This study determined the serotype-specific acquisition rates for pneumococcal colonization in a cohort of 1404 newborn infants followed intensively for 3 months. By observing pneumococcal carriage in family members, we were able to determine serotype-specific transmission probabilities between relatives.
Abstract
Background. Herd protection and serotype replacement disease following introduction of pneumococcal conjugate vaccine (PCV) are attributable to the vaccine's impact on colonization. Prior to vaccine introduction in Kenya, we did an epidemiological study to estimate the rate of pneumococcal acquisition, by serotype, in an uncolonized population.
Methods. Nasopharyngeal swab specimens were taken from newborns aged ≤7 days and weekly thereafter for 13 weeks. Parents, and siblings aged <10 years, were swabbed at monthly intervals. Swabs were transported in skim milk-tryptone-glucose-glycerin and cultured on gentamicin blood agar. Pneumococci were serotyped by the Quellung reaction. We used survival analysis and Cox regression analysis to examine serotype-specific acquisition rates and risk factors and calculated transmission probabilities from the pattern of acquisitions within the family.
Results. Of 1404 infants recruited, 887 were colonized by 3 months of age, with the earliest acquisition detected on the first day of life. The median time to acquisition was 38.5 days. The pneumococcal acquisition rate was 0.0189 acquisitions/day (95% confidence interval, .0177–.0202 acquisitions/day). Serotype-specific acquisition rates varied from 0.00002–0.0025 acquisitions/day among 49 different serotypes. Season, coryza, and exposure to cigarettes, cooking fumes, and other children in the home were each significant risk factors for acquisition. The transmission probability per 30-day duration of contact with a carrier was 0.23 (95% CI, .20–.26).
Conclusions. Newborn infants in Kilifi have high rates of nasopharyngeal acquisition of pneumococci. Half of these acquisitions involve serotypes not included in any current vaccine. Several risk factors are modifiable through intervention. Newborns represent a consistent population of pneumococcus-naive individuals in which to estimate the impact of PCV on transmission.
Introduction of pneumococcal conjugate vaccines (PCVs) has reduced childhood invasive pneumococcal disease (IPD) caused by vaccine serotypes in all populations studied. However, the indirect effects of PCV, herd protection and serotype replacement disease, have been much more variable [1]. Serotype replacement disease has not occurred in some populations, while in others it has almost negated the beneficial direct effect of PCV [2, 3].
The GAVI Alliance is supporting PCV introduction in all developing countries, and the magnitude of the indirect effects of PCV will be a significant factor determining the value of these investments. The broad surveillance systems for IPD that have characterized the impact of PCV in developed countries [4, 5] were not established in developing countries, and prevaccine data on IPD incidence are scarce. However, because indirect vaccine effects are mediated by changes in nasopharyngeal carriage, and because carriage is more amenable to study than IPD, it may be possible to model the effect of vaccine introduction on disease by monitoring its effect on carriage and transmission [6, 7].
A 10-valent PCV (containing serotypes 1, 4, 5, 6B, 7F, 9V, 14, 18C, 19F, and 23F) was introduced into the childhood immunization program of Kenya in February 2011. Before introduction of the vaccine, we studied uninfected newborn infants to examine the baseline rate of acquisition of nasopharyngeal colonization. Because each pneumococcal serotype exhibits a different epidemiological pattern [8], we studied acquisition rates for the 28 most common serotypes. Acquisition rates describe the experience of the newborn without reference to the source of infection. To estimate the transmission probability, defined as the probability that contact between a colonized relative and an uninfected newborn results in colonization of the newborn [9], we also studied the carriage status of household family members.
METHODS
Study Population
The study was conducted at Kilifi District Hospital (KDH) on the Indian Ocean coast of Kenya among families who were residents of the Kilifi Health and Demographic Surveillance System (KHDSS) [10]. This is a longitudinal surveillance of approximately 250 000 people living in a well-defined geographic area around KDH, with an annual birth cohort of approximately 8000. Mother-infant pairs were recruited after delivery of the infant in the KDH maternity department, when the infant was registered in the KHDSS, or when the infant was brought to the KDH vaccination clinic, if the visit occurred ≤7 days after birth. We then visited the whole family at home and invited them to participate. Infants were excluded from the study if they had >6 siblings aged <10 years but were not excluded if their relatives declined to participate.
Study Design
We collected nasopharyngeal swab specimens from newborns twice weekly for 2 weeks and weekly thereafter. Follow-up ceased when a pneumococcus was cultured from an infant's swab or 13 weeks after study entry, whichever was sooner. Nasopharyngeal swab specimens were taken from the infant's mother, father, and siblings at recruitment and every 4 weeks thereafter until the index infant was colonized or reached 9 weeks of age, whichever was sooner. By questionnaire, we ascertained household size and location, socioeconomic variables, and results of human immunodeficiency virus (HIV) testing performed for the mother antenatal clinic. At each maternal contact, we ascertained the following information for the infant: the number of household members and siblings, breast-feeding status, cigarette and cooking smoke exposure, symptoms of upper respiratory tract infection, and antibiotic use.
Laboratory Assay
Specimens were collected and processed following standard methods [11]. Dacron-tipped flexible wire swabs were inserted into the posterior nasopharynx, rotated slowly for 1 second, and withdrawn. Swab tips were removed with wire cutters and transported to the laboratory within 8 hours in skim milk-tryptone-glucose-glycerin (STGG) medium, where they were cultured directly. The STGG was vortexed for 20 seconds to extract the nasopharyngeal specimen from the swab, and a 10-μL sample was inoculated onto blood agar with 2.5 μg/mL gentamicin and incubated overnight at 37°C in 5% CO2. Pneumococci were identified by α-hemolysis, optochin sensitivity, and presence of capsules. Pneumococci were serotyped by the Quellung reaction, using polyclonal rabbit antisera (Statens Seruminstitut, Copenhagen, Denmark). We serotyped 4 pneumococcal colonies per plate, selecting morphologically distinct colonies when possible. For the infant's relatives we analyzed multiple-serotype carriage because all colonizing serotypes constitute an infection risk to the infant, but for the infant we only analyzed the dominant colonizing serotype because the risk of simultaneous acquisition of two serotypes is small. We performed quality control of STGG and gentamicin blood agar to ensure sterility and the ability to support pneumococcal growth. Serotyping was monitored by a 2-monthly internal quality assurance scheme, using a library of standard pneumococci.
Analysis
The analyses were performed using Stata v11.2 (StataCorp, College Station, TX). Acquisition rates were estimated by survival analysis of infection-free periods, defined by the midpoint of the interval in which the swab result switched from negative to positive. Infants whose first swab specimen tested positive were assumed to have been negative for pneumococci at birth. Infants who were lost to follow-up throughout the study or who remained untraceable after 98 days were censored at the time their last swab specimen was collected.
We explored the risk factors for acquisition of pneumococcal colonization by use of Cox regression to examine both fixed covariates (eg, month of birth) and time-dependent covariates (eg, coryza at the time of swabbing). Variables with a significant association (P < .1) on univariate analyses were included in a backward, stepwise regression model and rejected at the P ≥ .05 level on the basis of likelihood ratio tests. We examined the proportional hazards assumption by testing the slope of Schoenfeld residuals over time and by identifying parallelism in log-log hazard plots.
Transmission Probability
Because pneumococci are poorly transmissible, we monitored family members at monthly intervals and set the interval for a relevant contact as 30 days of cohabitation. We identified these contact periods between relatives and newborns and followed the infection-free survival of the infant for up to 30 days. The start of each contact period was defined as the date on which a pneumococcus-positive swab sample was collected from the relative, and the end of the contact period was defined by the date on which the next swab specimen was collected from that same relative, the date on which acquisition of any pneumococcus was detected in the infant, the date on which the last pneumococcus-negative swab specimen was collected from the infant (if the infant was lost to follow-up), or 30 days after the collection of the initial swab specimen from the relative, whichever was sooner. We assumed that a relative colonized at the outset remained infectious throughout the contact period. Because the contact periods varied in duration (1–30 days), we used survival analysis to estimate the daily hazard of acquisition of a homologous serotype in the newborn. Analysis time began at the start of each contact period, and we measured hazard rates per 30 days. If the relative's initial swab specimen was positive for >1 serotype, we treated these serotypes as independent exposures and duplicated the contact period record. If an infant was simultaneously exposed to >1 relative with the same serotype, we weighted the record with the reciprocal of the number of simultaneously infected relatives. We explored variation in the transmission hazard rate by type of relative, age of sibling, and sex of newborn. We converted the hazard of homologous acquisition (λ) to transmission probabilities (P), using P = 1−e−λ.
The KEMRI National Ethical Review Committee and the Oxford Tropical Research Ethics Committee approved the study, and written informed consent was obtained from all adult participants and from the mothers of participating infants and their siblings.
RESULTS
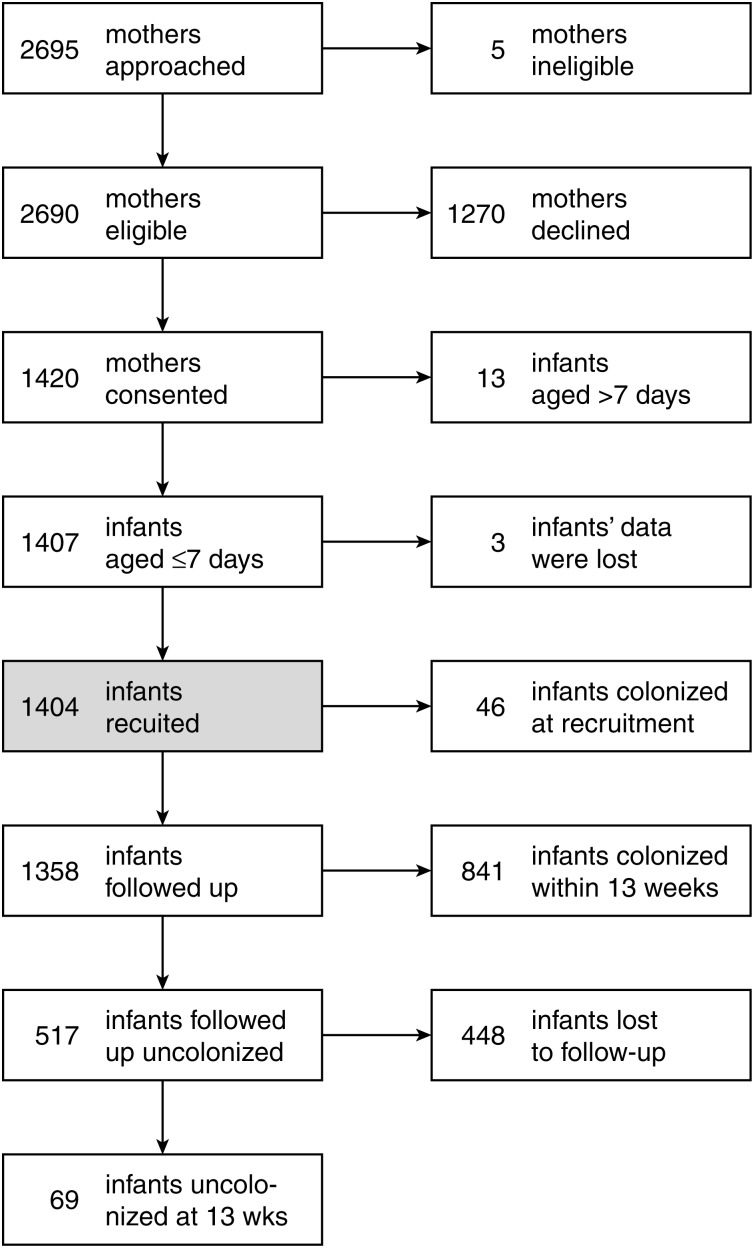
Recruitment began 29 June 2006 and ended 3 March 2009, and the last swab was obtained on 14 May 2009. In total, 2080 pneumococcal isolates were cultured from 12 610 swabs. Figure 1 shows the flow of recruitment and follow-up. The mean (median) age of infants at recruitment was 2.1 days (1 day). Of 1404 children recruited, 46 infants (3.3%) were already colonized at the time their first swab specimen was collected. The prevalence of existing colonization increased linearly, from 0.86% (5 of 583) on day 1 to 9.7% (10 of 103) on day 7 of life (Supplementary Table S1). We recruited 1372 mothers, 221 fathers, and 1412 siblings, of whom 1357 (99%), 189 (86%), and 1268 (90%), respectively, were recruited within 7 days of the infant. We were unable to locate 355 fathers, and 828 fathers declined to participate.
Figure 1.
Flow of subjects recruited into the study. The timing of acquisitions and losses to follow-up are detailed in Supplementary Table S2. Losses to follow-up were mainly due to withdrawal of consent.
Rates of Acquisition
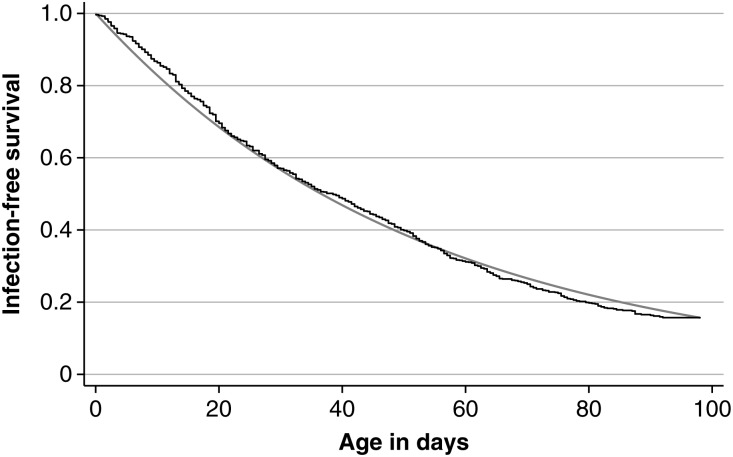
The mean (median) interval between swab collection among newborn participants was 7.04 days (7 days); the mean (median) interval used to estimate acquisition was 9.2 days (7 days). A total of 887 infections were observed during 46 947 days of risk, giving an acquisition hazard rate of 0.0189 per child per day (95% confidence interval [CI], .0177–.0202). The observed median time to acquisition was 38.5 days. Despite a good approximation to an exponential curve (Figure 2), the hazard rate was 25% higher (0.0225; 95% CI, .0197–.0257) in the second half of follow-up time than in the first half (0.0180; 95% CI, .0166–.0194; P = .0046). We used nonparametric survival techniques for all further analyses.
Figure 2.
Survival curve and exponential fit to infection-free duration among newborn infants followed up for pneumococcal colonization.
Serotype-specific Rates of Acquisition
Among 887 pneumococci acquired in the study, there were 49 different serotypes; one pneumococcus died before it could be serotyped. The hazard rate for acquisition of individual serotypes (Table 1 and Supplementary Table S3) varied from 0.0025/day for serotype 19F, the most common serotype, to 0.000021/day for the rarest serotypes. The acquisition rate for serotypes contained in the 13-valent PCV (which includes serotypes 3, 6A, and 19A, in addition to PCV10 serotypes) was half of the total pneumococcal acquisition rate (Table 1).
Table 1.
Rate of Pneumococcal Acquisition Among Newborn Infants, by Serotype, in Decreasing Order of Rates and in Groups of Serotypes Contained in Vaccine Formulations
| Serotype | Acquisitions (No.) | Hazard Rate (per Day) | 95% CI |
|---|---|---|---|
| 19F | 119 | 0.00254 | .00212–.00303 |
| 6A | 70 | 0.00149 | .00118–.00189 |
| 6B | 59 | 0.00126 | .00097–.00162 |
| 23F | 58 | 0.00124 | .00096–.00160 |
| 23B | 47 | 0.00100 | .00075–.00133 |
| 14 | 37 | 0.00079 | .00057–.00109 |
| 35B | 37 | 0.00079 | .00057–.00109 |
| 11A | 33 | 0.00070 | .00050–.00099 |
| 15B | 28 | 0.00060 | .00041–.00086 |
| 15C | 26 | 0.00055 | .00038–.00081 |
| 15A | 25 | 0.00053 | .00036–.00079 |
| 34 | 25 | 0.00053 | .00036–.00079 |
| 19A | 24 | 0.00051 | .00034–.00076 |
| 10A | 23 | 0.00049 | .00033–.00074 |
| 3 | 21 | 0.00045 | .00029–.00069 |
| 9V | 20 | 0.00043 | .00028–.00066 |
| 13 | 19 | 0.00041 | .00026–.00063 |
| 19B | 19 | 0.00041 | .00026–.00063 |
| 21 | 19 | 0.00041 | .00026–.00063 |
| 7C | 17 | 0.00036 | .00023–.00058 |
| 20 | 16 | 0.00034 | .00021–.00056 |
| 16F | 15 | 0.00032 | .00019–.00053 |
| 4 | 15 | 0.00032 | .00019–.00053 |
| 18C | 12 | 0.00026 | .00015–.00045 |
| 23A | 12 | 0.00026 | .00015–.00045 |
| 33B | 12 | 0.00026 | .00015–.00045 |
| 38 | 12 | 0.00026 | .00015–.00045 |
| 33D | 11 | 0.00023 | .00013–.00042 |
| PCV7 types | 320 | 0.00682 | .00611–.00761 |
| 1, 5, 7F | 9 | 0.00019 | .00010–.00037 |
| 3, 6A, 19A | 115 | 0.00245 | .00204–.00294 |
| All other types | 443 | 0.00944 | .00860–.01036 |
| All | 887 | 0.01889 | .01769–.02018 |
The total time at risk was 46 947 days. Analyses are shown for all serotypes in which >10 acquisitions were observed in the study. Serotypes contained in PCV7 are 4, 6B, 9V, 14, 18C, 19F, and 23F. The results of the analyses of all serotypes are shown in Supplementary Table S3.
Abbreviations: CI, confidence interval; PCV7, 7-valent pneumococcal conjugate vaccine.
Risk Factors for Acquisition
Univariate hazard ratios for potential risk factors are shown in Table 2. In the final model (Table 3), there was a single significant interaction between coryza observed at the previous visit and the number of siblings (P = .019, by the likelihood ratio test). Prior coryza did not have any effect on the hazard of acquisition in the absence of siblings but enhanced the hazard associated with siblings, at least for newborns with 1–4 siblings. The proportional hazards assumptions were met by all variables.
Table 2.
Univariate Risk Factors for Acquisition of Pneumococcal Colonization
| Risk factor | Acquisitions (No.) | Alla Infants (No.) | Hazard Rate | 95% CI | P |
|---|---|---|---|---|---|
| Study period (per month) | 887 | 1404 | 0.999 | .997–1.037 | <.00005 |
| Month | 887 | 1404 | <.00005 | ||
| Jan | … | … | 1.00 | ||
| Feb | … | … | 0.85 | .58–1.23 | |
| Mar | … | … | 0.88 | .62–1.25 | |
| Apr | … | … | 0.92 | .65–1.3 | |
| May | … | … | 0.91 | .64–1.3 | |
| Jun | … | … | 1.27 | .9–1.79 | |
| Jul | … | … | 1.77 | 1.29–2.42 | |
| Aug | … | … | 1.46 | 1.05–2.02 | |
| Sep | … | … | 1.50 | 1.07–2.1 | |
| Oct | … | … | 1.26 | .89–1.77 | |
| Nov | … | … | 0.94 | .65–1.35 | |
| Dec | … | … | 0.66 | .44–.99 | |
| Male sex | 887 | 1404 | 0.97 | .85–1.11 | .68 |
| Mother tested positive for HIV | 515 | 923 | 1.11 | .78–1.56 | .5792 |
| No. of smokers in the house | 835 | 1191 | .0276 | ||
| 0 | … | … | 1.00 | ||
| 1 | … | … | 1.26 | 1.08–1.48 | |
| 2 | … | … | 1.54 | .69–3.43 | |
| Type of fuel used for cooking | 814 | 1167 | .0018 | ||
| Firewood | … | … | 1.00 | ||
| Charcoal | … | … | 0.52 | .3–0.88 | |
| Paraffin | … | … | 0.77 | .61–.97 | |
| Gas | … | … | 2.11 | .94–4.72 | |
| Breast-feeding at this visit | 880 | 1401 | 1.21 | .65–2.27 | .5279 |
| Breast-feeding at last visit | 880 | 1402 | 1.03 | .58–1.83 | .907 |
| Cough observed at this visit | 887 | 1404 | 1.65 | 1.34–2.03 | <.00005 |
| Coryza observed at this visit | 887 | 1404 | 1.81 | 1.55–2.13 | <.00005 |
| Coryza observed at last visit | 887 | 1404 | 1.46 | 1.21–1.76 | .0001 |
| Obvious nasal mucous at NP swab | 887 | 1404 | 1.36 | 1.03–1.8 | .0354 |
| Caregiver reports history of cough in infant | 846 | 1400 | 1.97 | 1.42–2.73 | .0002 |
| Caregiver reports history of coryza in infant | 800 | 1395 | 1.64 | 1.23–2.18 | .0017 |
| No. of siblings aged <10 years | 887 | 1403 | <.00005 | ||
| 0 | … | … | 1.00 | ||
| 1–2 | … | … | 1.69 | 1.41–2.02 | |
| 3–4 | … | … | 2.03 | 1.66–2.47 | |
| 5–6 | … | … | 1.47 | 1.02–2.13 | |
| No. of others aged <10 years in household | 835 | 1194 | <.00005 | ||
| 0 | … | … | 1.00 | ||
| 1 | … | … | 1.40 | 1.11–1.76 | |
| 2 | … | … | 1.66 | 1.35–2.05 | |
| 3 | … | … | 1.72 | 1.37–2.15 | |
| 4 | … | … | 2.25 | 1.72–2.95 | |
| ≥5 | … | … | 1.93 | 1.33–2.79 | |
| No. of others aged >10 years in household | |||||
| 0 | 834 | 1190 | 1.00 | .697 | |
| 1 | … | … | 0.93 | .74–1.17 | |
| 2 | … | … | 1.03 | .86–1.24 | |
| 3 | … | … | 0.88 | .69–1.12 | |
| 4–5 | … | … | 0.95 | .74–1.21 | |
| 6–9 | … | … | 0.87 | .56–1.36 |
Abbreviations: CI, confidence interval; HIV, human immunodeficiency virus; NP, nasopharyngeal.
a Data are for all infants for whom the risk factor was known.
Table 3.
Final Model of Risk Factors for Acquisition of Pneumococcal Colonization
| Risk Factor | Adjusted Hazard Ratio | 95% CI |
|---|---|---|
| Month | ||
| Jan | 1.00 | |
| Feb | 0.90 | .61–1.33 |
| Mar | 0.89 | .61–1.30 |
| Apr | 0.96 | .66–1.40 |
| May | 0.96 | .66–1.39 |
| Jun | 1.40 | .97–2.02 |
| Jul | 1.72 | 1.22–2.41 |
| Aug | 1.59 | 1.12–2.25 |
| Sep | 1.53 | 1.07–2.18 |
| Oct | 1.35 | .94–1.94 |
| Nov | 1.06 | .72–1.57 |
| Dec | 0.65 | .42–1.00 |
| Cigarette smokers in household | ||
| Per smoker | 1.20 | 1.04–1.39 |
| Fuel used for cooking | ||
| Firewood | 1.00 | |
| Charcoal | 0.49 | .29–.83 |
| Paraffin | 0.75 | .59–.94 |
| Gas | 2.24 | .99–5.08 |
| Coryza observed this visit | ||
| No | 1.00 | |
| Yes | 1.73 | 1.46–2.06 |
| Coryza not observed at last visit | ||
| No. of siblings aged <10 years | ||
| 0 | 1.00 | |
| 1–2 | 1.53 | 1.23–1.89 |
| 3–4 | 1.80 | 1.41–2.32 |
| 5–6 | 1.36 | .89–2.09 |
| Coryza observed at last visit | ||
| No. of siblings aged <10 years | ||
| 0 | 1.02 | |
| 1–2 | 2.42 | 1.79–3.27 |
| 3–4 | 2.03 | 1.35–3.04 |
| 5–6 | 0.40 | .10–1.65 |
| No. of other children <10 years in household | ||
| Per child | 1.08 | 1.02–1.14 |
Abbreviation: CI, confidence interval.
Serotype Prevalence in Family Members
Among 4387 swab specimens taken from family members, 1120 (26%) were positive for pneumococci, with 170 of 2144 swab specimens (7.93%) from mothers, 35 of 284 (12%) from fathers, and 915 of 1959 (46.7%) from siblings aged <10 years yielding positive results. Two serotypes were observed simultaneously in swab specimens from 69 siblings, 3 fathers, and 1 mother. In total, we observed 1193 pneumococci in the families of index children. The distribution of serotypes varied substantially across the 3 groups (ie, newborns, siblings, and parents; Supplementary Table S4).
Transmission Probability Within Families
There were 874 periods of contact of 1–30 days in duration between relatives and newborns in which the relative was colonized at the outset. In 54, the relative was cocolonized with 2 serotypes, giving 928 exposure periods for potential serotype-specific transmission. The mean (median) duration of these episodes was 21.3 days (26.5 days), and they were terminated by homologous transmission, heterologous acquisition, or no acquisition in the newborn in 186, 329, and 413 episodes, respectively. In 60 episodes homologous transmission took place from 1 of 2 simultaneously colonized relatives, and in 9 episodes it took place from 1 of 3 simultaneously colonized relatives.
The baseline homologous acquisition hazard for contact with a colonized relative was 0.26 (95% CI .23–.31) per 30-day period, giving a calculated transmission probability of 0.23 (95% CI, .20–.26) per 30-day period (Table 4). The crude transmission probability (calculated as the number of infections divided by the number of exposure episodes) was 0.20 (186 infections per 928 exposure episodes), although some of these episodes were <30 days. Overall, the homologous acquisition hazard was slightly greater among female infants than male infants, but this varied with the type of potentially infecting contact. The hazard ratio for (male) sex was 1.49 in association with maternal contact periods and 0.74 in association with sibling contact periods. The study was not designed to test interactions between child sex and relative type, and the test for interaction was not significant (P = .10). In sibling contact periods, the age of the sibling did not affect the probability of homologous acquisition in the infant (P = .37). Transmission probabilities for individual serotypes are shown in Table 4.
Table 4.
Conditional Pneumococcal Acquisition Hazard Rates and Transmission Probabilities
| Subset of Observations | Contact Periods (No.) | Risk Interval (×30 Days) | Hazard Rate | 95% CI | Transmission Probability |
|---|---|---|---|---|---|
| All risk episodes | 928 | 571.9 | 0.26 | .23–0.31 | 0.23 |
| For female infants | 407 | 247.6 | 0.29 | .23–0.36 | 0.25 |
| For male infants | 521 | 324.3 | 0.24 | .20–0.30 | 0.22 |
| Fathers | 31 | 20.2 | 0.10 | .03–0.56 | 0.09 |
| Mothers | 128 | 87.5 | 0.27 | .19–0.40 | 0.24 |
| To female infants | 62 | 45.0 | 0.22 | .12–0.42 | 0.20 |
| To male infants | 66 | 42.4 | 0.33 | .20–0.55 | 0.28 |
| Siblings | 769 | 464.3 | 0.27 | .23–0.32 | 0.23 |
| To female infants | 333 | 193.3 | 0.32 | .25–0.40 | 0.27 |
| To male infants | 436 | 271.0 | 0.23 | .19–0.30 | 0.21 |
| Siblings, by age | |||||
| 0–1 year | 97 | 59.5 | 0.34 | .22–0.53 | 0.29 |
| 2–3 years | 350 | 213.1 | 0.26 | .21–0.34 | 0.23 |
| 4–5 years | 198 | 120.2 | 0.27 | .20–0.38 | 0.24 |
| 6–7 years | 89 | 52.2 | 0.19 | .11–0.35 | 0.17 |
| 8–9 years | 35 | 19.4 | 0.31 | .15–0.73 | 0.27 |
| Serotype | |||||
| 3 | 41 | 24.8 | 0.04 | .01–0.39 | 0.04 |
| 4 | 19 | 12.2 | 0.25 | .08–1.07 | 0.22 |
| 5 | 7 | 4.6 | 0.22 | .04–1.96 | 0.20 |
| 6A | 78 | 48.2 | 0.31 | .20–0.51 | 0.27 |
| 6B | 61 | 35.6 | 0.20 | .10–0.43 | 0.18 |
| 7C | 12 | 7.6 | 0.13 | .04–0.12 | 0.12 |
| 9V | 24 | 14.3 | 0.28 | .11–0.90 | 0.24 |
| 10A | 29 | 20.5 | 0.20 | .08–0.64 | 0.18 |
| 11A | 43 | 28.4 | 0.25 | .12–0.57 | 0.22 |
| 13 | 33 | 22.6 | 0.18 | .07–0.55 | 0.16 |
| 14 | 34 | 22.2 | 0.23 | .10–0.64 | 0.20 |
| 15A | 23 | 15.7 | 0.26 | .10–0.85 | 0.23 |
| 15B | 20 | 11.8 | 0.34 | .13–1.04 | 0.29 |
| 15C | 40 | 25.8 | 0.31 | .16–0.64 | 0.27 |
| 16F | 13 | 10.0 | 0.20 | .05–1.65 | 0.18 |
| 18C | 19 | 11.0 | 0.27 | .09–1.03 | 0.24 |
| 19A | 27 | 16.2 | 0.25 | .10–0.78 | 0.22 |
| 19B | 12 | 5.2 | 0.58 | .20–1.98 | 0.44 |
| 19F | 104 | 67.3 | 0.39 | .27–0.56 | 0.32 |
| 20 | 22 | 15.7 | 0 | ||
| 21 | 17 | 9.9 | 0 | ||
| 23A | 11 | 9.0 | 0 | ||
| 23B | 33 | 16.8 | 0.54 | .30–1.00 | 0.41 |
| 23F | 43 | 24.9 | 0.44 | .25–0.81 | 0.36 |
| 33B | 13 | 9.8 | 0.20 | .05–1.72 | 0.19 |
| 34 | 41 | 25.6 | 0.16 | .06–0.49 | 0.14 |
| 35B | 37 | 20.6 | 0.29 | .14–0.68 | 0.25 |
| 38 | 11 | 5.6 | 0.18 | .03–1.64 | 0.16 |
The hazard rates and transmission probabilities for infant acquisition of a homologous serotype reflect a 30-day contact period with a colonized family member. Only serotypes with at least 120 days of risk time (4 × 30 days) are shown here: data on all serotypes are shown in Supplementary Table S5. Crude transmission probabilities can be obtained as the ratio of acquisitions to exposure periods.
Abbreviation: CI, confidence interval.
DISCUSSION
In one of the largest carriage studies ever conducted, we have estimated the rate of acquisition of colonization of 28 serotypes of pneumococcus among a susceptible uninfected population—newborn infants—and used acquisition conditional on contact with colonized relatives to estimate serotype-specific transmission probabilities.
Evidence from vaccine studies [12–14] and randomized controlled trials [15] suggests that the indirect effects of PCV on IPD are brought about by changes in pneumococcal carriage. Because of the delayed impact of PCV on carriage prevalence, it is assumed that PCV reduces the acquisition of vaccine serotypes without necessarily affecting carriage duration [12, 16]. PCV also reduces the carriage density of vaccine serotypes in the nasopharynx [16]. In a population with high vaccine coverage, reduced acquisition of vaccine serotypes leads to reduced prevalence, and this, in turn, reduces transmission. This negative feedback cycle anticipates a decline to extinction that can be predicted by the transmission probability, contact probability, vaccine efficacy against carriage, and vaccine coverage. A similar approach can be taken to predict the commensurate rise in nonvaccine serotype carriage leading to serotype-replacement disease [17]. However, at present both approaches rely on limited empirical data, and most models are constrained to a handful of common serotypes [18, 19]. The objectives of this study were to provide acquisition rates and transmission probabilities for a wide range of serotypes in the prevaccine era to populate such models. We also aimed to describe the epidemiology of pneumococcal acquisition in a highly vulnerable population.
It has been known for 80 years that pneumococci can colonize infants on the day of birth [20]; between 1930–1950, 30% of European newborns acquired pneumococci by the age of 12–15 days [20, 21]. In America, in the 1980s, the mean age of initial pneumococcal colonization was 6 months [22]; in Papua New Guinea, by contrast, 60% of infants were colonized within 15 days of age, and all infants acquired infection by 3 months of age [23, 24]. Similarly, in The Gambia half of all newborns were colonized within 24 days [25]. Newborns in Kilifi were colonized less rapidly than in these other tropical environments: at 1 week of age, 10% of children were already colonized, and the median age at colonization was 38.5 days. The acquisition rate increased 25% between the first and second 6 weeks of life. This may be a function of follow-up losses (in 24% of infants), or it may simply signal that newborns are protected against acquisition either by passive maternal antibody or by behavioral factors limiting their exposure to infection.
The study provides relatively precise estimates of acquisition rates for 28 pneumococcal serotypes. The serotypes included in the 10- and 13-valent PCVs accounted for 37%–50% of all acquisitions in newborns. Serotype-specific acquisition rates in this study correlated well with those estimated for older children (age, 3–59 months) in the same setting (r = 0.87; P < .00005), although the rates in the older children are on average 50% greater [26]. Our expectation, at the outset, was that acquisition rates would be higher in uninfected newborns than in the general childhood population because intraspecies competition among individuals with prevalent carriage would lower population mean rates, compared with rates in the uninfected group [27].
Our results provide credible transmission probabilities for approximately 25 different serotypes. These vary from 0.04 per 30 contact-days for serotype 3 to 0.44 per 30 contact-days for serotype 19B. Because the contact periods varied in duration, we estimated transmission probabilities, assuming a constant rate of transmission over time. Time-dependent processes that influence transmission, like acquired immunity in the relative or declining passive immunity in the newborn, undermine this assumption. However, the major factors in transmission, such as the occurrence of a viral upper respiratory tract infection in the transmitter [28], are not associated with the existing duration of contact between the pairs.
Age and household size are strong determinants of mixing in social contact studies and are likely to impact transmission [29]. In our study, mothers and siblings were equally effective in transmitting pneumococci to infants, but siblings were more effective in transmitting to a female infant and mothers to a male infant. Toddlers are thought to be the engine of transmission because they have a high prevalence of carriage and poor hygiene, but we observed that carriers were equally effective transmitters from birth to 9 years of age. In Africa, this has important implications for the strategy of PCV introduction because the prevalence of carriage remains high through 9 years of age [30–32]. To vaccinate the majority of effective transmitters and establish herd protection would take nearly 10 years if immunization targeted only infants.
The risk factors for acquisition confirm that the household is an important vehicle for transmission and that children in the household, regardless of family membership, are the primary source. Coryza is a known risk for carriage prevalence [32], but it has not been possible to distinguish whether coryza enhances the risk of colonization or just the detection of colonization. Our results suggest that both interpretations apply. Coryza at the end of a risk period is associated with acquisition (higher detection), but so too is coryza at the beginning of a risk period. However, preexisting coryza only enhanced acquisition among infants who had 1–4 siblings, suggesting that the symptoms of coryza may facilitate pneumococcal attachment in exposed individuals–as has been suggested in animal models [33]. Smoky firewood, as compared to charcoal or paraffin, is associated with acquisition risk, as is cigarette smoke exposure; smoking is a known risk factor for pneumococcal carriage [34] and, like indoor cooking smoke [35], is amenable to intervention. Finally, the acquisition hazard illustrates a monthly seasonal pattern that mirrors the colonization prevalence among 3–59-month-old children in the same community very closely [32].
The study was unable to provide a comprehensive description of household transmission, particularly from fathers. In the KHDSS, adult males are present in the household relatively infrequently because they out-migrate for work [10]. Furthermore, fathers were much less likely than mothers to consent to provide a series of 3 swab specimens. Our study also ignores the role of the wider community in pneumococcal transmission, although elsewhere, community-based acquisition is trivial, compared with intrafamilial acquisition [18]. The study did seek multiple serotypes among colonized household members, but the methods used are less sensitive than more recently available techniques [36]. Populations of pneumococci that exist at much lower densities in the nasopharynx are likely to have a transmission probability that is proportionately lower, and this will minimize the impact of misclassification on the results.
This study has provided acquisition rates for 28 different pneumococcal serotypes in young infants; half of all acquisitions observed were of serotypes included in the 13-valent formulation of PCV. The acquisition rates are similar to those observed in older children in the same population [26], and the risk factors include cooking and cigarette smoke, season, contact with children in the household, and symptoms of upper respiratory tract infection. This is the first study to provide credible transmission probabilities for a wide range of serotypes. These parameters will be valuable to modelers attempting to describe and understand the indirect effects of conjugate vaccines. The study also sets the baseline for epidemiological observations of indirect vaccine effects following PCV introduction in Kenya.
Supplementary Data
Supplementary materials are available at Clinical Infectious Diseases online (http://cid.oxfordjournals.org/our_journals/cid). Supplementary materials consist of data provided by the author that are published to benefit the reader. The posted materials are not copyedited. The contents of all supplementary data are the sole responsibility of the authors. Questions or messages regarding errors should be addressed to the author.
Notes
Acknowledgements We thank the families of Kilifi District who took part in the study; the field staff, for their tremendous work; and Prof Marc Lipsitch, for helpful comments on a previous version of the manuscript. This article is published with the permission of the Director of the Kenya Medical Research Institute.
Financial support. This work was supported by a Wellcome Trust research fellowship (grant 081835 to A. S.). The KEMRI-Wellcome Trust Research Programme is supported by core funding from the Wellcome Trust (092654/Z/10/A).
Potential conflicts of interest. J. A. G. S. has received research grant funding from GlaxoSmithKline Biologicals. All other authors report no potential conflicts.
All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1.World Health Organization. Changing epidemiology of pneumococcal serotypes after introduction of conjugate vaccine: July 2010 report. Wkly Epidemiol Rec. 2010;85:434–6. [PubMed] [Google Scholar]
- 2.Lacapa R, Bliss SJ, Larzelere-Hinton F, et al. Changing epidemiology of invasive pneumococcal disease among White Mountain Apache persons in the era of the pneumococcal conjugate vaccine. Clin Infect Dis. 2008;47:476–84. doi: 10.1086/590001. [DOI] [PubMed] [Google Scholar]
- 3.Singleton RJ, Hennessy TW, Bulkow LR, et al. Invasive pneumococcal disease caused by nonvaccine serotypes among Alaska Native children with high levels of 7-valent pneumococcal conjugate vaccine coverage. JAMA. 2007;297:1784–92. doi: 10.1001/jama.297.16.1784. [DOI] [PubMed] [Google Scholar]
- 4.Direct and indirect effects of routine vaccination of children with 7-valent pneumococcal conjugate vaccine on incidence of invasive pneumococcal disease—United States, 1998–2003. MMWR Morb Mortal Wkly Rep. 2005;54:893–7. [PubMed] [Google Scholar]
- 5.Miller E, Andrews N, Waight P, Slack M, George R. Herd immunity and serotype replacement 4 years after seven-valent pneumococcal conjugate vaccination in England and Wales: an observational cohort study. Lancet Infect Dis. 2011;11:760–8. doi: 10.1016/S1473-3099(11)70090-1. [DOI] [PubMed] [Google Scholar]
- 6.Flasche S, Van Hoek AJ, Sheasby E, et al. Effect of pneumococcal conjugate vaccination on serotype-specific carriage and invasive disease in England: a cross-sectional study. PLoS Med. 2011;8:e1001017. doi: 10.1371/journal.pmed.1001017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Weinberger D, Malley R, Lipsitch M. Serotype replacement in disease after pneumococcal vaccination. Lancet. 2011;378:1962–73. doi: 10.1016/S0140-6736(10)62225-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Scott JA, Hall AJ, Dagan R, et al. Serogroup-specific epidemiology of Streptococcus pneumoniae: associations with age, sex, and geography in 7,000 episodes of invasive disease. Clin Infect Dis. 1996;22:973–81. doi: 10.1093/clinids/22.6.973. [DOI] [PubMed] [Google Scholar]
- 9.Halloran ME. Concepts of infectious disease epidemiology. In: Rothman KJ, Greenland S, editors. Modern epidemiology. Philadelphia: Lippincott, Williams & Wilkins; 1998. pp. 529–54. [Google Scholar]
- 10.Scott JA, Bauni E, Moisi J, et al. Profile: The Kilifi Health and Demographic Surveillance System (KHDSS) 2011 doi: 10.1093/ije/dys062. Int J Epidemiol In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.O'Brien KL, Nohynek H The WHO Pneumococcal Vaccine Trials Carriage Working Group. Report from a WHO working group: standard method for detecting upper respiratory carriage of Streptococcus pneumoniae. Pediatr Infect Dis J. 2003;22:133–40. doi: 10.1097/01.inf.0000048676.93549.d1. [DOI] [PubMed] [Google Scholar]
- 12.Dagan R, Givon-Lavi N, Zamir O, et al. Reduction of nasopharyngeal carriage of Streptococcus pneumoniae after administration of a 9-valent pneumococcal conjugate vaccine to toddlers attending day care centers. J Infect Dis. 2002;185:927–36. doi: 10.1086/339525. [DOI] [PubMed] [Google Scholar]
- 13.Mbelle N, Huebner RE, Wasas AD, Kimura A, Chang I, Klugman KP. Immunogenicity and impact on nasopharyngeal carriage of a nonavalent pneumococcal conjugate vaccine. J Infect Dis. 1999;180:1171–6. doi: 10.1086/315009. [DOI] [PubMed] [Google Scholar]
- 14.Obaro SK, Adegbola RA, Banya WA, Greenwood BM. Carriage of pneumococci after pneumococcal vaccination. Lancet. 1996;348:271–2. doi: 10.1016/s0140-6736(05)65585-7. [DOI] [PubMed] [Google Scholar]
- 15.Cheung Y-B, Zaman SMA, Nsekpong ED, et al. Nasopharyngeal carriage of Streptococcus pneumoniae in Gambian children who participated in a 9-valent pneumococcal conjugate vaccine trial and in their younger siblings. Pediatr Infect Dis J. 2009;28:990–5. doi: 10.1097/INF.0b013e3181a78185. [DOI] [PubMed] [Google Scholar]
- 16.O'Brien KL, Millar EV, Zell ER, et al. Effect of pneumococcal conjugate vaccine on nasopharyngeal colonization among immunized and unimmunized children in a community-randomized trial. J Infect Dis. 2007;196:1211–20. doi: 10.1086/521833. [DOI] [PubMed] [Google Scholar]
- 17.Lipsitch M. Vaccination against colonizing bacteria with multiple serotypes. Proc Natl Acad Sci U S A. 1997;94:6571–6. doi: 10.1073/pnas.94.12.6571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Auranen K, Arjay E, Leino T, Takala AK. Transmission of pneumococcal carriage in families: a latent Markov process model for binary longitudinal data. J Am Stat Assoc. 2000;95:1044–53. [Google Scholar]
- 19.Melegaro A, Choi Y, Pebody R, Gay N. Pneumococcal carriage in United Kingdom families: estimating serotype-specific transmission parameters from longitudinal data. Am J Epidemiol. 2007;166:228–35. doi: 10.1093/aje/kwm076. [DOI] [PubMed] [Google Scholar]
- 20.Gundel M, Schwarz FTK. Studien über die Bakterienflora der obern atmungswege Nergeborner (im Verleich mit der Mundhohlenflora der Mutter und des Pflegepersonals) unter besonderer Berucksichtigung ihrer Bedeuting fur das Pneumonieproblem [Studies of neonatal upper respiratory tract bacterial flora (in comparison with the oral flora of the mother and the nursing staff), especially with regard to its relevance to pneumonia] Z Hyg Infektionskr. 1932;113:411–36. [Google Scholar]
- 21.Landesman JB. A study of the pneumococcal carriage in infancy. Proc Roy Soc Med. 1953;46:61–3. [Google Scholar]
- 22.Gray BM, Converse GM, Dillon HCJ. Epidemiologic studies of Streptococcus pneumoniae in infants: acquisition, carriage, and infection during the first 24 months of life. J Infect Dis. 1980;142:923–33. doi: 10.1093/infdis/142.6.923. [DOI] [PubMed] [Google Scholar]
- 23.Gratten M, Gratten H, Poli A, Carrad E, Raymer M, Koki G. Colonisation of Haemophilus influenzae and Streptococcus pneumoniae in the upper respiratory tract of neonates in Papua New Guinea: primary acquisition, duration of carriage, and relationship to carriage in mothers. Biol Neonate. 1986;50:114–20. doi: 10.1159/000242576. [DOI] [PubMed] [Google Scholar]
- 24.Montgomery JM, Lehmann D, Smith T, et al. Bacterial colonization of the upper respiratory tract and its association with acute lower respiratory tract infections in Highland children of Papua New Guinea. Rev Infect Dis. 1990;12(Suppl 8):S1006–16. doi: 10.1093/clinids/12.supplement_8.s1006. [DOI] [PubMed] [Google Scholar]
- 25.Hill PC, Cheung YB, Akisanya A, et al. Nasopharyngeal carriage of Streptococcus pneumoniae in Gambian infants: a longitudinal study. Clin Infect Dis. 2008;46:807–14. doi: 10.1086/528688. [DOI] [PubMed] [Google Scholar]
- 26.Abdullahi O, Karani A, Mugo D, et al. The rates of acquisition and clearance of pneumococcal serotypes in the nasopharynges of children in Kilifi District, Kenya. 2011 doi: 10.1093/infdis/jis447. J Infect Dis In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lipsitch M, Abdullahi O, D'Amour A, et al. Rates of carriage acquisition and clearance and competitive ability for pneumococcal serotypes in Kilifi District, Kenya: application of a Markov transition model. Epidemiology. 2011. doi:10.1097/EDE.0b013e31824f2f32. [DOI] [PMC free article] [PubMed]
- 28.Gwaltney JMJ, Sande MA, Austrian R, Hendley JO. Spread of Streptococcus pneumoniae in families. II. Relation of transfer of S. pneumoniae to incidence of colds and serum antibody. J Infect Dis. 1975;132:62–8. doi: 10.1093/infdis/132.1.62. [DOI] [PubMed] [Google Scholar]
- 29.Mossong J, Hens N, Jit M, et al. Social contacts and mixing patterns relevant to the spread of infectious diseases. PLoS Med. 2008;5:e74. doi: 10.1371/journal.pmed.0050074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hill PC, Akisanya A, Sankareh K, et al. Nasopharyngeal carriage of Streptococcus pneumoniae in Gambian villagers. Clin Infect Dis. 2006;43:673–9. doi: 10.1086/506941. [DOI] [PubMed] [Google Scholar]
- 31.Abdullahi O, Nyiro J, Lewa P, Slack M, Scott JAG. The descriptive epidemiology of Streptococcus pneumoniae and Haemophilus influenzae nasopharyngeal carriage in children and adults in Kilifi district, Kenya. Pediatr Infect Dis J. 2008;27:59–64. doi: 10.1097/INF.0b013e31814da70c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Abdullahi O, Karani A, Mugo D, et al. The prevalence and risk factors for pneumococcal colonization of the nasopharynx among children in Kilifi District, Kenya. PLoS One. 2011;7:e30787. doi: 10.1371/journal.pone.0030787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.McCullers J, McAuley J, Browall S, Iverson A, Boyd K, Henriques Normark B. Influenza enhances susceptibility to natural acquisition of and disease due to Streptococcus pneumoniae in Ferrets. J Infect Dis. 2010;202:1287–95. doi: 10.1086/656333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lee C-C, Middaugh NA, Howie SRC, Ezzati M. Association of secondhand smoke exposure with pediatric invasive bacterial disease and bacterial carriage: a systematic review and meta-analysis. PLoS Med. 2010;7:e1000374. doi: 10.1371/journal.pmed.1000374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Smith KR, McCracken JP, Weber MW, et al. Effect of reduction in household air pollution on childhood pneumonia in Guatemala (RESPIRE): a randomised controlled trial. Lancet. 2011;378:1717–26. doi: 10.1016/S0140-6736(11)60921-5. [DOI] [PubMed] [Google Scholar]
- 36.Turner P, Hinds J, Turner C, et al. Improved detection of nasopharyngeal cocolonization by multiple pneumococcal serotypes by use of latex agglutination or molecular serotyping by microarray. J Clin Microbiol. 2011;49:1784–9. doi: 10.1128/JCM.00157-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.