Abstract
Alzheimer’s disease is commonly regarded as a loss of memory for past events. However, patients with Alzheimer’s disease seem not only to forget events but also to express false confidence in remembering events that have never happened. How and why false recognition occurs in such patients is currently unknown, and treatments targeting this specific mnemonic abnormality have not been attempted. Here, we used a modified object recognition paradigm to show that the tgCRND8 mouse—which overexpresses amyloid β and develops amyloid plaques similar to those in the brains of patients with Alzheimer’s disease—exhibits false recognition. Furthermore, we found that false recognition did not occur when tgCRND8 mice were kept in a dark, quiet chamber during the delay, paralleling previous findings in patients with mild cognitive impairment, which is often considered to be prodromal Alzheimer’s disease. Additionally, false recognition did not occur when mice were treated with the partial N-methyl-d-aspartic acid receptor antagonist memantine. In a subsequent experiment, we found abnormally enhanced N-methyl-d-aspartic acid receptor-dependent long-term depression in these mice, which could be normalized by treatment with memantine. We suggest that Alzheimer’s disease typical amyloid β pathology leads to aberrant synaptic plasticity, thereby making memory representations more susceptible to interfering sensory input, thus increasing the likelihood of false recognition. Parallels between these findings and those from the literature on Alzheimer’s disease and mild cognitive impairment suggest a mechanism underlying false recognition in these patients. The false recognition phenomenon may provide a novel paradigm for the discovery of potential therapies to treat the mnemonic dysfunction characteristic of this disease.
Keywords: Alzheimer’s disease, false recognition, amyloid β, object recognition, mouse
Introduction
Alzheimer’s disease is a progressive, neurodegenerative disease that is associated with an impairment in memory. While memory distortions are usually thought to be due to a failure to encode or recall specific events, a number of studies have shown that patients with Alzheimer’s disease or its prodromal condition, mild cognitive impairment, also express false memories for events they have never experienced (Hart et al., 1985; Budson et al., 2000; Gold et al., 2007; Hildebrandt et al., 2009; Plancher et al., 2009; Abe et al., 2011). How and why false memories occur in such patients is currently unknown, and the majority of clinical trials do not consider this specific mnemonic abnormality when assessing potential new treatments (Budson et al., 2002).
In this study, we first investigated whether elevated amyloid β levels can lead to false recognition. We tested a mouse model of Alzheimer’s disease-typical amyloid β pathology—the tgCRND8 mouse, which overexpresses amyloid precursor protein with the Swedish and Indiana mutations and develops amyloid plaques like those in the brains of patients with Alzheimer’s disease (Chishti et al., 2001)—on a modified version of the spontaneous object recognition paradigm (McTighe et al., 2010) (Fig. 1). In this ‘decoupled’ object recognition procedure, animals are allowed to explore an object during a study phase, and then, after a short delay, are allowed to explore the studied object or a novel object in a test phase. Normal rats and mice spend more time exploring a novel object than a repeated object. Crucially, this version of the recognition memory test allowed us to distinguish between false recognition, which is reflected in the reduced exploration of a novel object relative to controls (they erroneously think they have seen it before), and the loss or inaccessibility of information from memory, reflected in the enhanced exploration of the already-studied object relative to controls (they erroneously think they have not seen it before). The behaviour of the tgCRND8 mice clearly conformed to the former, and not the latter pattern, indicating false recognition.
Figure 1.
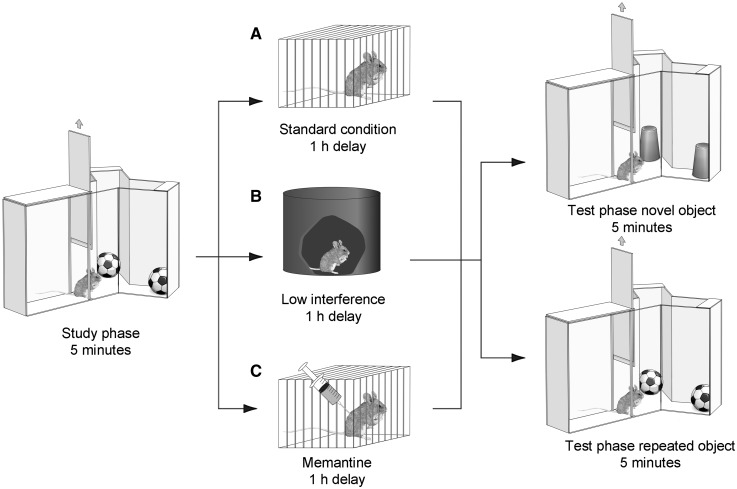
A modified version of the spontaneous object recognition task, in which exploration of the repeated and novel object is decoupled. All animals received a study exposure to two copies of object A for 5 min. After a delay of 1 h, animals received a test exposure of 5 min to either two copies of a novel object, object B (novel condition) or to two new copies of object A (repeated condition). During the delay, animals were put either (A) into an individual holding cage (standard condition) or (B) into a visually restricted environment (reduced interference condition). (C) A second cohort of animals were treated with the NMDA receptor antagonist memantine immediately after the study phase and then kept under standard conditions during the delay.
To test our hypothesis that this false recognition effect was due to enhanced encoding of interfering information, we tested performance of the mice under conditions of sensory restriction during the delay. It has previously been shown that a period in a dark quiet room, i.e. sensory restriction, enhanced recognition in participants with mild cognitive impairment, thought to be a prodromal stage of Alzheimer’s disease (Della Sala et al., 2005). This treatment restored performance of tgCRND8 mice to normal levels, indicating that interference is a likely mechanism underlying the false recognition effect. In a subsequent experiment, we treated mice with the partial N-methyl-d-aspartic acid (NMDA) receptor antagonist memantine during the delay. Interestingly, this treatment also restored performance of tgCRND8 mice to normal levels. Finally, long-term depression of synaptic transmission in the perirhinal cortex is thought to be a critical mechanism underlying recognition memory (Cho et al., 2000; Griffiths et al., 2008), and amyloid β leads to abnormally high levels of NMDA receptor long-term depression (Kim et al., 2001; Cheng et al., 2009). We therefore hypothesized that aberrant synaptic plasticity might comprise at least part of the mechanism underlying false recognition in the tgCRND8 mice. Indeed, we found enhanced NMDA receptor-dependent long-term depression in slices taken from these mice which, like the false recognition in the mice, could be normalized by treatment with memantine. Taken together, the behavioural and electrophysiological data support the suggestion that tgCRND8 mice demonstrate false recognition as a result of enhanced encoding of interfering information, and that this enhanced susceptibility to interference may be due to altered synaptic plasticity.
Materials and methods
Animals
Heterozygous tgCRND8 mice (Chishti et al., 2001) and wild-type littermates were received from Michael Coleman at the Babraham Institute, Cambridge and housed under standard conditions in groups of two or three on a 12 h light/12 h dark cycle (lights on 07:00). All behavioural testing was conducted during the light phase of the cycle. Food and water were available ad libitum throughout the experiment.
Animals of all cohorts were 8–10 weeks old at the onset of testing, correlating with the occurrence of the first amyloid β plaque deposits in the cortex (Adalbert et al., 2009). All experimentation was conducted in accordance with the UK Animals (Scientific Procedures) Act, 1986.
Object recognition task
Object recognition was conducted as previously described for rats (McTighe et al., 2010), using a Y-shaped apparatus adapted for mice (Bartko et al., 2007b) (Fig. 1). The Y-apparatus had high, homogeneous white walls constructed from Perspex to prevent the mouse from looking out into the room, thereby maximizing attention to the stimuli. All walls were 30 cm high, and each arm was 16 cm in length and 8 cm wide. A lamp illuminated the apparatus, and a white shelf, 50 cm from the top of the apparatus, created a ceiling on which a video camera was mounted to record trials. One arm was used as the start arm, and the other two arms were used to display the objects (randomly shaped junk objects, dimensions ∼10 × 4 × 4 cm). All mice were habituated to the apparatus in two consecutive daily sessions in which they were placed in the start arm and left to explore the empty Y-apparatus for 5 min.
The following task sessions were separated by a minimum of 48 h, which the animals spent under normal holding conditions in their home cages. Task sessions were performed in the morning (light cycle, 09:00–14:00) and consisted of a study phase and a test phase. In the study phase, two identical ‘object A’s’ were placed at the end of each arm. The animal was placed in the start arm and left to explore the objects for 5 min. After a delay of 1 h, which the animal spent either in its home cage (high interference) or in a dark, quiet chamber (low interference; Fig. 1), the procedure was repeated (test phase). However, in the test phase, animals were presented either with two new copies of object A, or two copies of a novel object B. After the test phase, animals were returned to the home cage until the onset of the next task session (at least 48 h later). Each animal received two test sessions for each trial type (repeated versus novel object), and the order of trial types as well as the designated study and novel objects for each pair (A → B versus B → A) were counterbalanced within and across groups.
The time spent exploring objects was assessed from video recordings of the study and test phases. Exploratory bouts were scored using a personal computer running a custom made program written in Visual Basic 6.0 (Microsoft). Times when an animal climbed or sat on an object were not counted. For the test phase, a discrimination score was calculated by dividing the exploration of the novel or repeated objects by the exploration time of the sample object. Therefore, a score of 1 corresponded to equal exploration of study and test object (no discrimination). The mean discrimination score across the two test sessions per condition was calculated for each animal.
Group mean discrimination scores were compared by repeated measures ANOVA with genotype as between-subjects factor, and interference level or drug treatment (sensory restriction/memantine versus standard condition) and trial type (novel versus repeated object) as within subject factors. Where appropriate, simple main effects analysis was performed for individual factors, adjusted after Sidak for multiple comparisons using SPSS 17 (SPSS Inc.; significance level P < 0.05).
Drug administration
Memantine (5 mg/kg) or vehicle (physiological saline) was systemically administered (intraperitoneal injection, counterbalanced across animals in a group and across conditions) immediately following the study phase, 15 min before the study phase or 15 min before the choice phase. The time of application was guided by previous studies reporting significant behavioural effects of acute memantine 15 min after intraperitoneal application in mice (Costa et al., 2008). To avoid accumulation effects, each dosing day was followed by three washout days.
Neuritic plaque histology
Mice (tgCRND8 and wild-type) at 2–3 months of age were sacrificed by overdose of sodium pentobarbital (Dolethal; Vetoquinol UK Ltd.) administered by intraperitoneal injection. The mice were then perfused transcardially with phosphate-buffered saline (pH 7.4) followed by 10% neutral-buffered formalin (pH 7.4). Following perfusion, brains were removed and post-fixed in 10% neutral-buffered formalin followed by cryoprotection in 20% (w/v) sucrose in phosphate-buffered saline. The tissue was then embedded in Jung Tissue Freezing Medium (Leica Microsystems Nussloch GmbH) and stored at −80°C until sectioning. Before sectioning, the tissue was transferred to −20°C to equilibrate for 24 h. Following equilibration, sectioning at 30 µm was performed using a microtome. Sections were collected and stored in phosphate-buffered saline until immunohistochemical processing.
The immunohistochemical protocol used was based on previously published methods (Ly et al., 2011). Briefly, sections were immersed in 0.5% hydrogen peroxide in 0.3% Triton X-100 in phosphate-buffered saline (PBST) for 30 min at room temperature, washed for 10 min in PBST three times and then blocked for 60 min at room temperature in 5% (w/v) non-fat skimmed milk in PBST (PBST-M). Sections were then incubated with 1:2000 biotinylated mouse anti-β amyloid 17–24 (4G8) primary antibody (Covance Inc.) in PBST-M overnight at 4°C. Following incubation, sections were washed in PBST three times for 10 min and then incubated with freshly prepared ABC solution (Vector Laboratories Ltd.) for 30 min. Three further 10 min washes in PBST were performed before the sections were incubated in freshly prepared 3,3′-diaminobenzidene solution (Vector Laboratories Ltd.) until colour development. Sections were then washed in PBST for 10 min a further three times before mounting on Superfrost® Plus microscope slides (Gerhard Menzel GmbH). Slides were allowed to dry overnight before immersion in cresyl violet solution for 60 min at 40°C. Slides were then briefly differentiated in 70% ethanol and dehydrated through an ethanol series before xylene clearing and mounting with Histomount (National Diagnostics). Slides were imaged using a densitometry imaging system (Interfocus Imaging Ltd.) and perirhinal cortex was identified by comparison to the Allen Institute for Brain Science Mouse Brain Reference Atlas (P56 coronal series; Allen Mouse Brain Atlas 2009; Lein et al., 2007).
Electrophysiology
Perirhinal cortex slices were prepared from tgCRND8 and wild-type mice. Experiments were carried out in accordance with the UK Animals Scientific Procedures Act of 1986. Animals were sacrificed by dislocation of the neck and then decapitated. The brain was rapidly removed and placed in ice-cold artificial CSF containing (in mM): 124 NaCl, 3 KCl, 26 NaHCO3, 1.25 NaH2PO4, 2 CaCl2, 1 MgSO4, 10 d-glucose (bubbled with 95% O2/5% CO2). A mid-sagittal section of the brain was made, with tissue removed from both rostral and caudal sections of the section, at ∼45° alignment to the dorsoventral axis. The sections were then fixed, by the caudal end, to a vibrotome stage (VT1000S, Leica). Slices (400 µm) were submerged in artificial CSF (20–25°C) and incubated for ∼1 h. As required, single slices were placed into a submerged recording chamber (27–29°C; flow rate ∼3 ml/min). Extracellular field potentials were recorded in the layer II/III of area 35 of the perirhinal cortex using glass electrodes containing NaCl (3 M). Stimulating electrodes were placed on either side of temporal and entorhinal input of the perirhinal cortex (Cho et al., 2000). Stimuli (constant voltage) were delivered alternately to the two electrodes (each electrode 0.016 Hz). Long-term depression was evoked by low frequency stimulation (1 Hz, 900 pulses). The peak amplitude of the evoked field potential responses was measured and expressed relative to the normalized preconditioning baseline. Data were recorded using an Axopatch 700B amplifier (Axon Instruments). Data were monitored and analysed online and re-analyzed offline using the WinLTP software (http://www.ltp-program.com).
Results
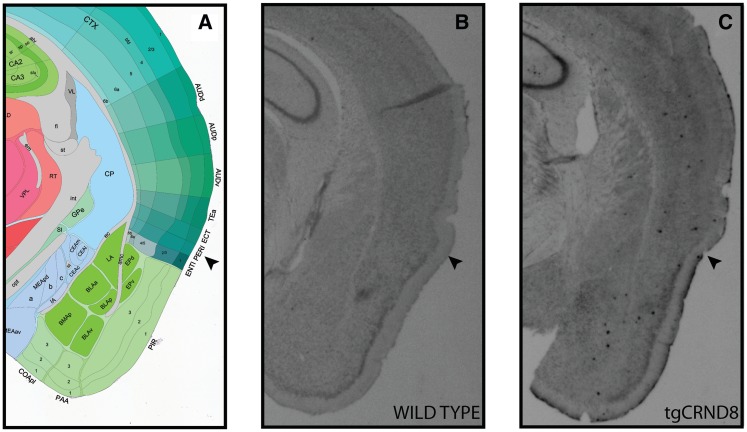
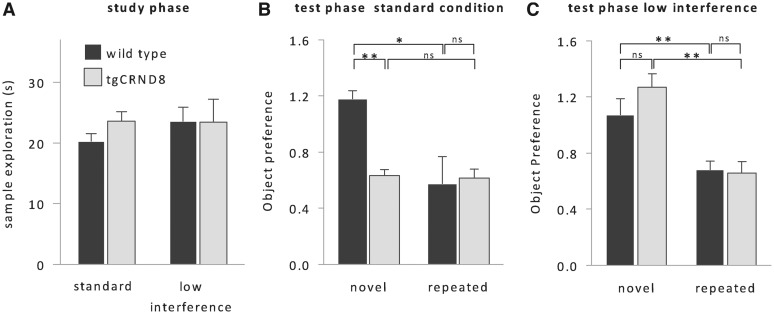
In agreement with previous reports of abundant amyloid β pathology in the cortex of 2- to 3-month-old tgCRND8 mice (Adalbert et al., 2009), immunohistochemical analysis of 2- to 3-month-old tgCRND8 tissue showed plaque-like amyloid β deposits in the perirhinal cortex (Fig. 2), a medial temporal lobe structure necessary for object recognition memory (Meunier et al., 1993; Mumby and Pinel, 1994; Winters et al., 2008). Consistent with previously described recognition memory deficits in mouse models of Alzheimer’s disease (Francis et al., 2012; Greco et al., 2010), tgCRND8 mice did not discriminate between novel and repeated objects and explored both types of objects to a similar degree (Fig. 3A and B; no effect of trial type: F < 1). Crucially, however, we found that the memory impairment was not due to these animals treating the repeated object as novel [i.e. a loss of the memory trace that would be reflected in enhanced exploration of the studied object (Fig. 3B); no main effect of genotype on repeated object exploration, F < 1], but rather was due to these animals treating the novel object as familiar [i.e. false recognition as reflected in the reduced exploration of the novel object; main effect of genotype on novel object exploration: F(1, 16) = 5.1; P < 0.05]. Thus, tgCRND8 mice paradoxically express recognition of objects they have never encountered, suggesting that false recognition is a direct consequence of Alzheimer’s disease typical amyloid β pathology. Importantly, exploration times of objects during the study phases were not significantly different between genotypes (Fig. 3A). Since the intervals between subsequent study phases were longer than the usual object memory retention span of a mouse (<24 h; Sik et al., 2003), this finding suggests that reduced exploration of the novel objects during the test phase was not related to a general, altered response to novelty regardless of mnemonic demands.
Figure 2.
Amyloid β deposits in and around the tgCRND8 perirhinal cortex at 2–3 months of age. (A) Diagrammatic representation of perirhinal cortex (PERI) and surrounding brain regions. Image adapted from: Allen Institute for Brain Science Mouse Reference Atlas (P56 coronal series) (Allen Mouse Brain Atlas; Lein et al., 2007). (B) Immunohistochemical processing of wild-type tissue using 4G8 anti-amyloid 17–24 antibody reveals no evidence of plaque pathology. (C) Plaques present in the perirhinal cortex of the tgCRND8 mouse as detected by the 4G8 antibody. Arrowhead indicates perirhinal cortex region.
Figure 3.
False recognition in tgCRND8 mice. (A) Exploration times during the study phase did not differ between genotypes or levels of visual interference, suggesting the absence of obvious genotype-related behavioural differences that might have influenced the initial object-encoding phase. (B) Under standard conditions, tgCRND8 mice (n = 7) did not discriminate between novel and repeated objects in the test phase and falsely treated novel objects as familiar. (C) If interfering visual input was minimized during the delay, performance of tgCRND8 mice was indistinguishable from performance of wild-type mice (n = 11). Object preference was calculated by dividing test object exploration by study object exploration time. Therefore, a score of 1 corresponded to equal exploration in the study and test phases, whereas a score of 0.5 corresponded to the mouse exploring half as much in the test phase as it had in the study phase [group mean + SEM, repeated measures ANOVA, genotype × interference level × trial type: F(1,16) = 4.3, P < 0.05, simple main effects: **P < 0.01, *P < 0.05]. ns = not significant.
In the next set of experiments, we aimed to investigate whether, as we found in a previous lesion experiment (McTighe et al., 2010), false recognition might be due to increased interference during the delay. In order to reduce sensory interference, we placed the animals in a dark, empty, quiet chamber during the interval between study and test phase (Fig. 1B). Strikingly, tgCRND8 mice kept under sensory restriction no longer falsely recognized novel objects, and like wild-type animals spent more time exploring the novel object than the studied object [Fig. 3A and C; effect of trial type: F(1, 16) = 9.9; P < 0.01; effect of visual interference on novel exploration: F(1,16) = 8.8; P < 0.01]. They performed identically to wild-type mice [no simple main effect of genotype: F(1, 16) = 1.8; P > 0.1], who were unaffected by sensory restriction (no simple main effect of interference level: F < 1). Thus, preventing visual and other sensory input after the study phase completely abolished false recognition, which suggests that amyloid β pathology can heighten susceptibility to interference.
It is thought that a principle mechanism underlying the encoding of object information is NMDA receptor-dependent long-term depression (Cho et al., 2000; Griffiths et al., 2008). As aberrant long-term depression has been observed previously in rodent models of Alzheimer’s disease (Kim et al., 2001; Cheng et al., 2009), we hypothesized that aberrant encoding of interfering information in these mice might be due to aberrant long-term depression. To test this idea, we next applied the low-affinity NMDA-type glutamate receptor antagonist memantine during the delay, immediately after the study phase (Fig. 1C). The time-course of memantine application in this and the following experiments was guided by previous studies reporting significant behavioural effects of acute memantine 15 min after intraperitoneal application in mice (Costa et al., 2008). Although the half-life of memantine in rodents is short (3–5 h; Parsons et al., 1999), each dosing day was followed by three washout days where the animals remained in their home cage.
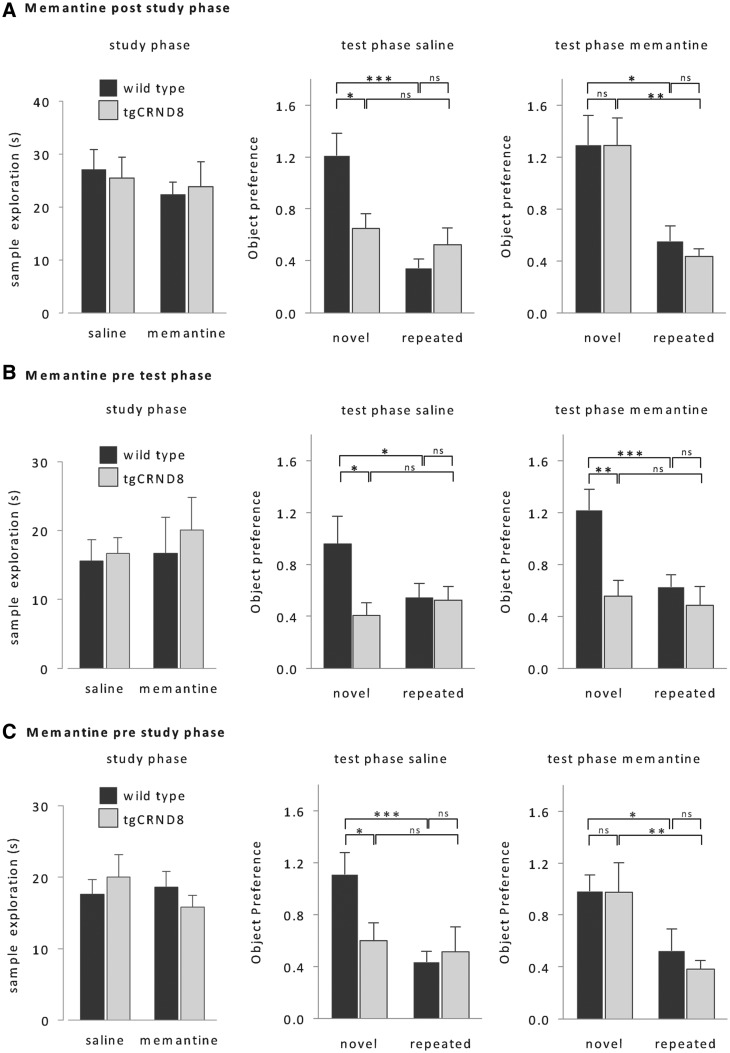
In contrast to saline-treated tgCRND8 mice (no effect of trial type: F < 1), memantine-treated tgCRND8 mice no longer treated novel objects as familiar and spent more time exploring the novel object than the previously studied object [Fig. 4A; effect of memantine on novel object exploration: F(1, 18) = 6.6; P < 0.05, main effect of trial type: F(1, 18) = 10.8; P < 0.01]. Their performance was indistinguishable from wild-type performance (no effect of genotype, F < 1), which was unaffected by the drug (no effect of memantine: F < 2.6; P > 0.1).
Figure 4.
Effect of memantine. (A) Memantine applied immediately after the study phase rescued false recognition in tgCRND8 mice without affecting wild-type performance [wild-type: n = 9, tgCRND8: n = 11, repeated measures ANOVA, genotype × memantine × trial type: F(1,18) = 4.7, P < 0.05; simple main effects: ***P < 0.005, **P < 0.01, *P < 0.05]. (B) Memantine applied only before to the test phase did not enhance tgCRND8 performance [same animals as in A, repeated measures ANOVA, genotype × trial type: F(1,18) = 3.7, P = 0.05; simple main effects: ***P < 0.005, **P < 0.01, *P < 0.05]. (C) Memantine injected before the study phase also rescued tgCRND8 performance, and had no effect on object discrimination performance of wild-type mice [new age matched cohort, wild-type: n = 11, tgCRND8: n = 10; repeated measures ANOVA, genotype × memantine × trial type: F(1,19) = 3.8, P = 0.05; simple main effects: ***P < 0.005, **P < 0.01, *P < 0.05]. Memantine had no effect on exploration times of the study phase (left, repeated measures ANOVA, all F < 1). All data are presented as mean + SEM. ns = not significant.
However, it is possible that the action of memantine could have persisted throughout the retrieval phase and thus, rather than preventing enhanced plasticity during the delay, may have somehow facilitated retrieval operations. To test this possibility, we next injected the drug prior to the test phase. Pretest phase memantine did not affect the recognition memory performance of tgCRND8 or control mice (Fig. 4B; no effect of memantine or interactions involving memantine: all F < 1.4; P > 0.1). Neither memantine-treated nor saline-treated tgCRND8 mice discriminated between novel and repeated objects (no effect of trial type or interactions involving trial type, all F < 1), and spent significantly less time exploring the novel object than wild-type controls. Thus, the reduction of false recognition by post-study phase memantine is not due to facilitated memory retrieval during the test phase.
Finally, we addressed whether memantine treatment not only prevented erroneous memories in tgCRND8 mice, but would also affect the normal formation of representations of salient novel objects. We administered memantine prior to the study phase, which had no effect on object exploration times during the study phase in either genotype (Fig. 4C). Prestudy memantine treatment also had no effect on object recognition in wild-type mice [Fig. 4C; no effect of memantine: F < 1; effect of trial type: F(1,19) = 4.8; P < 0.05]. In tgCRND8 mice, the effect of prestudy memantine was comparable to the effects of memantine administered after the study phase [Fig. 4A and C, simple main effect of memantine on novel object exploration: F(1,19) = 5.2; P < 0.05; simple main effect of trial type: F(1,19) = 8.6; P < 0.01]. Therefore, memantine did not abolish the encoding of representations of salient novel objects either in wild-type or in tgCRND8 mice. The implication is that whereas memantine can block the encoding of interfering incidental familiar object information, it is insufficient to block encoding of a salient novel object.
These behavioural–pharmacological findings with memantine are consistent with the idea that amyloid β pathology alters NMDA receptor-dependent synaptic plasticity such as long-term depression and that this altered synaptic plasticity leads to aberrant encoding of interfering information leading to false recognition. To test the plausibility of this mechanism at the cellular level, we made extracellular field recordings in perirhinal cortex slices from 10-week-old wild-type and tgCRND8 mice. We delivered a low-frequency long-term depression–induction protocol, which is known to induce reliable, NMDAR-dependent long-term depression in juvenile mice, but commonly fails to induce long-term depression in adult animals (low frequency stimulation: 1 Hz stimulation, 900 pulses; Kemp and Bashir, 2001; Massey et al., 2004; Massey and Bashir, 2007). Indeed, low frequency stimulation was unable to induce long-term depression in the wild-type mice (97 ± 2%, n = 6; Fig. 5A) but interestingly long-term depression was induced in tgCRND8 mice (83 ± 3%, n = 6; Fig. 5B), indicative of aberrant, more sensitive induction mechanisms of NMDAR long-term depression in these mice. Memantine treatment blocked the increased long-term depression in the tgCRND8 mice (97 ± 4%, n = 5; Fig. 5C). The parallel, normalizing actions of memantine on object recognition and perirhinal NMDA receptor-long-term depression in tgCRND8 mice strongly support the idea that false recognition and aberrant long-term depression in perirhinal cortex may be related, although causality cannot be assumed.
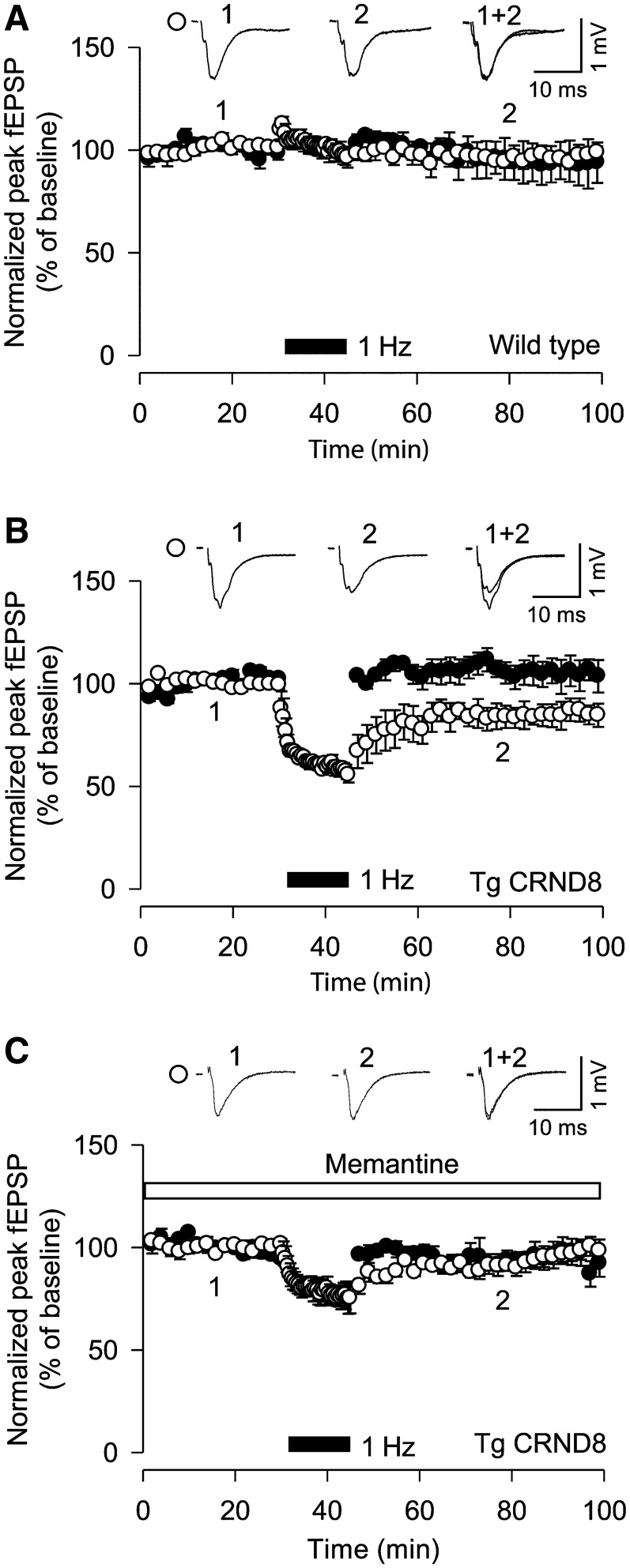
Figure 5.
Memantine prevents long-term depression in tgCRND8 mice. (A) Low frequency stimulation did not induce long-term depression in wild-type mice (n = 6). (B) Low frequency stimulation induced long-term depression in tgCRND8 mice (n = 6). (C) Treatment with memantine prevented the induction of long-term depression in tgCRND8 mice (n = 5). fEPSP = field excitatory postsynaptic potential.
Discussion
The general aim of the present study was to attempt to shed light on the recognition memory impairments present in Alzheimer’s disease and in preclinical models of Alzheimer’s disease. A major finding was that tgCRND8 mice, which model amyloid pathology in Alzheimer’s disease, are susceptible to false recognition. That is, these animals did not forget that they had encountered an object before, but instead falsely treated novel objects as if they had encountered them in the past, a pattern consistent with that seen in human patients with Alzheimer’s disease and mild cognitive impairment (Budson et al., 2003; Hildebrandt et al., 2009; Plancher et al., 2009), and with other amnestic perturbations in experimental animal models (Burke et al., 2010; McTighe et al., 2010).
To test our hypothesis that the false recognition effect was due to enhanced encoding of interfering information, we examined performance of the mice under conditions of sensory restriction during the delay (retention) interval. This treatment abolished the false recognition effect. Importantly, this treatment was effective when applied after the study phase, which suggests that Alzheimer’s disease-typical amyloid β pathology does not prevent the encoding of salient novel objects (because recognition memory under sensory restriction conditions was preserved), but instead increases susceptibility to interference. Internal controls, such as the normal behaviour of tgCRND8 mice on repeated object trials, indicate that these results are not due to gross changes in perceptual ability or exploration of objects. In addition, although tgCRND8 mice treated novel objects as familiar when followed by a study object presented 1 h earlier, they showed normal novel object exploration during the study phase (see further discussion below). This finding shows that tgCRND8 mice do not have any difficulty judging novelty per se. Instead, they appear to confuse the memory of the study object with the novel object, resulting in false recognition.
The pattern of false recognition and rescue with sensory restriction is similar to that observed with rats with lesions of the perirhinal cortex (McTighe et al., 2010; see also false recognition in ageing rats; Burke et al., 2010; cf. Albasser et al., 2011; however, these authors did not observe any impairment using a decoupled procedure following perirhinal lesions at a very short delay). Like tgCRND8 mice, perirhinal cortex-lesioned rats also falsely recognized novel objects, and false recognition could be prevented by sensory restriction after the study phase (McTighe et al., 2010). We previously concluded that in order to judge familiarity, animals with perirhinal cortex lesions cannot use the complex conjunctive representations housed within this region, and instead must rely on more basic, less complex visual features encoded in areas upstream of perirhinal cortex. Therefore, basic visual features common to many complex visual stimuli, which would be encountered during the delay, may provide a false signal of familiarity for the novel object (thus we have referred to such interference as ‘feature ambiguity’; for a more detailed account of this proposed mechanism, see Cowell et al., 2006; McTighe et al., 2010). The clear parallel with the findings described here suggests that dysfunctional perirhinal cortex or related structures may be responsible for false recognition in tgCRND8 mice, consistent with the observation that the perirhinal cortex of the tgCRND8 mouse contains amyloid deposits at 2–3 months of age.
It might at first seem that the differential treatment of novel objects by tgCRND8 mice in the test phase and the study phase is inconsistent: if the mechanism is increased interference as we suggest, then why is there not a similar amount of interference present prior to presentation of the study object, leading to tgCRND8 mice (and rats with perirhinal cortex lesions) treating those objects as familiar? The answer, we think, is that a significant degree of interference—or what we have previously termed ‘feature ambiguity’ (Bussey and Saksida, 2002; Saksida and Bussey, 2011)—during the test phase comes from the trace of the study object presented 1 h before, which is of course not present prior to the study phase. In the case of perirhinal cortex lesions, which yield a pattern of false recognition identical to that in the tgCRND8 mice (McTighe et al., 2010), if the studied and novel test objects are made more similar by sharing more features, the resulting feature ambiguity is sufficient to cause profound impairments even at short delays with little or no incidental inter-delay interference. When the objects are dissimilar, however, the degree of interference between shared features is not sufficient to cause impairment (Eacott et al., 1994; Bartko et al., 2007a). In this experiment, the studied and novel test objects were relatively dissimilar, and so the prediction is that the nature of the objects alone will not be enough to generate an effect. However, when incidental interference caused by stimuli present during the delay is added, the interference is sufficient to produce false recognition. Support for this interpretation is shown by the finding that when that interference is removed via sensory restriction, the effect is abolished (McTighe et al., 2010).
It is worth noting that an additional mechanism may also be at play that could exaggerate the interference during the test phase, which is preceded by object presentation, compared with the study phase, which is not preceded by object presentation. After being handled, placed in the apparatus and presented with an object, the animal is likely to be far more aroused than when undisturbed in the home cage prior to the presentation of the study object. This arousal could lead to increased encoding of interfering information. Indeed, there is empirical evidence to support this idea. Winters et al. (2006) found that infusions of the cholinergic antagonist scopolamine into perirhinal cortex during the delay period improved object recognition in rats (performance following vehicle infusion was relatively poor). The interpretation was that the activity accompanying vehicle infusion caused arousal, leading to increased encoding of interfering information during the delay. Infusion of scopolamine appeared to have blocked this deleterious encoding, an idea supported by the finding that scopolamine infusion prior to the encoding of the sample impaired subsequent object recognition, and that the interference caused by the interpolation during the delay of a perceptually similar object (Bartko et al., 2010) could be abolished by pre-interference infusions of scopolamine (Winters et al., 2007). This interpretation was tested by assessing rats under identical conditions but without the putatively arousing vehicle infusion. Performance under this condition increased to the same level as that following scopolamine infusion. Thus, there are several reasons to expect the false recognition effect to be reflected in decreased exploration of a novel object during the test phase in this paradigm, but not necessarily during the sample phase.
Electrophysiological data
The precise amyloid β-related mechanisms leading to cognitive dysfunction are still unknown, but rodent models of Alzheimer’s disease typical amyloid β pathology show aberrant plasticity mechanisms (Walsh et al., 2002; Hsieh et al., 2006; Shankar et al., 2008; Li et al., 2009; Jo et al., 2011). Long-term depression of synaptic transmission is thought to be a principle mechanism underlying recognition memory (Cho et al., 2000; Massey and Bashir, 2007; Griffiths et al., 2008), and amyloid β increases NMDA receptor-dependent long-term depression (Kim et al., 2001; Cheng et al., 2009). Furthermore, similar to sensory restriction, memantine administration rescued the false recognition effect in the tgCRND8 mice. Thus, we hypothesized that aberrant NMDA receptor-dependent long-term depression in these mice may have led to the aberrant encoding of interfering object information (see Discussion section above) leading to false recognition effects. Indeed, we found that NMDA receptor-dependent long-term depression was increased in these mice, and that memantine treatment blocked this increase. To summarize, the data allow us to infer causal relationships between (i) the alterations in these mice and false recognition; (ii) memantine (and sensory restriction) treatment and the amelioration of false recognition; (iii) the alterations in these mice and aberrant long-term depression; and (iv) memantine and the amelioration of aberrant long-term depression. In addition, we hypothesize a causal relationship between altered long-term depression and false memory. It should be noted, however, that such a causal relationship cannot be demonstrated; we offer this idea as a hypothesis only, which will require further examination.
We have made reference to perirhinal cortex lesions, which yield a false recognition effect identical to that seen in the tgCRND8 mice, and which can also be rescued by sensory restriction (McTighe et al., 2010). However, tgCRND8 mice are not like perirhinal-lesioned animals in the sense that they do not show a high degree of neuronal loss and instead show a more global pattern of amyloid β pathology (Chishti et al., 2001; Kobayashi et al., 2005; Adalbert et al., 2009). On the present genetic background, diffuse and dense core amyloid deposits and dystrophic neurites are detected as early as 2 months in the cortex and hippocampus, and affect most brain regions, including the cerebellum and brainstem by 8–9 months (Chishti et al., 2001; Kobayashi et al., 2005; Adalbert et al., 2009). Hence, it might seem surprising that such a global insult has cognitive effects that closely match those seen after very selective perirhinal cortex lesions in rats. It may be, as suggested above, that the effects observed in the CRND8 mice are due to pathology in perirhinal cortex. However, perirhinal cortex is not the only structure implicated in object recognition; other regions implicated in object recognition include the thalamus, and area TE (Ho et al., 2011), and so the possibility remains that other affected structures are contributing to these effects.
In both the case of perirhinal cortex lesions and the tgCRND8 mouse, the evidence suggests that false recognition is caused by increased susceptibility to interference. However, in the case of perirhinal damage, the interpretation is that animals have lost a high-level object representation that protects memory from interference from lower level representations (Bussey and Saksida, 2002; McTighe et al., 2010; Saksida and Bussey, 2010). In the case of the tgCRND8 mouse, our hypothesis is that the baseline level of interference is increased due to enhanced NMDA receptor-dependent plasticity and perhaps long-term depression in particular. On this account, the aberrant encoding of interfering information due to aberrant plasticity throughout regions that code object-related information (e.g. perirhinal cortex and other structures such area TE, Ho et al., 2011; area V2, Lopez-Aranda et al., 2009) may be sufficient to cause interference leading to false recognition. It is also possible, of course, that in the tgCRND8 mouse, both perirhinal (or other) dysfunction and aberrant encoding of more distributed object representations could come into play, perhaps in an additive manner.
Our findings that pathologically raised amyloid β levels lead to object recognition deficits are in agreement with previous reports of such impairments in tgCRND8 (Francis et al., 2012; Greco et al., 2010) and similar common mouse models of amyloid β pathology, such as Tg2576 and PDAPP mice (Dodart et al., 1999, 2002a, b; Mouri et al., 2007; Taglialaleta et al., 2009; Yuede et al., 2009). However, other studies have failed to see object recognition deficits in Tg2576 and PDAPP mice (Chen et al., 2000; Hale and Good, 2005). Thus, it appears that the precise nature of the object recognition paradigm, the type of amyloid precursor protein mutation and promoter, or the background mouse strain can have an influence on the degree of recognition memory impairment (Dodart et al., 2002a; Kobayashi et al., 2005; Dere et al., 2007). It is conceivable that the ‘decoupling’ procedure used in the present study, which allows an assessment of false recognition not possible with forced-choice methodology (McTighe et al., 2010), is more sensitive to detecting impairments than standard rodent object recognition procedures.
Relevance to Alzheimer’s disease in humans
It is often argued that data from mouse models may not directly translate to humans. However, initial evidence suggests that amyloid β may have a similar effect in patients with Alzheimer’s disease to that shown here in mice. False recognition has been demonstrated a number of times in patients with Alzheimer’s disease (Hart et al., 1985; Budson et al., 2000; Gold et al., 2007; Plancher et al., 2009; Abe et al., 2011) and the degree of false recognition appears to correlate with amyloid β levels in the CSF (Hildebrandt et al., 2009). More specifically, Abe et al. (2011) have recently shown that patients with Alzheimer’s disease show false recognition of pictorial stimuli, especially when the studied and target pictures are similar to each other [it is worth noting the similarity between this effect and the findings of Bartko et al. (2007a) discussed above]. Interestingly, repeated presentation of a perceptually similar studied picture, which would be expected to strengthen the representation of that stimulus, exacerbates the effect, a finding consistent with our suggestion that amyloid β increases the confusion of the study object with the test object. Perhaps even more strikingly, a period in a dark quiet room, i.e. sensory restriction, enhanced recognition in participants with mild cognitive impairment, a prodromal stage of Alzheimer’s disease (Della Sala et al., 2005). Although that study could not distinguish between misses (memory loss) and false alarms (false recognition), it certainly provides an indication that susceptibility to interference may be a critical component of the memory disruption seen in Alzheimer’s disease, paralleling our results in mice. However, such parallels, while encouraging, need to be considered with caution. False recognition in these patients may occur via different, or additional, mechanisms to those underlying false recognition in Alzheimer’s disease mice; for example, false recognition may occur in humans due to a compensatory shift in bias leading to false alarms (Werheid et al., 2011). Furthermore, confabulation can occur particularly when prefrontal pathology is present (Tallberg and Almkvist, 2001; Cooper et al., 2006; Attali et al., 2009). Finally, we are modelling recognition using a paradigm that some believe taps mostly ‘familiarity’ based, as opposed to context-dependent ‘recollection’ based recognition memory (Aggleton and Brown, 1999). To the extent that this idea is correct, our finding may be more relevant to the former. With further experimentation taking these various factors into account, an understanding of the phenomenon of false memory in Alzheimer’s disease will become more complete.
Memantine is one of only a handful of compounds (and the only non-cholinesterase inhibitor) approved for the treatment of Alzheimer’s disease. In our experiments, memantine not only rescued the false recognition effect but also normalized the aberrant long-term depression that we hypothesize might be part of the mechanism underlying that effect. Recently, however, the use of memantine as a treatment for mild Alzheimer’s disease and mild cognitive impairment has been criticized (Schneider et al., 2011), in part, because memantine treatment does not lead to broad improvements in cognition in Alzheimer’s disease. However, no study has yet investigated the effect of memantine on false recognition in Alzheimer’s disease. The encouraging parallels between the animal and clinical studies may be an indication that the specific phenomenon of false recognition could be capitalized upon (Budson et al., 2002), to provide a novel and perhaps more sensitive paradigm for the assessment of potential therapies to treat the mnemonic dysfunction characteristic of this disease.
Funding
Behavioural experiments were supported by grants to T.J.B. and L.M.S. from the Alzheimer's Research Trust and the Medical Research Council, UK. Electrophysiological experiments were funded by a grant to T.J.B., L.M.S., and K.C. from The Wellcome Trust, UK.
Acknowledgements
C.R., S.M., K.C., T.J.B., D.J.W. and L.M.S. conceived the experiments. Experiments were performed by C.R. and S.M. (behaviour), C.J.H. (histology) and D.J.W. (electrophysiology). The manuscript was co-written by all authors. We would like to thank O.F.X. Almeida for his advice on the manuscript, and David E. Theobald and Alan G. Lyon for assistance with plaque histology.
Glossary
Abbreviations
- NMDA
N-methyl-d-aspartic acid
References
- Abe N, Fujii T, Nishio Y, Iizuka O, Kanno S, Kikuchi H, et al. False item recognition in patients with Alzheimer's disease. Neuropsychologia. 2011;49:1897–902. doi: 10.1016/j.neuropsychologia.2011.03.015. [DOI] [PubMed] [Google Scholar]
- Adalbert R, Nogradi A, Babetto E, Janeckova L, Walker SA, Kerschensteiner M, et al. Severely dystrophic axons at amyloid plaques remain continuous and connected to viable cell bodies. Brain. 2009;132:402–16. doi: 10.1093/brain/awn312. [DOI] [PubMed] [Google Scholar]
- Aggleton JP, Brown MW. Episodic memory, amnesia, and the hippocampal-anterior thalamic axis. Behav Brain Sci. 1999;22:425–44; discussion 444–89. [PubMed] [Google Scholar]
- Allen Mouse Brain Atlas [Internet] Seattle (WA): Allen Institute for Brain Science; ©2009. Available from: http://mouse.brain-map.org (march 2012, date last accessed) [Google Scholar]
- Attali E, De Anna F, Dubois B, Dalla Barba G. Confabulation in Alzheimer's disease: poor encoding and retrieval of over-learned information. Brain. 2009;132:204–12. doi: 10.1093/brain/awn241. [DOI] [PubMed] [Google Scholar]
- Bartko SJ, Cowell R, Winters BD, Bussey TJ, Saksida LM. Heightened susceptibility to interference in an animal model of amnesia: impairment in encoding, storage, retrieval - or all three? Neuropsychologia. 2010;48:2987–97. doi: 10.1016/j.neuropsychologia.2010.06.007. [DOI] [PubMed] [Google Scholar]
- Bartko SJ, Winters BD, Cowell RA, Saksida LM, Bussey TJ. Perirhinal cortex resolves feature ambiguity in configural object recognition and perceptual oddity tasks. Learn Mem. 2007a;14:821–32. doi: 10.1101/lm.749207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bartko SJ, Winters BD, Cowell R, Saksida LM, Bussey TJ. Perceptual impairments following perirhinal cortex lesions in rats: zero-delay object recognition and simultaneous oddity discriminations. J Neurosci. 2007b;27:2548–59. doi: 10.1523/JNEUROSCI.5171-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Budson AE, Daffner KR, Desikan R, Schacter DL. When false recognition is unopposed by true recognition: gist-based memory distortion in Alzheimer's disease. Neuropsychology. 2000;14:277–87. doi: 10.1037//0894-4105.14.2.277. [DOI] [PubMed] [Google Scholar]
- Budson AE, Michalska KJ, Rentz DM, Joubert CC, Daffner KR, Schacter DL, et al. Use of a false recognition paradigm in an Alzheimer's disease clinical trial: a pilot study. Am J Alzheimers Dis Other Demen. 2002;17:93–100. doi: 10.1177/153331750201700204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Budson AE, Michalska KJ, Sullivan AL, Rentz DM, Daffner KR, Schacter DL. False recognition in Alzheimer disease: evidence from categorized pictures. Cogn Behav Neurol. 2003;16:16–27. doi: 10.1097/00146965-200303000-00003. [DOI] [PubMed] [Google Scholar]
- Burke SN, Wallace JL, Nematollahi S, Uprety AR, Barnes CA. Pattern separation deficits may contribute to age-associated recognition impairments. Behav Neurosci. 2010;124:559–73. doi: 10.1037/a0020893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bussey TJ, Saksida LM. The organization of visual object representations: a connectionist model of effects of lesions in perirhinal cortex. Eur J Neurosci. 2002;15:355–64. doi: 10.1046/j.0953-816x.2001.01850.x. [DOI] [PubMed] [Google Scholar]
- Chen G, Chen KS, Knox J, Inglis J, Bernard A, Martin SJ, et al. A learning deficit related to age and β-amyloid plaques in a mouse model of Alzheimer's disease. Nature. 2000;408:975–9. doi: 10.1038/35050103. [DOI] [PubMed] [Google Scholar]
- Cheng L, Yin WJ, Zhang JF, Qi JS. Amyloid beta-protein fragments 25-35 and 31-35 potentiate long-term depression in hippocampal CA1 region of rats in vivo. Synapse. 2009;63:206–14. doi: 10.1002/syn.20599. [DOI] [PubMed] [Google Scholar]
- Chishti MA, Yang DS, Janus C, Phinney AL, Horne P, Pearson J, et al. Early-onset amyloid deposition and cognitive deficits in transgenic mice expressing a double mutant form of amyloid precursor protein 695. J Biol Chem. 2001;276:21562–70. doi: 10.1074/jbc.M100710200. [DOI] [PubMed] [Google Scholar]
- Cho K, Kemp N, Noel J, Aggleton JP, Brown MW, Bashir ZI. A new form of long-term depression in the perirhinal cortex. Nat Neurosci. 2000;3:150–6. doi: 10.1038/72093. [DOI] [PubMed] [Google Scholar]
- Cooper JM, Shanks MF, Venneri A. Provoked confabulations in Alzheimer's disease. Neuropsychologia. 2006;44:1697–707. doi: 10.1016/j.neuropsychologia.2006.03.029. [DOI] [PubMed] [Google Scholar]
- Costa AC, Scott-McKean JJ, Stasko MR. Acute injections of the NMDA receptor antagonist memantine rescue performance deficits of the Ts65Dn mouse model of Down syndrome on a fear conditioning test. Neuropsychopharmacology. 2008;33:1624–32. doi: 10.1038/sj.npp.1301535. [DOI] [PubMed] [Google Scholar]
- Cowell RA, Bussey TJ, Saksida LM. Why does brain damage impair memory? A connectionist model of object recognition memory in perirhinal cortex. J Neurosci. 2006;26:12186–97. doi: 10.1523/JNEUROSCI.2818-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Della Sala S, Cowan N, Beschin N, Perini M. Just lying there, remembering: improving recall of prose in amnesic patients with mild cognitive impairment by minimising interference. Memory. 2005;13:435–40. doi: 10.1080/09658210344000387. [DOI] [PubMed] [Google Scholar]
- Dere E, Huston JP, De Souza Silva MA. The pharmacology, neuroanatomy and neurogenetics of one-trial object recognition in rodents. Neurosci Biobehav Rev. 2007;31:673–704. doi: 10.1016/j.neubiorev.2007.01.005. [DOI] [PubMed] [Google Scholar]
- Dodart JC, Bales KR, Gannon KS, Greene SJ, DeMattos RB, Mathis C, et al. Immunization reverses memory deficits without reducing brain Abeta burden in Alzheimer's disease model. Nat Neurosci. 2002a;5:452–7. doi: 10.1038/nn842. [DOI] [PubMed] [Google Scholar]
- Dodart JC, Mathis C, Bales KR, Paul SM. Does my mouse have Alzheimer's disease? Genes Brain Behav. 2002b;1:142–55. doi: 10.1034/j.1601-183x.2002.10302.x. [DOI] [PubMed] [Google Scholar]
- Dodart JC, Meziane H, Mathis C, Bales KR, Paul SM, Ungerer A. Behavioral disturbances in transgenic mice overexpressing the V717F beta-amyloid precursor protein. Behav Neurosci. 1999;113:982–90. doi: 10.1037//0735-7044.113.5.982. [DOI] [PubMed] [Google Scholar]
- Eacott MJ, Gaffan D, Murray EA. Preserved recognition memory for small sets, and impaired stimulus identification for large sets, following rhinal cortex ablations in monkeys. Eur J Neurosci. 1994;6:1466–1478. doi: 10.1111/j.1460-9568.1994.tb01008.x. [DOI] [PubMed] [Google Scholar]
- Francis BM, Kim J, Barakat ME, Fraenkl S, Yucel YH, Peng S, et al. Object recognition memory and BDNF expression are reduced in young TgCRND8 mice. Neurobiol Aging. 2012;33:555–63. doi: 10.1016/j.neurobiolaging.2010.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gold G, Giannakopoulos P, Herrmann FR, Bouras C, Kovari E. Identification of Alzheimer and vascular lesion thresholds for mixed dementia. Brain. 2007;130:2830–6. doi: 10.1093/brain/awm228. [DOI] [PubMed] [Google Scholar]
- Greco SJ, Bryan KJ, Sarkar S, Zhu X, Smith MA, Ashford JW, et al. Leptin reduces pathology and improves memory in a transgenic mouse model of Alzheimer's disease. J Alzheimers Dis. 2010;19:1155–67. doi: 10.3233/JAD-2010-1308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griffiths S, Scott H, Glover C, Bienemann A, Ghorbel MT, Uney J, et al. Expression of long-term depression underlies visual recognition memory. Neuron. 2008;58:186–94. doi: 10.1016/j.neuron.2008.02.022. [DOI] [PubMed] [Google Scholar]
- Hart SA, Smith CM, Swash M. Recognition memory in Alzheimer's disease. Neurobiol Aging. 1985;6:287–92. doi: 10.1016/0197-4580(85)90005-3. [DOI] [PubMed] [Google Scholar]
- Hildebrandt H, Haldenwanger A, Eling P. False recognition correlates with amyloid-beta (1-42) but not with total tau in cerebrospinal fluid of patients with dementia and mild cognitive impairment. J Alzheimers Dis. 2009;16:157–65. doi: 10.3233/JAD-2009-0931. [DOI] [PubMed] [Google Scholar]
- Ho JW, Narduzzo KE, Outram A, Tinsley CJ, Henley JM, Warburton EC, et al. Contributions of area Te2 to rat recognition memory. Learn Mem. 2011;18:493–501. doi: 10.1101/lm.2167511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsieh H, Boehm J, Sato C, Iwatsubo T, Tomita T, Sisodia S, et al. AMPAR removal underlies Abeta-induced synaptic depression and dendritic spine loss. Neuron. 2006;52:831–43. doi: 10.1016/j.neuron.2006.10.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jo J, Whitcomb DJ, Olsen KM, Kerrigan TL, Lo SC, Bru-Mercier G, et al. Aβ(1-42) inhibition of LTP is mediated by a signaling pathway involving caspase-3, Akt1 and GSK-3β. Nat Neurosci. 2011;14:545–7. doi: 10.1038/nn.2785. [DOI] [PubMed] [Google Scholar]
- Kemp N, Bashir ZI. Long-term depression: a cascade of induction and expression mechanisms. Prog Neurobiol. 2001;65:339–65. doi: 10.1016/s0301-0082(01)00013-2. [DOI] [PubMed] [Google Scholar]
- Kim JH, Anwyl R, Suh YH, Djamgoz MB, Rowan MJ. Use-dependent effects of amyloidogenic fragments of (beta)-amyloid precursor protein on synaptic plasticity in rat hippocampus in vivo. J Neurosci. 2001;21:1327–33. doi: 10.1523/JNEUROSCI.21-04-01327.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kobayashi DT, Chen KS. Behavioral phenotypes of amyloid-based genetically modified mouse models of Alzheimer's disease. Genes Brain Behav. 2005;4:173–96. doi: 10.1111/j.1601-183X.2005.00124.x. [DOI] [PubMed] [Google Scholar]
- Lein ES, Hawrylycz MJ, Ao N, Ayres M, Bensinger A, Bernard A, et al. Genome-wide atlas of gene expression in the adult mouse brain. Nature. 2007;445:168–76. doi: 10.1038/nature05453. [DOI] [PubMed] [Google Scholar]
- Li S, Hong S, Shepardson NE, Walsh DM, Shankar GM, Selkoe D. Soluble oligomers of amyloid Beta protein facilitate hippocampal long-term depression by disrupting neuronal glutamate uptake. Neuron. 2009;62:788–801. doi: 10.1016/j.neuron.2009.05.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lopez-Aranda MF, Lopez-Tellez JF, Navarro-Lobato I, Masmudi-Martin M, Gutierrez A, Khan ZU. Role of layer 6 of V2 visual cortex in object-recognition memory. Science. 2009;325:87–9. doi: 10.1126/science.1170869. [DOI] [PubMed] [Google Scholar]
- Ly PTT, Cai F, Song W. Detection of neuritic plaques in Alzheimer’s Disease mouse model. J Vis Exp. 2011;53:e2831. doi: 10.3791/2831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Massey PV, Bashir ZI. Long-term depression: multiple forms and implications for brain function. Trends Neurosci. 2007;30:176–84. doi: 10.1016/j.tins.2007.02.005. [DOI] [PubMed] [Google Scholar]
- Massey PV, Johnson BE, Moult PR, Auberson YP, Brown MW, Molnar E, et al. Differential roles of NR2A and NR2B-containing NMDA receptors in cortical long-term potentiation and long-term depression. J Neuro. 2004;24:7821–8. doi: 10.1523/JNEUROSCI.1697-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McTighe SM, Cowell RA, Winters BD, Bussey TJ, Saksida LM. Paradoxical false memory for objects after brain damage. Science. 2010;330:1408–10. doi: 10.1126/science.1194780. [DOI] [PubMed] [Google Scholar]
- Meunier M, Bachevalier J, Mishkin M, Murray EA. Effects on visual recognition of combined and separate ablations of the entorhinal and perirhinal cortex in rhesus monkeys. J Neurosci. 1993;13:5418–32. doi: 10.1523/JNEUROSCI.13-12-05418.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mouri A, Noda Y, Hara H, Mizoguchi H, Tabira T, Nabeshima T. Oral vaccination with a viral vector containing Abeta cDNA attenuates age-related Abeta accumulation and memory deficits without causing inflammation in a mouse Alzheimer model. FASEB J. 2007;21:2135–48. doi: 10.1096/fj.06-7685com. [DOI] [PubMed] [Google Scholar]
- Mumby DG, Pinel JP. Rhinal cortex lesions and object recognition in rats. Behav Neurosci. 1994;108:11–8. doi: 10.1037//0735-7044.108.1.11. [DOI] [PubMed] [Google Scholar]
- Palop JJ, Mucke L. Amyloid-beta-induced neuronal dysfunction in Alzheimer's disease: from synapses toward neural networks. Nat Neurosci. 2010;13:812–8. doi: 10.1038/nn.2583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parsons CG, Danysz W, Quack G. Memantine is a clinically well tolerated N-methyl-D-aspartate (NMDA) receptor antagonist - a review of preclinical data. Neuropharmacology. 1999;38:735–67. doi: 10.1016/s0028-3908(99)00019-2. [DOI] [PubMed] [Google Scholar]
- Plancher G, Guyard A, Nicolas S, Piolino P. Mechanisms underlying the production of false memories for famous people's names in aging and Alzheimer's disease. Neuropsychologia. 2009;47:2527–36. doi: 10.1016/j.neuropsychologia.2009.04.026. [DOI] [PubMed] [Google Scholar]
- Rosi S, Ramirez-Amaya V, Vazdarjanova A, Esparza EE, Larkin PB, Fike JR, et al. Accuracy of hippocampal network activity is disrupted by neuroinflammation: rescue by memantine. Brain. 2009;132:2464–77. doi: 10.1093/brain/awp148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saksida LM, Bussey TJ. The representational-hierarchical view of amnesia: translation from animal to human. Neuropsychologia. 2010;48:2370–84. doi: 10.1016/j.neuropsychologia.2010.02.026. [DOI] [PubMed] [Google Scholar]
- Schneider LS, Dagerman KS, Higgins JP, McShane R. Lack of evidence for the efficacy of memantine in mild Alzheimer disease. Arch Neurol. 2011;68:991–8. doi: 10.1001/archneurol.2011.69. [DOI] [PubMed] [Google Scholar]
- Selkoe DJ. Alzheimer's disease is a synaptic failure. Science. 2002;298:789–91. doi: 10.1126/science.1074069. [DOI] [PubMed] [Google Scholar]
- Sik A, van Nieuwehuyzen P, Prickaerts J, Blokland A. Performance of different mouse strains on an object recognition task. Behav Brain Res. 2003;147:49–54. doi: 10.1016/s0166-4328(03)00117-7. [DOI] [PubMed] [Google Scholar]
- Shankar GM, Li S, Mehta TH, Garcia-Munoz A, Shepardson NE, Smith I, et al. Amyloid-beta protein dimers isolated directly from Alzheimer's brains impair synaptic plasticity and memory. Nat Med. 2008;14:837–42. doi: 10.1038/nm1782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taglialatela G, Hogan D, Zhang WR, Dineley KT. Intermediate- and long-term recognition memory deficits in Tg2576 mice are reversed with acute calcineurin inhibition. Behav Brain Res. 2009;200:95–9. doi: 10.1016/j.bbr.2008.12.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tallberg IM, Almkvist O. Confabulation and memory in patients with Alzheimer's disease. J Clin Exp Neuropsychol. 2001;23:172–84. doi: 10.1076/jcen.23.2.172.1215. [DOI] [PubMed] [Google Scholar]
- Walsh DM, Klyubin I, Fadeeva JV, Cullen WK, Anwyl R, Wolfe MS, et al. Naturally secreted oligomers of amyloid beta protein potently inhibit hippocampal long-term potentiation in vivo. Nature. 2002;416:535–9. doi: 10.1038/416535a. [DOI] [PubMed] [Google Scholar]
- Werheid K, McDonald RS, Simmons-Stern N, Ally BA, Budson AE. Familiar smiling faces in Alzheimer's disease: understanding the positivity-related recognition bias. Neuropsychologia. 2011;49:2935–40. doi: 10.1016/j.neuropsychologia.2011.06.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winters BD, Bartko SJ, Saksida LM, Bussey TJ. Scopolamine infused into perirhinal cortex improves object recognition memory by blocking the acquisition of interfering object information. Learn Memory. 2007;14:590–6. doi: 10.1101/lm.634607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winters BD, Forwood SE, Cowell RA, Saksida LM, Bussey TJ. Double dissociation between the effects of peri-postrhinal cortex and hippocampal lesions on tests of object recognition and spatial memory: heterogeneity of function within the temporal lobe. J Neurosci. 2004;24:5901–8. doi: 10.1523/JNEUROSCI.1346-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winters BD, Saksida LM, Bussey TJ. Paradoxical facilitation of object recognition memory following infusion of scopolamine into perirhinal cortex: implications for cholinergic system function. J Neurol. 2006;26:9520–9. doi: 10.1523/JNEUROSCI.2319-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winters BD, Saksida LM, Bussey TJ. Object recognition memory: neurobiological mechanisms of encoding, consolidation and retrieval. Neurosci Biobehav Rev. 2008;32:1055–70. doi: 10.1016/j.neubiorev.2008.04.004. [DOI] [PubMed] [Google Scholar]
- Winters BD, Saksida LM, Bussey TJ. Implications of animal object memory research for human amnesia. Neuropsychologia. 2010;48:2251–61. doi: 10.1016/j.neuropsychologia.2010.01.023. [DOI] [PubMed] [Google Scholar]
- Yuede CM, Zimmerman SD, Dong H, Kling MJ, Bero AW, Holtzman DM, et al. Effects of voluntary and forced exercise on plaque deposition, hippocampal volume, and behavior in the Tg2576 mouse model of Alzheimer's disease. Neurobiol Dis. 2009;35:426–32. doi: 10.1016/j.nbd.2009.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]