Abstract
Objective To chart mothers’ trajectories of mental health from pregnancy to 36 months postpartum in order to investigate the association between infants’ congenital heart defects (CHD) and compromised maternal mental health. Methods Mothers of infants with mild, moderate, or severe CHD (n = 141) and mothers (n = 36,437) enrolled in the Norwegian Mother and Child Cohort Study were assessed at regular intervals from pregnancy up to 36 months postpartum, including measurements at 6 and 18 months, using an 8-item version of the Hopkins Symptom Checklist-25. Results Mean score trajectories of SCL-8 for mothers of infants with severe CHD deviated significantly from cohort controls 6, 18, and 36 months postpartum, indicating heightened symptoms of depression and anxiety. Conclusions Mothers of infants with severe CHD are at risk of compromised mental health from delivery to 36 months postpartum. Strain due to CHD-related interventions is identified as a possible partial mediator of the distress.
Keywords: anxiety, critically ill children, depression, longitudinal research, parent stress
Introduction
The postpartum period is widely considered to be a period of increased vulnerability to compromised maternal mental health (Cox, Murray, & Chapman, 1993; Eberhard-Gran, Eskild, Tambs, Samuelsen, & Opjordsmoen, 2002; Eberhard-Gran, Tambs, Opjordsmoen, Skrondal, & Eskild, 2003), and stressors associated with postpartum childcare or infant illness have been associated with symptoms of postpartum depression (Beck, 2001; O’Hara, Neunaber, & Zekoski, 1984). Within this context, most mothers of infants with congenital heart defects (CHD) are exposed to varying levels of postpartum distress in the first months after delivery (Van Horn, DeMaso, Gonzalez-Heydrich, & Erikson, 2001). While some forms of mild CHD (e.g., small ventricular septal defects) and moderate CHD (e.g., pulmonary stenosis or patent ductus arteriosus), that resolve within the first year of life, may add stress to the mothers’ first postpartum months, infants with severe forms of CHD (e.g., transposition of the great arteries or tetralogy of Fallot) might be clearly symptomatic at birth and in need of acute and prolonged interventions that might further affect their mothers’ mental health within and beyond the same time period. Indeed, previous studies have suggested that elevated levels of psychological distress might be apparent in as many as one-third of mothers and one-fifth of fathers the first weeks after delivery of a child with severe CHD (Doherty et al., 2009). Furthermore, although successful surgery may correct the heart defect, the surgical treatment itself can be highly stressful for both mother and child, and some children continue to struggle with postoperative problems such as impaired growth (Polat et al., 2011; Schuurmans, Pulles Heintzberger, Gerver, Kester, & Forget, 1998) and motor skills (Brandlistuen et al., 2010; Snookes et al., 2010), neurodevelopmental retardation (Dittrich et al., 2003; Donofrio & Massaro, 2010), digestive and/or breastfeeding problems (Clemente, Barnes, Shinebourne, & Stein, 2001; Tandberg, Ystrom, Vollrath, & Holmstrom, 2010), emotional reactivity, and/or impaired social development (Brandlistuen et al., 2010; Stene Larsen et al., 2010) beyond the immediate postpartum period. In line with this, previous research have suggested that parents in general report distress and poor adjustment due to caregiving demands associated with the special needs of a chronically ill child (Cohen, 1999; Wallander & Varni, 1998), identifying feelings of uncertainty, daily hassles, social isolation, and financial strain as additional stressors. Moreover, parents of children with CHD have been shown to report excessive parenting stress, especially related to characteristics of the infant that make them difficult to parent (Uzark & Jones, 2003) and many parents continue to feel either stressed or defensive about their infant, particularly if he/she exhibits behavioral difficulties (Majnemer et al., 2006). In other words, some mothers might be unable to adapt to their infant, possibly leading to disturbed or lower levels of positive affect and engagement than in healthy mother–infant dyads, possibly causing disordered/disrupted mother–infant interaction (Gardner, Freeman, Black, & Angelini, 1996). On the other hand, a recent study by our research group found that mothers of infants with CHD showed the same level of life satisfaction and joy as mothers of children without CHD, both during pregnancy and 6 months postpartum (Dale et al., 2011), possibly indicating that the effect of the infant’s illness is temporary or that life satisfaction as a whole might be maintained, even though the mothers experience parallel symptoms of depression related to her more immediate situation.
Within this framework, a previous longitudinal study by our research group examined the relationship between the severity of infants’ CHDs and their mothers’ symptoms of depression and anxiety from pregnancy to 18 months postpartum (Solberg et al., 2011). In accordance with Doherty et al. (2009), that study revealed that mothers of infants with severe CHD were more distressed during this time frame than mothers of infants with mild to moderate CHD and cohort controls, reporting prolonged symptoms of both depression and anxiety beyond the expected symptom decline and/or well-being recovery 2–6 months postpartum that is usually associated with postnatal depression (Cooper & Murray, 1998).
The term “recovery” here connotes a mental health trajectory in which normal functioning temporarily gives way to, in our case, symptoms of depression or anxiety, usually for a period of a few months, and then gradually returns to predelivery levels. Still, according to Bonanno (2004) and Bonanno et al. (2002), different life stressors can have similar initial impact, but whereas some events directly cause temporary disruption in normal functioning, others appear to have a more indirect impact mediated by the stressors that accompany them. In other words, different negative life events not only vary in impact, they might also have different trajectories of recovery related to additional factors that coincide with the stressor. Accordingly, it is possible that the mothers in our previous study reported elevated symptoms of depression and anxiety up to 18 months postpartum in part because of ongoing medical and surgical treatments endured by their infants in addition to the CHD diagnosis. Supporting this interpretation, previous studies have found associations between ongoing or chronic stressful life events and prolonged chronicity in mental health problems (McGonagle & Kessler, 1990). In addition, studies of chronic and acute stress effects have demonstrated that chronic stress might be a stronger predictor of psychological maladjustment than acute stress (Avison & Turner, 1988; Eckenrode, 1984).
With the aforementioned in mind, one can easily assume that the broad CHD spectrum, which ranges from generally asymptomatic conditions that are left untreated, to conditions that can be treated within the first year of life using minimally invasive interventions, to life-threatening chronic conditions that require acute treatment and continuous follow-up beyond the infants’ first years of life, may result in distinct differences in terms of the mothers’ psychological adjustment and/or recovery trajectories several months or years after delivery. However, exactly how these diagnosis-specific differences manifest over time is not a trivial question. Whereas mothers of infants with mild forms of CHD might experience moderate levels of stress that causes early, temporary adjustment problems, mothers of infants with severe CHD might experience prolonged continuous high-level stress in addition to the acuteness of the situation. This could possibly lead to the development of longer-lasting problems and/or posttraumatic stress levels similar to those found in, mothers of children undergoing open heart surgery (Helfricht, Latal, Fischer, Tomaske, & Landolt, 2008) or mothers of children newly diagnosed with cancer (Landolt, Vollrath, Ribi, Gnehm, & Sennhauser, 2003). Previous efforts to explore this complex relationship further (Davis, Brown, Bakeman, & Campbell, 1998; Lawoko & Soares, 2002, 2003, 2006; Torowicz, Irving, Hanlon, Sumpter, & Medoff-Cooper, 2010) have utilized different methods for objective and comprehensive grouping of the CHD severity, but in general the defect alone has been employed as an illness severity index, in most cases failing to establish support for a graded link between CHD severity, time, and psychological impact on mothers and/or fathers.
Considering this, the aim of the present study was to further investigate the relationship between the severity of infants’ CHD and their mothers’ mental health trajectories beyond their infants’ first period of intensive and/or invasive treatment. Since diagnosis-specific differences might affect the mothers differently over time and/or at different points in time, regular measurement intervals were included at pregnancy, 6, 18, and 36 months after delivery in order to identify stability and/or possible peaks in mothers’ symptoms of depression and/or anxiety. Moreover, a large pregnancy cohort was included as controls for normative comparison. With this prospective design, we hypothesized that we would be able to isolate how the different categories of mild, moderate, and severe CHD in infants affect the mothers differently by creating independent, CHD category-specific maternal mental health trajectories over time. Specifically, we hypothesized that severe CHD in infants again would be a predictor of prolonged compromised maternal mental health up to 18 months postpartum, but since infants’ most stressful treatment interventions are concluded at 36 months, we expect this association to diminish or even remit to predelivery levels at the final measurement.
Method
Population
As in our previous research (Solberg et al., 2011), data were again obtained from the prospective Norwegian Mother and Child Cohort Study (MoBa) conducted at the Norwegian Institute of Public Health (Magnus et al., 2006). Women in MoBa were recruited using mailed invitations after they registered for routine prenatal ultrasound examination at their local hospitals at approximately gestational weeks 17–18. Participants were recruited from all over Norway from 1999 to 2008, and 38.5% of the invited women consented to participate. According to the 2010 MoBa update (http://www.fhi.no/dokumenter/ce2e768194.pdf), the cohort now includes approximately 108,000 children and 90,700 mothers. Participating women, who supplied written informed consent to enroll in the study, responded to mailed questionnaires at their 30th gestational week and 6, 18, and 36 months postpartum concerning mothers’ mental health, well-being, and social support, together with more descriptive data concerning age, income, and education. The response rates to the questionnaires were 91.0, 84.8, 72.4, and 59.3%, respectively. The Regional Committee for Medical Research and the Norwegian Data Inspectorate approved the study and the MoBa cohort has since been linked to the nation-wide Medical Birth Registry of Norway (MBRN) to record health information related to the pregnancy, delivery, and the child’s status at birth.
The present study (based on measurement wave 5 of MoBa, 2010) included mothers who had (a) identification information in the MBRN; (b) valid MoBa questionnaires from gestational week 30 to 36 months postpartum; and (c) valid data for infant weight, gestational age, and questionnaire items concerning mental health. Among these women, mothers of infants with CHD were identified in the country-wide CHD registry at the Department of Pediatric Cardiology at Oslo University Hospital, Norway, using the unique personal identification number from the Norwegian National Population Register and information from the MBRN. Significant live-born pediatric heart defects cases in Norway are recorded in the country-wide CHD registry. The registry includes data on diagnoses, interventions, surgeries, and detailed clinical outcomes obtained from medical records and information from other hospitals, outpatient clinics, and local physicians.
Procedures and Measurements
CHD Severity Classification
As described in Solberg et al. 2011 and previous research from our group (e.g., Brandlistuen et al., 2010; Dale et al., 2011), the classification of the different defects, using previously published guidelines for grouping (Hoffman & Kaplan, 2002), into the categories of mild, moderate, and severe CHD was done in order to systemize and ensure the quality of the data from the cardiac registry, including information from the medical records on syndromes and comorbid medical diagnoses. A detailed diagnoses chart for the severe CHD category is shown in Table I. Follow-up information on syndromes was available up to about 2–5 years after birth for several of the infants, ensuring information exceeding the actual timing of the study, enabling us to exclude neurological syndromes, detected at a later stage. For further CHD grouping details, see Solberg et al., 2011.
Table I.
Characteristics of the Severe CHD Group
| Diagnosis | N |
|---|---|
| Hypoplastic left heart syndrome | 3 |
| Univentricular heart defect | 1 |
| Double outlet of the right ventricle | 2 |
| Pulmonary atresia with intact ventricular septum | 2 |
| Tetralogy of Fallot. Pulmonary atresia with ventricular septal defect | 6 |
| Common arterial trunk (Truncus arteriosus communis) | 1 |
| Transposition of the great arteries | 13 |
| Atrioventricular septal defect | 2 |
| Severe pulmonary stenosis | 3 |
| Severe aortic stenosis | 5 |
| Coarctation of the aorta and interrupted aortic arch | 4 |
| Totally anomalous pulmonary venous drainage | 1 |
| Ventricular septal defect (complex) | 3 |
| Other | 1 |
| Total | 47 |
Maternal Mental Health
An 8-item version (SCL-8) of the Hopkins Symptom Checklist-25 was used to measure mental health trajectories from gestational week 30 to 36 months postpartum, including measurements at 6 and 18 months postpartum. For details concerning short form versions of SCL, see Solberg et al., 2011; Tambs & Moum, 1993; Strand, Dalgard, Tambs, & Rognerud, 2003. Items in the SCL are scored on a Likert scale ranging from 1 (“not at all”) to 4 (“very much bothered”) and, in the present study, Cronbach’s α for SCL-8 were 0.82, 0.84, 0.84, and 0.87 for the four assessments (week 30 and 6, 18, and 36 months postpartum), respectively. The SCL-8 incorporates two subscales, four items each, allowing separate analysis of depression and anxiety scores (SCL-4Dep and SCL-4Anx), with Cronbach’s α of 0.72, 0.77, 0.79, 0.82 and 0.71, 0.71, 0.73, 0.76, respectively.
Statistical Analysis
In order to minimize listwise deletion and preserve the number of mother–infant dyads, we used a maximum likelihood imputation procedure for missing data computation (Schafer & Graham, 2002). An Expectation Maximization (EM) algorithm (Dempster, Laird, & Rubin, 1977) was used to impute values for missing SCL-8 scores using SCL response parameters from gestational week 30, and 6, 18, and 36 months postpartum. The SCL-8 missing rate for each assessment period was reduced as follows: from 8.3 to 4.5% for gestational week 30, from 11.9 to 4.7% for 6 months, from 11.9 to 10.8% for 18 months, and from 5.0 to 3.9% for 36 months. Logarithmic transformation was also computed for each SCL item for all measurement times to minimize skewness (posttransformation values, 1.75, 1.89, 1.67, and 1.73, respectively) and kurtosis (posttransformation values, 3.76, 4.32, 3.28, and 3.27, respectively).
Mixed between-within subjects analyses of covariance, with the between factor “group” (control, mild, moderate, and severe CHD) and the within factor “time of measurement” (gestational week 30 and 6, 18, and 36 months postpartum) for the dependent variable SCL-8, were used to compute group differences in symptom trajectories over time. The variables “birth weight” and “gestational week” from MBRN were included as covariates in the analysis. Finally, separate, cross-sectional analyses were computed for 36 months measurement in order to further investigate the time of follow-up. A covariate controlling for recent CHD-related interventions was also included at this time. In this context, “recent CHD-related interventions” meant cardiac interventions (e.g., open heart surgery, catheterization, or other CHD-related interventions) carried out between 30 and 36 months postpartum, close to the time of our final measurement. Also, novel to the present study, we included an analysis of attrition due to decreasing participation rates in MoBa, utilizing Chi-square tests and two sample t-tests. The figure and tables presented in this article show standardized regression coefficients and standardized marginal means computed from the logarithmically transformed data.
Results
Study Population
In the present study, a case-match initially generated a sample of 293 women (0.66% of the MoBa cohort, n = 44,220) who had infants with CHD. However, 77 of these mothers were lost due to attrition in MoBa from 18 to 36 months, leaving 216 in the sample. In order investigate if this was a threat to the validity of our study, we analyzed whether these 77 mother–infant dyads differed from the mother–infant dyads that continued to participate, comparing them with respect to CHD severity grouping and SCL-8 mean scores. A Chi-square test revealed no differences between the attrition sample and the mothers-infant dyads who continued to participate in the study in respect to frequencies of mild, moderate, and severe CHD, χ2 (2, N = 293) = 3.522, p = .17, and a two-tailed, two sample t-test showed no difference in mean scores of SCL-8 at 18 months, t(142) = 0.59, p = .55.
Since we wanted to investigate the impact of CHD only, 41 infants with comorbid medical conditions (e.g., esophageal atresia, intestine malformations, cancer, and Down syndrome) were excluded from the analysis. Furthermore, due to listwise deletion and missing over the four SCL-8 measurements (gestational week 30 and 6, 18, and 36 months postpartum) and missing values in “birth weight” and “gestational week” variables, 34 additional mother–infant dyads were lost over time, leaving a total of 141 (Table II) in the final sample that we could follow with regular intervals from pregnancy to 36 months after delivery, including measurements at 6 and 18 months postpartum.
Table II.
Characteristics of the CHD Groups Compared to the Cohort Control Group
| Control group (N = 36437) | Mild CHD (N = 63) | Moderate CHD (N = 31) | Severe CHD (N = 47) | p-value* | |
|---|---|---|---|---|---|
| Mothers’ education (years) M ± SD | 14.8 ± 2.4 | 14.5 ± 2.6 | 14.8 ± 2.3 | 14.7 ± 2.8 | .725 |
| Mothers’ age at time of delivery (years) M ± SD | 30.3 ± 4.4 | 30.9 ± 4.8 | 30.2 ± 4.5 | 29.8 ± 3.9 | .627 |
| Mothers’ income (low/average/high, %) | 28.2/62.0/9.8 | 27.9/63.9/8.2 | 26.7/ 60.0/13.3 | 34.1/59.1/6.8 | .951 |
| Fathers’ income (low/average/high, %) | 10.4/59.6/30.0 | 8.6/60.3/31.0 | 6.7/63.3/30.0 | 7.1/52.4/40.5 | .804 |
| Social support at 36 months (yes, %) | 97.0 | 96.8 | 100.0 | 91.3 | .113 |
| Recent CHD-related interventions (yes, %) | 0.0b | 0.0 | 6.5 | 10.6a | .005 |
| Birth weight (g) M ± SD | 3603 ± 554 | 3676 ± 747 | 3438 ± 717 | 3371 ± 697a | .007 |
| Gestational age (weeks) M ± SD | 39.5 ± 1.8 | 39.3 ± 2.4 | 38.9 ± 2.5 | 38.6 ± 2.3a | .002 |
| Sex (Female, %) | 48.8 | 52.4 | 29.0 | 44.7 | .138 |
aSignificantly different from the control group, assessed with parameter estimates or Pearson chi-square.
bThis parameter is set to zero because it is redundant.
*p-values for testing between-subjects effects.
Maternal Mental Health Trajectories and Infant CHD Severity
Analysis of the full eight-item scale (SCL-8) with “birth weight” and “gestational week” as covariates showed a significant main effect of group (mild, moderate, severe, and controls) [F(3,36572) = 5.567, p < .001] and a significant interaction between Group and Time of measurement (gestational week 30, 6, 18, and 36 months postpartum) [F(9,109716) = 2.958, p < .002]. The overall effect of Time was not significant [F(3,109716) = 1.134, p = .334].
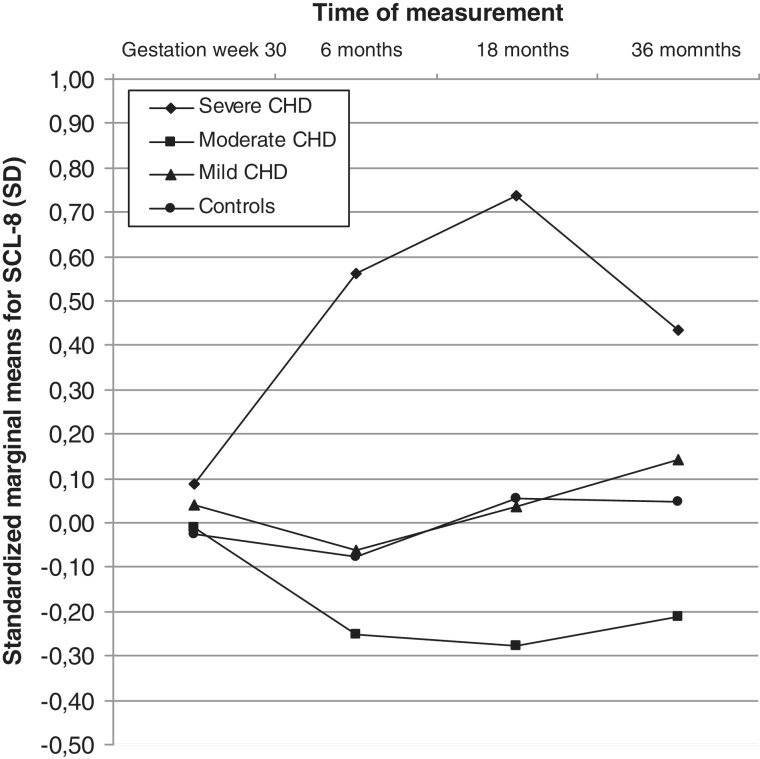
Parameter estimates revealed no difference in the mothers’ SCL-8 scores prenatally, but mothers of infants with severe CHD reported significantly higher scores than mothers in the control group 6, 18, and 36 months postpartum, with standard deviations of 0.64, 0.69, and 0.39, respectively, as shown in Table III. Standardized, adjusted marginal means are shown in Figure 1.
Table III.
Parameter Estimates Showing Standardized Regression Coefficients (b) With 95% Confidence Intervals For SCL-8, SCL-4Dep, and SCL-4Anx Across CHD Severity (Mild, Moderate, Severe) Compared to Cohort Controls From Gestational Week 30 to 36 Months Postpartum
| Time of measurement | Group | N | SCL-8 ba (CI) | SCL-4Depb ba (CI) | SCL-4Anxc ba (CI) |
|---|---|---|---|---|---|
| Gestational week 30 | Severe CHD | 47 | 0.11 (−0.15 to 0.38) | 0.16 (−0.10 to 0.42) | 0.04 (−0.25 to 0.32) |
| Moderate CHD | 31 | 0.01 (−0.32 to 0.35) | 0.05 (−0.27 to 0.37) | −0.03 (−0.38 to 0.32) | |
| Mild CHD | 63 | 0.07 (−0.16 to 0.30) | 0.03 (−0.19 to 0.26) | 0.10 (−0.15 to 0.35) | |
| Control | 36437 | 0d | 0d | 0d | |
| 6 months postpartum | Severe CHD | 47 | 0.64 (0.37 to 0.91) * | 0.62 (0.35 to 0.90) * | 0.52 (0.25 to 0.79) * |
| Moderate CHD | 31 | −0.17 (−0.51 to 0.16) | −0.25 (−0.59 to 0.09) | −0.04 (−0.37 to 0.29) | |
| Mild CHD | 63 | 0.01 (−0.22 to 0.25) | −0.04 (−0.28 to 0.19) | 0.08 (−0.15 to 0.31) | |
| Control | 36437 | 0d | 0d | 0d | |
| 18 months postpartum | Severe CHD | 47 | 0.69 (0.39 to 0.98) * | 0.71 (0.41 to 1.01) * | 0.51 (0.22 to 0.79) * |
| Moderate CHD | 31 | −0.33 (−0.69 to 0.03) | −0.26 (−0.62 to 0.11) | −0.36 (−0.71 to 0.00) * | |
| Mild CHD | 63 | −0.01 (−0.27 to 0.24) | −0.03 (−0.28 to 0.23) | 0.01 (−0.24 to 0.25) | |
| Control | 36437 | 0d | 0d | 0d | |
| 36 months postpartum | Severe CHD | 47 | 0.39 (0.08 to 0.70) * | 0.34 (0.04 to 0.65) * | 0.36 (0.05 to 0.66) * |
| Moderate CHD | 31 | −0.26 (−0.64 to 0.12) | −0.34 (−0.72 to 0.04) | −0.10 (−0.47 to 0.28) | |
| Mild CHD | 63 | 0.09 (−0.17 to 0.36) | 0.14 (−0.13 to 0.41) | 0.02 (−0.25 to 0.28) | |
| Control | 36437 | 0d | 0d | 0d |
aThe coefficients show deviations from the control mean in fractions of standard deviations.
bOverall group effect [F(3,36572) = 5.973, p < .0005] and interaction effect [F(9,109716) = 2.881, p < .002].
cOverall group effect [F(3,36572) = 3.586, p < .013] and interaction effect [F(9,109716) = 2.068, p < .029].
dThis parameter is set to zero because it is redundant.
*p < .05.
Figure 1.
Mothers’ standardized adjusted marginal means for SCL-8 according to the severity of the CHD (controls, mild, moderate, severe) from gestational week 30 to 36 months postpartum. The coefficients show adjusted mean deviations in fractions of standard deviations.
Cohen’s d for the adjusted marginal means showed a medium effect at 6 months (d = 0.60), a medium effect at 18 months (d = 0.65), and a small effect at 36 months (d = 0.37) when the severe CHD group was compared to controls. Bonferroni corrected, pairwise comparisons revealed that, overall, mothers of infants with severe CHD had significantly higher SCL-8 scores than mothers of infants with mild or moderate CHD, (p < .046 and p < .004, respectively).
Cross-Sectional Analysis at 36 Months
To further investigate the effects of severe CHD at 36 months, cross-sectional analyses were carried out. Analysis of the full 8-item scale (SCL-8) showed a significant effect of group (mild, moderate, severe, and controls) [F(3,36572) = 2.731, p < .042]. Parameter estimates revealed that mothers of infants with severe CHD reported significantly higher scores than mothers in the control group, and 14.9% of these women reported scores above a moderate to severe cut-off that corresponded to the 95th percentile of the MoBa cohort. In comparison, only 3.2% of the mothers in the mild and moderate CHD group scored above the same threshold.
Furthermore, when analyzing the four items in each subscale (SCL-4Dep and SCL-4Anx) separately, we found a significant main effect of group [F(3,36572) = 2.982, p < .030] for the items that measured depression. Here, too, the mothers of infants with severe CHD reported significantly higher scores than mothers in the control group, while the four items measuring anxiety were not significantly different [F(3,36572) = 1.854, p = .135]. Finally, when incorporating “recent CHD-related interventions” as a additional covariate in the full eight-item analysis, the already small overall effect for the severe CHD group 36 months postpartum declined to a nonsignificant level [F(3,36571) = 2.050, p = .105]. The between-subjects effect of recent CHD-related interventions for the CHD groups was significant [F(3,135) = 2.355, p = .035].
Discussion
Main Findings
As mentioned in the introduction, we expected mothers of infants with severe CHD to report declining levels of depression and anxiety symptoms 3 years after delivery. However, in accordance with our previous research (Solberg et al., 2011), the results from the present study revealed that severe CHD in infants had prolonged negative effects on the mothers’ mental health, identifying prevailing, heightened symptoms of depression and anxiety, compared to pregnancy cohort controls, at 6, 18, and finally at 36 months postpartum, beyond the conclusion of most of the infants’ initial intensive treatment programs. To our knowledge, this is the first time such an association has been demonstrated in a prospective case-cohort design, with stable, consistent measurements at regular intervals, incorporating predelivery, pregnancy baselines.
Moreover, the classification of the severity of the cardiac defects according to the guidelines of Hoffman and Kaplan (2002), assigning infants with CHD into mild, moderate, and severe categories, produced an advantage. Although slightly heterogeneous in defects, these three groups or categories of severity are more homogenous in important parameters, such as number of interventions, curability, and/or expected postoperative quality of life. This gave us larger, less specific CHD groups, but higher statistical power, enabling us to demonstrate differences in impact across a more general CHD severity spectrum. In doing so, we paralleled previous research with results demonstrating a link between infant CHD and compromised maternal mental health, showed consistency with the pediatric chronic illness literature, insofar that a defect alone does not predict maternal adjustment over time, but extended the understanding in isolating the independent association between severe CHD in infants and prolonged compromised maternal mental health.
Moreover, in order to investigate this association further, we performed a cross-sectional analysis of the 36-month data, taking ongoing or recent treatment interventions that might accompany the severe CHD diagnosis treatment program or schedule at 36 months into account. This accompanying factor was shown to have a significant impact on mothers’ mental health at this time, possibly partially explaining why the expected symptom decline at 36 months was not found in the prospective analysis.
Limitations
As in our previous research, the results of the present study are subject to limitations, such as oversimplification in the CHD severity grading, low participation rate, attrition, and the lack of clinical assessments of the mothers that might possibly have affected the results (see Solberg et al., 2011 for details). Furthermore, unique to the discussion in this study, we do not have data concerning additional possible confounding factors, such as the affected families access to care (travel distance, access to treatment locally etc.), the mothers prior knowledge about CHD and severity outcomes, mothers’ and fathers’ coping styles or strategies, and situational and self-resource appraisals, that possibly could affect the association between CHD severity and maternal mental health. Therefore, and since the illness brings infants and their families into healthcare settings under adverse and often life-threatening circumstances, a more conceptual model associated with the overall experience would have been desirable in order to further interpret the validity and impact of our findings.
Clinical Implications and Conclusions
In accordance with Solberg et al. (2011), the analyses in the present study again isolate the independent prolonged association between severe CHD in infants and compromised maternal mental health. Moreover, each category of CHD severity seems to predict an independent maternal mental health trajectory over time, and only when an infant suffers from severe CHD might stable symptoms of maternal distress be present 6, 18, and 36 months postpartum. Still, if the distress is due in part to the CHD-related interventions the severely ill infants undergo, the mothers’ mental health trajectories should possibly decline or even return eventually to predelivery symptom levels when CHD treatment is concluded.
With this in mind, and considering the possible negative effects of depression and anxiety on mother–infant interactions (Campbell et al., 2004; Feldman et al., 2009; Pearlstein, Howard, Salisbury, & Zlotnick, 2009), medical personnel should be made aware of the link between severe CHD and compromised maternal mental health. Since earlier studies have shown less postpartum distress in fathers (Skari et al., 2002; Skreden et al., 2008, 2010) and positive effects of paternal involvement (e.g., Gavin & Wysocki, 2006), screening and treatment interventions that include both parents at an early stage or close to CHD-related interventions, utilizing paternal resources, and support are recommended. Interventions should include programs that educate the fathers, mothers, and families in self-management skills and/or group counseling in order to reduce the risk of prolonged negative adjustment (McCusker et al., 2010). Moreover, future research should explore the possible impact of maternal and paternal compromised mental health on health outcomes of the developing infant or child within and beyond this time frame, incorporating accompanying factors such as the families access to care, knowledge about CHD and outcomes, and parents’ coping styles that possibly could affect this association, in order to identify possible key factors that generate or alleviate strain for both child, mother and family in a more comprehensive interpretative stress-coping model than the present study, due to its limitations, could apply.
Funding
The Norwegian Mother and Child Cohort Study is supported by the Norwegian Ministry of Health, National Institutes of Health/National Institute of Environmental Health Sciences (grant no. N01-ES-85433), National Institutes of Health/National Institute of Neurological Disorders and Stroke (grant no. 1 UO1 NS 047537-01), and the Norwegian Research Council/Functional Genomics (grant no. 151918/S10). The present study is supported by the Norwegian Research Council (grant no. 186031/v50).
Conflicts of interest: None declared.
Acknowledgments
The authors wish to thank Kim Stene-Larsen for valuable comments and insights during the conceptual elaboration; Bo Engdahl for his expert contribution to the statistical discussion prior to the final analysis of the data; and Sarah Hampson for valuable review comments prior to submission of the final manuscript. The authors are also grateful to all the participating families in Norway who took part in this cohort study.
References
- Avison W R, Turner R J. Stressful life events and depressive symptoms: Disaggregating the effects of acute stressors and chronic strains. Journal of Health and Social Behavior. 1988;29:253–264. [PubMed] [Google Scholar]
- Balluffi A, Kassam-Adams N, Kazak A, Tucker M, Dominguez T, Helfaer M. Traumatic stress in parents of children admitted to the pediatric intensive care unit. Pediatric Critical Care Medicine. 2004;5:547–553. doi: 10.1097/01.PCC.0000137354.19807.44. [DOI] [PubMed] [Google Scholar]
- Beck C T. Predictors of postpartum depression: An update. Nursing Research. 2001;50:275–285. doi: 10.1097/00006199-200109000-00004. [DOI] [PubMed] [Google Scholar]
- Board R, Ryan-Wenger N. Stressors and stress symptoms of mothers with children in the PICU. Journal of Pediatric Nursing. 2003;18:195–202. doi: 10.1053/jpdn.2003.38. [DOI] [PubMed] [Google Scholar]
- Bonanno G A. Loss, trauma, and human resilience: Have we underestimated the human capacity to thrive after extremely aversive events? American Psychologist. 2004;59:20–28. doi: 10.1037/0003-066X.59.1.20. [DOI] [PubMed] [Google Scholar]
- Bonanno G A, Wortman C B, Lehman D R, Tweed R G, Haring M, Sonnega J, Carr D, Nesse R. Resilience to loss and chronic grief: A prospective study from preloss to 18-months postloss. Journal of Personality and Social Psychology. 2002;83:1150–1164. doi: 10.1037//0022-3514.83.5.1150. [DOI] [PubMed] [Google Scholar]
- Brandlistuen R E, Stene-Larsen K, Holmstrom H, Landolt M A, Eskedal L T, Vollrath M E. Motor and social development in 6-month-old children with congenital heart defects. The Journal of Pediatrics. 2010;156:265–269. doi: 10.1016/j.jpeds.2009.08.035. [DOI] [PubMed] [Google Scholar]
- Campbell S B, Brownell C A, Hungerford A, Spieker S J, Mohan R, Blessing J S. The course of maternal depressive symptoms and maternal sensitivity as predictors of attachment security at 36 months. Development and Psychopathology. 2004;16:231–252. doi: 10.1017/s0954579404044499. [DOI] [PubMed] [Google Scholar]
- Clemente C, Barnes J, Shinebourne E, Stein A. Are infant behavioural feeding difficulties associated with congenital heart disease? Child: Care, Health & Development. 2001;27:47–59. doi: 10.1046/j.1365-2214.2001.00199.x. [DOI] [PubMed] [Google Scholar]
- Cohen M S. Families coping with childhood chronic illness: A research review. Families, Systems, & Health. 1999;17:149–164. [Google Scholar]
- Cooper P J, Murray L. Postnatal depression. British Medical Journal. 1998;316:1884–1886. doi: 10.1136/bmj.316.7148.1884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cox J L, Murray D, Chapman G. A controlled study of the onset, duration and prevalence of postnatal depression. The British Journal of Psychiatry. 1993;163:27–31. doi: 10.1192/bjp.163.1.27. [DOI] [PubMed] [Google Scholar]
- Dale M T G, Solberg O, Holmstrom H, Landolt M A, Eskedal L T, Vollrath M E. Mothers of infants with congenital heart defects: well-being from pregnancy through the child’s first six months. Quality of Life Research. 2011;21:115–122. doi: 10.1007/s11136-011-9920-9. [DOI] [PubMed] [Google Scholar]
- Davis C C, Brown R T, Bakeman R, Campbell R. Psychological adaptation and adjustment of mothers of children with congenital heart disease: Stress, coping, and family functioning. Journal of Pediatric Psychology. 1998;23:219–228. doi: 10.1093/jpepsy/23.4.219. [DOI] [PubMed] [Google Scholar]
- Dempster A P, Laird N M, Rubin D B. Maximum likelihood from incomplete data via the EM algorithm. Journal of the Royal Statistical Society. Series B (Methodological) 1977;39:1–38. [Google Scholar]
- Dittrich H, Buhrer C, Grimmer I, Dittrich S, Abdul-Khaliq H, Lange P E. Neurodevelopment at 1 year of age in infants with congenital heart disease. British Medical Journal. 2003;89:436–441. doi: 10.1136/heart.89.4.436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doherty N, McCusker C G, Molloy B, Mulholland C, Rooney N, Craig B, Sands A, Stewart M, Casey F. Predictors of psychological functioning in mothers and fathers of infants born with severe congenital heart disease. Journal of Reproductive and Infant Psychology. 2009;27:390–400. [Google Scholar]
- Donofrio M T, Massaro A N. Impact of congenital heart disease on brain development and neurodevelopmental outcome. International Journal of Pediatrics. 2010;2010:1–13. doi: 10.1155/2010/359390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eberhard-Gran M, Eskild A, Tambs K, Samuelsen S O, Opjordsmoen S. Depression in postpartum and non-postpartum women: Prevalence and risk factors. Acta Psychiatrica Scandinavica. 2002;106:426–433. doi: 10.1034/j.1600-0447.2002.02408.x. [DOI] [PubMed] [Google Scholar]
- Eberhard-Gran M, Tambs K, Opjordsmoen S, Skrondal A, Eskild A. A comparison of anxiety and depressive symptomatology in postpartum and non-postpartum mothers. Social Psychiatry and Psychiatric Epidemiology. 2003;38:551–556. doi: 10.1007/s00127-003-0679-3. [DOI] [PubMed] [Google Scholar]
- Eckenrode J. Impact of chronic and acute stressors on daily reports of mood. Journal of Personality and Social Psychology. 1984;46:907–918. doi: 10.1037//0022-3514.46.4.907. [DOI] [PubMed] [Google Scholar]
- Feldman R, Granat A, Pariente C, Kanety H, Kuint J, Gilboa-Schechtman E. Maternal depression and anxiety across the postpartum year and infant social engagement, fear regulation, and stress reactivity. Journal of the American Academy of Child & Adolescent Psychiatry. 2009;48:919–927. doi: 10.1097/CHI.0b013e3181b21651. [DOI] [PubMed] [Google Scholar]
- Fink P, Ornbol E, Hansen M S, Sondergaard L, De Jonge P. Detecting mental disorders in general hospitals by the SCL-8 scale. Journal of Psychosomatic Research. 2004;56:371–375. doi: 10.1016/S0022-3999(03)00071-0. [DOI] [PubMed] [Google Scholar]
- Fink P, Ornbol E, Huyse F J, De Jonge P, Lobo A, Herzog T, Slaets J P, Arolt V. A brief diagnostic screening instrument for mental disturbances in general medical wards. Journal of Psychosomatic Research. 2004;57:17–24. doi: 10.1016/S0022-3999(03)00374-X. [DOI] [PubMed] [Google Scholar]
- Gardner F V, Freeman N H, Black A M, Angelini G D. Disturbed mother-infant interaction in association with congenital heart disease. Heart. 1996;76:56–59. doi: 10.1136/hrt.76.1.56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gavin L, Wysocki T. associations of paternal involvement in disease management with maternal and family outcomes in families with children with chronic illness. Journal of Pediatric Psychology. 2006;31:481–489. doi: 10.1093/jpepsy/jsj043. [DOI] [PubMed] [Google Scholar]
- Helfricht S, Latal B, Fischer J E, Tomaske M, Landolt M A. Surgery-related posttraumatic stress disorder in parents of children undergoing cardiopulmonary bypass surgery: A prospective cohort study. Pediatric Critical Care Medicine. 2008;9:217–223. doi: 10.1097/PCC.0b013e318166eec3. [DOI] [PubMed] [Google Scholar]
- Hoffman J I E, Kaplan S. The incidence of congenital heart disease. Journal of the American College of Cardiology. 2002;39:1890–1900. doi: 10.1016/s0735-1097(02)01886-7. [DOI] [PubMed] [Google Scholar]
- Landolt M A, Vollrath M, Ribi K, Gnehm H E, Sennhauser F H. Incidence and associations of parental and child posttraumatic stress symptoms in pediatric patients. Journal of Child Psychology & Psychiatry & Allied Disciplines. 2003;44:1199–1207. doi: 10.1111/1469-7610.00201. [DOI] [PubMed] [Google Scholar]
- Lawoko S, Soares J J F. Distress and hopelessness among parents of children with congenital heart disease, parents of children with other diseases, and parents of healthy children. Journal of Psychosomatic Research. 2002;52:193–208. doi: 10.1016/s0022-3999(02)00301-x. [DOI] [PubMed] [Google Scholar]
- Lawoko S, Soares J J F. Quality of life among parents of children with congenital heart disease, parents of children with other diseases and parents of healthy children. Quality of Life Research. 2003;12:655–666. doi: 10.1023/a:1025114331419. [DOI] [PubMed] [Google Scholar]
- Lawoko S, Soares J J F. Psychosocial morbidity among parents of children with congenital heart disease: A prospective longitudinal study. Heart & Lung-The Journal of Acute and Critical Care. 2006;35:301–314. doi: 10.1016/j.hrtlng.2006.01.004. [DOI] [PubMed] [Google Scholar]
- Magnus P. The Norwegian Mother and Child Cohort Study (MoBa) New research possibilities. Norsk epidemiologi. 2009;17:107–110. [Google Scholar]
- Magnus P, Irgens L M, Haug K, Nystad W, Skjaerven R, Stoltenberg C. Cohort profile: the Norwegian mother and child cohort study (MoBa) International Journal of Epidemiology. 2006;35:1146–1150. doi: 10.1093/ije/dyl170. [DOI] [PubMed] [Google Scholar]
- Majnemer A, Limperopoulos C, Shevell M, Rohlicek C, Rosenblatt B, Tchervenkov C. Health and well-being of children with congenital cardiac malformations, and their families, following open-heart surgery. Cardiology in the Young. 2006;16:157–164. doi: 10.1017/S1047951106000096. [DOI] [PubMed] [Google Scholar]
- Marteau T M, Kidd J, Cook R, Johnston M, Michie S, Shaw R W, Slack J. Screening for Down's syndrome. British Medical Journal. 1988;297:1469. doi: 10.1136/bmj.297.6661.1469-b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCusker C G, Doherty N N, Molloy B, Rooney N, Mulholland C, Sands A, Stewart M, Casey F. A controlled trial of early interventions to promote maternal adjustment and development in infants born with severe congenital heart disease. Child: Care, Health and Development. 2010;36:110–117. doi: 10.1111/j.1365-2214.2009.01026.x. [DOI] [PubMed] [Google Scholar]
- McGonagle K A, Kessler R C. Chronic stress, acute stress, and depressive symptoms. American Journal of Community Psychology. 1990;18:681–706. doi: 10.1007/BF00931237. [DOI] [PubMed] [Google Scholar]
- Nilsen R M, Vollset S E, Gjessing H K, Skjaerven R, Melve K K, Schreuder P, Alsaker E R, Haug K, Daltveit A K, Magnus P. Self-selection and bias in a large prospective pregnancy cohort in Norway. Paediatric and Perinatal Epidemiology. 2009;23:597–608. doi: 10.1111/j.1365-3016.2009.01062.x. [DOI] [PubMed] [Google Scholar]
- O'Hara M, Neunaber D, Zekoski E. Prospective study of postpartum depression: Prevalence, course, and predictive factors. Journal or Abnormal Psychology. 1984;93:158–171. doi: 10.1037//0021-843x.93.2.158. [DOI] [PubMed] [Google Scholar]
- Pearlstein T, Howard M, Salisbury A, Zlotnick C. Postpartum depression. American Journal of Obstetrics and Gynecology. 2009;200:357–364. doi: 10.1016/j.ajog.2008.11.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Polat S, Okuyaz C, Hallioglu O, Mert E, Makharoblidze K. Evaluation of growth and neurodevelopment in children with congenital heart disease. Pediatrics International. 2011;53:345–349. doi: 10.1111/j.1442-200X.2010.03230.x. [DOI] [PubMed] [Google Scholar]
- Rona R J, Smeeton N C, Beech R, Barnett A, Sharland G. Anxiety and depression in mothers related to severe malformation of the heart of the child and foetus. Acta Paediatrica. 1998;87:201–205. doi: 10.1080/08035259850157679. [DOI] [PubMed] [Google Scholar]
- Schafer J L, Graham J W. Missing data: Our view of the state of the art. Psychological Methods. 2002;7:147–177. [PubMed] [Google Scholar]
- Schuurmans F M, Pulles Heintzberger C F M, Gerver W J M, Kester A D M, Forget P. Long-term growth of children with congenital heart disease: A retrospective study. Acta Paediatrica. 1998;87:1250–1255. doi: 10.1080/080352598750030933. [DOI] [PubMed] [Google Scholar]
- Skari H, Skreden M, Malt U F, Dalholt M, Ostensen A B, Egeland T, Emblem R. Comparative levels of psychological distress, stress symptoms, depression and anxiety after childbirth: A prospective population-based study of mothers and fathers. BJOG: An International Journal of Obstetrics & Gynaecology. 2002;109:1154–1163. doi: 10.1111/j.1471-0528.2002.00468.x. [DOI] [PubMed] [Google Scholar]
- Skreden M, Skari H, Bjork M D, Malt U F, Veenstra M, Faugli A, Åvitsland T L, Emblem R. Psychological distress in mothers and fathers of preschool children: A 5-year follow-up study after birth. BJOG: An International Journal of Obstetrics & Gynaecology. 2008;115:462–471. doi: 10.1111/j.1471-0528.2007.01631.x. [DOI] [PubMed] [Google Scholar]
- Skreden M, Skari H, Malt U F, Haugen G, Pripp A H, Faugli A, Emblem R. Long-term parental psychological distress among parents of children with a malformation-A prospective longitudinal study. American Journal of Medical Genetics Part A. 2010;152:2193–2202. doi: 10.1002/ajmg.a.33605. [DOI] [PubMed] [Google Scholar]
- Snookes S H, Gunn J K, Eldridge B J, Donath S M, Hunt R W, Galea M P, Shekerdemain L. A systematic review of motor and cognitive outcomes after early surgery for congenital heart disease. Pediatrics. 2010;125:818–827. doi: 10.1542/peds.2009-1959. [DOI] [PubMed] [Google Scholar]
- Solberg O, Gronning Dale M T, Holmstrom H, Eskedal L T, Landolt M A, Vollrath M E. Long-term symptoms of depression and anxiety in mothers of infants with congenital heart defects. Journal of Pediatric Psychology. 2011;36:179–187. doi: 10.1093/jpepsy/jsq054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stene Larsen K, Brandlistuen R E, Holmstrom H, Landolt M A, Eskedal L T, Vollrath M E. Emotional reactivity in infants with congenital heart defects: Findings from a large case cohort study in Norway. Acta Paediatrica. 2010;99:52–55. doi: 10.1111/j.1651-2227.2009.01515.x. [DOI] [PubMed] [Google Scholar]
- Strand B H, Dalgard O S, Tambs K, Rognerud M. Measuring the mental health status of the Norwegian population: A comparison of the instruments SCL-25, SCL-10, SCL-5 and MHI-5 (SF-36) Nordic Journal of Psychiatry. 2003;57:113–118. doi: 10.1080/08039480310000932. [DOI] [PubMed] [Google Scholar]
- Tandberg B S, Ystrom E, Vollrath M E, Holmstrom H. Feeding infants with CHD with breast milk: Norwegian mother and child cohort study. Acta Paediatrica. 2010;99:373–378. doi: 10.1111/j.1651-2227.2009.01605.x. [DOI] [PubMed] [Google Scholar]
- Tambs K, Moum T. How well can a few questionnaire items indicate anxiety and depression? Acta Psychiatrica Scandinavica. 1993;87:364–367. doi: 10.1111/j.1600-0447.1993.tb03388.x. [DOI] [PubMed] [Google Scholar]
- Torowicz D, Irving S Y, Hanlon A L, Sumpter D F, Medoff-Cooper B. Infant temperament and parental stress in 3-month-old infants after surgery for complex congenital heart disease. Journal of Developmental & Behavioral Pediatrics. 2010;31:202–208. doi: 10.1097/DBP.0b013e3181d3deaa. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uzark K, Jones K. Parenting stress and children with heart disease. Journal of Pediatric Health Care. 2003;17:163–168. doi: 10.1067/mph.2003.22. [DOI] [PubMed] [Google Scholar]
- Van Horn M, DeMaso D R, Gonzalez-Heydrich J, Erikson J D. Illness-related concerns of mothers of children with congenital heart disease. Journal of the American Academy of Child & Adolescent Psychiatry. 2001;40:847–854. doi: 10.1097/00004583-200107000-00020. [DOI] [PubMed] [Google Scholar]
- Wallander J L, Varni J W. Effects of pediatric chronic physical disorders on child and family adjustment. The Journal of Child Psychology and Psychiatry and Allied Disciplines. 1998;39:29–46. [PubMed] [Google Scholar]
- Wortman C B, Silver R C. The myths of coping with loss. Journal of Consulting and Clinical Psychology. 1989;57:349–357. doi: 10.1037//0022-006x.57.3.349. [DOI] [PubMed] [Google Scholar]