Abstract
Ornithischia (the ‘bird-hipped’ dinosaurs) encompasses bipedal, facultative quadrupedal and quadrupedal taxa. Primitive ornithischians were small bipeds, but large body size and obligate quadrupedality evolved independently in all major ornithischian lineages. Numerous pelvic and hind limb features distinguish ornithischians from the majority of other non-avian dinosaurs. However, some of these features, notably a retroverted pubis and elongate iliac preacetabular process, appeared convergently in maniraptoran theropods, and were inherited by their avian descendants. During maniraptoran/avian evolution these pelvic modifications led to significant changes in the functions of associated muscles, involving alterations to the moment arms and the activation patterns of pelvic musculature. However, the functions of these features in ornithischians and their influence on locomotion have not been tested and remain poorly understood. Here, we provide quantitative tests of bipedal ornithischian muscle function using computational modelling to estimate 3D hind limb moment arms for the most complete basal ornithischian, Lesothosaurus diagnosticus. This approach enables sensitivity analyses to be carried out to explore the effects of uncertainties in muscle reconstructions of extinct taxa, and allows direct comparisons to be made with similarly constructed models of other bipedal dinosaurs. This analysis supports some previously proposed qualitative inferences of muscle function in basal ornithischians. However, more importantly, this work highlights ambiguities in the roles of certain muscles, notably those inserting close to the hip joint. Comparative analysis reveals that moment arm polarities and magnitudes in Lesothosaurus, basal tetanuran theropods and the extant ostrich are generally similar. However, several key differences are identified, most significantly in comparisons between the moment arms of muscles associated with convergent osteological features in ornithischians and birds. Craniad migration of the iliofemoralis group muscles in birds correlates with increased leverage and use of medial femoral rotation to counter stance phase adduction moments at the hip. In Lesothosaurus the iliofemoralis group maintains significantly higher moment arms for abduction, consistent with the hip abduction mode of lateral limb support hypothesized for basal dinosaurs. Sensitivity analysis highlights ambiguity in the role of musculature associated with the retroverted pubis (puboischiofemoralis externus group) in ornithischians. However, it seems likely that this musculature may have predominantly functioned similarly to homologous muscles in extant birds, activating during the swing phase to adduct the lower limb through lateral rotation of the femur. Overall the results suggest that locomotor muscle leverage in Lesothosaurus (and by inference basal ornithischians in general) was more similar to that of other non-avian dinosaurs than the ostrich, representing what was probably the basal dinosaur condition. This work thereby contradicts previous hypotheses of ornithischian–bird functional convergence.
Keywords: bipedalism, Lesothosaurus, locomotion, modelling, moment arms
Introduction
Ornithischia is a diverse clade of non-avian dinosaurs that first appeared in the Late Triassic Period and became the dominant terrestrial vertebrate herbivores of the late Mesozoic Era (Butler et al. 2008). The most primitive ornithischians, such as Lesothosaurus diagnosticus from the Lower Jurassic of southern Africa (Thulborn, 1970, 1972; Sereno, 1991), were small (∼ 1 m long), bipedal animals with forelimbs displaying non-locomotor adaptations (e.g. grasping). However, during the 170 million years of their evolutionary history ornithischians diversified into a disparate range of body plans, shapes and sizes, and quadrupedality evolved in the clade on at least three independent occasions (Fig. 1; Sereno, 1999).
Fig. 1.
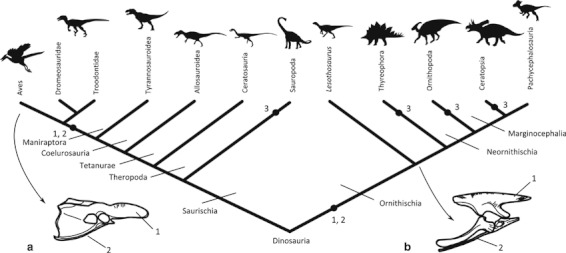
Dinosaurian relationships, showing major clades discussed in the text and the phylogenetic position of Lesothosaurus diagnosticus. Although Lesothosaurus has been recovered in a number of different positions in recent cladistic analyses (Sereno, 1999; Butler et al. 2008, 2010), this is to be expected from a taxon that displays few autapomorphies but shares numerous features with basal members of all ornithischian lineages, representing the ‘ancestral’ condition. We follow Sereno (1999) herein, in line with Maidment & Barrett (2011). a, avian pelvis in right lateral view; b, basal ornithischian pelvis in right lateral view; both after Maidment & Barrett (2011) and not to scale. 1, elongate preacetabular process of the ilium; 2, retroversion of the pubis; 3, habitual quadrupedal stance.
The pelvic anatomy of ornithischians exhibits several osteological characters that are considered to be synapomorphic for the clade: these features were established early in ornithischian evolutionary history and clearly distinguish them from the majority of saurischian dinosaurs and non-dinosaurian dinosauromorphs (Fig. 1). The most obvious of these is the possession of a retroverted, or opisthopubic, pubis (Seeley, 1887; Sereno, 1986; Butler et al. 2008). Among tetrapods, the primitive condition is for the pubis to project cranioventrally, and this anatomy is conserved in the majority of saurischian dinosaurs and dinosaur outgroups, and represents the primitive condition for Dinosauria (e.g. Romer, 1956). However, in ornithischians the pubis is rotated caudally, to lie parallel to the ischium (Sereno, 1986, 1999; Butler, 2010). A similar condition was acquired in non-avian maniraptoran theropod dinosaurs (therizinosauroids, dromaeosaurids, troodontids and their close relatives), and was inherited by their avian descendants (Fig. 1a,b: e.g. Gauthier, 1986). Another ornithischian synapomorphy, the possession of an elongate preacetabular process of the ilium (Sereno, 1986, 1999; Butler, 2010), also appeared convergently in birds (Hutchinson, 2001a,b; Fig. 1a,b). Extensive work on the maniraptoran to bird transition, involving work on both extinct non-avian theropod dinosaurs and extant birds, has demonstrated that these modifications in pelvic osteology led to significant changes in the function of associated muscles, involving the alteration of muscle moment arms and changing activation patterns of hip musculature along the avian stem-lineage (e.g. Gatesy, 1999; Hutchinson & Gatesy, 2000). Caudal expansion of the retroverted ischium and pubis and elongation of the preacetabular process are cited as key musculoskeletal adaptations underpinning the flexed femoral postures and rotation-based mode of lateral limb support that are characteristic of extant birds (Hutchinson & Gatesy, 2000).
In contrast, the effects of these pelvic modifications on ornithischian muscle function and locomotor behaviour have not been studied in detail. Although previous authors have proposed various functional hypotheses, all of these are based on qualitative comparisons with birds and crocodilians and were largely theoretical (e.g. Charig, 1972). None of these hypotheses has been subjected to rigorous biomechanical analysis or tested within a modern phylogenetic framework. As bipedal ornithischians retain many elements of the basal dinosauriform body plan (e.g. a large muscular tail and laterally compressed postacetabular pelvis), and also have derived features (e.g. retroverted pubis), they possessed a combination of musculoskeletal features that is not present in any extant tetrapod. Consequently, it is unlikely that simple comparisons with extant birds and reptiles will be adequate to fully understand the locomotion and patterns of neuromuscular control of the hip and hind limb musculature that would have occurred in these animals.
Correct interpretation of the structure–function relationships in basal bipedal ornithischians is important not only for understanding the disparity among, and convergence between, bipedal tetrapods, but is also critical for unravelling the evolution of ornithischian locomotion. All three major ornithischian lineages, Thyreophora, Ornithopoda and Marginocephalia, include clades characterized by the evolution of facultative or obligate quadrupedality (Fig. 1), a series of reversions to the primitive tetrapod condition that resulted in further profound changes in fore- and hind limb osteology and musculature (SCRM and PMB, unpublished data). Remarkably, this imposition of secondary quadrupedality on to a primitively bipedal bauplan has received very little attention, and as a consequence ornithischian dinosaur stance and locomotion remains poorly understood.
Reconstruction of soft-tissue features and behaviour in extinct animals (particularly those with no extant descendants) must be grounded in an explicit phylogenetic context, especially in those cases where osteological characteristics have become dramatically altered from the condition present in the basalmost taxa. Lesothosaurus diagnosticus represents the most completely known basal bipedal ornithischian taxon and offers the opportunity to determine the basal conditions for the pelvic and hind limb muscle architecture and muscle function in the clade (Maidment & Barrett, 2011). In his diagnosis of Lesothosaurus, Sereno (1991) considered that there was one autapomorphic feature in the pelvic region: a groove on the proximal ischium. However, this feature may actually be a symplesiomorphy with a wide distribution in archosaurs (see Maidment & Barrett, 2011). We currently recognize no autapomorphies in the pelvis of Lesothosaurus that are likely to affect interpretations of hind limb muscle function or limit the utility of this taxon as a model for basal bipedal ornithischians in general. Information on muscle function in Lesothosaurus will therefore provide a foundation upon which biomechanical studies of locomotion in derived bipedal and quadrupedal ornithischians can be based. However, we acknowledge that in the future, aspects of the pelvic anatomy of Lesothosaurus may be found to be autapomorphic and this may affect our understanding of the ‘basal condition’ for ornithischians.
Maidment & Barrett (2011; Fig. 2A) reconstructed the locomotor musculature of Lesothosaurus using the Extant Phylogenetic Bracket method (EPB: Bryant & Russell, 1992; Witmer, 1995), and proposed a series of hypotheses regarding the function of specific locomotor muscles based on osteology (constraints on degree of relative motion, positions of muscle scars), geometry (lines of action of limb muscles, ranges of motion) and the concepts of limb bone loading developed by Hutchinson & Gatesy (2000). Here, we explicitly and quantitatively test these hypotheses of muscle function and those of other authors (Romer, 1927; Galton, 1969; Charig, 1972; Coombs, 1979; Hutchinson et al. 2008) using computational modelling to estimate moment arms, and provide quantitative definitions of limb function in terms of joint rotation direction for specific muscles and muscle groups (Fig. 2B).
Fig. 2.
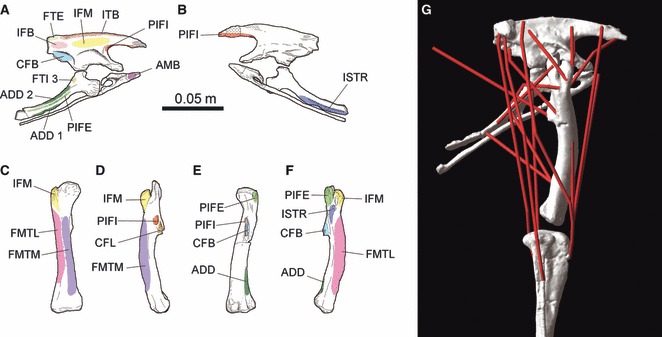
Myological reconstruction of the pelvis and hind limb of Lesothosaurus diagnosticus based on Maidment & Barrett (2011). (A,B) Pelvis in: (A) lateral; and (B) medial views. (C–F) Femur in: (C) cranial; (D) medial; (E) caudal; and (F) lateral views. (G) The 3D musculoskeletal model of Lesothosaurus in right lateral view. See Table 1 for muscle abbreviations. Scale bar equal to 0.05 m (a–f modified from Maidment & Barrett, 2011).
Computational modelling is a powerful tool partly because it is possible to undertake sensitivity analyses in order to determine, and explicitly define, the effects of uncertainties in musculature reconstructions: understanding and quantitatively defining these uncertainties is of key importance when attempting to comprehend the biology of an extinct animal. Direct comparisons with other taxa can be made because the technique is quantitative and repeatable, and it has previously been used in a number of studies to investigate muscle evolution in other bipedal archosaurs (e.g. Hutchinson et al. 2005, 2008; Bates et al. in press). Muscle dynamics are dictated primarily by their mass, architecture and contractile properties (Alexander, 2003), and moment arms may only provide an incomplete picture of muscle performance, joint forces and habitual gait. However, muscle masses and physiology are not directly measurable in extinct taxa, and these properties can only be broadly estimated (Hutchinson, 2004; Hutchinson et al. 2007; Allen et al. 2009; Bates et al. 2009a,b, 2010). An understanding of muscle force orientations, and the level of uncertainty in their reconstruction, represents an important methodological step prior to more challenging mechanical assessments (Hutchinson et al. 2005). This approach provides a robust foundation upon which more complex, mathematical models of ornithischian locomotion can be based (Hutchinson, 2004; Hutchinson et al. 2007; Sellers & Manning, 2007; Sellers et al. 2009; Bates et al. 2010).
This study addresses three questions crucial to understanding the basic muscular control of locomotion in basal ornithischians.
Do moment arms for specific muscles support the qualitative hypotheses of muscle function proposed by previous workers (Romer, 1927; Galton, 1969; Charig, 1972; Coombs, 1979; Hutchinson et al. 2008; Maidment & Barrett, 2011)?
Basal ornithischians and birds convergently acquired similar pelvic osteology, suggesting that basal ornithischians might have had a bird-like locomotor style. Do moment arms suggest that muscle function in Lesothosaurus was more similar to birds or to basal non-avian dinosaurs?
What are the effects of using alternative myological reconstructions on muscle function?
Materials and methods
Model construction
Pelvic and hind limb bones of the most complete specimen of Lesothosaurus [NHMUK (Natural History Museum, London, UK) RUB 17] were digitized using a high-resolution Polhemus laser scanner. The computer-aided design package Maya (http://www.autodesk.com) was used to digitally rearticulate hind limb bones in a standard neutral posture (see Hutchinson et al. 2005, 2008; Bates et al. in press) and to rig 3D muscle–tendon units and joint centre positions (Fig. 2B). The hip joint was modelled as a 3° of freedom ball-and-socket joint, with the flexion–extension axis perpendicular to the craniocaudal axis of the body (the x-axis in the global co-ordinate system of the model). Axes for the remaining degrees of freedom were defined as mutually orthogonal, so that adduction–abduction was aligned to the global z-axis, and long axis rotation to the global y-axis. Pelvic and femoral muscle attachments were based on Maidment & Barrett (2011; Fig. 2A; see Table 1 for muscle abbreviations used throughout), who reconstructed 14 muscles on the basis of osteological correlates of homologous origins and insertions in extant birds and crocodiles (the EPB approach; Bryant & Russell, 1992; Witmer, 1995). Numerous dissections of extant archosaurs and lepidosaurs carried out by the authors were used to constrain the reconstructions (described in Allen et al. 2009, 2010; Maidment & Barrett, 2011; Bates et al. in press). Intermediate or ‘via points’ (see Sellers et al. 2003) were used to guide 3D muscle paths from origin to insertion, and the location of via points was guided by information from homologous muscles in extant taxa (see discussion in Hutchinson et al. 2005; Bates et al. in press). Analysis of the model was carried out in GaitSym (http://www.animalsimulation.org; Sellers & Manning, 2007). The moment arm of each hip muscle for joint flexion/extension, abduction/adduction and long axis rotation was calculated across a wide spectrum of limb postures, varying both hip flexion–extension and abduction–adduction separately and simultaneously. Data in the graphs throughout the paper show moment arms across different flexion–extension angles, with the hip abducted 10°. Data from other postures are available from KTB.
Table 1.
Abbreviations used for musculature throughout the text.
| Muscle | Abbreviation |
|---|---|
| Adductor femoris 1 & 2 | ADD1&2 |
| Ambiens | AMB |
| Caudofemoralis brevis | CFB |
| Caudofemoralis longus | CFL |
| Flexor tibialis externus | FTE |
| Flexor tibialis internus 1 | FTI1 |
| Flexor tibialis internus 3 | FTI3 |
| Iliofibularis | IFB |
| Iliofemoralis externus | IFE |
| Iliofemoralis | IFM |
| Iliofemoralis, cranial part | IFMa |
| Iliofemoralis, caudal part | IFMp |
| Ischiotrochantericus | ISTR |
| Iliotibialis | ITB |
| Iliotibialis, cranial part | ITBa |
| Iliotibialis, caudal part | ITBp |
| Iliotrochantericus caudalis | ITC |
| Puboischiofemoralis externus 1 | PIFE1 |
| Puboischiofemoralis externus 2 | PIFE2 |
| Puboischiofemoralis internus 1 | PIFI1 |
| Puboischiofemoralis internus 2 | PIFI2 |
Sensitivity analysis
Reconstructing 3D muscle moment arms in extinct taxa inevitably involves a degree of subjectivity; while many muscles have well-constrained origins and insertions in extinct taxa and fairly conservative paths among living archosaurs, others are poorly constrained by osteology (see Hutchinson et al. 2005 for discussion). Maidment & Barrett (2011) used the EPB and information from direct observation of a wide range of basal archosaur and ornithischian taxa to build the least speculative (most parsimonious) reconstruction of the gross hind limb musculature of Lesothosaurus, as previous workers have done for other extinct archosaur taxa (e.g. Dilkes, 2000; Carrano & Hutchinson, 2002; Otero & Vizcaíno, 2008; Schachner et al. 2011; Bates et al. in press). However, questions surrounding the origins, insertions and even the presence/absence of some muscle groups remain. An alternative myological reconstruction of Lesothosaurus was built independently by one of us (VA). This reconstruction relied upon the same methodological principles, but was based more heavily on direct observation of theropod osteology and previous reconstructions of theropod myology (e.g. Hutchinson, 2001a,b; Carrano & Hutchinson, 2002; Hutchinson et al. 2005). In a sense, the model of Maidment & Barrett (2011) is ‘ornithischian-biased’, while the alternative model of VA is ‘theropod-biased’.
As might be expected given that they are based on the same extant comparative data, the two reconstructions are similar in many respects (Figs 2 and 3). However, differences were observed between the two reconstructions: (i) the origins of ADD1&2 on the ischium were both located more proximally in the ‘theropod-biased’ model (Fig. 3A,B); (ii) ISTR inserted more proximally on the lateral femur in the ‘theropod-biased’ model (Fig. 3C); (iii) the via points for ITB were located further laterally in the ‘theropod-biased’ model, suggesting a greater thigh muscle bulk (Fig. 3D); (iv) the origin of PIFE on the ischium was located more proximally in the ‘theropod-biased’ model (Fig. 3E); (v) the insertion of PIFI2 was located more cranially and more proximally on the femur in the ‘theropod-biased’ model (Fig. 3F); and (vi) the origin of PIFI2 was located on the dorsal vertebrae rather than on the medial side of the preacetabular process of the ilium in the ‘theropod-biased’ model (Fig. 3F). Five of these highlighted differences (1, 2, 4–6) relate specifically to difficulties reconstructing and/or defining the centroids of muscle attachments in extinct archosaurs, and particularly ornithischian dinosaurs, which lack any living descendants. Although approximate skeletal landmarks exist for these muscles, our analysis indicates they are sufficiently ambiguous that extra caution must be exercised when attempting quantitative reconstructions such as that presented here. The third difference (the lateral path of ITB) represents a purely subjective representation of the thigh muscle bulk, and thereby relates to a source of uncertainty common to reconstructions of all extinct animals.
Fig. 3.
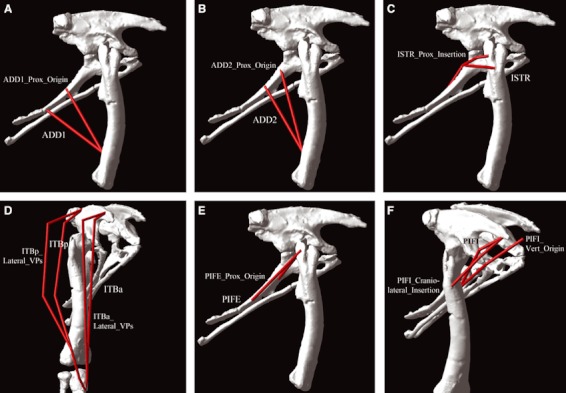
Sensitivity analysis for key muscles with poorly constrained origins, insertions and/or 3D paths in Lesothosaurus and other basal ornithischians. The sensitivity analysis investigated the differences between ‘ornithischian-biased’ and ‘theropod-biased’ reconstructions of the pelvic musculature of Lesothosaurus (see text). Specifically, relative to the original ‘ornithischian-biased’ model, the analysis tested the effect of a more proximal origin for (A) ADD1 and (B) ADD2, (C) a more proximal insertion for ISTR, (D) more lateral via points for ITBa and ITBp, (E) a more proximal origin for PIFE, and (F) a craniolateral insertion and also an origin from the dorsal vertebrae for PIFI2.
In order to examine the effects of this uncertainty on muscle moment arms, we produced two versions of the six contentious muscles in the GaitSym model of Lesothosaurus, thereby allowing direct comparisons between the ‘ornithischian-biased’ and ‘theropod-biased’ reconstructions. A comparison of estimated moment arm magnitudes between these two iterations is presented below.
Comparative analysis
To place our results from Lesothosaurus in a broader context, we compared them with those from similarly constructed models of other bipedal dinosaurs (Hutchinson et al. 2005, 2008; Bates & Schachner, in press; Bates et al. in press). These studies include three models constructed by the authors (Bates et al. in press) using the same procedures employed here for Lesothosaurus: two on non-avian theropod dinosaurs (Allosaurus fragilis, Struthiomimus sedens); and one on the extant ostrich (Struthio camelus). Data gleaned from these taxa include hip flexion–extension, adduction–abduction and long axis rotation moment arms across a wide spectrum of limb postures (Bates & Schachner, in press; Bates et al. in press). Additionally, estimates of hip flexion–extension moment arms for the non-avian theropods Tyrannnosaurus rex and Velociraptor mongoliensis are included for comparison, taken from Hutchinson et al. (2005, 2008). Although subjective worker-bias cannot ultimately be discounted (see Sensitivity analysis, above), the dataset and methodology used by Hutchinson and colleagues are essentially similar to those used here, and so the results should be comparable.
Rather than discuss the relationship between posture and 3D moment arms in every homologous muscle in these models (which would require comparison of over 250 muscles in total), we concentrate instead on gross comparisons and on those aspects that we consider to have the most significant implications for muscular control of the pelvis and hind limb in bipedal archosaurs. Note that summed moment arms were calculated by simply adding together individual muscle moment arms for a given moment polarity at each joint angle, meaning that this sum is unweighted. A sum weighted according to the sizes of the muscles would more accurately reflect the total torque possible about the hip joint, but muscle size is unavailable for fossil taxa (see treatment of limitations in the Discussion below). Also, note that in most graphs, flexion–extension moment arm data are presented at higher density than abduction–adduction and long axis rotation data, as the modelling software used in this study was only capable of sampling a single axis (in this case flexion/extension) at high density without multiple re-runs of the analysis.
Results
Lesothosaurus moment arms
Flexion–extension moment arms
Moment arms for hip flexion and extension varied considerably with joint angle for all 14 muscles in the model (Figs 4 and 5). In general, extensor moment arm magnitudes decreased with increasing hip flexion (e.g. Fig. 4b–d,h), with only ISTR and PIFE experiencing an increase in extensor moment arm magnitude with increasing femoral protraction (Fig. 5c,f). ADD1&2 have the highest extensor moment arms (0.03–0.05 m: Fig. 5a,b) in the model, slightly higher than the muscles of the caudofemorales (CFB, CFL: Fig. 4b,c) and flexor cruris (FTE, FTI3: Fig. 4d,e) groups. The joint angle at which individual muscles experienced their peak extensor moment arm showed substantial variation (Figs 4 and 5). In general extensor muscles originating caudodorsal to the hip joint (e.g. CFB, CFL, FTE, IFB, ITBp) had their peak moment arm at more extended hip angles (Figs 4b–d,f and 5e), while muscles originating caudoventrally (e.g. ADD1&2, FTI3, ISTR, PIFE) peaked at more flexed postures (Figs 4e and 5a–c,f), but peaks covered the full continuum of limb postures tested. Two muscles switched function at different joint angles; IFMp switched from hip flexion to hip extension at −10° hip flexion angle (Fig. 4h), while the moment arm of PIFE showed significant angular variation, having a relatively high extensor moment arm at flexed femoral postures, but an increasingly large flexion moment arm at angles more extended than −23° hip flexion (Fig. 5f, but see below). Most hip flexors had their peak moment arms at flexed joint angles, with the exception of AMB and PIFE (Figs 4a,i and 5d,f). Muscles with the most cranially positioned origins had the largest flexor moment arm magnitudes, notably AMB, ITBa and PIFI2 (Figs 4a and 5d,g).
Fig. 4.
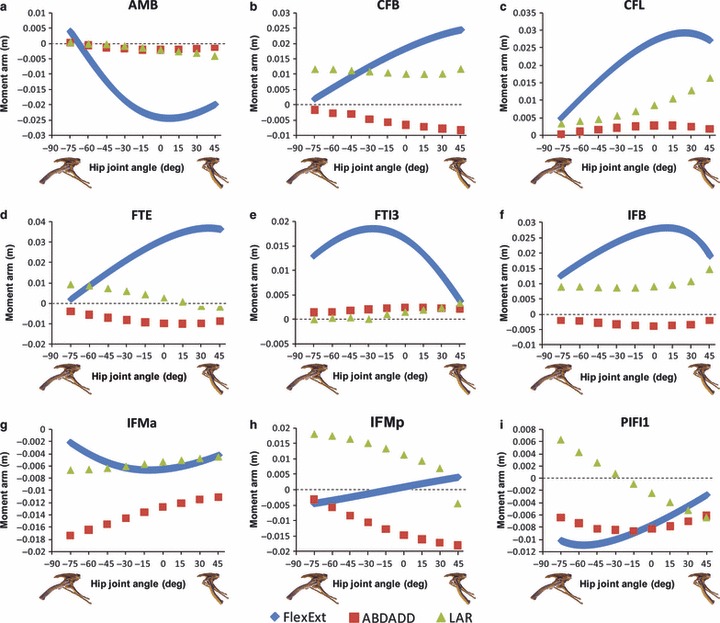
Hip muscle moment arm predictions for (a) AMB, (b) CFB, (c) CFL, (d) FTE, (e) FTI3, (f) IFB, (g) IFMa, (h) IFMp and (i) PIFI1 for a range of hip flexion/extension angles in Lesothosaurus. A positive hip joint angle (x-axis) indicates hip extension (femoral retraction), while a negative hip joint angle indicates hip flexion (femoral protraction), as shown by the small images of the pelvis of Lesothosaurus in the left lateral view along the x-axis of each graph. A negative moment arm (y-axis) for flexion/extension is a moment arm for flexion; a negative moment arm for abduction/adduction is a moment arm for abduction; a negative moment arm for long axis rotation is a moment arm for medial rotation. FlexExt, flexion/extension; ABDADD, abduction/adduction; LAR, long axis rotation.
Fig. 5.
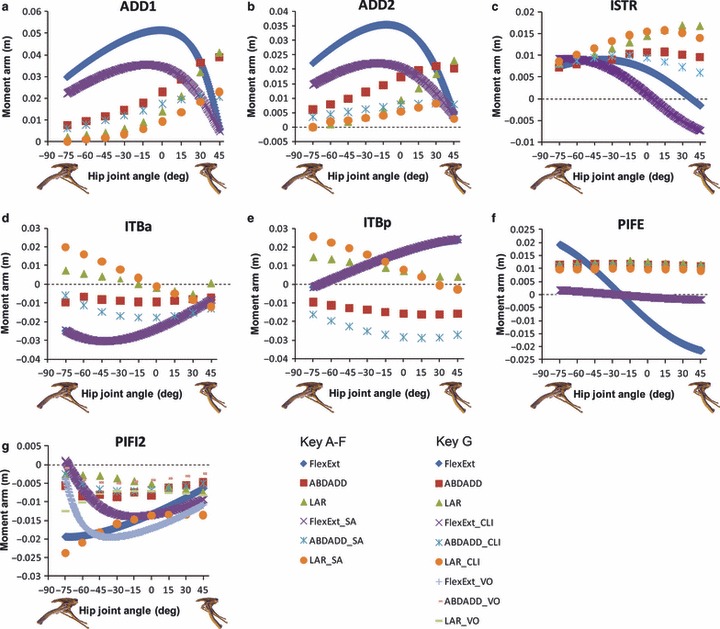
Hip muscle moment arm predictions for (a) ADD1, (b) ADD2, (c) ISTR, (d) ITBa, (e) ITBp, (f) PIFE, (g) PIFI2, the muscles for which the sensitivity analysis was performed, for a range of hip flexion/extension angles in Lesothosaurus. A positive hip joint angle (x-axis) indicates hip extension (femoral retraction), while a negative hip joint angle indicates hip flexion (femoral protraction), as shown by the small images of the pelvis of Lesothosaurus in the left lateral view along the x-axis of each graph. A negative moment arm (y-axis) for flexion/extension is a moment arm for flexion; a negative moment arm for abduction/adduction is a moment arm for abduction; a negative moment arm for long axis rotation is a moment arm for medial rotation. FlexExt, flexion/extension; ABDADD, abduction/adduction; LAR, long axis rotation. SA indicates the results of the sensitivity analysis in a–f; CLI indicates the results using the alternative insertion for PIFI2; VO indicates the results using the alternative origin for PIFI2 in g (see text for details).
Abduction–adduction moment arms
Muscles with origins and paths that are dorsal and lateral to the hip joint centre (CFB, FTE, IFB, IFMa, IFMp, ITBa, ITBp, PIFI1&2) consistently had abduction moment arms (Figs 4b,d,f–i and 5d–e,g), while muscles originating ventral and medial to the hip (ADD1&2, CFL, FTI3, ISTR, PIFE) provided leverage for adduction (Figs 4c,e and 5a–c,f). The adductor femores muscles (ADD1&2) had the largest adduction moment arms (Fig. 5a,b), while abductor muscle moment arms were more uniform (e.g. 0.008–0.017 m) for CFB, FTE, IFMa, IFMp, ITBa, ITBp and PIFI1&2 (Figs 4b,d,g–i and 5d,e,g). Abduction–adduction moment arm magnitudes varied with hip flexion–extension and abduction–adduction joint angles (Figs 4 and 5; Fig. S1 in Supporting Information). Adduction moment arms tended to increase with hip extension and adduction (e.g. ADD1&2 and ISTR; Fig. 5a–c), while abduction moment arms also tended to increase with hip abduction but showed more varied patterns with flexion–extension angle (Figs 4 and 5; moment arms at more abducted and adducted postures not shown, but available on request from KTB). For example, leverage for abduction in IFMa increased with increasing hip flexion (Fig. 4g), while the abduction moment arm of IFMp decreased with increasing hip flexion (Fig. 4h). All muscles retained the same function across the continuum of hip flexion–extension angles tested, but two adductors (ADD1, FTI3) did switch their moment polarity to become weak abductors at adducted (> 10°) and extended femoral postures (moment arms at more abducted and adducted postures not shown, but available on request from KTB).
Femoral long axis rotation moment arms
Three muscles (AMB, IFMa, PIFI2) had predominately medial femoral rotation moment arms, owing to their cranial paths around the hip joint centre (Figs 4a,g and 5d). Lateral rotators (ADD1&2, CFB, CFL, FTE, FTI3, IFB, IFMp, ISTR, ITBp, PIFE; Figs 4b–f,h and 5a–c,e,f) originated caudal to the hip joint and inserted lateral to the hip joint centre. ITBa and PIFI1 were medial rotators at extended hip joint angles, but switched to being lateral rotators at angles more flexed than −15 to 30° (Figs 4i and 5d). Moment arm magnitudes varied with joint angle in seven of the modelled muscles (ADD1&2, CFL, IFMp ISTR, ITBa, PIFI1; Figs 4 and 5). Muscles with origins caudal to the hip joint and lines of action at high angles to the femur in neutral pose (ADD1&2, CFL, ISTR) experienced peak lateral rotation moment arms when the hip was extended, and moments decreased rapidly as the hip was flexed (Figs 4c and 5a–c). This is because the line of action of these muscles is highly oblique to the femur when it is retracted; however, as protraction occurs, the line of action becomes increasingly parallel to the femur, resulting in little leverage for long axis rotation when the hip is flexed and these muscles are lying almost parallel to the femur. PIFI1, ITBa and IFMp (Figs 4h,i and 5d) experienced peak lateral rotation moments when the hip was flexed. Moment arms decreased significantly during hip extension and switched function to medial rotation in ITBa and PIFI1 (Figs 4i and 5d). This results from the line of action of these muscles passing caudal to the centre of rotation for femoral long axis rotation during hip flexion, but as the hip is extended the line of action closely approaches the centre of rotation and, for ITBa and PIFI1, passes cranial to it when the femur is retracted, resulting in a change to medial rotation.
Sensitivity analysis
Altering muscle origins, insertions or 3D paths according to specifically targeted uncertainties in the Lesothosaurus model (see ‘Sensitivity analyses’ in Materials and methods) had at least a modest effect on mechanical leverage in almost every muscle tested (Fig. 5). The smallest effects were observed in ITBa, ITBp and ISTR (Fig. 5c–e), in which relatively minor changes in magnitude occurred. Moving the via points of ITBa and ITBp laterally had no effect on flexion–extension moment arms, but did increase abduction–adduction and long axis rotation leverage at most joint angles (Fig. 5d,e). A more proximal insertion on the femur for ISTR had relatively little effect on adduction and lateral rotation moment arms of this muscle, but did cause a noticeably greater decline in extensor moment at extended postures, such that the muscle switched to exerting a flexor moment at 5° hip flexion (Fig. 5c). More proximal origins for the ADD1&2 had little effect on the lateral rotation moment arms of these muscles, but did considerably decrease leverage for hip extension and femoral adduction (Fig. 5a,b). However, a similar proximal shift in origin for PIFE had no effect on the adduction or lateral rotation leverage of this muscle, but did have a considerable impact on its flexion–extension moment arm (Fig. 5f). The original distal origin chosen for this muscle produced a large change in moment arm with hip joint angle, such that it had a relatively high extensor moment arm at flexed femoral postures (0.002 m at −75°), but an increasingly large flexion moment arm as the hip extended (−0.0022 m at 45°; Fig. 5f). A more proximal origin, which gives PIFE a more horizontal line of action, reduced the change in moment arm magnitude with change in joint angle (hereafter termed the ‘angular dependency’ of the moment arm). This increased angular dependency is demonstrated quantitatively by the greater range in magnitude when PIFE is reconstructed with a more distal origin (0.0042 m with a distal origin, vs. 0.0036 m with more proximal origin). PIFE still switched function from hip extension to flexion at around the same joint angle, and its peak magnitudes occurred at the postural extremes tested, but these magnitudes (peak extensor moment arm 0.0015 m; peak flexor moment arm −0.0019 m) were reduced by 19% and 11%, respectively (Fig. 5f).
Two alternative reconstructions of PIFI2 were tested; a craniolateral insertion (CLI) on the femur and a more craniomedial origin (VO) on the dorsal vertebrae. Both alternative reconstructions resulted in an abrupt and rapid decline in the flexor moment arm at postures more flexed than about −30° (Fig. 5g). This abrupt decline did not occur in the original reconstruction of PIFI2, which has its peak flexor moment arm magnitude at highly flexed joint angles (−0.02 m at −75°; Fig. 5g). Both alternative reconstructions yielded a decrease in the magnitude of the abduction moment arm (e.g. a decrease in the maximum and average values of 18% and 33% in CLI, 43% and 58% in VO). Origination from the dorsal vertebrae caused a slight increase in the medial rotation moment at flexed postures, whereas a craniolateral insertion resulted in a substantial increase in rotational leverage at all joint angles tested (e.g. an increase in the maximum and average values of 217% and 119% in CLI, 67% and 12% in VO; Fig. 5g).
Comparative analysis
Summed hip extensor moment arms in all of the bipedal non-avian dinosaurs modelled varied considerably with joint angle, but tended to decrease when the hip was strongly flexed and extended (Fig. 6a). The summed hip extensor moment arms peaked at a similar hip joint angle in the tetanurans Allosaurus, Tyrannosaurus and Struthiomimus (−15° to −19°), at −25° in the maniraptoran theropod Velociraptor, and at −20° in the ostrich Struthio. Lesothosaurus was the only taxon to peak with the hip slightly extended (6° extension; Fig. 6a). Lesothosaurus consistently had the lowest total hip flexor moment arms (normalized by femoral length) across the full range of postures tested (Fig. 6b). Non-avian theropods consistently had the highest summed flexor moment arms, while the ostrich showed significant angular dependency in moment arm magnitude (Fig. 6b). Again this is evidenced by the greater average change in moment arm per degree of joint flexion–extension (Lesothosaurus 0.003, Allosaurus 0.003, Struthiomimus 0.004, Tyrannosaurus 0.004, Velociraptor 0.006, ostrich 0.008).
Fig. 6.
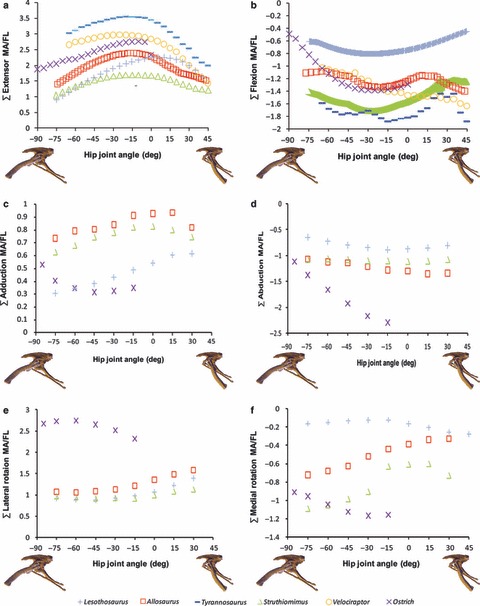
Sum of (a) hip extensor, (b) hip flexor, (c) adduction, (d) abduction, (e) lateral femoral rotation and (f) medial femoral rotation muscle moment arms normalized by segment length for Lesothosaurus and other dinosaurian bipeds (for further comparisons, see Fig. S1 in Supporting Information).
Total summed adductor moment arms decreased slightly with hip flexion (Fig. 6c) and hip adduction (data not shown; available on request from KTB) in all non-avian dinosaurs, while summed abductor moment arms increased slightly with hip extension (Fig. 6d) and hip adduction (data not shown). In the ostrich, summed moment arms for both adduction and abduction decreased slightly with hip flexion (Fig. 6c,d) and hip adduction (data not shown). Lesothosaurus and the ostrich had low summed moment arms for adduction, while those of Struthiomimus and Allosaurus were higher (Fig. 6c). Lesothosaurus had the lowest summed abductor moment arms, the non-avian theropods had intermediate summed abductor moment arms, while the ostrich had the highest values (Fig. 6d).
Summed long axis rotator moment arms varied little with long axis rotation of the femur (data not shown), and displayed a taxonomic signal with hip flexion and extension (Fig. 6e,f). Lesothosaurus had an extremely weak summed medial rotator moment arm (Fig. 6f) compared with those of theropods, while its summed moment arm for lateral rotation (Fig. 6e) was similar to the non-avian theropods in magnitude. The ostrich had significantly higher summed medial and lateral rotator moment arms than any other dinosaur modelled (Fig. 6e,f).
In general, homologous muscles are similar between Lesothosaurus and the other taxa modelled (see Fig. S1 in Supporting Information for graphic comparisons), so we do not explicitly discuss every single muscle-by-muscle comparison. However, Lesothosaurus did differ from the other taxa in the following ways: (i) CFB has an adduction moment arm in the theropods examined, while it has an abduction moment in Lesothosaurus (Fig. 7b); (ii) IFMp (ITC in birds) has a considerably larger moment arm for medial long axis rotation in the ostrich than in non-avian dinosaurs (Fig. 8c); (iii) PIFE has a lateral rotator moment in the ostrich and Lesothosaurus, while it has a medial rotator moment arm in the non-avian theropods (Fig. 9c,f); (iv) PIFE has an extensor moment arm in the ostrich and Lesothosaurus, but a flexor moment arm in other taxa (Fig. 9a,d). The implications of these differences will be discussed below.
Fig. 7.
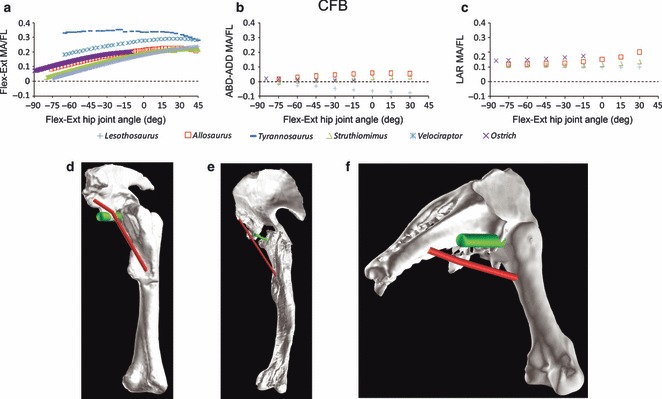
(a) CFB flexion–extension, (b) abduction–adduction and (c) long axis rotation moment arm predictions for Lesothosaurus and other dinosaurian bipeds over a range of hip joint flexion–extension angles. CFB is reconstructed as passing dorsolateral to the joint centre yielding a weak abduction moment arm in (d) Lesothosaurus, while in non-avian theropods like (e) Allosaurus and (f) ostrich it extends caudoventral to the hip joint centre producing a weak adduction moment arm. Green cylinder in d–f shows the abduction–adduction axis running through the hip joint centre. ABD-ADD, abduction/adduction; FL, femoral length; Flex-Ext, flexion/extension; LAR, long axis rotation; MA, moment arm.
Fig. 8.
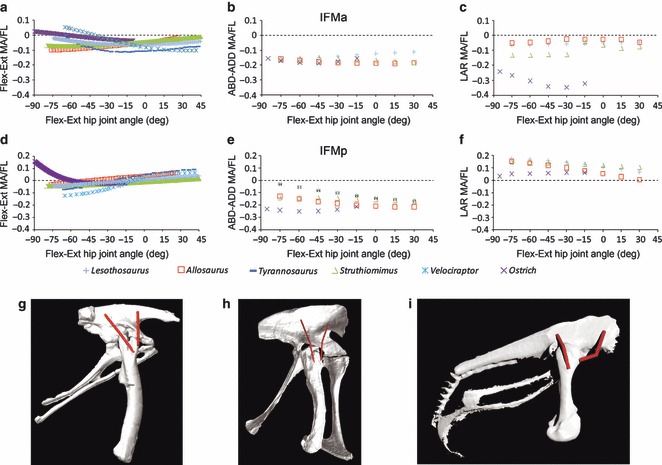
Predicted Iliofemoralis group muscle moment arms for hip (a, d) flexion–extension, (b, e) abduction–adduction and (c, f) long axis rotation in Lesothosaurus and other dinosaurian bipeds over a range of hip joint flexion–extension angles. The cranial part of iliofemoralis approximately corresponds to the avian iliotrochantericus caudalis; the caudal part of iliofemoralis approximately corresponds with the avian iliofemoralis externus. Pelvis, femur and IFM in right lateral view in (g) Lesothosaurus, (h) Allosaurus and (i) Struthio (not to scale). IFMa is located much farther cranial to the joint centre in the ostrich (i), causing higher medial rotation moment arms. ABD-ADD; abduction/adduction; FL, femoral length; Flex-Ext, flexion/extension; LAR, long axis rotation; MA, moment arm.
Fig. 9.
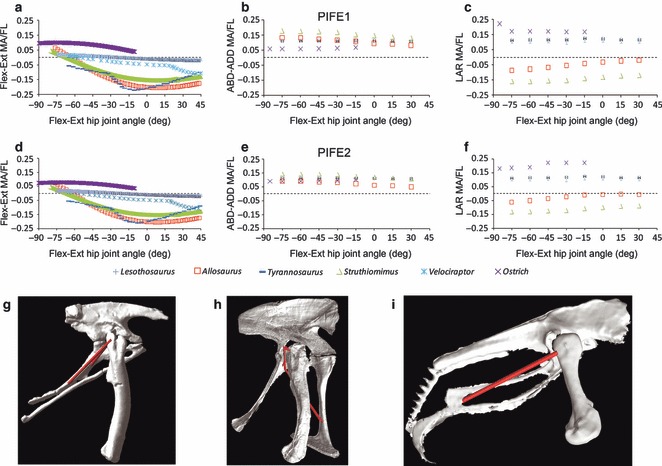
Predicted pubioischiofemoralis externus group muscle moment arms for hip (a, d) flexion–extension, (b, e) abduction–adduction and (c, f) long axis rotation in Lesothosaurus and other dinosaurian bipeds over a range of hip joint flexion–extension angles. (a–c) PIFI1; (d–f) PIFI2. Pelvis, femur and PIFI in right lateral view in (g) Lesothosaurus, (h) Allosaurus and (i) Struthio (not to scale). PIFI is located cranial to the joint centre in Allosaurus, but caudal to it in Lesothosaurus and ostrich due to retroversion of the pubis. ABD-ADD, abduction/adduction; FL, femoral length; Flex-Ext, flexion/extension; LAR, long axis rotation; MA, moment arm.
Discussion
Muscle moment arms in Lesothosaurus
During locomotion, muscle activation patterns routinely coincide or overlap to produce the desired 3D control of the limb (e.g. Gatesy & Dial, 1993, 1996; Gatesy, 1997, 1999). Individual muscle function therefore varies during the step cycle, and examination of the moment arm of a single muscle, independent of other variables and the muscles with which it may interact, does not provide full details of its function. Muscle moment arm magnitude also interacts with other muscle properties in ways not accounted for in this study. While a relatively large moment arm allows a relatively small muscle to exert a relatively large joint moment, it also reduces the ability of the muscle to accelerate the joint (and hence to move the limbs quickly). Similarly, relatively smaller moment arms are not always a signature of reduced muscle size or functional importance, as there is good evidence for muscles with small moment arms playing important roles in limb movement, particularly those responsible for producing higher joint velocities (Sacks & Roy, 1982; Alexander & Ker, 1990; Payne et al. 2006a,b; Smith et al. 2006, 2007; Allen et al. 2010). The dramatically larger moment arm of ADD1 vs. that of CFL (the latter likely to be much more massive muscle; e.g. Gatesy, 1990, 1995; Bates et al. in press; Hutchinson et al. in press) for hip extension is a good example of this (see Fig. 5).
Thus, the 3D moment arm estimates produced herein should not be interpreted as a direct indicator of specific muscle function during locomotion. However, they can indicate whether a specific muscle had the ability to actuate a joint motion (e.g. to effect joint extension, adduction, etc.), and so can be used to directly test general hypotheses of muscle function (e.g. ‘muscle X acts to extend the hip’). Also, because our moment arm estimates can indicate the ease with which a muscle may apply moments about a particular joint by comparing relative moment arm magnitude, they also have a bearing on hypotheses of the relative function of different muscles in the same broad functional category (e.g. ‘muscle X is a more important extensor of the hip than muscle Y’), with the same caveats as above. As such, our estimates provide the first quantitative, repeatable dataset against which the hypotheses of muscle function suggested by workers such as Romer (1927) and Maidment & Barrett (2011) may be tested.
Previous authors (Romer, 1927; Galton, 1969; Coombs, 1979; Norman, 1986; Maidment & Barrett, 2011) have suggested that musculature originating on the elongate postacetabular iliac process (e.g. CFB, FTE) would have functioned to retract the femur, extending the hip. This assertion is strongly supported by our Lesothosaurus model, in which the muscles of the caudofemoralis (CFB, CFL), flexor cruris (FTE, FTI3), the iliofibularis (IFB) and the caudal part of the triceps femoris (ITBp) have the highest leverage for hip extension, particularly when the hip is extended. Similarly, these authors variously hypothesized that musculature originating cranial to the centre of rotation for flexion–extension, such as those on the elongate preacetabular process of the ilium (ITBa) and the prepubis (AMB) would have had large moment arms for hip flexion (femoral protraction), and our data support this, with ITBa and AMB having high moment arms for hip flexion, particularly when the hip is flexed in the case of the former, and extended in the case of the latter.
Confusion over the homology of various parts of PIFI (Walker, 1977; Rowe, 1986) and debate over their origins and insertions (e.g. Romer, 1927; Galton, 1969) has meant that there has been little agreement about the likely function of this muscle complex in ornithischians. Maidment & Barrett (2011) proposed that it was the predominant femoral protractor (in agreement with in vivo data on the function of PIFI and its homologues in extant archosaurs: Gatesy, 1997, 1999), and this is supported by our ‘ornithischian-biased’ reconstruction. However, uncertainty in both the origin and insertion of PIFI2 results in a degree of functional ambiguity about its relative moment arms (Fig. 5g). The ‘ornithischian-biased’ model predicts PIFI2 had highest leverage for femoral protraction (hip flexion) except when the hip was fully flexed (Fig. 5g). In the ‘theropod-biased’ model, an alternative origin located on the lateral surfaces of the dorsal vertebrae also results in the highest moment arm of PIFI2 being hip flexion. However, the use of an alternative insertion on the cranial femur suggests that PIFI2 had greater muscle leverage for medial rotation, except when the femur was held relatively vertically, at which point its flexion and medial rotation moment arms are both reduced but approximately equal. This iteration does not necessarily preclude a primary flexor function, but indicates that flexor moments exerted by the PIFI during locomotion may be accompanied by medial rotation moments, which would need to be incorporated into locomotion or resisted by the action of other muscles.
Romer (1927) and Maidment & Barrett (2011) suggested that ADD1&2 were primarily femoral adductors and retractors, as has also been suggested for theropod dinosaurs (Hutchinson & Gatesy, 2000; Hutchinson et al. 2005; Bates & Schachner, in press; Bates et al. in press). Our results suggest that ADD1&2 only had large moment arms for adduction at extended joint angles. The largest moment arms of ADD1&2 were for hip extension (femoral retraction) in Lesothosaurus at all joint angles studied, except high angles of hip extension when extension moment arms became relatively small and were exceeded by those for adduction and lateral rotation (Fig. 5a,b).
Maidment & Barrett (2011) reconstructed the iliofemoral musculature as a ‘complex’ referred to as IFM because they could not identify osteological correlates to distinguish between the origin of the two distinct heads present in extant birds (ITC and IFE). These authors suggested that IFM would function primarily as an abductor in bipedal ornithischians. Romer (1927) and Galton (1969), who reconstructed both avian muscles, suggested IFE ‘stabilized’ the femoral head, preventing disarticulation of the hip, while Norman (1986) suggested that IFE was a femoral protractor in Mantellisaurus. We modelled a cranial head of IFM, (IFMa) and a caudal head (IFMp) to encompass the full extent of this muscle complex on the ilium, and these roughly correspond with ITC and IFE, respectively, in the theropod reconstructions. Both IFMa and IFMp have their highest moment arm magnitudes for femoral abduction, although IFMp also had relatively high hip flexion leverage at flexed hip joint angles (Fig. 4g,h).
Maidment & Barrett (2011) suggested that PIFE would have primarily had leverage for lateral femoral rotation, while Romer (1927) and Galton (1969) both suggested that PIFE may have been a femoral retractor. Our results highlighted ambiguity in the relative moment arm magnitudes of PIFE (Fig. 5f). The ‘ornithischian-biased’ model suggested that PIFE was a protractor at extended hip angles and a retractor at flexed hip angles (having a relatively high flexion–extension moment arms, but high degree of angular dependency; Fig. 5f), but maintained relatively high adduction and lateral rotation moment arms across this range of postures. However, the ‘theropod-biased’ model suggested that PIFE would have had a very small moment arm for flexion–extension regardless of hip joint angle (hence reduced angular dependency), and instead would have predominantly functioned to adduct and laterally rotate the femur.
Maidment & Barrett (2011) proposed that ISTR adducted and laterally rotated the femur. Our data suggest that ISTR primarily had leverage for lateral femoral rotation in Lesothosaurus, although at highly flexed joint angles it also had a relatively high moment arm for hip extension (Fig. 5c).
Comparative analysis
Moment arm polarities and magnitudes in Lesothosaurus and the other taxa modelled are very similar in many respects. This is perhaps to be expected because they also share many homologous myological and osteological features related to their shared ancestry, as well as functional constraints as obligate bipeds. Taxa compared in this study, and indeed bipedal archosaurs in general, are characterized by at least moderate post- and preacetabular expansion of their pelvic elements relative to the plesiomorphic archosaurian condition, as broadly represented by extant Alligator (Hutchinson & Gatesy, 2000). Thus, many pelvic muscle origins are shifted craniad and caudad relative to the hip joint centre, and their highest moment arms are for hip flexion and extension, as they are in Lesothosaurus (Figs 4 and 5; Fig. S1 in Supporting Information). In all of the taxa modelled, the ilium is dorsoventrally deep dorsal to the acetabulum, and musculature originating here (such as the muscles of the IFM complex) originates medial to the hip joint centre and extends over the hip to insert ventrolateral to it, abducting the femur. Likewise, in all taxa, the ischium extends caudoventrally from the acetabulum, so that musculature originating on it (e.g. ADD1&2; ISTR) extends cranially to insertions on the femur, and therefore acts to extend the hip.
Although the majority of comparisons between the taxa modelled revealed few qualitative differences in moment arms (and therefore provide no indications of differential muscle function), several key differences were observed, which can be related to previously observed trends in osteology and hypotheses of archosaur locomotor evolution. Pelvic osteology in Lesothosaurus and birds is convergent, with independent acquisition of the retroverted pubis and elongate preacetabular process (Fig. 1). It has been suggested, based on observations of gross morphological similarity, that the muscles associated with retroverted pelvic elements in basal ornithischians may have functioned more similarly to those of non-avian maniraptoran theropods and extant birds than those of other archosaurs (Romer, 1927; Hutchinson et al. 2008). If this is the case, moment arms estimated by the Lesothosaurus model would be expected to be more similar to the Velociraptor and ostrich models than to those of other non-avian archosaurs with cranially orientated pubis. Alternatively, the hypothesis that the pelvic elements of basal ornithischians and extant birds functioned similarly can be rejected if our quantitative approach suggests that the musculature of Lesothosaurus had mechanical leverages that differed from Velociraptor and ostrich.
Our results suggest that locomotor muscle leverage in Lesothosaurus (and by inference basal ornithischians in general; Maidment & Barrett, 2011) has a number of important distinctions from the ostrich, and generally shows greater similarity to that of other non-avian dinosaurs in our analysis, contradicting hypotheses of ornithischian–maniraptoran functional convergence. Below, we discuss the reasons for differences in the leverage of specific muscles in the taxa examined, as well as gross differences in the architecture of the hip musculature.
CFB is an abductor in Lesothosaurus, but an adductor in theropods
CFB originates on the caudoventral margin of the ilium in all taxa examined, and a robust osteological correlate for this muscle is present in dinosaurs (the brevis fossa: Hutchinson, 2001a). Although CFB has the greatest leverage for hip extension in all of the taxa examined (Fig. 7), it has a weak moment arm for abduction in Lesothosaurus, but a weak moment arm for adduction in non-avian theropods and the ostrich (Fig. 7). These differences arise from the location of the brevis shelf relative to the acetabulum: in Lesothosaurus the brevis shelf is dorsal to and level with the acetabulum (Fig. 7b), so that CFB passes dorsal to the hip joint. In contrast, in theropods (Fig. 7d), the brevis shelf and its equivalent surface on the ostrich ilium (Fig. 7e) is located more ventrally and more medially, so that CFB extends ventral to the centre of rotation. Thus, although the brevis shelf is present in basal ornithischians and theropods, the musculature extending from it appears to function differently in the two groups.
IFMa (ITC) has a considerably larger moment for medial rotation in the ostrich than non-avian dinosaurs
The posterior head of the IF group musculature (IFMp in Lesothosaurus, IFE in other taxa) has a similar moment arm (Fig. 8d–f), consistent with its conservative path relative to the hip joint (Fig. 8g–i). However, in the ostrich, the anterior head of the IF group (IFMa in Lesothosaurus, ITC in other taxa) has much higher leverage for medial femoral rotation relative to non-avian dinosaurs (Fig. 8c). In non-avian taxa the abduction moment arm of IFMa (ITC) is higher than its medial rotation moment arm, the converse of the situation in the ostrich (Fig. 8b,c). The origin of the avian homologue of IFMa (ITC) on the ilium is much more cranial in birds than in non-avian dinosaurs because of the development of the elongate preacetabular process, on which it originates (Hutchinson, 2001a). Although basal ornithischians convergently developed an elongate preacetabular process, there is no indication of muscle scarring on the lateral surface and no suggestion that IFM migrated onto this area, and it appears that this muscle retained its primitive position on the lateral surface of the ilium directly above the acetabulum (Maidment & Barrett, 2011; Figs 2 and 8).
PIFE is a lateral rotator and extensor in birds and Lesothosaurus, but a medial rotator and flexor in non-avian theropods
PIFE1&2 originate on the pubis in theropods (Hutchinson, 2001a); in birds retroversion of the pubis, and close approximation of the pubes and ischia has resulted in their homologues, the obturators, originating on the puboischiadic membrane (Hutchinson, 2001a; Gangl et al. 2004). In Lesothosaurus and other ornithischians, the postpubis, the homologue of the pubis, is extremely thin and delicate and it is unlikely that it could have supported musculature of practical size. Maidment & Barrett (2011) suggested that, if present and an active role in locomotion maintained, PIFE would probably have migrated onto the much more robust ischium following pubic rotation. The sensitivity analysis performed here suggests that ambiguity in the precise reconstruction of the anatomy of PIFE affects the degree to which this muscle is estimated to have been able to flex and extend the hip in Lesothosaurus, (Fig. 5f). However, the differences in magnitude of the ‘ornithischian-biased’ model vs. the ‘theropod-biased’ model are extremely small when compared with the range of magnitudes observed in the other taxa (Fig. 9a): PIFE had a very weak moment arm for flexion/extension in Lesothosaurus when compared with all other taxa, regardless of the myological reconstruction used.
Hutchinson et al. (2008) modelled the flexion/extension moment arm in the dromaeosaurid theropod Velociraptor (Fig. 9), and demonstrated that PIFE maintained a moment arm for hip flexion despite retroversion of the pubis. PIFE inserts on the greater trochanter in theropods, which projects slightly higher than the femoral head in Velociraptor (Hutchinson et al. 2008, fig. 3.2D), meaning that it inserts dorsal to the hip centre of rotation resulting in a weak flexor moment about the hip. In our ostrich and Lesothosaurus models, we also reconstruct PIFE inserting on the greater trochanter and its avian homologue, the trochanteric crest (Hutchinson, 2001b), but our insertions are located level with or slightly ventral to the joint centre (Fig. 9), and as a result PIFE extends the hip. This demonstrates how very slight repositioning of musculature that inserts close to the joint centre can result in changes in moment arm polarity, and highlights an area where caution is required when making functional interpretations.
Our comparisons with other taxa indicate that PIFE has a much weaker moment arm for flexion/extension in all three taxa with retroverted pubes, and the primary function of the muscle likely switched to adduction and lateral rotation (Fig. 9). Hutchinson & Gatesy (2000) hypothesized that the medial inflection of the femoral head in tetanuran theropods would have increased the lateral rotation moment arms of PIFE1&2 by shifting their insertion on the greater trochanter laterally. The implication of this hypothesis is that PIFE1&2 had functionally attained their derived neornithine condition in basal tetanuran theropods, although Hutchinson & Gatesy (2000) noted that with cranial origins maintained (i.e. non-retroverted pubes), some femoral protraction would have remained. Our results contradict this hypothesis and suggest that PIFE1&2 maintained a medial rotation moment arm in tetanuran theropods, despite medial inflection of the femoral head (Fig. 9). The hip joint centre in our models is assumed to be at the centre of a spheroid fitted around the most ‘ball-shaped’ (medial most) part of the femoral head. This places the hip medially offset from the femoral shaft and greater trochanter, resulting in PIFE1&2 passing lateral to the hip joint, and thereby producing medial, rather than lateral, rotation. Given this geometry, our models indicate that it would have been impossible for PIFE1&2 to induce lateral rotation while their pubic origin remained cranial to the hip joint centre. We therefore suggest that the derived neornithine lateral rotation function of PIFE1&2 did not evolve until pubic retroversion had occurred in maniraptoran theropods, rather than being present in basal tetanurans as postulated by Hutchinson & Gatesy (2000).
However, this hypothesis rests upon the assumption of a geometrically discrete ball-and-socket joint at the hip. The more open acetabulum of basal tetanurans and the presence of accessory articulations, such as the iliac antitrochanter (Gauthier, 1986; Sereno, 1991; Novas, 1996; Carrano, 2000; Hutchinson, 2001a,b), which are not accounted for in our models, may have allowed the long axis of the femur to rotate about an axis placed lateral to the geometric centre of the more spherical area of the femoral head. This in turn could reduce or remove hip long axis rotation moment arms from a cranially positioned PIFE1&2, leaving a moment arm for protraction. It is also possible that accessory articulations may have limited rotation, thereby negating any long axis rotation moments provided by this muscle group, but this remains speculative and untested. However, with a lack of comparative data from which to build robust, quantitative reconstructions of more complex hip joints, our hypothesis that PIFE1&2 caused medial, rather than lateral, femoral long axis rotation seems less speculative.
Summed (gross) extensor moment arms
Extant birds stand and move with ‘flexed’ postures (i.e. with a strongly cranially inclined femur; Gatesy, 1990, 1995, 1999; Rubenson et al. 2007). An increasingly well-supported hypothesis states that this flexed posture was acquired relatively gradually along the maniraptoran theropod lineage leading to modern birds (Gatesy, 1990, Hutchinson & Gatesy, 2000; Carrano, 1998, 2001). The available moment arm data for non-avian dinosaurs strongly suggest that hip extensor muscle moment arms are reduced as the hip becomes increasingly flexed (Figs 4–9; Fig. S1; Hutchinson et al. 2005, 2008; Bates et al. in press; Bates & Schachner, in press), thereby decreasing muscular capacity to support the hip in these postures. Various osteological and myological changes occurred during avian evolution that may have plausibly acted to alleviate this decrease in the magnitude of hip extensor moment arm (i.e. the angular dependency of the moment arm) in flexed postures.
Caudal elongation of the pelvis (Fig. S3 in Supporting Information), pubic retroversion and a reduction in femoral length relative to that of the ilium (Fig. S3 in Supporting Information) represent geometrical changes that potentially helped extant birds to reduce the strong angular dependency of hip extensor moment arms seen in more basal archosaurs (Fig. 6a). This geometric effect is demonstrated by our sensitivity analysis on the origins of muscles with pubic and/or ischial origins (i.e. ADD1&2, PIFE) in Lesothosaurus, which indicates that a more horizontal line of action reduces the angular dependency of hip extension moment arms through more horizontal muscle orientations; for example, a more proximal origin for PIFE, which produces a more horizontal line of action, reduced the angular dependency of its moment arm (Fig. 5f; note in this case by significantly reducing its flexion–extension moment arm due to the proximity of PIFE insertion to the hip joint). This effect may be important in ornithischian limb evolution; the presence of an elongate postacetabular process is a synapomorphy of Neornithischia (Butler et al. 2008), so it might be expected that the angular dependency of extensor muscles will be decreased in neornithischians relative to Lesothosaurus.
However, our data show that the peak in summed extensor moment arms is conservative along the theropod lineage leading to birds (Fig. 6a), although Lesothosaurus peaked at more extended postures. The models presented here also produce no evidence that the gross hip extensor moment of the ostrich is less angular dependant (i.e. no lesser decline with increasing hip flexion) than those of non-avian dinosaurs, with the relative average change in moment arm per degree of joint flexion–extension supporting conservatism (Lesothosaurus 0.011, Allosaurus 0.008, Struthiomimus 0.006, Tyrannosaurus 0.015, Velociraptor 0.015, ostrich 0.009). This suggests that overall gross pelvic geometry was not modified to reduce the overall angular dependency of hip extensor moment arms during avian evolution. This hypothesis should be tested further with additional models of theropod taxa, particularly those representing portions of the avian lineage not sampled by our analysis (e.g. basal Dinosauriformes, other non-avian coelurosaurs).
Summed (gross) flexion moment arms
The key hip flexors (AMB and ITBa; see Fig. S1 in Supporting Information) experience a large increase in their moment arm as the femur is held more vertically in all taxa in the sample, indicating strong angular dependency due to their almost vertical line of action from the pelvis to the knee. These muscles have by far the highest range in magnitudes (AMB 0.028 m and ITBa 0.022 m, vs. IFMa 0.005 m, IFMp 0.008 m, PIFE 0.004 m, PIFI1 0.013 m, PIFI2 0.008 m), confirming the relative postural trends visually apparent in the graphs (Fig. S1). Lesothosaurus has a consistently lower summed flexion moment arm than non-avian theropods, but is broadly similar to the ostrich at flexed hip joint angles (Fig. 6b). At more extended postures, the summed flexion moment arms in the ostrich increase significantly, reaching magnitudes similar to the non-avian theropods (Fig. 6b). Low summed flexion moment arms in Lesothosaurus relative to other dinosaurs are due to the loss of a hip flexor (PIFE) following pubic retroversion (see discussion of PIFE function above). This is further supported by a plot of the average summed flexor moment arms (Fig. S2 in Supporting Information), in which Lesothosaurus is much more similar to non-avian theropods, suggesting its lower total summed moment arms result from the non-avian theropods having a greater number of flexors, and not individual flexors with relatively greater leverage.
Summed (gross) adduction moment arms
Summed moment arms for adduction indicate further differences between Lesothosaurus, non-avian theropods and the ostrich (Fig. 6c,d). Lesothosaurus has low moment arms for adduction across all joint angles relative to non-avian dinosaurs, while the adductors of the ostrich display stronger angular dependency (Fig. 6d; average change in moment arm per degree of hip flexion–extension; Lesothosaurus 0.0026, Allosaurus 0.0019, Struthiomimus 0.0019, ostrich 0.0031). Examination of adductor muscles in Lesothosaurus on a muscle-by-muscle basis suggests that the key difference between it and theropods relates to the total number of adductor muscles in each. CFB is an abductor in Lesothosaurus, but an adductor in theropods (see above), while the theropods in the study are reconstructed with three PIFE muscles (see Hutchinson, 2001a,b; Carrano & Hutchinson, 2002; Hutchinson et al. 2005; Bates et al. in press). Retroversion of the pubis in Lesothosaurus coincided with reduction of PIFE (Maidment & Barrett, 2011), and only one part of PIFE is reconstructed here. This means that Lesothosaurus has three fewer adductors than theropods, and consequently a lower total adduction moment arm.
The extremely low adductor moment arms observed in the ostrich at all but the most flexed joint angles are due to loss of the ischial symphysis and the caudolateral expansion of the pelvis relative to other taxa in the study, which moved muscle origins close to, and in some cases lateral to, the hip joint, switching their function to hip abduction as the hip is extended (e.g. IFB, ADD1&2, FTI3; see Fig. S1 in Supporting Information).
Summed (gross) abduction moment arms
Lesothosaurus has the lowest summed moment arms for abduction in any of the taxa sampled (Fig. 6c). Examination of abduction moment arms on a muscle-by-muscle basis in Lesothosaurus indicates that muscles of the IFM complex are responsible for low abductor moment arms: IFMa is a weaker abductor at extended joint angles than in other dinosaurs, while IFMp is a weaker abductor at flexed joint angles than in other dinosaurs (Fig. 8).
The dinosaur taxa modelled in this study share an enlarged, barrel-like femoral head and neck that laterally offsets the proximal femur and its associated muscle insertions from the mediolateral plane of the hip joint and pelvic muscle origins. The origin of IFMp on the lateral surface of the ilium is noticeably more caudally positioned in Lesothosaurus than in any of non-avian theropods modelled here or by Hutchinson et al. (2005, 2008). In Lesothosaurus the origin of IFMp is situated some distance caudal to the acetabulum, while in non-avian theropods it lies either directly in-line with (i.e. dorsal to) or only slightly caudal to the hip joint (Fig. 8; see also Hutchinson et al. 2005, 2008). From the more caudally positioned origin in Lesothosaurus, the reconstructed muscle path wraps laterally over the femur across the greater trochanter in a more craniocaudal orientation when the femur is held vertically (in contrast to a more dorsoventral orientation over the lesser trochanter in non-avian theropods). As a result, IFMp has a relatively lower moment arm for abduction but slightly greater leverage for lateral long axis rotation than the non-avian theropod models (Fig. 8).
While pelvic and femoral anatomy (e.g. iliac depth, femoral head size, height of the femoral trochanters) might have genuinely contributed to differences in IFMa moment arm magnitudes, it is equally likely that the subjectivity inherent in muscle reconstructions is also playing some role in generating these results. In non-avian theropods the cranial head of the IF group (ITC) inserted onto the lesser trochanter, which is homologous with the anterior portion of the trochanteric shelf (Hutchinson, 2001b). The centroid of ITC insertion in the non-avian theropod models was placed on the lateral surface, while in Lesothosaurus the centroid was located on the medial surface, which would have had a small impact on the abduction moment arm magnitude. Manipulation of these models also leads us to believe that greater uncertainty (i.e. subjectivity) exists in the path of IFE and ITC in non-avian theropods owing to the relatively dorsoventrally deeper ilia in these taxa than in Lesothosaurus (KTB, personal observation). Sensitivity analysis of ITC paths in the non-avian theropod models, combined with a shift of IFMa insertion to the lateral surface of the lesser trochanter in Lesothosaurus, would likely account for the relatively higher abduction moment arm magnitude for IFMa in non-avian theropods.
Summed (gross) long axis rotation moment arms
The summed moment arms for lateral rotation in Lesothosaurus are similar to those of other dinosaurs (Fig. 6f), because they all share a barrel-like femoral head and neck that laterally offsets the proximal femur and its associated muscle insertions from the mediolateral plane of the hip joint and pelvic muscle origins, providing leverage for long axis rotation.
However, Lesothosaurus has lower summed moment arms for medial rotation than any other taxon (Fig. 6e). Examination of the moment arms of individual muscles for long axis rotation shows that PIFE, a medial rotator in non-avian theropods, functions as a lateral rotator in Lesothosaurus and the ostrich. PIFE originates on the cranioventrally directed pubis in theropods, inserting on the greater trochanter (Hutchinson, 2001b) lateral to the joint centre for long axis rotation, and therefore has a moment arm for medial long axis rotation. Retroversion of the pubis in ornithischians and birds (Fig. 1) results in a caudoventrally directed pubis, with PIFE originating on the ischium or puboischiadic membrane, respectively (Maidment & Barrett, 2011). PIFE therefore has a moment arm for lateral long axis rotation in these taxa, and is largely responsible for the reduction in the summed moment arms for medial rotation in Lesothosaurus.
PIFI1 is also a weaker medial rotator in Lesothosaurus than it is in other taxa (see Fig. S1 in Supporting Information). This difference appears to be due to the exact location of the insertion of this muscle on the femur: it inserts very close to the centre of rotation for long axis rotation, so very small changes in insertion can result in polarity differences in moment arms. This again emphasizes the sensitivity of our models to very small changes in soft tissue reconstruction for muscles that insert very close to centres of rotation.
The highest moment arms for medial rotation are observed in the ostrich (Fig. 6e). This is due to the development of the elongate preacetabular process of the ilium and the migration of the IFMa (ITC) and PIFI2 homologues onto it. These muscles, which have large abduction and flexion moment arms in non-avian dinosaurs (see Fig. S1 in Supporting Information) are therefore cranially offset in birds and orientated more perpendicular with respect to the long axis of the femur, resulting in large moments for medial rotation (Hutchinson & Gatesy, 2000).
Functional implications
Hutchinson & Gatesy (2000) conceptualized the functional evolution of the avian pelvis and hind limb from a quadrupedal archosaur common ancestor in a series of five incremental functional conditions. They stated that basal, bipedal dinosaurs, placing their standing foot medial to the hip joint, counteracted the adduction moment generated about the hip by the ground reaction force through an abduction moment generated by activation of the IF group musculature. During the subsequent evolution of theropods (including the origin of birds), abduction by IF was de-emphasized in favour of medial rotation, which also counteracts adduction in extant birds by outward rotation of the foot against the substrate when the femur is held sub-horizontally (Gatesy, 1999; Hutchinson & Gatesy, 2000). Medial rotation of the femora will only abduct the lower limb at flexed postures, suggesting that changes in medio-lateral control of the hip were synchronized with reduced habitual motion of the hip and evolution of the knee-based system of limb retraction (Gatesy, 1990, 1995; Hutchinson & Gatesy, 2000). The gradual adoption of flexed femoral postures and knee-based limb retraction are hypothesized to be reflected in the gradual changes in limb segment proportions (particularly reduced femoral length; Gatesy and Middleton, 1997; Carrano, 1998), and reduction in the size of tail-based hip extensors like the CFL (Gatesy, 1990, 1995) and overall tail length, the latter indicative of cranial migration of the centre of mass (Gatesy, 1995; Christiansen & Bonde, 2002). Although these changes occurred in an incremental or step-wise pattern in bird-line theropods, the most significant changes underpinning the evolution of this rotation-based system of medio-lateral support and knee-based system of limb retraction probably occurred in maniraptoran theropods (e.g. pubic retroversion, elongation of the preacetabular process; Hutchinson, 2001a,b; Hutchinson & Gatesy, 2000).
Our analysis of pelvic muscle moment arms in Lesothosaurus indicates that it has many of the features characteristic of the basal dinosaur condition as described qualitatively by Hutchinson & Gatesy (2000). The IF group, including its anterior head (IFMa, corresponding functionally with ITC of birds: Hutchinson & Gatesy, 2000) has highest leverage for abduction (Fig. 4g), in contrast with medial rotation in the ostrich, which resulted from cranial migration of the origins of this muscle group on the ilia (Fig. 8). The non-avian theropod models discussed herein are similar to Lesothosaurus in this respect, supporting Hutchinson & Gatesy's (2000) hypothesis of an abduction-based mode of lateral limb support in more basal taxa, as well as their suggestion that the medial rotation mechanism seen in extant birds may be related to cranial migration of the IF group during avian evolution (Bates & Schachner, in press).
All of these features suggest that Lesothosaurus is functionally more representative of a the condition qualitatively inferred for basal dinosaurs by Hutchinson & Gatesy (2000) in terms of pelvic muscle moment arms, despite the apparent convergence in basal ornithischian and avian pelvic osteology. Furthermore, although retroversion of the pubis in both birds and basal ornithischians would have resulted in similar reorganization of PIFE musculature, this appears to have had different effects on joint moments in basal ornithischians and birds. In Lesothosaurus, retroversion of the pubis led to lower overall flexor, adductor and medial rotator moment arms as PIFE leverage for these functions was reduced or lost. Instead, the PIFE of Lesothosaurus had leverage for adduction and lateral rotation but not for hip extension (Figs 4 and 9). In birds, PIFE also lost its leverage for flexion and medial rotation, and also offers considerable leverage for hip extension, in addition to adduction and lateral rotation (Fig. 9). The resulting reduction in summed moment arms for flexion, adduction and medial rotation seen in Lesothosaurus is not observed in the ostrich model because the cranial migration of ITC led to increased flexion and medial rotation leverage in this muscle (Hutchinson & Gatesy, 2000). Assessing PIFE function in basal ornithischians is therefore somewhat difficult. In Alligator, PIFE1&2 are active during swing to protract the femur, but also adduct the whole limb in late swing, returning the femur to a near-sagittal orientation for the next stance phase (Gatesy, 1997; Hutchinson & Gatesy, 2000). In birds, the homologues of PIFE1&2 (OL and OM, Table 1) are also active during swing, again adducting the lower limb but through lateral rotation of the femur (Gatesy, 1999; Hutchinson & Gatesy, 2000). With little or no moment arm for hip extension, it seems most parsimonious to infer that swing phase activation of the PIFE group was maintained in basal bipedal ornithischians and that this group continued to play a role in controlling swing phase abduction–adduction.
These new data therefore provide additional quantitative support to more qualitative anatomical traits that suggest the habitual gaits of basal, bipedal ornithischians were quite distinct from those of extant birds. Basal ornithischians retained long muscular tails seen in other basal dinosaur groups, housing large femoral retractor musculature (Maidment & Barrett, 2011). Femora also remained relatively long and gracile compared with the short, robustly proportioned avian femur, thought to be adapted for the high bending and torsional stresses incurred under a flexed ‘avian-like’ posture and a rotational-based system of muscular support (Carrano, 1998). Thus, basal, bipedal ornithischians likely employed a more upright posture and caudofemoralis-driven limb retraction quite unlike that of extant birds.
However, the pelvic muscle moment arms of Lesothosaurus clearly also differ from those of tetanuran theropods in several respects. CFB generates a weak abduction moment in Lesothosaurus because of the dorsal location of the brevis shelf relative to the acetabulum (Fig. 7). The peak in summed extensor moment arms occurs at more extended femoral postures in Lesothosaurus because modifications to the postacetabular ilium and pelvic proportions that occurred on the line to birds were not present (e.g. Fig. S3). Weaker summed abduction moment arms can be related to the primitive caudal origin of IFM. Basal dinosaurs have been the subject of fewer quantitative functional analyses than other clades, such as tetanuran theropods and extant birds, but it is possible that the features observed in Lesothosaurus are characteristic of basal dinosaur locomotor anatomy in general, and that Lesothosaurus represents the basal dinosaur condition.
Conclusions
Computational modelling of moment arms in 3D offers insights into muscle function, allows muscle leverage to be quantified at a range of different postures, and quantitative comparisons to be made between muscles in different taxa. This provides a fundamental framework for understanding the evolution of muscle function through time and across major functional transitions, as well as providing a foundation for more complex, dynamic models of locomotion in extinct animals (Hutchinson et al. 2005; Sellers & Manning, 2007). As the best preserved basal ornithischian, Lesothosaurus offers the opportunity to examine the basal condition for muscular control of the hip in ornithischians, and provides a foundation upon which further work on the quadrupedal locomotor mode of more derived ornithischians can be built.
Qualitative hypotheses of muscle function in Lesothosaurus derived from examination of osteology and myological reconstruction grounded in the EPB (Maidment & Barrett, 2011) are supported by our 3D models. Our sensitivity analysis indicates that small changes to myological origins and insertions can result in differing interpretations of muscle function, particularly when they originate or insert close to joint rotational centres, emphasizing the importance of studies such as this one as a stepping stone between comparative anatomy and biomechanical simulation.
The pelvic and hind limb anatomies of birds and ornithischian dinosaurs are highly convergent, with features such as the retroverted pubis and elongate preacetabular process appearing independently in each clade. Sensitivity analysis highlights ambiguity in the role of musculature associated with the retroverted pubis (PIFE1&2) in ornithischians. However, it seems likely that this musculature may have predominantly functioned similarly to homologous muscles in extant birds, activating during the swing phase to adduct the lower limb through lateral rotation of the femora. Craniad migration of the iliofemoralis group muscles in birds correlates with increased leverage and use of medial femoral rotation to counter stance phase adduction moments at the hip. In Lesothosaurus the iliofemoralis groups maintain significantly higher moment arms for abduction, consistent with the hip abduction mode of lateral limb support hypothesized for basal dinosaurs. Correlation of lower long axis rotation moment arms with other anatomical traits (such as limb segment proportions and retention of a long muscular tail) suggests basal, bipedal ornithischians employed a more upright posture and caudofemoralis-driven limb retraction quite unlike that of extant birds. We therefore find little overall support for functional convergence between basal bipedal ornithischians and birds in our analysis of muscle moment arms.
Acknowledgments
Bill Sellers (University of Manchester) is thanked for his support with GaitSym, and Peter Falkingham (University of Manchester) for advice in producing Figs 7–9. SCRM is funded by Natural Environment Research Council grant number NE/G001898/1 to PMB. Corwin Sullivan and another anonymous reviewer are thanked for comments that greatly improved the manuscript.
Author contributions
Study concept/design: KTB, SCRM. Acquisition of data: KTB, SCRM, VA. Data processing: KTB. Data interpretation/analysis: KTB, SCRM. Drafting manuscript: KTB, SCRM, VA, PMB. Critical revision of the manuscript and approval of the article: KTB, SCRM, VA, PMB.
Supporting Information
Fig. S1. Predicted muscle moment arms for hipflexion-extension (left), abduction-adduction (centre) and longaxis rotation (right) in Lesothosaurus and other archosaurian bipeds over a range of hip joint flexion-extension angles.
Fig. S2. Average (a) hip extensor, (b) hipflexor, (c) hip abduction, (d) adduction, (e) lateral femoralrotation and (f) medial femoral rotation muscle moment armsnormalized by femoral length for Lesothosaurus and other archosaurian bipeds.
Fig. S3. Bar charts illustrating relative iliac proportions in the bipedal archosaurs modelled in this study.
As a service to our authors and readers, this journal provides supporting information supplied by the authors. Such materials are peer-reviewed and may be re-organized for online delivery, but are not copy-edited or typeset. Technical support issues arising from supporting information (other than missing files) should be addressed to the authors.
As a service to our authors and readers, this journal provides supporting information supplied by the authors. Such materials are peer-reviewed and may be re-organized for online delivery, but are not copy-edited or typeset. Technical support issues arising from supporting information (other than missing files) should be addressed to the authors.
References
- Alexander RM. Principles of Animal Locomotion. Princeton: Princeton University Press; 2003. p. 371. [Google Scholar]
- Alexander RM, Ker RF. Running is priced by the step. Nature. 1990;346:220–221. doi: 10.1038/346220a0. [DOI] [PubMed] [Google Scholar]
- Allen V, Paxton H, Hutchinson JR. Variation in center of mass estimates for extant sauropsids and its importance for reconstructing inertial properties of extinct archosaurs. Anat Rec. 2009;292:1442–1461. doi: 10.1002/ar.20973. [DOI] [PubMed] [Google Scholar]
- Allen V, Ellsey R, Jones N, et al. Functional specialisation and ontogenetic scaling of limb anatomy in Alligator mississippiensis. J Anat. 2010;216:423–445. doi: 10.1111/j.1469-7580.2009.01202.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bates KT, Schachner ER. Disparity and convergence in bipedal archosaur locomotion. J R Soc Interface. doi: 10.1098/rsif.2011.0687. (in press) doi: 10.1098/rsif.2011.0687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bates KT, Manning PL, Hodgetts D, et al. Estimating mass properties of dinosaurs using laser imaging and 3D computer modeling. PLoS ONE. 2009a;4 doi: 10.1371/journal.pone.0004532. e4532. doi: 10.1371/journal.pone.0004532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bates KT, Falkingham PL, Breithaupt BH, et al. How big was ‘Big Al’? Quantifying the effect of soft tissue and osteological unknowns on mass predictions for Allosaurus (Dinosauria: Theropoda) Palaeontologia Electronica. 2009b;12:33. [Google Scholar]
- Bates KT, Manning PL, Margetts L, et al. Sensitivity analysis in evolutionary robotic simulations of bipedal dinosaur running. J Vert Paleontol. 2010;30:458–466. [Google Scholar]
- Bates KT, Benson RBJ, Falkingham PL. A computational analysis of muscle leverage and body mass evolution in Allosauroidea (Dinosauria: Theropoda) with implications for locomotion. Paleobiology. (in press) in press. [Google Scholar]
- Bryant HN, Russell AP. The role of phylogenetic analysis in the inference of unpreserved attributes of extinct taxa. Phil Trans R Soc Lond Ser B. 1992;337:405–418. [Google Scholar]
- Butler RJ. The anatomy of the basal ornithischian dinosaur Eocursor parvus from the lower Elliot Formation (Late Triassic) of South Africa. Zool J Linn Soc. 2010;160:648–684. [Google Scholar]
- Butler RJ, Upchurch P, Norman DB. The phylogeny of the ornithischian dinosaurs. J Syst Palaeontol. 2008;6:1–40. [Google Scholar]
- Butler RJ, Galton PM, Porro LB, et al. Lower limits of ornithischian dinosaur body size inferred from a new Upper Jurassic heterodontosaurid from North America. Proc R Soc Lond B. 2010;277:375–381. doi: 10.1098/rspb.2009.1494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carrano MT. Locomotion in non-avian dinosaurs: integrating data from hind limb kinematics, in vivo strains, and bone morphology. Paleobiology. 1998;24:450–469. [Google Scholar]
- Carrano MT. Homoplasy and the evolution of dinosaur locomotion. Paleobiology. 2000;26:489–512. [Google Scholar]
- Carrano MT. Implications of limb bone scaling, curvature and eccentricity in mammals and non-avian dinosaurs. J Zool Lond. 2001;254:41–55. [Google Scholar]
- Carrano MT, Hutchinson JR. Pelvic and hindlimb musculature of Tyrannosaurus rex (Dinosauria: Theropoda) J Morphol. 2002;253:207–228. doi: 10.1002/jmor.10018. [DOI] [PubMed] [Google Scholar]
- Charig AJ. The evolution of archosaur pelvis and hind limb, an explanation in functional terms. In: Joysey KA, Kemp TS, editors. Studies in Vertebrate Evolution. Edinburgh: Oliver and Boyd; 1972. pp. 121–151. [Google Scholar]
- Christiansen P, Bonde N. Limb proportions and avian terrestrial locomotion. J Für Ornithologie. 2002;143:356–371. [Google Scholar]
- Coombs W. Osteology and myology of the hindlimb in the Ankylosauria (Reptilia, Ornithischia) J Paleontol. 1979;53:666–684. [Google Scholar]
- Dilkes DW. Appendicular myology of the hadrosaurian dinosaur Maiasaura peeblesorum from the Late Cretaceous (Campanian) of Montana. Trans R Soc Edinb Earth Sci. 2000;90:87–125. [Google Scholar]
- Galton PM. The pelvic musculature of the dinosaur Hypsilophodon (Reptilia: Ornithischia) Postilla. 1969;131:1–64. [Google Scholar]
- Gangl D, Weissengruber GE, Egerbacher M, et al. Anatomical description of the muscles of the pelvic limb in the Ostrich (Struthio camelus. Anat Histol Embryol. 2004;33:100–114. doi: 10.1111/j.1439-0264.2003.00522.x. [DOI] [PubMed] [Google Scholar]
- Gatesy SM. Caudofemoralis musculature and the evolution of theropod locomotion. Paleobiology. 1990;16:170–186. [Google Scholar]
- Gatesy SM. Functional evolution of the hindlimb and tail from basal theropods to birds. In: Thomason JJ, editor. Functional Morphology in Vertebrate Paleontology. Cambridge: Cambridge University Press; 1995. pp. 219–234. [Google Scholar]
- Gatesy SM. An electromyographic analysis of hindlimb function in Alligator during terrestrial locomotion. J Morphol. 1997;234:197–212. doi: 10.1002/(SICI)1097-4687(199711)234:2<197::AID-JMOR6>3.0.CO;2-9. [DOI] [PubMed] [Google Scholar]
- Gatesy SM. Guineafowl hind limb function. II: electromyographic analysis of motor pattern evolution. J Morphol. 1999;240:127–142. doi: 10.1002/(SICI)1097-4687(199905)240:2<127::AID-JMOR4>3.0.CO;2-Q. [DOI] [PubMed] [Google Scholar]
- Gatesy SM, Dial KP. Tail muscle activity patterns in walking and flying pigeons (Columbia livia. J Exp Biol. 1993;176:55–76. [Google Scholar]
- Gatesy SM, Dial KP. Locomotor modules and the evolution of avian flight. Evolution. 1996;50:331–340. doi: 10.1111/j.1558-5646.1996.tb04496.x. [DOI] [PubMed] [Google Scholar]
- Gatesy SM, Middleton KM. Bipedalism, flight, and the evolution of theropod locomotor diversity. J Vert Paleontol. 1997;17:308–329. [Google Scholar]
- Gauthier J. Saurischian monophyly and the origin of birds. In: Padian K, editor. Memoirs of the California Academy of Sciences. Vol. 8. Berkeley: California Academy of Sciences; 1986. pp. 1–55. [Google Scholar]
- Hutchinson JR. The evolution of pelvic osteology and soft tissues on the line to extant birds (Neornithes) Zool J Linn Soc. 2001a;131:123–168. [Google Scholar]
- Hutchinson JR. The evolution of femoral osteology and soft tissues on the line to extant birds (Neornithes) Zool J Linn Soc. 2001b;131:169–197. [Google Scholar]
- Hutchinson JR. Biomechanical modeling and sensitivity analysis of bipedal running. II. Extinct taxa. J Morphol. 2004;262:441–461. doi: 10.1002/jmor.10240. [DOI] [PubMed] [Google Scholar]
- Hutchinson JR, Gatesy SM. Adductors, abductors, and the evolution of archosaur locomotion. Paleobiology. 2000;26:734–751. [Google Scholar]
- Hutchinson JR, Anderson FC, Blemker SS, et al. Analysis of hindlimb muscle moment arms in Tyrannosaurus rex using a three-dimensional musculoskeletal computer model: implications for stance, gait and speed. Paleobiology. 2005;31:676–701. [Google Scholar]
- Hutchinson JR, Ng-Thow-Hing V, Anderson FC. A 3D interactive method for estimating body segmental parameters in animals: application to the turning and running performance of Tyrannosaurus rex. J Theor Biol. 2007;246:660–680. doi: 10.1016/j.jtbi.2007.01.023. [DOI] [PubMed] [Google Scholar]
- Hutchinson JR, Miller CE, Fritsch G. The anatomical foundation for multidisciplinary studies of animal limb function: examples from dinosaur and elephant limb imaging studies. In: Frey R, Endo H, et al., editors. Anatomical Imaging Techniques: Towards a New Morphology. Berlin: Springer; 2008. pp. 23–38. [Google Scholar]
- Hutchinson JR, Bates KT, Molnar J, et al. A computational analysis of limb and body dimensions in Tyrannosaurus rex with implications for locomotion, ontogeny and growth. PLoS ONE. 2011;6(10):e26037. doi: 10.1371/journal.pone.0026037. doi: 10.1371/journal.pone.0026037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maidment SCR, Barrett PM. The locomotor musculature of basal ornithischian dinosaurs. J Vert Paleontol. 2011;31:1265–1291. [Google Scholar]
- Norman DB. On the anatomy of Iguanodon atherfieldensis (Ornithischia: Ornithopoda) Bull Inst R Sci Nat Belgique. 1986;56:281–372. [Google Scholar]
- Novas FE. Dinosaur monophyly. J Vert Paleontol. 1996;16:723–741. [Google Scholar]
- Otero A, Vizcaíno SF. Hindlimb musculature and function of Neuquensaurus australis (Sauropoda: Titanosauria) Ameghiniana. 2008;45:333–348. [Google Scholar]
- Payne RC, Crompton RH, Isler K, et al. Morphological analysis of the hindlimb in apes and humans I: muscle architecture. J Anat. 2006a;208:709–724. doi: 10.1111/j.1469-7580.2006.00563.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Payne RC, Crompton RH, Isler K, et al. Morphological analysis of the hindlimb in apes and humans II: moment arms. J Anat. 2006b;208:725–742. doi: 10.1111/j.1469-7580.2006.00564.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romer AS. The pelvic musculature of ornithischian dinosaurs. Acta Zool. 1927;8:225–275. [Google Scholar]
- Romer AS. Osteology of the Reptiles. Malabar: Krieger; 1956. p. 772. [Google Scholar]
- Rowe T. Homology and evolution of the deep dorsal thigh musculature in birds and other Reptilia. J Morphol. 1986;189:327–346. doi: 10.1002/jmor.1051890310. [DOI] [PubMed] [Google Scholar]
- Rubenson J, Lloyd DG, Besier TF, et al. Running in ostriches (Struthio camelus): three dimensional joint axes alignment and joint kinematics. J Exp Biol. 2007;210:2548–2562. doi: 10.1242/jeb.02792. [DOI] [PubMed] [Google Scholar]
- Sacks RD, Roy RR. Architecture of the hind limb muscles of cats: functional significance. J Morphol. 1982;173:185–195. doi: 10.1002/jmor.1051730206. [DOI] [PubMed] [Google Scholar]
- Schachner ER, Manning PL, Dodson P. Pelvic and hindlimb myology of the basal archosaur Poposaurus gracilis (Archosauria: Poposauridaea) J Morphol. 2011;272:1464–1491. doi: 10.1002/jmor.10997. [DOI] [PubMed] [Google Scholar]
- Seeley H. On a sacrum, apparently indicating a new type of Bird, Ornithodesmus cluniculus, Seeley, from the Wealden of Brook. Quart J Geol Soc Lond. 1887;42:206–211. [Google Scholar]
- Sellers WI, Manning PL. Estimating dinosaur maximum running speeds using evolutionary robotics. Proc R Soc B. 2007;274:2711–2716. doi: 10.1098/rspb.2007.0846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sellers WI, Dennis L, Crompton RH. Predicting the metabolic energy costs of bipedalism using evolutionary robotics. J Exp Biol. 2003;206:1437–1448. doi: 10.1242/jeb.00205. [DOI] [PubMed] [Google Scholar]
- Sellers WI, Manning PL, Lyson T, et al. Virtual palaeontology: gait reconstruction of extinct vertebrates using high performance computing. Palaeontol Electron. 2009;12(11A):26. [Google Scholar]
- Sereno PC. Phylogeny of the bird-hipped dinosaurs (order Ornithischia) Nat Geog Res. 1986;2:234–256. [Google Scholar]
- Sereno PC. Lesothosaurus, “fabrosaurids”, and the early evolution of Ornithischia. J Vert Paleontol. 1991;11:168–197. [Google Scholar]
- Sereno PC. The evolution of the dinosaurs. Science. 1999;284:2137–2147. doi: 10.1126/science.284.5423.2137. [DOI] [PubMed] [Google Scholar]
- Smith NC, Wilson AM, Jespers KJ, et al. Muscle architecture and functional anatomy of the pelvic limb of the ostrich (Struthio camelus. J Anat. 2006;209:765–779. doi: 10.1111/j.1469-7580.2006.00658.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith NC, Payne RC, Jespers KJ, et al. Muscle moment arms of pelvic limb muscles of the ostrich (Struthio camelus. J Anat. 2007;211:313–324. doi: 10.1111/j.1469-7580.2007.00762.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thulborn RA. The skull of Fabrosaurus australis, a Triassic ornithischian dinosaur. Palaeontology. 1970;13:414–432. [Google Scholar]
- Thulborn RA. The post-cranial skeleton of the Triassic ornithischian dinosaur Fabrosaurus australis. Palaeontology. 1972;15:29–60. [Google Scholar]
- Walker AD, Problems in Vertebrate Evolution Andrews SM, Miles RS, Walker AD, editors. Evolution of the pelvis in birds and dinosaurs. Linnean Soc Sympos Ser. 1977;4:319–358. [Google Scholar]
- Witmer LM. The extant phylogenetic bracket and the importance of reconstructing soft tissues in fossils. In: Thomason JJ, editor. Functional Morphology in Vertebrate Paleontology. Cambridge: Cambridge University Press; 1995. pp. 19–33. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


