Abstract
Background
In open-chest model of acute infarct, epicardial delivery of hepatocyte growth factor (HGF) gene improved LV function. This study was designed to test 1) the efficacy of HGF gene in infarct scar delivered under MR guidance and 2) the potential of multiple MR sequences in assessing the effects of pCK-HGF (treatment) and pCK-LacZ (control) genes on myocardial structure and function.
Methods and Materials
Swine (6 per group) were subjected to myocardial infarct, under X-ray fluoroscopy, developed LV remodelling at 5weeks. Multiple clinical MR imaging sequences were performed before delivery of gene (at 5 weeks after infarction) and 5 weeks after delivery of gene. Under MR-guidance, the active endovascular catheter was introduced into LV to transendocardially deliver 3.96×1011 viral copies of pCK-HGF or pCK-LacZ in the border and core of the infarct scar. Histological evaluation of the infarct scar was performed 5 weeks after delivery of gene.
Results
At 5weeks after infarction, there was no significant difference in measured cardiovascular MR parameters between the groups. PCK-HGF gene caused significant improvement in the following parameters (P<0.05 for these parameters): 3D strain (radial, circumferential, and longitudinal) , perfusion (maximum upslope, peak signal intensity, and time to peak) compared with control pCK-LacZ at 5 weeks after delivery of the genes. The ejection fraction was higher in pCK-HGF treated (43±1%) than pCK-LacZ control (37±1%, P<0.05). These changes are associated with a decrease in infarct scar size (11.3±2.0% in pCK-LacZ control and 6.7±1.3%, in pCK-HGF treated, P<0.01) and transmurality in 4 out of 5 infarct scar segments (P<0.05) on DE-MR imaging. Microscopic study confirmed the increase in capillary (P<0.05), and arteriole (P<0.05) density of infarct scar in pCK-HGF treated compared with pCK-LacZ control animals.
Conclusions
HGF gene delivered under MR-guidance into infarct scar ameliorated global function, 3D strain, increased regional perfusion, infarct resorption and enhanced angiogenesis/arteriogenesis. This feasibility study provides novel approach and analysis methods and instrumentation for delivering and evaluating new locally delivered therapies.
Keywords: Angiogenesis, gene therapy, myocardial infarction, magnetic resonance imaging, interventional cardiology
Congestive heart failure has become a widespread public health concern; there are approximately 5 million patients in the United States. 1. Following infarction, the left ventricle (LV) undergoes structural remodeling resulting in functional deterioration 2. Despite significant advances in pharmaceutical, surgical and interventional therapies, positive results in angiogenesis and cardiac repair are limited. Growing evidence from preclinical 3-5 and clinical 6-9 studies suggest that gene therapy has the potential to enhance angiogenesis and improve LV function. Assessment of LV function after gene therapy has hinged on invasive analysis and most of the focus was on the cellular and molecular changes 3, 4, 10-14. For example investigators established the fundamental mechanisms of action of hepatocyte growth factor (HGF) that include angiogenesis, arteriogenesis, myogenesis, reduction of collagen deposition and apoptosis 3, 4, 11-14.
Our group and others demonstrated in open-chest model the improved LV function and perfusion at 8 weeks after delivering pCK-HGF gene into the epicardium of acute infarct 5, 15, 16. More recently, a minimally invasive approach under MR–guidance has been introduced for delivering vascular endothelial growth factor gene in acute infarct 17. In routine clinic, a minimally invasive approach is critical for rapid recovery, reduction of morbidity and mortality and cost savings to the health care system. To our knowledge the efficacy of pCK-HGF and pCK-LacZ genes delivered transendocardially into the border and core of infarct scar, under MR-guidance, has not been demonstrated. Therefore, this study was designed to test 1) the efficacy of the effects of pCK-HGF and pCK-LacZ genes on infarct scar delivered under MR guidance and 2) the potential of multiple MR sequences in assessing the effects of these genes on myocardial structure and function.
MATERIALS AND METHODS
Therapeutic gene and delivery system
The phosphoenolpyruvate carboxykinase (pCK) vector, with hybrid hepatocyte growth factor (HGF) gene (VM202; ViroMed, Seoul, S-Korea) was used as a therapeutic agent and pCK-β-galactosidase (LacZ) gene as a control. The components of HGF gene and their functions have been previously described 5, 15.
Six steerable, deflectable guiding catheters with a retractable needle were used for transendocardial delivery of the genes (SurgiVision Inc. Baltimore, MD) 17. The endovascular catheter had a nitinol needle for puncturing the border and core of the infarct scar. The catheters were fitted with multiple MR radiofrequency receiver coils and were actively tracked to the target (infarct scar) under MR imaging (Figure 1).
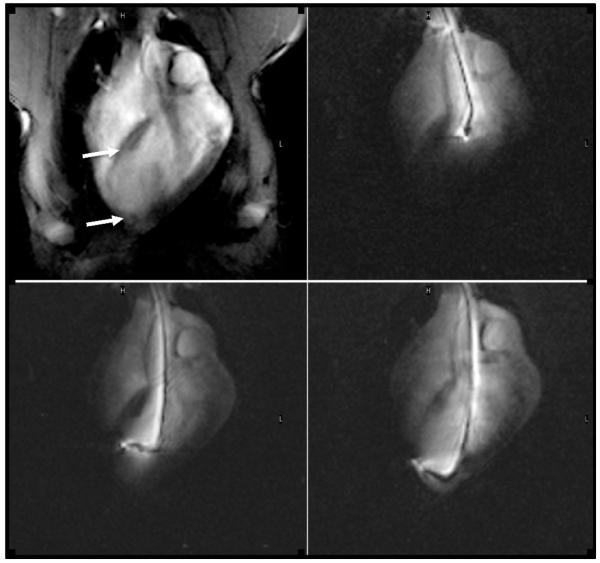
Figure 1.
MR fluoroscopic images show the navigation of the endovascular catheter in the ascending aorta (top left), LV chamber (top right) and the injections of a gene at two different infarct sites in this plan 5 weeks after infarction. The first image was acquired with external imaging coils showing the entire chest and the infarcted thin wall (arrows), while the latter three images were created with signal from the catheter showing the catheter shaft and tip.
Experimental protocol
The study conformed to the Guide for the Care and Use of Laboratory Animals (NIH Publication No 85-23, revised 1996) and approval was obtained from IACUC. Pigs (n=18, 30-32kg) were premedicated using acepromazine/ketamine and anesthetized using isoflurane/oxygen. A percutaneous balloon catheter (Boston Scientific, Natick, MA) was advanced distal to the 2nd diagonal branch under X-ray guidance and the artery was occluded for 90min followed by reperfusion 18. Coronary angiograms were repeatedly acquired to ensure complete occlusion/reperfusion.
A total of 18 animals were studied, where 2 animals died during the coronary occlusion/reperfusion and 4 died during the navigation of the endovascular catheter in LV at 5 weeks after infarction. The infarct was allowed to evolve for 5 weeks and the remaining animals were divided in a blinded fashion into 2 groups: pCK-HGF gene treated group (n=6) and pCK-LacZ control group (n=6). These 12 animals completed the study and follow-up. The specially designed endovascular catheter was used to deliver 3.96×1011 viral copies of the pCK-HGF (therapy, n=6) or pCK-LacZ (control, n=6). The number of injected viral copies in the border and core of infarct scar was 50% greater compared to those injected in acute infarct and the genes were delivered in over a larger infarct area by doubling the number of injection sites from 4 to 8 (0.3-0.4ml/site) at a rate of ~20s/site. 5, 15. All procedures were performed under sterile conditions. All animals received 3.3mg/kg lidocaine (IV, during occlusion/reperfusion and gene injection), 15 mg/kg trimethyl sulfa (PO, for 3 days after intervention).
Non-invasive clinical MR imaging sequences were performed at 5 weeks after infarction before delivery of gene and 5 weeks after delivery of the genes. The first MR imaging session was performed to establish a baseline. MR contrast media was delivered via the left ear vein for first pass perfusion (0.1mmol/kg Gd-DTPA, 3ml/s) using power injector. An additional dose of 0.05mmol/kg Gd-DTPA was delivered for delayed contrast enhanced MR imaging (DE-MR imaging).
Cardiovascular MR imaging
The hybrid XMR suite (Philips, Best, The Netherlands) featuring an X-ray catheterization lab and a 1.5T short-bore cardiovascular MR scanner with an in-room display monitor and console for viewing and interactive scanning enables LV catheterization under real-time imaging and for delivery of gene and its efficacy in infarct scar. Multiple MR sequences were simultaneously used in each session:
Cine MR imaging for measuring LV volumes, cardiac output, stroke volume, mass, wall thickness and % systolic wall thickening (radial strain);
Tagged MR imaging for measuring circumferential strain;
Phase-contrast velocity-encoded MR imaging for measuring longitudinal strain;
First-pass perfusion MR imaging for measuring regional myocardial perfusion;
DE-MR imaging for measuring infarct size and infarct transmurality.
MR-fluoroscopy for navigating the endovascular active catheter to the target.
Image analysis
Segment software was used by investigators blinded to treatment to determine LV volumes, ejection fraction, mass, perfusion, infarct size and transmurality and for measuring, longitudinal and systolic wall thickening (radial strain) 5, 17, 19. Regional wall thickness at end systole and end diastole of infarct scar (the target) and remote segments (LV free wall) were obtained, using DE-MR imaging as guidance. A 0° starting point at the posterio-septal groove was used in the above parameters for each individual slice in the set (clockwise direction). The software calculated the mean centerline of 8 LV segments (45° per segment). Percent systolic thickening at all time points were determined on three slices (8 segments per slice). A total of 576 segments was measured for segmental systolic wall thickening covering 5 weeks after infarction and 5 weeks after delivery of genes time points using Segment software, while HARP software was used blinded to treatment for measurement of peak systolic circumferential strain 20. For image registration of the circumferential strain the LV and the anterio-septal groove were used as the anatomic landmark 5, 19, 21. The radial, circumferential and longitudinal strain were assessed in the same apical slices containing the infarct, shown on DE-MR images. Automatic signal intensity threshold +2SD above remote myocardium was used to define the infarct and infarct transmurality 22.
Histopathology
After the last imaging session at 5 weeks after delivery of genes, the animals were euthanized and the freshly excised hearts were sliced and stained with triphenyltetrazolium chloride (TTC) to delineate the infarct scar. MR images were registered with TTC slices in both groups. Large tissue samples that include infarct scar (the target) and remote myocardium (free LV wall) were obtained. Following fixation and paraffin-embedding, 5μm thick tissue sections were stained with hematoxylin and eosin, Masson trichrome and biotinylated Bandeiria simplicifolia isolectin B4 (Vector Lab, Burlingame, Ca) 23. Standard immunoperoxidase method, biotinylated isolectin B4 localized vascular endothelial cells. Vascular density and myocyte diameter were determined in a blinded fashion using previously described methods 24, 25.
Statistical analysis
Differences in data obtained at at 5 weeks after infarction and 5 weeks after delivery of gene in both pCK-HGF gene treated and pCK-LacZ control groups were compared using one-way ANOVA for repeated measurements followed by post-hoc test when an overall significance was detected. The following parameters were compared using two-way ANOVA, followed by a Scheffe post-hoc analysis: 1) body weight, heart rate and arterial blood pressure, 2) LV function (ejection fraction, LV volumes, stroke volumes, cardiac output, 3D strain), 3) first pass perfusion parameters (maximum upslope, peak signal intensity and time to peak), 4) infarct transmurality and size and 5) capillary and arteriole densities as well as myocyte diameters. Data were expressed as mean±SEM. P<0.05 was considered statistically significant.
RESULTS
Cardiac interventions
Cardiac interventions were performed 1) under X-ray for coronary artery occlusion/reperfusion and 2) MR-guidance for transendocardial delivery of gene. Animals (n=16) showed myocardial infarct 5 weeks prior to delivery of gene and (n=12) 5 weeks after delivery of gene on DE-MR imaging. The pCK-HGF and pCK-LacZ animals showed no significant difference in body weight, heart rate, and mean arterial blood pressure at any time point (Table 1). The advancement of endovascular active catheter from the aorta into the LV chamber then to the target, is shown in figure 1. The brightness of endovascular catheter in the dark background LV chamber facilitates the navigation of the catheter to the target. The injection was performed after turning off the active coils to eliminate the heating produced by coil activation.
TABLE 1.
Body weight, heart rate and mean blood pressure measurements during the 10 weeks observation period.
| Groups | 5 weeks after infarction | 5 weeks after therapy | ||
|---|---|---|---|---|
|
| ||||
| Parameters | pCK-LacZ | pCK-HGF | pCK-LacZ | pCK-HGF |
| Body-weight (kg) | 43±2 | 41±1 | 54±1 | 52±1 |
| Heart rate (bpm) | 92±3 | 98±7 | 86±3 | 88±3 |
|
Mean blood pressure
(mmHg) |
69±5 | 67±4 | 70±4 | 69±3 |
LV mass and volumes
There was no significant difference in LV mass or wall structure (diastolic and systolic LV wall thickness) of remote myocardial segments at any time point between the groups. The segments with infarct scar were significantly thinner compared to remote myocardial segments in both groups at 5 weeks after infarction (Figure 2). Five weeks after delivery of pCK-HGF gene, there was a significant increase in wall thickness of segments with infarct scar at both diastole (from 5.7±0.5mm at 5 weeks after infarction to 6.6±0.3mm at 5 weeks after therapy, P<0.05) and systole (5.7±0.4mm to 8.1±0.5, P<0.05) compared with pCK-LacZ (from 5.7±0.4mm to 5.4±0.6mm at diastole and from 5.5±0.5mm to 5.4±0.5mm at systole).
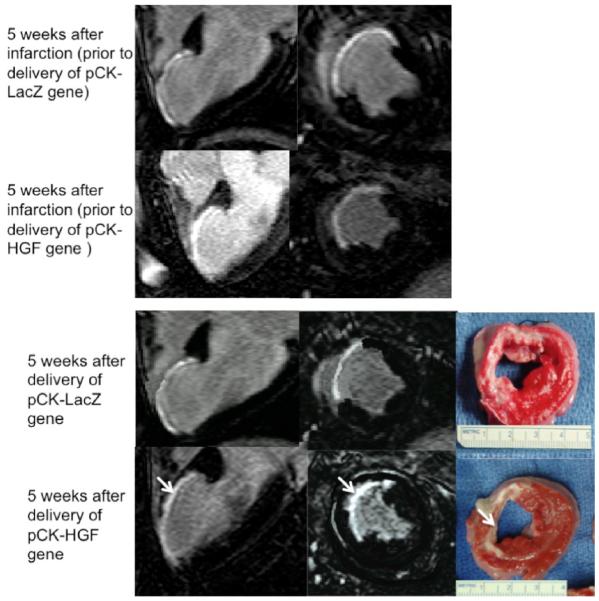
Figure 2.
Long and short axis views of DE-MR images illustrate the hyperenhanced infarct at 5 weeks after infarction of two representative animals (top block). The bottom block shows the effects of pCK-LacZ (top row) and pCK-HGF genes (bottom row) on infarct transmurality (arrow) and size 5 weeks after treatment. Histochemical TTC staining (right LV slices) confirms the smaller and non-transmural infarct (arrow) in the pCK-HGF treated animal compared with pCK-LacZ control animal.
Furthermore, end-diastolic, end-systolic, stroke volumes, cardiac output and ejection fraction were also not significantly different between the groups 5 weeks after infarction (Table 2). Animals treated with pCK-HGF gene also showed significant decrease in end systolic volume (from 1.27±0.05 at 5 weeks after infarction to 1.10±0.05ml/kg body weight at 5 weeks after delivery of gene, P<0.05) and an average increase of 5%, in ejection fraction (from 38±1 at 5 weeks after infarction to 43±1% at 5 weeks after delivery of gene, P<0.05). PCK-LacZ animals showed no significant change in end systolic volume (1.31±0.06 at 5 weeks after infarction to 1.28±0.05 ml/kg body weight at 5 weeks after delivery of gene, P=ns) or ejection fraction (38±2 at 5 weeks after infarction to 37±1% at 5 weeks after delivery of gene, P=ns), respectively. Furthermore, both end systolic volume and ejection fraction in pCK-HGF treated animals were significantly different from control pCK-LacZ at 5 weeks after gene delivery (Table 2).
TABLE 2.
Serial MR measurements of global LV function and mass before and after delivery of the genes. Note that the genes were derived 5 weeks after infarction.
| Groups | 5 weeks after infarction | 5 weeks after therapy | ||
|---|---|---|---|---|
|
| ||||
| Parameters | pCK-LacZ | pCK-HGF | pCK-LacZ | pCK-HGF |
| EDV (ml/kg) | 2.10±0.05 | 2.03±0.08 | 2.03±0.06 | 1.99±0.09 |
| ESV (ml/kg) | 1.31±0.06 | 1.27±0.05 | 1.28±0.05 | 1.10±0.05*+ |
| Stroke volume (ml) | 36±2 | 33±2 | 40±1 | 47±2*+ |
| Ejection fraction (%) | 38±2 | 38±1 | 37±1 | 43±1*+ |
| Cardiac output (L) | 3.3±0.2 | 3.2±0.3 | 3.4±0.2 | 4.2±0.3 |
| LV mass (g) | 96±3 | 102±5 | 117±4 | 123±4 |
| MR infarct scar (%LV) | 12.4±1.1 | 11.3±2.0 | 12.7±0.2 | 6.7±1.3**++ |
| TTC (%LV) | 12.1±0.7 | 7.2±0.9**++ | ||
P<0.05
P<0.01 compared to pCK-LacZ control animals at 5 weeks after treatment (unpaired t-test).
P<0.05
P<0.01 compared to 5 weeks prior treatment (paired t-test)
3D strain
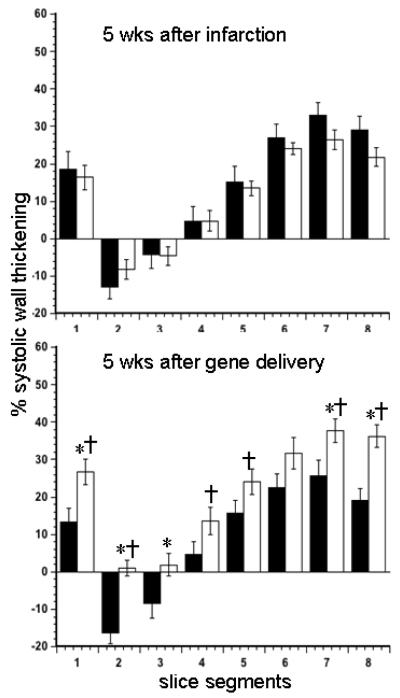
At 5 weeks after infarction, quantitative analysis of cine MR images revealed no significant difference between the groups in regional % systolic wall thickening (radial strain) (Figure 3). The segments with infarct scar were dyskinetic in pCK-LacZ control and akinetic in pCK-HGF animals at 5 weeks after delivery of the gene. However, there was significant improvement in systolic wall thickening of remote myocardial segments in pCK-HGF treated animals. In contrast, control animals showed a trend of further dysfunction in radial strain of remote myocardium at 5 weeks after pCK-LacZ delivery.
Figure 3.
Percent systolic wall thickening (radial strain) from 8 circumferential segments (average of 3 slices) of LV from pCK-LacZ control (black bars) and pCK-HGF treated (white bars) animals. The data were obtained at 5 weeks after infarction (top) and 5 weeks after delivery of gene (bottom). There was no significant difference between the groups at 5 weeks. However, the systolic wall thickening was markedly increased in treated compared with control animals. *P<0.05 measurements after pCK-HGF compared with pCK-LacZ at 5 weeks after delivery of gene and †P<0.05 5 weeks after delivery of gene compared with 5 week after infarction of the same animals.
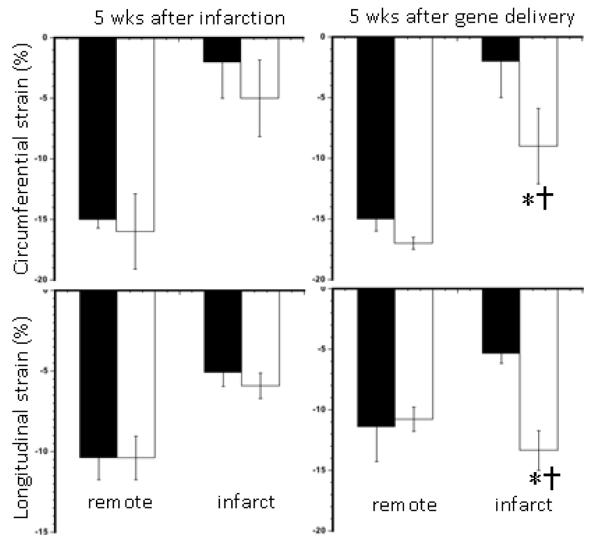
Data obtained from tagged and phase-contrast velocity-encoded MR images showed severe dysfunction in segments with infarct scar at 5 weeks after infarction in both groups (Figure 4). PCK-HGF, but not pCK-LacZ, treated animals showed significant improvement in both circumferential and longitudinal strain suggesting that these imaging sequences were sensitive enough to demonstrate the improvements at 5 weeks after delivery of gene. Figure 5 shows a pixel-by-pixel illustration of the difference in LV longitudinal strain between treated and control animals.
Figure 4.
Percent peak circumferential (top) and longitudinal (bottom) strain measurements obtained from remote myocardium and chronic infarct scar containing the infarct shown on DE-MR images for guidance. The circumferential and longitudinal strain were markedly increased in animals treated with pCK-HGF gene compared with pCK-LacZ gene. The data were obtained at 5 weeks after infarction (left blocks) and 5 weeks after delivery of gene (right blocks). Black bars represent animals injected with pCK-LacZ gene and white bars represent animals treated with pCK-HGF gene. *P<0.05-0.01 measurements compared with pCK-LacZ control animal data and †P<0.05 weeks prior to therapy.
Figure 5.
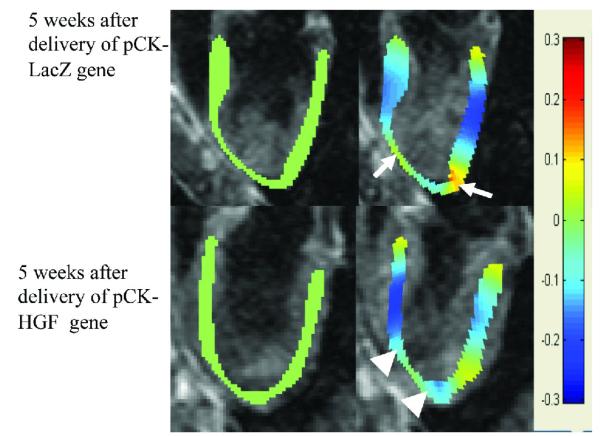
Longitudinal strain in pixel-by-pixel obtained from phase contrast velocity encoded MR images in animals treated with pCK-LacZ and pCK-HGF gene. Longitudinal strain at diastole is shown in green (left images) and at systole in blue. Top block demonstrates longitudinal strain at diastole (left images) and systole (right images) at 5 weeks after infarction. At 5 weeks after infarction, a marked decrease in longitudinal strain was noted in the apex of both animals (arrow) compared with remote myocardium. An improvement in strain was noticed at 5 weeks after delivery of pCK-HGF (arrowhead, right image in the bottom block), but not pCK-LacZ (arrow) gene.
Myocardial perfusion
At 5 weeks after infarction, first pass perfusion MR imaging showed the infarcted segments as hypoenhanced, due to the delayed arrival of the contrast medium, compared to remote myocardium. The perfusion parameters measured in the infarcted and remote segments were not significantly different between the groups (Table 3). At 5 weeks after delivery of HGF gene, however, maximum upslope, P<0.05, peak signal intensity, P<0.05 and time to peak, P<0.05 were improved compared to 5 weeks after infarction in the same animals. Furthermore, treated animals at 5 weeks after delivery of pCK-HGF gene showed better perfusion compared with pCK-LacZ control animals (Table 3).
TABLE 3.
Regional perfusion parameters obtained from first pass MR imaging.
| Groups | 5 weeks after infarction | 5 weeks after therapy | ||
|---|---|---|---|---|
|
| ||||
| Parameters | pCK-LacZ | pCK-HGF | pCK-LacZ | pCK-HGF |
| LV Blood | ||||
| Max upslope (s-1) | 3075±408 | 2959±682 | 2507±241 | 3233±365 |
| Peak signal intensity (au) | 6295±361 | 6021±503 | 6342±269 | 6081±282 |
| Time to the peak (s) | 5.2±0.3 | 4.5±0.1 | 4.4±0.3 | 5.2±1.3 |
| Remote myocardium | ||||
| Max upslope (s-1) | 376±42 | 318±34 | 260±43 | 290±46 |
| Peak signal intensity (au) | 1533±81 | 1291±129 | 1688±150 | 1375±142 |
| Time to the peak (s) | 12.3±1.0 | 13.5±0.8 | 7.6±0.4 | 7.9±0.9 |
| Infarct scar | ||||
| Max upslope (s-1) | 134±24 | 127±21 | 130±29 | 367±58*+ |
| Peak signal intensity (au) | 1058±58 | 1067±33 | 1249±141 | 1482±126*+ |
| Time to the peak (s) | 15.0±1.0 | 15.4±1.1 | 14.1±0.5 | 10.6±1.1*+ |
P<0.05 compared to pCK-LacZ control animals at 5 weeks after delivery of the genes and 5 weeks after infarction data point, respectively.
Infarct size and infarct transmurality
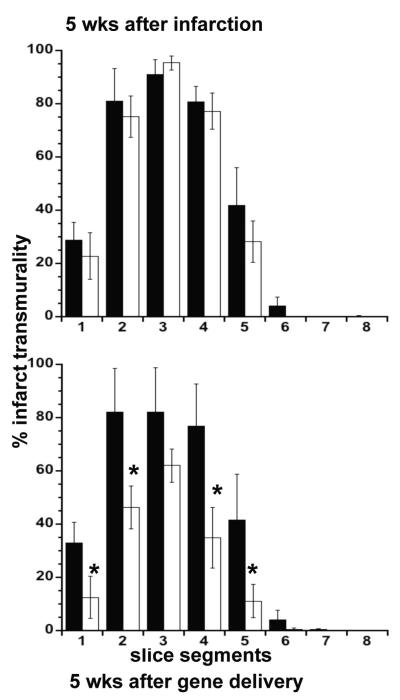
DE-MR imaging highlighted infarct scar (the target) in short and long axis views allowing precise delivery of the genes (Figure 2). At 5 weeks after infarction the infarct size was not significantly different between the groups (Table 2). PCK-HGF treated animals showed significantly smaller infarct size (6.7±1.3%) compared to pCK-LacZ control (11.3±2.0%) at 5 weeks after delivery (P<0.01). These changes are associated with a significant decrease in infarct transmurality in 4 of 5 segments with infarct scar (P<0.05) on DE-MR imaging (Figure 6). TTC infarct size at autopsy was not significantly different from MR infarct size (Table 2). MR images and TTC histochemical staining shows the non-transmural infarct at 5 weeks in pCK-HGF, but not in pCK-LacZ, group (Figure 2). There was no significant change in infarct transmurality in all segments with infarct scar in pCK-LacZ control animals.
Figure 6.
Percent infarct transmurality before (upper block) and after (lower block) delivery of pCK-LacZ (black bars) and pCK-HGF (white bars). Prior to therapy, there was no significant difference in infarct transmurality between the groups. pCK-HGF significantly reduced infarct transmurality, but not pCK-LacZ. Infarct transmurality did not change over the course of 5 week after delivery of pCK-LacZ. * P<0.05 compared with pCK-LacZ group and to 5 weeks prior to delivery of genes.
Histopathology
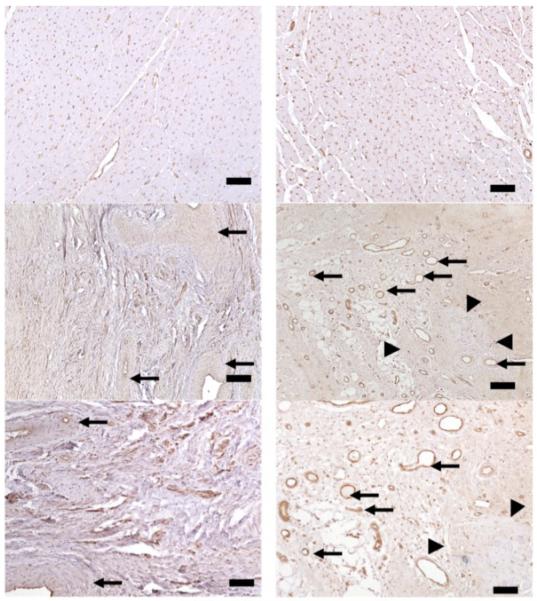
Masson trichrome stain showed homogeneous chronic infarct scar at 5 weeks after pCK-LacZ delivery. However, pCK-HGF treated animals showed different infarct architecture compared to controls. For example, Masson trichrome stain showed few viable islands/peninsulas at the border of segments with infarct scar, while isolectin B5 showed homogeneous increase in vascularity of infarct scar in pCK-HGF treated animals but disarray of vessels in control animals (Figure 7). The number of vessels in border and core of chronic infarct was greater in pCK-HGF treated (206±10 capillaries/mm2) compared with pCK-LacZ control animals (78±25 capillaries/mm2, P<0.05). A significant difference was also observed in the number of arterioles within the infarct scar in pCK-HGF (11.7±1.9 arterioles/mm2) and pCK-LacZ control (3.4±0.5 arterioles/mm2, P<0.05) animals. On the other hand, there was no significant difference in capillary and arterial densities in remote myocardium between treated (269±30 capillaries/mm2, 10.9±2.1 arterioles/mm2, respectively) and control animals (267±54 capillaries/mm2 and 10.7±1.1 arterioles/mm2, respectively). Myocyte diameters of remote myocardium in pCK-HGF (16.4±0.8μm) and pCK-LacZ control animals (18.3±0.2μm) were not significantly different, respectively. There was no evidence of inflammation in the segments with infarct scar in pCK-LacZ control and pCK-HGF treated animals.
Figure 7.
Histopathological heart sections stained with isolectin B5 obtained from two animals 5 weeks after delivery of pCK-LacZ (left) and pCK-HGF (right). Remote myocardium obtained from both animals (top row) showed no difference in vascular density or myocyte diameters. The pCK-LacZ control animal showed few old thick-walled blood vessels (left-center, arrows) embedded in the dense scar tissue (left-bottom), whereas pCK-HGF treated animals showed numerous brown thin-walled new blood vessels (right-center and bottom, arrows) surrounding the few islands/peninsulas of viable myocyte at the border zone (arrowheads). The data supports the notion that pCK-HGF has angiogenic and may be myogenic properties. Calibration bars=200 microns (top panels), 50 microns (center panels), 25 microns (bottom panels).
DISCUSSION
The major findings of this study were that pCK-HGF, but not pCK-LacZ, gene showed beneficial effects on myocardial structure, perfusion and function when delivered under MR-guidance into the border and core of infarct scar. The used multi MR sequences have the potential in discriminating the effects of pCK-HGF gene (VM202) from plasmid-LacZ control. Cine, tagged and phase-contrast velocity-encoded, and first pass perfusion MR imaging showed the improvement in radial, circumferential and longitudinal strain and perfusion parameters (max upslope, peak signal intensity and time to the peak) after delivery of plasmid pCK-HGF gene compared with plasmid-LacZ control. Furthermore, pCK-HGF gene reduced infarct transmurality and size as demonstrated in vivo on MR imaging and postmortem on TTC stain. At the microscopic level, pCK-HGF gene enhanced angiogenesis and arteriogenesis in the border and infarct core, thereby provided indirect evidence on the gene expression. The effects of pCK-HGF were demonstrated in this study at 5 weeks compared to 8 weeks in previous studies 5, 15, which may have clinical value in end-stage patients.
MR-guidance
The success of the pCK-HGF delivery of the genes in the border zone and core of the infarction under MR-guidance was evident from: 1) the improvement in global LV function, 2) increase in perfusion of the infarcted segments, 3) reduction in infarct transmurality, 4) homogeneous increase in vascular density in the border zone and core of the infarction and 5) presence of few viable islands/peninsulas in the border zone.
The signal derived from the catheter could either be activated or suppressed, depending on visualization needs. Positioning the catheter in the LV was made possible by rotating and deflecting the distal tip. The active coil at the tip of the needle, as well as short- and long-axis views of the LV, were valuable in guiding the catheter to the target. This study is the first to use endovascular MR-guided catheters for transendocardial delivery of the pCK-HGF gene and pCK-LacZ in multi-site targets. Prior to the clinical use of endovascular active catheter the following refinements are needed: 1) determining the safety issues, 2) reduction in catheter diameter to 6F, 3) increased flexibility of the catheter shaft and tip, and 4) addition of guide-wire to the catheter to avoid vascular or ventricular perforation.
Gene therapy
Unlike previous invasive pathophysiological studies 3, 4, 10-14, the current study used noninvasive MR imaging in validating global and regional (3D strain) LV function perfusion and viability. The novel aspects of this study are the use of: 1) HGF gene in arresting fibrosis model (chronic infarct scar) with established remodeled LV, 2) percutaneous MR-guided delivery approach and multiple injections and 3) serial MR evaluation of 3D strain analysis, perfusion and viability of chronic infarct scar. The scope of this MR study was not to define the mechanism(s) of action of HGF gene due to the current limitation of MR imaging. Furthermore, multiple laboratories established antifibrotic, antiapoptotic and cardiotrophic properties of HGF 3, 4, 10-14. Others found that HGF also increases the recruitment of bone marrow-derived endothelial progenitor cells and/or enhances the effect of these cells in myocardial infarct 12, 26. It is likely that preservation of myocardial function, perfusion and viability seen in the current study is derived from the multi-mechanism mentioned above.
3D strain
Echocardiographic studies in swine models (ischemic cardiomyopathy and LV dysfunction caused by doxorubicin) illustrated significant improvement in LV function after delivering HGF genes 3, 10. The used multiple MR sequences have the potential to comprehensively assess the changes in load-dependent parameters (such as ejection fraction, stroke volume and cardiac output) and regional 3D strain. PCK-HGF treatment reversed LV remodeling by increasing wall strain and infarct thickness. Tagged and phase-contrast velocity-encoded MR images were sensitive enough to demonstrate the improvements in circumferential and longitudinal strain after pCK-HGF, but not pCK-LacZ, injection. Figure 5 illustrates the clear difference in longitudinal strain at the pixel-by-pixel level between the groups. A previous study showed that the change in regional strain is linearly related to ejection fraction 27. The improvement in LV function cannot be attributed to differences in heart rate, blood pressure or LV mass, as these parameters were not significantly different between the groups (Table 1).
Myocardial perfusion
First pass parameters (maximum upslope, peak signal intensity and time to peak) were used to assess whether pCK-HGF gene increased regional perfusion. We found that pCK-HGF gene significantly improved perfusion in chronic infarct scar 5 weeks after delivery. The increase in perfusion is related to the increases in capillary and arteriole densities seen on histology and confirms the functionality of the new vessels. This study is consistent with a recent study that showed angiogenesis induced by VEGF or stem cell-based VEGF prevents demand-induced ischemia and improves LV ejection fraction 28.
Chronic infarct scar
MR imaging showed the decrease in transmural infarct extent and the increase in wall thickness in pCK-HGF gene treated animals compared with control. Hayakawa et al found that inhibition of apoptosis by HGF alters infarct tissue dynamics, making infarct scar thicker and rich in preserved cellular components 11. The increase in wall thickness in chronic infarct scar is important because wall stress is directly proportional to the diameter of LV chamber and is inversely related to wall thickening of remote myocardium 29. These changes could also be related to the inhibition of collagen I, III and TGF-beta expression 13.
This non-invasive MR study is the first to demonstrate spared epicardium on DE-MR imaging after delivery of pCK-HGF gene, a finding confirmed on postmortem. The presence of few islands/peninsula in the border zone in infarct has been previously documented after administration of HGF protein 4, 12, 30 and VEGF gene in mice and swine 25, 31.
Study limitations
This study comprised of a small number of animals because our institutional animal care and use committee imposed a restriction on all investigators, requiring the use of a minimum number of animals necessary to achieve significance. Thus we did not perform a power calculation; instead we based the number of animals in our study on the number of animals in previous studies 17, 32, 33. The number of animals in our study, however, was greater than the number of animals in recently published reports 32, 33.
We had 25% procedural failure due to arrhythmia, during the navigation of the catheter in the LV chamber. Other investigators also found in swine that electro-mapping and intramyocardial delivery of plasmid HGF using the NOGA is associated with transient ventricular ectopic activity 3. Other limitations include lack of: 1) measurement of HGF gene expression in myocardium or plasma because it has been documented in clinical 34 and experimental studies 3, 4, therefore, this study provides indirect evidence of gene expression 2) knowledge as to the origin of few islands/peninsulas at the border of chronic infarct scar, possibly by HGF mobilization of progenitor cells 14. The presence of islands/peninsulas at the border of infarct scar warrant additional electrophysiologic studies. Previous study indicated that such islands/peninsulas may create ventricular arrhythmia 35. In addition, the safety of using endovascular active catheters must also be addressed prior to clinical use.
Clinical implications
The used of noninvasive multiple imaging sequences and image analysis may provide new insights for future studies designed to characterize the arrest of ongoing fibrosis and cardiotrophic activity in remodelled LV. The combination of this minimally invasive MR-guided procedure and gene therapy may be useful in end stage patients. MR-guidance of local gene therapy enables a substantially reduced level of invasiveness compared with open-chest surgery, potentially resulting in treatment on an outpatient basis, rapid patient recovery, and cost savings to the health care system.
Conclusion
MR-guided transendocardial delivery of HGF gene in infarct scar ameliorated global function and 3D strain, increased perfusion, and reduced infarct size. At the microscopic level, pCK-HGF gene enhanced angiogenesis and arteriogenesis. This study provides novel imaging and analysis methods and instrumentation for delivering and evaluating locally delivered therapies.
Acknowledgments
Supported by a grant from the National Institutes of Health (R01HL72956) and a gift from ViroMed Company, Ltd, Seoul, S. Korea.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
No financial relationship between any of the authors and the subject matter.
References
- 1.Emond M, Mock MB, Davis KB, Fisher LD, Holmes DR, Jr., Chaitman BR, Kaiser GC, Alderman E, Killip T., 3rd Long-term survival of medically treated patients in the Coronary Artery Surgery Study (CASS) Registry. Circulation. 1994;90(6):2645–2657. doi: 10.1161/01.cir.90.6.2645. [DOI] [PubMed] [Google Scholar]
- 2.Kramer CM, Lima JA, Reichek N, Ferrari VA, Llaneras MR, Palmon LC, Yeh IT, Tallant B, Axel L. Regional differences in function within noninfarcted myocardium during left ventricular remodeling. Circulation. 1993;88(3):1279–1288. doi: 10.1161/01.cir.88.3.1279. [DOI] [PubMed] [Google Scholar]
- 3.Azuma J, Taniyama Y, Takeya Y, Iekushi K, Aoki M, Dosaka N, Matsumoto K, Nakamura T, Ogihara T, Morishita R. Angiogenic and antifibrotic actions of hepatocyte growth factor improve cardiac dysfunction in porcine ischemic cardiomyopathy. Gene Ther. 2006;13(16):1206–1213. doi: 10.1038/sj.gt.3302740. [DOI] [PubMed] [Google Scholar]
- 4.Chen XH, Minatoguchi S, Kosai K, Yuge K, Takahashi T, Arai M, Wang N, Misao Y, Lu C, Onogi H, Kobayashi H, Yasuda S, Ezaki M, Ushikoshi H, Takemura G, Fujiwara T, Fujiwara H. In vivo hepatocyte growth factor gene transfer reduces myocardial ischemia-reperfusion injury through its multiple actions. J Card Fail. 2007;13(10):874–883. doi: 10.1016/j.cardfail.2007.07.004. [DOI] [PubMed] [Google Scholar]
- 5.Saeed M, Martin A, Ursell P, Do L, Bucknor M, Higgins CB, Saloner D. MR assessment of myocardial perfusion, viability, and function after intramyocardial transfer of VM202, a new plasmid human hepatocyte growth factor in ischemic swine myocardium. Radiology. 2008;249(1):107–118. doi: 10.1148/radiol.2483071579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Fuchs S, Dib N, Cohen BM, Okubagzi P, Diethrich EB, Campbell A, Macko J, Kessler PD, Rasmussen HS, Epstein SE, Kornowski R. A randomized, double-blind, placebo-controlled, multicenter, pilot study of the safety and feasibility of catheter-based intramyocardial injection of AdVEGF121 in patients with refractory advanced coronary artery disease. Catheter Cardiovasc Interv. 2006;68(3):372–378. doi: 10.1002/ccd.20859. [DOI] [PubMed] [Google Scholar]
- 7.Losordo DW, Vale PR, Symes JF, Dunnington CH, Esakof DD, Maysky M, Ashare AB, Lathi K, Isner JM. Gene therapy for myocardial angiogenesis: initial clinical results with direct myocardial injection of phVEGF165 as sole therapy for myocardial ischemia. Circulation. 1998;98(25):2800–2804. doi: 10.1161/01.cir.98.25.2800. [DOI] [PubMed] [Google Scholar]
- 8.Rosengart TK, Lee LY, Patel SR, Sanborn TA, Parikh M, Bergman GW, Hachamovitch R, Szulc M, Kligfield PD, Okin PM, Hahn RT, Devereux RB, Post MR, Hackett NR, Foster T, Grasso TM, Lesser ML, Isom OW, Crystal RG. Angiogenesis gene therapy: phase I assessment of direct intramyocardial administration of an adenovirus vector expressing VEGF121 cDNA to individuals with clinically significant severe coronary artery disease. Circulation. 1999;100(5):468–474. doi: 10.1161/01.cir.100.5.468. [DOI] [PubMed] [Google Scholar]
- 9.Schumacher B, Pecher P, von Specht BU, Stegmann T. Induction of neoangiogenesis in ischemic myocardium by human growth factors: first clinical results of a new treatment of coronary heart disease. Circulation. 1998;97(7):645–650. doi: 10.1161/01.cir.97.7.645. [DOI] [PubMed] [Google Scholar]
- 10.Esaki M, Takemura G, Kosai K, Takahashi T, Miyata S, Li L, Goto K, Maruyama R, Okada H, Kanamori H, Ogino A, Ushikoshi H, Minatoguchi S, Fujiwara T, Fujiwara H. Treatment with an adenoviral vector encoding hepatocyte growth factor mitigates established cardiac dysfunction in doxorubicin-induced cardiomyopathy. Am J Physiol Heart Circ Physiol. 2008;294(2):H1048–1057. doi: 10.1152/ajpheart.01102.2007. [DOI] [PubMed] [Google Scholar]
- 11.Hayakawa K, Takemura G, Kanoh M, Li Y, Koda M, Kawase Y, Maruyama R, Okada H, Minatoguchi S, Fujiwara T, Fujiwara H. Inhibition of granulation tissue cell apoptosis during the subacute stage of myocardial infarction improves cardiac remodeling and dysfunction at the chronic stage. Circulation. 2003;108(1):104–109. doi: 10.1161/01.CIR.0000074225.62168.68. [DOI] [PubMed] [Google Scholar]
- 12.Miyagawa S, Sawa Y, Taketani S, Kawaguchi N, Nakamura T, Matsuura N, Matsuda H. Myocardial regeneration therapy for heart failure: hepatocyte growth factor enhances the effect of cellular cardiomyoplasty. Circulation. 2002;105(21):2556–2561. doi: 10.1161/01.cir.0000016722.37138.f2. [DOI] [PubMed] [Google Scholar]
- 13.Taniyama Y, Morishita R, Aoki M, Hiraoka K, Yamasaki K, Hashiya N, Matsumoto K, Nakamura T, Kaneda Y, Ogihara T. Angiogenesis and antifibrotic action by hepatocyte growth factor in cardiomyopathy. Hypertension. 2002;40(1):47–53. doi: 10.1161/01.hyp.0000020755.56955.bf. [DOI] [PubMed] [Google Scholar]
- 14.Yang ZJ, Xu SL, Chen B, Zhang SL, Zhang YL, Wei W, Ma DC, Wang LS, Zhu TB, Li CJ, Wang H, Cao KJ, Gao W, Huang J, Ma WZ, Wu ZZ. Hepatocyte growth factor plays a critical role in the regulation of cytokine production and induction of endothelial progenitor cell mobilization: a pilot gene therapy study in patients with coronary heart disease. Clin Exp Pharmacol Physiol. 2009;36(8):790–796. doi: 10.1111/j.1440-1681.2009.05151.x. [DOI] [PubMed] [Google Scholar]
- 15.Carlsson M, Osman NF, Ursell PC, Martin AJ, Saeed M. Quantitative MR measurements of regional and global left ventricular function and strain after intramyocardial transfer of VM202 into infarcted swine myocardium. Am J Physiol Heart Circ Physiol. 2008;295(2):H522–532. doi: 10.1152/ajpheart.00280.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cho KR, Choi JS, Hahn W, Kim DS, Park JS, Lee DS, Kim KB. Therapeutic angiogenesis using naked DNA expressing two isoforms of the hepatocyte growth factor in a porcine acute myocardial infarction model. Eur J Cardiothorac Surg. 2008;34(4):857–863. doi: 10.1016/j.ejcts.2008.05.045. [DOI] [PubMed] [Google Scholar]
- 17.Saeed M, Martin A, Jacquier A, Bucknor M, Saloner D, Do L, Ursell P, Su H, Kan YW, Higgins CB. Permanent coronary artery occlusion: cardiovascular MR imaging is platform for percutaneous transendocardial delivery and assessment of gene therapy in canine model. Radiology. 2008;249(2):560–571. doi: 10.1148/radiol.2491072068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Furtado AD, Carlsson M, Wintermark M, Ordovas K, Saeed M. Identification of residual ischemia, infarction, and microvascular impairment in revascularized myocardial infarction using 64-slice MDCT. Contrast Media Mol Imaging. 2008;3(5):198–206. doi: 10.1002/cmmi.253. [DOI] [PubMed] [Google Scholar]
- 19.Dicks DL, Carlsson M, Heiberg E, Martin A, Saloner D, Arheden H, Saeed M. Persistent decline in longitudinal and radial strain after coronary microembolization detected on velocity encoded phase contrast magnetic resonance imaging. J Magn Reson Imaging. 2009;30(1):69–76. doi: 10.1002/jmri.21773. [DOI] [PubMed] [Google Scholar]
- 20.Neizel M, Lossnitzer D, Korosoglou G, Schaufele T, Peykarjou H, Steen H, Ocklenburg C, Giannitsis E, Katus HA, Osman NF. Strain-encoded MRI for evaluation of left ventricular function and transmurality in acute myocardial infarction. Circ Cardiovasc Imaging. 2009;2(2):116–122. doi: 10.1161/CIRCIMAGING.108.789032. [DOI] [PubMed] [Google Scholar]
- 21.Fuchs S, Satler LF, Kornowski R, Okubagzi P, Weisz G, Baffour R, Waksman R, Weissman NJ, Cerqueira M, Leon MB, Epstein SE. Catheter-based autologous bone marrow myocardial injection in no-option patients with advanced coronary artery disease: a feasibility study. J Am Coll Cardiol. 2003;41(10):1721–1724. doi: 10.1016/s0735-1097(03)00328-0. [DOI] [PubMed] [Google Scholar]
- 22.Lund GK, Stork A, Saeed M, Bansmann MP, Gerken JH, Muller V, Mester J, Higgins CB, Adam G, Meinertz T. Acute myocardial infarction: evaluation with first-pass enhancement and delayed enhancement MR imaging compared with 201Tl SPECT imaging. Radiology. 2004;232(1):49–57. doi: 10.1148/radiol.2321031127. [DOI] [PubMed] [Google Scholar]
- 23.Theilmeier G, Verhamme P, Dymarkowski S, Beck H, Bernar H, Lox M, Janssens S, Herregods MC, Verbeken E, Collen D, Plate K, Flameng W, Holvoet P. Hypercholesterolemia in minipigs impairs left ventricular response to stress: association with decreased coronary flow reserve and reduced capillary density. Circulation. 2002;106(9):1140–1146. doi: 10.1161/01.cir.0000026805.41747.54. [DOI] [PubMed] [Google Scholar]
- 24.Jacquier A, Higgins CB, Martin AJ, Do L, Saloner D, Saeed M. Injection of adeno-associated viral vector encoding vascular endothelial growth factor gene in infarcted swine myocardium: MR measurements of left ventricular function and strain. Radiology. 2007;245(1):196–205. doi: 10.1148/radiol.2451061077. [DOI] [PubMed] [Google Scholar]
- 25.Laguens R, Meckert P Cabeza, Janavel G Vera, De Lorenzi A, Lascano E, Negroni J, Del Valle H, Cuniberti L, Martinez V, Dulbecco E, Melo C, Fernandez N, Criscuolo M, Crottogini A. Cardiomyocyte hyperplasia after plasmid-mediated vascular endothelial growth factor gene transfer in pigs with chronic myocardial ischemia. J Gene Med. 2004;6(2):222–227. doi: 10.1002/jgm.478. [DOI] [PubMed] [Google Scholar]
- 26.Duan HF, Wu CT, Wu DL, Lu Y, Liu HJ, Ha XQ, Zhang QW, Wang H, Jia XX, Wang LS. Treatment of myocardial ischemia with bone marrow-derived mesenchymal stem cells overexpressing hepatocyte growth factor. Mol Ther. 2003;8(3):467–474. doi: 10.1016/s1525-0016(03)00186-2. [DOI] [PubMed] [Google Scholar]
- 27.Weidemann F, Jamal F, Kowalski M, Kukulski T, D’Hooge J, Bijnens B, Hatle L, De Scheerder I, Sutherland GR. Can strain rate and strain quantify changes in regional systolic function during dobutamine infusion, B-blockade, and atrial pacing--implications for quantitative stress echocardiography. J Am Soc Echocardiogr. 2002;15(5):416–424. doi: 10.1067/mje.2002.116535. [DOI] [PubMed] [Google Scholar]
- 28.Wang X, Hu Q, Mansoor A, Lee J, Wang Z, Lee T, From AH, Zhang J. Bioenergetic and functional consequences of stem cell-based VEGF delivery in pressure-overloaded swine hearts. Am J Physiol Heart Circ Physiol. 2006;290(4):H1393–1405. doi: 10.1152/ajpheart.00871.2005. [DOI] [PubMed] [Google Scholar]
- 29.Yin FC. Ventricular wall stress. Circ Res. 1981;49(4):829–842. doi: 10.1161/01.res.49.4.829. [DOI] [PubMed] [Google Scholar]
- 30.Wang Y, Ahmad N, Wani MA, Ashraf M. Hepatocyte growth factor prevents ventricular remodeling and dysfunction in mice via Akt pathway and angiogenesis. J Mol Cell Cardiol. 2004;37(5):1041–1052. doi: 10.1016/j.yjmcc.2004.09.004. [DOI] [PubMed] [Google Scholar]
- 31.Guerrero M, Athota K, Moy J, Mehta LS, Laguens R, Crottogini A, Borrelli M, Corry P, Schoenherr D, Gentry R, Boura J, Grines CL, Raff GL, Shanley CJ, O’Neill WW. Vascular endothelial growth factor-165 gene therapy promotes cardiomyogenesis in reperfused myocardial infarction. J Interv Cardiol. 2008;21(3):242–251. doi: 10.1111/j.1540-8183.2008.00358.x. [DOI] [PubMed] [Google Scholar]
- 32.Ferrarini M, Arsic N, Recchia FA, Zentilin L, Zacchigna S, Xu X, Linke A, Giacca M, Hintze TH. Adeno-associated virus-mediated transduction of VEGF165 improves cardiac tissue viability and functional recovery after permanent coronary occlusion in conscious dogs. Circ Res. 2006;98(7):954–961. doi: 10.1161/01.RES.0000217342.83731.89. [DOI] [PubMed] [Google Scholar]
- 33.Kraitchman DL, Heldman AW, Atalar E, Amado LC, Martin BJ, Pittenger MF, Hare JM, Bulte JW. In vivo magnetic resonance imaging of mesenchymal stem cells in myocardial infarction. Circulation. 2003;107(18):2290–2293. doi: 10.1161/01.CIR.0000070931.62772.4E. [DOI] [PubMed] [Google Scholar]
- 34.Nakamura T, Mizuno S, Matsumoto K, Sawa Y, Matsuda H. Myocardial protection from ischemia/reperfusion injury by endogenous and exogenous HGF. J Clin Invest. 2000;106(12):1511–1519. doi: 10.1172/JCI10226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Yan AT, Shayne AJ, Brown KA, Gupta SN, Chan CW, Luu TM, Di Carli MF, Reynolds HG, Stevenson WG, Kwong RY. Characterization of the peri-infarct zone by contrast-enhanced cardiac magnetic resonance imaging is a powerful predictor of post-myocardial infarction mortality. Circulation. 2006;114(1):32–39. doi: 10.1161/CIRCULATIONAHA.106.613414. [DOI] [PubMed] [Google Scholar]