Abstract
Back pain constitutes a major problem in modern societies. Facet joints are increasingly recognised as a source of such pain. Knowledge about the internal morphology and its changes with age may make it possible to include the facets more in therapeutic strategies, for instance joint replacements or immobilisation. In total, 168 facets from C6/7 and L4/5 segments were scanned in a micro-computed tomography. Image analysis was used to investigate the internal morphology with regard to donor age and gender. Additional data from trabecular bone of the vertebral core allowed a semi-quantitative comparison of the morphology of the vertebral core and the facets. Porosity and pore spacing of the cortical sub-chondral bone does not appear to change with age for either males or females. In contrast, bone volume fraction decreases in females from approximately 0.4 to 0.2 , whereas it is constant in males. Trabecular thickness decreases during the ageing process in females and stays constant in males , whereas trabecular separation increases during the ageing process in both genders. The results of this study may help to improve the understanding of pathophysiological changes in the facet joints. Such results could be of value for understanding back pain and its treatment.
Keywords: cervical spine, lumbar spine, facet joints, micro-computed tomography, trabecular architecture
Introduction
Back pain constitutes a major problem in modern societies. With a yearly prevalence of up to 70% in Germany alone (Schmidt et al. 2007; Wenig et al. 2009) back pain is responsible for annual treatment costs of up 8.4 billion (Lange and Ziese, 2006). Facet joints, as one of the most important parts of a spinal segment, are increasingly recognised as a source of back pain (Schwarzer et al. 1994, 1995; Manchikanti and Singh, 2002; Manchikanti et al. 2004; Manchikanti et al. 2008; DePalma et al. 2011). They form the posterior column of the spinal segment and serve as a motion delimiter and guide the movement of the spinal segment. The anatomy of individual facet joints is highly dependent on their location in the spinal column. In the lower cervical spine for instance, the facet joints are set up like roofing (Panjabi et al. 1993). Such a configuration contributes to the high rotatory range of motion. In contrast, the lumbar facets are arranged approximately in the sagittal plane (Panjabi et al. 1993). Here, the lower facets are convex, whereas the upper facets are concave. This arrangement limits axial rotation and translations in the posterior-anterior direction.
Herniated intervertebral discs or other age-dependent degeneration processes can affect the facet joints indirectly. Those degenerative processes may lead to changing load situations in the facet joints (Galbusera et al. 2011) which may affect the cartilaginous layer and trigger arthrosis. This in turn may cause macroscopically observable degeneration such as osteophytes and hypertrophy that can lead to stenoses of the foramina, lateral and central spinal canals (Butler et al. 1990; Prescher, 1998; Fujiwara et al. 1999, 2001; Tischer et al. 2006; Kettler et al. 2007).
Currently, stenoses are treated surgically with a resection if conservative therapies such as transforaminal epidural steroid injections or physiotherapy fail (Meyer et al. 2008; Thome et al. 2008). The extent of the resection in those surgeries may range from mere windowing of the nerve canal up to resection of whole posterior columns along with the subsequent need for internal posterior fixation. Such widespread surgical procedures are a huge burden for the patient and for the surgeon. Furthermore, those interventions are known to cause a reduction in the range of motion in the segment of interest as well as degeneration in the neighbouring spinal segments (Park et al. 2004; Levin et al. 2007).
One way to minimise these problems involves minimal invasive approaches that aim to maintain the natural mobility of the spinal segment operated on. Modern facet joint implants aim to replace the articulating parts of the facet joint instead of replacing the whole joint by anatomical stabilisation systems to stop arthrosis-induced degenerative processes and try to re-establish the natural range of motion of the facets. As such, these implants are one component used to maintain or re-establish the healthy range of motion of the spinal segment (Khoueir et al. 2007; Büttner-Janz, 2010). If the facet joints are to be preserved, resurfacing technologies may be used in surgical treatments. These need to be anchored in the cortical shell and the sub-chondral bone. On the other hand, the facet joints may offer a possibility for minimal invasive segmental immobilisation to support segmental fusion. Such systems need to be anchored in the cortical shell and the sub-chondral bone as well. However, at present, little is known about these structures.
A former study investigated stress distributions within the sub-chondral bone of lumbar facet joints (Putz, 1985). It concluded that the internal morphology of the facet joints involves adaptations to lateral and medial bending loads. Another study investigated the macroscopic structure and the bone mineral density of lumbar facet joints (Müller-Gerbl, 1992). A limitation with that study was the coarse CT resolution of approximately 2 mm. A μ -computer tomography (μCT) – based study investigated the internal morphology of lumbar, superior facets with regard to differences as functions of spinal level and donor age (Drews et al. 2008). The limitation of that study was that the trabecular structure and the surrounding cortical shell were pooled in the analyses. None of these studies compared diffewrences with regard to gender, age or regions of the spinal column. As life expectancy rises, surgical procedures on elderly patients will becom much more likely. To guarantee the best outcome in those surgeries it is necessary to understand the bony structures that could be used for fixation.
This study aimed to investigate the morphology of cervical (C6/7) and lumbar (L4/5) facet joints with regard to age and gender. We hypothesise: (i) Cervical and lumbar facets specific to the studied levels have different internal morphology. (ii) The porosity of the sub-chondral cortical bone in the facets is similar in both sexes and will increase with age similarly for both genders. (iii) The trabecular bone morphology is different with regard to gender and develops differently with age in females and males.
Materials and methods
Cervical (C6/7) and lumbar (L4/5) spinal segments of 11 male and 12 female donors with a median age of 69 years (43–98 years) fixed in formalin were included in the study. After removing excess soft tissue, vertebral bodies and posterior arches were separated by a coronal cut through the pedicles with a bone saw. The remaining laminar arches were separated by a sagittal cut through the spinous process. The lumbar facet joints were subsequently separated in to superior and inferior parts. The cervical joints were left intact (Fig. 1). Subsequently, the facets were visually controlled to exclude any joint surface showing a pathology such as osteophytes and hypertrophy. This led to a final inclusion of 176 facets (96 female and 80 male; 31 C6, 32 C7, 32 superior, 31 inferior; 55 L4, 58 L5, 58 superior, 55 inferior; 92 right, 84 left).
Fig. 1.

Experimental protocol for μCT-investigation of the internal morphology of the facet joints. Specimens were prepared for μCT-scans from human vertebral bodies (top row). In contrast to lumbar specimens, the cervical superior and inferior specimens were not separated prior to scanning (indicated by the black star). After scanning the specimens were aligned with the dataset main axis (middle row). Subsequently, VOI were assigned, the data binarised and morphology analysed. Images describing the specimen preparation were adapted from Schünke et al. (2005).
Cylinders of 10 mm diameter were cored from the centre of the lumbar facets. A drill hole of 1 mm diameter and approximately 2 mm depth was set at the cranial end of the joint surface to allow the anatomical orientation in μCT – dataset to be controlled. The specimens were placed in holders submerged in formalin and scanned in a μCT (XCT FAN Beam μ -Scope; Stratec Medizintechnik GmbH, Pforzheim, Germany) at a spatial resolution of 30 μm in the case of the lumbar cylindrical cores or 35 μm in the case of the cervical joints. Specimens were stored at 4 ○C between preparation and scanning (Fig. 1).
μCT-datasets were further processed using (Version 1.43, National Institute of Health, USA). Misalignment errors of the cored lumbar cylinders were corrected by rotation of the datasets so that the cylindrical main axis was aligned with the dataset main axis. The vector for computing the rotation angles was determined using two contour-circles at the top and bottom ends of the cored cylinders (Fig. 1). Cervical specimens were rotated manually according to anatomical parameters such as the joint tip and the transition into the pedicle. Rotation was performed so that the joints were oriented along the main axis of the dataset. Subsequently, the joints were separated in silico. This approach assured that all specimens were analysed in a similar position, with the articulating surface oriented towards the top of the datasets and the superior/inferior parts of the joints aligned with the main axis of the dataset (Fig. 1).
Image analyses was performed with CT a Analyser (ctan, Version 1.9.3.2; SkyScan, Kontich, Belgium). Volumes of interest (VOI) were defined so that they contained a maximal specimen volume. Separate VOI were determined for the sub-chondral cortical bone and the trabecular core. The boundaries of the VOI were placed well away from the anatomic boundary of the trabecular core and/or the sub-chondral cortical bone. This ensured that no bony parts of one VOI could influence the measurements in the other and vice versa. Image data were binarised using a threshold that was determined with an automatic thresholding technique (Otsu, 1979) using imagej. After assignment of the VOI, five sub-VOI of equal size were defined, with the first at the cranial end of the VOI and the last at the caudal end of the articular process (Fig. 1).
The trabecular VOI and the cortical VOI were evaluated as a whole and the 20%-sub-VOI of these regions were evaluated separately. Bone volume fraction (BV/TV), bone surface to bone volume ratio (BS/BV), porosity (Po = 1–BV/TV), pore space (Po.Sp), trabecular separation (Tb.Sp), trabecular thickness (Tb.Th), trabecular number (Tb.N), degree of anisotropy (DA) and trabecular pattern factor (Tb.Pf) (Parfitt, 1988; Hildebrand and Rüegesegger, 1997) were evaluated to compare the morphology of cervical and lumbar facets.
To allow a semi-quantitative comparison of the morphology of facet trabeculae and trabeculae from the vertebral core, results from μCT-datasets from a prior study were used (Wolfram et al. 2011). Briefly, 251 cylindrical specimens from the cores of 104 human vertebrae (50 male, 54 female; 32 donors; median age 65 years, range 21–94 years; T1–L3) were scanned in a μCT (μCT 40, SCANCO Medical AG, Brüttisellen, Switzerland) at a spatial resolution of 12 μm. Datasets were analysed for the same parameters as the facet joints in this study using Image Processing Language (SCANCO Medical AG).
Statistical analyses were performed using pasw Statistics (Version 18.0.0; IBM, USA). Donors were assigned to five age groups that contained approximately the same number of donors and male and female groups of equal size. Outliers were excluded if BV/TV was below the 2.5 or above the 97.5 percentile, which applied for eight samples. Linear QQ-plots and equal variances were used to conduct an analysis of variance (anova). A significance level of P = 0.05 was used and significances were confirmed with Tukey's post-hoc test. In the case of DA, a test to determine whether the data were normally distributed failed. Therefore this parameter was tested with Mann–Whitney U-tests. Throughout the following sections the term significant denotes statistically significant.
Results
Generally, no significant difference was found between left and right facets and these facets were therefore pooled. All other investigated factors showed distinct differences (Table 1).
Table 1.
Investigated parameters (columns) and their behaviour (lines) are presented as results at a glance. The investigated parameters were: porosity (Po), pore space (Po.Sp), bone volume fraction (BV/TV), structural model index (SMI), trabecular pattern factor (Tb.Pf), trabecular separation (Tb.Sp), bone surface to bone volume (BS/BV), trabecular thickness (Tb.Th), trabecular number (Tb.N), and degree of anisotropy (DA). The expressions in brackets specify exceptions in the conclusions, where present. For example, >(L4) in the case of inf–sup means that it is significantly higher differences in the inferior joint part of L4. The following encoding was used.
| = | No statistically significant difference between groups | P≤0.05 |
| > | Statistically significant higher | P≤0.05 |
| < | Statistically significant lower | P≤0.05 |
| + | Statistically significantly increasing | P≤0.05 |
| − | Statistically significantly decreasing | P≤0.05 |
| C, L | Cervical, lumbar | |
| in f, sup | Inferior, superior | |
| le, ri | Left or right | |
| T, M, B | Top, middle, bottom of the facet surface |
| ♂–♀ | Age | Vertebra | C–L | inf–sup | ri–le | T–B–M | |
|---|---|---|---|---|---|---|---|
| Cortex | |||||||
| Po | < | = | = | = | =(L4) | = | = |
| Po.Sp | = | = | = | = | =(L4) | = | = |
| Trabeculae | |||||||
| BV/TV | > | =(♂)−(♀) | = | = | =(C)>(L) | = | −(T to M)+(M to B) |
| BS/BV | < | − | = | > | = | = | = |
| Tb.Th | > | =(♂)−(♀) | = | = | = | = | = |
| Tb.Sp | = | + | >(C7) | = | < | = | −(T to M)+(M to B) |
| Tb.N | = | =(♂)−(♀) | = | > | >(L4) | = | −(T to M)+(M to B) |
| DA | = | − | = | > | >(not L5) | = | −(T to M)+(M to B) |
| SMI | = | = | = | = | =(C)<(L) | = | = |
| Tb.Pf | = | =(♂)+(♀) | = | = | =(C)<(L) | = | = |
A significantly higher cortical porosity was found for females then males (P = 0.01). Po did not change significantly with age in either gender except for the third age group (60–78 years) of females (Fig. 2). No significantly different Po was found between superior and inferior joint parts except in L4. Furthermore,there was no significant difference between cervical and lumbar Po. Regarding the sub-VOI (20% sections of the VOI from cranial to caudal) Po seems to be constant. Strikingly, no significant difference regarding the Po.Sp was found in the tested segments except between the superior and inferior facets in L4 (Fig. 2).
Fig. 2.

Sub-chondral bone in facets: Porosity (Po) of the cortical sub-chondral bone was significantly higher in females than in males. No difference was found between the superior and inferior articulating parts except in L4. Pore size (Po.Sp), i.e. pore diameter, was not significantly different in the tested specimens except between superior and inferior joint parts in L4. Black stars indicate significant differences to P = 0.05.
In the trabecular VOI, significantly higher BV/TV was found in males than in females. In females, BV/TV decreased significantly with age (Fig. 3). No significant difference between cervical and lumbar segments could be observed. However, BV/TV was significantly higher in the inferior joint part in the lumbar segments. Whereas no significant differences in the sub-VOI of the inferior joint parts were found, BV/TV decreased significantly from the cranial to the caudal end (Fig. 3). The active surface (BS/BV) was significantly lower in males than in females in all age groups (Fig. 3). The significant increase in BS/BV over age seems to be similar for both males and females. No difference was found between superior and inferior joint parts but there was a difference between the cervical and lumbar segments.
Fig. 3.

Trabecular bone in facets: Bone volume fraction (BV/TV) was higher in males than in females. With increasing age Females are more affected by bone loss than males are. No difference was found between cervical and lumbar segments. However, lower BV/TV was found in the lumbar superior joint parts. Bone surface to bone volume ratio (BS/BV) was lower in males than in females but increased over age similarly for both genders. No significant difference was found for BS/BV between superior and inferior joint parts but between cervical and lumbar spine. Black stars indicate significant differences to P < 0.05.
Tb.Th decreases significantly with age over age in females. In contrast, no significant trabecular thinning over the ageing process was found in males. Neither the comparison of cervical and lumbar facets nor the comparison between inferior and superior joint parts showed significant differences in Tb.Th (Fig. 4). Furthermore, Tb.Th was constant over all sub-VOI. In contrast, Tb.Sp increased significantly with age for both genders but more strongly so in females. Tb.Sp was significantly higher in superior joint parts, equal between C6 and lumbar segments, and significantly higher in C7 (Fig. 4). Furthermore, Tb.Sp was statistically significantly higher in the middle sub-VOI than towards the cranial and caudal end. This was not the case for Tb.Th. which was constant throughout the sub-VOI. In the superior joint parts, Tb.Sp increased from cranial to caudal with a slight drop in the most caudal sub-VOI. Throughout the inferior parts, Tb.Sp increased from the first to the second sub-VOI and then decreased constantly to the caudal end.
Fig. 4.

Trabecular bone in facets: Trabecular thickness (Tb.Th) was constant over age in males but decreased significantly in females. No difference was found with regard to cervical and lumbar segments as well as to superior or inferior parts. Trabecular separation (Tb.Sp), however, increased significantly with age for both males and females. It was significantly higher in superior joint parts as well as in the cervical spine. Black stars indicate significant differences to P = 0.05.
Tb.N was constant over age in males and decreased significantly, almost linearly, with age from 1.5 1 mm−1 at 43–52 years to approximately 1 mm−1 at 93–98 years in females. No difference was found between inferior and superior joint parts in the cervical facets, whereas the lumbar specimens showed significantly greater values for the superior facet joint than for the inferior facet joint in L4. Overall, cervical joints showed significantly higher Tb.N than lumbar joints. The superior joint parts showed a significantly higher Tb.N in the cranial sub-VOI. Conversely, in the inferior parts the most caudal sub-VOI showed the highest Tb.N.
The trabeculae in the facets showed a high orientation (DA >2), with significantly higher DA in the cervical than in the lumbar facets. This was found for males and females over all ages. DA increased towards the centre of the articulating surface. The trabeculae showed a, rather plate-like structure as indicated by a mean SMI of 0.64 ± 0.59 in males and females. SMI was constant over the age groups. The connectivity of the trabecular structure did not diminish in males, as indicated by a constant Tp.Pf of around 0. In females, Tb.Pf was raised significantly over the age groups from approximately −1.5 to approximately 2, indicating decreasing connectivity.
Discussion
Motivated by the increasing attention that is being paid to the facet joints as a source of back pain as well as a target structure for new implant technologies, this study aimed to increase knowledge about their internal anatomy. Such knowledge- is a pre-requisite for developing tissue-preserving surgical technologies that use the facet joints to fix small devices. Therefore, facet joints from cervical (C6/7) and lumbar (L4/5) spinal segments were investigated. The results suggest that: (i) cervical and lumbar spines have different internal morphology; (ii) the porosity of the sub-chondral cortical bone is different in males and females and increases with age for females; (iii) gross trabecular bone morphology is different with regard to gender and develops differently with age in females and males. The results can be qualitatively summarised using two extreme cases (Fig. 5).
Fig. 5.
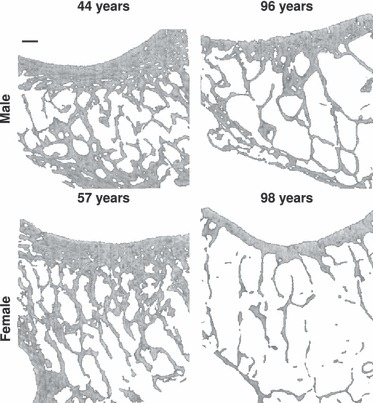
Trabecular bone in facets: The differences in the internal structure of the facet joint becomes obvious when comparing facets from L5 of four representative young vs. old male and female donors. Taken together, trabecular bone in facets of females decreases more than in males. Spatial resolution of the images is 30 μm and the scale bar in the top left image represents 0.9 mm.
The sub-chondral cortex does not seem to be affected by age or gender and does not show great variations between spinal locations. This may be due to a slower remodelling rate in cortical bone. As cortical bone offers less free surface in comparison with the trabecular structures for remodelling, it maintains its structure better during the ageing process. This makes the cortical shell of the facets a reliable structure for possible fixation. In the results presented here, the inferior joint part in L4 showed greater porosity and pore size.
This could suggest that a special load situation is present in the lumbar spine. In L4/5, the facets are rotated less transversely compared with the lumbar-sacral transition, which could be due to the potentially higher shear forces in the L4/5 segment. In fact, the facets in C6/7 and L5 are transversely oriented to withstand compressive forces (Putz, 1981) rather than forces resulting from rotations or anterior-posterior shear.
Similar to vertebral trabecular (Fig. 6) bone, BV/TV of the facets joints seems to decrease more with age in females than in males. Although it is known that this decrease in BV/TV differs between various anatomical sites (Eckstein et al. 2007) the results suggest that facets and cores are affected similarly. However, no reduction in BV/TV was found in the pedicles (Inçeoglu et al. 2005). The higher BV/TV in the facets when compared to the vertebrae (Figs 2 and 6) and the pedicles (Inçeoglu et al. 2005) may be due to the high forces that act on the joint during motion.However, the higher BV/TV in the inferior lumbar joint parts may be explained by the ellipsoidal shape of the facet. The semi-shell shape of the superior joint part may provide less room for trabeculae but a stronger cortex. BV/TV decreases cranialy to caudally in these semi-shells, which could be due to the fact that the force contact point slides over the concave surface of the inferior part. This further supports findings that the main point of application of the joint reaction force is located rather more cranially (Drews et al. 2008). The inferior joint moves with a fairly constant bending motion and changes in the superior joint could further explain the differences found. Strikingly, BV/TV was similar in cervical and lumbar segments even though these segments have nearly completely different load histories and modes.
Fig. 6.

Trabecular bone in vertebral bodies: To provide a semi-quantitative comparison of trabecular bone from the facets to vertebral trabecular bone, cylindrical specimens of vertebral trabecular bone were analysed for the same morphological parameters. Black stars indicate significant differences to P = 0.05.
BS/BV rises with age in the facet, which increases the free surface in the trabecular structure. The rise seems to be slightly stronger than in vertebral trabecular bone (Fig. 6). This is somewhat puzzling that the facets are loaded during motions of the segment. However, this could reflect degeneration in the spinal segment that constricts motion and, thus, reduce the load acting on the facets. On the other hand, BS/BV was slightly lower than in vertebral trabecular bone (Fazzalari et al. 2001).
As in vertebral trabecular bone, Tb.Sp rises with age, whereas Tb.Th remains almost constant (Figs 3 and 6) with no great differences between genders. In combination with the results found for BV/TV, this suggests that BV/TV and Tb.Sp decrease as a result of trabecular loss rather than thinning. In the case of Tb.Sp within the facets, females seem to be more affected by the ageing process. In contrast to another study (Drews et al. 2008), no difference in Tb.Th was found between the superior and inferior joint parts. In their study, Drews et al. pooled trabecular core and cortical shell, which might be the source of the difference. The constant Tb.Th supports the assumption that trabecular loss rather than thinning is responsible for the increase in BV/TV and Tb.Sp. Tb.Th seems to be similar to trabecular bone from the vertebral core, but Tb.Sp appears to be smaller, which explains the higher BV/TV in the facets. These findings match with those in the literature (Inçeoglu et al. 2005; Drews et al. 2008) and indicate that the facets are a dense tissue that is well equipped to sustain high loads. Bearing in mind that the mechanical properties of the underlying bone itself do not seem to change with age (Wolfram et al. 2010), the facets seem to provide proper bone for fixation systems.
Tb.N showed values between 1 and 1.5 mm−1 which is higher than in vertebral trabecular bone (0.95 mm−1). This illustrates again the density and morphological potential of the tissue in comparison to the vertebral core. Tb.N decreases with age in females more than in males. The decrease, supports the assumption that trabeculae are lost rather than thinned. A similar deterioration is known from other anatomical sites (Cvijanovic et al. 2004; Chen et al. 2008).
The anisotropy of the facets decreases over age continuously without much differences in gender, but comparable to that of the vertebral core (DA = 1.46). Higher DA was found in the inferior, cervical joint parts, which could be due to a higher and more ventrally oriented force acting on the joint (Putz and Müller-Gerbl, 1996). DA increases towards the centre of the articulating joint surface for both cervical and lumbar facets, indicating that the reaction force becomes more unidirectional towards the articulation centre.
Trabecular structure in the tissue is rather plate-like (SMI = 0.64 ± 0.59) compared to the vertebral core (SMI = 1.52 ± 0.39), where the trabecular structure is more rod-like. This could as well be due to the high loads that necessitate more plate-like supportive structures (Bevill et al. 2006). The trabecular structure changes from plates to rods in the ageing skeleton (Grote et al. 1995) and differs in different anatomical sites (Eckstein et al. 2007). In the facets, this change cannot be shown for females or for males. This contrasts with the assumptions that trabeculae are lost rather than thinned because it would necessitate losing whole plates. On the other hand, if the loss is high enough, Tb.Th would not be greatly affected due to the distance transformation used (Hildebrand and Rüegesegger, 1997), in contrast to BV/TV and Tb.Sp.
The high number of excluded facet joints compared to the number of donors was partly due to the fact that full body donors from an anatomy course were used. Damage due to preparation training as well as pathologies such as deformities led to the exclusion of some joints. Other vertebrae had already been prepared for other experiments so that the facets had damaged. Ideally,to provide results that are statistically valid with regard to the basic population at least 32 donors should have been included in the investigation (4 vertebrae × 2 genders × 2 sites × 2 heights = 32 donors). Including the age groups, this number would have been even greater. Due to limited resources, the necessary amount of donor material was not available. Unfortunately, this also led to an unequal distribution of donors in the age groups. Nevertheless, the number of facets used (168) is fairly high in comparison with other studies (Müller-Gerbl, 1992; Drews et al. 2008) and should allow a reasonable estimation of trends regarding the internal facet morphology. Post-hoc power analysis yielded in an anova of >0.95.
The μCT used only allowed for resolutions of 30 and 35 μm; newer machines would enable higher resolutions. At the time this study was performed, no better machine was available at the institute. However, with a Tb.Th of approximately 200 μm, the resolution was more than a factor of five better. Thus, it should have been high enough for the investigations and conclusions. The separation of the scans into 30 μm (lumbar facets) and 35 μm (cervical facets) was necessary to decrease the scanning time of around 12 h per specimen.
VOI were placed so that at least five characteristic internal lengths (such as trabecular spacing) were contained (Harrigan et al. 1988). This ensured enough material for the investigations carried out. The boundaries of the VOI were placed away from the transition zone between sub-chondral cortex and trabecular core to minimise possible influences. The most sensible step was the binarisation (Hara et al. 2002; Buie et al. 2007; Hangartner, 2007). Therefore, an automatic adaptive thresholding algorithm was used in the present study (Otsu, 1979). This, further ensures reproducibility and avoids intra- and inter-observer errors.
In summary, this study provides insights in to the internal morphology of human facet joints with regard to age and gender and may help to understand changes in their internal morphology. Furthermore, the results may provide additional knowledge necessary to improve the understanding of pathophysological changes in the facet joints with regard to low back pain, as well as an understanding of how to treat those pathophysiological changes.
Acknowledgments
The study was financially supported by the Deutsche Forschungsgemeinschaft (German Research Foundation), grant number WI 1352/14–1. The authors would like to thank the Department for Molecular and Cellular Anatomy of Ulm University (Ulm, Germany) for providing donor material and preparation facilities.
Conflict of interest
None.
References
- Büttner-Janz K. Status quo of facet joint replacement. Orthopäde. 2010;39:609–622. doi: 10.1007/s00132-009-1588-2. [DOI] [PubMed] [Google Scholar]
- Bevill G, Eswaran S, Gupta A, Papadopoulos P, Keaveny T. Influence of bone volume fraction and architecture on computed large deformation failure mechanisms in human trabecular bone. Bone. 2006;39:1218–1225. doi: 10.1016/j.bone.2006.06.016. [DOI] [PubMed] [Google Scholar]
- Buie H, Campbell G, Klinck R, MacNeil J, Boyd S. Automatic segmentation of cortical and trabecular compartments based on a dual threshold technique for in vivo micro-CT bone analysis. Bone. 2007;41:505–515. doi: 10.1016/j.bone.2007.07.007. [DOI] [PubMed] [Google Scholar]
- Butler D, Trafimow JH, Andersson GB, McNeill TW, Huckman MS. Discs degenerate before facets. Spine. 1990;15:111–113. doi: 10.1097/00007632-199002000-00012. [DOI] [PubMed] [Google Scholar]
- Chen H, Shoumura S, Emura S, Bunai Y. Regional variations of vertebral trabecular bone microstructure with age and gender. Osteoporos Int. 2008;19:1473–1483. doi: 10.1007/s00198-008-0593-3. [DOI] [PubMed] [Google Scholar]
- Cvijanovic O, Bobinac D, Zoricic S, Ostojic Z, Maric I, Crncevic-Orlic Z, Kristofic I, et al. Age- and region-dependent changes in human lumbar vertebral bone: a histomorphometric study. Spine. 2004;29:2370–2375. doi: 10.1097/01.brs.0000143620.95267.39. [DOI] [PubMed] [Google Scholar]
- DePalma MJ, Ketchum JM, Saullo T. What is the source of chronic low back pain and does age play a role. Pain Med. 2011;12:224–233. doi: 10.1111/j.1526-4637.2010.01045.x. [DOI] [PubMed] [Google Scholar]
- Drews S, Matsuura M, Putz R. The trabecular architecture of the superior articular process of the lumbar spine (L2-S1) Surg Radiol Anat. 2008;30:209–213. doi: 10.1007/s00276-008-0317-6. [DOI] [PubMed] [Google Scholar]
- Eckstein F, Matsuura M, Kuhn V, Priemel M, Müller R, Link TM, Lochmüller EM, et al. Sex differences of human trabecular bone mi373 crostructure in aging are site-dependent. J Bone Miner Res. 2007;22:817–824. doi: 10.1359/jbmr.070301. [DOI] [PubMed] [Google Scholar]
- Fazzalari NL, Manthey B, Parkinson IH. Intervertebral disc disor- ganisation and its relationship to age adjusted vertebral body morphometry and vertebral bone architecture. Anat Rec. 2001;262:331–339. doi: 10.1002/1097-0185(20010301)262:3<331::AID-AR1044>3.0.CO;2-H. [DOI] [PubMed] [Google Scholar]
- Fujiwara A, Tamai K, Yamato M, An HS, Yoshida H, Saotome K, Kurihashi A, et al. The relationship between facet joint osteoarthritis and disc degeneration of the lumbar spine: an MRI study. Eur Spine J. 1999;8:396–401. doi: 10.1007/s005860050193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujiwara A, Tamai K, An HS, Lim TH, Yoshida H, Kurihashi A, Saotome K, et al. Orientation and osteoarthritis of the lumbar facet joint. Clin Orthopaed Rel Res. 2001;395:88–94. doi: 10.1097/00003086-200104000-00015. [DOI] [PubMed] [Google Scholar]
- Galbusera F, Schmidt H, Neidlinger-Wilke C, Wilke H-J. The effect of degenerative morphological changes of the intervertebral disc on the lumbar spine biomechanics: a poroelastic finite element investigation. Comp Methods Biomech Biomed Engin. 2011;14:729–739. doi: 10.1080/10255842.2010.493522. [DOI] [PubMed] [Google Scholar]
- Grote HJ, Amling M, Vogel M, Hahn M, Posl M, Delling G. Intervertebral variation in trabecular microarchitecture throughout the normal spine in relation to age. Bone. 1995;16:301–308. doi: 10.1016/8756-3282(94)00042-5. [DOI] [PubMed] [Google Scholar]
- Hangartner TN. Thresholding technique for accurate analysis of density and geometry in QCT, pQCT and microCT images. J Musculoskelet Neuronal Interact. 2007;7:9–16. [PubMed] [Google Scholar]
- Hara T, Tanck E, Homminga J, Huiskes R. The influence of microcomputed tomography threshold variations on the assessment of structural and mechanical trabecular bone properties. Bone. 2002;31:107–109. doi: 10.1016/s8756-3282(02)00782-2. [DOI] [PubMed] [Google Scholar]
- Harrigan TP, Jasty M, Mann RW, Harris WH. Limitations of the continuum assumption in cancellous bone. J Biomechan. 1988;21:269–275. doi: 10.1016/0021-9290(88)90257-6. [DOI] [PubMed] [Google Scholar]
- Hildebrand T, Rüegesegger P. A new method for the model-independent assessment of thickness in three-dimensional images. J Microsc. 1997;185:67–75. [Google Scholar]
- Inçeoglu S, Burghardt A, Akbay A, Majumdar S, McLain RF. Trabecular architecture of lumbar vertebral pedicle. Spine. 2005;30:1485–1490. doi: 10.1097/01.brs.0000168373.24644.9f. [DOI] [PubMed] [Google Scholar]
- Kettler A, Werner K, Wilke H-J. Morphological changes of cervical facet joints in elderly individuals. Eur Spine J. 2007;16:987–992. doi: 10.1007/s00586-006-0275-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khoueir P, Kim KA, Wang MY. Classification of posterior dynamic stabilization devices. Neurosurg Focus. 2007;22:E3. doi: 10.3171/foc.2007.22.1.3. [DOI] [PubMed] [Google Scholar]
- Lange C, Ziese T. Gesundheitsberichtersattung des Bundes–Gesund heit in Deutschland. Dtsch Gesundheitssurvey. 2006;225:34–40. [Google Scholar]
- Levin DA, Hale JJ, Bendo JA. Adjacent segment degeneration following spinal fusion for degenerative disc disease. Bull NYU Hosp Jt Dis. 2007;65:29–36. [PubMed] [Google Scholar]
- Müller-Gerbl M. The role of the vertebral joint for the kinetics of the moving segment. Ann Anat. 1992;174:48–53. [PubMed] [Google Scholar]
- Manchikanti L, Singh V. Review of chronic low back pain of facet joint origin. Pain Physician. 2002;5:83–101. [PubMed] [Google Scholar]
- Manchikanti L, Boswell MV, Singh V, Pampati V, Damron K, Beyer CD. Prevalence of facet joint pain in chronic spinal pain of cervical thoracic and lumbar regions. BMC Musculoskelet Disord. 2004;5:15. doi: 10.1186/1471-2474-5-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manchikanti L, Manchikanti KN, Cash KA, Singh V, Giordano J. Age-related prevalence of facet-joint involvement in chronic neck and low back pain. Pain Physician. 2008;11:67–75. [PubMed] [Google Scholar]
- Meyer F, Borm W, Thome C. Degenerative cervical spinal stenosis: current strategies in diagnosis and treatment. Dtsch Ärzteblatt Int. 2008;105:366–372. doi: 10.3238/arztebl.2008.0366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Otsu N. A threshold selection method from grey level histograms. IEEE Trans Syst Man Cybern. 1979;9:62–66. [Google Scholar]
- Panjabi MM, Oxland T, Takata K, Goel V, Duranceau J, Krag M. Articular facets of the human spine. quantitative three-dimensional anatomy. Spine. 1993;18:1298–1310. doi: 10.1097/00007632-199308000-00009. [DOI] [PubMed] [Google Scholar]
- Parfitt AM. Bone histomorphometry: proposed system for standardization of nomenclature, symbols, and units. Calcf Tissue Int. 1988;42:284–286. doi: 10.1007/BF02556360. [DOI] [PubMed] [Google Scholar]
- Park P, Garton HJ, Gala VC, Hoff JT, McGillicuddy JE. Adjacent segment disease after lumbar or lumbosacral fusion: review of the literature. Spine. 2004;29:1938–1944. doi: 10.1097/01.brs.0000137069.88904.03. [DOI] [PubMed] [Google Scholar]
- Prescher A. Anatomy and pathology of the aging spine. Eur J Radiol. 1998;27:181–195. doi: 10.1016/s0720-048x(97)00165-4. [DOI] [PubMed] [Google Scholar]
- Putz R. Funktionelle Anatomie der Wirbelgelenke. Stuttgart, NY: Thieme; 1981. Vol. 43 of Normale und pathologische Anatomie. [PubMed] [Google Scholar]
- Putz R. The functional morphology of the superior articular processes of the lumbar vertebrae. J Anat. 1985;143:181–187. [PMC free article] [PubMed] [Google Scholar]
- Putz R, Müller-Gerbl M. The vertebral column–a phylogenetic failure? A theory explaining the function and vulnerability of the human spine. Clin Anat. 1996;9:205–212. doi: 10.1002/(SICI)1098-2353(1996)9:3<205::AID-CA12>3.0.CO;2-V. [DOI] [PubMed] [Google Scholar]
- Schünke M, Schulte E, Schumacher U. Rumpfwand–Knochen, Bän457 der und Gelenke. Stuttgart, NY: Thieme; 2005. Vol. 1. Auflage of Prometheus – Lernatlas der Anatomie. [Google Scholar]
- Schmidt C, Raspe H, Pfingsten M, Hasenbring M, Basler H, Eich W, Kohlmann T, et al. Back pain in the German adult population: prevalence, severity, and sociodemographic correlates in a multiregional survey. Spine. 2007;32:2005–2011. doi: 10.1097/BRS.0b013e318133fad8. [DOI] [PubMed] [Google Scholar]
- Schwarzer A, Aprill C, Derby R, Fortin J, Kine G, Bogduk N. Clinical features of patients with pain stemming from the lumbar zygapophysial joints. Is the lumbar facet syndrome a clinical entity. Spine. 1994;19:1132–1137. doi: 10.1097/00007632-199405001-00006. [DOI] [PubMed] [Google Scholar]
- Schwarzer AC, Wang SC, Bogduk N, McNaught PJ, Laurent R. Prevalence and clinical features of lumbar zygapophysial joint pain: a study in an Australian population with chronic low back pain. Ann Rheum Dis. 1995;54:100–106. doi: 10.1136/ard.54.2.100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thome C, Borm W, Meyer F. Degenerative lumbar spinal stenosis: current strategies in diagnosis and treatment. Dtsch Ärzteblatt Int. 2008;105:373–379. doi: 10.3238/arztebl.2008.0373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tischer T, Aktas T, Milz S, Putz R. Detailed pathological changes of human lumbar facet joints L1–L5 in elderly individuals. Eur Spine J. 2006;15:308–315. doi: 10.1007/s00586-005-0958-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wenig C, Schmidt C, Kohlmann T, Schweikert B. Costs of back pain in Germany. Eur J Pain. 2009;13:280–286. doi: 10.1016/j.ejpain.2008.04.005. [DOI] [PubMed] [Google Scholar]
- Wolfram U, Wilke H-J, Zysset PK. Rehydration of vertebral tra becular bone: Influences on its anisotropy, its stiffness and the indentation work with a view to age, gender and vertebral level. Bone. 2010;46:348–354. doi: 10.1016/j.bone.2009.09.035. [DOI] [PubMed] [Google Scholar]
- Wolfram U, Wilke H-J, Zysset PK. Damage accumulation in vertebral trabecular bone depends on loading mode and direction. J Biomechan. 2011;44:1164–1169. doi: 10.1016/j.jbiomech.2011.01.018. [DOI] [PubMed] [Google Scholar]


