Abstract
The objective of the study was to assess cerebral mass, based on head circumference measurements in neonates exposed to tobacco smoke in utero, and to determine the relative proportions of the cerebral and body mass. The study included 147 neonates born in the period 2003–2004 at the Princess Anna Mazowiecka University Hospital and admitted to the Neonatal and Intensive Care Department of the Medical University in Warsaw. Subjects were divided into three groups on the basis of maternal status as active, passive, or nonsmokers determined by maternal urinary cotinine concentration and a questionnaire. Neonates whose mothers were active smokers throughout the whole period of pregnancy had a lower head circumference and in consequence a lower cerebral mass significantly more frequently when compared with those whose mothers were nonsmokers, P= 0.002. (Median difference in cerebral mass was 48.27 g.) The risk of lower cerebral mass was 3.9 (1.4–10.8, CI 95%) in the group of neonates whose mothers actively smoked cigarettes during pregnancy. A negative correlation was seen between cerebral mass and maternal urinary cotinine concentration (correlation coefficient r=−23, P= 0.006). The ratio of the cerebral to body mass was similar for neonates in all three groups. Active smoking during pregnancy had a negative effect on the cerebral mass of the neonate, however no such effect was observed in neonates whose mothers were passive smokers. The deficiency in cerebral mass increased with greater smoking intensity. Active smoking by the mother during pregnancy inhibits the growth of the brain as well as that of the body mass of the neonate.
Keywords: Active smoking, cerebral mass, cotinine, neonate, passive smoking, smoker mother, urine
Introduction
Smoking during pregnancy causes neurological disorders in the neonate, which are manifested by increased muscle tension, increased excitability, limb tremor, sleep disturbance (nicotine withdrawal), and changes in the neurological development of children (Law et al. 2003; Godding et al. 2004; Pichini and Garcia-Algar 2006). General impairment of intellectual ability of the child, a lower IQ, behavioral disturbance, and attention deficit hyperactivity disorder (ADHD) are also associated with the harmful effects of tobacco (Thapar et al. 2003; Batty et al. 2006; Linnet et al. 2006; Martin et al. 2006).
Maternal tobacco smoking is one of the main risk factors for sudden infant death syndrome (SIDS). This syndrome occurs four times more frequently among neonates exposed to tobacco smoke in utero and postpartum and twice as frequently in neonates whose mothers did not smoke in pregnancy but did so postpartum (Schoendorf and Kiely 1992). It is estimated that 80% of deaths due to SIDS are associated with maternal cigarette smoking (Anderson et al. 2005). It has been demonstrated that SIDS in children exposed to tobacco smoke may be caused by a disorder in the development of the brain, namely the anatomical and functional changes in the brain stem and the associated tendency for the occurrence of central apnea (Matturri et al. 2006).
Materials and Methods
The study included 147 neonates born during the period 2003–2004 at the Princess Anna Mazowiecka University Hospital in Warsaw and admitted to the Neonatal and Intensive Care Unit of the Medical University in Warsaw.
Inclusion in the study was conditional upon voluntary consent by the mother and the completion of a questionnaire in which mothers assessed their degree of exposure to tobacco smoke during pregnancy. Live neonates from singleton births were included in the study. The study protocol was approved by the ethical committee of the Medical University in Warsaw—no. 34/2003 on 18 February 2003. The study was conducted in accordance with the 1975 Helsinki declaration. Neonates were divided into three groups based upon the response to the questionnaire on exposure to tobacco smoke and on the concentration of maternal urinary cotinine (nicotine metabolite).
There were 58 subjects born to mothers who declared that they were active smokers with maternal urinary cotinine concentration of >200 ng/mg of creatinine. Neonates whose mothers declared passive exposure to tobacco smoke during pregnancy numbered 64 (maternal urinary cotinine concentration 5–200 ng/mg of creatinine). The third group included 25 subjects whose mothers declared no exposure to tobacco smoke during pregnancy and whose urinary cotinine concentration was <5 ng/mg of creatinine. In case of discrepancy between declared lower exposure to tobacco smoke and maternal urinary cotinine concentration, assignment to the appropriate group was based upon the latter. Twelve neonates whose mothers had declared either passive exposure (5) or no exposure (7) to tobacco smoke and for whom maternal urinary cotinine concentration was >200 ng/mg of creatinine were assigned to the active smoker group. However, 19 women who had declared no exposure to tobacco smoke were identified as passive smokers on the basis of a urinary cotinine concentration of 5–200 ng/mg of creatinine. On the first day postpartum, 5 mL of urine was taken from the mothers. Urine samples were frozen at a temperature of −80°C and stored until urinary cotinine measurement was performed. Urinary cotinine concentration was measured by high-performance liquid chromatography with spectrophotometric detection and norephedrine was used as an internal marker, following earlier liquid–liquid extraction. The concentration of urinary cotinine was measured in ng/mL and then expressed as mg of creatinine (ng/mg of creatinine), in order to avoid errors associated with excessive dilution or concentration of the urine. Creatinine concentration was measured by spectrophotometry.
The fetal age of the neonates in the study ranged from 26 to 42 weeks of gestation and the average birth age of neonates was 38 weeks of gestation. The majority (114) were term births (77.55%). There were 33 (22.45%) preterm births. There was no statistically significant difference between the groups of subjects in terms of sex, method of delivery, or numbers of preterm births (Table 1). Measurement of the head circumference was made immediately after birth with the aid of a tape measure with an accuracy of up to 0.5 cm. The head circumference was measured by passing the tape measure over the opisthocranion and metopion. The cerebral mass was calculated according to the following equation: cerebral mass (g) = 0.037 × head circumference (cm)2.57 (Lindley et al. 2000).
Table 1.
Characteristics of the neonates.
| Characteristic | Neonates with active smoker mothers n= 58 | Neonates with passive smoker mothers n= 64 | Neonates with nonsmoker mothers n= 25 | P-value |
|---|---|---|---|---|
| Sex | ||||
| Male | 32 (55.2%) | 38 (59.4%) | 15 (60.0%) | n.s. |
| Female | 26 (44.8%) | 26 (40.6%) | 10 (40.0%) | |
| Gestational age | ||||
| ≥37 weeks | 42 (72.4%) | 52 (81.3%) | 20 (80.0%) | n.s. |
| <37 weeks | 16 (27.6%) | 12 (18.7%) | 5 (20.0%) | |
| Delivery | ||||
| Spontaneous labor | 32 (55.2%) | 35 (54.7%) | 12 (48.0%) | n.s. |
| Vacuum extractor | 1 (1.7%) | 0 (0.0%) | 1 (4.0%) | |
| Cesarean section | 25 (43.1%) | 29 (45.3%) | 12 (48.0%) | |
P-value compared with newborns of nonsmoking mothers.
n.s., not statistically significant.
In order to determine the proportion of cerebral mass to body mass, the brain body ratio (BBR) was calculated according to the following equation: BBR = 100 × [0.037 × head circumference (cm)2.57]/body mass (g) (Lindley et al. 2000).
The following tools were used for the statistical analysis:
Descriptive statistics—average values, standard deviations, medians, and ranges for quantitative variables.
The Shapiro–Wilk test for testing the null hypothesis of no difference between the distribution of the subjects and the normal distribution.
The Kruskal–Wallis one-way analysis of variance for the comparison of groups for quantitative variables with a non-Gaussian distribution.
Wilcoxon's test for analysis of the differences between two groups for quantitative variables with a non-Gaussian distribution.
Fisher's exact test for analysis of the relationship between quantitative variables.
The multi-dimensional logistic regression model (GLIMMIX) was used to calculate the odds ratio for the different patient groups as well as the multi equivalent risk ratio in the groups under comparison.
The relationship between maternal urinary cotinine concentration and the cerebral mass of the neonates was confirmed by estimating Spearman's rank correlation coefficient. A P-value of <0.05 was taken as statistically significant. Calculations were made using the SAS system.
Results
The median measurement of head circumference for neonates whose mothers were active smokers was 2 cm smaller than for neonates whose mothers did not smoke and this difference was statistically significant at P= 0.002. The median head circumference measurement of neonates whose mothers were passive smokers differed by 1 cm only, which was not statistically significant (Table 2).
Table 2.
Median head circumference, brain weight, brain body ratio (BBR).
| Variable | Median | Minimal value | Maximal value | Mean ± SD | P-value |
|---|---|---|---|---|---|
| Head circumference (cm) | |||||
| NN n= 25 | 35 | 32 | 38 | 34 ± 2 | |
| NP n= 64 | 34 | 27 | 37 | 34 ± 2 | n.s.* |
| NA n= 58 | 33 | 25 | 38 | 33 ± 2 | 0.002* |
| Brain weight (g) | |||||
| NN n= 25 | 343.92 | 273.17 | 424.86 | 330.54 ± 42.40 | |
| NP n= 64 | 319.23 | 176.53 | 396.72 | 312.43 ± 52.42 | n.s.* |
| NA n= 58 | 295.65 | 144.85 | 396.72 | 289.44 ± 53.41 | 0.002* |
| BBR | |||||
| NN n= 25 | 9.56 | 7.68 | 12.11 | 9.70 ± 1.36 | |
| NP n= 64 | 9.26 | 6.61 | 16.16 | 9.80 ± 1.83 | n.s.* |
| NA n= 58 | 9.56 | 7.51 | 15.60 | 10.22 ± 2.03 | n.s.* |
P-value against newborns of nonsmoking mothers.
n.s., not statistically significant; NN, neonates with nonsmoker mothers; NP, neonates with passive smoker mothers; NA, newborns with active smoker mothers.
Neonates of mothers who were active smokers had significantly lower cerebral mass P= 0.002. The median cerebral mass for children in this group was 295.65 g, and was 23.58 g lower than the cerebral mass of neonates whose mothers were passive smokers (319.23 g) and 48.27 g lower than the cerebral mass of neonates of non-smoker mothers (343.92 g). The cerebral mass of neonates whose mothers were passive smokers was 24.69 g lower than for neonates of nonsmoker mothers; however, this difference was not statistically significant (Table 2).
There was a statistically significant higher risk of lower cerebral mass 3.9 (1.4–10.8, CI 95%) in neonates whose mothers were active smokers when compared with those of nonsmoker mothers. This risk was also higher in the neonates whose mothers were passive smokers 1.9 (0.7–5.2, CI 95%); however, this difference was not statistically significant (Table 3).
Table 3.
Odds ratio (OR) (95% confidence interval [CI]) for brain weight according to the smoker status of the mother, complications of pregnancy, sex, and gestational age of the neonate.
| Independent variable | Brain weight OR (95% CI) |
|---|---|
| Active smoker versus nonsmoker | 3.9 (1.4–10.8) |
| Passive smoker versus nonsmoker | 1.9 (0.7–5.2) |
| Complications of pregnancy No/Yes | 1.5 (0.8–3.0) |
| Newborn sex male/female | 0.5 (0.3–0.9) |
| Gestational age <37 weeks/≥37 weeks | 21.8 (8.2–57.8) |
A negative correlation was evident between the maternal urinary concentration of cotinine and the cerebral mass of neonates whose mothers were active smokers. The correlation coefficient (r) was negative and was –0.23 with P= 0.006, which means that the neonatal cerebral mass fell with a rise in maternal urinary cotinine concentration (Fig. 1).
Figure 1.
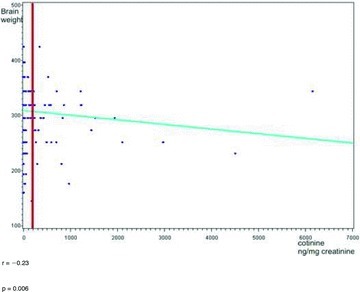
Correlation coefficient (r) between cotinine concentration in the mother's urine and brain weight of the neonates.
There was no statistically significant difference in the BBR between the neonates whose mothers were active and those whose mothers were passive smokers (9.56 and 9.26), and this ratio was identical in neonates whose mothers were active smokers and those whose mothers were nonsmokers (9.56) (Table 2).
Discussion
Tobacco smoking throughout pregnancy affects the growth of all anthropometric features (body mass, length, head, and chest circumference); however, the ponderal index, that is the relative proportions of the body, remains unchanged, which is evidence of symmetrical retardation of fetal interuterine growth (Cliver et al. 1995; Roquer et al. 1995). It is difficult to assess the direct impact of tobacco smoke on the increase in cerebral mass. Many authors use the measurement of head circumference to assess the development of the brain. In their studies, Jaddoe et al. (2007) and Roza et al. (2007) measured the weekly increase in the head circumference of fetuses of smoker compared with nonsmoker mothers. Growth retardation in terms of head circumference was −0.56 mm (−0.73, −0.40, CI 95%) and −0.13 mm (−0.18, −0.09, CI 95%), respectively (Jaddoe et al. 2007; Roza et al. 2007).
Many authors underline the significant difference in the head circumference of neonates whose mothers are active smokers and nonsmokers and this difference ranges from 0.2 to 1.1 cm (Olds et al. 1994; Cliver et al. 1995; Roquer et al. 1995; Zaren et al. 1996). In this study, the neonates whose mothers were active smokers during pregnancy had statistically smaller head circumference in comparison with those whose mothers were nonsmokers. Based upon head circumference, it is possible to estimate the cerebral mass of the neonate [cerebral mass (g) = 0.037 × head circumference (cm)2.57] (Lindley et al. 2000).
In this study, the median cerebral mass of the neonates whose mothers were active smokers during pregnancy was statistically significantly lower than the cerebral mass of neonates of nonsmoker mothers. The cerebral mass of neonates whose mothers were passive smokers was also lower when compared with neonates born to nonsmoker mothers, however this difference was not statistically significant. What is significant is that the weight indicator BBR, which determines the proportion of cerebral to body mass, was identical in the groups of neonates of active smoker and nonsmoker mothers and was 9.56, which indicates the symmetrical retardation of growth of the whole body. Mild reduction of this indicator was observed in the group of neonates whose mothers were passive smokers (9.26), but this difference was not statistically significant.
Similarly in the study by Pichini et al. (2003), this indicator was almost identical in the group of neonates whose mothers were active, passive, or nonsmokers and these values were 10.5, 10.4, and 10.2, respectively.
According to Lindley, the average value of the BBR in neonates whose mothers were nonsmokers was 9.45 and decreased by 0.074 [−0.031, −0.117, CI 95%] in the group of neonates whose mothers smoked throughout the duration of pregnancy, if the mother smoked less than 10 cigarettes/day and by 0.046 [−0.001, −0.091, CI 95%] if the mother smoked ≥10 cigarettes/day, indicating that the head circumference decreases in neonates whose mothers are smokers. Lindley showed that stopping smoking up to the 32 week of gestation results in the same BBR in neonates whose mothers are smokers and those who have not smoked throughout the whole pregnancy (Lindley et al. 2000).
The negative correlation between cerebral mass and maternal urinary cotinine concentration (a rise in cotinine concentration was accompanied by a decrease in cerebral mass) demonstrated in this study is important evidence for the influence of nicotine on the retardation of the development of the brain. Impaired development of the fetal central nervous system in smoker mothers is associated with a decrease in the delivery of nutrients and oxygen to the fetus as well as the direct effect of nicotine and its metabolites on brain cells. Cigarette smoking by the mother has been shown to alter the expression of nicotinic and muscarinic receptors in the brain stem and cerebellum of human fetuses, which impairs the development of the cholinergic system (Falk et al. 2005).
Slotkin's studies demonstrate that nicotine administered in doses smaller than those that impair fetal growth, damages and reduces irreversibly the number of brain cells, and damages the activity of neural synapses (Slotkin 1998). Nicotine reaches the fetal brain from the maternal circulation crossing through the blood/brain barrier without hindrance and damages the nicotine receptors in the human fetal brain as early as the first trimester of pregnancy (Cairns and Wonnacott 1988).
Lower total cerebral mass was detected in neonatal rats exposed to the effect of nicotine (dose 20–60 ng/mL). Histological changes were also registered in brain tissue in the form of impaired maturation of pyramidal neurons, reduction of the pyramidal area, and narrowing of the cortical layer (Lambers and Clark 1996). The changes described above in the brain flows and the histological changes in the structure of animal fetal brains subjected to the effects of nicotine can be extrapolated to changes in humans. Albuquerque et al. (2004), who studied the flow in the middle cerebral artery (MCA) in human fetuses, did not show any difference between the values of the resistance index in the MCA between fetuses of smoker and nonsmoker mothers. They did however find a statistically significant higher resistance index in the MCA of mothers who smoked >10 cigarettes per 24 h, which is evidence of increased resistance of cerebral vasculature and of poorer cerebral blood supply (Albuquerque et al. 2004).
The investigations of Matturri et al. (2006) provide interesting observations. They carried out histological examination the brains of neonates who had died from SIDS and found hypoplastic changes in the nuclei (nucleus arcuatus) of the important centers for the circulatory–respiratory system including the chemoreceptors as well as functional changes in the brain stem centers. The morphological and functional changes of the brainstem were associated with maternal smoking in 91.36% of cases. The authors suggest that fetal exposure to tobacco smoke, and therefore to the effect of carboxyhemoglobin and chronic oxygen insufficiency, impairs the formation of the nervous system (Matturri et al. 2006). MRI examination undertaken in a group of premature babies (<1500 g or <32 weeks of gestation) who were exposed to the effect of nicotine in utero showed statistically significant reduction in the volume of the frontal lobes and cerebellum, which in consequence leads to impairment of their function and disturbance in emotional control, behavior, and concentration in children (Ekblad et al. 2010). A decrease of the thickness of the cerebral cortex in the fronto-orbital, midfrontal, and hippocampus region was shown in children and adolescents who were exposed to the effect of nicotine in fetal life (Toro et al. 2008). Gale et al. (2004) showed that brain growth in the neonatal period and early childhood has a greater effect on cognitive function in children then during fetal life. In their study, the IQ increased by 1.98 [0.34–3.62, CI 95%] for each additional standard deviation in the increase in head circumference at nine months of life and by 2.87 [1.05–4.69 CI 95%] for each additional standard deviation in the nine years of life. They did not show any association with the IQ at 18 weeks of gestation or immediately after birth (Gale et al. 2004).
Conclusion
Active smoking during pregnancy reduces the cerebral mass of neonates; this effect was not evident in the case of passive exposure to tobacco smoke.
The deficiency in cerebral mass in neonates whose mothers were smokers increased in conjunction with the intensity of cigarette smoking.
Active smoking of cigarettes by mothers during pregnancy retards both brain growth and increase in body mass in neonates.
References
- Albuquerque CA, Smith KR, Johnson C, Chao R, Harding R. Influence of maternal tobacco smoking during pregnancy on uterine, umbilical and fetal cerebral artery blood flows. Early Hum. Dev. 2004;80:31–42. doi: 10.1016/j.earlhumdev.2004.05.004. [DOI] [PubMed] [Google Scholar]
- Anderson ME, Johnson DC, Batal HA. Sudden infant death syndrome and prenatal maternal smoking: rising attributed risk in the back to sleep era. BMC Med. 2005;3:1–7. doi: 10.1186/1741-7015-3-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Batty GD, Der G, Deary IJ. Effect of maternal smoking during pregnancy on offspring's cognitive ability: empirical evidence for complete confounding in the US National Longitudinal Survey of Youth. Pediatrics. 2006;118:943–950. doi: 10.1542/peds.2006-0168. [DOI] [PubMed] [Google Scholar]
- Cairns NJ, Wonnacott S. [3H](-) nicotine binding sites in fetal human brain. Brain Res. 1988;475:1–7. doi: 10.1016/0006-8993(88)90192-8. [DOI] [PubMed] [Google Scholar]
- Cliver SP, Goldenberg RL, Cutter GR, Hoffman HJ, Davis RO, Nelson KG. The effect of cigarette smoking on neonatal anthropometric measurements. Obstet. Gynecol. 1995;85:625–630. doi: 10.1016/0029-7844(94)00437-I. [DOI] [PubMed] [Google Scholar]
- Ekblad M, Parkkola R, Lapinleimu H, Haataja L, Lehtonen L PIPARI Study Group. Maternal smoking during pregnancy and region brain volumes in preterm infants. J. Pediatr. 2010;156:185–190. doi: 10.1016/j.jpeds.2009.07.061. [DOI] [PubMed] [Google Scholar]
- Falk L, Nordberg A, Seiger A, Kj´aeldgaard A, Hellström-Lindahl E. Smoking during early pregnancy affects the expression pattern of both nicotinic and muscarinic acetylcholine receptors in human first trimester brainstem and cerebellum. Neuroscience. 2005;132:389–397. doi: 10.1016/j.neuroscience.2004.12.049. [DOI] [PubMed] [Google Scholar]
- Gale CR, O'Callaghan FJ, Godfrey KM, Law CM, Martyn CN. Critical periods of brain growth and cognitive function in children. Brain. 2004;127:321–329. doi: 10.1093/brain/awh034. [DOI] [PubMed] [Google Scholar]
- Godding V, Bonnier C, Fiasse L, Michel M, Longueville E, Lebecque P, Robert A, Galanti L. Does in utero exposure to heavy maternal smoking induce nicotine withdrawal symptoms in neonates? Pediatr. Res. 2004;55:645–651. doi: 10.1203/01.PDR.0000112099.88740.4E. [DOI] [PubMed] [Google Scholar]
- Jaddoe VWV, Verburg BO, Ridder MAJ, Hofman A, Mackenbach JP, Moll HA, Steegers EAP, Witteman JCM. Maternal smoking and fetal growth characteristics in different periods of pregnancy. Am. J. Epidemiol. 2007;165:1207–1215. doi: 10.1093/aje/kwm014. [DOI] [PubMed] [Google Scholar]
- Lambers DS, Clark KE. The maternal and fetal physiologic effects of nicotine. Semin. Perinatol. 1996;20:115–126. doi: 10.1016/s0146-0005(96)80079-6. [DOI] [PubMed] [Google Scholar]
- Law KL, Stroud LR, LaGasse LL, Niaura R, Liu J, Lester BM. Smoking during pregnancy and newborn neurobehavior. Pediatrics. 2003;111:1318–1323. doi: 10.1542/peds.111.6.1318. [DOI] [PubMed] [Google Scholar]
- Lindley AA, Becker S, Gray RH, Herman AA. Effect of continuing or stopping smoking during pregnancy on infant birth weight, crown-heel length, head circumference, ponderal index and brain:body weight ratio. Am. J. Epidemiol. 2000;152:219–225. doi: 10.1093/aje/152.3.219. [DOI] [PubMed] [Google Scholar]
- Linnet KM, Obel C, Bonde E, Thomsen PH, Secher NJ, Wisborg K, Henriksen TB. Cigarette smoking during pregnancy and hyperactive-distractible preschooler's: a follow-up study. Acta Paediatr. 2006;95:694–700. doi: 10.1080/08035250500459709. [DOI] [PubMed] [Google Scholar]
- Martin RP, Dombrowski SC, Mullis C, Wisenbaker J, Huttunen MO. Smoking during pregnancy: association with childhood temperament, behavior, and academic performance. J. Pediatr. Psychol. 2006;31:490–500. doi: 10.1093/jpepsy/jsj041. [DOI] [PubMed] [Google Scholar]
- Matturri L, Ottaviani G, Lavezzi AM. Maternal smoking and sudden infant death syndrome: epidemiological study related to pathology. Virchows Arch. 2006;449:697–706. doi: 10.1007/s00428-006-0308-0. [DOI] [PubMed] [Google Scholar]
- Olds DL, Henderson CR, Tatelbaum R. Intellectual impairment in children of women who smoke cigarettes during pregnancy. Pediatrics. 1994;93:221–227. [PubMed] [Google Scholar]
- Pichini S, Garcia-Algar O. In utero exposure to smoking and newborn neurobehavior. How to assess neonatal withdrawal syndrome? Ther. Drug Monit. 2006;28:288–290. doi: 10.1097/01.ftd.0000211809.81816.1b. [DOI] [PubMed] [Google Scholar]
- Pichini S, Garcia-Algar O, Munoz L, Vall O, Pacifici R, Figueroa C, Pascual JA, Diaz D, Sunyer J. Assessment of chronic exposure to cigarette smoke and its change during pregnancy by segmental analysis of maternal hair nicotine. J. Expo. Anal. Environ. Epidemiol. 2003;13:144–151. doi: 10.1038/sj.jea.7500264. [DOI] [PubMed] [Google Scholar]
- Roquer JM, Figueras J, Botet F, Jimenez R. Influence on fetal growth of exposure to tobacco smoke during pregnancy. Acta Paediatr. 1995;84:118–121. doi: 10.1111/j.1651-2227.1995.tb13592.x. [DOI] [PubMed] [Google Scholar]
- Roza SJ, Verburg BO, Jaddoe VWV, Hofman A, Mackenbach JP, Steegers EAP, Witteman JCM, Verhulst FC, Tiemeier H. Effects of maternal smoking in pregnancy on prenatal brain development. The Generation R Study. Eur. J. Neurosci. 2007;25:611–617. doi: 10.1111/j.1460-9568.2007.05393.x. [DOI] [PubMed] [Google Scholar]
- Schoendorf KC, Kiely JL. Relationship of sudden infant death syndrome to maternal smoking during and after pregnancy. Pediatrics. 1992;90:905–908. [PubMed] [Google Scholar]
- Slotkin TA. Fetal nicotine or cocaine exposure: which one is worse? J. Pharmacol. Exp. Ther. 1998;285:931–945. [PubMed] [Google Scholar]
- Thapar A, Fowler T, Rice F, Scourfield J, Bree M, Thomas H, Harold G, Hay D. Maternal smoking during pregnancy and attention deficit hyperactivity disorder symptoms in offspring. Am. J. Psychiatry. 2003;160:1985–1989. doi: 10.1176/appi.ajp.160.11.1985. [DOI] [PubMed] [Google Scholar]
- Toro R, Leonard G, Lerner JV, Lerner RM, Perron M, Pike GB, Richer L, Veilette S, Pausova Z, Paus T. Prenatal exposure to maternal cigarette smoking and the adolescent cerebral cortex. Neuropsychopharmacology. 2008;33:1019–1027. doi: 10.1038/sj.npp.1301484. [DOI] [PubMed] [Google Scholar]
- Zaren B, Lindmark G, Gebre-Medhin M. Maternal smoking and body composition of the newborn. Acta Paediatr. 1996;85:213–219. doi: 10.1111/j.1651-2227.1996.tb13995.x. [DOI] [PubMed] [Google Scholar]


