Abstract
Objective To examine genetic and environmental contributions to stability and change in sleep problems (SP) in early childhood. Methods The sample comprised over 300 twin pairs assessed at ages 2 and 3 years. Parents rated SP on the Sleep Problems subscale of the Child Behavior Checklist for ages 1.5–5 years. Results Longitudinal quantitative genetic analyses indicated that SP were genetically influenced at both ages. The stability of SP from ages 2 to 3 years was largely due to genetic factors common to both ages. Nonshared environmental influences displayed modest continuity across age. New genetic and nonshared environmental factors emerged at age 3 years. Conclusions Genetic factors contribute to the stability in SP, whereas change is due to both genetic and nonshared environmental influences. Early interventions on SP and individualized treatments based on children’s unique environmental experiences may be fruitful.
Keywords: development, heritability, sleep problems, toddlerhood, twins
Introduction
Sleep problems (SP) are one of the most common complaints that parents report about their children and are costly for health services (Mindell & Owens, 2003; Morris, St James-Roberts, Sleep, & Gillhamc, 2001). About 25–40% of children experience some type of SP at some point during their development, including insomnia (i.e., difficulty initiating and maintaining sleep), hypersomnia (i.e., excessive sleepiness), and parasomnia (i.e., abnormal behavior or activity during sleep) (Lam, Hiscock, & Wake, 2003; Mindell & Owens, 2003; Owens, 2005). In addition to the high prevalence, SP are linked to many other disorders. For example, SP have strong relations to other child behavioral and emotional problems (Gregory & O’Connor, 2002; Gregory, Van der Ende, Willis, & Verhulst, 2008; Reid, Hong, & Wade, 2009), and are highly comorbid with developmental disorders, such as autism spectrum disorders (Williams, Sears, & Allard, 2004). Furthermore, SP can influence cognitive functioning, such as executive function (Friedman, Corley, Hewitt, & Wright, 2009), working memory (Steenari et al., 2003), verbal creativity and abstract thinking (Randazzo, Muehlbach, Schweitzer, & Walsh, 1998), and therefore are associated with children’s academic achievement (Curcio, Ferrara, & De Gennaro, 2006).
Besides having detrimental influences on children themselves, SP also have an impact on their parents. That is, children’s SP are related to poorer parental mental and physical health, and overall well-being (Bayer, Hiscock, Hampton, & Wake, 2007; Meltzer & Mindell, 2007). Indeed, the remediation of children’s SP has been shown to result in the improvement of family functioning, and parental psychological well-being (Eckerberg, 2004; Hall, Clauson, Carty, Janssen, & Saunders, 2006).
The high prevalence of SP and the impacts of SP on children and their families suggest the need for thorough understanding of the etiology of SP and the factors that contribute to stability and change in SP across age. Knowledge of the mechanisms underlying SP and developmental changes in SP is essential for researchers and clinicians to identify effective interventions, and may also inform about comorbid disorders. To that end, the present study explores genetic and environmental influences on individual differences in SP at ages 2 and 3 years, and on the developmental change in SP across the transition from infancy to early childhood. Understanding the etiology of SP in toddlerhood is especially important given the empirical evidence which demonstrates considerable persistence in SP from early to later childhood and links between early SP and later adjustment (Gregory et al., 2008; Lam et al., 2003; Pollock, 1992, 1994).
Genetic and Environmental Influences on Sleep Problems
Although not well studied, the etiology of individual differences in SP is likely to be multiply determined. To date, most research exploring possible sources of SP in young children has focused on environmental mechanisms with particular attention on parenting practices. Parenting laxness has been related to more SP in children (Owens-Stively et al., 1997). Parental “hardiness” is related to SP in preschool-aged children (Johnson & McMahon, 2008). That is, parents who are more authoritative, have children with fewer SP. Longitudinal analyses suggest that maladaptive parental behaviors after children’s night awakenings (e.g., giving food/drink, cosleeping in mother’s bed, comforting the child out of bed) in toddlers may be associated with continued sleep disturbances in early school-age children (Simard et al., 2008). While these studies do suggest that child SP and parenting are related, they do not account for all of the variance in early SP. Moreover, there is substantial evidence that parenting behaviors are genetically influenced (Ulbricht & Neiderhiser, 2009). These genetic effects on parenting behaviors are child-driven effects in that they represent the genetic contributions of children to their parents’ behavior, and suggest that parents are responding to genetically influenced characteristics of their children (i.e., genotype-environment correlations). For example, it is likely that parents’ behaviors toward their children are, in part, in response to their children’s genetically influenced temperaments or personality (Saudino & Wang, in press). Links between parenting and child SP, therefore, do not rule out the possible genetic contribution to individual differences in SP.
Molecular genetic studies in adults suggest that sleep disorders have a genetic component (Tafti, 2009). Several candidate genes have been identified for specific sleep disorders. For example, human leukocyte antigen (HLA) gene located on chromosome 6 is associated with several sleep disorders such as delayed sleep phase syndrome (Hohjoh et al., 1999), narcolepsy (Ellis et al., 1997), sleep walking (Lecendreux et al., 2003), and REM sleep behavior disorder (Schenck, Garcia-Rill, Segall, Noreen, & Mahowald, 1996).
The molecular genetic studies with adults suggest that genetic influences might be an important aspect of liability toward developing SP in childhood, but research in this area is relatively rare. To our knowledge, there have been no molecular genetic studies of SP in children and only a handful of quantitative genetic twin studies parsing the variance of SP in child into genetic and environmental sources. The two twin studies that explore genetic influences on parent-rated SP in toddlers yield inconsistent findings regarding the relative contributions of genetic and shared environmental effects. Individual differences in SP in 3-year-olds in the Netherlands Twin Registry (NTR) were largely due to genetic influences (Van den Oord, Boomsma, & Verhulst, 2000). Heritability was estimated as 56%, and shared environmental variance as 17%, with the remaining variance due to nonshared environmental influences. By contrast, in the Twins Early Development Study (TEDS), the heritability of SP averaged across 3 and 4 years of age was much lower (21%), and shared environmental factors much higher (72%) (Gregory, Eley, O’Connor, & Plomin, 2004). A possible explanation for the difference in results between the two studies might lie in the measures used to assess SP. In the NTR, parents rated SP on the Child Behavior Checklist (CBCL; Achenbach, 1992). The SP subscale of the CBCL consists of seven items rated on a 3-point scale. Parents in TEDS reported SP on a four-item scale rated “yes” or “no.” It is possible that the TEDS measure with its more limited ratings was less sensitive to differences between twins. Interestingly, different results emerged in a follow-up study using a subsample of TEDS in middle childhood (8–10 years old). SP were assessed with a broader measure (33 items rated on a 3-point scale) and yielded results that were similar to the NTR findings. That is, individual differences in SP were influenced by substantial genetic factors (61–66%), and modest shared environmental factors (4.3–12%) (Gregory, Rijsdijk, Dahl, McGuffin, & Eley, 2006; Gregory, Rijsdijk, Lau, Dahl, & Eley, 2009). Although it is possible that the differences within the TEDS sample reflect changes in factors involved in SP from early childhood to middle childhood, it remains unclear as to the relative contributions of genetic factors and shared environmental influences on individual differences in SP in young children. Therefore, the first goal of the current study was to clarify the magnitude of genetic and shared environmental influences on individual differences in SP of toddlers. We hypothesized that the inconsistency in findings across studies in toddlerhood, and across age in the TEDS sample, reflected measurement issues. That is, the lower heritability for SP in early childhood in the TEDS sample may be due to using a more narrow measure of SP that was less sensitive to individual differences between twins. We therefore predicted that SP as assessed by parent ratings on the CBCL at both ages 2 and 3 years would replicate the findings of the NTR (Van den Oord et al., 2000) for their sample of 3-year-olds (i.e., that familial resemblance in SP would largely be due to genetic influences).
Development of Sleep Problems
There is a misconception that SP are transient (Mindell & Owens, 2003). Although the mean level of SP decreases with age in childhood (Gregory & O’Connor, 2002; Laberge et al., 2001), there is stability in individual differences in SP over time. Research consistently demonstrates that children who have SP in infancy tend to have SP in early and middle childhood (Lam et al., 2003; Pollock, 1992, 1994). Genetic and/or environmental factors may predispose children to have persistent SP (Mindell & Owens, 2003).
To date, only one behavioral genetic study has investigated genetic and environmental contributions to the continuity and change of SP. In TEDS, both genetic and nonshared environmental factors contributed to the continuity and change of SP from ages 8–10 years (Gregory et al., 2009). Forty-six percent of the genetic effects on SP at age 10 years were same as those that operated at age 8 years (i.e., approximately 52% of genetic effects at age 10 years were independent of those at age 8 years). Similarly, 37% of the nonshared environmental influences at age 10 years overlapped with those at age 8 years. The TEDS study provides important information about sources of continuity and change in SP in middle childhood, but no research has explored this question in infancy or early childhood—a period during which there are substantial developmental changes in sleep (Mindell & Owens, 2003). The factors that influence change in one developmental period may not be the same that influence change in another and a second goal of the present study was to address this gap in the literature by exploring the development of SP in early childhood. We hypothesized that SP at ages 2 and 3 years would be genetically influenced and that there would be some overlap in the genetic factors that operate at both ages.
Method
Sample
All procedures were approved by the Boston University Institutional Review Board. Twins were recruited via mail and telephone from the greater Boston area through Boston University Twin Project. Twins were selected preferentially for higher birth weight and gestational age. No twins with birth weights less than 1,750 g or with gestational ages less than 34 weeks were included in the study. After obtaining informed consent from the parents, assessments were made at 2 and 3 years of age. Data were available for 313 same-sex twin pairs at 2 years (144 monozygotic [MZ] and 169 dzygotic [DZ]; age = 2.07 ± .05 years), and 299 pairs at 3 years (138 MZ and 161 DZ; age = 2.99 ± .08 years). No differences in twins’ sex, zygosity, or SP, were observed between families who left the study after 2 years and those who remained participating at age 3 years (sex: χ2 = 2.43, p = .12; zygosity: χ2 = .12, p = .73; SP: t = −1.26, p = .22). Ethnicity was generally representative of the Massachusetts population (85.4% Caucasian, 3.2% Black, 2% Asian, 7.3% Mixed, 2.2% Other). Socioeconomic status according to the Hollingshead Four Factor Index (1975) ranged from low to upper middle class (range = 20.5–66; M = 50.9, SD = 14.1). Zygosity was determined via DNA analyses using DNA obtained from cheek swab samples. In the cases where DNA was not available (n = 3), zygosity was determined using parents’ responses on physical similarity questionnaires which have been shown to be more than 95% accurate when compared to DNA markers (Price, Freeman, Craig, Ebersole, & Plomin, 2000).
Measures
At both ages, SP were assessed on the SP subscale from the Child Behavior Checklist for ages 1.5–5 years (CBCL/1.5–5; Achenbach, 1991). The CBCL has demonstrated good reliability and validity (Achenbach & Rescorla, 2000). It has been shown to differentiate referred children from nonreferred children (Achenbach & Rescorla, 2000) and the SP subscale has shown strong positive correlations with other parent-report measures of SP (e.g., Hall, Scher, Zaidman-Zait, Espezel, & Warnock, 2011). Parents were given the questionnaires when they visited the lab and asked to return those 48 h later at a second visit to the lab. In most cases (94%), the parent completing the questionnaire was the mother. The SP subscale consist of seven items (i.e., “Doesn’t want to sleep alone,” “Has trouble getting to sleep,” “Nightmares,” “Resists going to bed at night,” “Sleeps less than most kids during day and/or night,” “Talks or cries out in sleep,” and “Wakes up often at night”). Parents were asked to indicate on a 3-point scale how well each item described their children’s behavior within the past 2 months (0 = “not true of their child”, 1 = “somewhat or sometimes true”, 2 = “very true or often true”). An overall sleep problem composite score was calculated as the sum of the seven items. The SP scale displayed good internal consistency in our sample (age 2 years: Cronbach’s α = .78; age 3 years: Cronbach’s α = .79).
Statistical Analyses
Data Transformation
Because of deviations from normality, sleep problem scores were rank transformed. Twin correlations can be inflated by variance due to gender, so all scores were residualized for gender effects (McGue & Bouchard, 1984). These residualized scores were used in all behavioral genetic analyses.
Correlational Analyses
Twin intraclass correlations indicating co-twin similarity were calculated using the double-entry method. If MZ twins are more similar than DZ twins, genetic influences are indicated. To evaluate genetic and environmental contributions to phenotypic continuity across age, cross-twin cross-age correlations were calculated. Cross-twin correlations are the essence of a multivariate analysis of covariance. For the present analyses, the cross-twin cross-age correlation involved correlating the score of Twin A for SP at age 2 years with score of Twin B for SP at age 3 years, and vice versa, within the same family. Genetic contributions to the covariance between two ages are implied when the MZ cross-twin correlation is greater than the DZ cross-twin correlation.
Model-Fitting Analyses
Longitudinal genetic models decompose both the variance of a phenotype at each age and the covariances between the phenotypes at multiple ages into additive genetic effects (A), shared environmental effects (C), and nonshared environmental effects (E) (Plomin, 1989). Additive genetic influences refer to the sum of the average effect of all genes that influence a phenotype. Based on the degree of genetic relatedness, the A factors correlate 1.0 and 0.5 for MZ and DZ twins, respectively. The C factors refer to the influence of shared rearing environments on twin resemblance. Because all twins were reared in the same family, shared environments correlate 1.0 for both MZ and DZ twins. Finally, the E factors reflect nonshared environmental influences that are unique to each member of a twin pair, including measurement error.
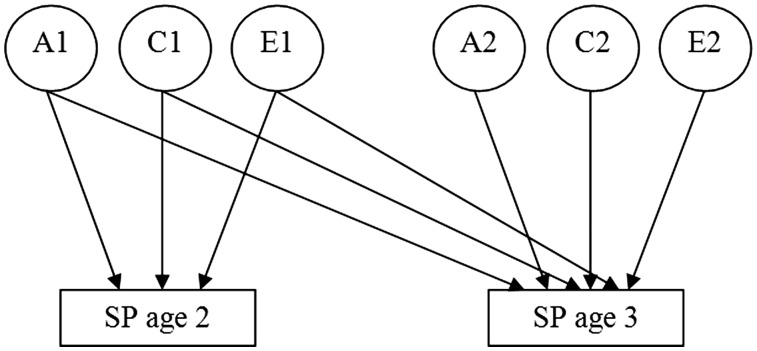
A bivariate Cholesky decomposition model was used to investigate sources of variance in SP within each age, and sources of covariance across age (see Figure 1). The model included two groups of factors: the first group of factors are additive genetic (A1), shared environmental (C1), and nonshared environmental factors (E1) influencing SP at ages 2 and 3 years; the second group of factors (A2, C2, and E2) represent genetic and environmental factors that are unique to SP at age 3 years. Using this model, we can estimate the proportion of variance due to genetic and environmental influences at each age, the genetic and environmental contributions to the phenotypic across-age correlation (i.e., stability), and genetic and environmental correlations across age (i.e., indexing the extent of overlap in genetic and environmental effects across age). We examined two main models: (i) the full ACE model, in which all of the paths were estimated; and (ii) a reduced model, in which the nonsignificant paths in the full model were set at zero.
Figure 1.
Bivariate Cholesky Model. The full model includes additive genetic (A), shared environmental (C), and nonshared environmental (E) factors. SP = sleep problems.
Models were fit to raw data using a maximum likelihood pedigree approach implemented in Mx structural equation modeling software (Neale, Boker, Xie, & Maes, 2003). This approach allows the inclusion of participants with incomplete data. The overall fit of a model can be assessed by calculating the difference between the negative log-likelihood (−2LL) of the model and that of a saturated model (i.e., a model in which the variance/covariance structure is not estimated and all variances and covariances for MZ and DZ twins are estimated). The difference in −2LL is asymptotically distributed as χ2 with degrees of freedom (df) equal to the difference in the number of parameters in the model and that in the saturated model. A reduced model, dropping nonsignificant parameters was compared to a full model in which all genetic and environmental parameters were estimated. The relative fit of the reduced model was determined by the χ2 difference (Δχ2) between full model and the reduced model, and corresponding change in degrees of freedom (Δdf). A nonsignificant change in chi-square between the full and reduced models indicates that the nonsignificant parameters can be eliminated from the model without a decrement in the overall fit of the model. Two additional fit indices, Akakie’s information criterion (AIC; AIC = Δχ2 – 2*Δdf) and the root mean square error of approximation (RMSEA), were used to assess models’ fits (Neale and Cardon, 1992). Negative AIC values indicate good fit of the model to the observed data, and the model that minimizes AIC is a better-fitting model (Akaike, 1987). The RMSEA values should be .05 or less for very good fit, or between .05 and .10 for good fit (Neale et al., 2003).
Results
Descriptive Statistics
Means and standard deviations (SD) of SP by age, gender and zygosity are presented in Table I. For the full sample the average SP score was 1.92 (SD = 2.40, range 0–13) and 2.30 (SD = 2.56, range 0-14) at ages 2 and 3 years, respectively. As compared to the CBCL nonreferred normative sample (M = 2.9, SD = 2.4; Achenbach & Rescorla, 2000), our obtained mean was slightly lower at age 2 years (p < .05), but not at age 3 years (p > .05). More important to our analyses, there was considerable variability in the measure at both ages and our obtained variances were similar to the normative sample. To test mean differences in SP across age, gender and zygosity, the Proc Mixed procedure in SAS was used for linear mixed effect models with repeated measures and unstructured covariance structures. Family number was the group effect and individual twins within a pair represented repeated observations. There were no significant mean differences in SP between ages 2 and 3 years (p > .05). When we included gender and zygosity as covariates in the model, the main effects of age, gender, and zygosity, as well as the interactions among variables were nonsignificant (p > .05).
Table I.
Means (SDs) for Sleep Problems at the Ages of 2 and 3 Years by Sex and Zygosity
| Males |
Females |
Effect size |
|||||
|---|---|---|---|---|---|---|---|
| Age | MZ twins | DZ twins | MZ twins | DZ twins | Age | Sex | Zygosity |
| Age 2 | 1.64 (1.99) | 2.09 (2.71) | 1.84 (2.41) | 2.08 (2.37) | −.15 | .01 | −.19 |
| n | 147 | 183 | 138 | 152 | |||
| Age 3 | 2.14 (2.43) | 2.53 (2.87) | 1.82 (2.01) | 2.62 (2.69) | |||
| n | 138 | 174 | 133 | 145 | |||
Note. Effect size was estimated by Cohen’s d. SP = sleep problems; MZ = monozygotic; DZ = dizygotic.
Correlations
SP at ages 2 and 3 years were highly correlated (r = .55, p < .001), indicating considerable stability in SP in toddlerhood. Twin intraclass correlations and cross-twin cross-age correlations are presented in Table II. For both ages, the intraclass correlations for MZ twins exceeded those for DZ twins, suggesting genetic influences on SP. DZ correlations were slightly higher than half the magnitude of the MZ correlation, suggesting possible shared environmental influence. Cross-twin cross-age correlations for MZ twins were slightly higher than those for DZ twins, so genetic and shared environmental influences may contribute to the phenotypic correlation between SP at ages 2 and 3 years, which can be tested by more powerful multivariate genetic model-fitting analyses.
Table II.
Twin Intraclass Correlations, and Cross-Twin Cross-Age Correlations
| Univariate Intraclass Correlations |
||
|---|---|---|
| Variables | MZ twins | DZ twins |
| SP age 2 | .67 | .40 |
| SP age 3 | .69 | .38 |
| Cross-Twin Cross-Age Correlations | ||
| SP age 2–SP age 3 | .41 | .37 |
Note. All correlations are significant at p < .001. SP = sleep problems; MZ = monozygotic; DZ = dizygotic.
Model-Fitting Analyses
Table III presents the fit statistics for the bivariate models. In the full model all shared environmental parameters were nonsignificant and comparative model fitting showed that the model fit could be improved by dropping all nonsignificant paths without a significant deterioration in fit (p > .05, as indicated by the ‘Relative fit of model’ p-value for the reduced model). Thus, shared environment did not contribute significantly to the observed variability in SP at ages 2 and 3 years, nor did it contribute to the covariance between ages. There were, however, significant additive genetic effects and nonshared environmental effects. The reduced model including genetic and nonshared environmental influences (i.e., the AE model) was the best fitting model in terms of relative fit, AIC, and RMSEA.
Table III.
Fit Statistics for Models of Sleep Problems Across Ages of 2 and 3 years
| Overall fit of modela |
Relative fit of modelb |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| −2LL | df | χ2 | Δdf | p | RMSEA | AIC | Δχ2 | Δdf | p | |
| Saturated model | 2863.395 | 1182 | ||||||||
| Full model | 2886.906 | 1193 | 23.511 | 11 | .015 | .063 | 1.511 | |||
| Reduced model | 2888.992 | 1196 | 25.597 | 14 | .029 | .054 | −2.403 | 2.086 | 3 | .555 |
Note. −2LL = log-likelihood statistic; df = degree of freedom; χ2 = chi-square fit statistic = −2LL difference between a model and the saturated model; AIC = Akaike's information criterion; RMSEA = root mean square error of approximation; Δχ2 = chi-square difference between reduced model and full model. Best fitting model indicated in bold.
aOverall fit of the model is determined by the difference in −2LL (χ2) of each model and that of the saturated model.
bRelative fit of the model determined by the χ2 difference (Δχ2) between the full model and the reduced model.
Parameter estimates are presented both for the full ACE model and the reduced model in Table IV to provide the reader with maximum information. On the basis of the best-fitting model (i.e., reduced model), genetic influence accounted for 69% and 71% of the variance, and nonshared environment 31% and 29% of the variance for SP at ages 2 and 3 years, respectively. This model suggested that the phenotypic correlations between two ages were due to genetic and nonshared environmental effects. The estimated genetic correlation between SP at ages 2 and 3 years was .69, and the nonshared environmental correlation was .23. The genetic correlation accounted for 87.3% of the phenotypic correlation across age, and the remainder was due to overlapping nonshared environmental effects. In addition, there was significant independent genetic variance for SP at age 3 years, indicating new age-specific genetic factors. Based on the reduced model, 52% of the genetic influences on SP at age 3 years are independent of genetic effects at age 2 years. The cross-age stability effects for nonshared environmental factors were more modest. Only a small proportion (i.e., 5%) of nonshared environmental influences at age 3 years was common to age 2 years.
Table IV.
Estimates of Genetic and Environmental Variances and Covariances (95% CI) From the Full Model and Reduced Model
| Full Model |
Reduced Model |
|||||
|---|---|---|---|---|---|---|
| Variance Within Age | h2 | c2 | e2 | h2 | c2 | e2 |
| SP age 2 | .55 (.32 to.74) | .14 (.00 to .33) | .31 (.24 to .41) | .69 (.60 to .76) | – | .31 (.24 to .40) |
| SP age 3 | .57 (.36 to .76) | .13 (.00 to .32) | .29 (.23 to .39) | .71 (.62 to .78) | – | .29 (.22 to .38) |
| Covariance across age | rg | rc | re | rg | rc | re |
| SP age 2 —SP age 3 | .62 (.40 to .76) | 1.00 (−1.00 to 1.00) | .25 (.08 to .41) | .69 (.58 to .79) | – | .23 (.06 to .38) |
Note. SP = sleep problems; h2 = genetic variance; c2 = shared environmental variance; e2 = nonshared environmental variance; CI = confidence interval. rg, rc, and re denote the genetic, shared-environmental, and nonshared environmental correlations, respectively.
Discussion
This was the first study to investigate the genetic and environmental etiology underling the development of SP from ages 2–3 years. SP in toddlerhood, as rated by parents, show substantial heritability and moderate nonshared environmental influence. In our sample, shared environmental influences were modest and not significant. The stability from ages 2 to 3 years is largely due to genetic factors common to both ages although there were some modest nonshared environmental effects that persisted across age. Both new genetic and nonshared environmental influences emerged at age 3 years thus contributed to rank-order change in SP from 2 to 3 years.
The present findings suggest that genetic factors, rather than shared environmental factors, play a more primary role in the etiology of toddlerhood SP. SP as assessed on the CBCL in early childhood are substantially heritable and shared environmental influences are negligible. Our estimates of genetic and environmental variances are remarkably similar to the findings from the NTR study (Van den Oord et al., 2001) that also used the CBCL to assess SP. This replication of the NTR results with the same measure suggests that measure differences likely account for the difference in outcomes between our study, the NTR study, and TEDS early childhood study (Gregory et al., 2004). In TEDS, SP at 4 years of age were assessed using only four items including “hard to get to sleep,” “frequent wakings,” “nightmares,” and “early waking” each rated “yes/no.” Yes/no response formats may inflate co-twin resemblances because they are less sensitive to differences between twins (e.g., within a pair a twin who displays the problem once would get the same rating as a twin who displays the problem multiple times). This methodological problem would apply to both MZ and DZ twins and consequently, could lead to underestimates of heritability and overestimates of shared environmental influences. The CBCL SP scale is broader both in content and response format, and therefore, may be more sensitive to behavioral differences between twins. Of course it is also possible that the additional items on the CBCL tap aspects of SP that are more heritable than the four items on the TEDS measure. Nonetheless, taken together these studies highlight the need to consider the measures used to assess SP. Different measures can yield different results, and replication is essential.
The present finding of genetic effects mainly influencing the stability of SP in toddlerhood is consistent with previous research in middle childhood (Gregory et al., 2009). Almost half of the genetic influences at a later age are same as those at the earlier age. These findings for strong genetic influences on stability of SP might become useful at a practical level if we can identify specific genes that account for this stability. Identifying these specific genes might make it possible to identify children who are at genetic risk for developing of SP and to provide early interventions and prevent problems’ further progressing.
The new genetic effects influencing SP at age 3 years are intriguing and may be related to the rapid brain development from ages 2–3 years. The patterns of sleep have a neurophysiologic base that undergoes maturational change during toddlerhood. For instance, there are shifts in the length of the rapid eye movement (REM) and non-REM sleep (NREM). REM sleep becomes proportionally shorter, and the percentage of NREM sleep increases as children mature (Kahn & Fischer, 1973). The maturation of REM–NREM sleep cycles reflects the maturation of internal central nervous system (Stores, 2001). Many brain areas are engaged in the control of ultradian rhythms of REM–NREM sleep alternation, as well as circadian sleep–wake rhythms (Pace-Schott & Hobson, 2002). SP, such as nightmares occurring in REM sleep, may behaviorally reflect the neurophysiological aspect of sleep. Thus, the development of SP involves neuronal networks which are rapidly developing during toddlerhood. Genetic mechanisms underlie these neuronal networks that are involved in sleep (Pace-Schott & Hobson, 2002). It is possible that some new genes are expressed at developing neuronal-network dynamics at age 3 years, and ultimately exert their effects on behavior such as SP. It is also possible that the new genetic effects reflect different developmental processes influencing SP at age 3 years, rather than new genes being expressed.
Besides intrinsic maturation, several possible nonshared environmental factors are also important for the development of SP. Transitional objects, the inanimate objects such as teddy-bears, dolls, blankets, and pillows, are often used by toddlers to help them fall sleep (Wolf & Lozoff, 1989). Thus using a transitional object may influence whether children have bedtime struggles or sleep-onset problems. Additionally, parent–child relationship can also have an effect on sleep disturbance (Anders, Halpern, & Hua, 1992). Evidence shows that treatment for children with SP focusing on daytime mother–child interactions can improve children’s sleep pattern (Minde, Faucon, & Falkner, 1994). Thus, different parent–child interactions may give rise to individual differences in SP. It is possible that parents’ interactions with their children have some changes across time that lead to change of children’s SP. Notably, parent–child interactions may not be a pure environmental factor, but can be influenced by genetic factors (Deater-Deckard & O'Connor, 2000). Children’s genes may predispose them to expose to certain parent–child interactions. Future research can examine whether relations between parent–child interactions and SP are due to same genetic factors having effects on both of them. Furthermore, some unexpected physical illness may cause SP, such as gastrointestinal disorders, allergies, atopic dermatitis, asthma, seizures, migraine headache, and chronic pain (Mindell & Owens, 2003), which may only appear at a certain age, and constitute the age-specific nonshared environmental influences on SP.
The few behavioral genetic studies of SP have all relied on parental reports, and thus the accuracy of information may be limited. For example, parents may judge how many night wakening children have based on different criteria (e.g., three night awakenings may be a lot for some parents, but not for the others). Similarly, parents may be not clearly aware of how many total sleep hours that children need, and thus may over-report or under-report on less sleep for their children. When considering how long their children sleep, some parents may think only about when their children get up, but ignore when their children go to bed (Owens & Burnham, 2009). Moreover, parents may not notice children’s night awakenings if they are not disturbed, leading to under-reports of awakenings. It is also possible that the seven items in SP subscale of the CBCL do not capture all facets of SP. For example, there is no item for hypersomnia. Consequently, our findings may be interpreted with caution in case of hypersomnia. It should be noted, however, that the CBCL provides the most extensive measure of SP in behavior genetic studies to date. Our use of the CBCL allows us to replicate and extend earlier research. Nonetheless, the limitations of subjective parental reports highlight the need for using objective measures, such as actigraphy, and polysomnography, in future studies. Careful consideration of the definition of the phenotype is also required.
Our findings are consistent with prior research indicating that SP in a nonclinical population are genetically influenced, but it remains a question as to whether these findings will generalize to clinical populations. It is possible that different etiological factors underlie variation in extreme and normal ranges of SP. Research with clinical samples is needed. Nonetheless, given that most children fall within the nonclinical range, our findings explaining variation in SP in a normative sample will have relevance to those interested in understanding individual differences in normal development. Additionally, brain maturation during childhood and possible age-related differences in the quality and quantity of SP suggest that the present results may not apply to other ages. It will be helpful for future studies to examine the relative roles of genetic and/or environmental factors in SP and developmental change in SP in other age groups—especially across the transition from early to middle childhood, an age group for which there has been no behavioral genetic studies of SP.
Although quantitative behavioral genetic analyses cannot inform about specific types of interventions, they can indicate the types of environments (shared or nonshared) that influence the behaviors understudy and hence may yield broad avenues for intervention. Our findings show that shared environments (i.e., those that are family-wide) are not significantly associated with variation in SP, therefore specific family-wide effects are unlikely to explain why some children experience SP and others do not. The environments that do influence SP and developmental change in SP are those that are unique to each member of a family (i.e., experiences that are not shared between twins or siblings). Consequently, researchers and clinicians should consider these types of environmental influences (e.g., focus on such things as differential experiences, accidents, illnesses, etc.) as explanatory mechanisms for SP, and program interventions accordingly.
In conclusion, we confirm the major contribution of genetic factors to SP in toddlerhood when using the Sleep Problems subscale from the CBCL. We also extend our knowledge of the stability of SP in toddlerhood which is mainly due to genetic effects. That is, particular sets of genes influence SP across age. Despite this, there are some genetic factors that have age-specific influences. Unique environmental effects are also important for SP within each age, but show less cross-age stability. Our findings have implications for both research and clinical practice. For researchers, the findings indicate that identifying the specific genetic loci relevant to SP across time can be a focus of attention in future to advance our knowledge on the stability of SP across time. For clinicians, although the findings suggest that the persistence of SP in toddlerhood is largely due to genetic factors, change is due to both genes and nonshared environments. The finding of nonshared environmental influences on change in SP suggests that the most efficacious targets for intervention should focus on the types of environmental influences/experiences that are specific to each child within the family rather than family-wide environmental influences.
Funding
The Boston University Twin Project (BUTP) is supported by the National Institute of Mental Health (MH062375).
Conflicts of interest: None declared.
References
- Achenbach T M. Manual for the Child Behavior Checklist and 1991 Profile. Burlington, VT: University of Vermont; 1991. [Google Scholar]
- Achenbach T M. Manual for the Child Behavior Checklist/2-3 and 1992 Profile. Burlington, VT: University of Vermont; 1992. [Google Scholar]
- Achenbach T M, Rescorla L A. Manual for the ASEBA Preschool Forms and Profiles. Burlington, VT: University of Vermont, Research Center for Children, Youth, and Families; 2000. [Google Scholar]
- Anders T F, Halpern L F, Hua J. Sleeping through the night: A developmental perspective. Pediatrics. 1992;90:554–560. [PubMed] [Google Scholar]
- Bayer J K, Hiscock H, Hampton A, Wake M. Sleep problems in young infants and maternal mental and physical health. Journal of Pediatrics and Child Health. 2007;43:66–63. doi: 10.1111/j.1440-1754.2007.01005.x. [DOI] [PubMed] [Google Scholar]
- Curcio G, Ferrara M, De Gennaro L. Sleep loss, learning capacity and academic performance. Sleep Medicine Reviews. 2006;10:323–337. doi: 10.1016/j.smrv.2005.11.001. [DOI] [PubMed] [Google Scholar]
- Deater-Deckard K, O'Connor T G. Parent-child mutuality in early childhood: two behavioral genetic studies. Developmental Psychology. 2000;36:561–570. doi: 10.1037/0012-1649.36.5.561. [DOI] [PubMed] [Google Scholar]
- Eckerberg B. Treatment of sleep problems in families with young children: Effects of treatment on family well-being. Acta Paediatrica. 2004;93:126–134. doi: 10.1080/08035250310007754. [DOI] [PubMed] [Google Scholar]
- Ellis M C, Hetisimer A H, Ruddy D A, Hansen S L, Kronmal G S, McClelland E, Quintana L, Drayna D T, Aldrich M S, Mignot E. HLA class II haplotype and sequence analysis support a role for DQ in narcolepsy. Immunogenetics. 1997;46:410–417. doi: 10.1007/s002510050295. [DOI] [PubMed] [Google Scholar]
- Friedman N P, Corley R P, Hewitt J K, Wright K P. Individual differences in childhood sleep problems predict later cognitive executive control. Sleep. 2009;32:323–333. doi: 10.1093/sleep/32.3.323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gregory A M, Eley T C, O'Connor T G, Plomin R. Etiologies of associations between childhood sleep and behavioral problems in a large twin sample. Journal of the American Academy of Child and Adolescent Psychiatry. 2004;43:744–751. doi: 10.1097/01.chi/0000122798.47863.a5. [DOI] [PubMed] [Google Scholar]
- Gregory A M, O'Connor T G. Sleep problems in childhood: A longitudinal study of developmental change and association with behavioral problems. Journal of the American Academy of Child and Adolescent Psychiatry. 2002;41:964–971. doi: 10.1097/00004583-200208000-00015. [DOI] [PubMed] [Google Scholar]
- Gregory A M, Rijsdijk F V, Dahl R E, McGuffin P, Eley T C. Associations between sleep problems, anxiety, and depression in twins at 8 years of age. Pediatrics. 2006;118:1124–1132. doi: 10.1542/peds.2005-3118. [DOI] [PubMed] [Google Scholar]
- Gregory A M, Rijsdijk F V, Lau J F, Dahl R E, Eley T C. The direction of longitudinal associations between sleep problems and depression symptoms: a study of twins aged 8 and 10 years. Sleep. 2009;32:189–199. doi: 10.1093/sleep/32.2.189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gregory A M, Van der Ende J, Willis T A, Verhulst F C. Parent-reported sleep problems during development predict self-reported anxiety/ depression, attention problems and aggressive behavior later in life. Archives of Pediatrics and Adolescent Medicine. 2008;162:330–335. doi: 10.1001/archpedi.162.4.330. [DOI] [PubMed] [Google Scholar]
- Hall W A, Clauson M, Carty E M, Janssen P A, Saunders R A. Effects on parents of an intervention to resolve infant behavioral sleep problems. Pediatric Nursing. 2006;32:243–250. [PubMed] [Google Scholar]
- Hall W A, Scher A, Zaidman-Zait A, Espezel H, Warnock F. A community-based study of sleep and behaviour problems in 12- to 36-month-old children. Child: Care, Health and Development. 2011 doi: 10.1111/j.1365-2214.2011.01252.x. doi: 10.1111/j.1365-2214.2011.01252.x. [DOI] [PubMed] [Google Scholar]
- Hohjoh H, Takahashi Y, Hatta Y, Tanaka H, Akaza T, Tokunaga K, Honda Y, Juji T. Possible association of human leucocyte antigen DR1 with delayed sleep phase syndrome. Psychiatry and Clinical Neurosciences. 1999;53:527–529. doi: 10.1046/j.1440-1819.1999.00574.x. [DOI] [PubMed] [Google Scholar]
- Hollingshead A B. The four-factor index of social status. 1975. Unpublished manuscript, Yale University, New Haven, CT. [Google Scholar]
- Johnson N, McMahon C. Preschoolers sleep behavior: Associations with parental hardiness, sleep-related cognitions and bedtime interactions. Journal of Child Psychology and Psychiatry. 2008;49:765–773. doi: 10.1111/j.1469-7610.2007.01871.x. [DOI] [PubMed] [Google Scholar]
- Kahn E, Fischer C. 24-Hour sleep patterns. A comparison between 2- to 3-year-old and 4- to 6-year-old children. Archives of General Psychiatry. 1973;29:380–385. doi: 10.1001/archpsyc.1973.04200030068010. [DOI] [PubMed] [Google Scholar]
- Laberge L, Petit D, Simard C, Vitaro F, Tremblay R E, Montplaisir J. Development of sleep patterns in early adolescence. Journal of Sleep Research. 2001;10:59–67. doi: 10.1046/j.1365-2869.2001.00242.x. [DOI] [PubMed] [Google Scholar]
- Lam P, Hiscock H, Wake M. Outcomes of infant sleep problems: A longitudinal study of sleep, behavior, and maternal well-being. Pediatrics. 2003;111:203–207. doi: 10.1542/peds.111.3.e203. [DOI] [PubMed] [Google Scholar]
- Lecendreux M, Bassetti C, Dauvilliers Y, Mayer G, Neidhart E, Tafti M. HLA and genetic susceptibility to sleepwalking. Molecular Psychiatry. 2003;8:114–117. doi: 10.1038/sj.mp.4001203. [DOI] [PubMed] [Google Scholar]
- McGue M, Bouchard T J., Jr Adjustment of twin data for the effects of age and sex. Behavior Genetics. 1984;14:325–343. doi: 10.1007/BF01080045. [DOI] [PubMed] [Google Scholar]
- Meltzer L J, Mindell J A. Relationship between child sleep disturbances and maternal sleep, mood, and parenting stress: A pilot study. Journal of Family Psychology. 2007;21:67–73. doi: 10.1037/0893-3200.21.1.67. [DOI] [PubMed] [Google Scholar]
- Minde K, Faucon A, Falkner S. Sleep problems in toddlers: Effects of treatment on their daytime behavior. Journal of the American Academy of Child & Adolescent Psychiatry. 1994;33:1114–1121. doi: 10.1097/00004583-199410000-00007. [DOI] [PubMed] [Google Scholar]
- Mindell J A, Owens J A. A clinical guide to pediatric sleep. Philadelphia, PA: Lippincott, Williams & Wilkins; 2003. [Google Scholar]
- Morris S, St. James-Roberts I, Sleep J, Gillham P. Economic evaluation of strategies for managing crying and sleeping problems. Archives of Disease in Childhood. 2001;84:15–19. doi: 10.1136/adc.84.1.15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neale M C, Boker S M, Xie G, Maes H H. Mx: Statistical modeling. 6th ed. Richmond, VA: Department of Psychiatry; 2003. [Google Scholar]
- Neale M C, Cardon L R. Methodology for genetic studies of twins and families. Dordrecht, NL: Kluwer Academic Publishers; 1992. [Google Scholar]
- Owens J A. Epidemiology of sleep disorders during childhood. In: Sheldon S H, Ferber R, Kryger M H, editors. Principles and practices of pediatric sleep medicine. Philadelphia, PA: Elsevier Health Sciences; 2005. pp. 27–34. [Google Scholar]
- Owens J A, Burnham M M. Sleep disorders. In: Zeanah C H, editor. Handbook of infant mental health. New York: Guilford; 2009. pp. 362–376. [Google Scholar]
- Owens-Stively J, Frank N, Smith A, Hagino O, Spirito A, Arrigan M, Alario A J. Child temperament, parenting discipline style, and daytime behavior in childhood sleep disorders. Journal of Developmental & Behavioral Pediatrics. 1997;18:314–321. doi: 10.1097/00004703-199710000-00005. [DOI] [PubMed] [Google Scholar]
- Pace-Schott E F, Hobson J A. The neurobiology of sleep: Genetics, cellular physiology and subcortical networks. Nature Reviews Neuroscience. 2002;3:591–605. doi: 10.1038/nrn895. [DOI] [PubMed] [Google Scholar]
- Plomin R. Developmental behavioral genetics: Stability and instability. In: Bornstein M H, Krasnegor N A, editors. Stability and continuity in mental development. Hillsdale, NJ: Lawrence Erlbaum Associates; 1989. pp. 273–291. [Google Scholar]
- Pollock J I. Predictors and long-term associations of reported sleeping difficulties in infancy. Journal of Reproductive and Infant Psychology. 1992;10:151–168. [Google Scholar]
- Pollock J I. Night-waking at five years of age: Predictors and prognosis. Journal of Child Psychology and Psychiatry and Allied Disciplines. 1994;35:699–708. doi: 10.1111/j.1469-7610.1994.tb01215.x. [DOI] [PubMed] [Google Scholar]
- Price T S, Freeman B, Craig I, Ebersole L, Plomin R. Infant zygosity can be assigned by parent questionnaire data. Twin Research. 2000;3:129–133. doi: 10.1375/136905200320565391. [DOI] [PubMed] [Google Scholar]
- Randazzo A C, Muehlbach M J, Schweitzer P K, Walsh J K. Cognitive function following acute sleep restriction in children ages 10-14. Sleep. 1998;21:861–868. [PubMed] [Google Scholar]
- Reid G J, Hong R Y, Wade T J. The relation between common sleep problems and emotional and behavioral problems among 2- and 3-year-olds in the context of known risk factors for psychopathology. Journal of Sleep Research. 2009;18:49–59. doi: 10.1111/j.1365-2869.2008.00692.x. [DOI] [PubMed] [Google Scholar]
- Saudino K J, Wang M. Quantitative and molecular genetic studies of temperament. In: Zentner M, Shiner R, editors. Handbook of temperament. New York: Guilford Press; in press. [Google Scholar]
- Schenck C H, Garcia-Rill E, Segall M, Noreen H, Mahowald M W. HLA class II genes associated with REM sleep behavior disorder. Annals of Neurology. 1996;39:261–263. doi: 10.1002/ana.410390216. [DOI] [PubMed] [Google Scholar]
- Simard V, Nielsen T A, Tremblay R E, Boivin M, Montplaisir J Y. Longitudinal study of preschool sleep disturbance: The predictive role of maladaptive parental behaviors, early sleep problems, and child/mother psychological factors. Archives of Pediatrics & Adolescent Medicine. 2008;162:360–367. doi: 10.1001/archpedi.162.4.360. [DOI] [PubMed] [Google Scholar]
- Steenari M R, Vuontela V, Paavonen E J, Carlson S, Fjallberg M, Aronen E. Working memory and sleep in 6-13 year old school children. Journal of the American Academy of Child and Adolescent Psychiatry. 2003;42:85–92. doi: 10.1097/00004583-200301000-00014. [DOI] [PubMed] [Google Scholar]
- Stores G. A clinical guide to sleep disorders in children and adolescents. Cambridge: Cambridge University Press; 2001. [Google Scholar]
- Tafti M. Genetic aspects of normal and disturbed sleep. Sleep Medicine. 2009;10:S17–S21. doi: 10.1016/j.sleep.2009.07.002. [DOI] [PubMed] [Google Scholar]
- Ulbricht J A, Neiderhiser J M. Genotype-environment correlation and family relationships. In: Kim Y K, editor. Handbook of behavior genetics. New York: Springer; 2009. [Google Scholar]
- Van den Oord E J C G, Boomsma D I, Verhulst F C. A study of genetic and environmental effects on the co-occurrence of problem behaviors in three-year old twins. Journal of Abnormal Psychology. 2000;109:360–372. doi: 10.1037/0021-843X.109.3.360. [DOI] [PubMed] [Google Scholar]
- Williams P G, Sears L L, Allard A. Sleep problems in children with autism. Journal of Sleep Research. 2004;13:265–268. doi: 10.1111/j.1365-2869.2004.00405.x. [DOI] [PubMed] [Google Scholar]
- Wolf A W, Lozoff B. Object attachment, thumb-sucking and the passage to sleep. Journal of the American Academy of Child and Adolescent Psychiatry. 1989;28:287–292. doi: 10.1097/00004583-198903000-00024. [DOI] [PubMed] [Google Scholar]