Abstract
Heparan sulfate proteoglycans are abundant matrix and membrane molecules. Smooth muscle specific deletion of one Heparan sulfate biosynthetic enzyme, N-deacetylase-N-sulfotransferase1 leads to decreased vascular smooth muscle cell proliferation, and vascular wall thickness. We hypothesized that this may lead to changes in blood pressure in conscious mice. Blood pressure was measured via telemetry in SM22αCre+Ndst1−/−(n=4) and wild type (n=8) mice. Aorta and thoracodorsal artery luminal area is significantly smaller in SM22αCre+Ndst1−/− (n=4–8, P=0.02, P=0.0002) compared to wild type (n=7) mice. Diurnal differences were observed in both cohorts for systolic, diastolic, mean arterial blood pressure, and heart rate (P<0.001 from T-Test). No significant differences were found in the above parameters between the cohorts in either light or dark times using a linear mixed model. In conclusion, deletion of N-deacetylase-N-sulfotransferase1 in smooth muscle did not influence any of the blood pressure parameters measured despite significant decrease in aorta and thoracodorsal artery luminal area.
Keywords: blood pressure, Ndst1, vascular, VSMC, Heparan Sulfate Proteoglycan
Introduction
Heparan sulfate proteoglycans (HSPG) are highly abundant molecules on the cell membrane as well as in the extracellular matrix [1]. HSPG molecules have the ability to interact with a variety of ligands, influencing the bioactivities of many substances such as lipids, chemokines and growth factors [2–3]. HSPGs regulate key steps in vasculogenesis [4] and angiogenesis through interactions with vascular endothelial growth factor A (VEGF A), transforming growth factor beta (TGFβ) or basic fibroblast growth factors (bFGF) [5–7] among others.
HSPGs are composed of long polysaccharide chains attached covalently to a core protein [8]. The synthesis of heparan sulfate chains involves a series of enzymatic reactions. Ndst1 is one of the enzymes that are important in the initial steps of HS synthesis [8–9]. Previously, we have reported that genetic ablation of N-deacetylase-N-sulfotransferase1(Ndst1) in smooth muscle resulted in smaller femoral artery comprised of a thinner vascular wall with significantly fewer vascular smooth muscle cells (VSMC) [10–11]. However, it is not clear how decreased number of vascular smooth muscle cells, and decreased medial wall thickness in macrovessels may contribute to blood pressure. Hence, the rationale of this study. The goal of the present study was to test the hypothesis that alterations in HS fine structure influenced arterial blood pressure in conscious mice.
The major methods of measuring blood pressure and related parameters are tail cuff, implanted fluid catheters, Millar solid state catheters and implanted telemetry systems [12]. The advantages of using the telemetry system over the other methods include long term simultaneous measurements of systolic blood pressure (SBP), diastolic blood pressure (DBP), mean arterial pressure (MAP), pulse pressure (PP) and heart rate (HR) in unrestrained and conscious animals [13 , 14].
Significant interest persists in the ability to deliver small molecules to target HSPG in the settings of atherosclerosis, inflammation, angiogenesis and oncology [15]. Genetic mouse models have provided better understanding of the biological role of HSPG in normal development and in the pathogenesis of diseases [15–22]. Hence, these studies are important for future steps in drug discovery for designing small molecules that target HSPG as therapies to enhance or slow specific cellular processes beneficial to patients in the clinical setting.
Materials and Methods
Generation of Ndst1 deficient mouse models
Ndst1flox/flox mice (gift from Dr. Esko, [23] were mated with male SM22α-cre mice (gift from Dr. M. Parmacek) [24–25]. F1 SM22αcre+Ndst1wt/flox males were mated with Ndst1flox/flox females to generate mice with smooth muscle specific deletion of Ndst1 (SM22αcre+Ndst1−/−). All the mice including the control mice used for this study were of the C57BL6 strain. The genotype of wild type (WT) control mice was Cre−Ndst1wt. The following primers were used to genotype the Ndst1flox, Ndst1wt and Ndst1−/− alleles: Ndst1 (1-10R) 5’ccagggcgtcagggcctcctg-3’, Ndst1 (1-16R) 5’-catcctctgaggtgaccgc3’, Ndst1 (1-17F) 5’-tcccacatggcgagactgaggttc-3’ [26]. Ndst1wt and Ndst1flox alleles were identified by primer pair 1-10R and 1-17F while Ndst1−/− allele was genotyped using 1-16R and 1-17F. Mice harboring smooth muscle specific deletion of the Ndst1 allele were also genotyped for Cre using primers: 5’ ccaatttactgaccgtacacc-3’; 5’-gtttcactatccaggttacgg-3’}[26].
Telemetry
All surgical procedures were according to the University of Minnesota IACUC policy and best practice for small animal surgery. WT and SM22αCre+Ndst1−/− male mice of 16–18 wk of age were surgically implanted with a telemetric probe and data were collected for 48 hours.
DBP, SBP, MAP, PP and HR were measured in WT (n=8) and SM22αCre+Ndst1−/− (n=4) male mice using implantable telemetric transmitters (DSI, Minneapolis, MN). The transmitters were implanted according to the manufacturer’s instructions. Briefly, telemetric probe was placed in the aortic arch of an anesthetized mouse via a catheter through the left carotid artery. Mice were allowed to recover for seven days before any measurements were made. Post-operative recovery was carefully monitored. Out of fifteen mice, three did not survive the implantation due to post-surgical complications. The mice were then synchronized to a light/dark cycle of 12/12 h with lights on at 5:30AM and data were collected for 48 hours. The location of the catheter tip was verified upon explantation.
Luminal Area Measurements
Thoracic Aortas
Mice were sacrificed using CO2 asphyxia. The thorax was exposed and the aortic arch was located. From the arch, the first 4 mm of the vessel was embedded in paraffin and 7μm sections were cut using a microtome. At least 4 sections from each animal were measured. The sections were then stained with Hematoxylin and Eosin (H&E). Area measurements were done by a blinded observer and pictures were taken using the Zeiss Axio Imager M1 Upright Microscope. All measurements were performed using Axiovision LE 4.7 software. The internal elastic lamina (IEL) was considered as the boundary between the lumen and the muscle layer.
Thoracodorsal Arteries
Mice were sacrificed using CO2 asphyxia, the thoracodorsal artery (TDA) was isolated and placed in Krebs-HEPES containing (in mM) NaCl 118.4, KCl 4.7, MgSO4 1.2, NaHCO3 4, KH2PO4 1.2, CaCl2 2, Hepes 10, glucose 6 and supplemented with 1% BSA. Arteries were free of surrounding tissue, were placed in an arteriograph (Danish MyoTechnology, DMT) where they were cannulated at both ends with glass micropipettes and secured with 10–0 nylon monofilament suture. Arteries were perfused with Krebs-HEPES supplemented with 1% BSA and superfused with a calcium free Krebs-HEPES containing ethylenbis-(oxyethylenenitrolo) tetra-acetic acid (EGTA, 2 mmol/L) and sodium nitroprussiate (10 μmol/L). The arteriograph was placed on an Olympus IX-71 microscope, the TDAs were visualized with a 20× objective and were subjected to a gradient of pressure from 10 to 140 mmHg with a 5 minute stabilization period for each pressure to measure the passive diameter using the Slidebook software. The intraluminal pressure was increased from 10 mmHg to 140 mmHg by steps of 20 mmHg and the lumen diameter was measured in μm using the DMT vessel acquisition suite. The lumen area was calculated in μm2, for each pressure step, using the lumen diameter values according to the formula: lumen area = π × lumen diameter2 / 4.
Statistics
Student T-test: Statistical significance between the two cohorts was done by Student T-test and a P value of less than 0.05 was considered significant.
Linear mixed-effects models were fitted to investigate the SM22αCre+Ndst1−/− deletion effect on each of the five response variables, SBP, DBP, MAP, PP, and HR. Each of the 5 response variables were measured every 15 seconds for two days. To reduce data dimension, we used 10-minute average values; we checked that the results were not sensitive to this choice. The 3 covariates are: 1) day effects with day=1 or 2, referring to one of the two days; 2) diurnal effects with dark =1 for dark time, =0 for light time, where we considered 5:30am to 5:30pm as light time, and 5:30pm to 5:30am as dark time; 3) SM22αCre+Ndst1−/− deletion effect with group=0 or 1 for the WT or SM22αCre+Ndst1−/− . To account for possible correlations among the repeated blood pressure measurements on the same mouse, we used a random intercept term to represent the mouse-specific effect, and also used a first-order autoregressive, i.e. AR(1), correlation structure for the residual error (i.e. the correlation between any two residual errors on a mouse decreases by ρ times as 1 time lag increases). Specifically, our model on mouse has the following form:
In the model, within a day, b3 detects the possible blood pressure difference of SM22αCre+Ndst1−/− versus WT in light times, and b3 + b4 detects the difference between SM22αCre+Ndst1−/− versus WT in dark times.
Results
Body weight was significantly different between WT (27.37 ± 0.60 g) and SM22αCre+Ndst1−/− (24.82 ± 0.12 g*, *P=0.005). Area measurements revealed that SM22αcre+Ndst1−/− have a significantly smaller luminal area in both thoracic aorta (P=0.02) and thoracodorsal artery (P=0.0002) (Table 1).
Table 1.
Luminal area of WT and SM22αCre+Ndst1−/− on thoracic aorta and thoracodorsal artery (TDA).
| Genotypes | Luminal Area (μm2) | |
|---|---|---|
| Aorta | TDA | |
| WT | 199507±19952 (n=7) | 16211±1060 (n=7) |
| SM22αCre+Ndst1−|− | 125382±14160 (n=4) | 11056±423 (n=8) |
| P Value | 0.02 | 0.0002 |
Vales are mean±SE. SM22αCre+Ndst1−/− luminal area on aorta and TDA are both significantly smaller compared to WT.
Using student’s T-test, SBP (P<0.001), DBP (P<0.001), MAP (P<0.001) and HR(P<0.001) were significantly different between dark and light times in both the cohorts (Table 2).
Table 2.
Pressure, pulse pressure and heart rate were measured in WT and SM22aCre+ Ndst1−1− using Telemetry.
| Parameters | WT (n=8) | SM22aCre+ Ndst1−1− (n=4) | ||||
|---|---|---|---|---|---|---|
| Light (12h) | Dark(12h) | P-value | Light(12h) | Dark(12h) | P-value | |
| SBP | 115±3.5 | 128±2.5 | <.001 | 114±3.4 | 126±3.7 | <.001 |
| DBP | 92±1.8 | 103±2.4 | <.001 | 89±4.6 | 99±5.1 | <.001 |
| MAP | 104±2.6 | 116±2.1 | <.001 | 102±3.6 | 113±4.1 | <.001 |
| PP | 23±2.4 | 24±2.5 | 0.200 | 25±3.5 | 27±3.6 | 0.284 |
| HR | 544±18.1 | 594±17.2 | <.001 | 559±11.0 | 603±14.7 | <.001 |
Values presented are mean ± SE. P<0.001 (as assessed by student’s T-Test) on SBP,DBP,MAP,and HR (between Light [5:30 am to 5:30 pm] and Dark times [5:30 pm to 5:30 am]).
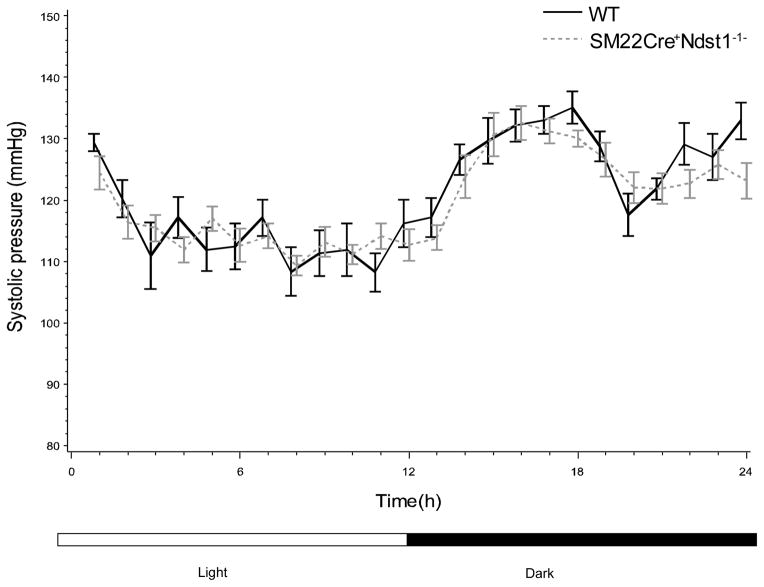
Using a mixed-random linear intercept model, and adjusting for light and dark times, we did not observe any significant SM22αCre+Ndst1−/− deletion effect in all five parameters measured (Figures 1 and 2).
Figure 1.
Systolic pressure in WT (n=8) and SM22αCre+Ndst1−/− (n=4) was measured using Telemetry. Light period was set from 5:30 AM to 5:30PM while dark period was set from 5:30PM to 5:30AM. SBP was not significantly different between the cohorts as assessed by the linear intercept model.
Figure 2.
Diastolic pressure in WT (n=8) and SM22αCre+Ndst1−/− (n=4) was measured using telemetry. Light period was set from 5:30 AM to 5:30PM while dark period was set from 5:30PM to 5:30AM. DBP was not significantly different between the cohorts as assessed by the linear intercept model.
Discussion
Increased blood pressure is an established risk factor for cardiovascular diseases [27–28]. Previous reports from our laboratory demonstrated that deletion of Ndst1, a HSPG synthetic enzyme, results in a reduction in vascular wall area and a decreased number of VSMC [10]. In this study, we hypothesized that the smaller vessels in SM22αCre+Ndst1−/− mice would alter blood pressure. Telemetry, an established method for measuring blood pressure in conscious and unrestrained mice [12] was employed to test this hypothesis.
We have previously shown that the SM22αCre+Ndst1−/− mice exhibited a significant decrease in transcript abundance of Ndst1 in smooth muscle cells [10]. In parallel, we demonstrated a significant loss of N- and 2-O- sulfated disaccharides as assessed by HPLC [10].
Our results indicate an increase in SBP, DBP MAP, and HR during dark times in both cohorts. This is in concordance with previous studies [10, 29]. The diurnal difference that we observed indicates and further confirms that telemetry is a reliable method for discerning blood pressure and related parameters in mice.
Our results suggest that smooth muscle specific deletion of Ndst1 decreases luminal area in both small vessel, (TDA) and conduit or large vessels (aorta). However, there were no significant differences in any of the five parameters (SBP, DBP, PP, MAP, and HR) between SM22αCre+Ndst1−/− and WT in either dark or light times. This is in concordance with a previous report from our laboratory which indicated that left ventricular end-diastolic pressure and ejection fraction did not differ in anaesthetized WT or SM22αCre+Ndst1−/− mice [10]. This data indicates that although deletion of Ndst1 in smooth muscle of mice results to fewer VSMC’s and a smaller luminal area, blood pressure still remains unchanged.
A previous study in mice lacking Ndst1, Ndst3 or both, indicates that these two enzymes probably are regulating a common pathway [30]. Deletion of both enzymes show a more extreme modification in HS sulfation compared to a deletion of either one although deletion of either enzyme still results in modified HS chains [30]. Previous work from our lab has shown that Ndst3 expression in VSMC is undetectable and is unchanged in response to deletion of Ndst1[10] . Thus it seems unlikely that Ndst3 is compensating for the loss of Ndst1 in SM22αCre+Ndst1−/− .
It is possible that the elasticity/compliance properties of the vessels have been altered to compensate and restore blood pressure in these mice. Studies are underway in our laboratory to test compliance in both small and large vessels. It is also possible that circulating neurohormones or growth factors that affect blood pressure are altered in this murine model. In conjunction, because HSPG serve as co-receptors to a variety of hormones and growth factors, the activities of the receptors that these compounds bind to could also have been altered in this murine model.
In summary, this study suggests that deletion of Ndst1 in smooth muscle does not affect SBP, DBP or HR.
Significant interest lies in the ability to detect genetic mutations that may deem an individual or a population pre-disposed for arterial stiffness, hypertension and other cardiovascular diseases. As such, studies like ours are essential in our understanding of genes, mutations and their influences in cardiovascular health.
References
- 1.Esko JD, Selleck SB. Order out of chaos: assembly of ligand binding sites in heparan sulfate. Ann Rev Biochem. 2002;71:435–71. doi: 10.1146/annurev.biochem.71.110601.135458. [DOI] [PubMed] [Google Scholar]
- 2.Bishop JR, Schuksz M, Esko JD. Heparan sulphate proteoglycans fine-tune mammalian physiology. Nature. 2007;446(7139):1030–7. doi: 10.1038/nature05817. [DOI] [PubMed] [Google Scholar]
- 3.Bernfield M, Gotte M, Park PW, Reizes O, Fitzgerald ML, Lincecum J, Zako M. Functions of cell surface heparan sulfate proteoglycans. Annu Rev Biochem. 1999;68:729–77. doi: 10.1146/annurev.biochem.68.1.729. [DOI] [PubMed] [Google Scholar]
- 4.Olsson A-K, Dimberg A, Kreuger J, Claesson-Welsh L. VEGF receptor signalling ? in control of vascular function. Nat Rev Mol Cell Biol. 2006;7(5):359–371. doi: 10.1038/nrm1911. [DOI] [PubMed] [Google Scholar]
- 5.Iozzo RV, San Antonio JD. Heparan sulfate proteoglycans: heavy hitters in the angiogenesis arena. The Journal of Clinical Investigation. 2001;108(3):349–355. doi: 10.1172/JCI13738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Jakobsson L, Kreuger J, Holmborn K, Lundin L, Eriksson I, Kjellen L, Claesson-Welsh L. Heparan sulfate in trans potentiates VEGFR-mediated angiogenesis. Dev Cell. 2006;10(5):625–34. doi: 10.1016/j.devcel.2006.03.009. [DOI] [PubMed] [Google Scholar]
- 7.Kirn-Safran CB, D'Souza SS, Carson DD. Heparan sulfate proteoglycans and their binding proteins in embryo implantation and placentation. Semin Cell Dev Biol. 2008;19(2):187–93. doi: 10.1016/j.semcdb.2007.07.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kirkpatrick CA, Selleck SB. Heparan sulfate proteoglycans at a glance. J Cell Sci. 2007;120(Pt 11):1829–32. doi: 10.1242/jcs.03432. [DOI] [PubMed] [Google Scholar]
- 9.Rudd TR, Yates EA. A highly efficient tree structure for the biosynthesis of heparan sulfate accounts for the commonly observed disaccharides and suggests a mechanism for domain synthesis. Molecular BioSystems. 2012 doi: 10.1039/c2mb25019e. [DOI] [PubMed] [Google Scholar]
- 10.Adhikari N, Basi DL, Townsend D, Rusch M, Mariash A, Mullegama S, Watson A, Larson J, Tan S, Lerman B, Esko JD, Selleck SB, Hall JL. Heparan sulfate Ndst1 regulates vascular smooth muscle cell proliferation, vessel size and vascular remodeling. Journal of Molecular and Cellular Cardiology. 2010;49(2):287–293. doi: 10.1016/j.yjmcc.2010.02.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Adhikari N, Rusch M, Mariash A, Li Q, Selleck SB, Hall JL. Alterations in Heparan Sulfate in the Vessel in Response to Vascular Injury in the Mouse. J Cardiovasc Transl Res. 2008;1(3):236–240. doi: 10.1007/s12265-008-9047-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zhao X, Ho D, Gao S, Hong C, Vatner DE, Vatner SF. Current Protocols in Mouse Biology. John Wiley & Sons, Inc; 2011. Arterial Pressure Monitoring in Mice. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Desjardins F, Lobysheva I, Pelat M, Gallez B, Feron O, Dessy C, Balligand J-L. Control of blood pressure variability in caveolin-1-deficient mice: role of nitric oxide identified in vivo through spectral analysis. Cardiovascular Research. 2008;79(3):527–536. doi: 10.1093/cvr/cvn080. [DOI] [PubMed] [Google Scholar]
- 14.Kramer K, Kinter LB. Evaluation and applications of radiotelemetry in small laboratory animals. Physiol Genomics. 2003;13(3):197–205. doi: 10.1152/physiolgenomics.00164.2002. [DOI] [PubMed] [Google Scholar]
- 15.Fan G, Xiao L, Cheng L, Wang X, Sun B, Hu G. Targeted disruption of NDST-1 gene leads to pulmonary hypoplasia and neonatal respiratory distress in mice. FEBS Lett. 2000;467:7–11. doi: 10.1016/s0014-5793(00)01111-x. [DOI] [PubMed] [Google Scholar]
- 16.Ringvall M, Ledin J, Holmborn K, van Kuppevelt T, Ellin F, Eriksson I, Olofsson AM, Kjellen L, Forsberg E. Defective heparan sulfate biosynthesis and neonatal lethality in mice lacking N-deacetylase/N-sulfotransferase-1. J Biol Chem. 2002;75:25926–30. doi: 10.1074/jbc.C000359200. [DOI] [PubMed] [Google Scholar]
- 17.Forsberg E, Kjellen L. Heparan sulfate: lessons from knockout mice. J Clin Invest. 2001;108(2):175–80. doi: 10.1172/JCI13561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Francis DJ, Parish CR, McGarry M, Santiago FS, Lowe HC, Brown KJ, Bingley JA, Hayward IP, Cowden WB, Campbell JH, Campbell GR, Chesterman CN, Khachigian LM. Blockade of vascular smooth muscle cell proliferation and intimal thickening after balloon injury by the sulfated oligosaccharide PI-88: phosphomannopentaose sulfate directly binds FGF-2, blocks cellular signaling, and inhibits proliferation. 2003;92(8):e70. doi: 10.1161/01.RES.0000071345.76095.07. [DOI] [PubMed] [Google Scholar]
- 19.Grobe K, Inatani M, Pallerla SR, Castagnola J, Yamaguchi Y, Esko JD. Cerebral hypoplasia and craniofacial defects in mice lacking heparan sulfate Ndst1 gene function. Development. 2005;132:3777–86. doi: 10.1242/dev.01935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kinnunen T, Huang Z, Townsend J, Gatdula MM, Brown JR, Esko JD, Turnbull JE. Heparan 2-O-sulfotransferase, hst-2, is essential for normal cell migration in Caenorhabditis elegans. Proc Natl Acad Sci U S A. 2005;102(5):1507–12. doi: 10.1073/pnas.0401591102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Pan Y, Woodbury A, Esko JD, Grobe K, Zhang X. Heparan sulfate biosynthetic gene Ndst1 is required for FGF signaling in early lens development. Development. 2006;133(24):4933–44. doi: 10.1242/dev.02679. [DOI] [PubMed] [Google Scholar]
- 22.Pallerla SR, Pan Y, Zhang X, Esko JD, Grobe K. Heparan sulfate Ndst1 gene function variably regulates multiple signaling pathways during mouse development. Dev Dyn. 2007;236(2):556–63. doi: 10.1002/dvdy.21038. [DOI] [PubMed] [Google Scholar]
- 23.Wang L, Fuster M, Sriramarao P, Esko JD. Endothelial heparan sulfate deficiency impairs L-selectin- and chemokine-mediated neutrophil trafficking during inflammatory responses. Nat Immunol. 2005;6(9):902–10. doi: 10.1038/ni1233. [DOI] [PubMed] [Google Scholar]
- 24.Lepore JJ, Cheng L, Min Lu M, Mericko PA, Morrisey EE, Parmacek MS. High-efficiency somatic mutagenesis in smooth muscle cells and cardiac myocytes in SM22alpha-Cre transgenic mice. Genesis. 2005;41(4):179–84. doi: 10.1002/gene.20112. [DOI] [PubMed] [Google Scholar]
- 25.Frutkin AD, Shi H, Otsuka G, Leveen P, Karlsson S, Dichek DA. A critical developmental role for tgfbr2 in myogenic cell lineages is revealed in mice expressing SM22-Cre, not SMMHC-Cre. J Mol Cell Cardiol. 2006;41(4):724–31. doi: 10.1016/j.yjmcc.2006.06.067. [DOI] [PubMed] [Google Scholar]
- 26.Grobe K, Inatani M, Pallerla SR, Castagnola J, Yamaguchi Y, Esko JD. Cerebral hypoplasia and craniofacial defects in mice lacking heparan sulfate Ndst1 gene function. Development. 2005;132(16):3777–86. doi: 10.1242/dev.01935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Franklin SS, Larson MG, Khan SA, Wong ND, Leip EP, Kannel WB, Levy D. Does the Relation of Blood Pressure to Coronary Heart Disease Risk Change With Aging? : The Framingham Heart Study. Circulation. 2001;103(9):1245–1249. doi: 10.1161/01.cir.103.9.1245. [DOI] [PubMed] [Google Scholar]
- 28.Gillum RF. The association of body fat distribution with hypertension, hypertensive heart disease, coronary heart disease, diabetes and cardiovascular risk factors in men and women aged 18–79 years. Journal of chronic diseases. 1987;40(5):421–8. doi: 10.1016/0021-9681(87)90175-5. [DOI] [PubMed] [Google Scholar]
- 29.Levy O, Dayan T, Kronfeld-Schor N. The Relationship between the Golden Spiny Mouse Circadian System and Its Diurnal Activity: An Experimental Field Enclosures and Laboratory Study. Chronobiology International. 2007;24(4):599–613. doi: 10.1080/07420520701534640. [DOI] [PubMed] [Google Scholar]
- 30.Pallerla SR, Lawrence R, Lewejohann L, Pan Y, Fischer T, Schlomann U, Zhang X, Esko JD, Grobe K. Altered heparan sulfate structure in mice with deleted NDST3 gene function. J Biol Chem. 2008;283(24):16885–94. doi: 10.1074/jbc.M709774200. [DOI] [PMC free article] [PubMed] [Google Scholar]