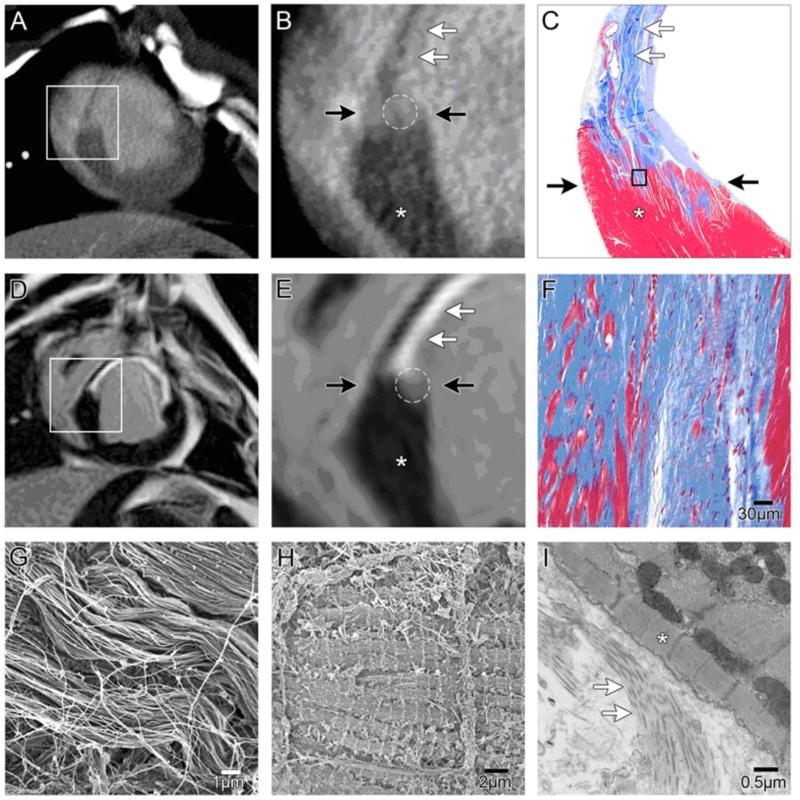
Figure 2. Characterization of tissue heterogeneity in peri-infarct zone by MDCT.
Example of matching 8mm slice section in chronic infarcts for de-MDCT (A, B) and de-MRI (D,E).(A, B) The viable myocardium(*) shows lower attenuation values, than enhanced scar tissue (white arrows). The PIZ is visualized between two black arrows by intermediate signal intensity (white circle) in de-MDCT images.
(D,E) Viable myocardium (*), delayed enhanced infarct scar (white arrows), and the PIZ (white circle) show different signal intensity in de- MRI. The viable myocardium is darker in the MRI acquisition, and the PIZ appears larger in de-MDCT images compared to de-MRI. C, F) Masson’s trichrome stain depicts viable myocardium in red (*) from non-viable tissue in blue. At higher magnification the densely packed collagenous extra-cellular matrix (white arrows) in the chronic infarct scar can be appreciated. Island of viable myocytes (red) within the scar tissue are visualized demonstrating the heterogeneity of the PIZ (area between black arrows). (G, H) Scanning electron microscope images of the densely packed collagen fibers six months post MI (G) and viable myocytes (H) characterize the ultra-structure of the chronic infarct and the PIZ. (I) Transmission electron microscopy of the PIZ demonstrates the clear delineating of the collagenous scar (white arrows) and viable tissue (*).
Effect of Slice Thickness on the PIZ
Slice thickness had a marked effect on the calculated mass of the PIZ for both in-vivo and ex-vivo de-MRI experiments (Figure 3), suggesting a strong partial volume effect. For ex-vivo MRI, the PIZ mass decreased from 0.9±0.2mg to 0.2±0.1mg comparing 8mm and 1mm thickness, respectively (p=0.0001). The de-MDCT PIZ volume showed a trend towards increasing values with decreasing slice thickness (Figure 4). Additionally, the relative proportion of the PIZ expressed as a percentage of the total infarct core changed significantly with slice thickness (Table 3 and Figure 5). These results were consistent with increased CNR and decreased SNR in the PIZ with thinner slices by visual and quantitative assessment (Figure 6B and C).
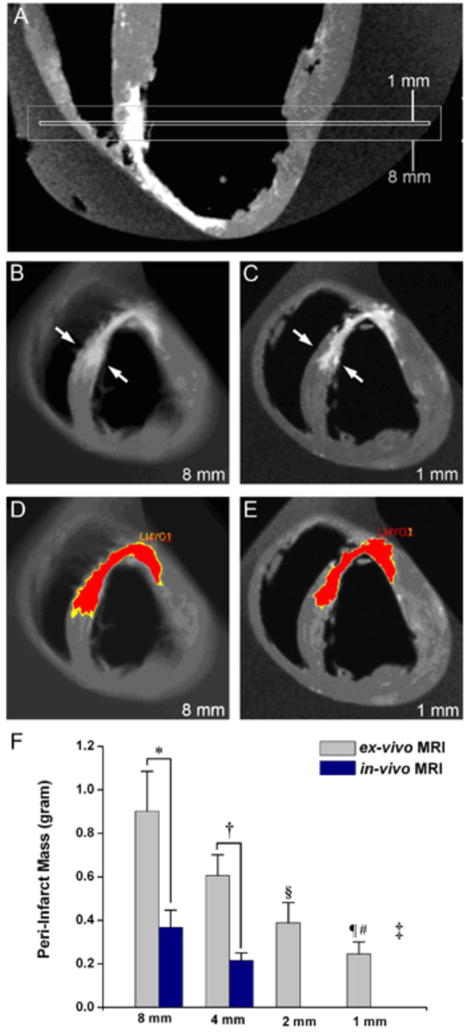
Figure 3. Peri-infarct zone assessment by ex-vivo MRI (n=5).
(A) Long-axis orientation showing the extent of 1 and 8mm slice thickness for short axis images. The presence of both viable and non-viable tissues in the 8 mm slice results in partial volume averaging. (B) For the 8mm slice thickness, the septal infarct appears transmural (arrows) and the PIZ is less defined with a wide range of gray values. (C) In the 1mm short axis slice at the same region it can be appreciated that infarct is not transmural and has well-delineated borders (D, E) The same myocardial slices with computer –generated mask depicting the core infarct (red) and PIZ (yellow). The PIZ is larger at 8mm. (F) Ex-vivo and in-vivo MRI showed different assessment of the PIZ at 8mm and 4 mm *p< 0.01 and †p= 0.01, respectively)
The PIZ mass in ex-vivo MRI acquisitions as a function of slice thickness showed marked differences (‡p=0.004) and decreased with thinner slice thickness; 2mm versus 8mm §p= 0.01, and 1mm vs 4 and 8mm, ¶p< 0.01 and #p< 0.001, respectively
Figure 4. Comparison of PIZ assessment with ex-vivo de-MRI and de-MDCT.
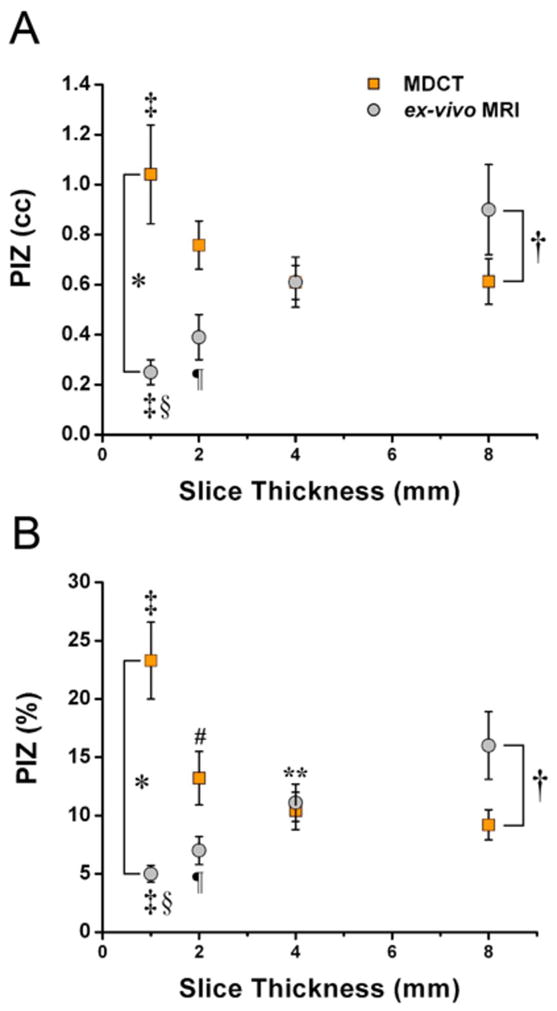
The volume of the PIZ (A) and PIZ expressed as percentage of the total infarct size (B) decreases in a linear fashion with reduced slice thickness evaluated by ex-vivo MRI suggesting a pronounced partial volume effect, while the amount of PIZ volume is less affected by slice thickness in MDCT acquisitions until a slice thickness of 1mm is reached. This implies that MDCT assessment of the PIZ is less susceptible to partial volume effects, but affected by image noise at 1mm. There are marked differences in the PIZ volume and percentage assessment between MDCT and ex-vivo MRI assessment at 1 and 8mm *p<0.01 and †p<0.001, respectively. Differences between slice thicknesses: 1mm versus 4 and 8mm, ‡ p< 0.01 and §p< 0.001, respectively; 2mm versus 8mm, ¶p= 0.01 for ex-vivo MRI, and 8mm versus 2 mm 4 mm # p< 0.05 and **p< 0.05, respectively
Table 3.
Infarct Parameters at Different Slice Thickness by MDCT (n=12)
| 8mm | 4mm | 2mm | 1mm | p-value | ||
|---|---|---|---|---|---|---|
| Infarct [%] LV | (%) | 17.3 ± 1.1 | 16.4 ± 1.4 | 16.0 ± 1.5 | 14.3 ± 1.8*† | 0.025 |
| Total volume | (gram) | 7.0 ± 0.6 | 6.8 ± 0.6 | 6.8 ± 0.7 | 5.9 ± 0.8 | 0.059 |
| Core volume | (gram) | 6.4 ± 0.6 | 6.1 ± 0.6 | 6.0 ± 0.7 | 4.8 ± 0.7*‡§ | 0.001 |
| (%) | 90.8 ± 1.3 | 89.6 ± 1.6 | 86.9 ± 2.1 | 66.5 ± 3.38*‡§ | <0.001 | |
| PIZ volume | (gram) | 0.6 ± 0.1 | 0.6±0.1 | 0.8±0.1 | 1.1±0.2 | 0.056 |
| (%) | 9.2 ± 1.3 | 10.4 ± 1.6 | 13.2 ± 2.3¶ | 23.3 ± 3.3*‡§ | 0.001 | |
| LVED mass | (gram) | 39.2 ± 1.6 | 39.7 ± 1.8 | 40.4 ± 1.9 | 38.9 ± 1.6 | 0.093 |
p<0.001; compared with 8mm slice thickness
p<0.05; compared with 4mm slice thickness
p<0.01; compared with 4mm slice thickness
p<0.01; compared with 2mm slice thickness
p<0.05; compared with 8mm slice thickness
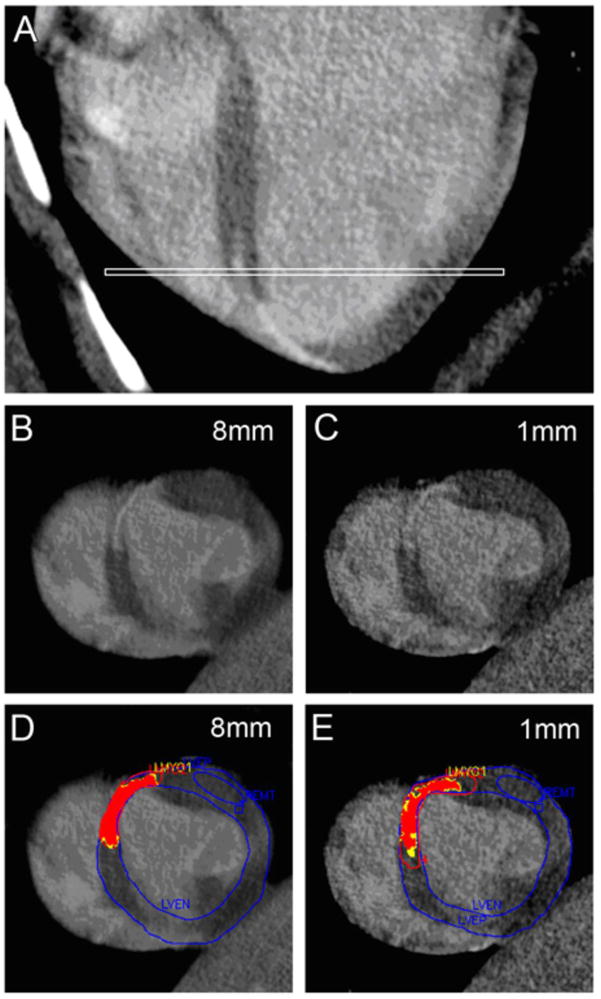
Figure 5. Effect of Slice Thickness on de-MDCT PIZ.
(A) Long-axis orientation of de-MDCT showing the location of the short axis images (white line). (B, C) De-MDCT images in short axis reconstructed at 8 and 1 mm. Note the smooth appearance of the tissue versus the more heterogeneous appearance at 1 mm. (D,E) The same myocardial slices with computer –generated mask depicting the core infarct (red) and the peri-infarct zone (PIZ) (yellow). The increase of the PIZ (yellow) can be appreciated with decreased slice thickness.
Figure 6. Effect of slice thickness on de-MDCT density and signal to noise ratios (SNR) and contrast to noise ratios (CNR).(.
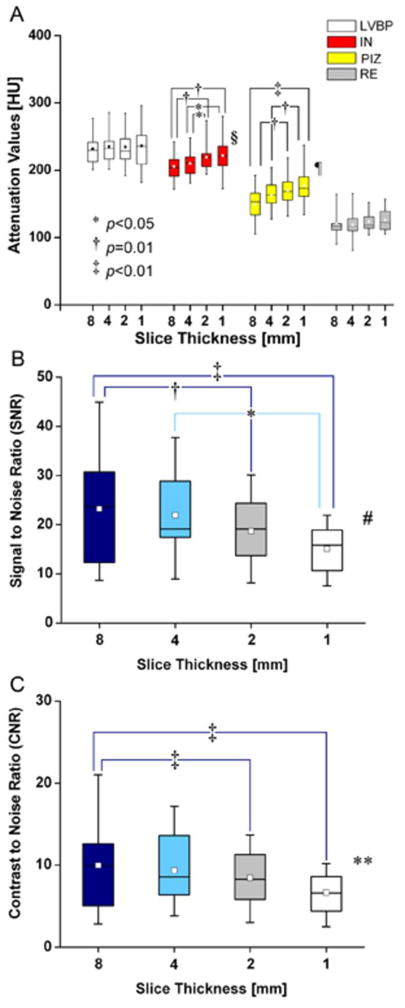
A) Signal density in Hounsfield Units (HU) for the left ventricular blood pool (LVBP), infarcted myocardium (IN), the peri-infarct zone (PIZ) and the remote myocardium (RE), LVBP and RE do not show different attenuation values at different slice thickness, while IN values (*p<0.05, 1 versus 4mm and 2 versus 4mm; † p=0.01,1 versus 8mm, 2 versus 8mm) and PIZ values (‡ p<0.01, 1 versus 4mm and 8mm, and 2 mm versus 8mm)change with the reconstructed slice thickness parameters(§p=0.007 and ¶ p=0.002, respectively). (B) Mean MDCT SNR (8 versus 2mm and 1mm, († p=0.01 and ‡ p<0.01 respectively; and 4 versus 1mm, *p<0.05; ‡ p=0.003) and (C) mean MDCT CNR (8 versus 2mm and 1mm, ‡ p<0.01; ** p=0.008) change with the reconstructed slice thickness, which reflects reduced imaging quality of thinner reconstructed slices.
Discussion
To the best of our knowledge this is the first report of de-MDCT and de-MRI PIZ imaging with post-mortem pathology in dense remodeled scar six months post-MI. The major findings of this study are that de-MDCT is superior to MRI in its ability to detect and quantify the peri-infarct zone (PIZ) after contrast delivery in chronic MI. Furthermore, we characterize the influence of partial volume effects on the measurement of the PIZ by imaging methods and provide detailed comparisons against histopathology.
Scar Enhancement by MDCT – Comparison with MRI
Delayed hyper-enhancement in chronic collagenous myocardial scar results from an accumulation of contrast media in the interstitial space between collagen fibers, resulting in an increased volume of contrast distribution in the scar tissue compared with that of tightly packed myocytes. (7) The accumulation of contrast material in the collagenous scar matrix is a passive process and the timing between administration of contrast agents and imaging is crucial to differentiate blood pool from scar and the PIZ. De-MDCT density values are somewhat unique and determined by the physical properties of individual constituents of the heart including blood, viable and non-viable myocardium that result from direct x-ray attenuation by iodine. While not a perfect binary system, such imaging is theoretically well suited to characterize infarct heterogeneity.
De-MRI is a highly attractive modality for myocardial viability imaging due to its high contrast-to-noise ratio and lack of ionizing radiation. This approach is based upon gadolinium-induced alterations of water relaxivity and thus represents a surrogate measure of the amount and distribution of contrast in the extra-cellular space. Recent studies have shown that de-MDCT and de-MRI infarct size compare well in patients (14, 24), despite the lower image quality of de-MDCT exams. The lower image quality of de-MDCT is likely related to technical factors such as temporal resolution as well as differences in the mechanisms of hyperenhacement between de-MDCT and de-MRI (e.g., the lack of iodine-water interactions that modulate signals). The quality of acute de-MDCT infarct imaging is improved over that of scar imaging since iodine is able to access the intracellular space rather than the limited extracellular space generated by a collagen matrix (15). Other features unique to de-MRI data acquisition such as inversion recovery based myocardial nulling further improve scar visualization over de-MDCT but may also partly explain the overestimation of infarct size by de-MRI in our chronic infarcts and in the acute setting (25).
Partial Volume Effects and the Appearance of the PIZ
The appearance of the PIZ by de-MDCT and de-MRI can be explained by two possible orientations of the normal myocardium with respect to scar: 1) an area of uniformly dense scar adjacent to preserved myocardium with a sharp binary transition, or 2) the intermingling of bundles of fibrotic scar with viable myocytes. Partial volume effects are related to the three-dimensional (3D) spatial resolution of a tomographic image. If a given voxel at the infarct periphery contains both infarct and non-infarct tissue, the two different signal intensities will be averaged, and this particular voxel will be represented by an intermediate attenuation value. For standard clinical de-MRI, voxel volumes are typically greater than 13 mm3 (1.3 × 1.3 × 8.0 mm3) and thus approximately 200,000 cells at the interface between infarcted and viable tissue in the PIZ can occur in a single voxel. To quantify the effect of partial volume averaging for de-MRI, we acquired high resolution ex-vivo MR images at 0.4 mm slice thickness using a 3D sequence with an isotropic voxel size 0.39 × 0.39 × 0.39 mm3 resulting in a pixel volume 59 μm3 which allowed for reproduction of various slice thicknesses. The measured PIZ decreased 350% when comparing a clinically used slice thickness (8 mm) to 1 mm slices. We also observed a similar decrease in the PIZ when comparing in vivo de-MRI images. These data suggest a strong partial volume effect that greatly decreases local contrast to noise ratio in the PIZ and support the hypothesis that the PIZ measured by de-MRI may overestimate the actual PIZ.
In MDCT imaging with 64-detector row scanners, raw data can be acquired and displayed at a high isotropic resolution with a voxel volume of 43 μm3 (0.35 × 0.35 × 0.35 mm3) which is two orders of magnitude smaller than that of in-vivo MRI. During the MDCT reconstruction process it is possible to reconstruct and analyze multiple slice thicknesses from the original data set which allows partial volume effects to be quantified. At thinner slices, partial volume averaging decreases and CNR increases at the expense of decreased SNR. Unlike the de-MRI derived PIZ, the de-MDCT PIZ volume increased with increasing slice thickness. This is likely explained by an increase in CNR in the PIZ that allows better differentiation of viable cells and scar as partial volume averaging is greatly decreased. These advantages, however, are partly off-set by increased image noise that may limit PIZ assessment.
Overall, the PIZ in these experiments in chronic infarct scars was small. This is partly due to the lack of collateral coronary circulation in swine species that generates well delineated infarcts with sharp borders. Further, the iodine wash-out in the chronic porcine model is more rapid compared to our previous experiments in a canine model of acute MI (15). The rich collateral supply in dog hearts facilitates the passive accumulation and distribution of contrast material in the infarct area, and delays the clearance of iodine creating a more intense infarct signal and greater PIZ. However, our PIZ size correlates well with human data reported for patients with small PIZ volumes (10).
Clinical Implications
The potential value of imaging the PIZ has been recently reported (9, 10). This very pathologically complex region contains a mixture of both viable and non-viable electrically inactive scar that is thought to provide substrate for ventricular tachycardia via macroreentry mechanisms. Thus, accurate characterization and phenotyping of the PIZ may allow selection of optimal candidates for implantable defibrillators and identify targets for ablation therapy during interventional electrophysiology procedures. We are currently using de-MDCT of the PIZ for this purpose at our institution in a porcine model of ventricular tachycardia.
Standardized low radiation de-MDCT protocols are required for ultimate translation of PIZ assessment into the clinical setting. We have recently described a prospectively gated protocol for high resolution de-MDCT imaging that lowers radiation dose by an order of magnitude (26). The focus of such protocols is achieving a reasonable balance of SNR and CNR for visual and quantitative infarct measurements while minimizing partial volume effects. Based upon the results of these studies, a slice thickness of 1–2mm appears to provide this balance. Further studies are required to determine optimal threshold cut offs and determine if the evaluation of the PIZ with de-MDCT has prognostic value as suggested for MR imaging (9, 10). The ability to image healed myocardial infarction in heart failure with de-MDCT and to quantify the PIZ may enhance identification of patients susceptible to life-threatening arrhythmias and sudden cardiac death
Limitations
We applied previously published signal density threshold algorithms to define the infarct core and peri-infarct region. However, specific optimal cutoff values for normal, PIZ, and core zones of myocardium are unknown and are likely dependent upon study quality. Additionally, due to the passive contrast kinetics of iodinated contrast and the limited collateral circulation in pigs, de-MDCT imaging of collagenous scar in this model requires a relatively large dose of iodine to cause a sufficient change in the volume of distribution in that myocardial bed. In this study we used 1.5 times a human equivalent dose although successful viability imaging in humans has been reported in several studies with standard contrast volumes used for coronary CT angiography (24) A further limitation of the study is the lack of quantitative histological gold-standards to index the degree of heterogeneity of the PIZ.
Conclusions
De- MDCT provides an accurate measure of the spatial extent of chronic collagenous infarct scars relative to MRI and post mortem pathology. The improved spatial resolution of de-MDCT minimizes partial volume averaging and allows for quantification of complex tissue substrates in the PIZ.
Acknowledgments
This work was supported by NIH grant U54 HL081028 (Specialized Center for Cell Therapeutics), and The Donald W. Reynolds foundation. The authors want to thank Michael Delannoy, M.S. and Carol Cooke, M.S. at the JHMI Microscope Facility for excellent technical assistance performing the electron microscopy studies.
The authors also would like to thank Norman J. Barker, M.S., R.B.P. for his expertise and helpful suggestions obtaining pathology and histology images.
Abbreviation List
- CNR
contrast-to-noise ratio
- de
delayed contrast-enhancement
- Gd - DTPA
Gadolinium diethylenetriamine penta-acetic acid
- MDCT
multi-detector computed tomography
- MI
myocardial infarction
- MRI
magnetic resonance imaging
- PIZ
peri-infarct zone
- SNR
signal-to-noise ratio
- SEM
scanning electron microscopy
- TEM
transmission electron microscopy
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Reference List
- 1.American Heart Association. Heart and Stroke Statistics - 2008 Update. [cited 10 June 2008]. Available at. [Google Scholar]
- 2.Burt RW, Perkins OW, Oppenheim BE, et al. Direct comparison of fluorine-18-FDG SPECT, fluorine-18-FDG PET and rest thallium-201 SPECT for detection of myocardial viability. J Nucl Med. 1995;36:176–9. [PubMed] [Google Scholar]
- 3.Mahrholdt H, Wagner A, Judd RM, Sechtem U. Assessment of myocardial viability by cardiovascular magnetic resonance imaging. Eur Heart J. 2002;23:602–19. doi: 10.1053/euhj.2001.3038. [DOI] [PubMed] [Google Scholar]
- 4.Sicari R, Pasanisi E, Venneri L, Landi P, Cortigiani L, Picano E. Stress echo results predict mortality: a large-scale multicenter prospective international study. J Am Coll Cardiol. 2003;41:589–95. doi: 10.1016/s0735-1097(02)02863-2. [DOI] [PubMed] [Google Scholar]
- 5.Iskandrian AS, Heo J, Schelbert HR. Myocardial viability: methods of assessment and clinical relevance. Am Heart J. 1996;132:1226–35. doi: 10.1016/s0002-8703(96)90467-6. [DOI] [PubMed] [Google Scholar]
- 6.Kim RJ, Wu E, Rafael A, et al. The use of contrast-enhanced magnetic resonance imaging to identify reversible myocardial dysfunction. N Engl J Med. 2000;343:1445–53. doi: 10.1056/NEJM200011163432003. [DOI] [PubMed] [Google Scholar]
- 7.Wu KC, Lima JA. Noninvasive imaging of myocardial viability: current techniques and future developments. Circ Res. 2003;93:1146–58. doi: 10.1161/01.RES.0000103863.40055.E8. [DOI] [PubMed] [Google Scholar]
- 8.Kim RJ, Albert TS, Wible JH, et al. Performance of delayed-enhancement magnetic resonance imaging with gadoversetamide contrast for the detection and assessment of myocardial infarction: an international, multicenter, double-blinded, randomized trial. Circulation. 2008;117:629–37. doi: 10.1161/CIRCULATIONAHA.107.723262. [DOI] [PubMed] [Google Scholar]
- 9.Schmidt A, Azevedo CF, Cheng A, et al. Infarct tissue heterogeneity by magnetic resonance imaging identifies enhanced cardiac arrhythmia susceptibility in patients with left ventricular dysfunction. Circulation. 2007;115:2006–14. doi: 10.1161/CIRCULATIONAHA.106.653568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Yan AT, Shayne AJ, Brown KA, et al. Characterization of the peri-infarct zone by contrast-enhanced cardiac magnetic resonance imaging is a powerful predictor of post-myocardial infarction mortality. Circulation. 2006;114:32–9. doi: 10.1161/CIRCULATIONAHA.106.613414. [DOI] [PubMed] [Google Scholar]
- 11.Poon M, Rubin GD, Achenbach S, et al. Consensus update on the appropriate usage of cardiac computed tomographic angiography. J Invasive Cardiol. 2007;19:484–90. [PubMed] [Google Scholar]
- 12.Schroeder S, Achenbach S, Bengel F, et al. Cardiac computed tomography: indications, applications, limitations, and training requirements: Report of a Writing Group deployed by the Working Group Nuclear Cardiology and Cardiac CT of the European Society of Cardiology and the European Council of Nuclear Cardiology. Eur Heart J. 2007 doi: 10.1093/eurheartj/ehm544. [DOI] [PubMed] [Google Scholar]
- 13.Budoff MJ, Achenbach S, Blumenthal RS, et al. Assessment of coronary artery disease by cardiac computed tomography: a scientific statement from the American Heart Association Committee on Cardiovascular Imaging and Intervention, Council on Cardiovascular Radiology and Intervention, and Committee on Cardiac Imaging, Council on Clinical Cardiology. Circulation. 2006;114:1761–91. doi: 10.1161/CIRCULATIONAHA.106.178458. [DOI] [PubMed] [Google Scholar]
- 14.Gerber BL, Belge B, Legros GJ, et al. Characterization of acute and chronic myocardial infarcts by multidetector computed tomography: comparison with contrast-enhanced magnetic resonance. Circulation. 2006;113:823–33. doi: 10.1161/CIRCULATIONAHA.104.529511. [DOI] [PubMed] [Google Scholar]
- 15.Lardo AC, Cordeiro MA, Silva C, et al. Contrast-enhanced multidetector computed tomography viability imaging after myocardial infarction: characterization of myocyte death, microvascular obstruction, and chronic scar. Circulation. 2006;113:394–404. doi: 10.1161/CIRCULATIONAHA.105.521450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mahnken AH, Koos R, Katoh M, et al. Assessment of myocardial viability in reperfused acute myocardial infarction using 16-slice computed tomography in comparison to magnetic resonance imaging. J Am Coll Cardiol. 2005;45:2042–7. doi: 10.1016/j.jacc.2005.03.035. [DOI] [PubMed] [Google Scholar]
- 17.George RT, Silva C, Cordeiro MA, et al. Multidetector computed tomography myocardial perfusion imaging during adenosine stress. J Am Coll Cardiol. 2006;48:153–60. doi: 10.1016/j.jacc.2006.04.014. [DOI] [PubMed] [Google Scholar]
- 18.George RT, Jerosch-Herold M, Silva C, et al. Quantification of myocardial perfusion using dynamic 64-detector computed tomography. Invest Radiol. 2007;42:815–22. doi: 10.1097/RLI.0b013e318124a884. [DOI] [PubMed] [Google Scholar]
- 19.Schuleri KH, Boyle AJ, Centola M, et al. Novel Use of the Adult Göttingen Minipig as a Chronic Heart Failure Model post Myocardial Infarction: Focus on Cardiovascular Imaging and Regenerative Therapies. Comp Med. 2008;58:568–579. [PMC free article] [PubMed] [Google Scholar]
- 20.Dewey M, Laule M, Krug L, et al. Multisegment and halfscan reconstruction of 16-slice computed tomography for detection of coronary artery stenoses. Invest Radiol. 2004;39:223–9. doi: 10.1097/01.rli.0000115201.27096.6e. [DOI] [PubMed] [Google Scholar]
- 21.Slavin GS, Saranathan M. FIESTA-ET: high-resolution cardiac imaging using echo-planar steady-state free precession. Magn Reson Med. 2002;48:934–41. doi: 10.1002/mrm.10321. [DOI] [PubMed] [Google Scholar]
- 22.Simonetti OP, Kim RJ, Fieno DS, et al. An improved MR imaging technique for the visualization of myocardial infarction. Radiology. 2001;218:215–23. doi: 10.1148/radiology.218.1.r01ja50215. [DOI] [PubMed] [Google Scholar]
- 23.Amado LC, Gerber BL, Gupta SN, et al. Accurate and objective infarct sizing by contrast-enhanced magnetic resonance imaging in a canine myocardial infarction model. J Am Coll Cardiol. 2004;44:2383–9. doi: 10.1016/j.jacc.2004.09.020. [DOI] [PubMed] [Google Scholar]
- 24.Nieman K, Shapiro MD, Ferencik M, et al. Reperfused myocardial infarction: contrast-enhanced 64-Section CT in comparison to MR imaging. Radiology. 2008;247:49–56. doi: 10.1148/radiol.2471070332. [DOI] [PubMed] [Google Scholar]
- 25.Saeed M, Wendland MF, Bremerich GL, Weinmann HJ, Higgins CB. Assessment of myocardial viability using standard extracellular and necrosis specific MR contrast media. Acad Radiol. 2002;9 (Suppl 1):S84–S87. doi: 10.1016/s1076-6332(03)80406-3. [DOI] [PubMed] [Google Scholar]
- 26.Chang HJ, George RT, Schuleri KH, et al. Prospectively ECG-gated delayed enhanced multi-detector computed tomography accurately quantifies infarct size and reduces radiation exposure by an order of magnitude. J Am Coll Cardiol Img. 2009 doi: 10.1016/j.jcmg.2008.12.019. in press. [DOI] [PubMed] [Google Scholar]