A 13-valent pneumococcal conjugate vaccine (PCV13), recently approved for use in adults, induced an overall superior functional antibody response compared with the 23-valent pneumococcal polysaccharide vaccine. PCV13 elicits immunological memory and provides a new approach to preventing pneumococcal disease in adults.
Abstract
A 13-valent pneumococcal conjugate vaccine has been studied in adults aged ≥50 years to compare the immune response to that induced by the 23-valent pneumococcal polysaccharide vaccine, which has been the standard of care over the past 30 years. The results demonstrate that adults, regardless of whether they are naive or previously vaccinated with the polysaccharide vaccine, have an overall superior antibody response when vaccinated with the conjugate vaccine compared with the pneumococcal polysaccharide vaccine. More importantly, the nature of the response is indicative of a T-cell–dependent response that elicits immunological memory and, therefore, primes the immune system for either natural exposure or subsequent booster vaccination with either conjugate or polysaccharide vaccine. The conjugate vaccine, which has been successful in reducing pneumococcal disease in children, now provides a new approach to preventing pneumococcal disease, including community-acquired pneumonia, in adults.
(See the Vaccines Invited Article by Grabenstein, on pages 255–8, and the Editorial Commentary by Musher, on pages 265–7.)
INTRODUCTION
Capsular polysaccharide conjugate vaccines directed at invasive bacteria have had a significant impact on the burden of disease in children since their introduction over 2 decades ago [1]. The success of these vaccines reflects their ability to induce a functional antibody response directed at the bacterial capsule that is T-cell dependent, resulting not only in a robust initial response but also in the establishment of immunological memory [2]. This memory is an important mechanism for protection upon exposure to the pathogen and for renewing immunity with subsequent immunizations [1]. Over the past decade, a 7-valent pneumococcal conjugate vaccine (PCV7; pneumococcal serotypes 4, 6B, 9V, 14, 18C, 19F, and 23F) has been introduced into childhood vaccination programs globally, resulting in a significant reduction in both invasive pneumococcal disease (IPD) and mucosal disease (including community-acquired pneumonia [CAP] and otitis media) [3].
However, in adults aged ≥50 years, the burden of pneumococcal disease remains high. Recent estimates suggest that in the United States, the annual burden is as high as 30 000 cases of IPD, 500 000 cases of CAP, and 25 000 deaths [4]. Multivalent, pneumococcal-free polysaccharide vaccines have been available for over 20 years [5]. In the United States, a 23-valent pneumococcal polysaccharide vaccine (PPSV23) has been recommended for all adults aged ≥65 years, and over the past decade the vaccination rate has been around 60% [6, 7]. Nevertheless, there has been little impact on disease caused by the serotypes that are unique to that vaccine [8]. This lack of impact is likely due to the T-cell–independent nature of the immune response to free polysaccharides that results in short-lived B-cell responses. In addition, memory B cells are not produced in response to most free polysaccharide vaccines and, in fact, may be depleted postvaccination resulting in hyporesponsiveness (a blunted immune response) to future vaccine doses [1]. Furthermore, PPSV23 efficacy against CAP has been difficult to document [5, 9] and a recent Cochrane analysis concluded that “the meta-analysis does not provide compelling evidence to support the routine use of pneumococcal polysaccharide vaccine to prevent all-cause pneumonia or mortality” [10].
THE RATIONALE FOR A PNEUMOCOCCAL CONJUGATED POLYSACCHARIDE VACCINE FOR ADULTS
A 13-valent pneumococcal conjugate vaccine (PCV13) was developed and recently licensed for use in children [11]. PCV13 contains conjugates for pneumococcal serotypes 1, 3, 4, 5, 6A, 6B, 7F, 9V, 14, 18C, 19A, 19F, and 23F. Since introduction, combined effectiveness against the 6 additional serotypes (1, 3, 5, 6A, 7F, and 19A) in this vaccine has been demonstrated for invasive disease [12, 13], and for carriage in children with otitis media, including evidence of cross-protection against serotype 6C [14]. A number of immunogenicity studies have been performed with PCVs in adults and have generally concluded that PCV7 elicits a superior response in adults when compared with PPSV23. These studies have been recently summarized [9]. However, in Malawi, a randomized controlled trial with PCV7 administered to adults infected with human immunodeficiency virus (HIV) following hospitalization for IPD demonstrated a 74% reduction in IPD [15], whereas a prior trial with PPSV23 in an HIV-positive population in Uganda did not demonstrate protection [16].
As a result of the success of conjugated pneumococcal polysaccharide vaccines in children and the encouraging, but limited, data from clinical studies with PCV7 in adults, we sought to test the ability of PCV13 to induce a response in adults that was quantitatively and qualitatively different from that seen with PPSV23. The clinical program was designed to show that PCV13 could induce a T-cell–dependent response that could then be recalled or boosted by either natural exposure or a subsequent vaccination, or both. Pivotal phase 3 clinical trials have been performed in 2 populations of adults aged ≥50 years: those who were naive to previous vaccination (Study 004) [17, 18] and those who had been previously vaccinated with PPSV23 but not within the past 5 years (Study 3005) [19]. The results from these trials have recently been presented and details will be submitted for publication in the near future. The results are briefly summarized here.
The design and objectives of the 2 pivotal trials in these populations are shown in Figures 1 and 2. In both trials, adults were randomized to receive either PCV13 or PPSV23, and the response after a single dose was evaluated. The subjects were subsequently administered a second immunization with one of the vaccines either 1 year later (Study 3005) or 3–4 years later (Study 004). The antipneumococcal immune responses were measured using a functional opsonophagocytic activity (OPA) assay, and these responses were generally concordant with the immunoglobulin G responses. As OPA is known to be the mechanism of protection against pneumococcal disease [5], it is the most appropriate assay for comparing antibody responses elicited by vaccines as well as for comparing the responses to various dosing regimens. To compare groups, a geometric mean titer ratio was determined; if the lower 95% confidence interval of the ratio was above 0.5 for serotypes in common, then the response was considered similar or noninferior. If, however, the lower 95% confidence interval was above 1.0, then the response was considered to be statistically significantly higher or superior.
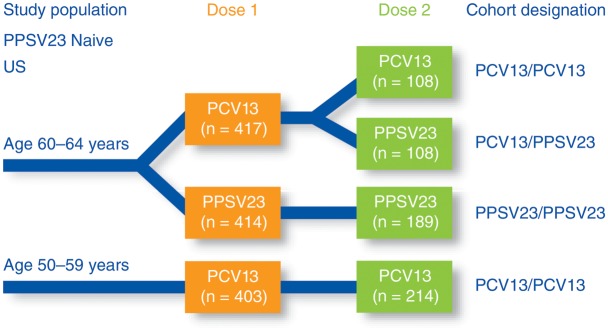
Figure 1.
Design of a phase 3, randomized, modified double-blind, active-controlled, multicenter trial (Study 004) conducted in the United States in subjects naive to 23-valent pneumococcal polysaccharide vaccine (PPSV23). Subjects aged 60–64 years were randomized to receive either 13-valent pneumococcal conjugate vaccine (PCV13) or PPSV23 as a first dose; subjects aged 50–59 years received PCV13 only as a first dose. All groups received a second vaccination with either PCV13 or PPSV23, 3–4 years after the first dose. Opsonophagocytic activity was measured at the time of the first dose, 1 and 12 months after the first dose, at the time of the second dose, and 1 month after the second dose. Abbreviations: PCV13, 13-valent pneumococcal conjugate vaccine; PPSV23, 23-valent pneumococcal polysaccharide vaccine.
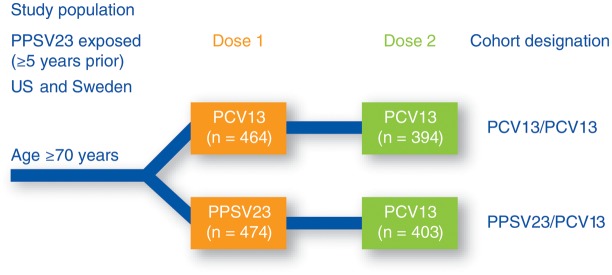
Figure 2.
Design of a phase 3, randomized, modified double-blind, active-controlled multicenter trial (Study 3005) conducted in the United States and Sweden in healthy subjects aged at least 70 years who received a previous vaccination with 23-valent pneumococcal polysaccharide vaccine (PPSV23) at least 5 years before study enrollment. Subjects were randomized to receive either 13-valent pneumococcal conjugate vaccine (PCV13) or PPSV23 as a first dose, with both groups receiving a dose of PCV13 1 year after the first dose. Opsonophagocytic activity was measured at the time of each dose and 1 month after each dose. Abbreviations: PCV13, 13-valent pneumococcal conjugate vaccine; PPSV23, 23-valent pneumococcal polysaccharide vaccine.
RESPONSE TO PCV13 IN NAIVE ADULTS
Study 004 [17, 18] was conducted in 2 age cohorts who had never received a pneumococcal vaccine previously: those aged 50–59 years and 60–64 years (Figure 1). At the time of enrollment and randomization, subjects had very low OPA titers near the lower limit of detection in the assay in both age groups. At 1 month following the first dose in those aged 60–64 years (mean age, 62 years), the response to a single dose of PCV13 was at least as good as the response to PPSV23 for all of the serotypes (noninferior) and statistically significantly higher for 9 of the 13 serotypes. The overall superior response to a single dose of PCV13 was seen consistently throughout the clinical program. The response to a dose of PCV13 in those aged 50–59 years (mean age, 54 years) was superior to the response in the 60–64 years cohort for 9 of 13 serotypes, indicating the importance of age as a factor in the immune response. The fact that 2–4 µg of conjugate vaccine induced a better response than 25 µg of free polysaccharide also likely reflects the different immune response pathway used by the T-cell–dependent antigens in PCV13.
A subset of the study subjects returned for a second immunization with either PCV13 or PPSV23 3–4 years (average, 3.7 years) after the initial dose of either PCV13 or PPSV23. Prior to the second immunization, antibody levels had waned in all groups. However, a second dose of PCV13 resulted in a renewed immune response, reflecting the response seen after the first PCV13 administration for all of the serotypes and, importantly, was statistically significantly higher for 6 of the 13 serotypes. A similar result was achieved when PCV13 was followed 3–4 years later by a dose of PPSV23. In this case, the response was statistically higher for 7 of the 13 serotypes. These data demonstrate that PCV13 primed the immune system for a booster response to subsequent vaccination with either vaccine.
This result was in stark contrast to the response seen when subjects received 2 doses of PPSV23. In this case, the second dose of PPSV23 elicited a response that was statistically significantly lower for 8 of the 12 serotypes common to both vaccines when compared with the response after the first dose of PPSV23. Of note, the response in subjects given 2 doses of PCV13 was significantly higher for all 12 serotypes common to both vaccines when compared with that seen for subjects receiving 2 doses of PPSV23.
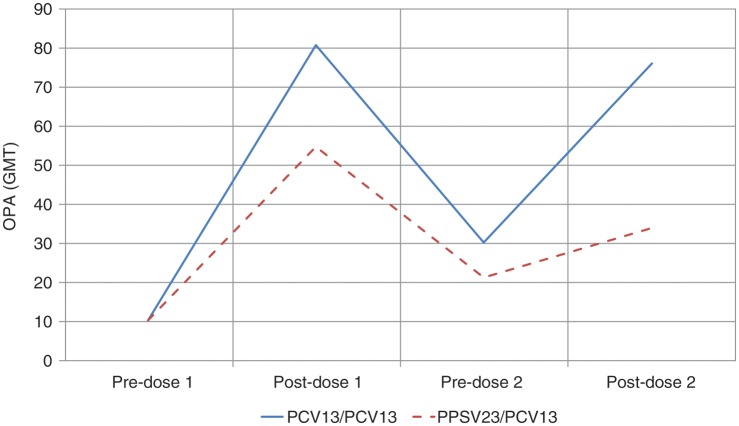
The blunted immune state illustrated by the lower response to subsequent immunization, observed in Study 004 for PPSV23, confirms previous findings of other investigators [20]. Studies of group A and C meningococcal polysaccharide vaccines and repeated doses of PPSV23 in adults and children have shown that a state of immune tolerance or hyporesponsiveness can develop to repeated polysaccharide vaccine antigen exposures [21]. The mechanism of hyporesponsiveness has remained undefined for many years, but recently, Brynjolfsson and colleagues [22] demonstrated in an animal study with meningococcal C polysaccharide that hyporesponsiveness is caused by apoptosis of memory B cells. Figure 3 shows the pattern of the immune response to serotype 1, which is illustrative of the sequences for the majority of serotypes. The conjugate vaccine induces an immune response that is superior to the free polysaccharide vaccine after the first dose but, more importantly, has primed the immune system for a second dose of either vaccine. In contrast, the PPSV23 vaccine inhibits the response to a second dose of the same vaccine, and this was seen for all of the serotypes.
Figure 3.
Functional immune responses for pneumococcal serotype 1 (geometric mean titer) in the pivotal noninferiority trial (Study 004) measured pre- and postvaccination using a functional opsonophagocytic activity assay. Antibodies were determined before first vaccination (pre-dose 1), 1 month after vaccination (1 month post), and 12 months after first vaccination (12 months post); and before the second vaccination 3–4 years later (pre-dose 2) and 1 month after the second vaccination (post-dose 2). Abbreviations: GMT, geometric mean titer; OPA, opsonophagocytic activity; PCV13, 13-valent pneumococcal conjugate vaccine; PPSV23, 23-valent pneumococcal polysaccharide vaccine.
RESPONSE TO PCV13 IN ADULTS WHO HAD PREVIOUSLY RECEIVED PPSV23
It was important to show that PCV13 could also prime the immune system in individuals previously immunized with PPSV23. Study 3005 [19] enrolled PPSV23 exposed individuals (mean age, 77 years) to receive either PCV13 or PPSV23 again (Figure 2). The pattern of the immune response was essentially the same as that seen in the PPSV23-naive adults (Study 004), although the magnitude of the response was somewhat lower in these older individuals. The response to PCV13 was at least as good as the response to PPSV23 and was statistically significantly higher for 11 of the 13 serotypes. Subjects were reimmunized 1 year later with PCV13, regardless of whether they had received PCV13 or PPSV23 the previous year. Subjects who had previously received a dose of PCV13 had a response that was at least noninferior to the first dose. In contrast, subjects who had received PPSV23 a year previous had a significantly reduced response to PCV13. Although Study 004 in vaccine-naive adults showed that PPSV23 blunted the response to a second dose of the same vaccine, Study 3005 showed that PPSV23 also blunted the response to a dose of the conjugate vaccine. Figure 4 shows the pattern of responsiveness to serotype 1 as an example.
Figure 4.
Functional immune responses for pneumococcal serotype 1 (geometric mean titer) in the pivotal noninferiority trial (Study 3005) measured pre- and postvaccination using a functional opsonophagocytic activity assay. Antibodies were determined before first vaccination (pre-dose 1), 1 month after vaccination (post-dose 1), before the second vaccination (pre-dose 2), and 1 month after the second vaccination (post-dose 2). Abbreviations: GMT, geometric mean titer; OPA, opsonophagocytic activity; PCV13, 13-valent pneumococcal conjugate vaccine; PPSV23, 23-valent pneumococcal polysaccharide vaccine.
CONCLUSION
PCV13 is a new tool for meeting the significant unmet medical need to prevent pneumococcal disease in adults. It induces a T-cell–dependent immune response in adults that can be recalled or boosted by a subsequent dose of PCV13 or free polysaccharide vaccine. The latter observation may be considered a surrogate for exposure to the polysaccharide during a “natural” infection. Thus, by priming the immune system, PCV13 provides the potential to significantly enhance and prolong immune protection by response to either natural exposure or to booster vaccination. The conjugate vaccine may also provide an important option for previously immunized adults in whom revaccination with a polysaccharide is generally not recommended.
Notes
Acknowledgments. I would like to thank my Pfizer colleagues Rene Reinert, Gail Rodgers, Emilio Emini, William Gruber, and Diana Morgenstern for their thoughtful review of the manuscript. Editorial support was provided by Nancy Price of Excerpta Medica and was funded by Pfizer Inc.
Potential conflict of interest. P. R. P. is an employee of and holds stock in Pfizer, Inc.
The author has submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1.Pollard AJ, Perrett KP, Beverley PC. Maintaining protection against invasive bacteria with protein-polysaccharide conjugate vaccines. Nat Rev Immunol. 2009;9:213–20. doi: 10.1038/nri2494. [DOI] [PubMed] [Google Scholar]
- 2.Richmond P, Borrow R, Miller E, et al. Meningococcal serogroup C conjugate vaccine is immunogenic in infancy and primes for memory. J Infect Dis. 1999;179:1569–72. doi: 10.1086/314753. [DOI] [PubMed] [Google Scholar]
- 3.Reinert RR, Paradiso P, Fritzell B. Advances in pneumococcal vaccines: the 13-valent pneumococcal conjugate vaccine received market authorization in Europe. Expert Rev Vaccines. 2010;9:229–36. doi: 10.1586/erv.10.6. [DOI] [PubMed] [Google Scholar]
- 4.Weycker D, Strutton D, Edelsberg J, Sato R, Jackson LA. Clinical and economic burden of pneumococcal disease in older US adults. Vaccine. 2010;28:4955–60. doi: 10.1016/j.vaccine.2010.05.030. [DOI] [PubMed] [Google Scholar]
- 5.Jackson LA, Neuzil KM. Pneumococcal polysaccharide vaccines. In: Plotkin SA, Orenstein WA, Offit PA, editors. Vaccines. 5th ed. Philadelphia: Saunders, Elsevier; 2008. pp. 570–604. [Google Scholar]
- 6.Centers for Disease Control and Prevention. Prevention of pneumococcal disease: recommendations of the Advisory Committee on Immunization Practices (ACIP) MMWR Recomm Rep. 1997;46:1–24. [PubMed] [Google Scholar]
- 7.Chowdhury P, Balluz L, Town M, et al. Surveillance of certain health behaviors and conditions among states and selected local areas—Behavioral Risk Factor Surveillance System, United States, 2007. MMWR Surveill Summ. 2010;59:1–220. [PubMed] [Google Scholar]
- 8.Pilishvili T, Lexau C, Farley MM, et al. Sustained reductions in invasive pneumococcal disease in the era of conjugate vaccine. J Infect Dis. 2010;201:32–41. doi: 10.1086/648593. [DOI] [PubMed] [Google Scholar]
- 9.Metersky ML, Dransfield MT, Jackson LA. Determining the optimal pneumococcal vaccination strategy for adults: is there a role for the pneumococcal conjugate vaccine? Chest. 2010;138:486–90. doi: 10.1378/chest.10-0738. [DOI] [PubMed] [Google Scholar]
- 10.Moberley SA, Holden J, Tatham DP, Andrews RM. Vaccines for preventing pneumococcal infection in adults. Cochrane Database Syst Rev. 2008:CD000422. doi: 10.1002/14651858.CD000422.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Paradiso PR. Advances in pneumococcal disease prevention: 13-valent pneumococcal conjugate vaccine for infants and children. Clin Infect Dis. 2011;52:1241–7. doi: 10.1093/cid/cir142. [DOI] [PubMed] [Google Scholar]
- 12.Miller E, Andrews NJ, Waight PA, Slack MP, George RC. Effectiveness of the new serotypes in the 13-valent pneumococcal conjugate vaccine. Vaccine. 2011;29:9127–31. doi: 10.1016/j.vaccine.2011.09.112. [DOI] [PubMed] [Google Scholar]
- 13.Moore M, Link-Gelles R, Farley M, et al. Impact of 13-valent pneumococcal conjugate vaccine on invasive pneumococcal disease among children <2 years old, U. S, 2010 [abstract G1–538] Programs and abstracts of the 51st Interscience Conference on Antimicrobial Agents and Chemotherapy; September 16–20, 2011; Chicago, Illinois. [Google Scholar]
- 14.Cohen R, Levy C, Bingen E, et al. Impact of 13-valent pneumococcal conjugate vaccine (PCV13) on nasopharyngeal (NP) flora in children with acute otitis media (AOM) [abstract G3–1709] Programs and abstracts of the 51st Interscience Conference on Antimicrobial Agents and Chemotherapy; September 16–20, 2011; Chicago, Illinois. [Google Scholar]
- 15.French N, Gordon SB, Mwalukomo T, et al. A trial of a 7-valent pneumococcal conjugate vaccine in HIV-infected adults. N Engl J Med. 2010;362:812–22. doi: 10.1056/NEJMoa0903029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.French N, Nakiyingi J, Carpenter LM, et al. 23-valent pneumococcal polysaccharide vaccine in HIV-1 infected Ugandan adults: double blind, randomised and placebo controlled trial. Lancet. 2000;355:2106–11. doi: 10.1016/s0140-6736(00)02377-1. [DOI] [PubMed] [Google Scholar]
- 17.Jackson LA, Gurtman A, van Cleeff M, et al. Immunogenicity and safety of a 13-valent pneumococcal conjugate vaccine in pneumococcal vaccine naïve adults, 50–64 years of age [abstract O426] Clin Microbiol Infect. 2011;17(suppl S4):S85. [Google Scholar]
- 18.Jackson LA, Gurtman A, van Cleeff M, et al. 13-valent Pneumococcal conjugate vaccine (PCV13) enhances the response to subsequent PCV13 and 23-valent pneumococcal polysaccharide (PPSV23) vaccinations in adults 50 years and older [abstract LB-3] Programs and abstracts of the 49th Annual Meeting of the Infectious Diseases Society of America; October 20–23, 2011; Boston, Massachusetts. [Google Scholar]
- 19.Jackson LA, Gurtman A, Rice K, et al. Immunogenicity and safety of a 13-valent pneumococcal conjugate vaccine in adults 70 years of age and older previously vaccinated with 23-valent pneumococcal polysaccharide vaccine [abstract O425] Clin Microbiol Infect. 2011;17(suppl S4):S85. doi: 10.1016/j.vaccine.2013.05.010. [DOI] [PubMed] [Google Scholar]
- 20.Törling J, Hedlund J, Konradsen HB, Ortqvist A. Revaccination with the 23-valent pneumococcal polysaccharide vaccine in middle-aged and elderly persons previously treated for pneumonia. Vaccine. 2003;22:96–103. doi: 10.1016/s0264-410x(03)00521-8. [DOI] [PubMed] [Google Scholar]
- 21.O'Brien KL, Hochman M, Goldblatt D. Combined schedules of pneumococcal conjugate and polysaccharide vaccines: is hyporesponsiveness an issue? Lancet Infect Dis. 2007;7:597–606. doi: 10.1016/S1473-3099(07)70210-4. [DOI] [PubMed] [Google Scholar]
- 22.Brynjolfsson SF, Henneken M, Bjarnarson SP, Mori E, Del Giudice G, Jonsdottir I. Hyporesponsiveness following booster immunization with bacterial polysaccharides is caused by apoptosis of memory B cells. J Infect Dis. 2012;205:422–30. doi: 10.1093/infdis/jir750. [DOI] [PubMed] [Google Scholar]