Abstract
Fluency tasks have been widely used to tap the voluntary generation of responses. The anatomical correlates of fluency tasks and their sensitivity and specificity have been hotly debated. However, investigation of the cognitive processes involved in voluntary generation of responses and whether generation is supported by a common, general process (e.g. fluid intelligence) or specific cognitive processes underpinned by particular frontal regions has rarely been addressed. This study investigates a range of verbal and non-verbal fluency tasks in patients with unselected focal frontal (n = 47) and posterior (n = 20) lesions. Patients and controls (n = 35) matched for education, age and sex were administered fluency tasks including word (phonemic/semantic), design, gesture and ideational fluency as well as background cognitive tests. Lesions were analysed by standard anterior/posterior and left/right frontal subdivisions as well as a finer-grained frontal localization method. Thus, patients with right and left lateral lesions were compared to patients with superior medial lesions. The results show that all eight fluency tasks are sensitive to frontal lobe damage although only the phonemic word and design fluency tasks were specific to the frontal region. Superior medial patients were the only group to be impaired on all eight fluency tasks, relative to controls, consistent with an energization deficit. The most marked fluency deficits for lateral patients were along material specific lines (i.e. left—phonemic and right—design). Phonemic word fluency that requires greater selection was most severely impaired following left inferior frontal damage. Overall, our results support the notion that frontal functions comprise a set of specialized cognitive processes, supported by distinct frontal regions.
Keywords: fluency tests, selection, fluid intelligence, energization, frontal cortex
Introduction
Fluency tasks have long been used to tap one of the executive functions implemented by the frontal lobes; namely, the voluntary generation of non-overlearned responses. The classic verbal generation task takes two forms, phonemic or semantic word fluency, each of which requires the generation of multiple single words from a single cue within a given time. Non-verbal generation analogues to word fluency have been developed (Jones-Gotman and Milner, 1977). Typically, these are design fluency tasks involving free or constrained conditions. In the free condition, subjects are required to invent novel drawings that do not represent and are not derived from real objects. In the constrained condition, subjects are asked to generate drawings that consist of four lines. Three other fluency tasks have been sporadically used to assess voluntary response generation: ideational fluency (also referred to as the Alternate Uses test and the Uses of Objects test), gesture fluency and motor movement generation. Ideational and gesture fluency require generation of either known or novel uses of objects (ideational, e.g. uses of a brick: conventional—build a house; unconventional—paperweight) or finger positions (gesture: meaningless and meaningful; Jason, 1985). Motor movement generation involves making random movements with a joystick and has been used to investigate movement selection in the context of neuroimaging (Deiber et al., 1991).
Fluency tasks and anatomical correlates
Voluntary generation is widely thought to be a frontal lobe process and prefrontal damage is known to result in a lack of initiation (Fuster, 2008). However, the evidence of frontal specialization (i.e. sensitivity and specificity) or localization within the frontal cortex for fluency tasks is inconsistent or sparse. Since the seminal study of Milner (1964), many lesion studies have demonstrated reduced word fluency following frontal lobe lesions (Baldo and Shimamura, 1998; Rogers et al., 1998; Stuss et al., 1998; Troyer et al., 1998; Schwartz and Baldo, 2001), compared to both posterior patients and controls (Perret, 1974; Pendleton et al., 1982). However, the lack of definitive sensitivity and specificity is reflected in the absence of a frontal semantic fluency deficit reported in some studies (Newcombe, 1969; Martin et al., 1990) while other studies report equivalent frontal and posterior impairments (Newcombe, 1969; Coslett et al., 1991) or a posterior deficit (Vilkki and Holst, 1994; Stuss et al., 1998). A meta-analysis of phonemic and semantic fluency revealed large and comparable frontal deficits, whereas temporal patients showed a larger semantic than phonemic fluency deficit (Henry and Crawford, 2004; for a similar finding in semantic dementia, see Hodges et al., 1992). In contrast, a lesion mapping study of left-hemisphere stroke patients revealed that semantic and phonemic fluency deficits correlated with temporal and frontal lesions, respectively (Baldo et al., 2006). This was considered by these authors to be consistent with the idea that temporal cortex supports word retrieval constrained by semantics while frontal regions support word retrieval constrained by phonology. Within the frontal cortex, a number of studies suggest word fluency is more reduced following left than right frontal lesions (Milner, 1964; Newcombe, 1969; Miceli et al., 1981; Pendleton et al., 1982; Vilkki and Holst, 1994), especially for phonemic fluency (Milner, 1964; Benton, 1968; Perret, 1974; Stuss et al., 1998). However, reduced word fluency has been reported in right lesions (Perret, 1974; Martin et al., 1990; Loring et al., 1994), although other studies suggest right frontals perform comparably with controls (Milner, 1964; Newcombe, 1969).
Design fluency has generally been associated with the frontal region (Tucha et al., 1999; Baldo et al., 2001). Specifically, Jones-Gotman and Milner (1977) reported an impairment for this task (i.e. reduced and perseverative output) in a relatively small group of right frontal and right fronto-central patients. A further study of eight patients has corroborated the right frontal deficit (Ruff et al., 1994), but frontal lateralization and more detailed localization remain controversial. Several studies have found an equally severe left and right frontal deficit (Tucha et al., 1999; Baldo et al., 2001). Notably, the failure to include a posterior patient control group means that the specificity of a design fluency deficit to the frontal lobes has yet to be established. The three other fluency tasks (ideational fluency, gesture fluency and motor movement generation) are little investigated and poorly understood. An ideational fluency deficit for novel uses was documented in 10 frontal patients with widely distributed lesions (compared to a small number of controls, basal ganglia and posterior patients; Eslinger and Grattan, 1993). For gesture fluency, Jason (1985) reported a left frontal deficit (meaningful and meaningless gestures) and a right frontal deficit (only meaningful gestures). We previously reported a dynamic aphasic patient (CH) who, despite severely impaired verbal generation skills, including word fluency, performed normally on a range of gesture fluency and motor movement generation tasks. Patient CH had focal left inferior frontal and temporal atrophy in the context of non-fluent progressive aphasia (Robinson et al., 2005). There are no reports of frontal lateralization for ideational fluency.
In a review of the sensitivity and specificity of phonemic word fluency to frontal lobe lesions, Alvarez and Emory (2006) concluded that this task is sensitive to frontal lobe lesions but its specificity remained a question. That is, phonemic word fluency may also be sensitive to non-frontal lesions. These authors highlighted several limitations of lesion studies that may have contributed to the lack of specificity including the lack of an appropriate control group (Benton, 1968), sparse lesion localization details, the use of written rather than oral word fluency tasks (Milner, 1964; Pendleton et al., 1982) and the provision of only minimal details about the presence (or absence) of dysphasia. Further limitations such as the small number of patients in many lesion studies (Baldo and Shimamura, 1998) and the variety of procedures adopted for grouping frontal patients according to lesion site are also relevant.
There are generally two main procedures used for grouping patients: standard approaches (i.e. frontal/posterior, left/right/bi-frontal; for examples of the method, see Milner, 1964; Benton, 1968; Tucha et al., 1999; Baldo et al., 2001) and the more recent ‘hot-spotting’ procedure that employs a finer-grained approach (i.e. left/right lateral, superior/inferior medial; for examples of the method, see Stuss et al., 1998; Troyer, et al., 1998; for review, see Stuss, 2011). Standard approaches to grouping frontal patients are by far the most widely adopted procedures and form the basis of the review above. An exception is the study by Stuss et al. (1998) who used a finer-grained approach to identify ‘the most significant effect on letter-based [phonemic] fluency … was produced by left dorso-lateral frontal lesions … Brodmann’s areas 46, 45, 44, 6, 8, and 9’ (p. 274). These authors were uncertain whether ‘further anatomical differentiation can be identified within this region’ (p. 274). Nevertheless, despite identification of the frontal region as having sensitivity for phonemic fluency, Stuss et al. (1998) did not show specificity of this task as patients with posterior lesions were also impaired, relative to healthy controls. Thus, the question of whether the word fluency task is specific to frontal lobe lesions remains unresolved.
In sum, it is well documented that all four fluency tasks (word, design, gesture and ideational) are sensitive to frontal lobe damage. However, evidence for the specificity of fluency tasks to the frontal lobes remains equivocal. In addition, questions remain regarding any further differentiation within this area for voluntary non-overlearned response generation as measured by fluency tasks.
Fluency tasks and cognitive processes
To generate responses for all types of fluency tasks, sustained activation for the duration of the time period is required. Indeed, a deficit in sustaining activation has been implicated in reduced performance in fluency tasks, including word fluency (Stuss et al., 1998; Reverberi et al., 2006). However, more specific processes may come into play for some fluency tasks, namely, mechanisms for selection between possible outputs and for the creation of new responses.
A selection mechanism comes into play when multiple verbal responses are linked to the same cue and so compete for generation. This produces an analogous situation within semantic memory to those in episodic memory, which give rise to the cue-overload phenomenon (Watkins and Watkins, 1975). The selection demands of fluency tasks may vary depending on the type of responses and the degree of conflict due to competition. A selection mechanism may be more necessary for phonemic rather than semantic fluency (Perret, 1974). This is because the former involves competition due to the habitual use of words with respect to their meaning in addition to the use of words with respect to the first letter (or sound).
We have previously documented that a selection deficit at the conceptual level accounted for reduced verbal generation (words, phrases and sentences) in patients with dynamic aphasia with left inferior frontal gyrus (LIFG) damage (Robinson et al., 1998, 2005). We have also reported that generation of sentences was only impaired when selection is required in patients with LIFG lesions (Robinson et al., 2010). Moreover, Thompson-Schill et al. (1997), on the basis of a functional imaging study of normal subjects, have argued that the LIFG is critical for selection. Since these studies, the role of the LIFG in selection has been debated (Hirshorn and Thompson-Schill, 2006; Heim et al., 2009; Schnur et al., 2009). Thus, it can be hypothesized that any fluency task with greater selection demands resulting from multiple response competition will be impaired following LIFG damage. However, a very different and considerably more general perspective has recently been put forward. In a large study of frontal patients, Roca et al. (2010) interpreted their impaired verbal fluency (i.e. phonemic word fluency) performance as resulting from a deficit in fluid intelligence, thought to ‘reflect current ability for abstract thought and reasoning’ (p. 235; see also Duncan et al., 1995). For a group of tasks including verbal fluency, the authors state that ‘… differences between patients and controls can be entirely explained by g …’ (Roca et al., 2010, p. 243).
Another type of mechanism appears to be important for design, ideational and gesture fluency tasks, but possibly not for word fluency tasks. The first three types of tasks have considerable novelty demands; that is they require the creation of new responses. If this is the key factor, then an impairment may result in the generation of an insufficient number of possible responses. Thus, little competition may occur such that minimal or no selection is necessary. One can then hypothesize that LIFG lesions may not be critical for these types of tasks.
The present study aimed to investigate a range of verbal and non-verbal generation tasks (word, design, gesture and ideational fluency) in patients with focal frontal and posterior lesions and matched healthy controls to address anatomical and theoretical issues.
Anatomical issues
The anatomical issues are frontal specialization and localization within the frontal cortex. Which fluency tasks are sensitive to frontal lobe damage (i.e. compared to healthy controls), and which tasks are specific to frontal lobe damage (i.e. compared to posterior patients)? We term these comparisons the standard analyses. Which fluency tasks rely on processes that can be localized more specifically within the frontal cortex? We selected regions thought to be relevant for key processes involved in the different fluency tasks. We investigated whether patients with lesions within specific frontal regions performed worse than healthy controls and patients with lesions in other parts of the frontal lobes. However, we did not compare a set of frontal regions only with each other. Such a comparison implicitly presupposes that only one such region contains all the processes required for a given fluency task; there are no grounds for making this assumption. More specifically, we investigated whether individual fluency tasks show a lateralization effect (i.e. left frontal compared to right frontal). Furthermore, can localization within the frontal cortex be revealed using a finer-grained lesion identification method (i.e. lateral—left/right, medial—superior/inferior)? We termed these comparisons the lateralization and finer-grained analyses, respectively. Moreover, adopting different grouping procedures is necessary in order to investigate whether effects found in previous studies replicate.
Theoretical issues
Is verbal generation supported by a common, general process (e.g. fluid intelligence) based on multiple demand regions (Roca et al., 2010) or does generation instead or as well involve specific cognitive processes supported by particular regions within the frontal cortex? In particular, we consider selection and the role of the LIFG: is there evidence that patients with LIFG lesions (compared with non-LIFG patients) show a deficit on fluency tasks with high selection requirements (e.g. phonemic versus semantic fluency)?
Materials and methods
Subjects
Seventy-two patients with focal frontal or posterior lesions primarily due to brain tumour and stroke were recruited from the National Hospital for Neurology and Neurosurgery. A description of patients’ lesion location, aetiology, lesion extent and chronicity is available in Supplementary Table 1 and full details of inclusion and exclusion criteria are detailed in Robinson et al. (2010) since the same sample was recruited for both studies. Thus, 67 patients with focal lesions (47 frontal; 20 posterior: 12 left and 8 right) were compared with 35 healthy adult controls with no neurological or psychiatric history, matched as was possible for age, gender and education.
The National Hospital for Neurology and Neurosurgery and the Institute of Neurology Joint Research Ethics Committee and the University College London Hospitals NHS Trust Research and Development Directorate approved the study.
Lesion analyses
A neurologist (M.B.) who was blind to the history of each patient reviewed the hard copies of MRI scans (or CT where MRI was unavailable, n = 14). Brain MRI was obtained on systems operated at 1.5 T and included the acquisition of an axial dual-echo and an axial and coronal T1-weighted scan. CT scans were all obtained using a spiral CT system (SOMATOM PLUS 4, Siemens). Axial images were collected with an effective slice thickness of 5 mm and pitch of 1.5. Both MRI and CT data were used as our goal was to enable the recruitment of a large number of patients. The exclusion criteria and lesion assessment guidelines were rigorous and based on detailed anatomical localization using standard atlases (Duvernoy, 1991). Of note, all frontal lesions were entirely within the frontal lobe except for two right frontal patients with vascular lesions that extended to the postcentral gyrus (analyses were conducted with and without these two patients with the results being unchanged). The lesion localization method is described in detail in Robinson et al. (2010). Briefly, each frontal patient was coded for the presence of lesion and oedema in each hemisphere in the anterior and posterior portion of nine left and right frontal regions (18 areas in total). An area was only coded as damaged if at least 25% was affected.
Four analyses (standard, lateralization, finer-grained and theoretically driven) for grouping frontal patients on the basis of their lesions were adopted. These four procedures were used for the behavioural analyses. They allowed comparison to be made with previous studies as well as replication and extension of previously reported effects.
In the standard analysis (frontal versus posterior versus controls), all 18 regions were collapsed together to establish a general frontal effect (frontal: n = 47; posterior: n = 20; controls: n = 35). This analysis was critical because subsequent analyses were carried out if, and only if, there was a significant frontal deficit compared to controls. This is because the aim was to be more specific anatomically on the localization of key systems responsible for an already established frontal lesion effect. Thus, following the first standard analysis, the lateralization analysis was carried out by collapsing all the patients with left and right frontal lesions and contrasting them with controls (left frontal: n = 18, right frontal: n = 22, controls: n = 35; seven frontal patients with bilateral lesions were excluded).
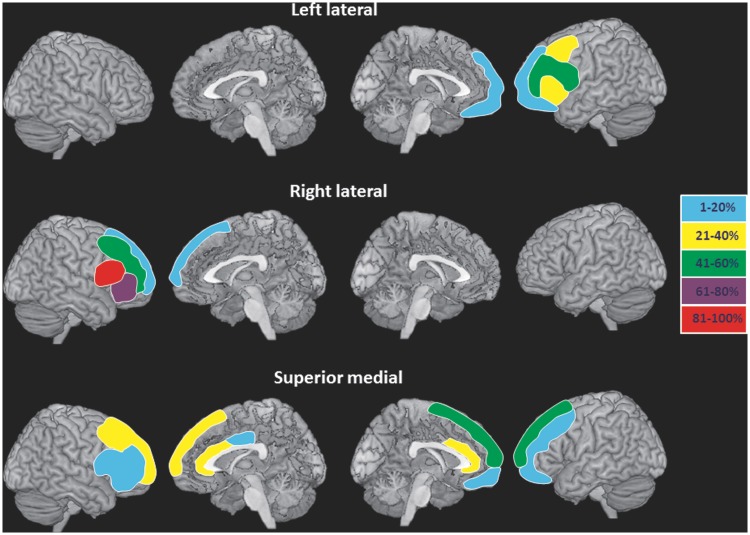
The finer-grained analysis was carried out in parallel with the lateralization analysis. This third type of analysis used the grouping previously adopted by Stuss et al. (1998) contrasting the left lateral versus right lateral versus superior medial versus controls (left lateral n = 10, right lateral n = 8, inferior and middle frontal gyri; superior medial n = 12, superior frontal gyri; controls n = 35; for examples of the method, see Picton et al., 2006; Turner et al., 2007). Primary lesion site was determined by a lesion falling entirely within one site or, when it extended over two sites, the lesion was required to affect >75% of the primary site and <30% of the secondary site. Seventeen frontal patients were excluded from this third finer-grained analysis as the primary site of lesion fit more than one subgroup (n = 13) or because of the small number of patients available for the inferior medial group (n = 4; cingulate and orbital cortex) (Fig. 1). For the word fluency results, we directly compared the left lateral and right lateral patients in order to attempt to replicate the effects previously documented by Stuss et al. (1998) and Troyer et al. (1998).
Figure 1.
Lesion location for patients with lesions predominantly affecting the left lateral, right lateral and superior medial regions. The shaded areas represent the proportion of patients within each group who have lesions affecting at least 25% of the depicted region.
The fourth theoretically driven analysis was used to investigate two hypotheses in relation to phonemic word fluency. The first is that the LIFG plays a specific role in verbal conceptual selection (Robinson et al. 2010). The second hypothesis is that verbal generation involves specific cognitive processes rather than a common, general process such as fluid intelligence (Roca et al., 2010). Thus, frontal patients with lesions to the LIFG (n = 12) were directly compared with frontal patients with lesions not including the LIFG (non-LIFG: n = 35).
Statistical analyses
ANOVA was used for the standard, the lateralization and the finer-grained analyses. Age was included as a covariate as the right frontal group was significantly older than the left frontal group. Adopting the methods of previous studies (e.g. Stuss et al., 1998; Troyer et al., 1998; Tucha et al., 1999), significant results were followed by pairwise comparisons (standard and lateralization analyses: between patient groups and controls; finer-grained analysis: each frontal sub-group compared to controls). Bonferroni’s correction was applied to all analyses (e.g. frontal versus posterior versus control; left frontal versus right frontal versus controls; left lateral/right lateral/superior medial versus controls; all P ≤ 0.017) and only significant results are reported. For word and design fluency, including the theoretically driven analyses, t-tests were used to directly compare the frontal patient groups for reasons discussed above (i.e. left frontal versus right frontal; left lateral versus right lateral; LIFG versus non-LIFG). If error variances were unequal (i.e. Levene’s test was significant), non-parametric statistics were applied (Kruskal–Wallis test; followed by pairwise Mann–Whitney U-tests—for similar method, see Turner et al., 2007).
Generation tests and procedure
Word fluency
Phonemic (‘S’) and semantic (fruit/vegetables) word fluency tasks were administered (Milner, 1964; Benton, 1968; Hodges et al., 1990). Subjects were asked to orally generate words for 1 min without producing proper nouns or numbers (phonemic fluency) or repeating words. The total number of correct words generated was recorded. Thus, perseverative responses and rule break errors (e.g. Susan for ‘S’ and bread for ‘fruit/vegetables’) were excluded. For each patient, a word fluency ratio was calculated to contrast the number generated for the two tasks: phonemic/(phonemic + semantic). The lower the ratio, the more reduced the performance on the phonemic relative to the semantic task.
Design fluency
Free and fixed design fluency tasks, as detailed by Jones-Gotman and Milner (1977), were given. Subjects were asked to generate as many drawings as possible in two conditions: (i) free—5 min to complete drawings that neither represented real objects nor were derived from such objects and (ii) fixed—4 min as in the free condition, except that each drawing had to consist of four lines, straight or curved. Subjects were provided with a pencil and blank A4 paper. The total number of correct designs generated was recorded. Perseverative responses (i.e. a repeat or partial repeat of a previous response) and errors (i.e. if it clearly broke the rules given) were excluded.
Gesture fluency
The gesture fluency tests, and instructions, were based on Jason (1985). Subjects were asked to generate as many movements as possible with the upper limbs in 2 min under two conditions: (i) meaningless movements and (ii) meaningful movements. The total number of correct responses generated was recorded and scored, aided by video camera. Perseverative responses and rule break errors (e.g. waving in the meaningless condition) were excluded from the analysis.
Ideational fluency
This task was based on the Uses of Objects (or Alternate Uses) Test (Lezak et al., 2004). Subjects were asked to generate possible uses of an object in 90 s (i) brick and (ii) table knife, under two conditions: (i) conventional uses (e.g. build a house and butter bread) and (ii) unconventional uses (e.g. paperweight and open letters). The total number of correct responses generated was recorded. Perseverative responses (complete or partial) and errors (e.g. a screwdriver for conventional uses of a table knife) were excluded. The responses from the two objects [(i) and (ii)] were combined and analysed together in each condition.
All fluency and baseline cognitive tests were administered by an experienced clinical neuropsychologist (G.R.), as part of a larger set of tests.
Results
Descriptive characteristics and baseline cognitive test summary
Descriptive characteristics and baseline cognitive test scores of the patients (posterior and left/right frontal; for the total frontal group scores, see Supplementary Table 2) and the healthy controls are presented in Table 1. Control and patient groups were matched (i.e. P > 0.05) for sex, age and pre-morbid intelligence, apart from the older age of right frontal than left frontal patients (U = 91.000, P < 0.01). The frontal sub-groups were equivalent in the time since the lesion occurred. However, posterior patients were more acute than frontal patients [posterior: mean = 4.5 months, SD = 10.4; frontal: mean = 18.3 months, SD = 34.6; U = 169.000, P < 0.01].
Table 1.
Descriptive characteristics and cognitive baseline scores for healthy controls and patient groups for the standard, lateralization analysis (posterior and frontal) and the finer-grained analysis
| Healthy controls | Posterior patients | Frontal patients |
Frontal sub-groups |
||||
|---|---|---|---|---|---|---|---|
| n = 12 | Left | Right | LL | RL | SM | ||
| n = 35 | n = 20 | n = 18 | n = 22 | n = 10 | n = 8 | ||
| Sex (M:F) | 18:17 | 10:10 | 14:4 | 11:11 | 8:2 | 3:5 | 6:6 |
| Mean lesion extent (no. frontal areas) | – | – | 4.3 | 3.4 | 3.5 | 3.1 | 3.3 |
| (2.8) | (2.0) | (2.2) | (1.9) | (1.8) | |||
| Mean age | 47.8 | 54.1 | 42.3 | 55.4**a | 45.0 | 47.9 | 48.3 |
| (15.5) | (9.9) | (9.0) | (15.2) | (10.2) | (17.2) | (12.5) | |
| Mean NARTb-derived premorbid IQ | 109.5 | 109.6 | 101.1 | 104.3 | 104.8 | 108.1 | 100.3 |
| (12.4) | (9.7) | (14.5) | (13.6) | (13.2) | (9.7) | (14.9) | |
| Advanced Progressive Matricesc (/12) | 7.6 | 7.3 | 8.2 | 6.1 | 7.7 | 7.1 | 7.7 |
| (3.0) | (2.4) | (2.3) | (2.8) | (2.6) | (2.4) | (3.1) | |
| Recognition Memory Testd Words (/50) | 45e | 44.7 | 46.4 | 44.2 | 46.8 | 46.1 | 46.3 |
| (4.7) | (3.3) | (5.7) | (3.1) | (2.7) | (3.6) | ||
| Recognition Memory Testd Faces (/50) | 44e | 41.1 | 43.1 | 37.7*a | 42.6 | 39.4 | 40.5 |
| (6.0) | (3.5) | (6.8) | (3.7) | (5.3) | (5.2) | ||
| Incomplete Letters Testf(/20) | 19.9 | 19.3** | 19.8 | 19.3* | 19.8 | 19.4 | 19.8 |
| (0.4) | (0.9) | (0.6) | (0.8) | (0.4) | (0.5) | (0.5) | |
| Graded Naming Testg (/30) | 22.3 | 17.8* | 17.9 | 18.9 | 19.2 | 20.9 | 20.5 |
| (4.9) | (7.4) | (4.8) | (5.9) | (5.2) | (5.2) | (3.9) | |
| Synonyms Testh (/50) | 43.1 | 43.4 | 36.9* | 41.5 | 39.0 | 41.8 | 39.4 |
| (6.8) | (5.2) | (7.6) | (6.8) | (6.0) | (4.9) | (7.3) | |
| Hayling overall scorei,j | 6.2 | 5.5 | 4.3** | 3.6*** | 4.0*** | 3.4** | 4.8* |
| (1.1) | (1.4) | (2.1) | (2.0) | (1.8) | (2.3) | (1.9) | |
Frontal patients total n = 47; LL = left lateral; RL = right lateral; SM = superior medial.
acompared with the left frontal patients.
bNART = National Adult Reading Test (Nelson and Willison, 1991).
estandardized sample 50th percentile (40–54 years) and healthy controls not included in statistical analysis.
jscaled score is 1–10, 6 is average; Scores with significant P - values are in bold; *P < 0.05; **P < 0.01; ***P < 0.001, compared with healthy controls.
For cognitive baseline measures, the three basic groups in the standard analysis did not differ in their performance on a test of general intelligence [Advanced Progressive Matrices: F(2,88) = 1.225, P > 0.05]. The mean score for the frontal and posterior patient groups was in the normal range for verbal memory and in the lower end of the normal range for visual memory (published normative data: Warrington, 1984) [Recognition Memory Test Words: t(52) = −0.02, P > 0.05; Faces: t(52) = −0.53, P > 0.05; comparison is between frontal and posterior patients as not all controls performed this standard test]. On the verbal memory test, 92% of both frontal and posterior patients scored above the 5% cut-off. For the visual memory test, 73% of frontal patients and 77% of posterior patients scored above the 5% cut-off. The three basic groups differed significantly on the naming and comprehension measures [Graded Naming Test: F(2,97) = 4.955, P < 0.01; Synonyms Test: F(2,91) = 4.315, P < 0.05]. Mild nominal deficits were present in frontal and posterior patients. Specifically, 85% of both frontal and posterior patients performed above the 5% cut-off on the naming test and 92% of frontal and all posterior patients performed above the 5% cut-off on the word comprehension test. The left frontal patients scored lower than right frontal patients only on the comprehension measure [lateralization analysis Graded Naming Test: F(2,69) = 4.14, P < 0.05; Synonyms Test: F(2,66) = 3.94, P < 0.05] (Table 1). On the Incomplete Letters Test of visual perception, all patients scored above the 5% cut-off, although the groups differed [non-parametric Kruskal–Wallis test: χ2(2) = 8.225, P < 0.05]. On the Hayling Test of frontal ‘executive’ function, the three basic groups differed significantly [non-parametric Kruskal–Wallis test: χ2(2) = 21.25, P < 0.001]. Posterior patients were unimpaired relative to controls (P > 0.05). In striking contrast, the Hayling Test overall scaled score of the frontal patient group (and each frontal sub-group) was significantly impaired, relative to controls, on the Hayling Test [non-parametric Kruskal–Wallis test: lateralization analysis: χ2(2) = 22.94, P < 0.001; finer-grained analysis: χ2(4) = 20.99, P < 0.001] (Table 1).
Generation tests
Word fluency
In the standard analysis, the three basic groups differed significantly for number of words generated on both the phonemic and semantic tasks [phonemic: F(2,98) = 16.328, P < 0.001; semantic: F(2,98) = 15.732, P < 0.001]. For the two word fluency tasks, frontal patients were severely impaired in comparison with controls while the posterior patients performed comparably to controls on the phonemic but not the semantic fluency task (Table 2). Moreover, frontal patients were impaired compared to posterior patients only on the phonemic task (i.e. frontal and posterior patients were equivalent for the semantic task). In the lateralization analysis, there was a significant effect for phonemic fluency [F(2,71) = 28.418, P < 0.001], with left frontal patients more impaired when directly compared to right frontal patients [t(38) = −3.920, P < 0.001]. For semantic fluency, both left frontal and right frontal patient groups were impaired, relative to controls [F(2,71) = 15.179, P < 0.001], but not compared to each other [t(38) = −0.956, P > 0.05]. The finer-grained analysis revealed significant effects for both phonemic and semantic fluency [non-parametric Kruskal–Wallis test for phonemic fluency: χ2(3) = 23.067, P < 0.001; semantic fluency: F(3,60) = 7.057, P < 0.001]. For phonemic fluency, the left lateral and the superior medial groups were significantly impaired compared to controls but the right lateral group was not (Table 2). When directly compared to right lateral patients, the left lateral patients were more impaired [t(38) = −3.920, P < 0.001]. For semantic fluency all three groups were significantly impaired, relative to controls, and left lateral and right lateral patients did not differ when directly compared [t(16) = −0.567, P > 0.05].
Table 2.
Word, design, gesture and ideational fluency: mean total number generated and standard deviation (SD) for healthy controls and patient groups for the standard, lateralization analysis (posterior and frontal) and finer-grained analysis (frontal sub-groups)
| Healthy controls | Posterior patients | Frontal patients |
Frontal sub-groups |
||||
|---|---|---|---|---|---|---|---|
| Left | Right | LL | RL | SM | |||
| n = 35 | n = 20 | n = 18 | n = 22 | n = 10 | n = 8 | n = 12 | |
| Word fluency | |||||||
| Phonemic | 16.9 | 13.8 | 6.8*** a | 12.6* | 7.8*** a | 14.8 | 11.2** |
| (4.7) | (5.8) | (5.2) | (4.2) | (6.1) | (2.4) | (4.1) | |
| Semantic fruit/vegetables | 20.5 | 14.2** | 12.4*** | 14.3** | 13.2*** | 15.0* | 15.3** |
| (5.5) | (5.2) | (6.1) | (6.0) | (6.2) | (7.3) | (4.0) | |
| Design fluency | |||||||
| Free | 17.9 | 14.2 | 10.1** | 5.8***a | 10.6* | 6.1*** | 7.8*** |
| (8.3) | (10.1) | (6.7) | (5.1) | (8.5) | (6.5) | (4.4) | |
| Fixed | 21.3 | 16.8 | 15.8 | 8.6***a | 13.8 | 8.1*** | 13.5* |
| (8.7) | (8.9) | (8.4) | (6.5) | (7.6) | (6.3) | (9.6) | |
| Gesture fluency | |||||||
| Meaningless movements | 17.9 | 13.4 | 12.8* | 11.3* | 11.7* | 12.8 | 10.2** |
| (8.2) | (5.3) | (7.1) | (7.0) | (6.9) | (7.5) | (6.1) | |
| Meaningful movements | 15.1 | 13.4 | 10.9** | 10.8* | 12.2 | 11.3 | 10.6* |
| (4.7) | (4.8) | (6.1) | (3.8) | (7.1) | (4.0) | (4.2) | |
| Ideational fluency | |||||||
| Conventional uses | 15.2 | 10.0** | 9.9* | 10.0** | 12.8 | 11.4 | 9.8** |
| (6.3) | (4.2) | (4.5) | (4.9) | (4.4) | (5.6) | (3.7) | |
| Unconventional uses | 13.7 | 9.9* | 6.9*** | 7.0*** | 9.4 | 7.3** | 7.4*** |
| (4.8) | (6.0) | (5.1) | (3.9) | (5.4) | (3.4) | (4.6) | |
Frontal patients total n = 47; LL = left lateral; RL = right lateral; SM = superior medial; Scores with significant P - values are in bold with significance indicated by *P < 0.05; **P < 0.01; ***P < 0.001, compared to healthy controls. asignificant comparing left frontal versus right frontal (lateralization analysis) and left lateral versus right lateral (finer-grained analysis).
The theoretically driven analysis (LIFG versus non-LIFG) revealed that LIFG patients were more severely impaired than non-LIFG patients for phonemic word fluency [t(45) = −2.860, P < 0.01] (Table 3). In contrast, the LIFG and non-LIFG patients did not differ significantly for semantic fluency [t(45) = −1.787, P > 0.05]. For the word fluency ratio analysis, there was a significant difference between LIFG and non-LIFG patients [t(45) = −2.479, P < 0.05]. The lower ratio of the LIFG patients reflects a disproportionately reduced performance on phonemic compared to semantic word fluency, relative to non-LIFG patients.
Table 3.
Word fluency: Number generated and mean ratio (and SD) for LIFG and Non-LIFG patients
| Frontal sub-groups |
||
|---|---|---|
| LIFG | Non-LIFG |
|
| n = 12 | n = 35 | |
| Word fluency | ||
| Phonemic | 6.0 (5.8)** | 11.2 (5.4) |
| Semantic | 10.3 (5.9) | 14.0 (6.4) |
| Contrast ratio [Phonemic/(Phonemic + Semantic)] | 0.30 (0.16)* | 0.45 (0.18) |
Scores with significant P - values are in bold with significance indicated by *P < 0.05; **P < 0.01.
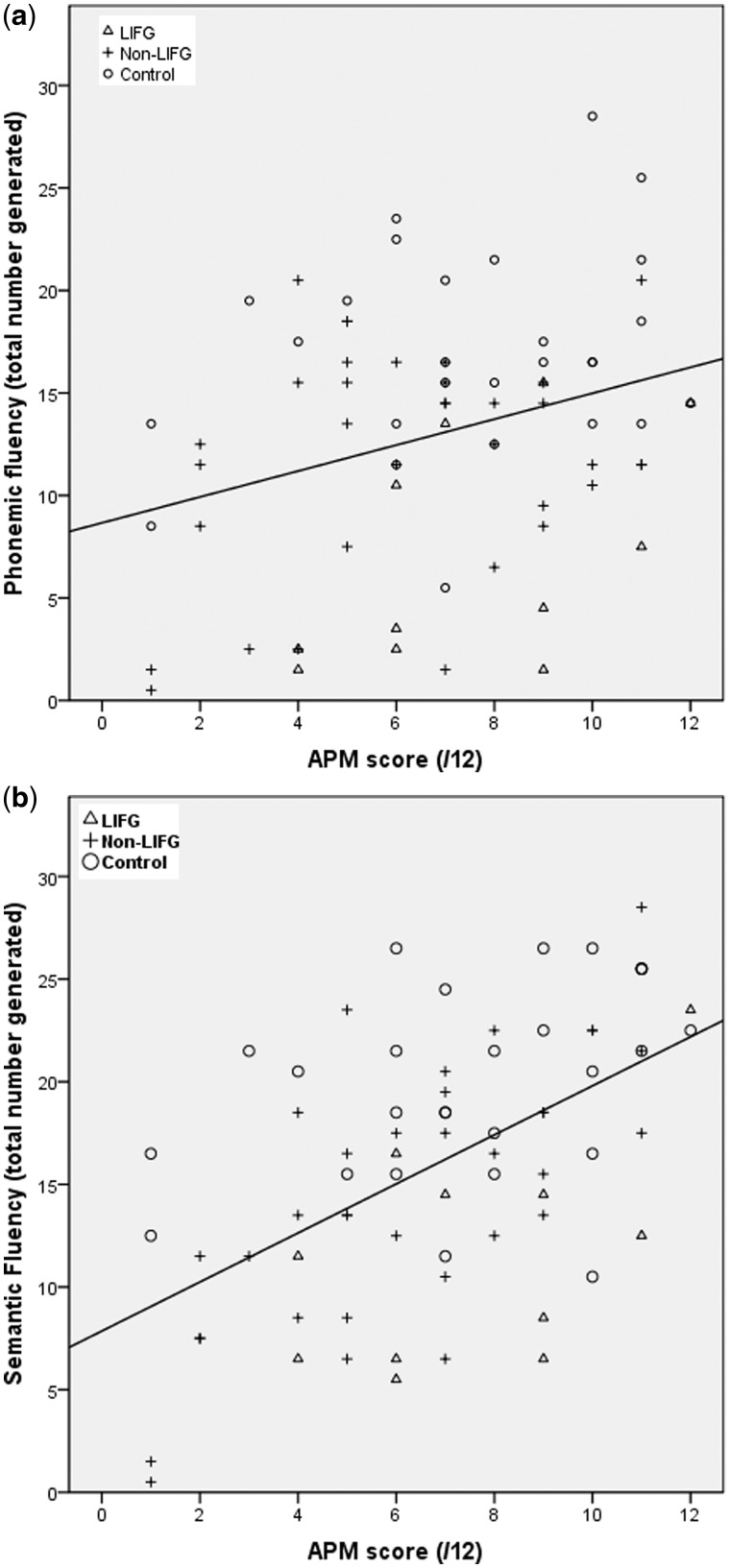
The relationship between word fluency and fluid intelligence was investigated by performing correlations between the two word fluency tasks and the fluid intelligence measure [number of words generated in word fluency regressed on Advanced Progressive Matrices score using combined frontal patient and control data]. The correlation between fluid intelligence and phonemic word fluency score was not significant (r = 0.246, P > 0.05). In contrast, semantic fluency score was positively correlated to the fluid intelligence measure (r = 0.609, P < 0.001). Scatterplots for the word fluency and fluid intelligence measures are shown in Fig. 2A and B. Interestingly, of the 11 frontal patients who performed below the lowest scoring control on the phonemic fluency task, six had LIFG lesions. Of the remaining five poorly performing patients, one had oedema (but not a lesion) to the LIFG, one had a left lateral lesion and three had superior medial lesions. This suggests that LIFG patients produced a low number of words in the phonemic task regardless of whether their fluid intelligence score was high or low.
Figure 2.
Regressions of phonemic (a) and semantic (b) word fluency on Advanced Progressive Matrices score. Regression line is calculated from combined patient and control data with LIFG patients, non-LIFG patients and controls shown.
Design fluency
In the standard analysis, the three basic groups differed significantly [free: F(2,93) = 15.380, P < 0.001; fixed: F(2,93) = 12.978, P < 0.001]. Frontal patients were impaired for number of designs generated in both the free and fixed conditions (except the left frontal patients who were unimpaired on the fixed task), compared to both controls and the posterior group who were not impaired (Table 2). The lateralization analysis revealed a significant effect for both tasks [free: F(2,69) = 20.490, P < 0.001; fixed: F(2,69) = 16.135, P < 0.001] with right frontal patients more impaired when directly compared to left frontal patients [free: t(36) = 2.274, P < 0.05; fixed: t(36) = 3.011, P < 0.01]. The finer-grained analysis revealed significant frontal effects [free: F(3,58) = 8.810, P < 0.001; fixed: F(3,58) = 6.863, P < 0.001]. However, left lateral and right lateral patients did not differ when directly compared [free: t(14) = 1.190, P > 0.05; fixed: t(14) = 1.651, P > 0.05].
Gesture fluency
The standard analysis showed a significant difference between the three groups for both meaningless and meaningful movements [meaningless: F(2,91) = 6.663, P < 0.05; meaningful: F(2,91) = 9.158, P < 0.001]. The frontal, but not the posterior, group was significantly impaired compared to controls, with no difference between frontal and posterior patients and the left frontal and right frontal patients being as equally impaired, relative to controls [meaningless: F(2,68) = 5.758, P < 0.01; non-parametric Kruskal–Wallis test used for meaningful: χ2(2) = 13.363, P < 0.01] (Table 2). The finer-grained analysis revealed a superior medial impairment for both tasks and a mild left lateral deficit for meaningless gestures, relative to controls [meaningless: F(3,57) = 4.549, P < 0.01; meaningful: F(3,57) = 3.201, P < 0.05] (Table 2). Interestingly, there was no impairment in the right lateral group.
Ideational fluency
The standard analysis revealed a significant difference between the three basic groups in the conventional and unconventional uses of objects [conventional: F(2,93) = 12.501, P < 0.001; unconventional: F(2,93) = 18.621 P < 0.001]. Frontal (left and right) and posterior patients generated fewer uses of objects than controls on both tasks, although there was no difference between frontal and posterior patients (Table 2). The lateralization analysis revealed that left and right frontal patients were equally impaired on both tasks, relative to controls [conventional: F(2,69) = 8.732, P < 0.001; unconventional: F(2,69) = 19.200, P < 0.001]. The finer-grained analysis revealed a superior medial deficit on both tasks and a right lateral deficit only for the unconventional uses task [conventional: F(3,57) = 3.309, P < 0.05; unconventional: F(3,57) = 7.9, P < 0.001]. The left lateral group showed no significant deficit.
Generation tests and baseline cognitive tests
To investigate the relationship between the eight fluency tests, correlations were performed within the frontal patient group. A significant positive relationship was found between almost all fluency tasks except for that between phonemic word fluency and free design, fixed design and meaningless movement fluency tasks (Table 4). For the baseline cognitive tests, only the semantic word fluency score was related to all cognitive measures. The fixed design fluency score was related to all cognitive measures apart from the synonyms test. Interestingly, phonemic word fluency was only related to verbal measures (verbal version of the Recognition Memory Test, Graded Naming Test and Synonyms Test). In contrast, the free design fluency score was only related to visual measures (Advanced Progressive Matrices and non-verbal version of the Recognition Memory Test).
Table 4.
Correlations (r) between word, design, gesture and ideational fluency tests and between fluency and cognitive baseline tests for the frontal patient group (n = 47)
| Word |
Design |
Gesture |
Ideational |
|||||
|---|---|---|---|---|---|---|---|---|
| Phonemic | Semantic | Free | Fixed | Meaningless movements | Meaningful movements | Conventional uses | Unconventional uses | |
| Word fluency | ||||||||
| Phonemic | – | – | – | – | – | – | – | – |
| Semantic | 0.689 | |||||||
| P < 0.001 | ||||||||
| Design fluency | ||||||||
| Free | 0.147 | 0.329 | ||||||
| P > 0.05 | P < 0.05 | |||||||
| Fixed | 0.224 | 0.527 | 0.638 | |||||
| P > 0.05 | P < 0.001 | P < 0.001 | ||||||
| Gesture fluency | ||||||||
| Meaningless movements | 0.202 | 0.164 | 0.521 | 0.542 | ||||
| P > 0.05 | P > 0.05 | P < 0.001 | P < 0.001 | |||||
| Meaningful movements | 0.552 | 0.679 | 0.400 | 0.512 | 0.618 | |||
| P < 0.001 | P < 0.001 | P < 0.01 | P < 0.01 | P < 0.001 | ||||
| Ideational fluency | ||||||||
| Conventional uses | 0.593 | 0.575 | 0.379 | 0.318 | 0.362 | 0.642 | ||
| P < 0.001 | P < 0.001 | P < 0.05 | P < 0.05 | P < 0.05 | P < 0.001 | |||
| Unconventional uses | 0.474 | 0.609 | 0.489 | 0.448 | 0.396 | 0.694 | 0.566 | |
| P < 0.001 | P < 0.001 | P < 0.01 | P < 0.01 | P < 0.05 | P < 0.001 | P < 0.001 | ||
| Advanced Progressive Matrices | 0.246 | 0.609 | 0.342 | 0.602 | 0.278 | 0.541 | 0.313 | 0.553 |
| P > 0.05 | P < 0.001 | P < 0.05 | P < 0.001 | P > 0.05 | P < 0.001 | P < 0.05 | P < 0.001 | |
| Recognition Memory Test Words | 0.397 | 0.560 | 0.232 | 0.423 | 0.135 | 0.340 | 0.447 | 0.418 |
| P < 0.05 | P < 0.001 | P > 0.05 | P < 0.01 | P > 0.05 | P < 0.05 | P < 0.01 | P < 0.01 | |
| Recognition Memory Test Faces | 0.217 | 0.413 | 0.462 | 0.652 | 0.215 | 0.292 | 0.250 | 0.244 |
| P > 0.05 | P < 0.01 | P < 0.01 | P < 0.001 | P > 0.05 | P > 0.05 | P > 0.05 | P > 0.05 | |
| Graded Naming Test | 0.548 | 0.566 | 0.175 | 0.323 | −0.057 | 0.270 | 0.195 | 0.455 |
| P < 0.001 | P < 0.001 | P > 0.05 | P < 0.05 | P > 0.05 | P > 0.05 | P > 0.05 | P < 0.01 | |
| Synonyms Test | 0.399 | 0.472 | 0.038 | 0.151 | 0.208 | 0.044 | −0.019 | 0.315 |
| P < 0.01 | P < 0.01 | P > 0.05 | P > 0.05 | P > 0.05 | P > 0.05 | P > 0.05 | P < 0.05 | |
| Hayling Test | 0.240 | 0.548 | 0.305 | 0.551 | 0.425 | 0.576 | 0.300 | 0.631 |
| P > 0.05 | P < 0.001 | P > 0.05 | P < 0.001 | P < 0.01 | P < 0.001 | P > 0.05 | P < 0.001 | |
r = Pearson product-moment correlation coefficient.
Discussion
To the best of our knowledge, this is the first time that a range of verbal and non-verbal fluency tasks have been administered to a large population of patients with focal frontal and posterior lesions. Our standard and finer-grained lesion analyses allowed us to address the sensitivity and specificity of the frontal lobes for each fluency task, compare performance across these tasks and take into account factors such as the relative proportion of frontal regions affected. Our study aimed to overcome the limitations of previous studies that have left the specificity of (word) fluency tasks elusive. In particular, we included healthy and patient control groups, used only oral word fluency tasks, provided lesion localization details, reported language measures so the presence/absence of dysphasia is clear and we recruited a large number of patients. Finally, we adopted both standard and finer-grained procedures for grouping frontal patients, so our results can be easily compared to previous studies.
We documented verbal and non-verbal fluency deficits in our frontal patients in all eight fluency tasks. The verbal fluency (word and ideational) deficits cannot be explained purely on the basis of dysphasia, as only 15% of our frontal (and posterior) patients presented with significant nominal deficits, whereas a much higher number of frontal patients were impaired on verbal fluency tasks. For instance, for phonemic fluency, 41% of frontal patients performed below the 5% cut-off. Similarly, our non-verbal fluency (design and gesture) deficits cannot be explained purely on the basis of significant perceptual defects as visual perception skills were intact for all subjects.
Anatomical issues
In the literature, there are conflicting findings for word and design fluency and virtually no relevant studies for gesture and ideational fluency. To the best of our knowledge, our study is the first to administer the same range of fluency tasks to patients with posterior lesions and patients with frontal lesions localized in differing regions and compare the performance of patients to that of healthy controls. Our methods allowed us to draw some powerful conclusions about fluency tasks, the procedures used for grouping frontal patients when analysing behavioural performance and the nature of frontal lobe functions.
Frontal specialization
Our frontal group was impaired on all eight fluency tasks (verbal and non-verbal), when compared with healthy controls. However, when compared with posterior patients, only phonemic word and design fluency tasks were selectively impaired in the frontal patients. Semantic word, ideational and gesture fluency performance did not differ significantly between frontal and posterior patients. Thus, all fluency tasks are sensitive to frontal damage, but only phonemic word and design fluency tasks show specificity and hence are specialized for the diagnosis of focal frontal lobe impairments.
Lateralization and localization within the frontal cortex
The lateralization and finer-grained analyses revealed a degree of lateralization within the frontal cortex, along material specific lines (i.e. left—verbal and right—non-verbal). Thus, we found a severe phonemic word fluency deficit for left frontal patients, compared to right frontal patients. This was further refined to the left lateral patients, compared to right lateral patients, and to patients with LIFG lesions, relative to frontal patients without LIFG lesions. This specificity can be seen from the lesion sites of the 11 frontal patients who performed worse than any healthy control on the phonemic fluency task. Thus, 6 of the 11 patients had LIFG lesions, one had oedema but not a lesion in the left inferior frontal region, three had superior medial lesions and one had a left lateral (but not a LIFG) lesion.
In contrast, our large right frontal patient group showed severe design fluency deficits (free and fixed), compared to left frontal patients. Our severe design fluency deficit in this large right frontal group (n = 22) is a robust and clear finding compared to the hint of a right frontal deficit (or no deficit) in previous studies with small numbers (e.g. n = 9, Jones-Gotman and Milner, 1977; n = 8, Ruff et al., 1994). The most severe design fluency effects were specific to right lateral patients. Right frontal patients were older than left frontal patients. However, this was not so for the considerably smaller right lateral subgroup who were completely intact on one fluency task (phonemic) and grossly impaired on the two design fluency tasks. This strongly suggests that the lateralization effect is unlikely to be due to the age differences. Given this difference in lateralization, it was not surprising that performance on the phonemic word fluency task was not correlated with either design fluency task despite the fact that almost all fluency tasks were positively correlated (Table 4). Our findings support the notion that phonemic word fluency and design fluency are underpinned by different cortical substrates.
Our results allowed us to provide further differentiation within the frontal region regarding the areas implicated in word and design fluency tasks. Our word fluency results precisely replicate the findings reported by Stuss et al. (1998) and Troyer et al. (1998) using the same finer-grained frontal grouping procedure, namely, a severe phonemic fluency deficit following left lateral damage. However, in their studies, left lateral lesions included the left inferior and middle frontal gyri. We documented the most severe phonemic word fluency deficit in patients with LIFG lesions.
Our right frontal design fluency deficit provides evidence for both the sensitivity and specificity of this task given that our posterior group was unimpaired. Moreover, our right lateral design fluency deficit provides evidence for a smaller critical region than that available in previous reports that only identify the right frontal region as a whole as relevant (Jones-Gotman and Milner, 1977).
In contrast to phonemic word and design fluency, semantic word fluency lacks utility for identifying critical lesions as we found equivalent deficits in all patients (left and right frontal, posterior). It is noteworthy that phonemic fluency is the most useful clinical test for detecting left frontal damage due to both its sensitivity and specificity to frontal damage and speed of administration (i.e. 1 min. cf. design fluency 4–5 min).
Ideational and gesture fluency are sensitive to frontal lobe lesions. However, we did not find specificity for these tasks and our finer-grained analysis did not reveal further anatomical differentiation within the frontal region. A possible explanation for the lack of specificity lies in the processing requirements of the two tasks, namely, imagining the use of objects (ideational fluency) and the execution of actions (gesture fluency). Although both tasks draw on semantic representations, they may also recruit other action-related representations that involve activation of a somewhat different basic network (i.e. fronto-parietal network; Fridman et al., 2006). Thus, ideational and gesture fluency tasks may then contrast with phonemic fluency in relying on less specific basic networks. It is of interest that both these fluency tasks, like all the other word and design fluency tasks, involve a superior medial deficit. For gesture fluency, the superior medial deficit we observed is more specific than the associated region described in the only previous report documenting frontal impairment (left frontal and right frontal; Jason, 1985). Therefore, our results suggest that a smaller region may be critically involved in gesture fluency.
Theoretical issues
We will consider evidence on the functional specificity of different prefrontal cortex regions. The most basic question is whether a general cognitive process, such as fluid intelligence, underpins generation on all fluency tasks or whether there are a number of sub-processes that implement the different fluency tasks. In support of the first notion, Roca et al. (2010) found that their left frontal patients’ word fluency deficit could be explained by their fluid intelligence performance. Moreover, for several ‘executive’ tasks including phonemic word fluency, Roca et al. (2010) found no specific association with particular regions of prefrontal damage. For some of the fluency tasks we used, an explanation along the lines that Roca et al. (2010) propose is plausible. Thus, the ideational and gesture fluency tasks do not show any specific localization within the frontal lobes. All four of these tasks correlate significantly, in most cases very strongly with each other, and, with one exception, with Raven’s Advanced Progressive Matrices (our fluid intelligence measure).
In our study, however, this is not the case for phonemic word fluency. Our findings are in striking contrast to those of Roca et al. (2010). Specifically, we did not find a significant correlation between performance for phonemic word fluency and our fluid intelligence measure. Moreover, we documented a frontal deficit for phonemic word fluency that was most severe in the LIFG patients. In these patients, phonemic word fluency performance, unlike that for semantic word fluency, was reduced regardless of their performance on the Advanced Progressive Matrices (Fig. 2). It is noteworthy that our right lateral patients were unimpaired on the phonemic word fluency task. Indeed, our LIFG finding may account for the difference with Roca et al.’s (2010) result. In the Roca et al. (2010) study, the frontal group included only two patients with left inferior frontal lesions. Our findings suggest that left inferior frontal damage critically underlies a phonemic word fluency deficit, a position first put forward by Milner (1964) more than 40 years ago.
Our results support the view that there are a number of functionally and anatomically distinct cognitive processes that may be selectively impaired following specific frontal damage. We hypothesize that performance on fluency tasks involves at least three sets of cognitive processes: sustained activation (or energization); selection and creation of novel responses. Fluency tasks have some similar core processes such as sustained activation, reflected in the positive correlation between most fluency tasks.
Energization processes
All fluency tasks require generation of new series of responses, rather than the production of a well-learned sequence, as well as sustained activation. Both processes are held to depend on the process of energization. Shallice et al. (2008) describe the ‘processes of energization (cognitive effort) [as] necessary to activate operations not directly triggered in an overlearned fashion by perceptual and motivational inputs’ (p. 82). Energization processes are thought to be localized in the superior medial region (for review, see Stuss and Alexander, 2007; Shallice et al., 2008; Stuss, 2011). This is consistent with the long held view that this region is critical in conditions such as apathy and akinetic mutism. Interestingly, our study reports for the first time a fluency deficit across all eight verbal and non-verbal fluency tasks in one of the four frontal regions, namely, the superior medial one. Our results are therefore consistent with the suggestion that the superior medial region plays a critical role in energization.
Selection processes
It has been argued that phonemic fluency requires greater selection demands than semantic fluency due to it producing more competition among associated stored words that are inappropriate given the specific task demands (Perret, 1974). In our study, word fluency was indeed more reduced for phonemic than semantic fluency in our LIFG patients, compared to non-left inferior frontal patients. This was supported by a significantly lower word fluency ratio; that is, the performance of left inferior frontal patients was significantly more reduced for phonemic than semantic fluency compared with frontal patients without left inferior frontal damage. Our word fluency findings converge with evidence from dynamic aphasia patients, left inferior frontal patients and neuroimaging studies suggesting that the LIFG plays a critical role in verbal generation when selection demands are high (Thompson-Schill et al., 1997, 1998; Robinson et al., 1998, 2005, 2010; Schnur et al., 2009).
Creation of novel responses
Design fluency, ideational fluency (novel uses) and gesture fluency (meaningless gestures) tasks place a very high demand on novelty in order to create new responses (for similar discussion, see Turner, 1999). We suggest that these fluency tasks with the greatest novelty component activate fewer previously existing responses. This results in little competition and a lower need for selection. Indeed, the processes of creating new responses and selection perhaps show an inverse relationship. In this context, we note that the LIFG, thought to be involved in selection, plays no specific role in these generation tasks requiring the creation of novel responses. Indeed, our findings do not point to any specific region of the prefrontal cortex as being most critically involved in these processes. This is compatible with Roca et al.’s (2010) general position we discussed above.
Conclusion
Our findings suggest the frontal lobes are critical for generation, as measured by fluency tasks. In particular, we provide clear evidence for the sensitivity of verbal and non-verbal fluency tasks to frontal lobe damage and for the specificity of the phonemic word and design fluency tasks to this region. There is a degree of lateralization within the frontal cortex for verbal and non-verbal material. Thus, we replicate word fluency effects established by a finer-grained procedure for grouping frontal patients and we extend this by applying the procedure to a range of verbal and non-verbal fluency tasks in a large sample. Moreover, our findings do not support the view proposing that a general, common frontal process underpins performance on all fluency tasks. On the contrary, our findings suggest the LIFG plays a crucial role in selection and the superior medial region plays a role in energization.
Funding
G.R. is supported by an Australian Research Council Discovery Early Career Researcher Award (DE120101119), the Australian National Stroke Foundation (small project grant) and the NEWRO Foundation. L.C. and T.S. hold a Welcome Trust Grant: 089231/A/09/Z.
Supplementary material
Supplementary material is available at Brain online.
Glossary
Abbreviations
- LIFG
left inferior frontal gyrus
- APM
advanced progressive matrices
References
- Alvarez JA, Emory E. Executive function and the frontal lobes: a meta-analytic review. Neuropsychol Rev. 2006;16:17–42. doi: 10.1007/s11065-006-9002-x. [DOI] [PubMed] [Google Scholar]
- Baldo JV, Shimamura AP. Letter and category fluency in patients with frontal lobe lesions. Neuropsychology. 1998;12:259–67. doi: 10.1037//0894-4105.12.2.259. [DOI] [PubMed] [Google Scholar]
- Baldo JV, Shimamura AP, Delis DC, Kramer J, Kaplan E. Verbal and design fluency in patients with frontal lobe lesions. J Int Neuropsychol Soc. 2001;7:586–96. doi: 10.1017/s1355617701755063. [DOI] [PubMed] [Google Scholar]
- Baldo JV, Schwartz S, Wilkins D, Dronkers NF. Role of frontal versus temporal Cortex in verbal fluency as revealed by voxel-based lesion symptom mapping. J Int Neuropsychol Soc. 2006;12(6):896–900. doi: 10.1017/S1355617706061078. [DOI] [PubMed] [Google Scholar]
- Benton AL. Differential behavioural effects in frontal lobe disease. Neuropsychologia. 1968;6:53–60. [Google Scholar]
- Burgess P, Shallice T. Bury St Edmunds, UK: Thames Valley Test Company; 1997. The Hayling and Brixton tests: test manual. [Google Scholar]
- Coslett HB, Bowers D, Verfaellie M, Heilman KM. Frontal verbal amnesia: phonological amnesia. Arch Neurol. 1991;48:949–55. doi: 10.1001/archneur.1991.00530210075027. [DOI] [PubMed] [Google Scholar]
- Deiber MP, Passingham RE, Colebatch JG, Friston KJ, Nixon PD, Frackowiak RS. Cortical areas and the selection of movement: a study with positron emission tomography. Exp Brain Res. 1991;84:393–402. doi: 10.1007/BF00231461. [DOI] [PubMed] [Google Scholar]
- Duncan J, Burgess P, Emslie H. Fluid intelligence after frontal lobe lesions. Neuropsychologia. 1995;33:261–8. doi: 10.1016/0028-3932(94)00124-8. [DOI] [PubMed] [Google Scholar]
- Duvernoy HM. New York: Springer-Verlag; 1991. The human brain: structure, three-dimensional sectional anatomy and MRI. [Google Scholar]
- Elfgren CI, Risberg J. Lateralized frontal blood flow increases during fluency tasks: influence of cognitive strategy. Neuropsychologia. 1998;36:505–12. doi: 10.1016/s0028-3932(97)00146-2. [DOI] [PubMed] [Google Scholar]
- Eslinger PJ, Grattan LM. Frontal lobe and frontal-striatal substrates for different forms of human cognitive flexibility. Neuropsychologia. 1993;31:17–28. doi: 10.1016/0028-3932(93)90077-d. [DOI] [PubMed] [Google Scholar]
- Fridman EA, Immisch I, Hanakawa T, Bohlhalter S, Waldvogel D, Kansaku K, et al. The role of the dorsal stream for gesture production. Neuroimage. 2006;29:417–28. doi: 10.1016/j.neuroimage.2005.07.026. [DOI] [PubMed] [Google Scholar]
- Fuster JM. The prefrontal cortex. 4th edn. London: Academic Press; 2008. [Google Scholar]
- Heim S, Friederici AD, Schiller NO, Ruschemeyer SA, Amunts K. The determiner congruency effect in language production investigated with functional MRI. Brain Mapp. 2009;30:928–40. doi: 10.1002/hbm.20556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henry JD, Crawford JR. A meta-analytic review of verbal fluency performance following focal cortical lesions. Neuropsychology. 2004;18:284–95. doi: 10.1037/0894-4105.18.2.284. [DOI] [PubMed] [Google Scholar]
- Hirshorn EA, Thompson-Schill SL. Role of the left inferior frontal gyrus in covert word retrieval: neural correlates of switching during verbal fluency. Neuropsychologia. 2006;44:2547–57. doi: 10.1016/j.neuropsychologia.2006.03.035. [DOI] [PubMed] [Google Scholar]
- Hodges JR, Salmon DP, Butters N. Differential impairment of semantic and episodic memory in Alzheimer’s and Huntington’s diseases: a controlled prospective study. J Neurol Neurosurg Psychiatry. 1990;53:1089–95. doi: 10.1136/jnnp.53.12.1089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hodges JR, Patterson K, Oxbury S, Funnell E. Semantic dementia. Progressive fluent aphasia with temporal lobe atrophy. Brain. 1992;115(6):1783–1806. doi: 10.1093/brain/115.6.1783. [DOI] [PubMed] [Google Scholar]
- Jason GW. Gesture fluency after focal cortical lesions. Neuropsychologia. 1985;23:463–81. doi: 10.1016/0028-3932(85)90002-8. [DOI] [PubMed] [Google Scholar]
- Jones-Gotman M, Milner B. Design fluency: the invention of nonsense drawings after focal cortical lesions. Neuropsychologia. 1977;15:653–74. doi: 10.1016/0028-3932(77)90070-7. [DOI] [PubMed] [Google Scholar]
- Lezak MD, Howieson DB, Loring DW. Neuropsychological assessment. 4th edn. Oxford: Oxford University Press; 2004. [Google Scholar]
- Loring DW, Meador KJ, Lee GP. Effects of temporal lobectomy on generative fluency and other language functions. Arch Clin Neuropsychol. 1994;9:229–38. [PubMed] [Google Scholar]
- Martin RC, Loring DW, Meador KJ, Lee GP. The effects of lateralized temporal lobe dysfunction on formal and semantic word fluency. Neuropsychologia. 1990;28:823–9. doi: 10.1016/0028-3932(90)90006-a. [DOI] [PubMed] [Google Scholar]
- McKenna P, Warrington EK. Testing for nominal dysphasia. J Neurol Neurosurg Psychiatry. 1980;43:781–8. doi: 10.1136/jnnp.43.9.781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miceli G, Caltagirone C, Gainotti G, Masullo C, Silveri MC. Neuropsychological correlates of localized cerebral lesions in non-aphasic brain-damaged patients. J Clin Neuropsychol. 1981;3:53–63. doi: 10.1080/01688638108403113. [DOI] [PubMed] [Google Scholar]
- Milner B. Some effects of frontal lobectomay in man. In: Warren JM, Akert K, editors. The frontal granular cortex and behaviour. New York: McGraw-Hill; 1964. pp. 313–34. [Google Scholar]
- Nelson HE, Willison JR. Windsor, UK: NFER-Nelson; 1991. The revised national adult reading test—test manual. [Google Scholar]
- Newcombe F. London: Oxford University Press; 1969. Missile wounds of the brain. [Google Scholar]
- Pendleton MG, Heaton RK, Lehman RA, Hulihan D. Diagnostic utility of the Thurstone Word Fluency Test in neuropsychological evaluations. J Clin Neuropsychol. 1982;4:307–17. doi: 10.1080/01688638208401139. [DOI] [PubMed] [Google Scholar]
- Perret E. The left frontal lobe of man and the suppression of habitual responses in verbal categorical behaviour. Neuropsychologia. 1974;12:323–30. doi: 10.1016/0028-3932(74)90047-5. [DOI] [PubMed] [Google Scholar]
- Picton TW, Stuss DT, Shallice T, Alexander MP, Gillingham S. Keeping time: effects of focal frontal lesions. Neuropsychologia. 2006;44:1195–209. doi: 10.1016/j.neuropsychologia.2005.10.002. [DOI] [PubMed] [Google Scholar]
- Price CJ. The anatomy of language: a review of 100 fMRI studies published in 2009. Ann NY Acad Sci. 2010;1191:62–88. doi: 10.1111/j.1749-6632.2010.05444.x. [DOI] [PubMed] [Google Scholar]
- Raven JC. Oxford, UK: Oxford Psychologists Press; 1976. Advanced progressive matrices, Set 1. [Google Scholar]
- Reverberi C, Laiacona M, Capitani E. Qualitative features of semantic fluency performance in mesial and lateral frontal patients. Neuropsychologia. 2006;44:469–78. doi: 10.1016/j.neuropsychologia.2005.05.011. [DOI] [PubMed] [Google Scholar]
- Robinson G, Blair J, Cipolotti L. Dynamic aphasia: an inability to select between competing verbal responses? Brain. 1998;121:77–89. doi: 10.1093/brain/121.1.77. [DOI] [PubMed] [Google Scholar]
- Robinson G, Shallice T, Cipolotti L. A failure of high level verbal response selection in progressive dynamic aphasia. Cognitive Neuropsychol. 2005;22(6):661–94. doi: 10.1080/02643290442000239. [DOI] [PubMed] [Google Scholar]
- Robinson G, Shallice T, Bozzali M, Cipolotti L. Conceptual proposition selection and the LIFG: neuropsychological evidence from a focal frontal group. Neuropsychologia. 2010;48:1652–63. doi: 10.1016/j.neuropsychologia.2010.02.010. [DOI] [PubMed] [Google Scholar]
- Roca M, Parr A, Thompson R, Woolgar A, Torralva T, Antoun N, et al. Executive function and fluid intelligence after frontal lobe lesions. Brain. 2010;133:234–47. doi: 10.1093/brain/awp269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rogers RD, Sahakian BJ, Hodges JR, Polkey CE, Kennard C, Robbins TW. Dissociating executive mechanisms of task control following frontal lobe damage and Parkinson’s disease. Brain. 1998;121:815–42. doi: 10.1093/brain/121.5.815. [DOI] [PubMed] [Google Scholar]
- Ruff RM, Allen CC, Farrow CE, Niemann H, Wylie T. Figural fluency: differential impairment in patients with left versus right frontal lobe lesions. Arch Clin Neuropsychol. 1994;9:41–55. [PubMed] [Google Scholar]
- Schnur TT, Schwartz MF, Kimberg DY, Hirshorn EA, Coslett HB, Thompson-Schill SL. Localizing interference during naming: convergent neuroimaging and neuropsychological evidence for the function of Broca’s area. Proc Natl Acad Sci USA. 2009;106:322–7. doi: 10.1073/pnas.0805874106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwartz S, Baldo J. Distinct patterns of word retrieval in right and left frontal lobe patients: a multidimensional perspective. Neuropsychologia. 2001;39:1209–17. doi: 10.1016/s0028-3932(01)00053-7. [DOI] [PubMed] [Google Scholar]
- Shallice T, Stuss DT, Picton TW, Alexander MP, Gillingham S. Mapping task switching in frontal cortex through neuropsychological group studies. Frontiers Neurosci. 2008;2:79–85. doi: 10.3389/neuro.01.013.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stuss DT. Functions of the frontal lobes: relation to executive functions. J Int Neuropsychol Soc. 2011;17:759–65. doi: 10.1017/S1355617711000695. [DOI] [PubMed] [Google Scholar]
- Stuss DT, Alexander MP, Hamer L, Palumbo C, Dempster R, Binns M, et al. The effects of focal anterior and posterior brain lesions on verbal fluency. J Int Neuropsychol Soc. 1998;4:265–78. [PubMed] [Google Scholar]
- Stuss DT, Alexander MP. Is there a dysexecutive syndrome? Philos Trans R Soc Lond B Biol Sci. 2007;362:901–15. doi: 10.1098/rstb.2007.2096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson-Schill SL, D'Esposito M, Aguirre GK, Farah MJ. Role of left inferior prefrontal cortex in retrieval of semantic knowledge: a reevaluation. Proc Natl Acad Sci USA. 1997;94:14792–7. doi: 10.1073/pnas.94.26.14792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson-Schill SL, Swick D, Farah MJ, D'Esposito M, Kan IP, Knight RT. Verb generation in patients with focal frontal lesions: a neuropsychological test of neuroimaging findings. Proc Natl Acad Sci USA. 1998;95:15855–60. doi: 10.1073/pnas.95.26.15855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Troyer AK, Moscovitch M, Winocur G, Alexander MP, Stuss D. Clustering and switching on verbal fluency: the effects of focal frontal- and temporal-lobe lesions. Neuropsychologia. 1998;36:499–504. doi: 10.1016/s0028-3932(97)00152-8. [DOI] [PubMed] [Google Scholar]
- Tucha OW, Smely CW, Lange KW. Verbal and figural fluency in patients with mass lesions of the left or right frontal lobes. J Clin Exp Neuropsychol. 1999;21:229–36. doi: 10.1076/jcen.21.2.229.928. [DOI] [PubMed] [Google Scholar]
- Turner MA. Generating novel ideas: fluency performance in high-functioning and learning disabled individuals with autism. J Child Psychol Psychiatry. 1999;40:189–201. [PubMed] [Google Scholar]
- Turner MS, Cipolotti L, Yousry T, Shallice T. Qualitatively different memory impairments across frontal lobe subgroups. Neuropsychologia. 2007;45:1540–52. doi: 10.1016/j.neuropsychologia.2006.11.013. [DOI] [PubMed] [Google Scholar]
- Vilkki J, Holst P. Speed and flexibility on word fluency tasks after focal brain lesions. Neuropsychologia. 1994;32:1257–62. doi: 10.1016/0028-3932(94)90107-4. [DOI] [PubMed] [Google Scholar]
- Warrington EK. Windsor, UK: NFER-Nelson; 1984. Recognition memory test. [Google Scholar]
- Warrington EK, James M. Bury St. Edmunds, UK: Thames Valley Test Company; 1991. The visual object and space perception battery. [Google Scholar]
- Warrington EK, McKenna P, Orpwood L. Single word comprehension: a concrete and abstract word synonym test. Neuropsychol Rehabil. 1998;8:143–54. [Google Scholar]
- Watkins OC, Watkins MJ. Build-up of proactive inhibition as a cue-overload effect. J Exp Psychol Hum Learn Mem. 1975;104:442–52. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.