Abstract
Roaring in rutting Iberian red deer stags Cervus elaphus hispanicus is unusual compared to other subspecies of red deer, which radiated from the Iberian refugium after the last glacial maximum. In all red deer stags, the larynx occupies a permanent low mid-neck resting position and is momentarily retracted almost down to the rostral end of the sternum during the production of rutting calls. Simultaneous with the retraction of the larynx, male Iberian red deer pronouncedly protrude the tongue during most of their rutting roars. This poses a mechanical challenge for the vocal tract (vt) and for the hyoid apparatus, as tongue and larynx are strongly pulled in opposite directions. This study (i) examines the vocal anatomy and the acoustics of the rutting roars in free-ranging male C. e. hispanicus; (ii) establishes a potential mechanism of simultaneous tongue protrusion and larynx retraction by applying a two-dimensional model based on graphic reconstructions in single video frames of unrestrained animals; and (iii) advances a hypothesis of evaporative cooling by tongue protrusion in the males of a subspecies of red deer constrained to perform all of the exhausting rutting activities, including acoustic display, in a hot and arid season.
Keywords: Cervidae, evaporative cooling, larynx retraction, respiratory tract, ruminants, rutting roars, sexual selection, thermoregulation, vocalization
Introduction
Rutting red deer stags (Cervus elaphus) use vocal displays both for deterring rival males and for attracting receptive females (Reby et al. 2005; Charlton et al. 2007a). Patterns of rutting roars differ markedly between subspecies (Tembrock, 1965; Nikol'skii et al. 1979; Reby & McComb, 2003a; Feighny et al. 2006; Kidjo et al. 2008). This vocal divergence may result from different phylogeographic origins of the respective subspecies (Ludt et al. 2004; Zachos & Hartl, 2011), from different vocal morphologies (Riede et al. 2010), from sexual selection by female preferences for certain roar acoustics (Charlton et al. 2007a; Reby et al. 2010), or from environmental factors acting on the stags during the rut.
During the last Pleistocene glacial maximum, red deer distribution in Europe was restricted to four isolated sites: the Iberian Peninsula/Southern France, Italy, the Balkans and the Carpathians (Zachos & Hartl, 2011). The Iberian C. e. hispanicus, Scottish C. e. scoticus, and Norwegian C. e. elaphus subspecies originate from the same Iberian/Southern France refuge and share the same mitochondrial cytochrome b A-haplotype group. In contrast, the B-haplotype group of Corsican red deer C. e. corsicanus and C. e. barbarus suggests a distinctive origin of these subspecies (Skog et al. 2009; Niedzialkowska et al. 2011; Zachos & Hartl, 2011).
Red deer re-colonization in Europe after the last Ice Age was influenced by ecological factors: climate, landscape, food availability and habitat acoustics. These factors accounted for the divergence in fur cover, body size, behaviour and acoustic communication between subspecies. In Spanish red deer stags the neck mane is lacking, whereas in red deer inhabiting the colder parts of Europe, the neck region is accented by a heavy mane (Wagenknecht, 1983; Fitch & Reby, 2001), suggesting some sort of thermoregulatory function, in addition to the ‘strong neck performance’ that is applied during the rut to pretend to have larger neck muscles, i.e. better fighting ability, by ruffling up the neck hair during lateral display (cf. Frey et al. 2007). While female defence is the usual mating strategy of red deer stags (Clutton-Brock et al. 1982), the mating system may vary from female defence to territory defence even in the same habitats within Spain, depending on resource dispersion (Carranza et al. 1990, 1995; Carranza & Valencia, 1999). In Middle Europe, the beginning of the rut is in September/October, mostly peaking in mid-October (Clutton-Brock et al. 1982; Wagenknecht, 1983), whereas in Spain and Corsica, C. e. hispanicus and C. e. corsicanus stags start roaring already in August–September, mostly peaking in mid-to-end September (Carranza et al. 1990; Kidjo et al. 2008). This variation of the rutting period may relate to plant phenology (Post et al. 2003), because the earlier rutting period in Southern Europe shifts the calving to an earlier date in the next year when high quality fresh forage is still available for hinds and calves before onset of the summer drought. Contrary to the northern subspecies, the season with the poorest food supply for C. e. hispanicus is not winter but the late summer and early autumn, owing to the drying up of the vegetation. As a consequence, C. e. hispanicus stags have to perform their straining rutting activities in a period when water supply is low and food is scarce, while incident solar radiation and ambient temperatures are still high (Carranza et al. 1990).
The acoustics of the rutting roars of red deer stags are the result of an interplay of source and filter characteristics represented on the one hand by the fundamental frequency (f0) that is produced by the vibration of the vocal folds in the larynx and, on the other hand, by the formants, i.e. the resonances of the vocal tract (vt) rostral to the vocal folds (Reby & McComb, 2003a,b; Taylor & Reby, 2010). In adult male C. elaphus, the larynx has a low resting position (Fitch & Reby, 2001) and is additionally retracted almost down to the sternum during their rutting roars (C. e. scoticus: Reby & McComb, 2003a; C. e. canadensis: Feighny et al. 2006; C. e. corsicanus: Kidjo et al. 2008). This entails a pronounced momentary elongation of the vt and a corresponding decrease of the vt resonances (formants), which inversely correlate with the vt length (Fitch & Reby, 2001; Reby & McComb, 2003a,b). Pronounced momentary retractions of the larynx during the rutting calls of male polygynous ruminants probably evolved through the selection pressure for acoustically exaggerating own body size in contests where optical assessment of a rival was difficult under twilight or nocturnal conditions (Fitch & Reby, 2001; Fitch & Hauser, 2002; Reby & McComb, 2003a,b; McElligott et al. 2006; Frey et al. 2008a,b, 2011). However, similar retraction of the larynx in North American/Asian subspecies of red deer produce tonal high-frequency bugles instead of the usual low-frequency roars and do not reveal their formant structure (Riede et al. 2010; Titze & Riede, 2010). Among European red deer, the minimum distance between formants, e.g. 256.3 Hz in C. e. corsicanus (Kidjo et al. 2008) and 243.5 Hz in C. e. scoticus (Reby & McComb, 2003a), is larger in the smaller subspecies and thus reliably reflects the differences in body size between subspecies. In contrast, the fundamental frequency (f0) of 40 Hz in C. e. corsicanus (Kidjo et al. 2008), of 107 Hz in C. e. scoticus (Reby & McComb, 2003a) and of over 1 kHz in C. e. nelsoni and C. e. canadensis (Feighny et al. 2006; Riede & Titze, 2008; Titze & Riede, 2010), is lowest in the smallest and highest in the largest subspecies, i.e. counter-intuitive to what we would normally expect from size-dependent acoustic features.
A conspicuous feature of the acoustic rutting display in male C. e. hispanicus consists in a pronounced protrusion of the tongue from the wide open mouth with a markedly retracted mouth angle (Fig. 1). It poses a mechanical challenge for the vocal organs as both the tongue and the larynx are connected to the hyoid apparatus but during those ‘tongue-roars’ are strongly pulled in opposite directions. As a neck mane is lacking, the laryngeal and hyoid prominences and their movements are clearly visible during the roars in this subspecies. A geometrical model of the main anatomical structures involved in laryngeal and lingual mobility was established to elucidate their mutual relationships and mechanical interplay in the process of simultaneous tongue protrusion and larynx retraction. This study (i) provides a detailed analysis of the vocal anatomy and the acoustics of the rutting roars in male C. e. hispanicus and (ii) establishes a potential mechanism of simultaneous tongue protrusion and larynx retraction based on graphic reconstructions in single video frames of unrestrained animals.
Fig. 1.

Typical posture of three Iberian red deer stags when producing a ‘tongue roar’. The tongue is pronouncedly protruded, the mouth angles are retracted and the mouth is wide open. Owing to the lack of a neck mane, certain anatomical features are externally visible: the lateral bulging of the rostral mouth floor caused by the contracting geniohyoid muscles, the hyoid prominence caused by the protracted basihyoid, the constriction of the neck circumference at the level of the ear caused by contraction of the parotidoauriculares muscles, the laryngeal prominence caused by the retracted larynx, the bulging of the caudoventral neck skin caused by the contracting sternothyroid muscles, the approximate contour and insertion of the extended sternomandibular portion of the sternocephalic muscle. Maximal protraction of the hyoid apparatus expands the skin of the mouth floor and throat region into a rhomboid region ventral to the lower jaw. Original single video frames: (A,B) lateral view, (C) frontal view.
Materials and methods
Subjects, sites and dates of work
Head-and-neck specimens of two adult male and one adult female C. e. hispanicus, were collected in 2007 at the estate Azagala (39°10′N, 6°50′W, Andalusia, Spain). The upper head of the males was sectioned off dorsal to the tongue and the skull base, to secure the trophies for the hunters, but to leave most of the hyoid apparatus and the vocal organs undamaged. The specimens were deep frozen shortly after death and kept so up to the dissections.
Audio and video recordings of free-ranging unmarked adult male C. e. hispanicus were collected in Andalusia and Extremadura, Spain. Audio recordings of rutting calls were made of about 75 and video recordings of about 50 male C. e. hispanicus. Audio and video recordings were made at four study sites: (i) at the natural reserve Coto Doñana (37°01′N, 6°26′W), 6–11 September 2007; (ii) at the Finca ‘La Ruda y el Moro’ (39°14N’, 6°23′W) on 13 September 2007; (iii) at the Finca ‘Las Monteras’ (38°06N’, 5°15′W) on 22–25 September 2007 and (iv) at the Finca ‘Bonagua’ near Villaviciosa, about 30 km northwest of Córdoba (38°01′N, 5°02′W) on 27 September 2007. Audio recordings were made between 21:00–03:00 h and between 06:00–13:00 h, and video recordings were made from 18:00 h up to sunset. In total, 14 sessions of recordings were made.
The recordings were made from a car, during short-term stops on a field path coursing close to the intact territories of resident males, or from observation towers. At Coto Doñana the recordings were made using a field path coursing close to the intact territories of resident males. The distance of the animals to the microphone and to the camcorder varied from 30 to 150 m. At the estates (fincas) the animals received supplementary food from the game warden at different locations of the area and approached the surroundings of the car so that the natural spatial pattern of territories could not be observed. In this setting, the distance of the animals was 10–80 m.
Audio analyses
For the audio recordings (48 kHz, 16 bit), we used a Fostex Field Recorder FR – 2LE (Fostex Company, Tokyo, Japan) and a Sennheiser MKH 70 P48 condenser directional microphone (Sennheiser, Wedemark, Germany). Only calls of good quality, with clearly visible spectral structure and not superimposed by wind, were used for the acoustical analyses. Before analysis, the calls were down-sampled to 11 025 Hz.
Earlier reports showed that the rutting vocal repertoire of C. e. scoticus and C. e. corsicanus stags includes five vocalizations, differing in structure and behavioural context: common roars, harsh roars and grunt roars, produced in bouts, as well as chase barks, produced in series, and single barks (Reby & McComb, 2003a,b; Kidjo et al. 2008). For acoustic analyses, we took only the most usual bouts consisting of roars; the much more rarely occurring barks were not analysed. We registered a call sequence as a bout only if we were sure that all calls of the sequence came from the same animal and did not contain concurrently produced calls of other stags. We analysed 1146 bouts containing a total of 2928 roars.
We measured the duration of each roar and its position within a bout and we selected the longest roars within bouts to analyse them separately as ‘main roars’ of bouts. Following Reby & McComb (2003a,b) and Kidjo et al. (2008), main roars were classified according to their acoustic structure into two types: either common roars (with a clearly visible f0 and its harmonics) or harsh roars (without a clearly visible f0). Sections of deterministic chaos or subharmonics (Wilden et al. 1998) could account for up to 50% of the duration of common roars and for 50–100% of the duration of harsh roars (Figs 2 and 3).
Fig. 2.

(A) Common roar (‘tongue roar’) and (B) harsh roar of the same young adult Iberian red deer stag which at the time of the recording did not have access to females. The two roars were produced in immediate succession close to a site where two older adult males displayed frequently to their respective hinds.
Fig. 3.
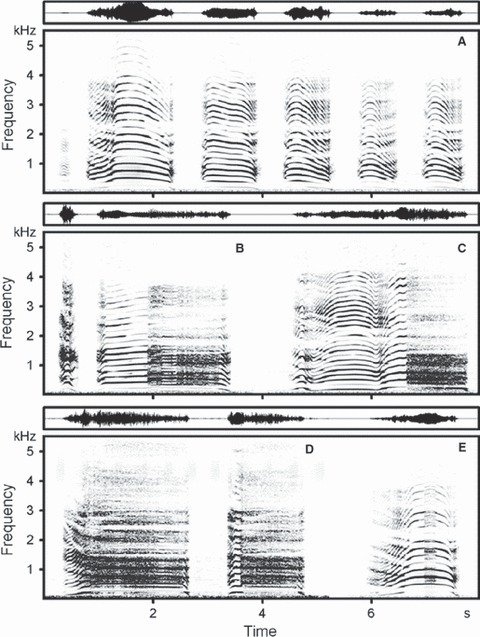
Spectrogram (below) and wave-form (above), of natural bouts of rutting roars of Iberian red deer stags (Supporting Information Audio S1). (A) A six-call bout exhibiting low amplitudes in the lower parts of the call spectra typical for Iberian red deer stags. As a consequence, the bands of fundamental frequency and first harmonic are barely visible. The main common roar is in second place. (B) A two-call bout with the main harsh roar in second place; compared to the harmonics, the fundamental frequency band is barely visible. (C) A single-call bout consisting of a common roar with a large section of deterministic chaos approximately at 6.4–7.0 s. (D) A two-call bout with clearly visible descending formants at the beginning of the main harsh roar in the first place. (E) A single-call bout consisting of a common roar with a small section of deterministic chaos in the middle of the roar. Compared to the harmonics, the fundamental frequency band is poorly visible, whereas the descending formants are clearly visible at the beginning of the roar. The spectrogram was created at 11 025 Hz sampling frequency, Hamming window, FFT 1024, frame 50%, overlap 93.75%.
We measured the duration for each of the 2928 roars on the screen with the standard marker cursor in the spectrogram window (Hamming window, FFT 1024 points, frame 50% and overlap 96.87%) using Avisoft saslab pro software (Avisoft Bioacoustics, Berlin, Germany). We measured the f0 maximum (f0 max) for 1182 of the 2928 roars with the harmonic cursor in the power spectrum, created in the 100-ms section of the f0 maximum area of the roar. All measurements were exported automatically to Microsoft excel (Microsoft Corp., Redmond, WA, USA).
We measured f0 variables in 645 common roars according to the methods of Reby & McComb (2003a) and Kidjo et al. (2008) using praat dsp package v. 5.2.07 (P. Boersma & D. Weenink, University of Amsterdam, Netherlands, http://www.praat.org). The f0 contour was extracted using a cross-correlation algorithm [to Pitch (cc) command in praat]. The time step in the analysis was 0.05 s, and the specified range for the lower and upper limits of the expected range of f0 was 60 and 360 Hz, respectively. These limits were determined by visual assessment of the f0 variation of the calls in the spectrograms. The lower limit was set at 60 Hz, as the f0 in this part of the call spectrum was indistinguishable from the background noise owing to the very low energy flow of the roars of C. e. hispanicus stags. Besides, a preliminary visual analysis of the spectrograms showed that the lower limit of 60 Hz was lower than the minimum f0 for most of the roars.
Spurious values and octave jumps in the f0 contour were corrected manually on the basis of the spectrograms. If a common roar contained sections of deterministic chaos, they were left unmarked. Values of minimum f0 (f0 min), maximum f0 (f0 max), average f0 (f0 mean) and the depth of frequency modulation f0 (Δf0) were taken automatically using Pitch info command in the Pitch edit window. Two different methods (one using Avisoft and one using praat) that were applied to the same roars, provided very close values of f0 max (r = 0.999, R2 = 0.998). Differences between the two measurements did not exceed 3 Hz in 628 of 645 (97.4%) calls. Therefore, we could use the Avisoft measurements of f0 max for those common roars in which the f0 contour extraction was difficult, and also for some of the harsh roars.
As most common roars of the C. e. hispanicus stags had high values of f0 and therefore widely spaced harmonics (see below), formant frequencies could not be measured in this type of call. However, we could measure formant frequencies in harsh roars and in common roars with long sections of deterministic chaos (103 roars in total). In these types of calls we measured the first eight formants (F1–F8) using linear prediction coding (LPC) with praat. Vt length measurements in the dissected specimen served to establish the settings for LPC. The selected LPC parameters for creating the formant tracks, Burg analysis, time step 0.05 s; window analysis 0.1 s; 8–11 formants and maximum formant frequency 1900–2100 Hz, mainly followed Reby & McComb (2003a), Charlton et al. (2007b, 2008) and Kidjo et al. (2008). Formant frequencies were measured from those call parts exhibiting clearly visible formants, which were produced with a fully retracted larynx, i.e. where formant tracks reached their minimum values and were nearly horizontal. The position of the formants was verified by superposition against the spectrogram. In each roar, we analysed the formants in the 0.5 s sections, taking 10 sequential point values separated by a time interval of 0.05 s. Point values of the formant tracks were extracted and exported to excel, where the value of each formant of a given call was calculated as the average value of all the extracted point values of the track.
Applying the model of a straight uniform tube closed at one end, we calculated the minimum formant dispersion (ΔF) for roars of the C. e. hispanicus stags using linear regression according to Reby & McComb (2003a). Based upon the formant frequencies of the roars, the maximum vt length during roars was calculated by the equation: vt length = c/2ΔF, where c is the speed of sound in air, approximated as 350 ms−1.
Video analyses
We used a Sony HDR-HD1E camcorder with conversion lens ×2.0 Sony VCL-HG2037Y (Sony Corp., Tokyo, Japan) for the video recordings. Single video frames were analysed by means of dvgate plus software (Sony Corp., Tokyo, Japan), in a PC. This procedure was used to distinguish successive steps of simultaneous tongue protrusion and larynx retraction, to trace the appearance and movement of the externally visible hyoid and laryngeal prominences and to register expiratory movements of the flanks.
Statistical analyses
Statistical analyses were done with statistica, v. 6.0 (StatSoft, Tulsa, OK, USA); all means are given as mean ± SD. Significance levels were set at 0.05, and two-tailed probability values quoted. As only one of 24 distributions of the values of the acoustic variables differed from normality (Kolmogorov–Smirnov test), we used a one-way anova to compare the acoustics of roars from bouts containing different numbers of roars. We used Student's t-test to compare the acoustics between common and harsh roars and between common roars that differed in position within a bout.
Computer tomographic investigation
The deep frozen or freshly dead specimens were scanned in a 64-slice spiral Computer Tomograph Aquilion CX (Toshiba Medical Systems Corp., 1385 Shimoishigami, Otawara-shi, Tochigi, Japan) at the IZW. The postmortem in situ positions of the vocal organs were registered in black-and-white virtual serial sections (MPRs) and in 3D-reconstructions with appropriate software (vitrea 2).
Anatomy
Specimens were dissected using the in-water method (Frey & Hofmann 2000) and consecutive dissection steps were photographically documented by means of a Nikon D70S digital camera (Nikon Corp., Tokyo, Japan) on a Compact Flash card. The images were fed to a PC and graphically processed (Adobe photoshop 5.5 and CS4; Adobe Systems Inc., San Jose, CA, USA) to identify the individual components of the vocal organs and to clarify mutual anatomical relationships. Anatomical terms are in accordance with the latest edition of the Nomina Anatomica Veterinaria (NAV) http://www.wava-amav.org/Downloads/nav_2005.pdf.
Graphical reconstructions and 2D-model
Graphical reconstructions were obtained using single video frames selected for best representation of the resting position and the maximally retracted position of the larynx, respectively. Integration of the results of behavioural observations, CT investigations and anatomical dissections was then attempted as an overlay of the involved anatomical structures and by hypothesizing on the possible mechanisms of tongue protrusion and larynx retraction in a 2D-model.
Results
Behaviour
Like other subspecies of red deer, C. e. hispanicus stags emit their roars while standing in a typical roaring posture but, in contrast to other subspecies, the stags protrude their tongue spectacularly during most of their common roars (Fig. 1). Typically, the tip of the pronouncedly protruded tongue is ventrally flexed around the chin region. During such a ‘tongue-roar’ the mouth is kept wide open and, in lateral view, the mouth angle is retracted up to a point about mid-way between the nostril and the eye. Regularly, white flakes of mucus covering the moist surface of the tongue can be observed. The hyoid prominence, observable between the branches of the lower jaw, creates a bulging of the pliable skin of the throat region and becomes remarkably prominent during a ‘tongue-roar’. In lateral view, the ventral throat contour is tightly expanded to a rhomboid shape (Fig. 1). At maximal throat expansion, the hyoid prominence is located approximately at the level of the lateral eye angle. In addition, a small lateral bulging of the expanded throat skin can often be observed between the hyoid prominence and the chin. During harsh roars, which are produced from a similar calling posture and in a similar way as in other subspecies, the mouth angles are protracted and the tongue is retained inside the mouth cavity (Fig. 2B).
As in all studied subspecies of red deer, C. e. hispanicus stags pronouncedly retract the larynx almost down to the thoracic entrance both during ‘tongue-roars’ and harsh roars. This can be easily observed owing to the lack of a neck mane in the Iberian subspecies as a downward movement of the laryngeal prominence. The laryngeal prominence is produced by the larynx that causes bulging of the flexible skin of the ventral neck region. During a ‘tongue-roar’, maximal retraction of the larynx coincides with maximal protraction of the tongue. At maximal retraction of the larynx, a further prominence develops between the laryngeal prominence and the rostral end of the sternum as some kind of a ‘swelling’ and disappears after the end of a roar, when the larynx regains its low mid-neck resting position (Figs 1B and 2).
Swallowing, together with deglutition or not, involves a slight upward movement of the larynx, from its low mid-neck resting position towards the angle of the mandibula and down again.
Acoustics
Bouts of roars consisted of 1–12 roars per bout (2.11 ± 1.71, n = 1146). Single-call bouts constituted 34.38% and two-call bouts 24.35% of the total number. Among the 752 bouts consisting of two and more roars, main roars were in first position in 49.87% of the bouts, in last position in 29.65% of the bouts, and intermediate in the remaining 20.48% of the bouts (Fig. 3). The average duration of the main roar of a bout was 1.90 ± 0.50 s; min–max 0.83–3.86 s. In a total of 1146 main roars, 1021 (89.1%) were common roars and 125 (10.9%) were harsh roars. Compared to main common roars, main harsh roars were significantly longer (2.12 ± 0.49 s vs. 1.88 ± 0.50 s, t = 5.05, df = 1144, P < 0.001) and higher in f0 max (235.7 ± 28.7 Hz vs. 222.7 ± 34.5 Hz, t = 3.31, df = 736, P < 0.001). Main harsh roars occurred more often in bouts containing two or more calls (96 of 752, 12.8%) than in single-call bouts (29 of 394, 7.4%).
A distribution of roar durations in a pooled call sample had two peaks (Fig. 4), one at 0.2–0.3 s and a second at 1.5–1.9 s, separated by an intermediate depression at 0.8 s. This depression corresponded to the median of this distribution (0.86 s). Thus, the right peak of this distribution constituted the main roars of the bouts. The left peak comprised bout calls shorter than 0.8 s, i.e. either short common roars or grunt roars.
Fig. 4.
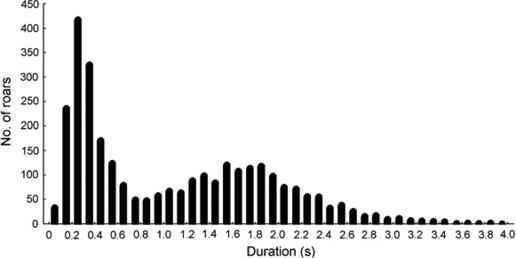
Distribution of the rutting roars of Iberian red deer stags according to duration, n = 2928 roars from 1146 bouts.
A comparison of f0max among roars within bouts (579 roars, 135 bouts containing 3–9 roars per bout, mean 4.35 ± 1.52) showed that the longest (i.e. main) roars were highest in f0 max in 127 (94.1%) of the bouts. In the remaining eight bouts the longest roars were not highest in f0 max. Thus, the overwhelming majority of main roars in bouts were highest in f0.
For bouts consisting of two and more roars, the one-way anova did not reveal any effects of the number of roars per bout on the acoustics of the main common roars (duration –F8,390 = 0.86, P = 0.55; f0 mean –F8,390 = 1.04, P = 0.41; f0 min –F8,390 = 1.37, P = 0.21; f0 max –F8,390 = 0.65, P = 0.74; Δf0–F8,390 = 0.86, P = 0.55). However, the acoustics of main common roars depended on their position within a bout: first roars in bouts were longer in duration, higher in f0 mean and f0max and had a more pronounced frequency modulation than intermediate or last roars in bouts (2.88 < t < 10.10, df = 397, P < 0.01 for all comparisons). No significant differences were found for f0 min (t = 1.90, df = 397, P = 0.06) (Table 1). The acoustics of main common roars that were in first position within bouts containing two or more calls did not differ from those of common roars of single-call bouts (0.08 < t < 1.84, df = 459, P > 0.05 for all comparisons) (Table 1).
Table 1.
Duration and fundamental frequency (f0) variables for main common roars of Iberian red deer stags (mean ± SD; min–max).
| Bout character | Roar position in bout | n calls | Duration, s | f0 mean, Hz | f0 min, Hz | f0 max, Hz | Δf0, Hz |
|---|---|---|---|---|---|---|---|
| Single-call bout | 246 | 2.04 ± 0.45 | 186.7 ± 27.8 | 104.9 ± 17.3 | 226.6 ± 35.6 | 121.7 ± 28.5 | |
| Two or more calls per bout | First call | 215 | 2.13 ± 0.47 | 189.0 ± 26.6 | 106.3 ± 17.1 | 228.2 ± 34.4 | 122.0 ± 31.0 |
| Intermediate call | 78 | 1.65 ± 0.40 | 183.3 ± 23.5 | 111.0 ± 14.3 | 211.9 ± 28.7 | 100.9 ± 27.5 | |
| Last call | 106 | 1.68 ± 0.47 | 180.1 ± 27.7 | 108.4 ± 17.8 | 210.0 ± 31.3 | 101.6 ± 23.0 | |
| Overall | 645 | 1.96 ± 0.49; 0.82–3.81 | 186.0 ± 27.0; 106.9–269.9 | 106.7 ± 17.1; 61.7–185.5 | 222.7 ± 34.5; 129.0–338.1 | 116.0 ± 29.8; 31.1–218.5 |
Formant dispersion of the roars, calculated as mean values of the eight formants by linear regression, was 228.15 Hz, providing an estimated maximum vt length of 767 mm (Fig. 5). Distances between neighbouring formants of roars were uneven, suggesting non-uniformity of the vt. The smallest distances were between F2 and F3 and between F7 and F8, and the largest between F4 and F5 (Fig. 5).
Fig. 5.
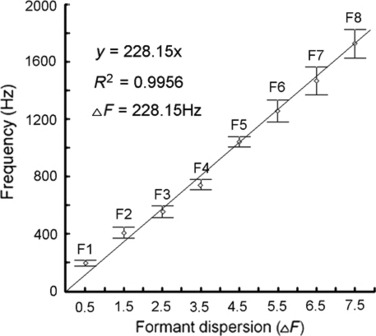
Estimation of minimum formant dispersion (ΔF) in the roars (n = 103) of Iberian red deer stags using linear regression according to Reby & McComb (2003a). The estimated minimum formant dispersion of 228.15 Hz corresponds to an estimated maximum vt length of 767 mm during the roars. Central points show the means of the first eight formants (F1–F8), whiskers show the SD.
Anatomy
Hyoid apparatus, thyrohyoid ligament
The hyoid apparatus of the Iberian red deer subspecies consists of the 11 elements (five paired, one unpaired) typical for ruminants (Fig. 6). The connection to the larynx is not established by a thyrohyoid articulation but instead by a long and elastic thyrohyoid ligament (Fig. 7). The caudally expanding, funnel-shaped caudal end of this ligament enwraps the rostral third of the rostral horn and in addition medially attaches to the middle portion of the pharynx (M. hyopharyngeus) by loose connective tissue. The resting length of the thyrohyoid ligament, between the thyrohyoid and rostral horn, is about 120 mm. Resting length was measured in the course of the dissections (Fig. 7).
Fig. 6.
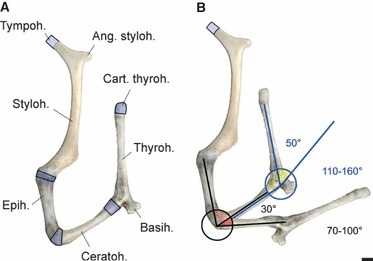
Left half of the hyoid apparatus of an adult male Iberian red deer. (A) Approximate resting position and components; cartilaginous parts marked in blue. (B) Resting position and maximally extended position. In the excised and demusculated hyoid apparatus, the epi-ceratohyoid connection can be extended by 30° (70–100°) and the cerato-basihyoid connection by 50° (110–160°) approximately. Scale bar: 10 mm. Left lateral view.
Fig. 7.
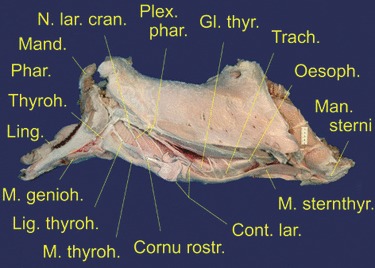
Dissected lower head-and-neck specimen of an adult male Iberian red deer; upper head lacking. Mylohyoid muscle and sternomandibular portion of sternocephalic muscle removed; pharynx, pharyngeal nerves, thyrohyoid, thyrohyoid ligament and strap muscles exposed. Contours of the larynx included as overlay. Scale bar: 50 mm. Left lateral view.
Pharynx, soft palate
As a consequence of the low mid-neck resting position of the larynx, the pharynx is of considerable length. The distance from the angle of the mandibula down to the rostral edge of the thyroid lamina is about 200 mm. The major muscular components of this wide, hose-like middle portion of the pharynx are the M. hyopharyngeus and the M. thyropharyngeus (Table 2). The circular and oblique bands of these muscles, together with their connective tissue links, allow for a substantial longitudinal elasticity of the pharynx.
Table 2.
Vocal tract muscles and laryngeal muscles of male Cervus elaphus hispanicus.
| Portion | Origin | Termination | Remarks | |
|---|---|---|---|---|
| M. parotidoauricularis | Lateroventral angle of auricular cartilage (Cart. anularis, Cart. meatus acustici) | Medioventrally on contralateral muscle | Muscle band, slightly expanding ventrally and hammock-like embracing the retromandibular fossa and the throat region including the rostral parts of the sternomandibular and omohyoid muscles | |
| M. mylohyoideus | Rostral portion | Mylohyoid line, medial surface of mandibula, mental angle to level of second lower cheek tooth (P3) | Ventromedian raphe, basihyoid | Termination of rostral portion covered by caudal portion, fibres running in oblique caudoventral direction |
| An additional slender band embraces the rostral portion at the level of the first lower cheek tooth (P2) | ||||
| Caudal portion | Mylohyoid line, medial surface of mandibula, level of fifth lower cheek tooth (M2) to level caudally beyond last lower molar (M3) | Ventromedian raphe, basihyoid | Hammock-like embracing the basihyoid and the terminations of the muscles attaching to it; any ventral excursion of the basihyoid will expand the mylohyoid muscle; fibres running in transverse direction | |
| M. sternocephalicus | Pars mandibularis | Sternal manubrium, lateral to sternomastoid portion | Rostral edge of masseter muscle and lateral surface of mandibula at the level of the caudal portion of the mylohyoid muscle | Covers sternomastoid portion laterally; thick powerful muscle coursing immediately ventral to external jugular vein; terminal portion dorsally bordered by ventral contours of parotid gland and masseter muscle |
| Pars mastoidea | Sternal manubrium, medial to sternomandibular portion | Mastoid process of temporal bone, muscular tubercle at skull base | From its ventral edge, from the throat region along most of the ventral neck contour caudally, extends a tough connective tissue layer that envelops the underlying muscles forming some sort of a gliding sheath for the omohyoid, thyrohyoid, sternothyroid and the vestigial sternohyoid muscles, thereby allowing independent action of these muscles and the overlying sternomandibular portion | |
| M. omohyoideus | Fascia lateral to cervical vertebrae 3–5 | Basihyoid, laterally covering the caudal portion of the mylohyoid muscle and the termination of the stylohyoid muscle | Fibers converge considerably towards termination inducing a strongly triangular muscle shape; from its caudoventral edge another connective tissue sheath extends and envelops the thyrohyoid, sternothyroid and the vestigial sternohyoid muscle | |
| M. stylohyoideus | Caudal angle of stylohyoid | Basihyoid | Splitting into two slender muscle bands with slit-like opening in between through which runs the intermediate tendon of the digastric muscle; raises the basihyoid | |
| M. sternohyoideus | Sternal manubrium together with sternothyroid muscle; no tendinous insertion visible | Together with sternothyroid muscle on ventral surface of thyroid cartilage | This muscle does not reach its typical origin on the basihyoid; instead, only its caudal portion is retained and, together with the sternothyroid muscle, terminates at the caudal end of the thyroid cartilage | |
| Owing to the derived insertion on the thyroid cartilage, this muscle has been transformed into a second or auxiliary ‘sternothyroid’ muscle. Functionally, it has become an additional retractor of the larynx and enhances the power of retraction | ||||
| M. sternothyroideus | Sternal manubrium together with sternohyoid muscle; no tendinous insertion visible | Thyroid cartilage | Main retractor of the larynx; reinforced by assimilation of the caudal part of the sternohyoid muscle | |
| M. thyrohyoideus | Thyroid cartilage | Thyrohyoid, ventral two thirds of caudal edge | Of considerable length owing to the low mid-neck resting position of the larynx; protractor of the larynx | |
| M. occipitohyoideus | Paracondylar process | Dorsal end of stylohyoid; | Origin not dissected; pulls stylohyoid caudally and, thereby, assists in moving the basihyoid, the root of the tongue and the thyrohyoids caudally | |
| M. stylopharyngeus rostralis | Medioventrally from stylohyoid | Pharyngeal raphe | Not reliably dissected, as it had been partly destroyed in the process of specimen sampling | |
| M. stylopharyngeus caudalis | Stylohyoid, dorsomedially | Thyroid cartilage, caudodorsal edge | Of considerable length owing to the low mid-neck resting position of the larynx; fibres diverge towards termination; retracts the stylohyoid or protracts the larynx; aids in suspending the larynx and in re-shortening the pharynx after extension has ended. In view of the derived insertion on the dorsal edge of the thyroid cartilage in ruminants, the name of this muscle is misleading and it should be better called a ‘stylothyroid’ muscle in respective taxa | |
| M. hyopharyngeus | Most of its fibres have lost their typical origin from cerato- and thyrohyoid and, instead, originate from the lateroventral wall of the pharynx | Pharyngeal raphe | Owing to the low mid-neck resting position of the larynx, this muscle has extended its rostrocaudal length; its circular fibres form the muscular wall of the pharynx between the thyrohyoid and the caudal pharyngeal constrictors; in its caudolateral half this muscle loosely connects to the thyrohyoid ligament; constricts middle portion of pharynx, aids in suspending the larynx and re-shortening the pharynx | |
| Mm. constr. phar. caudd. | Owing to the low mid-neck resting position of the larynx, these muscles have a more caudal position than in those species with undescended larynx; constrict caudal portion of pharynx | |||
| M. thyropharyngeus | Thyroid cartilage, caudally, ventrally, and rostrally | Pharyngeal raphe | Enveloping the larynx, most caudal fibres dorsally terminating with separate short tendon; caudal fibres coursing circularly, rostral fibres in strongly oblique dorsorostral direction; aids in suspending the larynx and in re-shortening the pharynx after extension has ended; can depress corniculate processes | |
| M. cricopharyngeus | Cricoid arch, dorsolaterally | Pharyngeal raphe | Two short and slender muscle bundles, caudally covering the dorsal part of the cricothyroid muscle; flanked by the thyropharyngeus muscle laterally and by the oesophagus medially | |
| M. levator veli palatini | Tympanic part of temporal bone | Enters soft palate to fuse with contralateral muscle in the median plane | Origin not dissected; medial to M. tensor veli palatini; raises the soft palate | |
| M. tensor veli palatini | Tympanic part of temporal bone | Palatine aponeurosis | Origin not dissected; tenses and straightens the rostral part of the soft palate | |
| M. palatopharyngeus | Palatine aponeurosis | Pharyngeal raphe | Of considerable length owing to the long soft palate of red deer; courses laterally along the pharyngeal wall; sphincter of the nasopharynx and of the intra-pharyngeal ostium; aids in suspending the larynx and in re-shortening of the pharynx | |
| M. hyoepiglotticus | Basihyoid and ceratohyoid | Rostroventrally on epiglottic cartilage | Of considerable length owing to the low mid-neck resting position of the larynx; depressor of the epiglottis, aids in suspending and protracting the larynx after retraction has ended | |
| M. styloglossus | Stylohyoid, lateroventral quarter | Lateroventral surface of the tongue up to its apex | Most lateral extrinsic lingual muscle that shortens and retracts the tongue, elevates the tip, or pulls the tip of the tongue laterally | |
| M. hyoglossus | Basihyoid, dorsorostrally; ventral third of thyrohyoid, rostrally; lateral to ceratohyoid muscle | Enters root of tongue between genioglossus muscle medially and styloglossus muscle laterally; its fibres extend towards the torus of the tongue | Courses lateral to the root of the tongue; retracts and depresses the tongue; antagonist of genioglossus muscle; with basihyoid fixed, simultaneous action of hyoglossus and genioglossus muscle depresses the tongue | |
| M. genioglossus | Incisival part of mandibula, mediocaudally | Enters the tongue ventrally in a paramedian plane, lateral to the lingual septum; owing to fan-shaped course of its fibres, its termination extends caudally up to the level of the basihyoid | It protrudes the tongue by pulling it rostrally and ventrally | |
| M. geniohyoideus | Incisival part of mandibula, mediocaudally, lateral to the genioglossus muscle | Basihyoid | This muscle is covered by the mylohyoid muscle; draws basihyoid and, with it, the tongue rostrally | |
| M. ceratohyoideus | Epi-, ceratohyoid, caudal edge | Thyrohyoid, rostral edge of dorsal half | Reduces the angle between cerato- and thyrohyoid; aids in suspending and protracting the larynx | |
| M. cricothyroideus | Cricoid arch, lateral surface | Thyroid cartilage, caudal edge and caudomedial surface; cricothyroid articulation; caudal horn of thyroid cartilage, ventral edge | Pivots cricoid and thyroid cartilage closer together, thereby tensing the vocal folds; pivot: cricothyroid joint | |
| M. cricoarytenoideus lateralis | Cricoid arch, rostrodorsally | Muscular process of arytenoid cartilage, laterocaudal surface | Pulls muscular process ventrolaterally; aids in closing the glottis | |
| M. cricoarytenoideus dorsalis | Dorsolateral half of cricoid lamina | Muscular process of arytenoid cartilage, dorsocaudal surface | Pulls muscular process dorsolaterally; opens the glottis | |
| M. arytenoideus transversus | Muscular process of arytenoid cartilage, rostrolateral surface | Contralateral muscle, mediodorsally | Adducts arytenoid cartilages; aids in closing the glottis and, possibly, also in opening the glottis | |
| M. thyroarytenoideus | Rostral portion | Lateroventral edge of epiglottis and adjacent lateroventral wall of laryngeal vestibulum | Arcuate crest of arytenoid cartilage, rostrally adjacent to termination of middle portion | Certain amount of twisting, fibres originating most rostrally, terminate most caudally and vice versa; similar to ‘aryepiglottic muscle’ of humans |
| Middle portion | Rostral fibres from lateroventral wall of laryngeal vestibulum, caudally adjacent to origin of rostral portion; | Rostral fibres to arcuate crest of arytenoid cartilage, caudally adjacent to termination of rostral portion | ||
| Caudal fibres paramedially from dorsal aspect of thyroid cartilage; partly covering vocal ligament laterally | Caudal fibres to lateral surface of arytenoid cartilage, rostral to its muscular process | |||
| Caudal portion | Paramedially from dorsal aspect of thyroid cartilage, caudally adjacent to origin of caudal fibres of middle portion; partly covering vocal ligament laterally | Muscular process of arytenoid cartilage, ventrally adjacent to termination of lateral cricoarytenoid muscle | At origin, fibres fan out from a point at the ventrocaudal end of the vocal ligament, partly by short joint tendons; most medial bundle terminates ellipsoid-shaped on vocal process of arytenoid cartilage at dorsal end of vocal ligament |
Like the pharynx, the soft palate is of extraordinary length. It extends from the choanae down to the intrapharyngeal ostium, positioned dorsally opposite to the laryngeal entrance (Fig. 8). The length of the soft palate is about 250 mm. Accordingly, the M. palatopharyngeus that forms the lateral edge of the soft palate, is of corresponding length. In other words, the naso- and oropharynx are completely separated from each other by this particularly long soft palate all the way down to the mid-neck region.
Fig. 8.
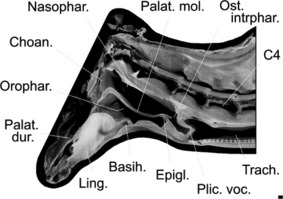
Virtual paramedian section of the lower head and neck of an adult male Iberian red deer, illustrating the elongated soft palate that completely subdivides the elongated pharynx from the choanae down to the intrapharyngeal ostium at the level of the third cervical. As the soft palate is laterally fused to the pharynx wall, the sole caudal communication between the nasal and oral portion of the pharynx is via the intrapharyngeal ostium. In the normal breathing position, the laryngeal entrance protrudes into the nasal portion while the larynx is retracted for the production of an oral call. In the latter case the intrapharyngeal ostium is mostly closed by contraction of the surrounding palatopharyngeal muscle. Scale bar: 10 mm. Medial view.
Larynx
The larynx is suspended at a low mid-neck resting position at a level fluctuating from the mid of the third neck vertebra to the rostral end of the fourth neck vertebra. This position has been ascertained by combining results from single video frames, CT images and dissections.
The basic data on the five intrinsic laryngeal muscles are given in Table 2. The thyroarytenoid muscle consists of three portions, of which the rostral one is the weakest; the latter does not originate from the thyroid cartilage but lateroventrally from the epiglottis and from the wall of the laryngeal vestibulum. Thus, this rostral portion seems to correspond with the aryepiglottic muscle in the human larynx (Duncker, 1985, p. 338f). The stronger middle and caudal portions cover the vocal ligament laterally. The most medial bundle of the caudal portion of the thyroarytenoid muscle terminates in a conspicuous, ellipsoid-shaped area on the tip of the vocal process, caudally adjacent to the dorsal end of the vocal ligament (Figs 9 and 10).
Fig. 9.
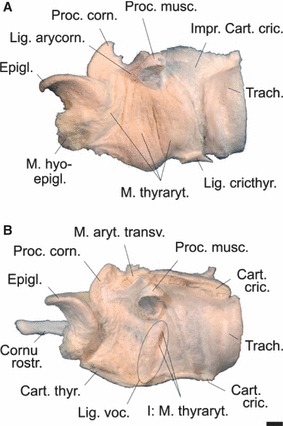
(A) The three portions of the thyroarytenoid muscle and (B) the vocal ligament of an adult male Iberian red deer. (A) Left halves of thyroid and cricoid cartilage removed. (B) Thyroarytenoid muscle removed, exposing the lateral surface of the left vocal ligament and the insertion of the most medial bundle of the caudal portion of the thyroarytenoid muscle on the tip of the vocal process. Scale bar: 10 mm. Left lateral view.
Fig. 10.
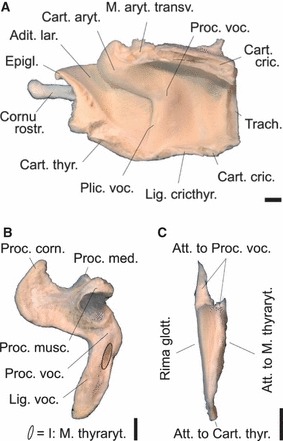
(A) Right half of larynx with right vocal fold, (B) left arytenoid cartilage plus vocal ligament and (C) excised left vocal ligament of an adult male Iberian red deer. No laryngeal ventricle. Dorsoventral vocal fold length is about 30 mm. Scale bar: 10 mm. (A) Medial view, (B) left lateral view, (C) frontal view.
The mucous membrane relief of the larynx and the laryngeal cartilages are presented photographically in Figs 10 and 11. The vocal process of the arytenoid cartilage is short. The vocal fold has a flexible rostral edge that is set at an angle of about 80° against the longitudinal axis of the larynx. The dorsoventral length of the vocal fold in the two adult males is about 30 mm, respectively. The vocal fold is supported by a strong vocal ligament extending from the vocal process of the arytenoid cartilage to the dorsal surface of the thyroid cartilage. The dorsoventral length of the vocal ligament exceeds that of the vocal fold (35 mm), and its maximal rostrocaudal length is 12 mm. Transverse diameters of the vocal ligament are 6 mm close to the vocal process, 4 mm at half length, and 1 mm close to the thyroid cartilage. Maximal transverse diameter in the dorsal third is 7 mm. A laryngeal ventricle is missing.
Fig. 11.
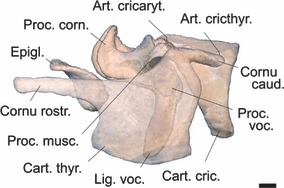
Laryngeal cartilages of an adult male Iberian red deer assembled in near-natural position. Left half of thyroid cartilage made translucent to reveal the contours of the underlying parts. Left arytenoid cartilage with attached vocal ligament and without (other soft parts removed) to expose its short vocal process. Scale bar: 10 mm. Left lateral view.
Overall length of the larynx is about 130 mm from the rostral tip of the rostral horn up to the caudal edge of the cricoid plate in the two male specimens and the female. Therefore, in our minimal sample size a sexual dimorphism of larynx length did not occur.
In the males, the rostral horn of the thyroid cartilage is of considerable length: 42% of the overall length of the thyroid cartilage (measured from tip of rostral horn to the tip of caudal horn –Fig. 11). It is connected to the hyoid apparatus by the highly elastic thyrohyoid ligament (see above).
Vocal tract musculature
A complete account of the dissected vt muscles is given in Table 2. In the following, only those muscles are shortly featured that, as a consequence of the low mid-neck resting position of the larynx, are unusual in size and shape and may have specific functions in the context of laryngeal mobility. These morphofunctional issues will be taken up again in the Discussion section.
The M. stylohyoideus is a double muscle, i.e. it consists of two slender muscle bands with common respective origin (angle of stylohyoid) and termination (basihyoid). Through a slit-like opening between these two muscle bands runs the intermediate tendon of the digastric muscle. The M. stylohyoideus raises the basihyoid. The M. ceratohyoideus fills the triangle between the epi- and ceratohyoid and the thyrohyoid. The M. ceratohyoideus aids in suspending the larynx in its low mid-neck resting position and in protracting the larynx after retraction has ended by pulling the thyrohyoid dorsorostrally. Remarkably, the M. sternohyoideus does not reach the basihyoid. Instead, it terminates together with the M. sternothyroideus at the thyroid cartilage. Along its entire course, from the sternal manubrium to the thyroid cartilage, the M. sternohyoideus remains a slender, weak bundle of muscle fibres that is fused to the ventral edge of the M. sternothyroideus. Accordingly, its termination is on the caudoventral surface of the thyroid cartilage. The fused M. sternothyroideus and M. sternohyoideus are the main larynx retractors. The M. stylopharyngeus caudalis, owing to the low mid-neck resting position of the larynx, has considerably increased its rostrocaudal length. Originating from the dorsomedial surface of the stylohyoid, its fibres diverge to terminate on the caudodorsal edge of the thyroid cartilage. The M. stylopharyngeus caudalis aids in suspending and protracting the larynx after retraction has ended and in re-shortening the pharynx. Most of the fibres of the M. hyopharyngeus have lost their typical origin from cerato- and thyrohyoid, and instead originate from the lateroventral wall of the pharynx. As a result of the low mid-neck resting position of the larynx and the concomitant permanent elongation of the pharynx, this muscle has considerably extended its rostrocaudal length. Its more or less circular fibres form a major component of the muscular wall of the pharynx, or, in a way, the muscular envelope of the middle portion of the pharynx. The M. hyopharyngeus aids in suspending the larynx and in re-shortening the pharynx after extension during larynx retraction has ended. In addition, it can constrict the middle portion of the pharynx. In contrast to the M. cricopharyngeus, which is small, the M. thyropharyngeus has considerably increased in length and size. First, it almost completely envelops the thyroid cartilage, i.e. its origin has extended ventrally almost down to the ventromedian line. Secondly, its elongated rostral fibres take a strongly oblique course in dorsorostral direction, providing a strong muscular connection between the larynx and the middle portion of the pharynx. The M. thyropharyngeus aids in suspending the larynx and in re-shortening the pharynx after extension during larynx retraction has ended. In addition, it can constrict the caudal portion of the pharynx and exert pressure on the corniculate processes. The M. palatopharyngeus, owing to the long soft palate, has considerably increased its rostrocaudal length. As a powerful muscle band it courses laterally within the pharyngeal wall down to the intrapharyngeal ostium, i.e. to the low mid-neck resting position of the larynx. The M. palatopharyngeus aids in suspending the larynx and in re-shortening the pharynx after extension during larynx retraction has ended. In addition, it constricts the intrapharyngeal ostium. The great distance between the basihyoid and the resting position of the larynx entailed a considerable increase in the length of the M. hyoepiglotticus. The M. hyoepiglotticus depresses the epiglottis. In addition, it aids in suspending the larynx and in protracting the larynx after retraction has ended.
Vocal tract length
The resting length of the vt was measured during dissection, in parasagittal virtual sections of CT scans and in single video frames of rutting males. The average from these combined data produced a resting vtl of 460 ± 37 mm. The analysis of single video frames of four stags exhibiting maximal retraction of the larynx in a lateral view during a tongue roar yielded a vtl of 715 ± 16 mm. Accordingly, the vt in the Iberian red deer stag can be elongated by about 55% during a tongue roar. The resting length of the fused sternothyroid and sternohyoid muscles (main larynx retractors) was about 400 mm. An increase of vtl by 250 mm at maximal larynx retraction, i.e. at maximal contraction of the above muscles, would require a shortening of about 2.5 times (62.5%). Gain in vtl by protruding the mouth angles during a harsh roar was roughly estimated to be 50 mm, i.e. around 10% of the resting length.
Nerves of the pharyngeal region
Remarkably, the more or less interconnecting nerves of the pharyngeal region undulated considerably on their way to their respective target fields. As a consequence of the low mid-neck resting position of the larynx and of the considerably elongated pharynx, the pharyngeal nerve plexus (Plexus pharyngeus) has correspondingly extended its range of supply in caudal direction. The undulatory pattern was particularly observed in the pharyngeal branches of the cranial cervical ganglion, the pharyngeal and esophageal branches of the vagus nerve, the pharyngeal branch of the glossopharyngeal nerve, the cranial laryngeal nerve and the ventral branch of the first cervical (spinal) nerve. The final portions of the cranial laryngeal nerve, i.e. its branch to the caudal constrictors of the pharynx, its external branch supplying the cricothyroid muscle, and its internal branch supplying the laryngeal mucosa, not only have to adjust to the low mid-neck resting position of the larynx. In addition, they have to follow the repeated pronounced extensions of the pharynx and the caudal movements of the larynx, which is achieved by stretching of their multiple reserve loops (Fig. 12). As a consequence of the low mid-neck resting position of the larynx, the recurrent laryngeal nerve and its end portion, the caudal laryngeal nerve, do not rise as far cranially as in species with an ancestral high larynx position, e.g. in domestic pigs, ruminants and horses (Nickel et al. 2004; see also Laitman & Reidenberg, 1988; Fitch, 2010).
Fig. 12.
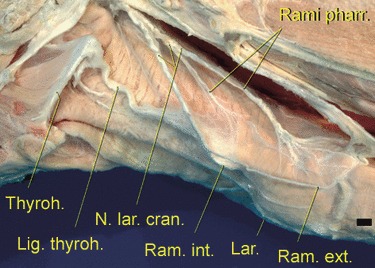
Multiple reserve loops of the branches of the cranial pharyngeal nerve along the elongated pharynx between the hyoid apparatus and the larynx, the latter being completely enveloped by the thyropharyngeus muscle (one of the caudal pharyngeal constrictors). The loops serve to adjust the nerve length to the pronounced extensions of the pharynx and the caudal movements of the larynx, Scale bar: 10 mm. Left lateral view.
Roaring with protruded tongue: a 2D model of the potential mechanism
Apart from the pronounced retraction of the larynx, Iberian red deer stags produce many common roars while pronouncedly protruding their tongue. The graphic reconstruction (Figs 13 and 14) suggests that a simultaneous maximal retraction of the larynx and maximal protrusion of the tongue will depend mainly on contraction of the sternothyroid muscle and the co-operative, antagonistic action of the geniohyoid and genioglossal muscles. To allow pronounced protrusion of the tongue, the hyoid apparatus must be kept from being retracted to any greater extent by the caudally moving larynx, which is connected to the hyoid apparatus via the thyrohyoid ligament. The necessary protraction of the hyoid apparatus, balancing the caudal pull exerted by the powerfully contracting sternothyroid (and sternohyoid) muscles via the larynx and the extended and tensed thyrohyoid ligament, is probably achieved by maximal contraction of the geniohyoid muscle, as indicated by the small lateral bulging of the throat skin between the hyoid prominence and the chin. Additionally, the rostral fibres of the genioglossus muscle have to contract strongly, thus effecting maximal protrusion of the tongue from the wide open mouth. Probably, the caudal fibres of the genioglossus muscle also contract, thereby drawing the root and torus of the tongue ventrally to allow free passage of the exhalatory airstream producing the roar. Any pronounced caudal movement of the basihyoid during sternothyroid (and sternohyoid) muscle contraction is prevented not only by antagonistic contraction of the geniohyoid muscle but also by the high resilience of the thyrohyoid ligament, which allows a considerable retraction of the larynx before transmitting the complete tractive power on the hyoid apparatus.
Fig. 13.
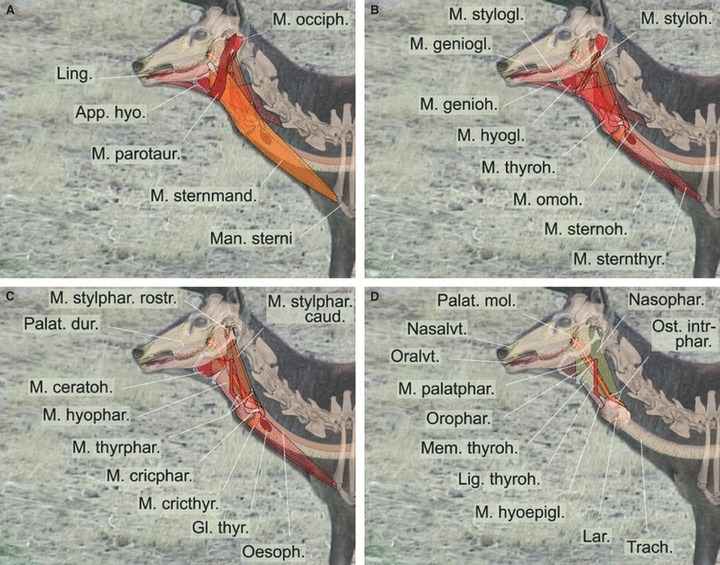
Graphic 2D-reconstruction of the resting position of the larynx and vocal tract structures in an adult male Iberian red deer. Overlays including single video frame, original skeletal parts and reconstructed soft parts. (A–D) Different layers, proceeding from superficial to deep.
Fig. 14.
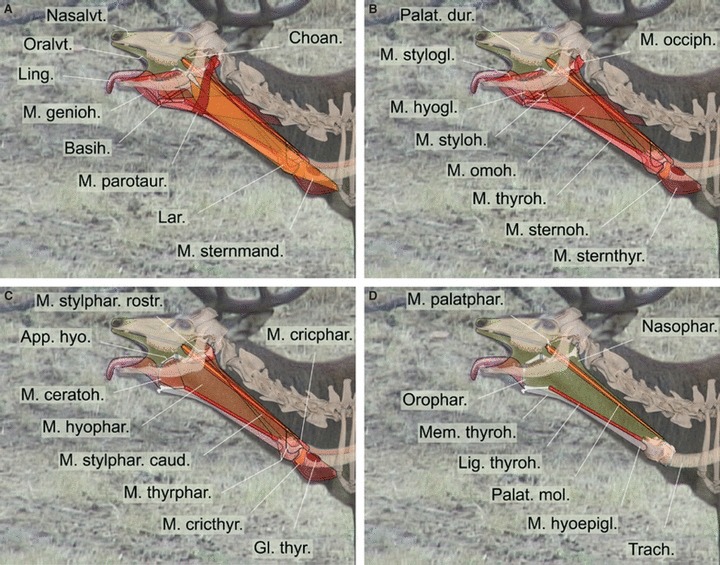
Graphic 2D-reconstruction of major changes involved in retraction of the larynx and simultaneous protraction of the tongue during emission of a ‘tongue’ rutting roar. Overlays including single video frame, original skeletal parts and reconstructed soft parts. Same individual as in Fig. 13. (A–D) Different layers, proceeding from superficial to deep.
In the following, special features of the vt muscles (cf. Table 2) are shortly commented on with regard to their potential respective functions in the context of laryngeal mobility. The double-banded stylohyoid muscle may increase control of the basi- and thyrohyoid positions, which can be expected to vary considerably between the resting position of the hyoid apparatus and its position under maximal tension, i.e. when the larynx is retracted and the tongue protracted simultaneously. Better control, compared with a one-banded muscle, can be expected to result from integrated differential contractions of the rostral and caudal portions while using the intermediate tendon of the digastric muscle as a slide bearing. The sternohyoid muscle, basically a slender vestigial muscle band that is ventrally fused to the sternothyroid muscle and has lost its rostral portion, typically terminating on the basihyoid, co-operates with and enhances the function of the strong sternothyroid muscle in momentary powerful retraction of the larynx. The caudal stylopharyngeal muscle (in ruminants actually a ‘stylothyroid’ muscle, Table 2) can be expected to aid in suspending the larynx at its low mid-neck resting position and, eventually, in protraction of the larynx up to its resting position after the concerted contraction of the sternothyroid and sternohyoid muscles has ended. Similarly, the thyropharyngeal muscle, by virtue of its long oblique bundles, may assist in suspension of the larynx in the resting position and also in returning it to the resting position. The palatopharyngeal muscle has been dramatically elongated as a result of the evolution of an enormously long soft palate. It can shorten the soft palate and has retained its function of a sphincter of the intrapharyngeal ostium, essential for breathing and swallowing. Thereby, compared with the ancestral situation, transition of food into the oesophagus has shifted from a position at the caudal end of the oral cavity, close to the root of the tongue, all the way down to a low mid-neck position.
Discussion
Larynx retraction and tongue protrusion
The capacious rhomboid expansion of the ventral throat skin (Fig. 1) probably arose as a consequence of the powerful caudoventrally directed force, exerted indirectly on the basihyoid by the contracting sternothyroid muscles via a paired converging angle lever (both thyrohyoids) and the rostrodorsally directed force exerted directly on the basihyoid by the contracting geniohyoid muscles. Together with the funnel-shaped rostral end of the vt and the wide open mouth, this will tend to physically enlarge those surfaces of the upper vt available for evaporative cooling. As the sternohyoid muscle does not reach the basihyoid but is fused to the sternothyroid muscle and also inserts to the thyroid cartilage, the ventral neck contour between the hyoid prominence and the laryngeal prominence is not a straight line but is rostrally curved. In contrast, the throat contour between the hyoid prominence and the chin forms a straight line owing to the contracted geniohyoid muscles that directly connect the basihyoid to the mandibula.
The additional ‘swelling’, caudal to the laryngeal prominence during maximal retraction, might be caused by the powerful contraction of the main larynx retractor, the sternothyroid muscle plus the vestigial band of the sternohyoid muscle fused to it. Powerful contraction involves extreme shortening and an increase of the muscle's cross-section so that it will tend to cause bulging of the ventral neck skin between the retracted larynx and the rostral end of the sternum (Figs 1 and 2). As these strap muscles are long and slender, one can expect a large number of sarcomeres arranged in series within each myofibril. Therefore, a shortening of 60–65% seems realistic and is close to textbook readings (50–60%) on general muscle biomechanics (Nordin & Frankel, 2001). Specific evolutionary adaptations or seasonal modifications of strap muscle structure to the extreme requirements of multiple momentary larynx retractions during the rut may exist but these were not addressed in the present work.
Furthermore, pronounced larynx retraction almost down to the thoracic aperture requires considerable resilience or yielding of those structures bridging the distance between the skull and the larynx, particularly the pharynx, the blood vessels and the nerves. In the case of the pharynx this is achieved by its muscular wall, including its connective tissue components. The walls of the blood vessels are of inherent high elasticity and the nerves in that region yield to the strong caudal pulling by stretching their relaxed undulated portions. In contrast, non-muscular ventral neck structures caudal to the larynx will be exposed to compressive forces. The trachea supposedly can absorb only part of this longitudinal compression by intrinsic shortening, and most of its length that is caudally pulled down the neck contour would have to be accommodated within the thorax, most probably by the elasticity of lung tissue and bronchi. Post-laryngeal blood vessels are hypothesized to absorb momentary longitudinal compression by intrinsic elasticity of their walls and, finally, by bending. Post-laryngeal nerves, particularly the recurrent laryngeal nerve, might react to momentary longitudinal compression by adopting an undulating pattern or by forming larger loops.
As a consequence of the extremely elongated soft palate and the long, almost completely subdivided pharynx, there is no need to protract the larynx fully from its low mid-neck resting position all the way up to the root of the tongue in order to accomplish the swallowing procedure. Instead, a slight rostral movement of the larynx suffices, most probably to secure positioning of the laryngeal entrance in the intrapharyngeal ostium to avoid swallowing the wrong way. With fixed basihyoid, the slight rostral movement of the larynx might be effected by a short contraction of the thyrohyoid muscle, possibly assisted by corresponding contractions of the caudal stylopharyngeus (‘stylothyroid’) muscle and the caudal sphincter muscles of the pharynx.
Our data suggest that the tongue protrusion display poses a mechanical challenge for the vt and the hyoid apparatus and may be energetically costly. This imposes a handicap on the rutting Iberian red deer stags in addition to the costs of the emission of several hundred roars per day, concomitant larynx retractions, abdominal contractions and the emission of large volumes of air from the lungs reported for Scottish red deer stags (Clutton-Brock et al. 1982; Reby & McComb, 2003b). Concurrently, the tongue protrusion behaviour with wide open mouth may provide benefits in terms of intermittent evaporative cooling from the respiratory tract surfaces, as this is an effective mechanism of heat dissipation under high metabolic and environmental heat loads (Robertshaw, 1968, 2006; Hales & Brown, 1974; Schmidt-Nielsen, 1997; Vesterdorf et al. 2011). In Córdoba, Andalusia, the average daily temperature maximum in September is 31.7 °C, with a mean of 24.0 °C and minimum of 16.2 °C (http://en.wikipedia.org/wiki/C%C3%B3rdoba,_Spain). Thus considerable environmental heat loads persist for much of the night (cf. Hillel, 1998; Ochsner et al. 2007), which may imply the danger of body overheating in physically strained rutting stags.
There are two avenues of evaporative cooling: sweating via the body surface and heat loss via the moist upper respiratory surfaces (Dmi'el & Robertshaw, 1983). Like sheep and cattle, red deer have sweat glands on their trunk surface and are capable of both sweating and panting (Johnson, 1971; Johnson et al. 1972; Sokolov, 1982). Sweating is favoured by direct solar radiation, which heats the body surface and thereby increases cutaneous moisture loss (Dmi'el & Robertshaw, 1983). At the same time, exposure to high air temperatures increases the proportion of respiratory cooling as a result of higher arterial and hypothalamic, but lower skin temperatures (Dmi'el & Robertshaw, 1983). In domestic sheep, respiratory cooling by panting may account for 60% of total heat loss when exposed to air temperatures above 25 °C (Hales & Brown, 1974). Freshly shorn sheep shift their thermoregulatory response from panting to sweating, indicating the influence of prevailing conditions on the relative proportions of panting and sweating (Johnson, 1971).
Consistently, in an experimental set-up, high ambient temperatures (heat stress) forced red deer stags of an unidentified subspecies to increase their respiratory frequency to over 200 breaths per minute, then exhibiting open-mouthed panting (Johnson et al. 1972; Jenkinson, 1973). Tongue protrusion was not explicitly reported in that study. Cineradiographic analysis of panting in domestic dogs clearly identified the soft palate, the hyoid apparatus and the tongue as the major active components of panting (Biewener et al. 1985). In Norwegian reindeer (Rangifer tarandus tarandus), the air flow is bidirectional during both high-frequent but shallow close-mouthed and open-mouthed panting. Interestingly, nasal airflow is drastically reduced during open-mouthed panting so that most of the evaporative cooling occurs via the surfaces of the oral cavity and the richly vascularized tongue (Schmidt-Nielsen et al. 1970; Biewener et al. 1985; Aas-Hansen et al. 2000; Blix et al. 2011). In Iberian red deer stags, the airflow during the common roars is unidirectional and the roars are produced during strong expiration along the extended tongue through the wide-open mouth.
Notwithstanding this, the common roars of Iberian red deer stags, involving the protrusion of the tongue and exposing large areas of the mucous membranes of the oral cavity, may have a thermoregulatory function in addition to their acoustic function of announcing own quality to conspecifics. Compared to panting, less frequent roaring with a protruded and salivated tongue might open up an alternative thermolytic strategy avoiding excessive water loss in a dried up habitat and the danger of hypocapnia and respiratory alkalosis (cf. Bell et al. 1983; Robertshaw, 2006). This seems all the more plausible as panting implies energy costs by recruiting additional blood flow to and activity of the respiratory muscles. In animals at rest, these costs are compensated for by reducing blood flow to non-respiratory muscles (Hales, 1973; Robertshaw, 2006) but this might not be possible during intense male rutting behaviour. Therefore, common roars, which have to be produced frequently during the rut anyway, might decrease the energy costs for evaporative cooling in Iberian red deer stags.
Tongue protrusion might be a general thermoregulatory response of red deer to prevent overheating. Possibly, tongue protrusion during common roars of Iberian red deer stags derive from this physiological behaviour.
Non-uniform vocal tract
Along most of its length, the walls of the oral vt consist of soft tissue, mainly musculature and connective tissue (Figs 13 and 14). Therefore, in addition to changes of vtl, cross-sectional changes of vt shape, i.e. expansions and constrictions, are likely to occur and will influence the vocal signal on its way through the vt.
Constrictions during vocalization can be expected at the following sites by contractions of the following muscles (given in brackets): laryngeal vestibulum (rostral portion of thyroarytenoid muscle); laryngeal entrance and caudal pharynx region (thyropharyngeal muscle); mid-pharynx region (hyopharyngeal muscle). Expansions during vocalization can be observed in the most rostral portion of the vt by extension of the hyoid apparatus (geniohyoid muscle, indirectly via thyrohyoid ligament: sternothyroid and sternohyoid muscles), by opening of the mouth (digastric muscle) and by depression of the tongue (genioglossus muscle).
These integrated cross-sectional changes of the vt might account for the observed uneven distribution of formants. Potential constrictions and expansions at several sites of the vt also demonstrate that the uniform tube model, used for our acoustic calculations, only very roughly approaches a naturally occurring vt and needs to be refined to achieve a more realistic modelling of vocalizations.
Effects of tongue protrusion on f0 and formants
Interestingly, Iberian red deer stags do not protrude the tongue during their harsh roars, which are emitted less frequently than common roars. Harsh roars are only applied in particularly challenging situations in which maximal acoustic impression towards a rival male (by maximal vt extension) is intended (Fig. 2). Apparently, the thermoregulatory function is largely suppressed in harsh roars. Mostly, the acoustic patterns of harsh roars of C. e. hispanicus are more similar to those of C. e. corsicanus (Kidjo et al. 2008) than to those of C. e. scoticus (Reby & McComb, 2003a,b). The C. e. scoticus stags produce harsh roars with maximally retracted larynx, so formants are nearly non-modulated (Reby & McComb, 2003a,b). In the stags of C. e. corsicanus (Kidjo et al. 2008) and C. e. hispanicus (Fig. 3D), however, the formants of harsh roars are often modulated, reflecting vt length adjustments. Achieving the lowest possible formants requires maximal vt elongation, which is effected by maximal retraction of the larynx at the caudal end and a more closed, O-shaped mouth opening by maximal protraction of the mouth angles at the rostral end of the vt. Apparently, this vocal gesture is incompatible with a wide-open mouth, a retraction of the mouth angles and a pronounced protrusion of the tongue. Therefore, in the perspective of the thermoregulation hypothesis, the Iberian red deer stags seem to quickly abandon evaporative cooling via the surfaces of the upper vt during the emission of harsh roars for the sake of higher acoustic competitiveness. So far, tongue protrusion behaviour has not been reported for other Southern subspecies of red deer.
In Iberian red deer, formants could be measured in harsh roars but tongue protrusion occurred only during common roars, where formants could not be measured because of widely spaced harmonics and a lack of acoustic energy in the lower part of the call spectrum that seems to be a characteristic of male C. e. hispanicus vocal production (Fig. 3). This dilemma prevented comparison of the lower formant positions of Iberian red deer stags with those in other red deer subspecies for the purpose of clarifying the potential acoustic effects of tongue protrusion.
We could not directly estimate the effect of tongue protrusion on f0, as the acoustic recordings were collected separately from the video recordings. However, tongue protrusion does not seem to increase f0 as the f0 was higher in harsh roars, during which the Iberian red deer stags do not protrude their tongues. Further analysis of simultaneous audio and video recordings of the common roars of the same individual stag, both with and without tongue protrusion, can be expected to elucidate the effects of tongue protrusion on the roar acoustics.
Comparison of vocal anatomy and related acoustics among subspecies
In male C. e. hispanicus, the larynx occupies a low-resting position in the mid-neck region (Figs 7 and 13) and, during male roars, can be additionally retracted by about 255 mm, i.e. by 55% of the vt resting length. The resting vtl in the C. e. hispanicus stags was 460 mm, consistent with data on vtls in C. e. nelsoni (473 mm, Titze & Riede, 2010) but distinctive from data on vtls in C. e. scoticus (370 mm, Fitch & Reby, 2001). Our video-based estimates of the maximally elongated vt length yielded 715 mm, plus 50 mm contributed by protruding the mouth angles during harsh roars, i.e. 765 mm in total. This coincided well with a maximal vt length of 767 mm calculated on the basis of minimum formant dispersion in the rutting roars of Iberian red deer stags (Fig. 5).
The vocal fold length in the two male C. e. hispanicus specimens of this study (30 mm) was in the same range as that of other subspecies: European red deer, 27 ± 1 mm and C. e. nelsoni, 30 ± 3 mm (Riede & Titze, 2008).
Following the methods applied in detailed studies of the roar acoustics of C. e. scoticus and C. e. corsicanus (Reby & McComb, 2003a; Kidjo et al. 2008), we obtained comparative data on the roar acoustics of Iberian red deer stags. Although the C. e. scoticus and C. e. hispanicus originate from the same glacial refugium and thus diverged only 10 000 years ago or later (Ludt et al. 2004), the roars of C. e. hispanicus compared to those of C. e. scoticus have considerably higher maximum and mean f0 frequencies (f0 max = 223 vs. 137 Hz; f0 mean = 186 vs. 107 Hz), but much more similar formant frequencies (ΔF = 228 vs. 243.5 Hz) (Reby & McComb, 2003a; this study). Similarly high f0 values (ranging from 170 to 300 Hz according to the presented spectrograms) were found in the roars of rutting red deer stags of the Cansiglio Forest (NE Italy; Favaretto et al. 2006). The f0 values in the roars of Austrian/Hungarian red deer, which were introduced to Argentina at the beginning of the 20th century, ranged from 116 to 140 Hz (Hurtado et al. 2011), i.e. they were lower than in C. e. hispanicus (this study) but higher than in C. e. scoticus (Reby & McComb, 2003a).
In rutting red deer, the maximum vt length correlates with body size and body mass of the callers (Reby & McComb, 2003a). Consistently, C. e. corsicanus stags, the smallest red deer subspecies (average body weight 88 kg), produce roars with the widest formant dispersion (256 Hz, Kidjo et al. 2008), whereas the larger male C. e. scoticus (average body weight 125 kg) produce roars with a formant dispersion of 243.5 Hz (Reby & McComb, 2003a). The C. e. hispanicus stags, which are of comparable size to C. e. scoticus (average body weight 125 ± 7 kg, n = 103, collected in 2000–2010 from stags of 6–9 years, see Carranza et al. 2004) produce roars with a formant dispersion of 228 Hz (Fig. 5). Thus, the relatively small differences between the formant dispersions in the rutting roars of C. e. corsicanus, C. e. scoticus and C. e. hispanicus roughly reflect differences in body size in these subspecies.
Enhancing the f0 of roars demands an increase of subglottal pressure and results in a faster expulsion of air from the lungs (Fitch & Hauser, 2002; Titze & Riede, 2010). Accordingly, one would not expect the highest f0 to be associated with the longest roars. However, in Iberian red deer stags, the longest roars within bouts were also highest in f0, suggesting that the stags put maximum effort in achieving roars of the highest possible fundamental frequency. At the same time, only a small minority of harsh roars and common roars of Iberian red deer stags had much energy in the lower part of the call spectra, so that the first formant (F1) usually was not accented (Fig. 3). This was not an artefact induced by the audio equipment or by the recording method, as the low energy in the lower part of the spectrum differed between individuals and human comments in this frequency range were clearly visible in the spectrogram. In addition, the high fundamental frequency in most of the common roars of Iberian red deer stags does not allow accenting formants. Thus, our data support a hypothesis that advocates opposing and conflicting selection pressures on the roar acoustics of red deer: one for low formants and one for a high f0 (Titze & Riede, 2010).
The demands for accenting formants and for achieving a high f0 can not be combined in the same roar pattern and will result in an evolutionary dichotomy of roar acoustics in red deer (Titze & Riede, 2010). Both rutting calls with low formants and rutting calls with a high f0 may serve as honest signals, as they reflect different physical qualities of the caller. The formants of roars advertise body size (Fitch & Reby, 2001; Reby & McComb, 2003a), while the high f0 of bugles may advertise muscular strength and endurance (Titze & Riede, 2010). In red deer and fallow deer (Dama dama), calls with low formants better deter rival males and attract females (Reby et al. 2005; Charlton et al. 2007a; Vannoni & McElligott, 2008). At the same time, red deer females preferred roars with a higher f0 at the peak of induced oestrus (Reby et al. 2010) but not during other stages of oestrus (McComb, 1991; Charlton et al. 2008). The evolutionary shift from roaring towards high-frequency vocalization may be assisted by better acoustic radiation of a high-frequency call and by potentially achieving higher sound amplitudes due to the lower airflow required for aerodynamic power (Titze & Riede, 2010).
As C. e. scoticus and C. e. hispanicus are very closely related subspecies, as evidenced by currently applied genetic markers (Skog et al. 2009; Niedzialkowska et al. 2011; Zachos & Hartl, 2011), the distinctive f0 of their roars, along with morphological traits, may be used as a diagnostic tool for their discrimination. Discrimination between subspecies is crucial in game biology because of multiple translocations of red deer subspecies across Europe in the past (review: Zachos & Hartl, 2011). Additional studies of the roars of hybrids is necessary, however, to test the accuracy of this potential diagnostic tool, as earlier studies suggested intermediate call characteristics in cross-breeds between subspecies of red deer (Nikol'skii et al. 1987).
Acknowledgments
We would like to thank Doñana Biological Station (EBD), the staff working at Doñana Biological Reserve for providing facilities and support and the Spanish and Portuguese helpers there: Marta Villasón Barroso, Mónica Expósito Granados, Francisco Javier Jimenez Moreno, Anna Marcia Barbosa and at Cáceres: Javier Pérez González for assistance in the field work and logistic support. We are very grateful to the owners, managers and guards of all the estates, where materials for this study have been collected, and to the ‘Consejería de Medio Ambiente’ of ‘Junta de Andalucía’. We also thank the Director of Tierpark Berlin, Dr. B. Blaszkiewitz, for providing two specimens of the Middle European subspecies (C. e. elaphus) for comparison and Andrey Bushuev for providing literature on bioenergetics, We would also like to thank Dr Gabriela Galateanu and Vet. Med. Guido Fritsch (both IZW) for CT-scanning of the specimens and assistance in further processing of the obtained data. We sincerely thank our reviewers, David Reby and Tobias Riede, for their detailed and instructive comments that helped greatly in improving this manuscript. During our work, we adhered to the ‘Guidelines for the treatment of animals in behavioural research and teaching’ (Anim. Behav., 2006, 71, 245–253) and to the laws of Spain, Germany, and the Russian Federation, the countries where the research was conducted. No animal has suffered, and their social structure was not destroyed as a result of the behavioural observations and recordings. This study was supported by the Russian Foundation for Basic Research, grant 12-04-00260 (for I.V. and E.V.) and Spanish projects CGL2007-63594 and CGL2010-17163 (for J.C. and J.T.).
Glossary
- Adit. lar.
Laryngeal entrance
- Ang. styloh
Angle of stylohyoid
- App. hyo.
Hyoid apparatus
- Art. cricaryt.
Cricoarytenoid articulation
- Art. cricthyr.
Cricothyroid articulation
- Att. to Cart. thyr.
Attached to thyroid cartilage
- Att. to M. thyraryt.
Attached to thyroarytenoid muscle
- Att. to Proc. voc.
Attached to vocal process
- Basih.
Basihyoid
- C4
Fourth cervical vertebra
- Cart. aryt.
Arytenoid cartilage
- Cart. cric.
Cricoid cartilage
- Cart. thyr.
Thyroid cartilage
- Cart. thyroh.
Thyrohyoid cartilage
- Ceratoh.
Ceratohyoid
- Choan.
Choanae (internal nares)
- Cont. lar.
Contours of larynx
- Cornu caud.
Caudal horn of thyroid cartilage
- Cornu rostr.
Rostral horn of thyroid cartilage
- Epigl.
Epiglottis
- Epih.
Epihyoid
- Gl. thyr.
Thyroid gland
- I: M. thyraryt.
Insertion of medial bundle of thyroarytenoid muscle
- Impr. Cart. cric.
Imprint of (removed) cricoid cartilage
- Lam. cric.
Cricoid lamina
- Lar.
Larynx
- Lig. arycorn.
Arycorniculate ligament
- Lig. cricthyr.
Cricothyroid ligament
- Lig. thyroh.
Thyrohyoid ligament
- Lig. voc.
Vocal ligament
- Ling.
Tongue
- Man. sterni
Sternal manubrium
- Mand.
Mandibula (dentary)
- Mem. thyroh.
Thyrohyoid membrane
- M. aryt. transv.
Transverse arytenoid muscle
- M. ceratoh.
Ceratohyoid muscle
- M. cricphar.
Cricopharyngeal muscle
- M. cricthyr.
Cricothyroid muscle
- M. geniogl.
Genioglossus muscle
- M. genioh.
Geniohyoid muscle
- M. hyoepigl.
Hyoepiglottic muscle
- M. hyogl.
Hyoglossus muscle
- M. hyophar.
Hyopharyngeal muscle
- M. occiph.
Occipitohyoid muscle
- M. omoh.
Omohyoid muscle
- M. palatphar.
Palatopharyngeal muscle
- M. parotaur.
Parotidoauricularis muscle
- M. sternmand.
Mandibular portion of sternocephalic muscle
- M. sternoh.
Sternohyoid muscle
- M. sternthyr.
Sternothyroid muscle
- M. stylogl.
Styloglossus muscle
- M. styloh.
Stylohyoid muscle
- M. stylphar. caud.
Caudal stylopharyngeal muscle
- M. stylphar. rostr.
Rostral stylopharyngeal muscle
- M. thyraryt.
Thyroarytenoid muscle
- M. thyroh.
Thyrohyoid muscle
- M. thyrphar.
Thyropharyngeal muscle
- Nasalvt.
Nasal vocal tract
- Nasophar.
Nasal part of pharynx
- N. lar. cran.
Cranial laryngeal nerve
- Oesoph.
Oesophagus
- Oralvt.
Oral vocal tract
- Orophar.
Oral part of pharynx
- Ost. intrphar.
Intrapharyngeal ostium
- Palat. dur.
Hard palate
- Palat. mol.
Soft palate
- Phar.
Pharynx
- Plex. phar.
Pharyngeal plexus
- Plic. voc.
Vocal fold
- Proc. corn.
Corniculate process of arytenoid cartilage
- Proc. med.
Medial process of arytenoid cartilage
- Proc. musc.
Muscular process of arytenoid cartilage
- Proc. voc.
Vocal process of arytenoid cartilage
- Ram. ext.
External branch of cranial laryngeal nerve
- Ram. int.
Internal branch of cranial laryngeal nerve
- Rami pharr.
Pharyngeal branches of cranial laryngeal nerve
- Rima glott.
Glottic cleft
- Styloh.
Stylohyoid
- Thyroh.
Thyrohyoid
- Trach.
Trachea
- Tympoh.
Tympanohyoid
Supporting Information
Audio S1. Natural bouts of rutting roars of Iberian red deer stags, as presented in Fig. 3.
As a service to our authors and readers, this journal provides supporting information supplied by the authors. Such materials are peer-reviewed and may be re-organized for online delivery, but are not copy-edited or typeset. Technical support issues arising from supporting information (other than missing files) should be addressed to the authors.
As a service to our authors and readers, this journal provides supporting information supplied by the authors. Such materials are peer-reviewed and may be re-organized for online delivery, but are not copy-edited or typeset. Technical support issues arising from supporting information (other than missing files) should be addressed to the authors.
References
- Aas-Hansen Ø, Folkow LP, Blix AS. Panting in reindeer (Rangifer tarandus. Am J Physiol Regul Integr Comp Physiol. 2000;279:R1190–R1195. doi: 10.1152/ajpregu.2000.279.4.R1190. [DOI] [PubMed] [Google Scholar]
- Bell AW, Hales JRS, King RB, et al. Influence of heat stress on exercise-induced changes in regional blood flow in sheep. J Appl Physiol. 1983;55:1916–1923. doi: 10.1152/jappl.1983.55.6.1916. [DOI] [PubMed] [Google Scholar]
- Biewener AA, Soghikian GW, Crompton AW. Regulation of respiratory airflow during panting and feeding in the dog. Respir Physiol. 1985;61:185–195. doi: 10.1016/0034-5687(85)90125-2. [DOI] [PubMed] [Google Scholar]
- Blix AS, Walløe L, Folkow LP. Regulation of brain temperature in winter-acclimatized reindeer under heat stress. J Exp Biol. 2011;214:3850–3856. doi: 10.1242/jeb.057455. [DOI] [PubMed] [Google Scholar]
- Carranza J, Valencia J. Red deer females collect on male clumps at mating areas. Behav Ecol. 1999;10:525–532. [Google Scholar]
- Carranza J, Alvarez F, Redondo T. Territoriality as a mating strategy in red deer. Anim Behav. 1990;40:79–88. [Google Scholar]
- Carranza J, Garcia-Muńos AJ, De Dios Vargas J. Experimental shifting from harem defence to territoriality in rutting red deer. Anim Behav. 1995;49:551–554. [Google Scholar]
- Carranza J, Alarcos S, Sánchez-Prieto CB, et al. Disposable-soma senescence mediated by sexual selection in an ungulate. Nature. 2004;432:215–218. doi: 10.1038/nature03004. [DOI] [PubMed] [Google Scholar]
- Charlton BD, Reby D, McComb K. Female red deer prefer the roars of larger males. Biol Lett. 2007a;3:382–385. doi: 10.1098/rsbl.2007.0244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Charlton BD, Reby D, McComb K. Female perception of size-related formant shifts in red deer, Cervus elaphus. Anim Behav. 2007b;74:707–714. [Google Scholar]
- Charlton BD, Reby D, McComb K. Effect of combined source (F0) and filter (formant) variation on red deer hind responses to male roars. J Acoust Soc Am. 2008;123:2936–2943. doi: 10.1121/1.2896758. [DOI] [PubMed] [Google Scholar]
- Clutton-Brock TH, Guinness F, Albon SD. Red Deer: Behavior and Ecology of Two Sexes. Chicago: The University of Chicago Press; 1982. p. 378. [Google Scholar]
- Dmi'el R, Robertshaw D. The control of panting and sweating in the black bedouin goat: a comparison of two modes of imposing a heat load. Physiol Zool. 1983;56:404–411. [Google Scholar]
- Duncker HR. Der Atemapparat (Apparatus respiratorius) In: Fleischhauer K, Staubesand J, Zenker W, editors. Benninghoff – Anatomie. Makroskopische und mikroskopische Anatomie des Menschen. Vol. 2. Munich: Urban & Schwarzenberg; 1985. pp. 307–388. (in German) [Google Scholar]
- Favaretto A, De Battisti R, Pavan G, et al. Acoustic features of red deer (Cervus elaphus) stags vocalizations in the Cansiglio Forest (NE Italy, 2001–2002). Advances in Bioacoustics 2, Dissertationes Classis IV: Historia Naturalis, Slovenian Academy of Sciences and Arts (Ljubljana) 2006;47:125–138. [Google Scholar]
- Feighny JJ, Williamson KE, Clarke JA. North American elk bugle vocalizations: male and female bugle call structure and context. J Mammal. 2006;87:1072–1077. [Google Scholar]
- Fitch WT. The Evolution of Language. Cambridge: Cambridge University Press; 2010. p. 625. [Google Scholar]
- Fitch WT, Hauser MD. Unpacking ‘honesty’: vertebrate vocal production and the evolution of acoustic signals. In: Simmons A, Fay RR, Popper AN, editors. Acoustic Communication, Springer Handbook of Auditory Research. New York: Springer; 2002. pp. 65–137. [Google Scholar]
- Fitch WT, Reby D. The descended larynx is not uniquely human. Proc R Soc Lond B. 2001;268:1669–1675. doi: 10.1098/rspb.2001.1704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frey R, Gebler G, Fritsch G, et al. Nordic rattle: the hoarse vocalization and the inflatable laryngeal air sac of reindeer (Rangifer tarandus. J Anat. 2007;210:131–159. doi: 10.1111/j.1469-7580.2006.00684.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frey R, Gebler A, Olson KA, et al. Mobile larynx in Mongolian gazelle: retraction of the larynx during rutting barks in male Mongolian gazelle (Procapra gutturosa Pallas, 1777) J Morphol. 2008a;269:1223–1237. doi: 10.1002/jmor.10656. [DOI] [PubMed] [Google Scholar]
- Frey R, Gebler A, Olson KA. Head anatomy of male and female Mongolian gazelle – a striking example of sexual dimorphism. In: Endo H, Frey R, et al., editors. Anatomical Imaging – Towards a New Morphology. Tokyo: Springer; 2008b. pp. 1–13. [Google Scholar]
- Frey R, Hofmann RR. Larynx and vocalization of the takin (Budorcas taxicolor Hodgson, 1850 – Mammalia, Bovidae) Zool Anz. 2000;239:197–214. [Google Scholar]
- Frey R, Volodin I, Volodina E, et al. Descended and mobile larynx, vocal tract elongation and rutting roars in male goitred gazelles (Gazella subgutturosa Güldenstaedt, 1780) J Anat. 2011;218:566–585. doi: 10.1111/j.1469-7580.2011.01361.x. doi: 10.1111/j.1469-7580.2011.01361.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hales JRS. Effects of heat stress on blood flow in respiratory and non-respiratory muscles in sheep. Pflügers Arch. 1973;345:123–130. doi: 10.1007/BF00585835. [DOI] [PubMed] [Google Scholar]
- Hales JRS, Brown GD. Net energetic and thermoregulatory efficiency during panting in the sheep. Comp Biochem Physiol A Comp Physiol. 1974;49:413–422. doi: 10.1016/0300-9629(74)90557-x. [DOI] [PubMed] [Google Scholar]
- Hillel D. Environmental Soil Physics. Amsterdam: Academic Press; 1998. p. 771. ISBN-13: 978-0-12-348525-0. [Google Scholar]
- Hurtado AM, Smith-Flueck JM, Black-Decima P. Description of vocalizations in exotic European red deer stags (Cervus elaphus) during the rut in northwestern Patagonia (Argentina) Anim Prod Sci. 2011;51:cvi–cxiii. [Google Scholar]
- Jenkinson DM. Comparative physiology of sweating. Br J Dermatol. 1973;88:397–406. doi: 10.1111/j.1365-2133.1973.tb07573.x. [DOI] [PubMed] [Google Scholar]
- Johnson KG. Sweating and panting in Welsh mountain sheep. Int J Biometeorol. 1971;15:281–285. doi: 10.1007/BF01803912. [DOI] [PubMed] [Google Scholar]
- Johnson KG, Maloiy GMO, Bligh J. Sweat gland function in the red deer (Cervus elaphus. Am J Physiol. 1972;223:604–607. doi: 10.1152/ajplegacy.1972.223.3.604. [DOI] [PubMed] [Google Scholar]
- Kidjo N, Cargnelutti B, Charlton BD, et al. Vocal behaviour in the endangered Corsican deer: description and phylogenetic implications. Bioacoustics. 2008;18:159–181. [Google Scholar]
- Laitman JT, Reidenberg JS. Advances in understanding the relationship between the skull base and larynx with comments on the origin of speech. Human Evol. 1988;3:99–109. [Google Scholar]
- Ludt CJ, Schroeder W, Rottmann O, et al. Mitochondrial DNA phylogeography of red deer (Cervus elaphus. Mol Phylogenet Evol. 2004;31:1064–1083. doi: 10.1016/j.ympev.2003.10.003. [DOI] [PubMed] [Google Scholar]
- McComb KE. Female choice for high roaring rates in red deer, Cervus elaphus. Anim Behav. 1991;4:79–88. [Google Scholar]
- McElligott AG, Birrer M, Vannoni E. Retraction of the mobile descended larynx during groaning enables fallow bucks (Dama dama) to lower their formant frequencies. J Zool. 2006;270:340–345. [Google Scholar]
- Nickel R, Schummer A, Seiferle E. Stuttgart: Parey bei MVS (Medizinverlage Stuttgart); 2004. Lehrbuch der Anatomie der Haustiere. Band II: Eingeweide. 9. unveränd. Auflage; p. 482. [Google Scholar]
- Niedzialkowska M, Jędrzejewska B, Honnen A-C, et al. Molecular biogeography of red deer Cervus elaphus from Eastern Europe: insights from mitochondrial DNA sequences. Acta Theriol. 2011;56:1–12. doi: 10.1007/s13364-010-0002-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nikol'skii AA, Pereladova OB, Rutovskaja MV, et al. The geographical variability of rut calls in red deer males. Bull Moscow Soc Nature Explorers. 1979;84:46–55. Dept. of Biology (in Russian) [Google Scholar]
- Nikol'skii AA, Yu I, Frommolt J, et al. Acoustic diagnostics of subspecies’ specifity of red deers (Cervus elaphus, Cervidae, Artiodactyla) from Transbaikalia. Zool Anz. 1987;219:25–32. (in German with short English abstract) [Google Scholar]
- Nordin M, Frankel VH. Basic Biomechanics of the Musculoskeletal System, Chapter 4: Biomechanics of Skeletal Muscle. 3rd edn. Baltimore: Lippincott Williams & Wilkins; 2001. pp. 44–65. [Google Scholar]
- Ochsner TE, Sauer TJ, Horton R. Soil heat storage measurements in energy balance studies. Agron J. 2007;99:311–319. [Google Scholar]
- Post E, Boving PS, Pederson C, et al. Synchrony between caribou calving and plant phenology in depredated and non-depredated populations. Can J Zool. 2003;81:1709–1714. [Google Scholar]
- Reby D, McComb K. Anatomical constraints generate honesty: acoustic cues to age and weight in the roars of red deer stags. Anim Behav. 2003a;65:519–530. [Google Scholar]
- Reby D, McComb K. Vocal communication and reproduction in deer. Adv Stud Behav. 2003b;33:231–264. [Google Scholar]
- Reby D, McComb K, Cargnelutti B, et al. Red deer stags use formants as assessment cues during intra-sexual agonistic interactions. Proc Biol Sci. 2005;272:941–947. doi: 10.1098/rspb.2004.2954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reby D, Charlton BD, Locatelli Y, et al. Oestrous red deer hinds prefer male roars with higher fundamental frequencies. Proc Biol Sci. 2010;277:2747–2753. doi: 10.1098/rspb.2010.0467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riede T, Titze IR. Vocal fold elasticity of the Rocky Mountain elk (Cervus elaphus nelsoni) – producing high fundamental frequency vocalization with a very long vocal fold. J Exp Biol. 2008;211:2144–2154. doi: 10.1242/jeb.017004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riede T, Lingle S, Hunter E, et al. Cervids with different vocal behavior demonstrate different visco-elastic properties of their vocal folds. J Morphol. 2010;271:1–11. doi: 10.1002/jmor.10774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robertshaw D. The pattern and control of sweating in the sheep and goat. J Physiol. 1968;198:531–539. doi: 10.1113/jphysiol.1968.sp008623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robertshaw D. Mechanisms for the control of respiratory evaporative heat loss in panting animals. J Appl Physiol. 2006;101:664–668. doi: 10.1152/japplphysiol.01380.2005. [DOI] [PubMed] [Google Scholar]
- Schmidt-Nielsen K. Animal Physiology: Adaptation and Environment. 5th edn. Cambridge: Cambridge University Press; 1997. p. 612. ISBN 0-521-57098-0 (hbk.) [Google Scholar]
- Schmidt-Nielsen K, Bretz WL, Taylor CR. Panting in dogs: unidirectional air flow over evaporative surfaces. Science. 1970;169:1102–1104. doi: 10.1126/science.169.3950.1102. [DOI] [PubMed] [Google Scholar]
- Skog A, Zachos FE, Rueness EK, et al. Phylogeography of red deer (Cervus elaphus) in Europe. J Biogeogr. 2009;36:66–77. [Google Scholar]
- Sokolov VE. Mammal Skin. Berkeley: University of California Press; 1982. p. 695. ISBN-0-520-03198-9. [Google Scholar]
- Taylor AM, Reby D. The contribution of source–filter theory to mammal vocal communication research. J Zool. 2010;280:221–236. [Google Scholar]
- Tembrock G. Untersuchungen zur intraspezifischen Variabilität von Lautäußerungen bei Säugetieren. Z Säugetierkd. 1965;30:257–273. (in German) [Google Scholar]
- Titze IR, Riede T. A cervid vocal fold model suggests greater glottal efficiency in calling at high frequencies. PLoS Comput Biol. 2010;6:e1000897. doi: 10.1371/journal.pcbi.1000897. doi: 10.1371/journal.pcbi.1000897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vannoni E, McElligott AG. Low frequency groans indicate larger and more dominant fallow deer (Dama dama) males. PLoS ONE. 2008;3:e3113. doi: 10.1371/journal.pone.0003113. doi: 10.1371/journal.pone.0003113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vesterdorf K, Blache D, Maloney SK. The cranial arterio-venous temperature difference is related to respiratory evaporative heat loss in a panting species, the sheep (Ovis aries. J Comp Physiol B. 2011;181:277–288. doi: 10.1007/s00360-010-0513-7. [DOI] [PubMed] [Google Scholar]
- Wagenknecht E. Der Rothirsch (Cervus elaphus. Lutherstadt Wittenberg: A. Ziemsen Verlag; 1983. p. 148. 2. Auflage. Die Neue Brehm Bücherei No. 129. ISBN 0138-1423 (in German) [Google Scholar]
- Wilden I, Herzel H, Peters G, et al. Subharmonics, biphonation, and deterministic chaos in mammal vocalization. Bioacoustics. 1998;9:171–196. [Google Scholar]
- Zachos FE, Hartl GB. Phylogeography, population genetics and conservation of the European red deer Cervus elaphus. Mammal Review. 2011;41:138–150. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


