Abstract
Although progressive supranuclear palsy is defined by its akinetic rigidity, vertical supranuclear gaze palsy and falls, cognitive impairments are an important determinant of patients’ and carers’ quality of life. Here, we investigate whether there is a broad deficit of modality-independent social cognition in progressive supranuclear palsy and explore the neural correlates for these. We recruited 23 patients with progressive supranuclear palsy (using clinical diagnostic criteria, nine with subsequent pathological confirmation) and 22 age- and education-matched controls. Participants performed an auditory (voice) emotion recognition test, and a visual and auditory theory of mind test. Twenty-two patients and 20 controls underwent structural magnetic resonance imaging to analyse neural correlates of social cognition deficits using voxel-based morphometry. Patients were impaired on the voice emotion recognition and theory of mind tests but not auditory and visual control conditions. Grey matter atrophy in patients correlated with both voice emotion recognition and theory of mind deficits in the right inferior frontal gyrus, a region associated with prosodic auditory emotion recognition. Theory of mind deficits also correlated with atrophy of the anterior rostral medial frontal cortex, a region associated with theory of mind in health. We conclude that patients with progressive supranuclear palsy have a multimodal deficit in social cognition. This deficit is due, in part, to progressive atrophy in a network of frontal cortical regions linked to the integration of socially relevant stimuli and interpretation of their social meaning. This impairment of social cognition is important to consider for those managing and caring for patients with progressive supranuclear palsy.
Keywords: progressive supranuclear palsy, voxel-based morphometry, social cognition, theory of mind, emotion perception
Introduction
When Richardson et al. (1963) first described progressive supranuclear palsy, they described a set of characteristic features that remain the principal diagnostic criteria: a vertical supranuclear gaze palsy and postural instability with falls (Litvan et al., 2003). Generally, patients also have akinetic rigidity and progressive dysarthria and dysphagia. However, significant cognitive problems are common, including apathy and a dysexecutive syndrome (Grafman et al., 1995; Bak and Hodges, 1998; Millar et al., 2006; Brown et al., 2010) and psychiatric comorbidity including depression (Schrag et al., 2003; Herting et al., 2007; Bak et al., 2010) or anxiety (Litvan et al., 1996b; Aarsland et al., 2001; Borroni et al., 2008). Half of patients also report social impairments as a negative influence on their quality of life (Schrag et al., 2003).
Recently, we reported deficits in basic emotion recognition in progressive supranuclear palsy (Ghosh et al., 2009). We proposed that these deficits were part of a wider deficit in social cognition that may contribute to the common difficulties patients experienced in social interactions (Schrag et al., 2003). However, social cognition is not a unitary phenomenon. At one level, it requires the ability to recognize basic emotions (e.g. happy, sad, angry, disgust and surprise) [‘emotion knowledge’ (Izard, 1971)]. It also encompasses an appreciation of more complicated emotions (e.g. sarcasm and humour) and higher order social inferences. These require greater understanding and representation of other people’s mental states in a given context, known as ‘Theory of Mind’ (Premack and Woodruff, 1978).
Several neurological disorders affect social cognition, including emotion recognition and theory of mind. In addition to progressive supranuclear palsy (Ghosh et al., 2009), these include behavioural variant frontotemporal degeneration (Keane et al., 2002; Rankin et al., 2005), Parkinson’s disease (Sprengelmeyer et al., 2003; Lawrence et al., 2007; Gray and Tickle-Degnen, 2010) and Huntington’s disease (Sprengelmeyer et al., 1996; Calder et al., 2010). Basic emotion recognition deficits in these degenerative disorders have been investigated using static pictures of faces showing basic emotions (Keane et al., 2002; Rosen et al., 2002b, 2004; Diehl-Schmid et al., 2007; Kipps et al., 2009a; Rankin et al., 2009); sounds expressing emotion (Keane et al., 2002; Rankin et al., 2009); and videos of emotions (Werner et al., 2007; Kipps et al., 2009b; Rankin et al., 2009). Theory of mind abilities in these clinical populations have been assessed using both static and dynamic tests such as the Awareness of Social Inference Test (TASIT) (Kipps et al., 2009b; Rankin et al., 2009); the Mind in the Eyes Test (Baron-Cohen et al., 2001; Gregory et al., 2002; Stone et al., 2003; Torralva et al., 2007; Hirao et al., 2008); cartoons of social situations (Snowden et al., 2003; Lough et al., 2006; Rankin et al., 2009); and faux pas stories (Stone et al., 1998, 2003; Gregory et al., 2002; Torralva et al., 2007; Roca et al., 2010).
The critical neural structures supporting these social cognitive functions have been studied with structure–function correlations such as voxel-based morphometry of MRI (Rosen et al., 2002b; Henley et al., 2008; Kipps et al., 2009b; Rankin et al., 2009); lesion studies (Adolphs et al., 1996, 2000, 2002; Channon and Crawford, 2000; Roca et al., 2010; Shamay-Tsoory et al., 2009) and functional MRI (Hennenlotter et al., 2004; Brunet-Gouet and Decety, 2006; Carrington and Bailey, 2009). These complimentary methods reveal a partial convergence onto a frontotemporal network for social cognition.
A functional anatomical distinction appears to exist between unimodal and supramodal representations of emotion. For example, discrete emotion associations have been found between the insula and disgust (Phillips et al., 1997; Calder et al., 2000; Kipps et al., 2007) and between the amygdala and fear (Adolphs et al., 1994; Calder et al., 1996, 2001). Other simple associations are less consistent, for example, between anger and the medial temporal gyrus (Sprengelmeyer et al., 1998) or happiness and the middle temporal gyrus and amygdala (Johnstone et al., 2006; Kipps et al., 2007).
For the supra- or cross-modal recognition of emotion stimuli, several critical regions have been proposed, including the inferior frontal gyrus (Sprengelmeyer et al., 1998; Nakamura et al., 1999; Schirmer and Kotz, 2006; Beaucousin et al., 2007; Philippi et al., 2009; Leitman et al., 2010), the orbitofrontal cortex (Keane et al., 2002; Hornak et al., 2003; Paulmann et al., 2010) and the medial frontal cortex (Peelen et al., 2010). Other regions have been associated with the coding of multiple emotions but within a single sensory domain, such as the auditory domain (Murphy et al., 2003; Wildgruber et al., 2005; Ethofer et al., 2006; Schirmer and Kotz, 2006).
For higher order aspects of social cognition, including empathy and theory of mind, a frontotemporal cortical network has been implicated that includes regions affected by progressive supranuclear palsy pathology. For example, the inferior frontal gyrus together with components of the emotional network have been linked to emotional empathy—the ability to ‘feel’ the emotion of others. This may be mediated by its putative role in the mirror neuron system (Schulte-Ruther et al., 2007; Bastiaansen et al., 2009; Shamay-Tsoory et al., 2009). Such ‘emotional empathy’ can be contrasted with ‘cognitive empathy’, the determination of what others think, feel or intend without necessarily sharing the ‘feeling’.
On the basis of this prior knowledge of the functional anatomy of social cognition, one can use a region of interest approach to increase sensitivity and localization of pathological changes correlated with empathy. It can be difficult to isolate empathic aspects of the task and control for the mode of testing (for example, videos versus cartoons). However, there is a consensus that cognitive empathy is associated with the medial prefrontal cortex, orbitofrontal cortex, posterior superior temporal sulcus, the temporoparietal junction and striatum (Gregory et al., 2002; Frith and Frith, 2003; Saxe and Kanwisher, 2003; Snowden et al., 2003; Amodio and Frith, 2006; Gilbert et al., 2006; Hirao et al., 2008; Carrington and Bailey, 2009; Kipps et al., 2009b; Rankin et al., 2009; Shamay-Tsoory et al., 2009). Of these, the correlation with the medial prefrontal cortex is most consistent (Carrington and Bailey, 2009). Therefore, it is relevant that with progressive supranuclear palsy, the foci of cerebral atrophy and highest pathological burden overlap with these regions associated with social cognition (Brenneis et al., 2004; Price et al., 2004; Padovani et al., 2006; Paviour et al., 2006b; Nilsson et al., 2007; Josephs et al., 2008; Nicoletti et al., 2008; Rizzo et al., 2008; Schofield et al., 2011), especially in the frontal areas.
Based on these associations between structure, function and pathology, we formed three specific hypotheses. These were tested in a cohort of patients with progressive supranuclear palsy using voxel-based morphometry of MRI in conjunction with neuropsychological assessment of social cognition. Our hypotheses were that: (i) progressive supranuclear palsy causes a multimodal deficit in emotion recognition, such that patients have deficits in basic emotion recognition in auditory and visual modalities. Demonstration of deficits in an auditory test would additionally overcome a potential confound in previous visual emotion studies, arising from disordered eye movements; (ii) progressive supranuclear palsy impairs higher order social cognition, including theory of mind and (iii) greater social cognition dysfunction in progressive supranuclear palsy correlates with more severe regional atrophy of the inferior frontal gyrus and medial frontal cortex.
Materials and methods
Participants
Twenty-three patients were recruited prospectively from a specialist neurological clinic for patients with progressive supranuclear palsy and related disorders between 2007 and 2009. Clinical diagnostic criteria (Litvan et al., 2003) were used by an experienced neurologist. To date, nine patients have subsequently undergone post-mortem examination; all nine had progressive supranuclear palsy. Patients were excluded if they had another significant neurological illness. Twenty-two age- and education-matched controls were recruited from the panel of volunteers at the Medical Research Council’s Cognition and Brain Sciences Unit or from spouses of patients. Control participants had normal hearing and corrected vision and did not have significant neurological or psychiatric comorbidity. Not all participants were able to complete all tests. Three patients had either poor visual acuity or intercurrent illness and therefore could not complete all the neuropsychological test sessions. Two controls and one patient were unable to complete the volumetric MRI scan.
Patients and controls underwent the same testing protocol. Examination included the motor section of the Unified Parkinson Disease Rating Scale (UPDRS) (Fahn, 1986), the Progressive Supranuclear Palsy Rating Scale (PSPRS) (Golbe and Ohman-Strickland, 2007) and the Brixton test of executive function. The Brixton test is an untimed test of executive function with minimal motor demands (Burgess and Shallice, 1997). Visual face perception was assessed with the famous faces test (Calder et al., 1996). In this test, pictures of the faces of 30 famous people and 10 unfamiliar people are intermixed. Participants were asked to state which faces were familiar and to state their occupation and name. Scores were given for correct answers for each of the four aspects of the test (i.e. correctly stating someone was famous, giving their correct occupation and name and correctly rejecting those who were not famous).
Auditory thresholds were assessed in each ear with an automated program with five frequencies. Pure tones (250, 500, 1000, 2000 and 4000 Hz) were presented through a calibrated sound card on a Dell Latitude D520 laptop with Sony MDR-7506 headphones.
Social cognition testing
The ability to recognize emotion in voices was tested using the voice emotion recognition task, developed from the Montreal Affective Voices (Belin et al., 2008). Seventy vocal ‘affect bursts’ (Juslin and Scherer, 2005) recorded by 10 different actors (five male and five female) were played to the participants. These sounds were short, ranging from 240 ms to 4310 ms and portrayed the emotion in some way—for example, a retch for disgust. The stimuli have been previously rated as representing one of the six basic emotions, or a neutral sound devoid of emotion (Belin et al., 2008). The names of the six basic emotions (happy, sad, fear, anger, disgust, surprise and neutral) were displayed on the screen and participants were asked to choose the best descriptor for the sound heard. The sounds were played through a Dell Latitude D520 laptop and Sony MDR-7506 headphones. Stimuli were presented using E-Prime (Psychology Software Tools, Inc.).
Theory of mind was tested with TASIT (McDonald et al., 2002, 2003). This presents participants with short videos of exchanges between actors. The actors exhibit sincerity, sarcasm or paradoxical sarcasm in these exchanges. Paradoxical sarcasm refers to an exchange where the words do not make sense unless the observer is aware that the actor is being sarcastic (Supplementary material). Participants were asked to relay the meaning behind the exchanges in response to fixed questions asked by the tester. Videos typically lasted a minute and were played more than once if requested. The videos were played using Windows Media Player on a Dell Latitude D520 laptop. Performance of each social cognition subtest is given in Supplementary Tables 1 and 2.
Statistics
Statistical analysis of behavioural data used SPSS v15 (SPSS Inc.). Parametric data for patients and controls were compared with t-tests or repeated measures ANOVA with post hoc t-tests, and Greenhouse–Geisser correction where necessary. Non-parametric data were investigated with Mann Whitney or χ2 tests if categorical. Significantly skewed data were transformed using arcsin transformations. Bonferroni correction was used for multiple comparisons where appropriate. Pearson’s correlations were used for correlations, with correlations only being carried out between patients. When voice emotion was used as a composite measure, such as in correlations or voxel-based morphometry, this was the average of all emotional stimuli without neutral. Similarly, references to TASIT scores as a composite measure refer to the average score on the sarcastic stimuli, without the sincere stimuli.
Voxel-based morphometry
The voxel-based morphometry (Ashburner, 2009) analysis used T1-weighted MPRAGE images (repetition time 2300 ms; echo time 2.86 ms; inversion time 900 ms; flip angle 9°; matrix dimensions 192 × 192 in 144 slices with isotropic voxels 1.25 mm) acquired on a 3 T Siemens MAGNETOM TrioTim Syngo MR B17 scanner (Siemens Medical Systems). Skull stripping and correcting for non-uniformities in the images can improve the performance of voxel-based morphometry in SPM5 (Acosta-Cabronero et al., 2008). Accordingly, the Hybrid Watershed Algorithm, using atlas information (Ségonne et al., 2004) (Freesurfer v.4.05, surfer.nmr.mgh.harvard.edu), was used to remove the skull and other non-brain tissue except CSF. Field inhomogeneities were corrected using a non-parametric non-uniform intensity normalization algorithm (N3 v.1.10 with Freesurfer default settings) (Sled et al., 1998). Venous sinuses and CSF were extracted using the brain extraction tool (BET) v.2.1 (Smith, 2002) in FSL v.4.1 (www.fmrib.ox.ac.uk/fsl). Fractional intensity threshold, f, was set to 0.2 and the vertical gradient, g, set to 1.
Subsequent preprocessing and analysis used SPM5 (www.fil.ion.ucl.ac.uk/spm). Images were spatially normalized and segmented into different tissue classes using the unified segmentation model in SPM5 (Ashburner and Friston, 2005); default settings were used throughout. The resulting modulated images were smoothed with a 16-mm full-width at half-maximum kernel to accommodate anatomical variation among subjects and enable Gaussian random field theory for statistical inferences.
Whole brain voxel-wise analyses used SPM5 with total intracranial volume as a nuisance covariate. Total intracranial volume was calculated by summing the number of voxels in each segmented tissue class over a threshold of 0.5 and multiplying the total by the voxel volume. This method is reproducible and accurate in older individuals (Pengas et al., 2009). An explicit mask was used in the grey and white matter analysis, respectively, averaged over all controls and patients in the ‘control versus patient’ contrasts, or patients only in the regression analyses, using a threshold of 0.1 for voxel inclusion.
Multiple regression analyses examined the relation between specified test scores and volumes of voxel grey or white matter. We predefined regions of interest based on prior studies of emotion recognition and theory of mind. For emotion recognition, the regions of interest included the left and right amygdala, bilateral insula and the right inferior frontal gyrus (Wildgruber et al., 2005; Schirmer and Kotz, 2006).
The definition of the regions of interest for the theory of mind task was based on two converging lines of evidence. First, we recognize that there are a multitude of different theory of mind tasks with different modes of presentation (Gregory et al., 2002; Frith and Frith, 2003; Saxe and Kanwisher, 2003; Snowden et al., 2003; Amodio and Frith, 2006; Gilbert et al., 2006; Hirao et al., 2008; Carrington and Bailey, 2009; Kipps et al., 2009b; Rankin et al., 2009; Shamay-Tsoory et al., 2009). However, there is a consensus that the medial frontal cortex, the temporoparietal area and the posterior superior temporal sulcus are core areas associated with theory of mind. In a review of 40 studies of theory of mind, Carrington and Bailey (2009) found that the medial frontal cortex was implicated in 88% of studies, with the posterior superior temporal sulcus and the temporoparietal area found in 45%. Secondly, MRI-based measures of atrophy in progressive supranuclear palsy commonly identify the frontal rather than temporal areas (Cordato et al., 2000, 2002, 2005; Brenneis et al., 2004; Paviour et al., 2004). Therefore, we hypothesized that atrophy in the medial frontal cortex would be a significant contributor to theory of mind deficits and used the anterior rostral medial frontal cortex [as defined by Amodio and Frith (2006)] as our region of interest. We used the WFU pickatlas toolbox (Lancaster et al., 1997, 2000; Tzourio-Mazoyer et al., 2002; Maldjian et al., 2003, 2004) to define the regions of interest with x = 0 ± 20 mm, as in other studies (Supplementary Fig. 1; Gilbert et al., 2006; Carrington and Bailey, 2009; Van Overwalle, 2009).
Two statistical thresholds are reported. First, at P < 0.05 with family wise error (FWE) correction for multiple comparisons and second, an exploratory threshold of P < 0.001 (uncorrected). For region of interest analysis, only areas reaching significance with FWE correction for multiple comparisons are reported.
Results
Participants
Demographic data are detailed in Table 1. There were no differences between patients and controls in gender (χ2 = 0.262, df = 1, P > 0.6), age [t(43) < 1, P > 0.9] or education years (U = 182.5, P > 0.1, r = −0.24). The Brixton test of executive function was significantly different between patients and controls (U = 133.5, P = 0.03, r = −0.3).
Table 1.
Demographic data for patients and controls
| Group | n | M:F | Age | Education (years) | Disease duration | UPDRS | PSPRS | Brixton | TIV (ml) |
|---|---|---|---|---|---|---|---|---|---|
| Patients | 23 | 14:9 | 71.1 (8.6) | 13 (9–20) | 2.5 (1–17) | 33.8 (15.7) | 38.2 (18.1) | 2 (1–7) | 1435 (272) |
| Controls | 22 | 15:7 | 71.4 (7.6) | 11 (9–19) | n/a | n/a | n/a | 5.5 (1–7) | 1383 (176) |
Mean values are given for age, UPDRS, PSPRS and total intracranial volume (TIV) with standard deviation in parentheses. Median values are given for education years, estimated symptomatic disease duration and Brixton with range in parentheses. F = female; M = male.
There were no differences between patients and controls for any of the perceptual control tasks. In the famous faces test, a 2 (group) × 4 (familiar faces, occupations, names, unfamiliar faces) repeated measures ANOVA of arcsin transformed scores showed a main effect for the test [F(1.75,71.7) = 39.3, P < 0.001, n = 21] but no main effect for group [F(1,41) = 2.4, P > 0.1] or Test × Group interaction [F(1.75,71.7) = 3.1, P = 0.06]. The summed proportion of famous faces correctly recognized and unfamiliar faces correctly rejected did not significantly differ between patients and controls [t(41) = 1.6, P > 0.1]. For auditory thresholds, repeated measures ANOVA showed no main effect for group [F(1,43) = 2.5, P > 0.1, n = 23] or Group × Frequency interaction [F(4.6,196.1) = 1.5, P > 0.1].
Social cognition
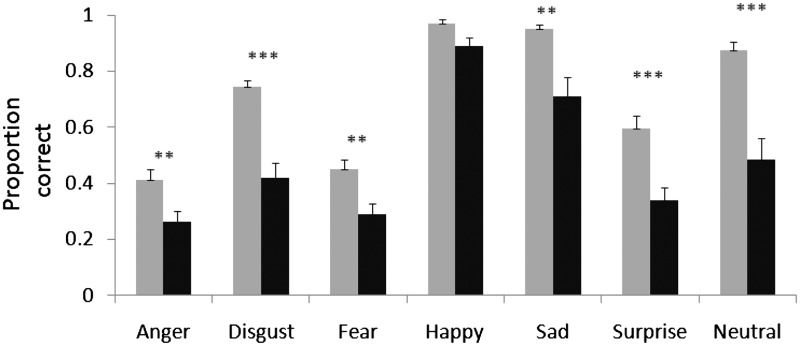
Patients with progressive supranuclear palsy were impaired on the social cognition tasks. For the voice emotion recognition task, repeated measures ANOVA of arcsin transformed scores showed a main effect for group [F(1,43) = 40.8, P < 0.001], a main effect of emotion type [F(4.1,176.4) = 86.6, P < 0.001] and an Emotion × Group interaction [F(4.1,176.4) = 3.9, P = 0.004]. Post hoc analysis showed no significant difference (Bonferroni corrected) in the happy condition but significant deficits for other emotions (each P < 0.007; Fig. 1).
Figure 1.
Voice emotion recognition test, shown separately for each emotion. Progressive supranuclear palsy deficits are seen in a wide range of emotions. Controls are shown in light grey and patients in black. Significance is indicated by asterisks. **Significant difference using Bonferroni correction for seven tests (P < 0.007); and ***P < 0.001. Error bars show standard error.
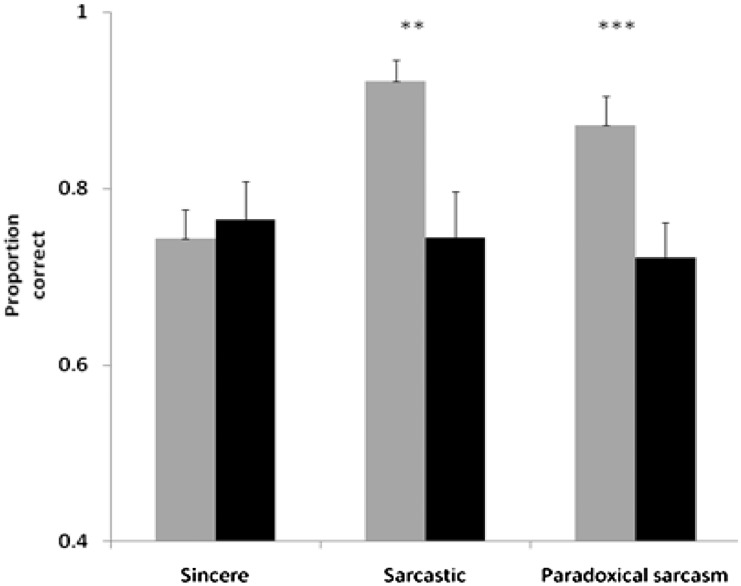
ANOVA of the TASIT (arcsin transformed) confirmed impaired performance by patients with progressive supranuclear palsy [main effect for group, F(1,40) = 15.9, P < 0.001, main effect for context (sincere, sarcastic and paradoxical sarcasm), F(1.7,66.7) = 4.3, P = 0.02 and a Group × Context interaction, F(1.7,66.7) = 5.8, P = 0.007]. Post hoc analysis showed patients were not worse at understanding sincere scenarios [t(33.4) = −0.8, n.s.], but were worse at understanding sarcastic [t(40) = 3.4, P = 0.001] and paradoxically sarcastic scenarios [t(38.9) = 3.5, P < 0.001; Fig. 2].
Figure 2.
Theory of mind on TASIT test. Sincere, sarcastic and paradoxical sarcasm categories from TASIT test. Controls are shown in light grey and patients in black. Using Bonferroni correction for three tests, ** indicates a significant result (P < 0.01); *** P < 0.001. Error bars show standard error.
The voice emotion recognition test was highly correlated with the TASIT (r = 0.73, P < 0.001, n = 20). Voice emotion also correlated with the motor section of the UPDRS (r = −0.49, P = 0.02, n = 22) and the PSPRS (r = −0.44, P = 0.047, n = 21). However, TASIT did not correlate with either UPDRS (r = −0.16, P = 0.5, n = 20) or the PSPRS (r = −0.17, P = 0.5, n = 19). When the correlation between TASIT and voice emotion was reassessed, partialling out UPDRS or PSPRS as a proxy for disease severity, the correlation was still significant [UPDRS (r = 0.74, P < 0.001, df = 17) and PSPRS (r = 0.72, P = 0.001, df = 16)].
We investigated the possible role of executive function in the social cognition tests by carrying out a repeated measure ANOVA for each of the social cognition tests as the dependant variables and the Brixton test as a covariate. The social cognition tests remained significantly different between patients and controls. For voice emotion (arcsin transformed), there was a main effect for group [F(1,39) = 24.5, P < 0.001], main effect for voice emotion test [F(4.2,162.5) = 18.0, P < 0.001], no interaction between voice emotion test and Brixton [F(4.2,162.5) = 1.2, P = 0.3] but a weak interaction between voice emotion test and group [F(4.2,162.5) = 2.4, P = 0.047]. For TASIT (arcsin transformed) there was a main effect for group [F(1,38) = 7.3, P = 0.01], but no main effect for TASIT [F(1.6,58.9) = 3.0, P = 0.07]. There was an interaction between TASIT and Brixton [F(1.6,58.9) = 4.1, P = 0.03] and an interaction between TASIT and group [F(1.6,58.9) = 3.9, P = 0.04)].
Voxel-based morphometry
Simple comparison between patients and controls
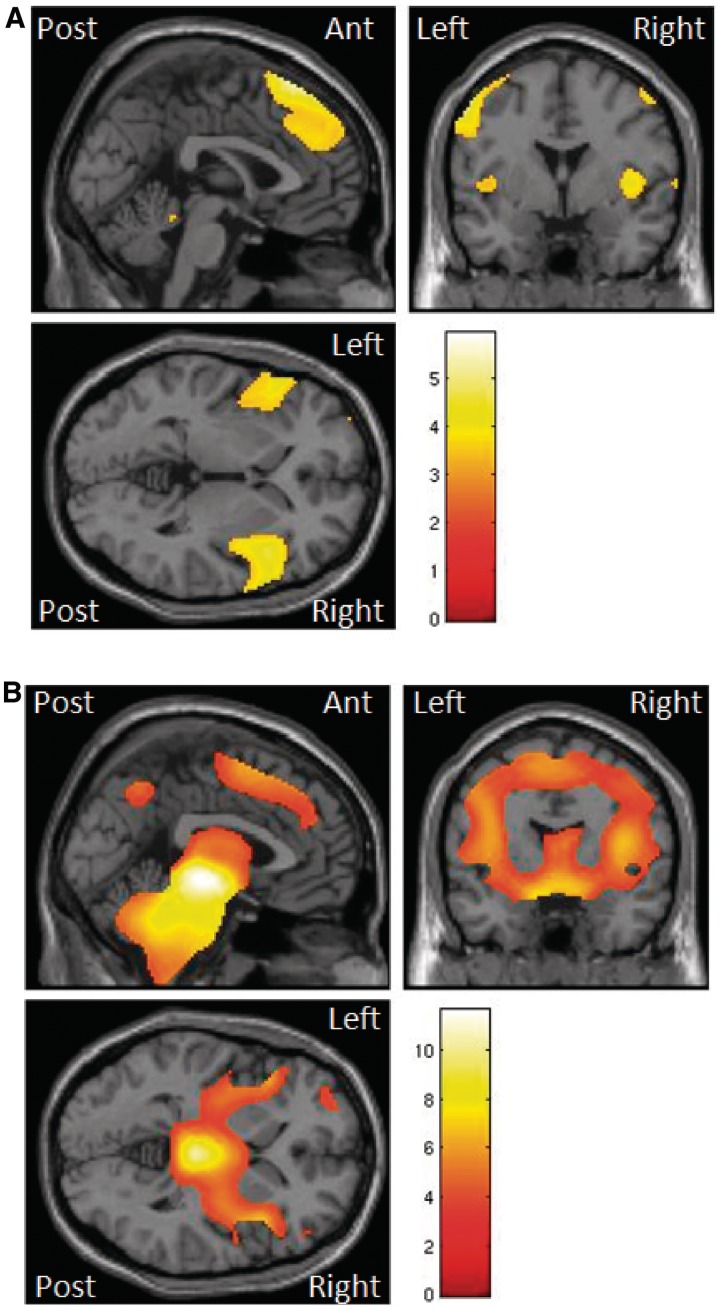
The results are summarized in Tables 2 and 3 and Fig. 3. Progressive supranuclear palsy was associated with grey matter atrophy in the right insula/frontal operculum and precentral gyrus, and in the left superior frontal gyrus, postcentral gyrus and superior parietal lobule. Bilateral or midline atrophy was seen in the vermis, middle frontal gyri and cerebellum. White matter atrophy was greatest in the midbrain, cerebral peduncles and cerebellar tracts, but also present in right orbitofrontal and superior frontal regions. This pattern of atrophy replicates previous reports (Brenneis et al., 2004; Price et al., 2004; Padovani et al., 2006; Paviour et al., 2006b; Nilsson et al., 2007; Josephs et al., 2008; Nicoletti et al., 2008; Rizzo et al., 2008).
Table 2.
Areas of grey matter atrophy in patients compared to controls
| Brain area | x | y | z | Peak Z-score |
|---|---|---|---|---|
| Superior frontal gyrus (L) | −12 | 26 | 66 | 4.98* |
| −24 | −16 | 76 | 3.6 | |
| Middle frontal gyrus (L) | −56 | 6 | 44 | 4.8* |
| Middle frontal gyrus (R) | 58 | 18 | 34 | 4.05 |
| Insula/frontal operculum (R) | 46 | 16 | 2 | 4.05 |
| 42 | −2 | 6 | 3.78 | |
| 64 | 12 | 0 | 3.64 | |
| Posterior cerebellum (L) | −34 | −88 | −44 | 4.04 |
| Posterior cerebellum (R) | 46 | −70 | −56 | 3.99 |
| 38 | −82 | −52 | 3.85 | |
| 24 | −92 | −42 | 3.84 | |
| Postcentral gyrus (L) | −40 | −36 | 68 | 3.68 |
| −8 | −66 | 66 | 3.24 | |
| Lateral cerebellum (L) | −46 | −44 | −48 | 3.51 |
| Lateral inferior cerebellum (L) | −48 | −60 | −58 | 3.38 |
| Precentral gyrus (R) | 36 | −22 | 72 | 3.35 |
| 24 | −22 | 76 | 3.13 | |
| Vermis of cerebellum (midline) | −8 | −40 | −18 | 3.32 |
| Superior parietal lobule (L) | −26 | −48 | 72 | 3.17 |
The table reports peak voxel location and Z-score, exceeding P < 0.001 (uncorrected). *Peaks at which P < 0.05 with a FWE correction for multiple comparison. Coordinates (x, y, z) are given according to standard anatomic space using the Montreal Neurological Institute template. L = left; R = right.
Table 3.
Areas of white matter atrophy in patients compared with controls
| Brain area | x | y | z | Peak Z-score |
|---|---|---|---|---|
| Upper brainstem | 2 | −20 | −8 | 7.6* |
| Cerebral peduncle (L) | −14 | −10 | −16 | 6.96* |
| Cerebellar tracts | 0 | −42 | −28 | 6.13* |
| Orbito-frontal region (R) | 12 | 58 | −18 | 3.43 |
| 8 | 40 | −24 | 3.21 | |
| 24 | 56 | −16 | 3.14 | |
| Superior frontal tracts (R) | 4 | −28 | 74 | 3.1 |
The table reports peak voxel location and Z-score, exceeding P < 0.001 (uncorrected). *Peaks at which P < 0.05 with a FWE correction for multiple comparison. Coordinates (x, y, z) are given according to standard anatomic space using the Montreal Neurological Institute template. L = left; R = right.
Figure 3.
SPM(t) of atrophy in grey (A) and white (B) matter in patients with progressive supranuclear palsy versus controls (threshold P < 0.001 uncorrected, overlaid on representative T1 brain in standard space using the MNI template).
Voxel-based morphometry correlates of neuropsychological performance
Voice emotion
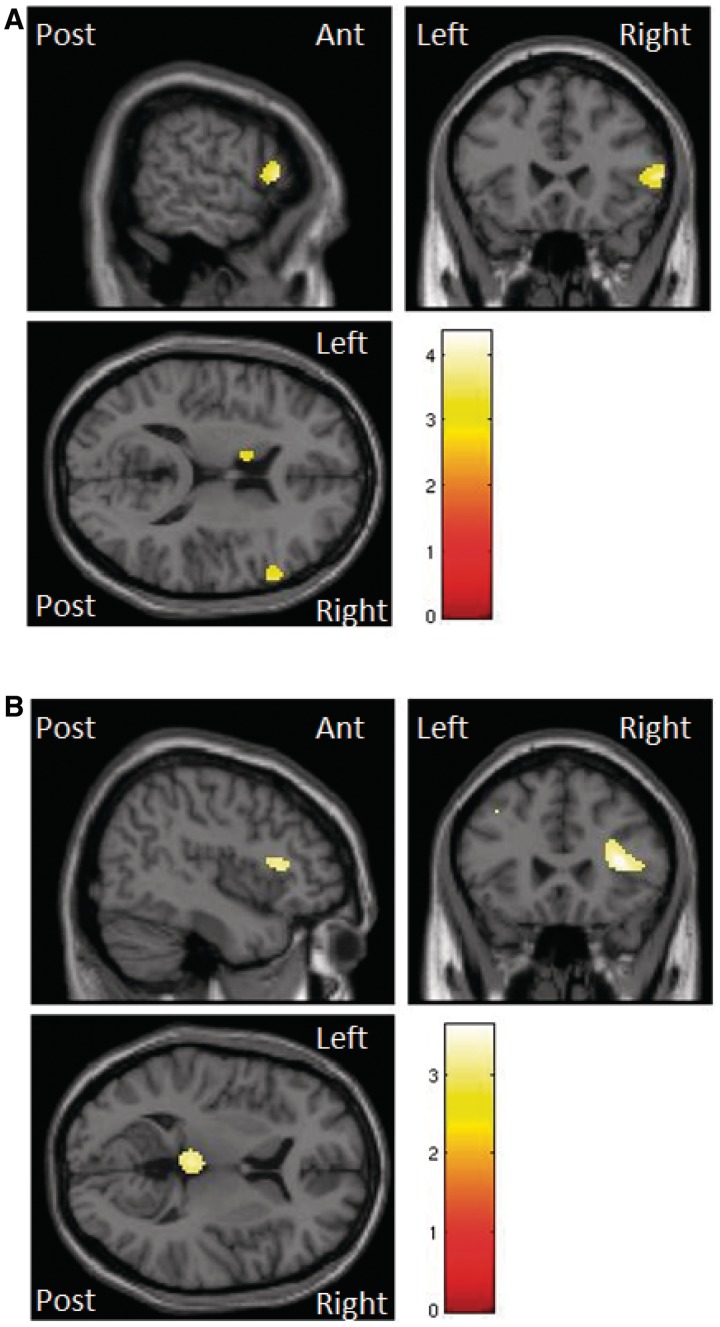
There were associations between averaged voice emotion performance and the right inferior frontal gyrus in both grey and white matter (significant at P < 0.001 uncorrected on whole brain analysis and P < 0.05 FWE corrected within regions of interest). In addition, grey matter atrophy correlated with voice emotion scores in the cerebellum and the left middle frontal gyrus (Table 4 and Fig. 4). There were no significant suprathreshold clusters that correlated with individual voice emotions in grey or white matter. This included a lack of significant correlations in the a priori regions of interest for fear (amydgala) and disgust (insula).
Table 4.
Areas of grey and white matter atrophy in patients when regressed with voice emotion scores
| Brain area | x | y | z | Peak Z-score |
|---|---|---|---|---|
| Grey matter | ||||
| Inferior frontal gyrus (R) | 62 | 24 | 8 | 3.19*,a |
| Middle frontal gyrus (L) | −42 | 14 | 32 | 3.16 |
| Lateral cerebellum (R) | 56 | −56 | −44 | 3.33 |
| White matter | ||||
| Base of inferior frontal gyrus (R) | 36 | 22 | 12 | 3.13 |
The table reports peak voxel location and Z-score, exceeding P < 0.001 (uncorrected). *Peaks at which P < 0.05 with a FWE correction for whole brain multiple comparison.
a The use of an a priori region of interest for Brodmann areas 45 and 47. Coordinates (x, y, z) are given according to standard anatomic space using the Montreal Neurological Institute template. L = left; R = right.
Figure 4.
SPM(t) map of atrophy in grey (A) and white (B) matter seen in patients with progressive supranuclear palsy when regressed with voice emotion scores. Illustrated at P < 0.001 uncorrected.
Theory of mind task
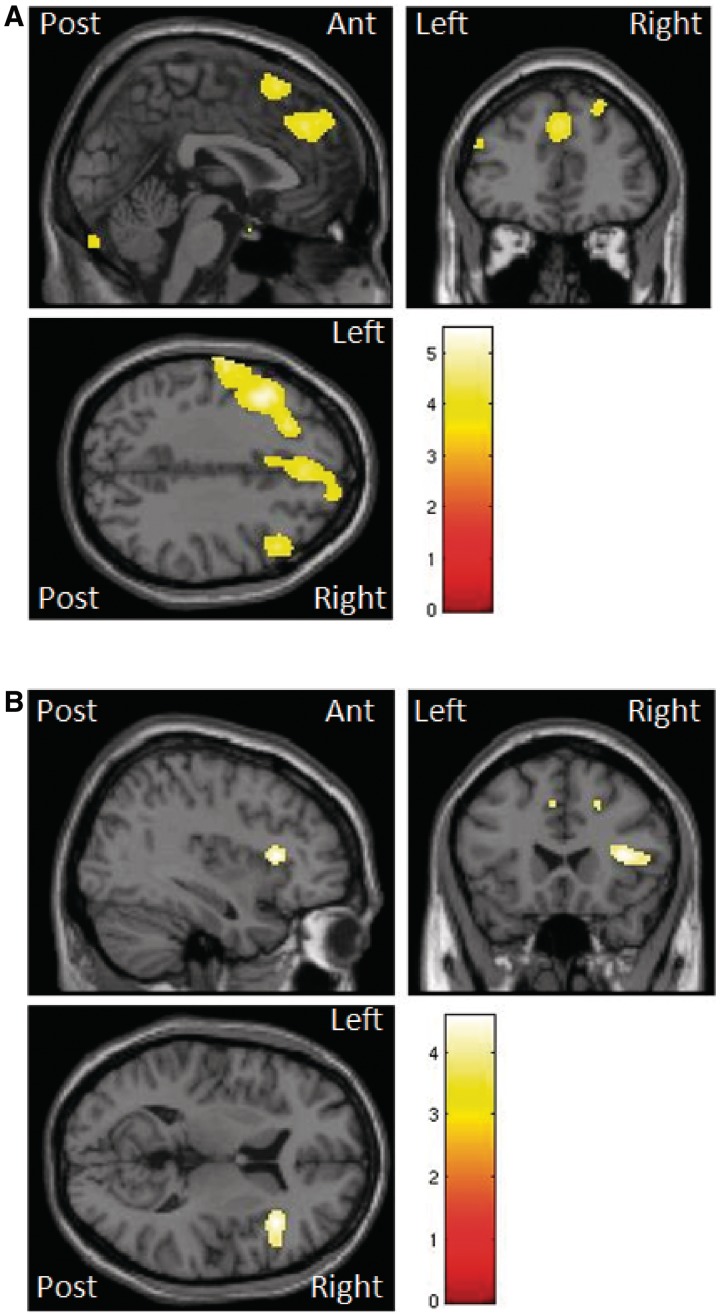
TASIT performance correlated negatively with grey matter atrophy in the anterior rostral medial frontal cortex (significant at P < 0.001 uncorrected on whole brain analysis and P < 0.05 FWE corrected within regions of interest; Fig. 5) as well as the right superior temporal gyrus and the left supramarginal gyrus (equivalent to the left temporoparietal junction; Table 5 and Fig. 5). An association was also seen with the right inferior frontal gyrus (as seen in the voice emotion analysis) and the middle and superior frontal gyri, postcentral gyrus and cerebellum (Table 5 and Fig. 5). There were suprathreshold white matter clusters (P < 0.001 uncorrected) correlated with TASIT scores, in the rostral medial frontal areas and lateral superior and inferior frontal areas on the right (Table 6 and Fig. 5). No areas in grey or white matter regressed with performance on the UPDRS or the PSPRS (P < 0.001).
Figure 5.
SPM(t) map of atrophy in grey (A) and white (B) matter that correlates negatively with TASIT scores. Illustrated uncorrected for multiple comparisons, threshold of P < 0.001 (Tables 5 and 6).
Table 5.
Areas of grey matter atrophy in patients when regressed with TASIT scores
| Brain area | x | y | z | Peak Z-score |
|---|---|---|---|---|
| Inferior frontal gyrus (R) | 62 | 24 | 8 | 4.1 |
| Middle frontal gyrus (L) | −46 | 16 | 32 | 4 |
| Middle frontal gyrus (R) | 26 | 28 | 58 | 3.97 |
| 50 | 24 | 42 | 3.65 | |
| Posterior cerebellum (R) | 44 | −76 | −52 | 3.86 |
| 12 | −88 | −32 | 3.5 | |
| Superior frontal gyrus (L) | −24 | 8 | 64 | 3.77 |
| 0 | 22 | 62 | 3.45 | |
| Postcentral gyrus (L) | −64 | −10 | 40 | 3.71 |
| Anterior medial frontal cortex (R) | 2 | 40 | 36 | 3.49*,a |
| 18 | 56 | 40 | 3.35*,a | |
| 6 | 54 | 42 | 3.29*,a | |
| Superior temporal gyrus (R) | 74 | −18 | −2 | 3.46 |
| Posterior cerebellum (L) | −42 | −60 | −62 | 3.41 |
| Supramarginal gyrus (L) | −52 | −44 | 56 | 3.38 |
| −56 | −52 | 52 | 3.31 |
The table reports peak voxel location and Z score, exceeding P < 0.001 (uncorrected).
*Peaks at which P < 0.05 with a FWE correction for multiple comparison.
a The use of an a priori region of interest for the anterior rostral medial frontal cortex (Amodio and Frith, 2006). Coordinates (x, y, z) are given according to standard anatomic space using the Montreal Neurological Institute template. L = left; R = right.
Table 6.
Areas of white matter atrophy in patients when regressed with TASIT scores
| Brain area | x | y | z | Peak Z-score |
|---|---|---|---|---|
| White matter to inferior frontal gyrus (R) | 38 | 22 | 10 | 3.65 |
| Cingulate area (L) | −2 | 16 | 44 | 3.35 |
| Anterior rostral medial frontal area (R) | 12 | 52 | 34 | 3.19*,a |
| White matter to superior frontal gyrus (R) | 24 | 18 | 38 | 3.14 |
| Posterior rostral medial frontal area (R) | 20 | 26 | 40 | 3.12 |
The table reports peak voxel location and Z score, exceeding P < 0.001 (uncorrected).
*Peaks at which P < 0.05 with a FWE correction for multiple comparison.
a The use of an a priori region of interest for the anterior rostral medial frontal cortex (Amodio and Frith, 2006). Coordinates (x, y, z) are given according to standard anatomic space using the Montreal Neurological Institute template. L = left; R = right.
Discussion
This study confirms that patients with progressive supranuclear palsy have significant impairments in multiple tests of social cognition. This study extends previous work (Ghosh et al., 2009) to show that emotion recognition is affected across modalities and that more complex theory of mind abilities are also impaired. In other words, progressive supranuclear palsy prevented the patients from properly understanding what another person is thinking or feeling. These cognitive deficits were not attributable to differences in simple visual or auditory perceptual functions, and are observed with both static and dynamic stimuli.
The social cognition deficits seen are similar to that seen in behavioural variant frontotemporal dementia, a closely related disorder to progressive supranuclear palsy, which often has a similar underlying tau neuropathology (Esiri et al., 2004; Lough et al., 2006; Kipps et al., 2009b; Rankin et al., 2009; Adenzato et al., 2010). However, in contrast to behavioural variant frontotemporal degeneration, the social cognitive deficits have been under-recognized in progressive supranuclear palsy. This is perhaps because the combination of physical disability, immobility, communication and cognitive problems (Litvan et al., 1996a; Bak and Hodges, 1998; Schrag et al., 2003), which were all observed in our patients, reduces the expression of socially inappropriate behaviours resulting from poor social cognition.
The analysis of MRI confirmed the group effect of progressive supranuclear palsy on regional brain structures, including grey matter atrophy of medial frontal and insula regions (Brenneis et al., 2004; Price et al., 2004; Padovani et al., 2006; Josephs et al., 2008) and severe white matter atrophy of the brainstem and cerebral and cerebellar peduncles (Brenneis et al., 2004; Price et al., 2004; Padovani et al., 2006; Paviour et al., 2006b; Nilsson et al., 2007; Nicoletti et al., 2008; Rizzo et al., 2008).
We extended the voxel-based morphometry approach to look specifically for the areas of atrophy that were associated with deficits in social cognition—both emotion recognition and theory of mind. We found that differential impairment of vocal emotion recognition was associated with atrophy of the right inferior frontal gyrus, an area critical for assessing vocal emotion (Adolphs et al., 2002; Schirmer and Kotz, 2006). Performance in the theory of mind task was also correlated with atrophy of the right inferior frontal gyrus, as well as the anterior rostral medial frontal cortex—an area instrumental in theory of mind capabilities (Amodio and Frith, 2006; Brunet-Gouet and Decety, 2006; Tavares et al., 2008; Carrington and Bailey, 2009; Van Overwalle, 2009).
Neuropsychology of social dysfunction
Progressive supranuclear palsy impaired the recognition of multiple emotions in the auditory (vocal) domain. In contrast to the deficits in the recognition of most emotions, happiness recognition was preserved. This might be because happiness is a positive emotion, the others being either negative (anger, disgust, sad, fear) or intermediate (surprise). It may also have been a ceiling effect, as happiness is least often confused with the other emotions when using visual stimuli (Ekman and Friesen, 1976; Scherer, 2003) and is more readily recognized than the other basic emotions in health (Russell, 1994). Nonetheless, the preservation of happiness recognition provides additional evidence that the patients understood the tasks and were able to respond appropriately.
Progressive supranuclear palsy also impaired the TASIT theory of mind task. Patients did not have difficulties interpreting sincere statements, suggesting that they understood the task, were able to engage with it and could manage its demands on working memory. However, they found the sarcastic statements difficult, particularly the paradoxical sarcasm. This suggests that they were unable to interpret the mental state of the protagonists in the video and have a theory of mind deficit.
Is there a role for executive dysfunction in the social cognition deficits? Some have argued that they are independent (Gregory et al., 2002; Lough et al., 2006; Torralva et al., 2007), while others have proposed that executive function is necessary to understand theory of mind tests (Channon and Crawford, 2000; Aboulafia-Brakha et al., 2011). To investigate this in our group, we used the Brixton test of executive function as it is a non-motor and untimed test of executive function. Although executive function was impaired in patients with progressive supranuclear palsy, we found that social cognition was still impaired when the Brixton test was included as a covariate. This suggests that executive functions are not sufficient to account for the social cognitive deficit in progressive supranuclear palsy, at least as measured by the tests used here.
The social cognition deficits that we have described are similar in range to those found in behavioural variant frontotemporal dementia. Patients with behavioural variant frontotemporal dementia have a profound deficit in both emotion recognition and theory of mind, which has been shown in a variety of tests including TASIT used here (Lough et al., 2006; Kipps et al., 2009b; Rankin et al., 2009; Adenzato et al., 2010; Shany-Ur et al., 2011). As pathology (Esiri et al., 2004) and patterns of atrophy (Rosen et al., 2002a; Brenneis et al., 2004; Price et al., 2004; Williams et al., 2005; Padovani et al., 2006; Josephs et al., 2008; Pereira et al., 2009) overlap between progressive supranuclear palsy and behavioural variant frontotemporal degeneration, it is likely that the aetiology of the social cognition deficits is similar in these two diseases.
Neural correlates of social cognition
A major aim of this study was to understand the neuroanatomical basis of social cognition deficits in progressive supranuclear palsy. The regression of grey and white matter volumes against voice emotion scores confirmed our prediction of a behaviourally relevant structure–function relationship in the right inferior frontal gyrus, as well as regressing with the left middle frontal gyrus. These areas have been associated with the explicit decoding of the prosody of sounds and non-linguistic vocalizations in order to determine their emotional content (George et al., 1996; Buchanan et al., 2000; Adolphs et al., 2002; Wildgruber et al., 2002, 2005; Gandour et al., 2003; Ethofer et al., 2006; Johnstone et al., 2006; Schirmer and Kotz, 2006; Alba-Ferrara et al., 2011). They have also been implicated as being important when interpreting low salience emotional stimuli (Leitman et al., 2010). Although our analysis was cross-sectional and not longitudinal, the result suggests that progressive atrophy of these areas contributes to the decline in the ability of patients with progressive supranuclear palsy to decode auditory emotional stimuli.
Despite our predictions, we did not find a correlation between the insula and disgust or the amygdala and fear stimuli. We estimated that we had sufficient statistical power to detect large or medium effects of association. One possible explanation is that these areas are not essential for recognizing emotion stimuli, despite activation in health. Alternatively, the atrophy in these regions may already have been too severe to show further correlations with performance: the group comparison confirmed that the insula and amygdala were severely atrophic in progressive supranuclear palsy, both in grey and surrounding white matter.
The regressions with TASIT performance revealed structural correlates of theory of mind in the anterior rostral medial frontal cortex. This area has been widely implicated in the function of theory of mind both by analysing theory of mind function in normal participants using functional MRI, as well as theory of mind deficits in patients using voxel-based morphometry (Amodio and Frith, 2006; Gilbert et al., 2006; Shamay-Tsoory et al., 2009). The finding of this association in our patients adds greater credence to our behavioural findings and adds further weight to the link between the anterior rostral medial frontal cortex and theory of mind ability.
In our study, the peak of association was on the superior border of the anterior rostral medial frontal cortex. A meta-analysis (Amodio and Frith, 2006) suggests that this border region of anterior rostral medial frontal cortex is used particularly when people judge actions or thoughts of unfamiliar others, as opposed to the inferior section of the anterior rostral medial frontal cortex, used when assessing the feelings of familiar others. In keeping with this anatomical specialization, TASIT involves assessing the intentions of unfamiliar people, and hence, our results are in keeping with this anatomical distinction.
Consistent with previous studies (Saxe and Kanwisher, 2003; Brunet-Gouet and Decety, 2006; Carrington and Bailey, 2009; Van Overwalle, 2009; Frith and Frith, 2010), the posterior superior temporal sulcus and the temporoparietal junction correlated with social cognition performance in progressive supranuclear palsy. These were not specified as a priori regions of interest for the purposes of ‘small volume correction’ of multiple comparisons. Their identification at the exploratory (uncorrected) threshold is nonetheless consistent with their posited role in theory of mind, and suggests that theory of mind deficits in our patients are linked to the atrophy of these areas seen pathologically (Schofield et al., 2011).
Interestingly, atrophy in the right inferior frontal gyrus and the left middle frontal gyrus correlated with TASIT score, as well as the voice emotion score. The voice emotion and TASIT tests were highly correlated with each other behaviourally. This correlation was still significant when the UPDRS or PSPRS, as proxy measures for general disease progression, were partialled out of the correlation. This suggests that performance on both social cognition tests may represent an underlying neuropsychological mechanism supported by the middle frontal gyrus and inferior frontal gyrus. One possibility could be that these areas are needed to interpret the auditory emotions from the stimuli in both the voice emotion recognition and theory of mind tasks. Previous research has shown that the ability to interpret voice prosody is important for the interpretation of sarcasm (Rockwell, 2007; Cheang and Pell, 2009), and that impairments in these two abilities are correlated in schizophrenia (Leitman et al., 2006). This provides a parsimonious explanation for the finding that atrophy in the right inferior frontal gyrus was correlated with both the voice emotion task and the theory of mind task. Both sets of stimuli necessitate the interpretation of prosody, a function which has been linked to the right inferior frontal gyrus (George et al., 1996; Buchanan et al., 2000; Adolphs et al., 2002; Wildgruber et al., 2002, 2005; Gandour et al., 2003; Ethofer et al., 2006; Johnstone et al., 2006; Schirmer and Kotz, 2006; Alba-Ferera et al., 2011).
The right inferior frontal gyrus might also be related to social cognition deficits as part of a putative mirror neuron system dysfunction. Mirror neurons are involved in the execution of an action, but are also activated while observing the same action when carried out by another (Gallese et al., 2011 for review). In progressive supranuclear palsy, mirror neurons have already been proposed to explain a deficit in naming objects that are manipulable, rather than those that are not (Chow et al., 2010). The authors relate this to patients’ progressive difficulty with manual dexterity and found that naming scores for manipulable items regressed with atrophy in left pre-motor areas. Several groups have gone further to show a link between deficits in the production and recognition of emotions (Wicker et al., 2003; Gallese et al., 2004). Other studies have looked at contextually specific, observed or heard motor tasks and found activation in cerebral areas that are necessary for carrying out social cognitive tasks (Gazzola et al., 2006; Kaplan and Iacoboni, 2006). Increased activation in the putative mirror neuron system has also been related to higher scores on empathy tests. For example, activity in the right inferior frontal gyrus increased when observed actions were correct contextually, suggesting the subjects used a ‘cognitive empathy’ approach for interpretation of the task (Kaplan and Iacoboni, 2006). In addition, that study found that the right inferior frontal gyrus activity correlated with several measures of empathy, including the ability to identify with characters in a fictional situation, clearly an ability that would be relevant in our assessment of theory of mind in progressive supranuclear palsy [but see Tavares et al. (2011) for contrasting findings, and Gallese et al. (2011) for broader issues with mirror neuron accounts of social cognition]. In our study, this interpretation is partially supported by the correlation of the voice emotion score to tests with a strong motor component, the UPDRS and the PSPRS (although we note that the TASIT did not correlate to either the UPDRS or the PSPRS).
Limitations of the study
There are several limitations to this study. First, we relied on clinical not pathological diagnosis, and this might in principal affect the specificity of the results. However, the clinical diagnostic criteria convey >90% accuracy in large trials (Bensimon et al., 2009) and in our local pathology series, so the associations we identify are unlikely to be driven by a pathology other than progressive supranuclear palsy. All nine of the patients who have undergone post-mortem examination by the time of writing had confirmation of progressive supranuclear palsy as the principal diagnosis. Second, we used a cross-sectional design, with patients who were both early and late in the disease. We cannot therefore make inferences about the within-subject progression of social cognition over time, or its relation to progressive atrophy. Regarding neuropsychological assessment, one must also consider confounds of performance, including fatigue. We took several steps to limit this, including short assessment periods over several days, preferentially in the morning or with frequent rest periods. The normal performance on control tasks/conditions speaks against a generic confound such as fatigue.
Voxel-based morphometry has potential confounds, including extra-axial tissue confounds, mis-registration to templates or tissue misclassification. To reduce these confounds, we used a voxel-based morphometry protocol that has been shown to be optimal in other age-related neurodegenerative diseases, and we replicated the simple group comparison results from previous studies of grey and white matter change in progressive supranuclear palsy (Brenneis et al., 2004; Price et al., 2004; Paviour et al., 2005, 2006a, b; Padovani et al., 2006; Nilsson et al., 2007; Josephs et al., 2008; Nicoletti et al., 2008; Rizzo et al., 2008). Our statistical approach was to emphasize control of type I error, and to use pre-specified regions of interest to enhance statistical power at the expense of anatomical coverage for key contrasts. Several areas, such as the temporal and temporoparietal cortex, were identified using the exploratory threshold. Although these areas have sometimes been associated with normal social cognition, we did not include them in our a priori regions of interest, highlighting the compromises inherent in region of interest analyses. We therefore also report at a more liberal threshold (0.001 uncorrected) as this may increase reliability across studies (Thirion et al., 2007). Larger groups would increase power, but our study was similar or larger than many voxel-based morphometry studies of progressive supranuclear palsy and related disorders (Whitwell et al., 2005; Kipps et al., 2007, 2009b; Noppeney et al., 2007; Werner et al., 2007; Rohrer et al., 2010).
We suggest that the deficits in social cognition arise from focal atrophy of critical brain regions and that they are likely to contribute to the reduced quality of life and psychiatric comorbidity among patients and carers (Schrag et al., 2003; Herting et al., 2007; Bak et al., 2010). However, in this study, we did not assess quality of life among our patient cohort, nor monitor the progression of these factors over time, and further work would be required to demonstrate any causal association.
Conclusion
We have shown that progressive supranuclear palsy impairs the recognition of emotion and impairs theory of mind. In addition to replicating grey and white matter atrophy patterns in progressive supranuclear palsy, we have found structural correlations with social cognition performance. Specifically, atrophy in the right inferior frontal gyrus correlated negatively with scores on both the voice emotion test and TASIT, and atrophy of the anterior rostral medial frontal cortex correlated with theory of mind impairments. These results, together with the preserved performance on control tasks, indicate a generic social cognition deficit due to progressive supranuclear palsy pathology in critical brain networks for social cognition.
Set in the context of previous research, showing that 50% of patients complain of a negative impact on their quality of life due to social impairment (Schrag et al. 2003) and research indicating that patients present with behavioural change, which includes disinhibition and aggressiveness (Donker Kaat et al., 2007), a social cognition deficit should be considered by those managing and caring for patients with progressive supranuclear palsy. Greater acknowledgment of the patients’ impaired ability to empathize with those around them may help to alleviate patient–carer relationship difficulties at a time when the patient needs them most.
Funding
This work was supported by the Medical Research Council [G0700503 to B.G., MC_US_A060_0017 to A.C.]; the Guarantors of Brain (to B.G.); the Raymond and Beverley Sackler Trust (to B.G.); Parkinson’s UK (to A.L. and P.P.). J.R. is supported by the Wellcome Trust [088324] and the Cambridge National Institute of Health Research Comprehensive Biomedical Research Centre. A.D.L. is supported by the Wales Institute of Cognitive Neuroscience (WICN).
Supplementary material
Supplementary material is available at Brain online.
Acknowledgements
We are grateful to Dr Nimmo-Smith of the MRC Cognition and Brain Sciences Unit for providing advice in relation to the statistics and to our patients for assisting us with this research.
Glossary
Abbreviations
- PSPRS
progressive supranuclear palsy rating scale
- TASIT
the awareness of social inference test
- UPDRS
Unified Parkinson Disease Rating Scale
References
- Aarsland D, Litvan I, Larsen JP. Neuropsychiatric symptoms of patients with progressive supranuclear palsy and Parkinson's disease. J Neuropsychiatry Clin Neurosci. 2001;13:42–9. doi: 10.1176/jnp.13.1.42. [DOI] [PubMed] [Google Scholar]
- Aboulafia-Brakha T, Christe B, Martory MD, Annoni JM. Theory of mind tasks and executive functions: a systematic review of group studies in neurology. J Neuropsychol. 2011;5:39–55. doi: 10.1348/174866410X533660. [DOI] [PubMed] [Google Scholar]
- Acosta-Cabronero J, Williams GB, Pereira JMS, Pengas G, Nestor PJ. The impact of skull-stripping and radio-frequency bins correction on grey-matter segmentation for voxel-based morphometry. Neuroimage. 2008;39:1654–65. doi: 10.1016/j.neuroimage.2007.10.051. [DOI] [PubMed] [Google Scholar]
- Adenzato M, Cavallo M, Enrici I. Theory of mind ability in the behavioural variant of frontotemporal dementia: an analysis of the neural, cognitive, and social levels. Neuropsychologia. 2010;48:2–12. doi: 10.1016/j.neuropsychologia.2009.08.001. [DOI] [PubMed] [Google Scholar]
- Adolphs R, Damasio H, Tranel D. Neural systems for recognition of emotional prosody: a 3-D lesion study. Emotion. 2002;2:23–51. doi: 10.1037/1528-3542.2.1.23. [DOI] [PubMed] [Google Scholar]
- Adolphs R, Damasio H, Tranel D, Cooper G, Damasio AR. A role for somatosensory cortices in the visual recognition of emotion as revealed by three-dimensional lesion mapping. J Neurosci. 2000;20:2683–90. doi: 10.1523/JNEUROSCI.20-07-02683.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adolphs R, Damasio H, Tranel D, Damasio AR. Cortical systems for the recognition of emotion in facial expressions. J Neurosci. 1996;16:7678–87. doi: 10.1523/JNEUROSCI.16-23-07678.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adolphs R, Tranel D, Damasio H, Damasio A. Impaired recognition of emotion in facial expressions following bilateral damage to the human amygdala. Nature. 1994;372:669–72. doi: 10.1038/372669a0. [DOI] [PubMed] [Google Scholar]
- Alba-Ferrara L, Hausmann M, Mitchell RL, Weis S. The neural correlates of emotional prosody comprehension: disentangling simple from complex emotion. PLoS One. 2011;6:e28701. doi: 10.1371/journal.pone.0028701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amodio DM, Frith CD. Meeting of minds: the medial frontal cortex and social cognition. Nat Rev Neurosci. 2006;7:268–77. doi: 10.1038/nrn1884. [DOI] [PubMed] [Google Scholar]
- Ashburner J. Computational anatomy with the SPM software. Magn Reson Imaging. 2009;27:1163–74. doi: 10.1016/j.mri.2009.01.006. [DOI] [PubMed] [Google Scholar]
- Ashburner J, Friston KJ. Unified segmentation. NeuroImage. 2005;26:839–51. doi: 10.1016/j.neuroimage.2005.02.018. [DOI] [PubMed] [Google Scholar]
- Bak TH, Crawford LM, Berrios G, Hodges JR. Behavioural symptoms in progressive supranuclear palsy and frontotemporal dementia. J NeurolNeurosurg Psychiatry. 2010;81:1057–9. doi: 10.1136/jnnp.2008.157974. [DOI] [PubMed] [Google Scholar]
- Bak TH, Hodges J. The neuropsychology of progressive supranuclear palsy. Neurocase. 1998;4:89–94. [Google Scholar]
- Baron-Cohen S, Wheelwright S, Hill J, Raste Y, Plumb I. The ‘Reading the Mind in the Eyes’ Test revised version: a study with normal adults, and adults with Asperger syndrome or high-functioning autism. J Child Psychol Psychiatry. 2001;42:241–51. [PubMed] [Google Scholar]
- Bastiaansen JA, Thioux M, Keysers C. Evidence for mirror systems in emotions. Phil Trans R Soc B. 2009;364:2391–404. doi: 10.1098/rstb.2009.0058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beaucousin V, Lacheret A, Turbelin M-R, Morel M, Mazoyer B, Tzourio-Mazoyer N. FMRI study of emotional speech comprehension. Cereb Cortex. 2007;17:339–52. doi: 10.1093/cercor/bhj151. [DOI] [PubMed] [Google Scholar]
- Belin P, Fillion-Bilodeau S, Gosselin F. The Montreal Affective Voices: a validated set of nonverbal affect bursts for research on auditory affective processing. Behav Res Methods. 2008;40:531–9. doi: 10.3758/brm.40.2.531. [DOI] [PubMed] [Google Scholar]
- Bensimon G, Ludolph A, Agid Y, Vidailhet M, Payan C, Leigh PN. Riluzole treatment, survival and diagnostic criteria in Parkinson plus disorders: the NNIPPS study. Brain. 2009;132:156–71. doi: 10.1093/brain/awn291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borroni B, Turla M, Bertasi V, Agosti C, Gilberti N, Padovani A. Cognitive and behavioral assessment in the early stages of neurodegenerative extrapyramidal syndromes. Arch Gerontol Geriatr. 2008;47:53–61. doi: 10.1016/j.archger.2007.07.005. [DOI] [PubMed] [Google Scholar]
- Brenneis C, Seppi K, Schocke M, Benke T, Wenning GK, Poewe W. Voxel based morphometry reveals a distinct pattern of frontal atrophy in progressive supranuclear palsy. J Neurol Neurosurg Psychiatry. 2004;75:246–9. [PMC free article] [PubMed] [Google Scholar]
- Brown RG, Lacomblez L, Landwehrmeyer BG, Bak T, Uttner I, Dubois B, et al. Cognitive impairment in patients with multiple system atrophy and progressive supranuclear palsy. Brain. 2010;133:2382–93. doi: 10.1093/brain/awq158. [DOI] [PubMed] [Google Scholar]
- Brunet-Gouet E, Decety J. Social brain dysfunctions in schizophrenia: a review of neuroimaging studies. Psychiatry Res Neuroimaging. 2006;148:75–92. doi: 10.1016/j.pscychresns.2006.05.001. [DOI] [PubMed] [Google Scholar]
- Buchanan TW, Lutz K, Mirzazade S, Specht K, Shah NJ, Zilles K, et al. Recognition of emotional prosody and verbal components of spoken language: an fMRI study. Cogn Brain Res. 2000;9:227–38. doi: 10.1016/s0926-6410(99)00060-9. [DOI] [PubMed] [Google Scholar]
- Burgess PW, Shallice T. The Hayling and Brixton tests. Bury St Edmunds: Thames Valley Test Company; 1997. [Google Scholar]
- Calder AJ, Keane J, Manes F, Antoun N, Young AW. Impaired recognition and experience of disgust following brain injury. Nat Neurosci. 2000;3:1077–8. doi: 10.1038/80586. [DOI] [PubMed] [Google Scholar]
- Calder AJ, Keane J, Young AW, Lawrence AD, Mason S, Barker RA. The relation between anger and different forms of disgust: implications for emotion recognition impairments in Huntington’s disease. Neuropsychologia. 2010;48:2719–29. doi: 10.1016/j.neuropsychologia.2010.05.019. [DOI] [PubMed] [Google Scholar]
- Calder AJ, Lawrence AD, Young AW. Neuropsychology of fear and loathing. Nat Rev Neurosci. 2001;2:352–63. doi: 10.1038/35072584. [DOI] [PubMed] [Google Scholar]
- Calder AJ, Young AW, Rowland D, Perrett DI, Hodges JR, Etcoff NL. Facial emotion recognition after bilateral amygdala damage: differentially severe impairment of fear. Cogn Neuropsychol. 1996;13:699–745. [Google Scholar]
- Carrington SJ, Bailey AJ. Are there theory of mind regions in the brain? A review of the neuroimaging literature. Hum Brain Mapp. 2009;30:2313–35. doi: 10.1002/hbm.20671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Channon S, Crawford S. The effects of anterior lesions on performance on a story comprehension test: left anterior impairment on a theory of mind-type task. Neuropsychologia. 2000;38:1006–17. doi: 10.1016/s0028-3932(99)00154-2. [DOI] [PubMed] [Google Scholar]
- Cheang HS, Pell MD. Acoustic markers of sarcasm in Cantonese and English. J Acoust Soc Am. 2009;126:1394–405. doi: 10.1121/1.3177275. [DOI] [PubMed] [Google Scholar]
- Chow ML, Brambati SM, Gorno-Tempini ML, Miller BL, Johnson JK. Sound naming in neurodegenerative disease. Brain Cogn. 2010;72:423–9. doi: 10.1016/j.bandc.2009.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cordato NJ, Duggins AJ, Halliday GM, Morris JG, Pantelis C. Clinical deficits correlate with regional cerebral atrophy in progressive supranuclear palsy. Brain. 2005;128:1259–66. doi: 10.1093/brain/awh508. [DOI] [PubMed] [Google Scholar]
- Cordato NJ, Halliday GM, Harding AJ, Hely MA, Morris JG. Regional brain atrophy in progressive supranuclear palsy and Lewy body disease. Ann Neurol. 2000;47:718–28. [PubMed] [Google Scholar]
- Cordato NJ, Pantelis C, Halliday GM, Velakoulis D, Wood SJ, Stuart GW, et al. Frontal atrophy correlates with behavioural changes in progressive supranuclear palsy. Brain. 2002;125:789–800. doi: 10.1093/brain/awf082. [DOI] [PubMed] [Google Scholar]
- Donker Kaat L, Boon AJ, Kamphorst W, Ravid R, Duivenvoorden HJ, van Swieten JC. Frontal presentation in progressive supranuclear palsy. Neurology. 2007;69:723–9. doi: 10.1212/01.wnl.0000267643.24870.26. [DOI] [PubMed] [Google Scholar]
- Diehl-Schmid J, Pohl C, Ruprecht C, Wagenpfeil S, Foerstl H, Kurz A. The Ekman 60 Faces Test as a diagnostic instrument in frontotemporal dementia. Arch Clin Neuropsychol. 2007;22:459–64. doi: 10.1016/j.acn.2007.01.024. [DOI] [PubMed] [Google Scholar]
- Ekman P, Friesen WV. Pictures of facial affect. Palo Alto, CA: Consulting Psychologists Press; 1976. [Google Scholar]
- Esiri MM, Lee VM-Y, Trojanowski JQ. The neuropathology of dementia. Cambridge: Cambridge University Press; 2004. [Google Scholar]
- Ethofer T, Anders S, Erb M, Herbert C, Wiethoff S, Kissler J, et al. Cerebral pathways in processing of affective prosody: a dynamic causal modeling study. NeuroImage. 2006;30:580–7. doi: 10.1016/j.neuroimage.2005.09.059. [DOI] [PubMed] [Google Scholar]
- Fahn S. Recent developments in Parkinson's disease. New York: Raven Press; 1986. [Google Scholar]
- Frith U, Frith CD. Development and neurophysiology of mentalizing. Phil Trans R Soc B Biol Sci. 2003;358:459–73. doi: 10.1098/rstb.2002.1218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frith U, Frith C. The social brain: allowing humans to boldly go where no other species has been. Phil Trans R Soc B Biol Sci. 2010;365:165–76. doi: 10.1098/rstb.2009.0160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gallese V, Gernsbacher MA, Heyes C, Hickok G, Iacoboni M. Mirror neuron forum. Perspect Psychol Sci. 2011;6:369–407. doi: 10.1177/1745691611413392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gallese V, Keysers C, Rizzolatti G. A unifying view of the basis of social cognition. Trends Cogn Sci. 2004;8:396–403. doi: 10.1016/j.tics.2004.07.002. [DOI] [PubMed] [Google Scholar]
- Gandour J, Wong D, Dzemidzic M, Lowe M, Tong Y, Li X. A cross-linguistic fMRI study of perception of intonation and emotion in Chinese. Hum Brain Mapp. 2003;18:149–57. doi: 10.1002/hbm.10088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gazzola V, Aziz-Zadeh L, Keysers C. Empathy and the somatotopic auditory mirror system in humans. Curr Biol. 2006;16:1824–9. doi: 10.1016/j.cub.2006.07.072. [DOI] [PubMed] [Google Scholar]
- George MS, Parekh PI, Rosinsky N, Ketter TA, Kimbrell TA, Heilman KM, et al. Understanding emotional prosody activates right hemisphere regions. Arch Neurol. 1996;53:665–70. doi: 10.1001/archneur.1996.00550070103017. [DOI] [PubMed] [Google Scholar]
- Ghosh BC, Rowe JB, Calder AJ, Hodges JR, Bak TH. Emotion recognition in progressive supranuclear palsy. J Neurol Neurosurg Psychiatry. 2009;80:1143–5. doi: 10.1136/jnnp.2008.155846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilbert SJ, Spengler S, Simons JS, Steele JD, Lawrie SM, Frith CD, et al. Functional specialization within rostral prefrontal cortex (area 10): a meta-analysis. J Cogn Neurosci. 2006;18:932–48. doi: 10.1162/jocn.2006.18.6.932. [DOI] [PubMed] [Google Scholar]
- Golbe LI, Ohman-Strickland PA. A clinical rating scale for progressive supranuclear palsy. Brain. 2007;130:1552–65. doi: 10.1093/brain/awm032. [DOI] [PubMed] [Google Scholar]
- Grafman J, Litvan I, Stark M. Neuropsychological features of progressive supranuclear palsy. Brain Cogn. 1995;28:311–20. doi: 10.1006/brcg.1995.1260. [DOI] [PubMed] [Google Scholar]
- Gray HM, Tickle-Degnen L. A meta-analysis of performance on emotion recognition tasks in Parkinson's disease. Neuropsychology. 2010;24:176–191. doi: 10.1037/a0018104. [DOI] [PubMed] [Google Scholar]
- Gregory C, Lough S, Stone V, Erzinclioglu S, Martin L, Baron-Cohen S, et al. Theory of mind in patients with frontal variant frontotemporal dementia and Alzheimer's disease: theoretical and practical implications. Brain. 2002;125:752–64. doi: 10.1093/brain/awf079. [DOI] [PubMed] [Google Scholar]
- Henley SMD, Wild EJ, Hobbs NZ, Warren JD, Frost C, Scahill RI, et al. Defective emotion recognition in early HD is neuropsychologically and anatomically generic. Neuropsychologia. 2008;46:2152–60. doi: 10.1016/j.neuropsychologia.2008.02.025. [DOI] [PubMed] [Google Scholar]
- Hennenlotter A, Schroeder U, Erhard P, Haslinger B, Stahl R, Weindl A, et al. Neural correlates associated with impaired disgust processing in pre-symptomatic Huntington's disease. Brain. 2004;127:1446–53. doi: 10.1093/brain/awh165. [DOI] [PubMed] [Google Scholar]
- Herting B, Beuthien-Baumann B, Pöttrich K, Donix M, Triemer A, Lampe JB, et al. Prefrontal cortex dysfunction and depression in atypical Parkinsonian syndromes. Mov Disord. 2007;22:490–7. doi: 10.1002/mds.21237. [DOI] [PubMed] [Google Scholar]
- Hirao K, Miyata J, Fujiwara H, Yamada M, Namiki C, Shimizu M, et al. Theory of mind and frontal lobe pathology in schizophrenia: a voxel-based morphometry study. Schizophrenia Res. 2008;105:165–74. doi: 10.1016/j.schres.2008.07.021. [DOI] [PubMed] [Google Scholar]
- Hornak J, Bramham J, Rolls ET, Morris RG, O'Doherty J, Bullock PR, et al. Changes in emotion after circumscribed surgical lesions of the orbitofrontal and cingulate cortices. Brain. 2003;126:1691–712. doi: 10.1093/brain/awg168. [DOI] [PubMed] [Google Scholar]
- Izard CE. The face of emotion. New York [S.l.]: Appleton; 1971. [Google Scholar]
- Johnstone T, van Reekum CM, Oakes TR, Davidson RJ. The voice of emotion: an FMRI study of neural responses to angry and happy vocal expressions. Soc Cogn Affect Neurosci. 2006;1:242–9. doi: 10.1093/scan/nsl027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Josephs KA, Whitwell JL, Dickson DW, Boeve BF, Knopman DS, Petersen RC, et al. Voxel-based morphometry in autopsy proven progressive supranuclear palsy and CBD. Neurobiol Aging. 2008;29:280–9. doi: 10.1016/j.neurobiolaging.2006.09.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Juslin P, Scherer K. Vocal expression of affect. In: Harrigan J, Rosentha R, Scherer K, editors. The new handbook of methods in nonverbal behavior research. New York: Oxford University Press; 2005. pp. 65–135. [Google Scholar]
- Kaplan JT, Iacoboni M. Getting a grip on other minds: mirror neurons, intention understanding, and cognitive empathy. Soc Neurosci. 2006;1:175–83. doi: 10.1080/17470910600985605. [DOI] [PubMed] [Google Scholar]
- Keane J, Calder AJ, Hodges JR, Young AW. Face and emotion processing in frontal variant frontotemporal dementia. Neuropsychologia. 2002;40:655–65. doi: 10.1016/s0028-3932(01)00156-7. [DOI] [PubMed] [Google Scholar]
- Kipps CM, Duggins AJ, McCusker EA, Calder AJ. Disgust and happiness recognition correlate with anteroventral insula and amygdala volume respectively in preclinical Huntington's disease. J Cogn Neurosci. 2007;19:1206–17. doi: 10.1162/jocn.2007.19.7.1206. [DOI] [PubMed] [Google Scholar]
- Kipps CM, Mioshi E, Hodges JR. Emotion, social functioning and activities of daily living in frontotemporal dementia. Neurocase. 2009a;15:182–9. doi: 10.1080/13554790802632892. [DOI] [PubMed] [Google Scholar]
- Kipps CM, Nestor PJ, Acosta-Cabronero J, Arnold R, Hodges JR. Understanding social dysfunction in the behavioural variant of frontotemporal dementia: the role of emotion and sarcasm processing. Brain. 2009b;132:592–603. doi: 10.1093/brain/awn314. [DOI] [PubMed] [Google Scholar]
- Lancaster JL, Summerln JL, Rainey L, Freitas CS, Fox PT. The Talairach Daemon, a database server for Talairach Atlas Labels. NeuroImage. 1997;5:S633. [Google Scholar]
- Lancaster JL, Woldorff MG, Parsons LM, Liotti M, Freitas CS, Rainey L, et al. Automated Talairach atlas labels for functional brain mapping. Hum Brain Mapp. 2000;10:120–31. doi: 10.1002/1097-0193(200007)10:3<120::AID-HBM30>3.0.CO;2-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lawrence AD, Goerendt IK, Brooks DJ. Impaired recognition of facial expressions of anger in Parkinson's disease patients acutely withdrawn from dopamine replacement therapy. Neuropsychologia. 2007;45:65–74. doi: 10.1016/j.neuropsychologia.2006.04.016. [DOI] [PubMed] [Google Scholar]
- Leitman DI, Wolf DH, Ragland JD, Laukka P, Loughead J, Valdez JN, et al. ‘It's not what you say, but how you say it’: a reciprocal temporo-frontal network for affective prosody. Front Hum Neurosci. 2010;4:19. doi: 10.3389/fnhum.2010.00019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leitman DI, Ziwich R, Pasternak R, Javitt DC. Theory of Mind (Theory of Mind) and counterfactuality deficits in schizophrenia: misperception or misinterpretation? Psychol Med. 2006;36:1075–83. doi: 10.1017/S0033291706007653. [DOI] [PubMed] [Google Scholar]
- Litvan I, Bhatia KP, Burn DJ, Goetz CG, Lang AE, McKeith I, et al. Movement Disorders Society Scientific Issues Committee report: SIC Task Force appraisal of clinical diagnostic criteria for Parkinsonian disorders. Mov Disord. 2003;18:467–86. doi: 10.1002/mds.10459. [DOI] [PubMed] [Google Scholar]
- Litvan I, Mangone CA, McKee A, Verny M, Parsa A, Jellinger K, et al. Natural history of progressive supranuclear palsy (Steele-Richardson-Olszewski syndrome) and clinical predictors of survival: a clinicopathological study. J Neurol Neurosurg Psychiatry. 1996a;60:615–20. doi: 10.1136/jnnp.60.6.615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Litvan I, Mega MS, Cummings JL, Fairbanks L. Neuropsychiatric aspects of progressive supranuclear palsy. Neurology. 1996b;47:1184–9. doi: 10.1212/wnl.47.5.1184. [DOI] [PubMed] [Google Scholar]
- Lough S, Kipps CM, Treise C, Watson P, Blair JR, Hodges JR. Social reasoning, emotion and empathy in frontotemporal dementia. Neuropsychologia. 2006;44:950–8. doi: 10.1016/j.neuropsychologia.2005.08.009. [DOI] [PubMed] [Google Scholar]
- Maldjian JA, Laurienti PJ, Burdette JH. Precentral gyrus discrepancy in electronic versions of the Talairach atlas. NeuroImage. 2004;21:450–5. doi: 10.1016/j.neuroimage.2003.09.032. [DOI] [PubMed] [Google Scholar]
- Maldjian JA, Laurienti PJ, Kraft RA, Burdette JH. An automated method for neuroanatomic and cytoarchitectonic atlas-based interrogation of fMRI data sets. NeuroImage. 2003;19:1233–9. doi: 10.1016/s1053-8119(03)00169-1. [DOI] [PubMed] [Google Scholar]
- McDonald S, Flanagan S, Rollins J. The awareness of social inference test. Bury St. Edmunds: Thames Valley Test Company; 2002. [Google Scholar]
- McDonald S, Flanagan S, Rollins J, Kinch J. TASIT: a new clinical tool for assessing social perception after traumatic brain injury. J Head Trauma Rehabil. 2003;18:219–38. doi: 10.1097/00001199-200305000-00001. [DOI] [PubMed] [Google Scholar]
- Millar D, Griffiths P, Zermansky AJ, Burn DJ. Characterizing behavioral and cognitive dysexecutive changes in progressive supranuclear palsy. Mov Disord. 2006;21:199–207. doi: 10.1002/mds.20707. [DOI] [PubMed] [Google Scholar]
- Murphy FC, Nimmo-Smith IAN, Lawrence AD. Functional neuroanatomy of emotions: a meta-analysis. Cogn Affect Behav Neurosci. 2003;3:207–233. doi: 10.3758/cabn.3.3.207. [DOI] [PubMed] [Google Scholar]
- Nakamura K, Kawashima R, Ito K, Sugiura M, Kato T, Nakamura A, et al. Activation of the right inferior frontal cortex during assessment of facial emotion. J Neurophysiol. 1999;82:1610–4. doi: 10.1152/jn.1999.82.3.1610. [DOI] [PubMed] [Google Scholar]
- Nicoletti G, Tonon C, Lodi R, Condino F, Manners D, Malucelli E, et al. Apparent diffusion coefficient of the superior cerebellar peduncle differentiates progressive supranuclear palsy from Parkinson's disease. Mov Disord. 2008;23:2370–6. doi: 10.1002/mds.22279. [DOI] [PubMed] [Google Scholar]
- Nilsson C, Bloch KM, Brockstedt S, Latt J, Widner H, Larsson E. Tracking the neurodegeneration of parkinsonian disorders: a pilot study. Neuroradiology. 2007;49:111–9. doi: 10.1007/s00234-006-0165-1. [DOI] [PubMed] [Google Scholar]
- Noppeney U, Patterson K, Tyler LK, Moss H, Stamatakis EA, Bright P, et al. Temporal lobe lesions and semantic impairment: a comparison of herpes simplex virus encephalitis and semantic dementia. Brain. 2007;130:1138–47. doi: 10.1093/brain/awl344. [DOI] [PubMed] [Google Scholar]
- Padovani A, Borroni B, Brambati SM, Agosti C, Broli M, Alonso R, et al. Diffusion tensor imaging and voxel based morphometry study in early progressive supranuclear palsy. J Neurol Neurosurg Psychiatry. 2006;77:457–63. doi: 10.1136/jnnp.2005.075713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paulmann S, Seifert S, Kotz SA. Orbito-frontal lesions cause impairment during late but not early emotional prosodic processing. Soc Neurosci. 2010;5:59–75. doi: 10.1080/17470910903135668. [DOI] [PubMed] [Google Scholar]
- Paviour DC, Price SL, Jahanshahi M, Lees AJ, Fox NC. Longitudinal MRI in progressive supranuclear palsy and multiple system atrophy: rates and regions of atrophy. Brain. 2006a;129:1040–9. doi: 10.1093/brain/awl021. [DOI] [PubMed] [Google Scholar]
- Paviour DC, Price SL, Jahanshahi M, Lees AJ, Fox NC. Regional brain volumes distinguish progressive supranuclear palsy, MSA-P, and PD: MRI-based clinico-radiological correlations. Mov Disord. 2006b;21:989–96. doi: 10.1002/mds.20877. [DOI] [PubMed] [Google Scholar]
- Paviour DC, Price SL, Stevens JM, Lees AJ, Fox NC. Quantitative MRI measurement of superior cerebellar peduncle in progressive supranuclear palsy. Neurology. 2005;64:675–9. doi: 10.1212/01.WNL.0000151854.85743.C7. [DOI] [PubMed] [Google Scholar]
- Paviour DC, Schott JM, Stevens JM, Revesz T, Holton JL, Rossor MN, et al. Pathological substrate for regional distribution of increased atrophy rates in progressive supranuclear palsy. J Neurol Neurosurg Psychiatry. 2004;75:1772–5. doi: 10.1136/jnnp.2003.033472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peelen MV, Atkinson AP, Vuilleumier P. Supramodal representations of perceived emotions in the human brain. J Neurosci. 2010;30:10127–34. doi: 10.1523/JNEUROSCI.2161-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pengas G, Pereira JMS, Williams GB, Nestor PJ. Comparative reliability of total intracranial volume estimation methods and the influence of atrophy in a longitudinal semantic dementia cohort. J Neuroimaging. 2009;19:37–46. doi: 10.1111/j.1552-6569.2008.00246.x. [DOI] [PubMed] [Google Scholar]
- Pereira JMS, Williams GB, Acosta-Cabronero J, Pengas G, Spillantini MG, Xuereb JH, et al. Atrophy patterns in histologic vs clinical groupings of frontotemporal lobar degeneration. Neurology. 2009;72:1653–60. doi: 10.1212/WNL.0b013e3181a55fa2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Philippi CL, Mehta S, Grabowski T, Adolphs R, Rudrauf D. Damage to association fiber tracts impairs recognition of the facial expression of emotion. J Neurosci. 2009;29:15089–99. doi: 10.1523/JNEUROSCI.0796-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phillips ML, Young AW, Senior C, Brammer M, Andrew C, Calder AJ, et al. A specific neural substrate for perceiving facial expressions of disgust. Nature. 1997;389:495–8. doi: 10.1038/39051. [DOI] [PubMed] [Google Scholar]
- Premack D, Woodruff G. Does the Chimpanzee have a theory of mind? Behav Brain Sci. 1978;1:515–26. [Google Scholar]
- Price S, Paviour D, Scahill R, Stevens J, Rossor M, Lees A, et al. Voxel-based morphometry detects patterns of atrophy that help differentiate progressive supranuclear palsy and Parkinson's disease. Neuroimage. 2004;23:663–9. doi: 10.1016/j.neuroimage.2004.06.013. [DOI] [PubMed] [Google Scholar]
- Rankin KP, Kramer JH, Miller BL. Patterns of cognitive and emotional empathy in frontotemporal lobar degeneration. Cogn Behav Neurol. 2005;18:28–36. doi: 10.1097/01.wnn.0000152225.05377.ab. [DOI] [PubMed] [Google Scholar]
- Rankin KP, Salazar A, Gorno-Tempini ML, Sollberger M, Wilson SM, Pavlic D, et al. Detecting sarcasm from paralinguistic cues: anatomic and cognitive correlates in neurodegenerative disease. Neuroimage. 2009;47:2005–15. doi: 10.1016/j.neuroimage.2009.05.077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richardson JC, Steele J, Olszewski J. Supranuclear ophthalmoplegia, pseudobulbar palsy, nuchal dystonia and dementia:a clinical report on eight cases of ‘Heterogenous System Degeneration’. Trans Am Neurol Assoc. 1963;88:25–9. [PubMed] [Google Scholar]
- Rizzo G, Martinelli P, Manners D, Scaglione C, Tonon C, Cortelli P, et al. Diffusion-weighted brain imaging study of patients with clinical diagnosis of corticobasal degeneration, progressive supranuclear palsy and Parkinson's disease. Brain. 2008;131:2690–700. doi: 10.1093/brain/awn195. [DOI] [PubMed] [Google Scholar]
- Roca M, Parr A, Thompson R, Woolgar A, Torralva T, Antoun N, et al. Executive function and fluid intelligence after frontal lobe lesions. Brain. 2010;133:234–47. doi: 10.1093/brain/awp269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rockwell P. Vocal features of conversational sarcasm: a comparison of methods. J Psycholinguist Res. 2007;36:361–9. doi: 10.1007/s10936-006-9049-0. [DOI] [PubMed] [Google Scholar]
- Rohrer JD, Ridgway GR, Modat M, Ourselin S, Mead S, Fox NC, et al. Distinct profiles of brain atrophy in frontotemporal lobar degeneration caused by progranulin and tau mutations. Neuroimage. 2010;53:1070–6. doi: 10.1016/j.neuroimage.2009.12.088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosen HJ, Gorno-Tempini ML, Goldman WP, Perry RJ, Schuff N, Weiner M, et al. Patterns of brain atrophy in frontotemporal dementia and semantic dementia. Neurology. 2002a;58:198–208. doi: 10.1212/wnl.58.2.198. [DOI] [PubMed] [Google Scholar]
- Rosen HJ, Pace-Savitsky K, Perry RJ, Kramer JH, Miller BL, Levenson RW. Recognition of emotion in the frontal and temporal variants of frontotemporal dementia. Dement Geriatr Cogn Disord. 2004;17:277–81. doi: 10.1159/000077154. [DOI] [PubMed] [Google Scholar]
- Rosen HJ, Perry RJ, Murphy J, Kramer JH, Mychack P, Schuff N, et al. Emotion comprehension in the temporal variant of frontotemporal dementia. Brain. 2002b;125:2286–95. doi: 10.1093/brain/awf225. [DOI] [PubMed] [Google Scholar]
- Russell JA. Is there universal recognition of emotion from facial expression? A review of the cross-cultural studies. Psychol Bull. 1994;115:102–41. doi: 10.1037/0033-2909.115.1.102. [DOI] [PubMed] [Google Scholar]
- Saxe R, Kanwisher N. People thinking about thinking people: the role of the temporo-parietal junction in ‘theory of mind’. NeuroImage. 2003;19:1835–42. doi: 10.1016/s1053-8119(03)00230-1. [DOI] [PubMed] [Google Scholar]
- Scherer KR. Vocal communication of emotion: a review of research paradigms. Speech Commun. 2003;40:227–256. [Google Scholar]
- Schirmer A, Kotz SA. Beyond the right hemisphere: brain mechanisms mediating vocal emotional processing. Trends Cogn Sci. 2006;10:24–30. doi: 10.1016/j.tics.2005.11.009. [DOI] [PubMed] [Google Scholar]
- Schofield EC, Hodges JR, Macdonald V, Cordato NJ, Kril JJ, Halliday GM. Cortical atrophy differentiates Richardson's syndrome from the parkinsonian form of progressive supranuclear palsy. Mov Disord. 2011;26:256–63. doi: 10.1002/mds.23295. [DOI] [PubMed] [Google Scholar]
- Schrag A, Selai C, Davis J, Lees AJ, Jahanshahi M, Quinn N. Health-related quality of life in patients with progressive supranuclear palsy. Mov Disord. 2003;18:1464–9. doi: 10.1002/mds.10583. [DOI] [PubMed] [Google Scholar]
- Schulte-Ruther M, Markowitsch HJ, Fink GR, Piefke M. Mirror neuron and theory of mind mechanisms involved in face-to-face interactions: a functional magnetic resonance imaging approach to empathy. J Cogn Neurosci. 2007;19:1354–72. doi: 10.1162/jocn.2007.19.8.1354. [DOI] [PubMed] [Google Scholar]
- Ségonne F, Dale AM, Busa E, Glessner M, Salat D, Hahn HK, et al. A hybrid approach to the skull stripping problem in MRI. NeuroImage. 2004;22:1060–75. doi: 10.1016/j.neuroimage.2004.03.032. [DOI] [PubMed] [Google Scholar]
- Shamay-Tsoory SG, Aharon-Peretz J, Perry D. Two systems for empathy: a double dissociation between emotional and cognitive empathy in inferior frontal gyrus versus ventromedial prefrontal lesions. Brain. 2009;132:617–27. doi: 10.1093/brain/awn279. [DOI] [PubMed] [Google Scholar]
- Shany-Ur T, Poorzand P, Grossman SN, Growdon ME, Jang JY, Ketelle RS, et al. Comprehension of insincere communication in neurodegenerative disease: lies, sarcasm, and theory of mind. Cortex. 2011 doi: 10.1016/j.cortex.2011.08.003. doi:10.1016/j.cortex.2011.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sled JG, Zijdenbos AP, Evans AC. A nonparametric method for automatic correction of intensity nonuniformity in MRI data. IEEE Trans Med Imaging. 1998;17:87–97. doi: 10.1109/42.668698. [DOI] [PubMed] [Google Scholar]
- Smith SM. Fast robust automated brain extraction. Hum Brain Mapp. 2002;17:143–55. doi: 10.1002/hbm.10062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snowden JS, Gibbons ZC, Blackshaw A, Doubleday E, Thompson J, Craufurd D, et al. Social cognition in frontotemporal dementia and Huntington's disease. Neuropsychologia. 2003;41:688–701. doi: 10.1016/s0028-3932(02)00221-x. [DOI] [PubMed] [Google Scholar]
- Sprengelmeyer R, Rausch M, Eysel UT, Przuntek H. Neural structures associated with recognition of facial expressions of basic emotions. Proc Biol Sci. 1998;265:1927–31. doi: 10.1098/rspb.1998.0522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sprengelmeyer R, Young AW, Calder AJ, Karnat A, Lange H, Homberg V, et al. Loss of disgust. Perception of faces and emotions in Huntington's disease. Brain. 1996;119(Pt 5):1647–65. doi: 10.1093/brain/119.5.1647. [DOI] [PubMed] [Google Scholar]
- Sprengelmeyer R, Young AW, Mahn K, Schroeder U, Woitalla D, Buttner T, et al. Facial expression recognition in people with medicated and unmedicated Parkinson's disease. Neuropsychologia. 2003;41:1047–57. doi: 10.1016/s0028-3932(02)00295-6. [DOI] [PubMed] [Google Scholar]
- Stone VE, Baron-Cohen S, Calder A, Keane J, Young A. Acquired theory of mind impairments in individuals with bilateral amygdala lesions. Neuropsychologia. 2003;41:209–20. doi: 10.1016/s0028-3932(02)00151-3. [DOI] [PubMed] [Google Scholar]
- Stone VE, Baron-Cohen S, Knight RT. Frontal lobe contributions to theory of mind. J Cogn Neurosci. 1998;10:640–56. doi: 10.1162/089892998562942. [DOI] [PubMed] [Google Scholar]
- Tavares P, Barnard PJ, Lawrence AD. Emotional complexity and the neural representation of emotion in motion. Soc Cogn Affect Neurosci. 2011;6:98–108. doi: 10.1093/scan/nsq021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tavares P, Lawrence AD, Barnard PJ. Paying attention to social meaning: an FMRI study. Cereb Cortex. 2008;18:1876–85. doi: 10.1093/cercor/bhm212. [DOI] [PubMed] [Google Scholar]
- Thirion B, Pinel P, Mériaux S, Roche A, Dehaene S, Poline J-B. Analysis of a large fMRI cohort: Statistical and methodological issues for group analyses. NeuroImage. 2007;35:105–20. doi: 10.1016/j.neuroimage.2006.11.054. [DOI] [PubMed] [Google Scholar]
- Torralva T, Kipps CM, Hodges JR, Clark L, Bekinschtein T, Roca M, et al. The relationship between affective decision-making and theory of mind in the frontal variant of fronto-temporal dementia. Neuropsychologia. 2007;45:342–9. doi: 10.1016/j.neuropsychologia.2006.05.031. [DOI] [PubMed] [Google Scholar]
- Tzourio-Mazoyer N, Landeau B, Papathanassiou D, Crivello F, Etard O, Delcroix N, et al. Automated anatomical labeling of activations in SPM using a macroscopic anatomical parcellation of the MNI MRI single-subject brain. Neuroimage. 2002;15:273–89. doi: 10.1006/nimg.2001.0978. [DOI] [PubMed] [Google Scholar]
- Van Overwalle F. Social cognition and the brain: a meta-analysis. Hum Brain Mapp. 2009;30:829–58. doi: 10.1002/hbm.20547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Werner KH, Roberts NA, Rosen HJ, Dean DL, Kramer JH, Weiner MW, et al. Emotional reactivity and emotion recognition in frontotemporal lobar degeneration. Neurology. 2007;69:148–55. doi: 10.1212/01.wnl.0000265589.32060.d3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitwell JL, Josephs KA, Rossor MN, Stevens JM, Revesz T, Holton JL, et al. Magnetic resonance imaging signatures of tissue pathology in frontotemporal dementia. Arch Neurol. 2005;62:1402–8. doi: 10.1001/archneur.62.9.1402. [DOI] [PubMed] [Google Scholar]
- Wicker B, Keysers C, Plailly J, Royet J-P, Gallese V, Rizzolatti G. Both of us disgusted in my insula: the common neural basis of seeing and feeling disgust. Neuron. 2003;40:655–64. doi: 10.1016/s0896-6273(03)00679-2. [DOI] [PubMed] [Google Scholar]
- Wildgruber D, Pihan H, Ackermann H, Erb M, Grodd W. Dynamic brain activation during processing of emotional intonation: influence of acoustic parameters, emotional valence, and sex. NeuroImage. 2002;15:856–69. doi: 10.1006/nimg.2001.0998. [DOI] [PubMed] [Google Scholar]
- Wildgruber D, Riecker A, Hertrich I, Erb M, Grodd W, Ethofer T, et al. Identification of emotional intonation evaluated by fMRI. NeuroImage. 2005;24:1233–41. doi: 10.1016/j.neuroimage.2004.10.034. [DOI] [PubMed] [Google Scholar]
- Williams GB, Nestor PJ, Hodges JR. Neural correlates of semantic and behavioural deficits in frontotemporal dementia. Neuroimage. 2005;24:1042–51. doi: 10.1016/j.neuroimage.2004.10.023. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.